1. Introduction
Xaa-Pro dipeptidase (XPD, EC 3.4.13.9), also called prolidase, proline dipeptidase, peptidase D (PEPD), imidodipeptidase or organophosphorus anhydrase (OPAA), is the only enzyme catalyzing the hydrolysis of iminopeptide bond in a trans Xaa-Pro dipeptide (Xaa represents any amino acid except proline) and belongs to subfamily M24B within clan MG of metallopeptidases in the version 12.5 MEROPS database (
https://www.ebi.ac.uk/merops/) [
1]. XPDs are found in all living organisms and have been isolated mainly from mammalian tissues, fungi, archaea and eubacteria [
2,
3]. Although the physiological roles of prolidases in bacteria and archaea remain unclear, they are thought to act along with other endo- and/or exopeptidases in proline recycling and antitoxin defense [
4,
5,
6]. In mammals, the physiological roles of XPDs are well established in different patho-physiological conditions. Moreover, human prolidases are involved in collagen turnover, matrix remodeling and protein metabolism [
2,
3], and they therefore play vital roles in several physiological processes including inflammation, carcinogenesis, wound healing, angiogenesis and cell proliferation, especially a rare hereditary syndrome called prolidase deficiency [
5,
7].
Xaa-Pro dipeptidases find extensive use in food, environmental protection and medical fields. Because they can hydrolyze proline-containing peptides responsible for bitter flavor in cheese, these enzymes have been used in dairy industry to lower cheese bitterness, thus enhancing its flavor [
8]. Organophosphorus (OP) cholinesterase-inhibiting compounds are constituents of many chemical nerve agents and pesticides. Some XPDs also exhibit OPAA activity and can detoxify these OP compounds by cleaving the P-F and P-O bonds, and this ability makes them serve as biosensors or environmentally friendly decontaminating agents to detect/degrade OP pesticides and chemical warfare nerve agents [
9,
10]. Currently, several approaches, such as immobilization [
11] and protein engineering [
12,
13], have also been adopted to improve the OPAA activities of several XPDs. Directed evolution of PlOPAA led to a 30-fold increase in paraoxon hydrolysis [
12] and 10000-fold increase in methyl-parathion hydrolysis [
13], respectively. In addition, human prolidase can be employed as prodrug therapeutic agents and potential biomarkers for prolidase deficiency [
14], cancer, diabetes, brain diseases, liver diseases, respiratory diseases, and many other disorders [
2,
7].
The fungi are always amongst the best enzyme producers. To date, however, only one fungal Xaa-Pro dipeptidase (GenBank accession No. CAC39600.1) has been molecularly and biochemically characterized from
Aspergillus nidulans [
15].
A. phoenicis is an interesting thermotolerant fungus which grows well at 42-45
oC. Due to its great hydrolytic potential,
A. phoenicis synthesizes many enzymes with various applications in the biotechnological industry, including carbohydrases, proteases, lipases and chitinases [
16,
17], as well as several oxidoreductases, such as laccases [
16], catalases [
18] and glucose dehydrogenases [
19]. In July 2018, the release of the genome sequence of
A. phoenicis ATCC 13157 has opened up opportunities to molecularly and biochemically characterize its proteolytic enzymes for industrial applications and investigate their exact physiological roles. However, only few of these proteases have been biochemically and functionally identified based on latest literature survey.
Examination of the genome sequence of A. phoenicis ATCC 13157 has revealed that it encodes at least one putative prolidase. In the present study, a putative Xaa-Pro dipeptidase gene (GenBank accession No. KZ851879.1) was cloned from A. phoenicis ATCC 14332 and expressed in Pichia pastoris. After partial purification, its biochemical characteristics were systematically investigated. Our results will provide a novel insight into fungal XPDs, and may facilitate further studies on the physiological roles of this prolidase.
2. Materials and Methods
2.1. Chemicals and Reagents
Restriction enzymes, T4 DNA ligase, PyrobestTM DNA polymerase, DNA Fragment Purification Kit, Agarose Gel DNA Extraction Kit, and Plasmid Purification Kit were purchased from TaKaRa Bio Inc. (Dalian, P.R. China). High Pure RNA Isolation Kit and Transcriptor High Fidelity cDNA Synthesis Kit were the products of Roche Molecular Biochemicals (QC, Canada). Tryptone and yeast extract were bought from OXOID (Basingstoke, UK). Casein, and prestained protein marker were supplied by Sigma-Aldrich (Shanghai, P.R. China), and Thermo Fisher Scientific (Shanghai, P.R. China), respectively. Dipeptides, tripeptides and tetrapeptides were purchased from Ontores Biotechnologies (Hangzhou, P.R. China). All other chemicals were commercially available in analytical grade.
2.2. Plasmids, Strains and Growth Conditions
Shuttle vector pPIC9K (Invitrogen; Carlsbad, CA, USA) was used for gene cloning and expression. A. phoenicis ATCC 14332 bought from ATCC (USA) served as a template for apxpd gene cloning. Escherichia coli JM109 and P. pastoris GS115 (Invitrogen) were employed as the hosts for plasmid amplification and gene expression, respectively. A. phoenicis ATCC 14332 and E. coli JM109 were respectively grown in PDA medium (20% (w/v) potato, 2% dextrose, 2% agar) containing 1% (w/v) casein and Luria-Bertani (LB) medium (1% (w/v) tryptone, 0.5% yeast extract, and 1% NaCl). To cultivate P. pastoris GS115 and its recombinants, yeast extract peptone dextrose (YPD) medium, minimal dextrose (MD) medium, the buffered minimal glycerol-complex (BMGY) medium, and the buffered minimal methanol-complex (BMMY) medium were prepared according to the manufacturer’s protocols.
2.3. Gene Cloning and Sequence Analysis
Total RNA of A. phoenicis ATCC 14332 was extracted and reverse transcribed into cDNA by the kits according to manufacturer’s instructions. Using the cDNA obtained as a template, apxpd gene was amplified by PCR with primers ApXPD1 (5’-GTACTGGACAAACAGACTGAGTCTCTCCC-3’) and ApXPD2 (5’-TGCTCTAGACTATGGGTCCCAAGGCCCATAT-3’; the XbaI restriction site is underlined). The PCR products were then cut by XbaI, purified with DNA Fragment Purification Kit, and ligated with SnaBI/AvrII-digested pPIC9K, generating the recombinant plasmid pPIC9K-apxpd. After this plasmid was transformed into E. coli JM109, positive clones were selected on LB plates supplemented with 100 μg/mL ampicillin. The correctness of the inserted sequence was subsequently confirmed by PstI-digested restriction pattern and DNA sequencing (Sangon Biotech Co., Ltd., Shanghai, P.R. China).
Based on the deduced full-length amino acid sequence of ApXPD, its phylogenic relationships with other known Xaa-Pro dipeptidases were investigated by an evolutionary tree which was constructed by program MEGA version X using the Neighbor-joining algorithm [
20]. Bootstrap resampling with 1000 pseudoreplicates was conducted to assess support for each individual branch. Prolidase family was designated by reference to the peptidase deposited in MEROPS database [
1]. The online server SignalP 5.0 (
https://services.healthtech.dtu.dk/service.php?SignalP-5.0) was used to predict the signal peptide cleavage sites of these XPDs. Multiple sequence alignment was performed with Clustal X2 and BioEdit 7.0.9 software packages. The three-dimensional structure of ApXPD was constructed by homology modeling using the Swiss-Model server (
http://swissmodel.expasy.org), with the crystal structure of Xaa-Pro dipeptidase from
Xanthomonas campestris pv.
campestris ATCC 33913 (XcXPD, PDB code: 5fcf) [
4] as a template. The fine structure of XPD was then generated and visualized by software Discovery Studio 3.5 (Accelrys, USA).
2.4. Expression of ApXPD in P. pastoris
After the correct recombinant plasmid pPIC9K-apxpd was linearized by SacI, it was electroporated into P. pastoris GS115 by a Gene Pulser X-cell II electroporation system (Bio-Rad, USA) under conditions of 1.5 kV, 200 Ω, and 25 μF. His+ transformants were selected on MD plates containing 0.5, 1.0, 1.5 and 2.0 mg/mL G418 with the replica plating method, and the genomic DNAs of these His+ transformants were then extracted and analyzed by PCR to confirm gene integration.
A single colony of the His+ transformants exhibiting resistance to 2.0 mg/mL G418 was inoculated into 25 mL BMGY medium and cultivated at 30oC and 200 rpm, until the optical density at 600 nm (OD600) reached 4-6. The growing cells were then harvested by centrifugation (10,000×g, 4oC; 10 min), resuspended in 25 mL BMMY medium to an OD600 of 1.0, and cultured at 30oC and 220 rpm for 120 h. To maintain induction, the cultures were fed with pure methanol to a final concentration of 0.5% (v/v) at 24-h intervals. Induction of GS115-9K cells (P. pastoris GS115 integrated with pPIC9K) was performed in parallel as a negative control. The supernatants were collected by centrifugation at 10,000×g and 4oC for 10 min, and used for the analysis of XPD activity. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was also performed to confirm the expression of ApXPD, using 12% (w/v) polyacrylamide gels (1 mm thickness).
2.5. Enzyme Purification
Unless otherwise stated, all purification procedures are performed at 4oC. The cell-free supernatant was fractionated with solid ammonium sulfate at 30-70% saturation. The pellet collected by centrifugation (10,000×g, 4oC; 20 min) was resuspended in a minimal volume of 20 mM Tris-HCl buffer (pH 8.0)/1 mM cobalt chloride and dialyzed extensively against the same buffer. The dialyzed sample was then concentrated and desalted using a VivaFlow 50 tangential flow filtration module (Vivascience, Sartorius group) with a polyethersulfone membrane (MWCO, 10 kDa). The resulting sample was used for enzyme assay and SDS-PAGE analysis.
2.6. Enzyme and Protein Assays
The ninhydrin method [
21] was slightly modified to determine the amount of proline liberated from the peptide substrates. Briefly, the reaction mixture consisted of 2 mM Lys-Pro or other dipeptides, 20 mM Tris-HCl buffer (pH 8.0) and 1 mM cobalt chloride. After the addition of enzyme solution, the reaction mixture was incubated at 55
oC for 10 min. An aliquot (200 μL) was withdrawn and mixed with 500 μL of glacial acetic acid and 500 μL of ninhydrin reagent (3% (w/v) ninhydrin, 60% (v/v) glacial acetic acid and 40% (v/v) phosphoric acid). After boiling for 10 min, the solution was cooled on ice. The resulting chromophore was quantified using 515 nm absorption. All measurements were performed in triplicate. One unit of Xaa-Pro dipeptidase activity is defined as the amount of enzyme liberating 1 μmol proline per minute under assay conditions. Protein concentrations were measured by the Bradford Protein Assay Kit (Beyotime Biotechnology, Nantong, China), using bovine serum albumin as a standard.
2.7. Biochemical Characterization of Recombinant ApXPD
The optimal temperature of the purified ApXPD was determined in reactions using fresh enzyme at various temperatures (20, 30, 35, 40, 45, 50 55, 60, 65 and 70oC). To determine its thermostability, recombinant ApXPD was pre-incubated in 20 mM Tris-HCl buffer (pH 8.0) containing 1 mM cobalt chloride at 20, 30, 40, 50, 60 or 70oC for 1 h, prior to standard enzyme assay. All determinations were carried out in triplicate, and the results were presented as the percentage of the activity measured compared with the maximum activity.
The optimum pH of recombinant ApXPD was examined using the following buffer solutions in place of the Tris-HCl buffer in the method described above: 20 mM sodium citrate buffer (pH 6.0-7.5), 20 mM Tris-HCl buffer (pH 7.5-9.0) and 20 mM glycine-NaOH buffer (pH 9.0-10.5). The pH stability was determined by pre-incubation of the purified enzyme in the abovementioned buffers at its optimum temperature for 1 h, followed by measurement of the residual activity under standard assay conditions.
To investigate the effects of various metal ions on the activity of recombinant ApXPD, they were added as the salts of chloride to the reaction mixtures at a final concentration of 0.1 mM or 1 mM. The procedure used to determine the effect of EDTA was the same as above described. The activity of recombinant ApXPD without any additions was set as 100%.
Relative substrate specificity of the recombinant ApXPD was determined in the presence of 1 mM Co
2+, using 2 mM various dipeptides (Lys-Pro, Gly-Pro, Ala-Pro, Pro-Pro, Cys-Gly, Lys-Ala, Lys-Ser, or Lys-Gly), tripeptides (Leu-Ala-Pro, Lys-Pro-Ala, or Gly-Pro-Ala), or tetrapeptides (Lys-Trp-Ala-Pro or Lys-Pro-Ala-Ala) as the substrates. The previously described thin-layer chromatography on Silica Gel 60 F254 [
22] were used to determine the activities of recombinant ApXPD towards dipeptides, tripeptides or tetrapeptides that don’t contain C-terminus prolines. All of the determinations were repeated three times.
Kinetic parameters of enzymatic activity for various substrates and substrate concentrations were studied in the presence of 1 mM Co2+ by non-linear regression, using software Origin 2021. The Michaelis constant (Km) and maximum velocity (Vmax) were determined by directly fitting the Michaelis-Menten kinetic equation, V= VmaxS/(Km+S) with the experimental data. Here V and S denote the reaction velocity and substrate concentration, respectively.
4. Discussion
To date, more than 20 microbial Xaa-Pro dipeptidases have been molecularly and/or biochemically characterized. Among them, the prolidase from
Aureohacterium esteraromaticum [
27] is possibly not a prolidase since it lacks the corresponding active site and metal-binding residues of prolidases as shown by multiple sequence alignment (data not shown). Previously, prokaryotic prolidases have been classified into two subfamilies (XPDxc-type and XPD48-type) according to their molecular weights and sequence relatedness [
4,
9,
25]. However, this classification system may cause confusions as observed for prolyl aminopeptidases [
28]. In the present study, based on sequence relatedness and structural similarity, the 16 Xaa-Pro dipeptidases that have been molecularly and biochemically characterized were reclassified into 4 subfamilies by the MEROPS classification system: M24.003, M24.007, M24.008 and one unassigned subfamily (
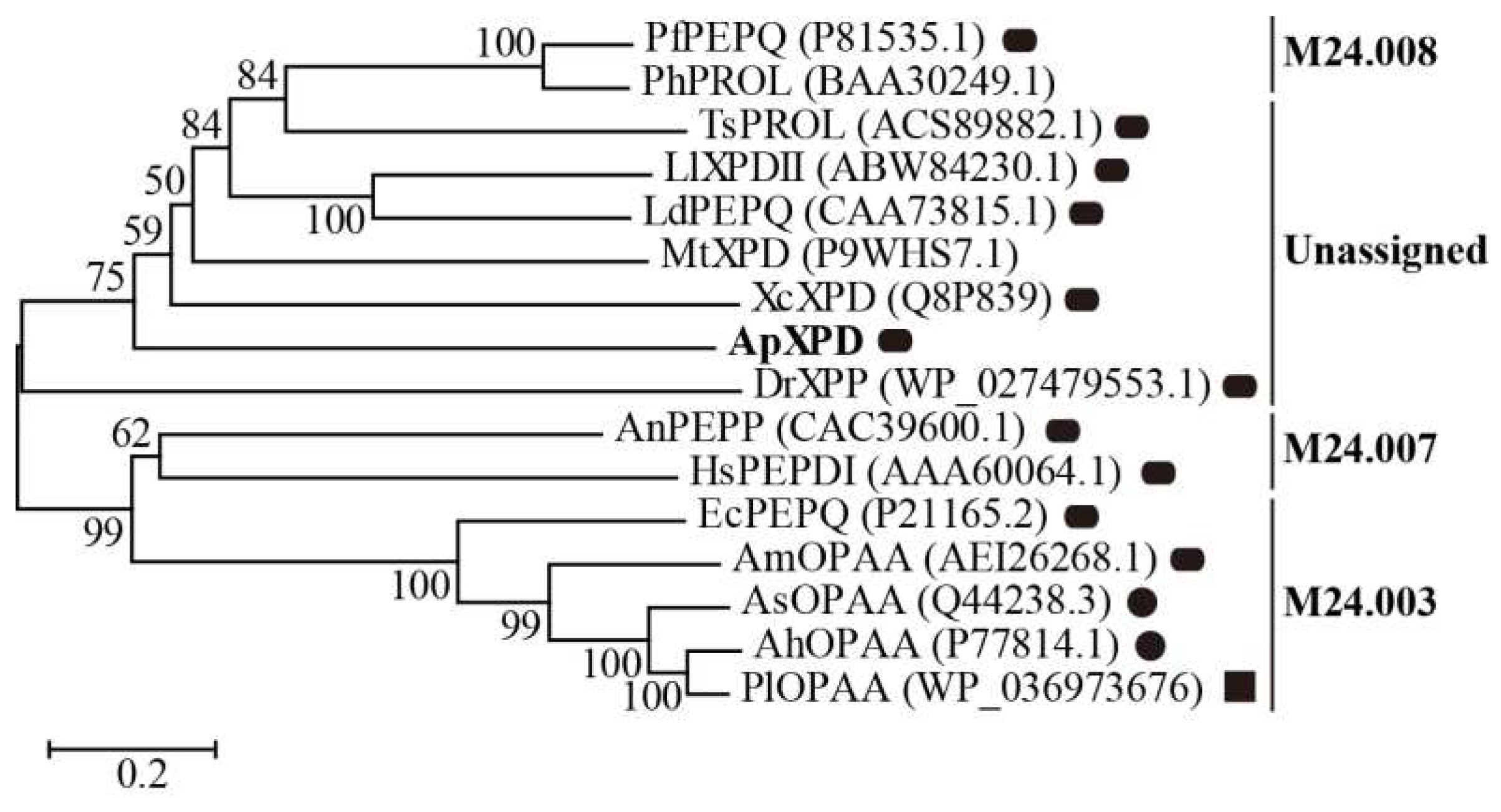
Figure 1). Prolidases from the unassigned subfamily and subfamilies M24.007 and M24.008 are mainly dimers, whereas subfamily M24.003 contains monomers, dimers and even a tetramer (
Figure 1 and
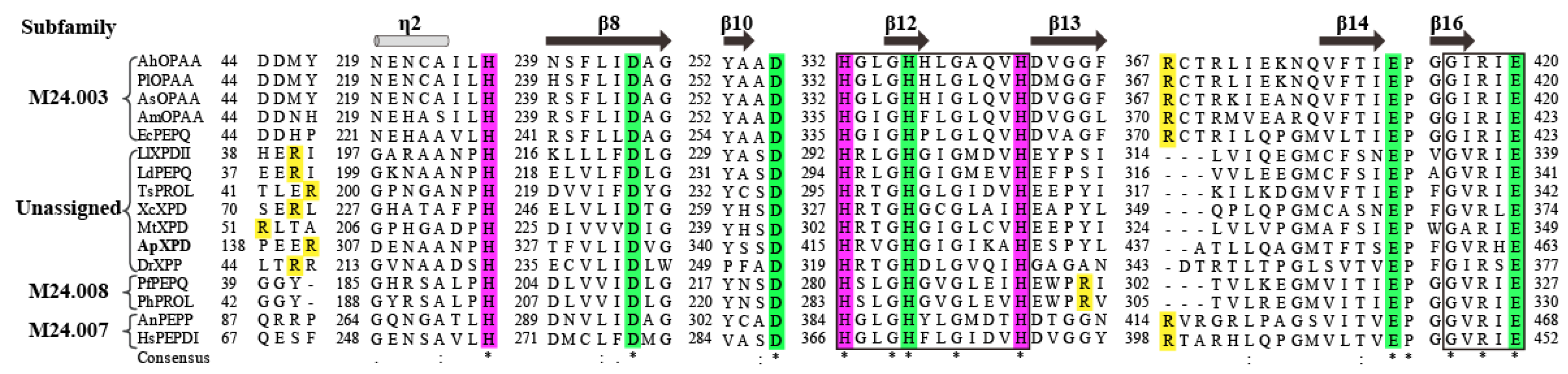
Table S1). Multiple sequence alignment demonstrated that four regions containing at least ten residues were present in prolidases from subfamilies M24.003 and M24.007 but absent in members from M24.008 and the unassigned subfamily (
Figure S1). In these regions, three residues (EcPEPQ numbering: residues Pro363, Leu369 and Arg370) are strictly conserved. Besides, an N-terminal loop extension has been found in prolidases from the M24.007 (AnPEPP and HsPEPDI) and unknown (XcXPD, MtXPD and ApXPD) subfamilies (
Figure S1). Xaa-Pro dipeptidases from subfamily M24.003 only exist in bacteria, and subfamily M24.007 contains prolidases from eukaryotes (fungi and human), while enzymes from subfamilies M24.008 proteins are prolidases from hyperthermophilic archaea. In the unassigned subfamily, all prolidases are from bacteria except ApXPD, which is the only reported fungal Xaa-Pro dipeptidase containing an additional region (PAPARLREKL, residues 361-370;
Figure S1). Interestingly, ApXPD also shares the highest sequence similarity with XcXPD [
4,
29], but not with the prolidase from the more related fungal species
A. nidulans (AnPEPP,
Figure 2). Therefore, XPD from
A. phoenicis ATCC 14332 represents a novel Xaa-Pro dipeptidase subfamily.
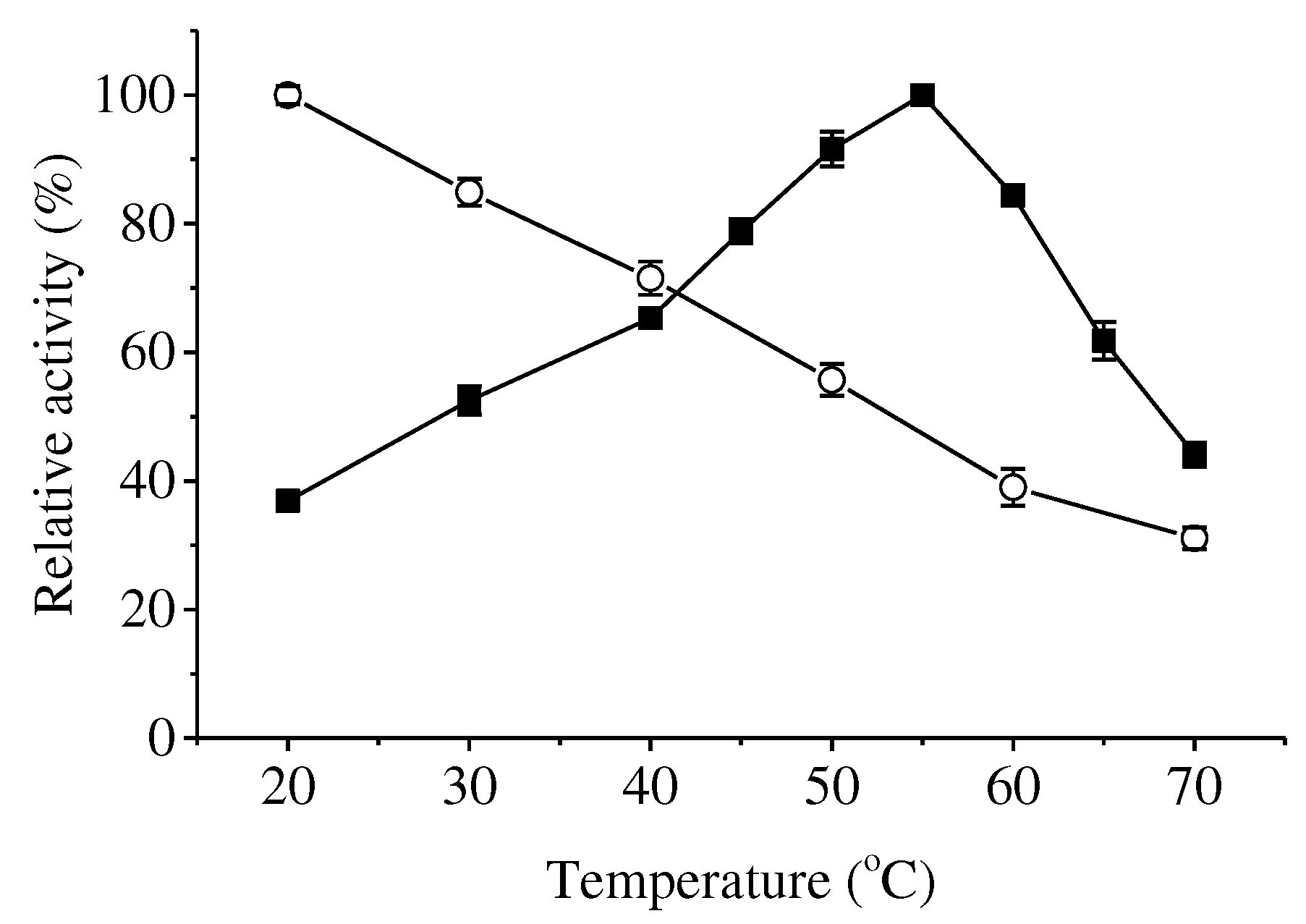
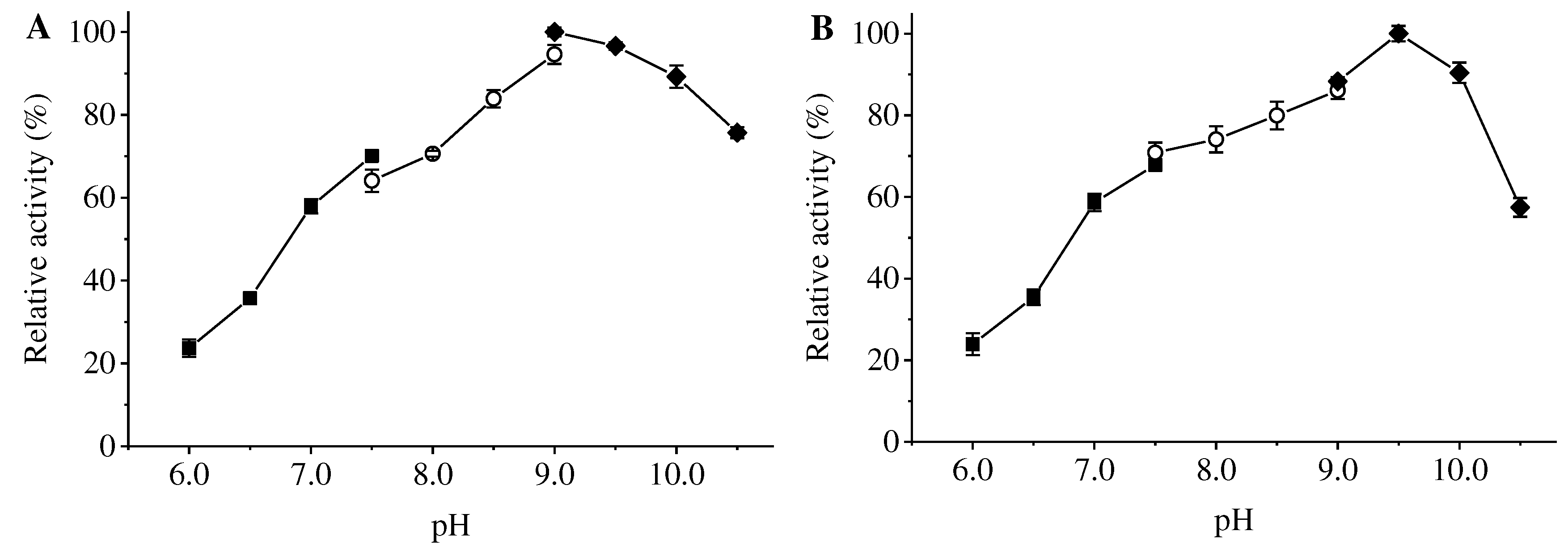
Xaa-Pro dipeptidases differ greatly in their optimal temperatures, optimal pHs, metal requirements and substrate specificities. The optimum temperature of ApXPD was 55
oC (
Figure 5), which was the same as or higher than those of prolidases from mesophilic sources [
2], but much lower than the optimal temperatures (100
oC) of prolidases derived from hyperthermophilic archaea
Pyrococcus ssp. [
21,
30]. Most XPDs are stable at low temperatures (20-50
oC), as is the case of XPD from
A. phoenicis ATCC 14332. Unlike most prolidases, which are most active under acidic or neutral pHs (pH 6.0-8.5), ApXPD acts optimally under more alkaline pH (pH 9.0) and is stable over a narrow pH range of 8.5-10.0 (
Figure 6).
XPDs require one or two divalent cations for enzyme activity, typically Mn
2+, Co
2+ or Zn
2+ [
31,
32].
In vitro studies have shown that most prolidases ever reported utilize Mn
2+ ion as cofactors (
Table S1) [
4,
24,
33,
34,
35,
36], whereas PfPEPQ, PhPROL and ScXPD require Co
2+ in their active sites under aerobic assay conditions [
21,
30,
37], as is the case of XPD from
A. phoenicis ATCC 14332 (
Table 1). Besides, the enzymatic activities of LdPEPQ, LlXPDII, and TsPROL are dependent on Zn
2+ [
22,
38,
39]. In contrast, the activities of AsOPAA, AuOPAA, LcXPD, PfpepQ, PhPROL and XmXPD were strongly or even completely inactivated by high concentrations of Zn
2+ (
Table S1), which is due to the fact that Zn binding decreases the volume of active site pocket for accumulating the substrates [
26]. Since the
in vivo concentrations of Mn
2+ and Co
2+ are much lower than that of Zn
2+, the metal ion requirements for XPDs
in vivo are quite different and a preference for Zn
2+ might be advantageous [
40]. Moreover, PfPEPQ and PhPROL require Fe
2+ rather than Co
2+ for maximal activity under anaerobic conditions, suggesting that their metal preferences
in vivo may be changed [
30,
31].
XPDs are highly specific in the type of substrate that they hydrolyze, and they usually prefer dipeptides to tripeptides and tetrapeptides. Prolidases from subfamilies M24.003, M24.007 and M24.008 hydrolyze both Xaa-Pro dipeptides and OP compounds, whereas those from the unassigned subfamily only cleave Xaa-Pro dipeptides (
Table S1). There are also some differences in dipeptide substrate specificities among the prolidases that have been biochemically characterized. Their main activity is Xaa-Pro dipeptides hydrolysis, although several prolidases can cleave dipeptides with proline at the N-terminus or dipeptides that do not contain a proline residue [
36,
39,
41]. Most prolidases show broad substrate specificities and prefer Xaa-Pro dipeptides containing N-terminal hydrophobic amino acids, whereas PfPEPQ [
21], PhPROL [
30] and EcPEPQ [
33] show affinity for negatively charged and/or hydrophilic amino acids at the N-terminus. The recombinant ApXPD characterized in this study, however, shows preference for positively charged Lys-Pro (
Table 2 and
Table 3). Besides, most prolidases don’t hydrolyze Pro-Pro and Gly-Pro except MtXPD [
4], XcXPD [
4], AnPEPP [
15], PlOPAA [
35], DrXPP [
39], and HsPEPDI [
42].
Typically, the substrate length of prolidases is restricted to dipeptides by the guanidinium side chain of an arginine residue that binds to the free carboxylate of dipeptides and prevents the binding of longer peptides [
4,
9,
25,
43]. Besides, the specific arginine residue can also increase the affinities of XPDs for substrates by introducing allosteric behavior and substrate inhibition [
4,
23,
44]. Prolidases from these four subfamilies vary greatly in their specific arginine residues. As shown by multiple sequence alignment in
Figure 2, the specific arginine residues of subfamily M24.003 prolidases are counterparts of those in XPDs from subfamily M24.007, such as Arg370 in AmOPAA [
9], Arg370 in EcPEPQ [
43] as well as Arg398 in HsPEPDI [
45], while the arginine residues in PfPEPQ and PhPROL from subfamily M24.008 are Arg295 and Arg298 [
38], respectively, which do not correspond to those in subfamilies M24.003 and M24.007 XPDs (
Figure 2). Unlike XPDs in other three subfamilies, however, prolidases from the unassigned subfamily use a novel arginine dependent mechanism for dipeptide selectivity. According to the results of structural, mutational and/or molecular dynamics simulation analyses, the corresponding arginine residues employed by XcXPD [
4], DrXPP [
39] and LlXPDII [
44] have been found to be Arg72, Arg46 and Arg40, respectively, while the equivalent residue in ApXPD is Arg141 (
Figure 2). Further understanding and study of the precise mechanism of prolidases from the unassigned subfamily used for dipeptide selectivity is highly warranted. Aside from structural differences in the substrate binding pocket, the variability of prolidase substrate specificity also depends on the catalytic metal cation [
4,
23,
38]. For example, in the presence of Mn
2+, LlXPDII shows low activity towards Pro-Pro, whereas it can’t hydrolyze this dipeptide in the presence of Zn
2+. Furthermore, its substrate preference changes from Leu-Pro to Arg-Pro in the presence of Mn
2+ [
38].
Figure 1.
Evolutionary tree describing the phylogenic relationships among XPD from A. phoenicis ATCC 14332 (ApXPD) and all the currently characterized prolidases from various organisms. Filled circles, rounded rectangles and filled squares represent monomers, dimmers and tetramers, respectively. PfPEPQ, PhPROL, TsPROL, LlXPDII, LdPEPQ, MtXPD, XcXPD, DrXPP, AnPEPP, HsPEPDI, EcPEPQ, AmOPAA, AsOPAA, AhOPAA and PlOPAAPlOPAA are prolidases from Pyrococcus furiosus DSM 3638, P. horikoshii OT3, Thermococcus sibiricus MM 739, Lactococcus lactis NRRL B-1821, L. delbrueckii subsp. bulgaricus CNRZ 397, Mycobacterium tuberculosis H37Rv, X. campestris pv. campestris ATCC 33913, Deinococcus radiodurans R1, A. nidulans WG312, Human sapiens, E. coli BL21 (DE3), Alteromonas macleodii NCIMN1963, Alteromonas sp. JD6.5, A. haloplanktis ATCC 23851 and Pseudoalteromonas lipolytica SCSIO04301, respectively. GenBank accession numbers of these polidases are given in parenthesis.
Figure 1.
Evolutionary tree describing the phylogenic relationships among XPD from A. phoenicis ATCC 14332 (ApXPD) and all the currently characterized prolidases from various organisms. Filled circles, rounded rectangles and filled squares represent monomers, dimmers and tetramers, respectively. PfPEPQ, PhPROL, TsPROL, LlXPDII, LdPEPQ, MtXPD, XcXPD, DrXPP, AnPEPP, HsPEPDI, EcPEPQ, AmOPAA, AsOPAA, AhOPAA and PlOPAAPlOPAA are prolidases from Pyrococcus furiosus DSM 3638, P. horikoshii OT3, Thermococcus sibiricus MM 739, Lactococcus lactis NRRL B-1821, L. delbrueckii subsp. bulgaricus CNRZ 397, Mycobacterium tuberculosis H37Rv, X. campestris pv. campestris ATCC 33913, Deinococcus radiodurans R1, A. nidulans WG312, Human sapiens, E. coli BL21 (DE3), Alteromonas macleodii NCIMN1963, Alteromonas sp. JD6.5, A. haloplanktis ATCC 23851 and Pseudoalteromonas lipolytica SCSIO04301, respectively. GenBank accession numbers of these polidases are given in parenthesis.
Figure 2.
Multiple sequence alignment of ApXPD with all the characterized XPDs from various organisms. Prolidases from subfamilies M24.003, M24.007 and M24.008 are indicated, while other enzymes are from the unassigned subfamily. (*), the conserved amino acids; (:), the conservative replacement; (.), semiconservative replacement. Xaa-Pro dipeptidase from A. phoenicis ATCC 14332 is in bold. The secondary structural elements of XcXPD are shown above the sequence. The strictly conserved motifs HXXGHXXGXXXH and GXRXE (X represents any amino acid) are boxed. The putative catalytic triads, metal ion binding residues and the specific arginine residues involved in dipeptide selection are colored in purple, green and yellow, respectively.
Figure 2.
Multiple sequence alignment of ApXPD with all the characterized XPDs from various organisms. Prolidases from subfamilies M24.003, M24.007 and M24.008 are indicated, while other enzymes are from the unassigned subfamily. (*), the conserved amino acids; (:), the conservative replacement; (.), semiconservative replacement. Xaa-Pro dipeptidase from A. phoenicis ATCC 14332 is in bold. The secondary structural elements of XcXPD are shown above the sequence. The strictly conserved motifs HXXGHXXGXXXH and GXRXE (X represents any amino acid) are boxed. The putative catalytic triads, metal ion binding residues and the specific arginine residues involved in dipeptide selection are colored in purple, green and yellow, respectively.
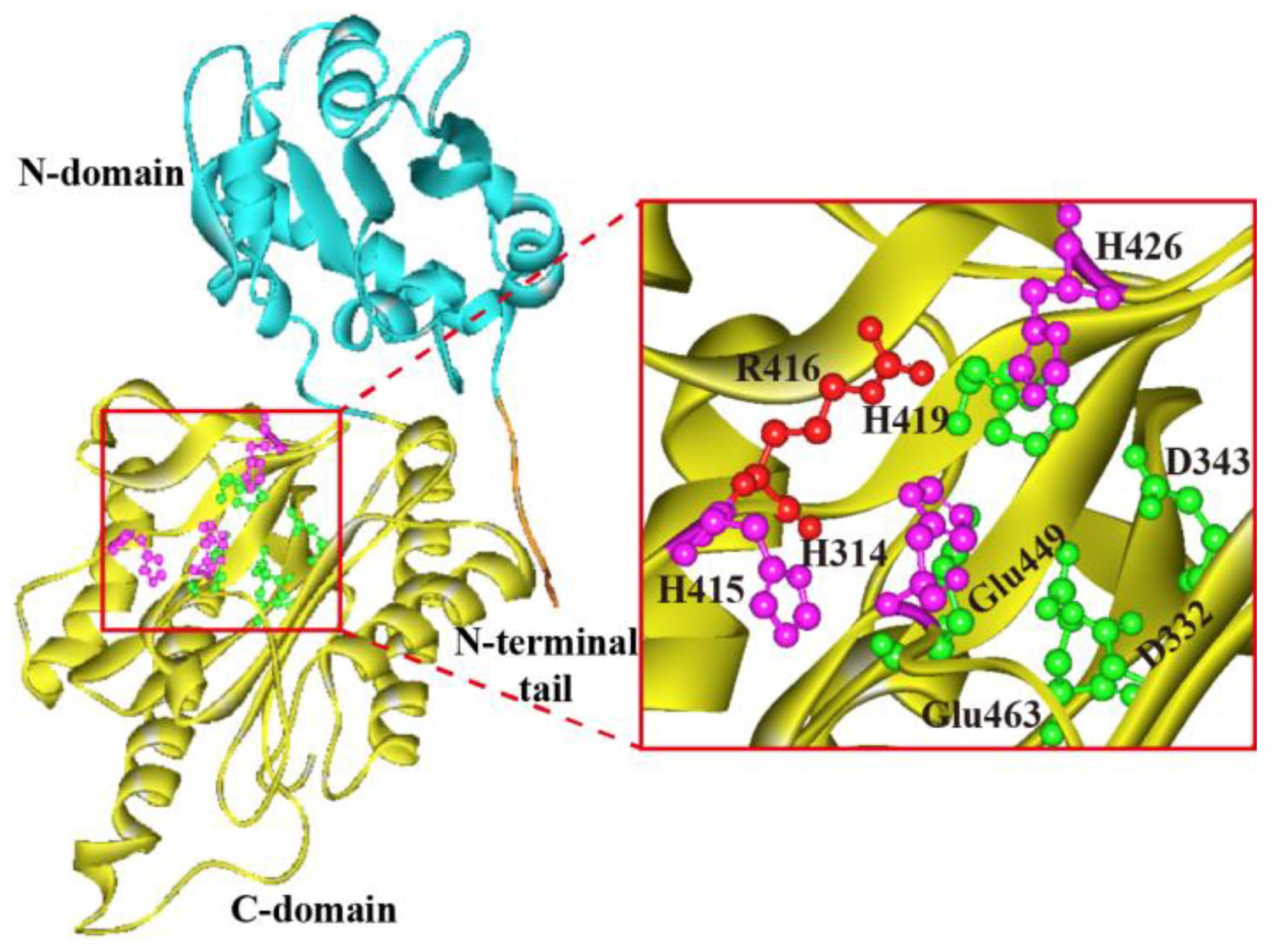
Figure 3.
Molded 3D structure of XPD from A. phoenicis ATCC 14332. Regions of N-terminal tail, N-domain, and C-domain are shown in orange, light blue and yellow colors, respectively. Putative catalytic triads (His314, His415 and H426), metal ion binding residues (Asp332, Asp343, His419, Glu449 and Glu463) and the specific arginine residue (Arg416) that determines the length of ligand are represented by purple, green, and red scaled ball and sticks, respectively.
Figure 3.
Molded 3D structure of XPD from A. phoenicis ATCC 14332. Regions of N-terminal tail, N-domain, and C-domain are shown in orange, light blue and yellow colors, respectively. Putative catalytic triads (His314, His415 and H426), metal ion binding residues (Asp332, Asp343, His419, Glu449 and Glu463) and the specific arginine residue (Arg416) that determines the length of ligand are represented by purple, green, and red scaled ball and sticks, respectively.
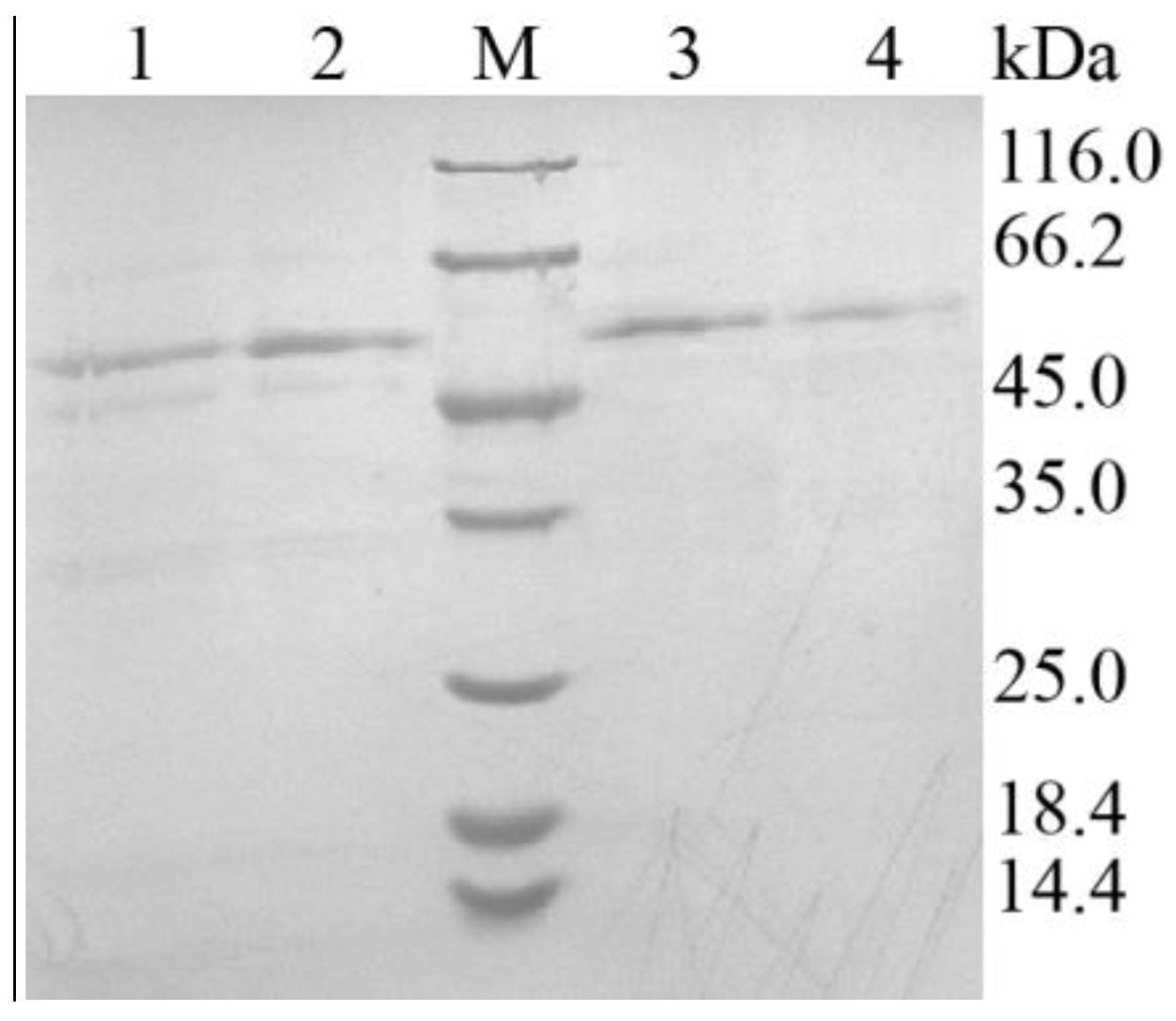
Figure 4.
SDS-PAGE analysis on the expression and purification of ApXPD. Lanes 1 and 2, cell-free supernatants of the recombinant strain; lanes 3 and 4, the purified ApXPD; M, molecular mass markers; the bands at approximately 55.0 kDa represent target proteins.
Figure 4.
SDS-PAGE analysis on the expression and purification of ApXPD. Lanes 1 and 2, cell-free supernatants of the recombinant strain; lanes 3 and 4, the purified ApXPD; M, molecular mass markers; the bands at approximately 55.0 kDa represent target proteins.
Figure 5.
Effects of temperature on the activity (closed squares) and stability (open circles) of ApXPD. The relative activity is calculated with the highest activity regarded as 100%.
Figure 5.
Effects of temperature on the activity (closed squares) and stability (open circles) of ApXPD. The relative activity is calculated with the highest activity regarded as 100%.
Figure 6.
Effects of pH on the activity (A) and stability (B) of ApXPD. The relative activity is calculated with the maximum activity set as 100%. Filled circles, open squares, and filled diamonds represent 20 mM sodium citrate buffer (pH 6.0-7.5), 20 mM Tris-HCl buffer (pH 7.5-9.0) and 20 mM glycine-NaOH buffer (pH 9.0-10.5), respectively.
Figure 6.
Effects of pH on the activity (A) and stability (B) of ApXPD. The relative activity is calculated with the maximum activity set as 100%. Filled circles, open squares, and filled diamonds represent 20 mM sodium citrate buffer (pH 6.0-7.5), 20 mM Tris-HCl buffer (pH 7.5-9.0) and 20 mM glycine-NaOH buffer (pH 9.0-10.5), respectively.
Table 1.
Effects of metal ions and chemicals on the activity of recombinant ApXPD.
Table 1.
Effects of metal ions and chemicals on the activity of recombinant ApXPD.
| Metal ions |
Concentration (mM) |
Relative Activity (%) |
| None |
0 |
100 |
| CaCl2
|
0.1 |
82.1 ± 0.4 |
| |
1 |
53.8 ± 0.9 |
| ZnCl2
|
0.1 |
102.6 ± 0.9 |
| |
1 |
89.7 ± 3.1 |
| MnCl2
|
0.1 |
114.7 ± 1.6 |
| |
1 |
104.5 ± 1.4 |
| CuCl2
|
0.1 |
64.7 ± 0.7 |
| |
1 |
25.6 ± 0.3 |
| CoCl2
|
0.1 |
136.5 ± 1.5 |
| |
1 |
300.6 ± 2.6 |
| SnCl2
|
0.1 |
203.2 ± 2.2 |
| |
1 |
166.0 ± 1.9 |
| FeCl3
|
0.1 |
96.8 ± 0.6 |
| |
1 |
87.8 ± 1.6 |
| EDTA |
0.1 |
92.1 ± 0.3 |
| |
1 |
76.5 ± 1.1 |
Table 2.
Substrate specificity of recombinant ApXPD.
Table 2.
Substrate specificity of recombinant ApXPD.
| Substrate |
Relative Activity (%) |
| Lys-Pro† |
100 ± 2.1 |
| Gly-Pro† |
86.3 ± 1.7 |
| Ala-Pro† |
32.7 ± 1.3 |
| Pro-Pro† |
ND |
| Cys-Gly﹡ |
- |
| Lys-Ala﹡ |
- |
| Lys-Ser﹡ |
- |
| Lys-Gly﹡ |
- |
| Leu-Ala-Pro† |
ND |
| Lys-Pro-Ala﹡ |
- |
| Gly-Pro-Ala﹡ |
- |
| Lys-Trp-Ala-Pro† |
ND |
| Lys-Pro-Ala-Ala﹡ |
- |
Table 3.
Kinetic parameters of recombinant ApXPD.
Table 3.
Kinetic parameters of recombinant ApXPD.
| Substrate |
Vmax (μmol min-1 mg-1) |
Km (mM) |
kcat (s-1) |
kcat/Km (mM-1 s-1) |
| Lys-Pro |
85.7 |
1.0 |
123.5 |
123.5 |
| Gly-Pro |
82.8 |
1.2 |
105.9 |
88.3 |