Introduction
Angina reflects a chest pain due to myocardial ischemia where, however, the structural context is extremely variable and may affect patient’s treatment and outcome. Indeed, angina may derive from anomalies of origin and course of coronary arteries often susceptible of surgical repair [
1], atherosclerotic lesions of epicardial vessels usually judging from PTCA and stenting and/or bypass [
2,
3], myocardial bridges and tunnels [
4,
5], potentially suitable of surgical correction [
5] and small vessel disease [
6], where the therapeutic impact is frequently disappointing. In the last instance, intramural vessels can be obstructed because of heritable diseases including sarcomeric as hypertrophic cardiomyopathy or non-sarcomeric gene mutations like Fabry disease [
7], or affected by acquired microvascular dysfunction as in coronary syndrome x [
8]. Sometime, however, despite major diagnostic efforts, angina may be of difficult pathophysiological definition. In the following report is described the case of persistent angina, refractory to high dose calcium antagonists, nitrates and betablockers due to microvascular coronary dysplasia. Cardiac affection is associated to similar microvascular obstruction causing renal microhematuria and recurrent transitory cerebral ischemic attaks. Extensive genetic study found out genetic variants in the ABCC6, MMP2 and XYLT1 genes.
Case Study
A 65-year old man had in the last year several hospital admission because of transitory ischemic brain attacks characterized at angio-Tac and magnetic resonance by multiple small foci of cerebral ischemia with normal great epi-aortic arteries, requiring infusion administration of mannitol and anti-platelet aggregation drugs. His history started at 30 years with spontaneous and effort angina which was investigated with multiple coronary angiographies showing systematically normal coronary arteries with remarkable slow flow (movies 1,2) in the absence of systemic artery hypertension and valvular heart disease. Echocardiogram showed normal cardiac parameters with preserved right and left ventricular function and normal left ventricular wall thickness. When he was forty, following a prolonged angina episode, ventriculography revealed the occurrence of micro-aneurysms at the base of right ventricle and in the left ventricular free wall. At that time the invasive study was implemented with bi-ventricular endomyocardial biopsy. At histology no evidence of myocarditis, infiltrative or storage disease was documented and PCR for the most common cardiotropic viruses was negative. Indeed, previous endomyocardial biopsy studies demonstrated in patients with syndrome X and resistant angina a microvscular dysfunction due to endothelial inflammation by endotheliotropic viral agents. [
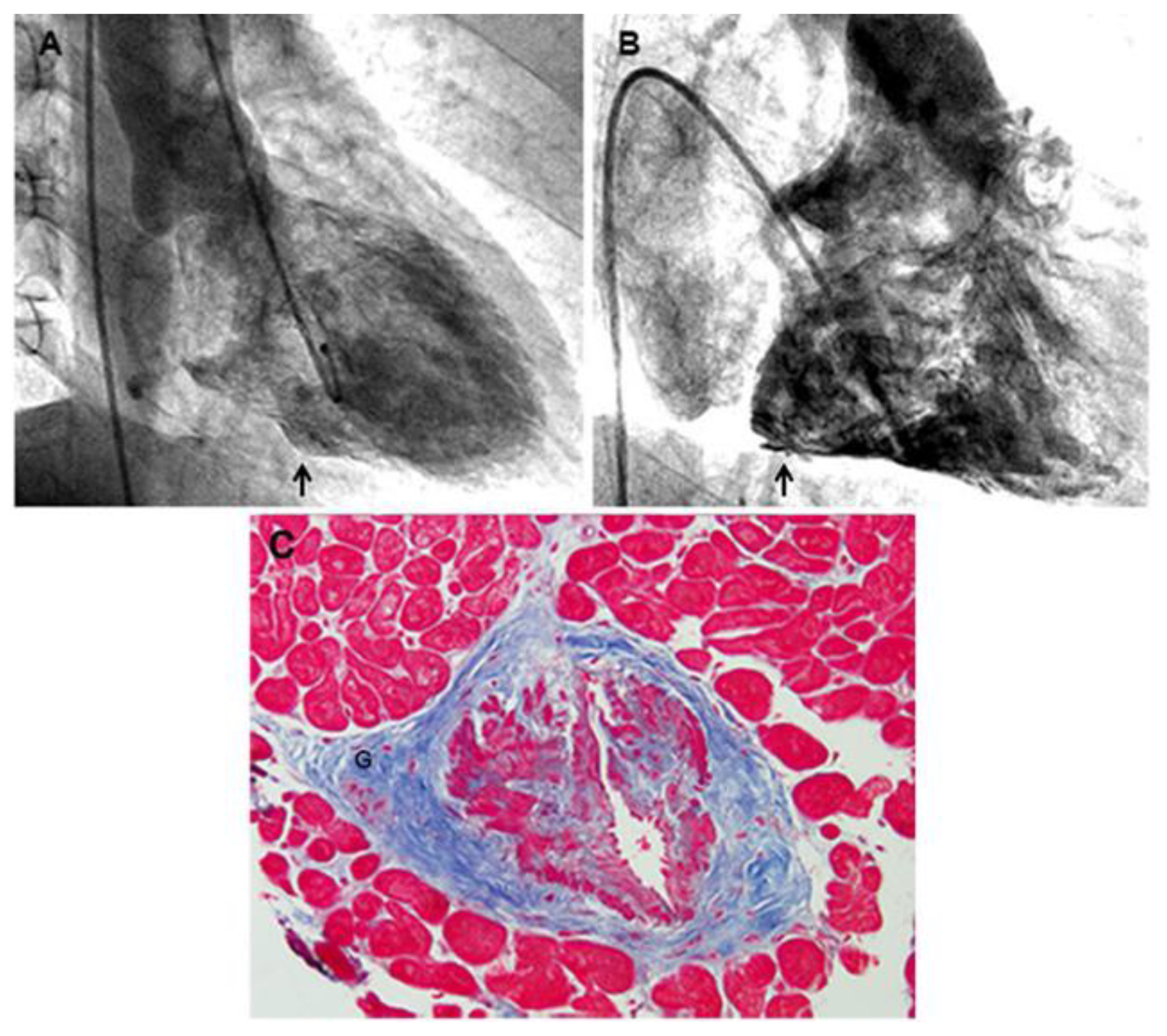
8]. Major abnormalities were documented in 5 of seven arterioles included in six biopsy fragments, showing a severe lumen obstruction due to an abnormal thickening of the muscular coat. The arterioles’ middle layer presented hypertrophy with hyperplasia and disorganization of smooth muscle cells that were focally replaced by fibrous tissue denoting a dysplastic process (
Figure 1). A prominence of adventitial ganglia (Fig 1) could have had a role in the hypertrophy and disarray of smooth muscle cells of the media through release of trophic medietors (Cathecolamines?).
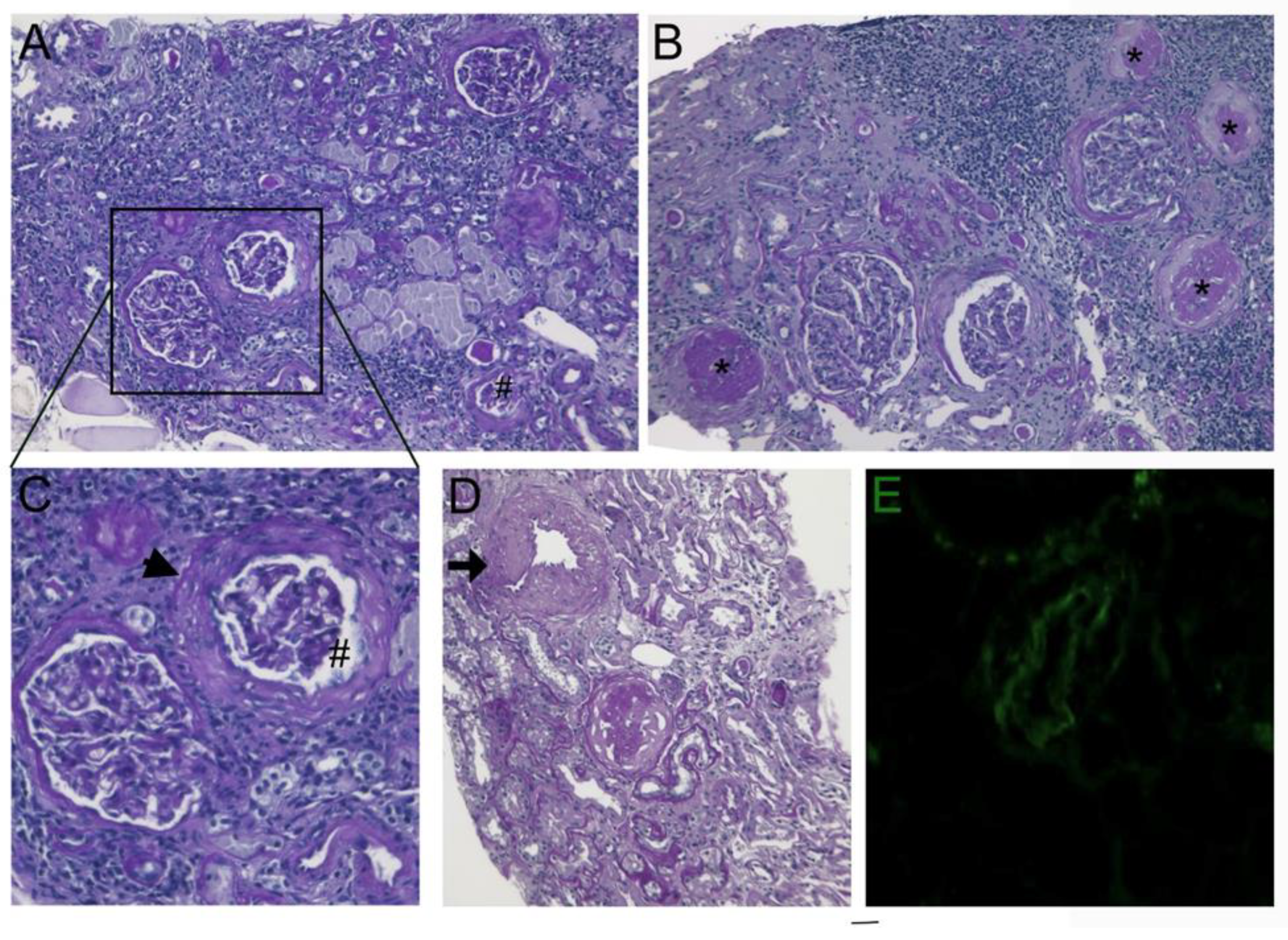
Anti-myocardial ischemia drug regimen included nitrates, betablockers, Ca antagonists and aspirin obtaining transitory attenuation of anginal symptoms. At the age of 60 years the patient complained of recurrent hematuria with normal values of renal echo and of creatinine clearance. A renal biopsy manifested histological changes similar to endomyocardial findings consisting in medial thickening of small and medial sized renal arteries associated to focal glomerular sclerosis, interstitial and replacement fibrosis (
Figure 2).
In order to identify possible gene variants associated with this condition, we used the SureSelect Custom Constitutional Panel 17Mb (CCP17) (Agilent), a targeted next-generation sequencing (NGS) panel for inherited disease comprising more than 5,000 genes, and selected those variants located in genes related to the patient’s clinical features, as they are defined by the “Human Phenotype Ontology, HPO”, which provides a standardized vocabulary of phenotypic abnormalities encountered in human disease. The following HPO terms were used: "hereditary hemorrhagic telangiectasia," "congenital vascular malformation, "vascular smooth muscle hypertrophy,"microhematuria”, “small vessel systems disease”, “transient ischemic attack”, “abnormal cerebral vascular morphology”, “abnormal renal vascular morphology”, “arteriovenous malformation”, “vascular systems disease”. Of note, bioinformatic analysis identified three functionally-related variants, as they affected genes that play pivotal roles in the extracellular matrix. The first variant was a heterozygous missense substitution (NM_001171.6:c.1171A>G; p.Arg391Gly) in the ABCC6 gene. The ABCC6 gene encodes an ATP-binding cassette (ABC) protein, which functions as an efflux transporter. Its specific substrate remains unknown, although it has been recently demonstrated that ABCC6 plays a significant role in the regulation of plasmatic inorganic pyrophosphate, a potent inhibitor of ectopic mineralization. The second variant was a missense substitution in the MMP2 gene (NM_004530.6:c.1483C>T; p.Arg495Trp), which encodes a member of the matrix metalloproteinase gene family, that are zinc-dependent enzymes capable of cleaving components of the extracellular matrix. The third variant was a missense change in the XYLT1 gene (NM_022166.4:c.2081G>A; p.Arg694His), which encodes an enzyme that catalyzes the transfer of UDP-xylose to serine residues of a substrate acceptor protein, a transfer reaction necessary for the biosynthesis of glycosaminoglycan chains.
There are several evidences correlating these three variants. Recessive loss-of-function variants of the ABCC6 gene [
9,
10] cause pseudoxanthoma elasticum, a multisystemic condition characterized by calcification and fragmentation of elastic fibers in the skin, eyes, and the cardiovascular system. Elevated production of MMP2 in pseudoxantoma elasticum fibroblasts and increased levels of MMP2 were observed in serum from pseudoxantoma elasticum patients. Moreover, variations in MMP2 gene were found as genetic co-factor for pseudoxantoma elasticum [
11]. Furthermore, high XYLT1 activity was found in the sera of affected patients with pseudoxantoma elasticum, reflecting a higher rate of proteoglycan biosynthesis in these patients.
This is the first description of a genetically-induced obstructive microvascular dysplasia causing ischemic damage of human brain, heart and kidney. The structural basis, provided by endomyocardial and kidney biopsies is mainly expressed in the media of arterioles showing hypertrophy with disarray and replacement fibrosis of smooth muscle cells causing severe narrowing of vessels ‘lumen. At brain level, it resulted in recurrent transient ischemic attacks; in the kidney in repeated episodes of hematuria and in the heart in resistant angina episodes causing biventricular micro-aneurysms. The best of vasoactive treatment only limited the severity of clinical manifestations.
2. Materials and Methods
Cardiac Studies
Cardiac investigations included noninvasive (ECG, Holter monitoring, 2D-echocardiography, and CMR) and invasive (coronary, left ventricular angiography, coronary sinus catheterization with atrial pacing and left ventricular EMB) was performed after receiving written informed consent. EMB is regularly performed in our institution whenever a symptomatic heart muscle disease remains undiagnosed by noninvasive procedures including echocardiography and cardiac magnetic resonance. Biopsy samples, 5–8 fragment, were cut and stored at −80 °C, or processed for histology, immunohistochemistry, and electron microscopy. Two frozen samples were processed for real-time PCR for the most common cardiotropic viruses in case of observation of overlapping myocarditis at histology.
The study complies with the Declaration of Helsinki, the locally appointed ethics committee (opinion number 6/2019) approved the research protocol, and informed consent was obtained from patient. The patient was strongly motivated to clarify the origin of symptoms and gave their written consent for the procedure.
Pathologists, echocardiographers, and CMR investigators were blind to the patient’ clinical and genetic background.
Cardiac Magnetic Resonance
CMR exams were performed on a 1.5 Tesla scanner (Avanto, Siemens). The CMR protocol included: (i) cine CMR sequence acquired during breath holds in the short-axis, 2-chamber, and 4-chamber; (ii) black blood T2-weighted short tau inversion recovery images on short-axis planes covering the entire left ventricle during 6 to 8 consecutive breath holds for myocardial edema detection; (iii) late gadolinium-enhanced imaging performed 15 min after injection of 0.2 nmol/kg of gadoterate meglumine and signal intensity value 2 SDs above the mean signal intensity of the remote normal myocardium were considered suggestive of myocardial fibrosis; (iv) native T1 mapping imaging was performed, when available, using the MOLLI sequence on three short-axis views (one basal and two midventricular); (v) a T2 map was obtained using a T2-prepared True-FISP prototype sequence producing 3 single-shot images with 3 different T2 pulse preparations. A nonrigid registration algorithm and the two-parametric automatic curve fitting were automatically applied to generate the map. CMR image analysis was performed as previously described, extending the analysis method for T1 maps also to T2 maps. In particular, the values of T1 and T2 global were defined as normal, reduced, or increased compared to a reference range developed on a multiage sample of 100 healthy subjects of both sexes (normal value nT1 < 970 ms, T2 > 49.7 ms).
Invasive and Endomyocardial Biopsy Studies
Cardiac catheterization with left ventricular and coronary angiography was obtained. EMB was performed in the septal–apical region of the left ventricle. Endomyocardial samples were blindly evaluated by the same pathologist. He was informed of clinical and genetic characteristics after morphological examination.
Histology and Immunohistochemistry
For histological analysis, the endomyocardial samples were fixed in 10% buffered formalin and paraffin-embedded. Five-micron-thick sections were stained with hematoxylin and eosin and Masson trichrome.
The histological diagnosis of myocarditis was based on the evidence of leukocyte infiltrates (≥14 leukocytes/2 mm) associated with necrosis of the adjacent myocytes, according to the Dallas criteria confirmed by immunohistochemistry. In particular, for the phenotypic characterization of inflammatory infiltrates, immunohistochemistry was performed for CD3, CD20, CD43, CD45RO, and CD68 (all Dako, Carpinteria, CA, USA).
Molecular Study
Definition of virus-negative, immune-mediated, overlapping myocarditis followed the presence at histology of ≥7 CD3+ T lymphocytes per lo- power field associated with focal necrosis of the adjacent myocytes . The molecular study was performed in the patient with Real Time PCR for the most common cardiotropic viruses (Adenovirus, Enterovirus, Influenza A and B virus, Epstein Barr virus, Parvovirus B19, Hepatitis C virus, Cytomegalovirus, Human Herpes virus 6, Herpes Simplex type 1 and 2) for the possible identification of viral genomes.
Angio-Tac and magnetic resonance were obtained to investigate the cerebral transitory ischemic attacks. by multiple small foci of cerebral ischemia with normal great epi-aortic arteries.
A renal biopsy was also undertaken because of the presence of microhematuria.
Genetic Study Methods
SureSelect Custom Constitutional Panel 17Mb (CCP17) Target Enrichment Library Preparation
Targeted enriched libraries were prepared using the SureSelect Custom Constitutional Panel 17Mb (CCP17), a targeted NGS panel designed to capture all the coding exons and intronic flanking regions (+/-25 bp) of more than 5,000 genes associated with inherited diseases (Agilent Technologies, Santa Clara, CA, USA).
In details, a genomic DNA sample was suspended in nuclease-free water to a final concentration of 25 ng/µl and final volume of 2 µl and enzymatically fragmented to achieve target peak positioned between 245 and 325 bp. Agilent's SureSelect Custom Constitutional Panel 17Mb (CCP17) Target Enrichment was used for library preparation according to the manufacturer's recommendations. Ten cycles of PCR were performed for amplification of the post-captured library, and the quality of the final DNA library was assessed using the High Sensitivity Agilent 2100 Bioanalyzer System (Agilent Technologies, Santa Clara, CA, USA). Per manufacturer's protocol, library peak size was in the range of 325 and 450 bp. Samples were then pooled and sequenced by using the NextSeq Mid Output Kit v2.5 (300 Cycles) (Illumina, San Diego, CA, USA) on NextSeq 500 (Illumina, San Diego, CA, USA) according to the manufacturer’s recommendations for paired-end 150-bp reads.
Variant Annotation, Filtering, Prioritization, and Classification
Generated reads were aligned to the human genome reference sequence (assembly GRCh37/hg19) by Bowtie 2 (version 2.3.0). BAM files were sorted by SAM tools (version 1.3.2) and purged from candidate PCR duplicates using Mark Duplicates tool from the Picard suite (version 2.9.0). The local realignment and base-quality-score recalibration functions were performed using the Genome Analysis Toolkit (GATK 4.0). Reads with mapping quality scores lower than 20 were filtered out. The GATK’s Haplotype Caller was used to identify single-nucleotide polymorphisms and insertions/deletions. Genetic variants were annotated by ANNOVAR and subjected to filtering. Common variants with a minor allele frequency (MAF) >1% in dbSNP [
https://www.ncbi.nlm.nih.gov/projects/SNP/], GO-ESP [
https://esp.gs.washington.edu/drupal/] and GnomAD [
http://gnomad.broadinstitute.org/] databases were excluded. Variants phenotype—based prioritizaton of candidate genes was conducted using Geneyx Analysys Software (Geneyx Genomex). Validation and segregation analyses were performed by Sanger sequencing using the ABI Prism BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). Sanger sequencing results were analyzed by Mutation Surveyor V5.0.0 software (SoftGenetics, State College, PA, USA). Variants classification was determined using the American College of Medical Genetics and Genomics - Association for Molecular Pathology (ACMG-AMP) standards and guidelines for the interpretation of sequence variants [
12].
Figure 1.
A) Presence of a micro-aneurysm in the inferior LV wall (arrow). B) Evidence of micro-aneurysms in the posterior RV free wall (arrow). C) Hypertrophy, hyperplasia and disorganization of smooth muscle cells, focally replaced by fibrous tissue, is observed. (Masson Trichrome, 200x). A prominent ganglion is shown in the adventitia (G).
Figure 1.
A) Presence of a micro-aneurysm in the inferior LV wall (arrow). B) Evidence of micro-aneurysms in the posterior RV free wall (arrow). C) Hypertrophy, hyperplasia and disorganization of smooth muscle cells, focally replaced by fibrous tissue, is observed. (Masson Trichrome, 200x). A prominent ganglion is shown in the adventitia (G).
Figure 2.
(A-B-C) Deflated (#) and globally sclerosed glomeruli (asterisks) associated with capsule thickening and pericapsular fibrosis (arrow head). Atrophic tubules and interstitial fibrosis with some lymphocytic infiltrates are also present (PAS original magnification, X100). D) Medium-sized artery showed thickening of the media (arrow) (PAS original magnification, X100). E) Immunofluorescence of arteriole staining with C3 antiserum (C3 antiserum X400).
Figure 2.
(A-B-C) Deflated (#) and globally sclerosed glomeruli (asterisks) associated with capsule thickening and pericapsular fibrosis (arrow head). Atrophic tubules and interstitial fibrosis with some lymphocytic infiltrates are also present (PAS original magnification, X100). D) Medium-sized artery showed thickening of the media (arrow) (PAS original magnification, X100). E) Immunofluorescence of arteriole staining with C3 antiserum (C3 antiserum X400).
VIDEO
- 1)
Movie 1: Coronary angiography of left coronary artery showing pronounced slow flow with persistence of contrast medium.
- 2)
Movie 2: Coronary angiography of right coronary artery showing pronounced slow flow with persistence of contrast medium.
Author Contributions
AF designed the study, participated in and lead the categorization of the subjects, and wrote the manuscript, RV,CB and DMC categorized and organized the data and experiment. AF, CR and ADL discussed and verified the cases, performed the statistics and collected the data. All authors participated in drafting the manuscript and approved the final manuscript.
Funding
This study was supported by an Investigator Initiated Research grant from Takeda Pharmaceuticals International AG, a member of the Takeda group of companies (IISR-2018-104317) and partially by the Italian Health Ministry (IRCCS San Raffaele Roma – Ricerca Corrente #2020/1) and Fondazione Roma (MEBIC # 18/6/2019). Mebic San Raffaele Pisana, and partially by the Italian Health Ministry Fondazione Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy – Ricerca Corrente #2020–2022 to ADL. This study was supported by the Italian Health Ministry I.N.M.I. L. Spallanzani IR.C.C.S, IRCCS San Raffaele Roma – Ricerca Corrente #2020/1 and Fondazione Roma (MEBIC # 18/6/2019) Mebic San Raffaele Pisana; and the Italian Health Ministry Fondazione Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy – Ricerca Corrente #2020–2022 to ADL.
Institutional Review Board Statement
This study was approved by the ethics committee, opinion number 6/2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
All authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Angelini P:. Coronary artery anomalies- current clinical issues: definitions, classification, incidence, clinical relevance and treatment guidelines. Tex Heart Inst J 2002, 29, 271–8.
- Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O'Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA/SCAI 2005 Guidelines Update for Percutaneous Coronary Intervention- summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation 2006, 113, 156–75.
- Cram P, Rosenthal GE, Vaughan-Sarrazin MS. Cardiac revascularization in specialty and general hospitals. N Engl J Med 2005, 352, 1454–1462.
- Basso C, Thiene G, Mackey Bojack S, Frigo AC, Corrado D, Maron BJ. Myocardial bridging, a frequent component of the hypertrophic cardiomyopathy phenotype, lacks systematic association with sudden cardiac death. Eur Heart J 2009, 30, 1627–34.
- Olivotto I, Cecchi F, Bini R, Favilli S, Murzi B, El-Hamamsy I, Yacoub MH. Tunneled left anterior descending artery in a child with hypertrophic cardiomyopathy. Nat Clin Pract Med 2009, 6, 134–9.
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007, 356, 830–840.
- Chimenti C, Morgante E, Tanzilli G, et al. Angina in Fabry disease reflects coronary small vessel disease. Circulation: Heart fail 2008, 1, 161–69.
- Chimenti C, Sale P, Verardo R, et al. High prevalence of intramural coronary infection in patients with drug-resistant cardiac syndrome X: comparison with chronic stable angina and normal controls [published correction appears in Heart. 2010 Dec;96,2045]. Heart. 2010, 96, 1926–1931. [CrossRef]
- Nollet L, Campens L, De Zaeytijd J, et al. Clinical and subclinical findings in heterozygous ABCC6 carriers: results from a Belgian cohort and clinical practice guidelines. J Med Genet. 2022, 59, 496–504. [CrossRef]
- Campens L, Vanakker OM, Trachet B, et al. Characterization of cardiovascular involvement in pseudoxanthoma elasticum families. Arterioscler Thromb Vasc Biol. 2013, 33, 2646–2652. [CrossRef]
- Zarbock R, Hendig D, Szliska C, Kleesiek K, Götting C. Analysis of MMP2 promoter polymorphisms in patients with pseudoxanthoma elasticum. Clin Chim Acta. 2010, 411, 1487–1490. [CrossRef]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015, 17, 405–424. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).