Submitted:
19 October 2023
Posted:
19 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Virus Preparation
2.2. Experimental Infection
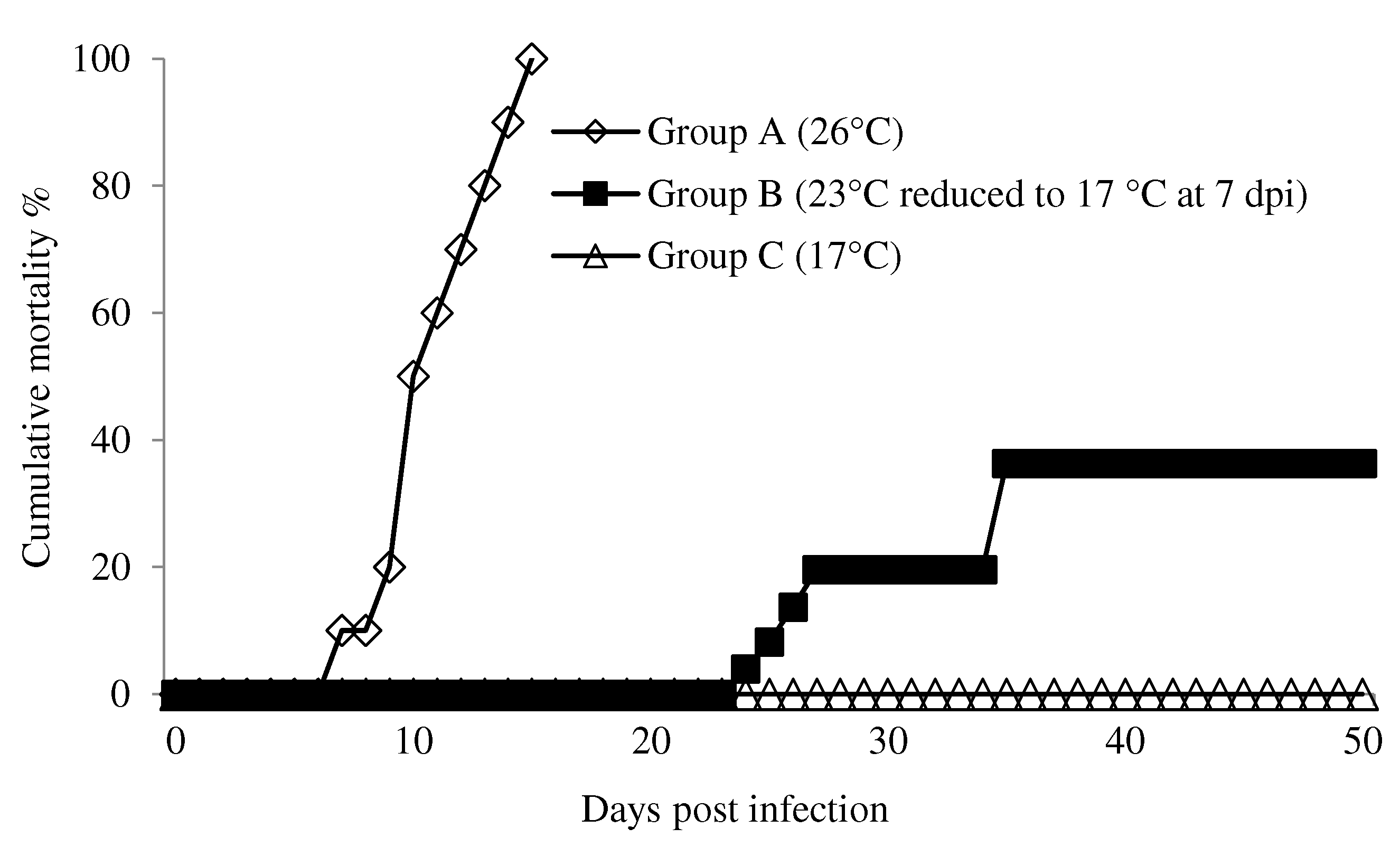
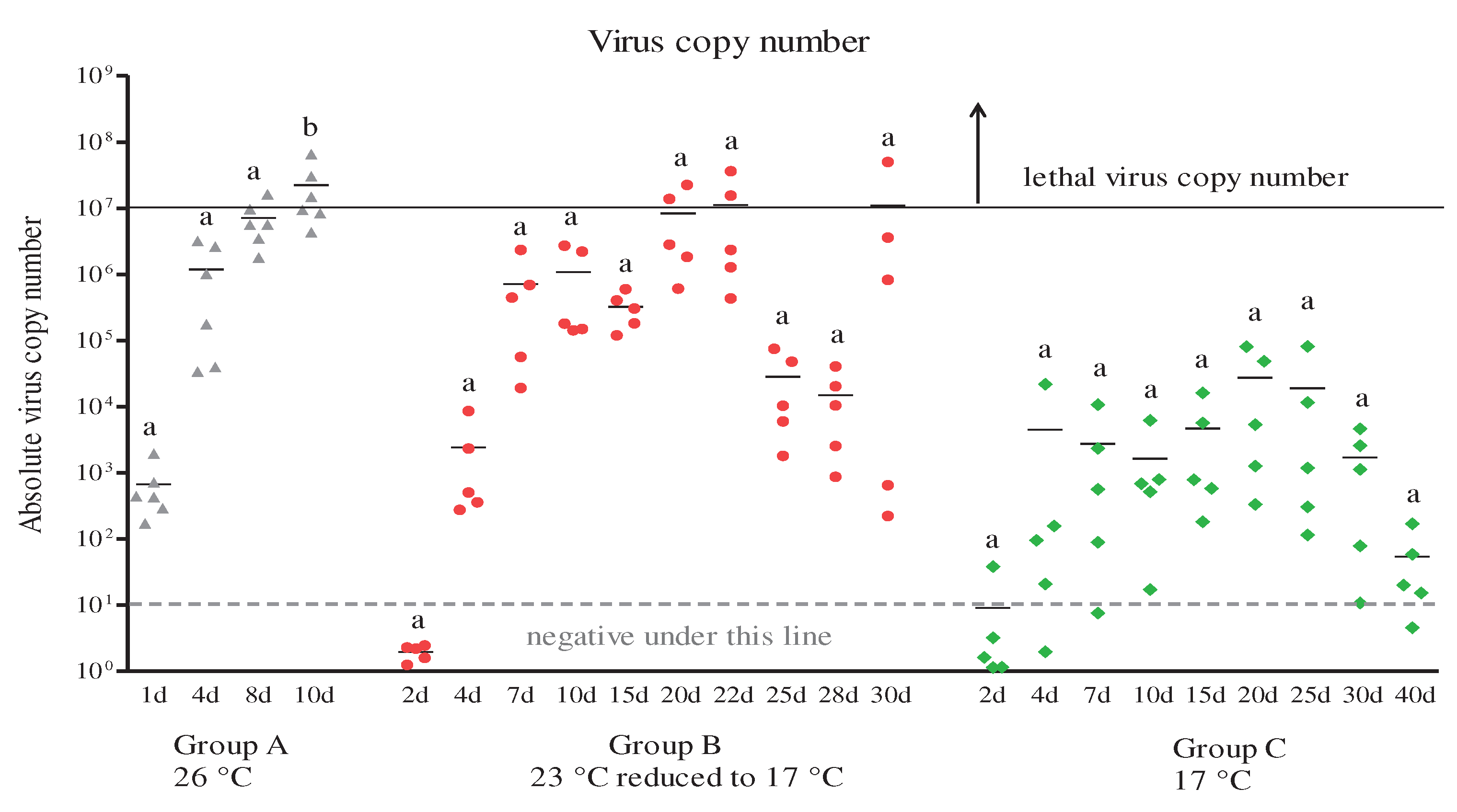
2.2.1. High-Mortality Condition (water temperature fixed at 26 °C; group A)
2.2.2. Low-Mortality Condition (water temperature Shifted from 23 °C to 17 °C; group B)
2.2.3. No-Mortality Condition (water temperature fixed at 17 °C; group C)
2.3. Quantitative expression of immune genes and determination of viral copy numbers
3. Results
3.1. RBIV Levels and Fish Mortality
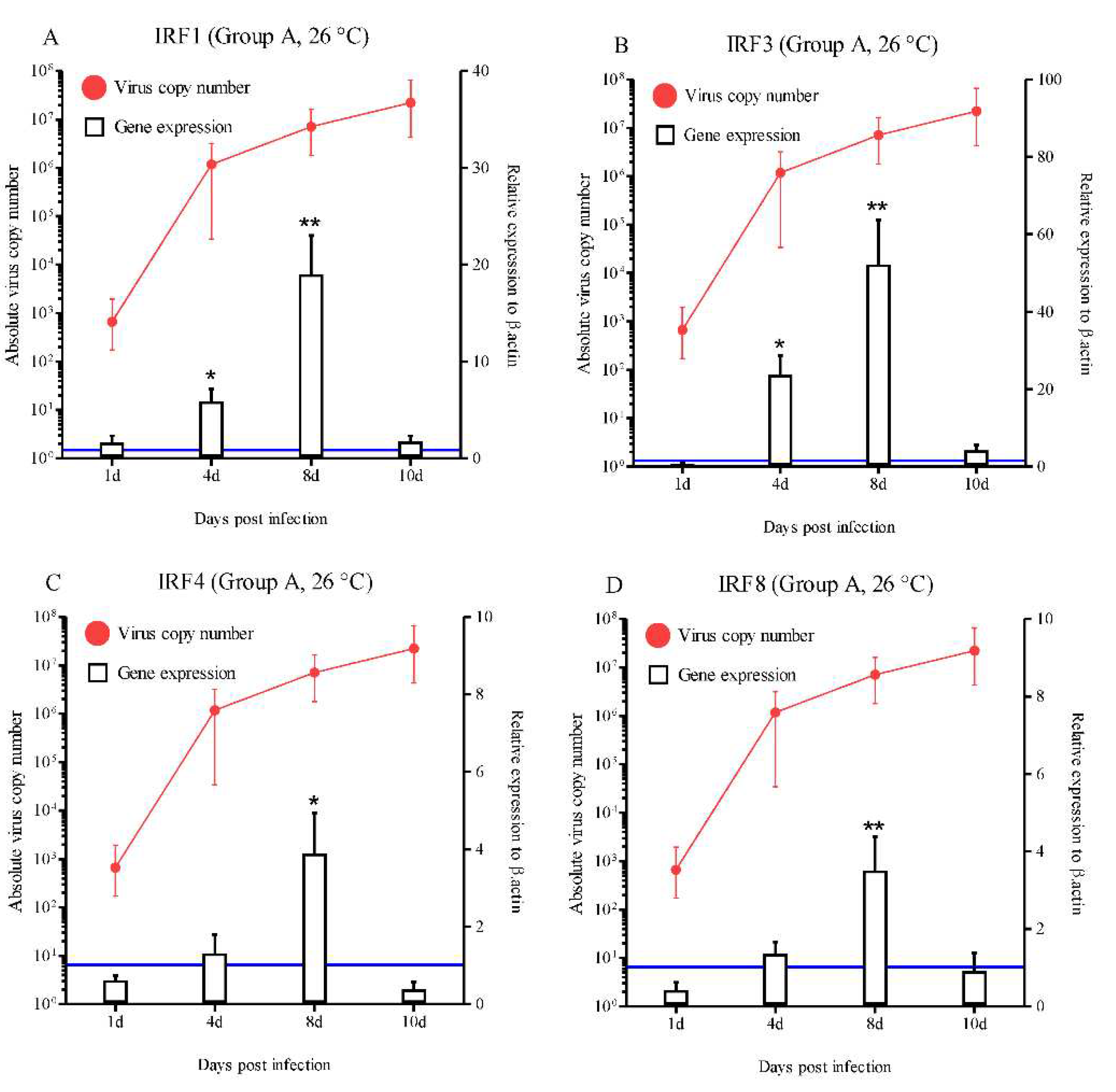
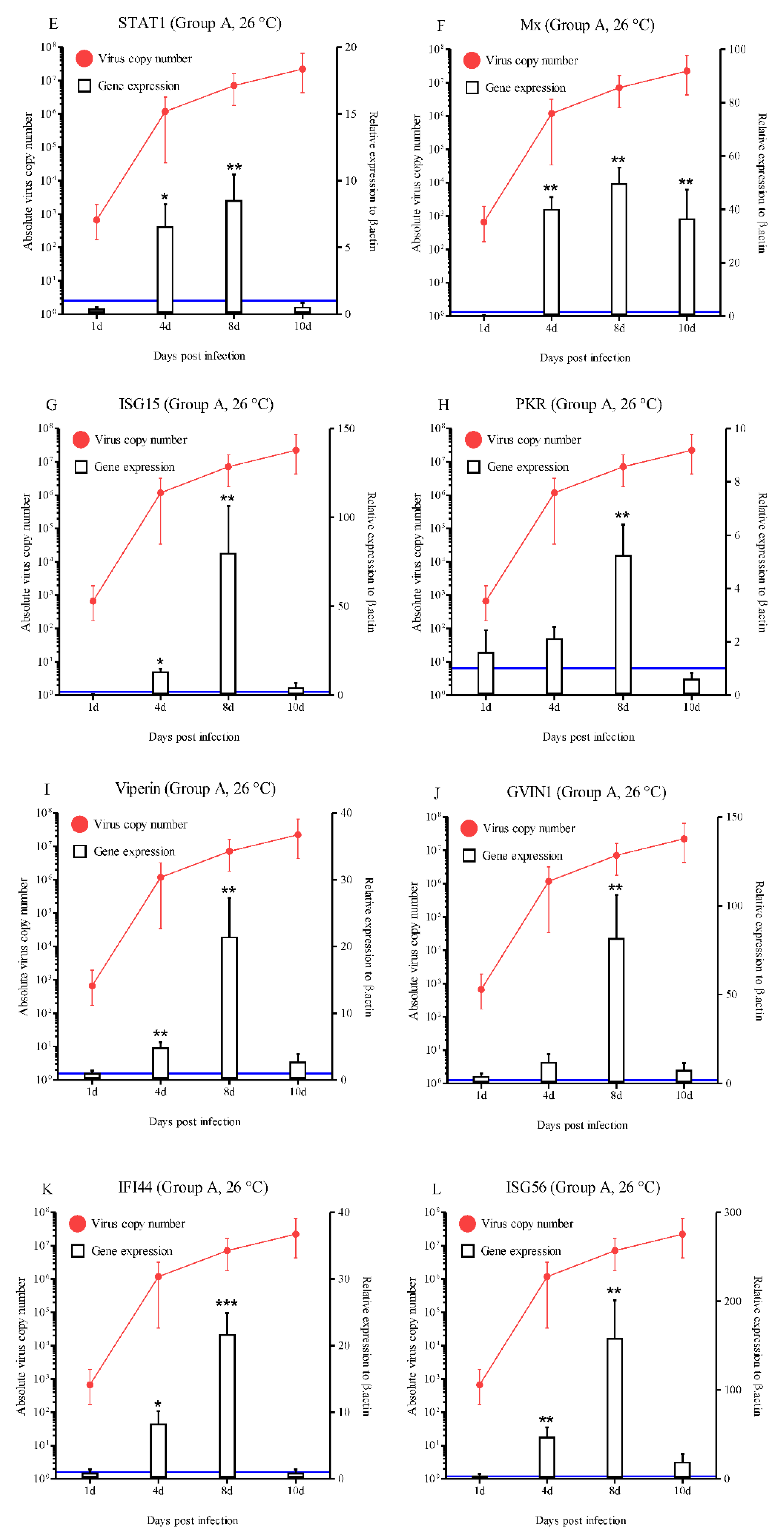
3.2. IFN-related Genes and Their Expression at Different Stages of RBIV Progression
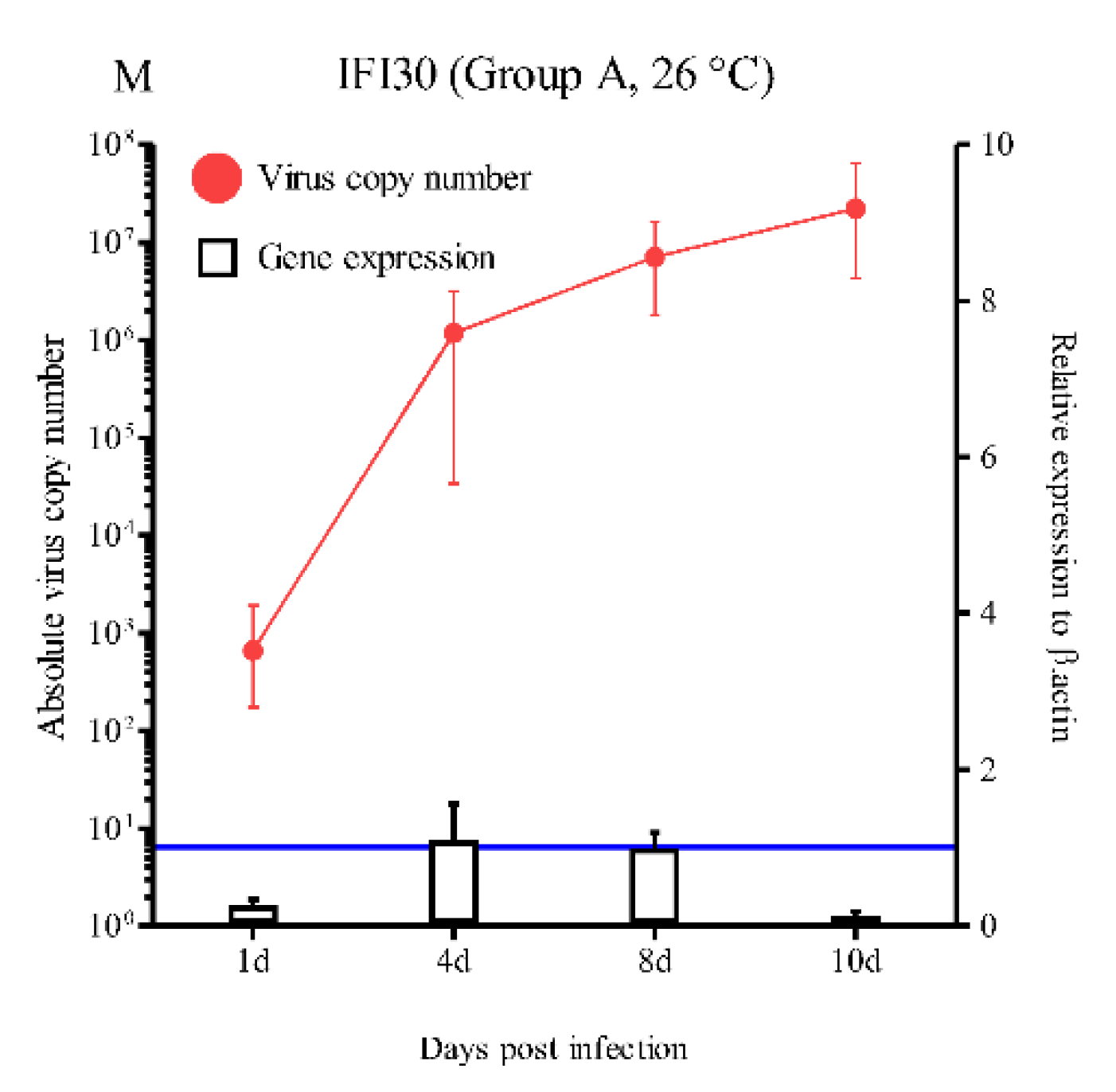
3.2.1. Expression in Group A
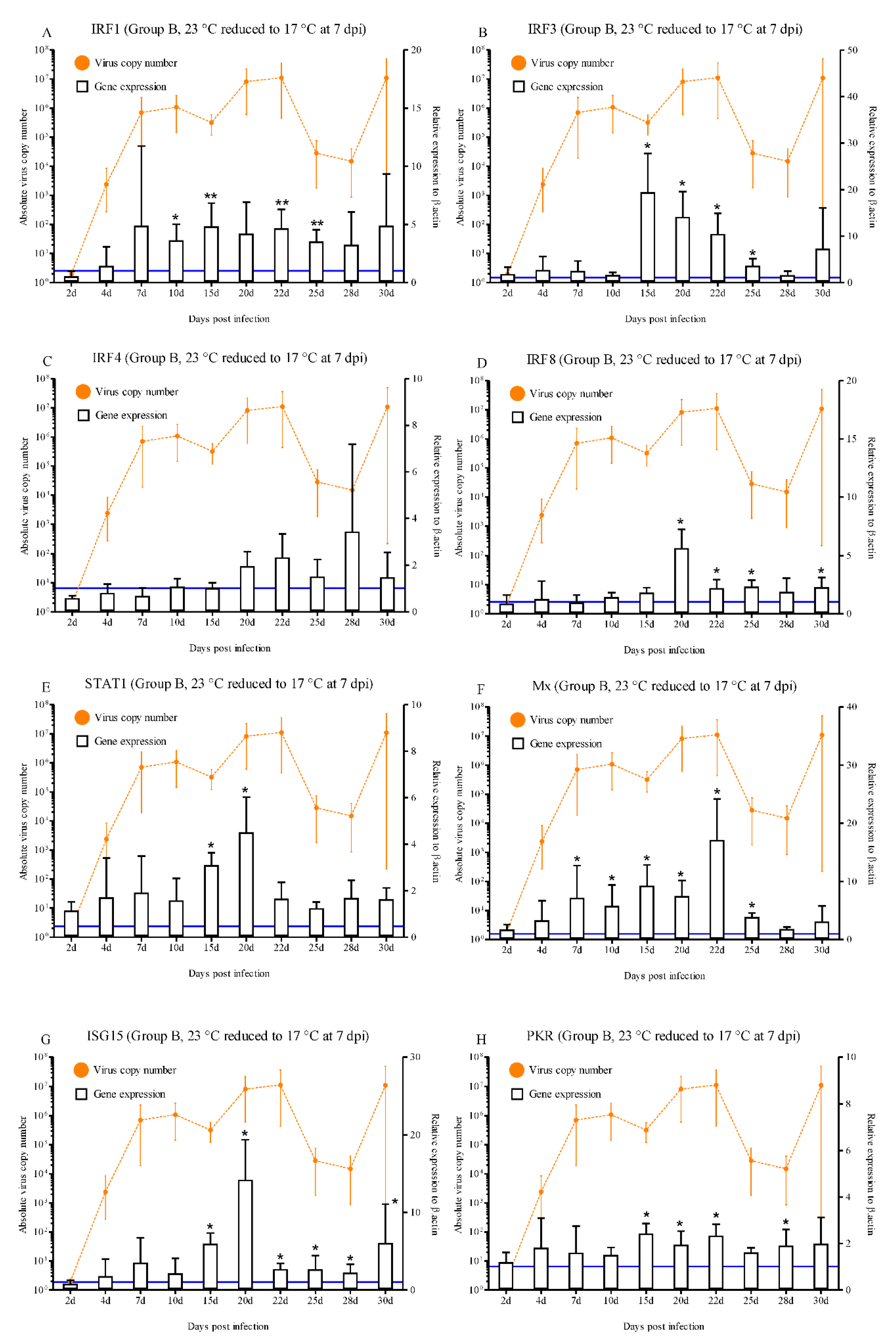
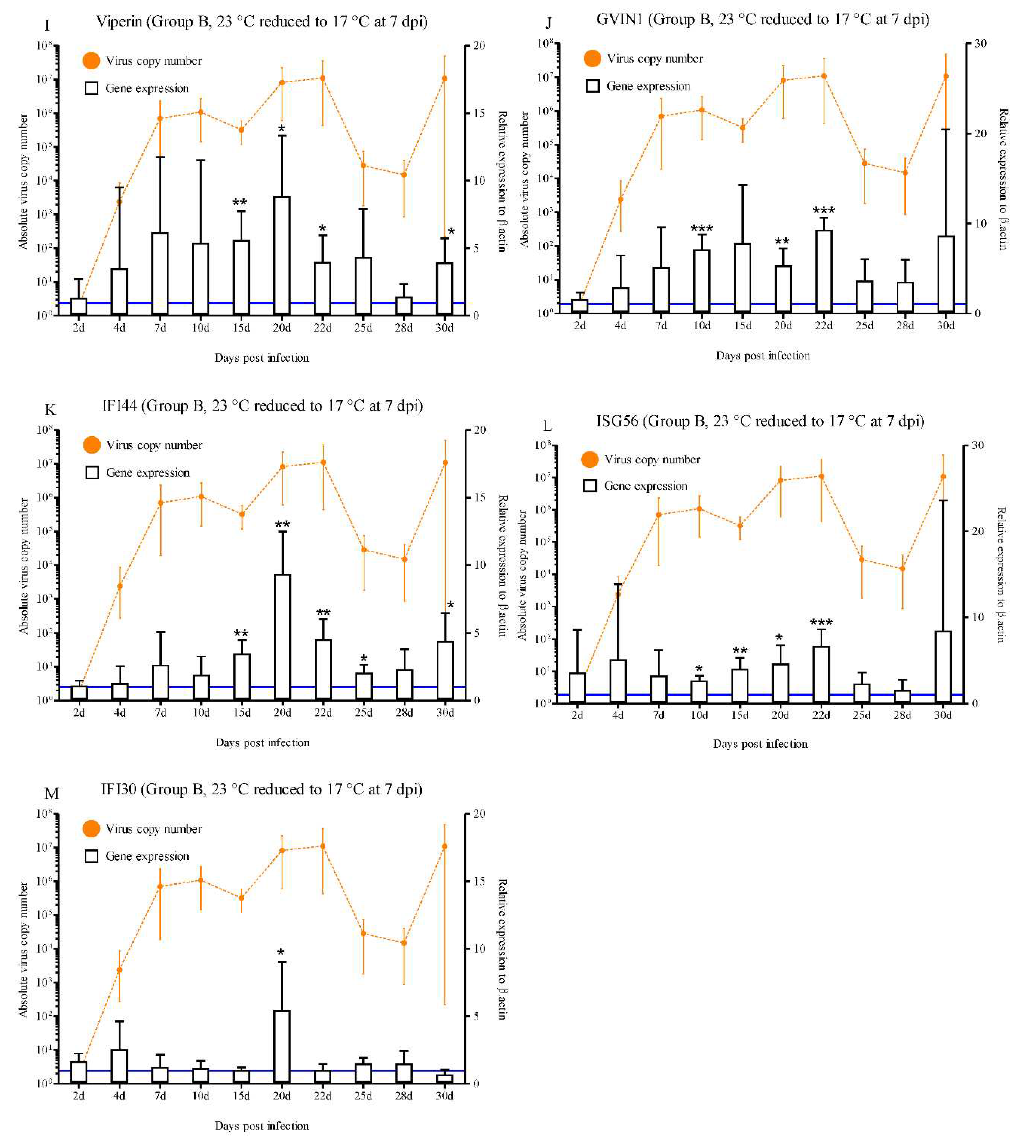
3.2.2. Expression in Group B
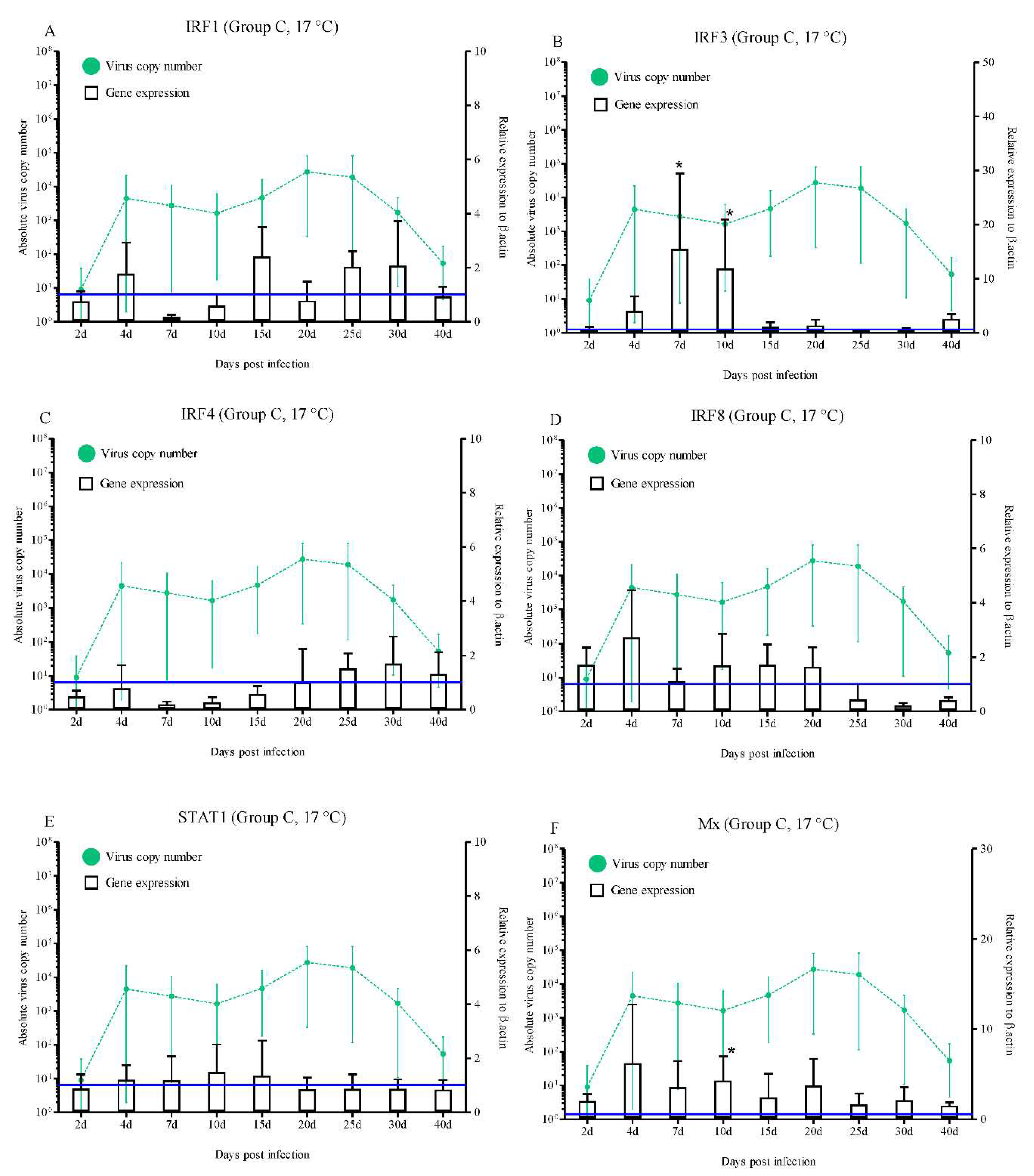
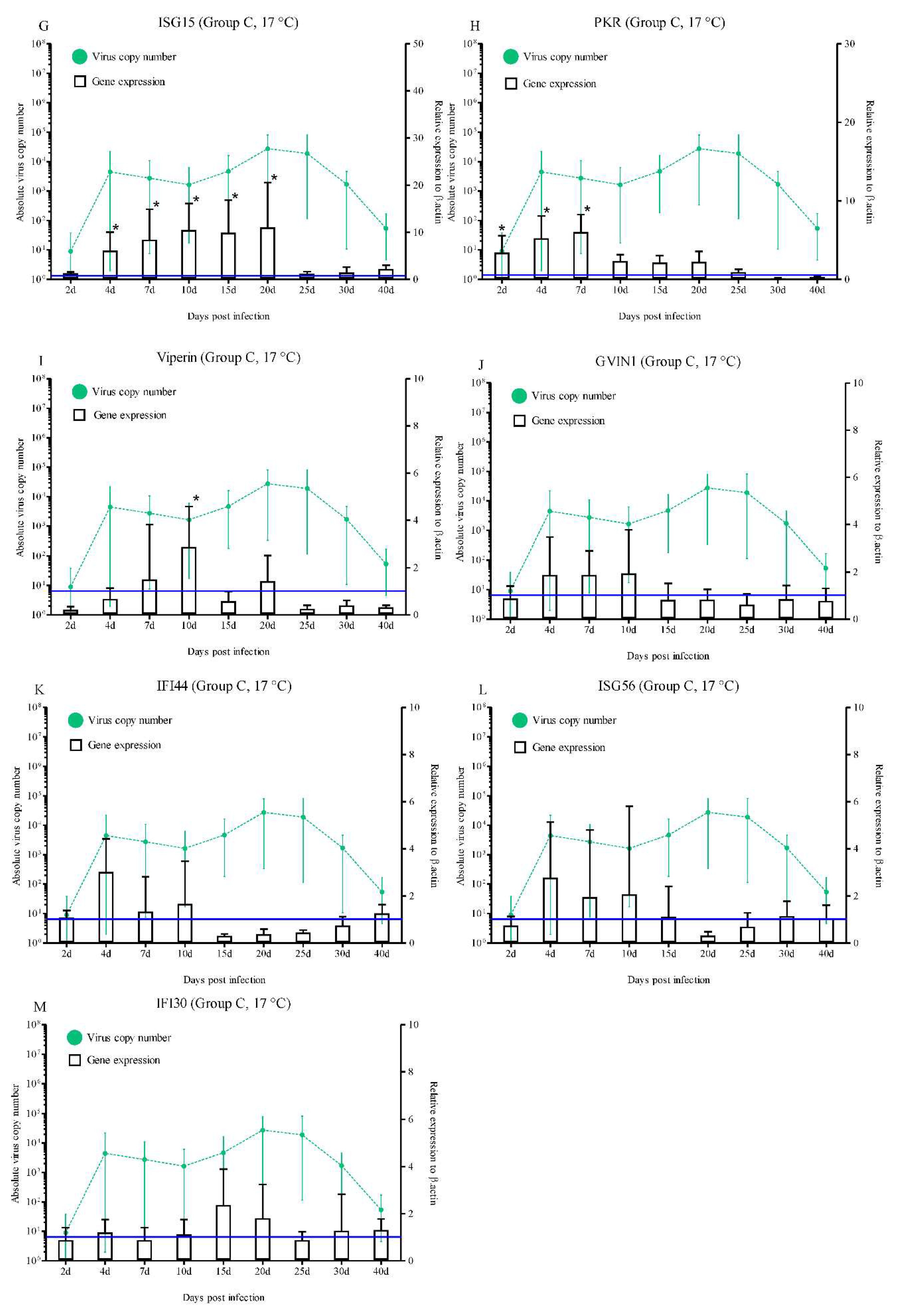
3.2.3. Expression in Group C
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurita, J.; Nakajima, K. Megalocytiviruses. Viruses. 2012, 4, 521–538. [Google Scholar] [CrossRef]
- Jung, S.J.; Oh, M.J. Iridovirus-like infection associated with high mortalities of striped beakperch, Oplegnathus fasciatus (Temminck et Schlegel), in southern coastal areas of the Korean peninsula. J Fish Dis. 2000, 23, 223–226. [Google Scholar] [CrossRef]
- Jung, M.H.; Jung, S.J.; Vinay, T.N.; Nikapitiya, C.; Kim, J.O.; Lee, J.H.; Lee, J.H.; Oh, M.J. Effects of water temperature on mortality in Megalocytivirus-infected rock bream Oplegnathus fasciatus (Temminck et Schlegel) and development of protective immunity. J Fish Dis. 2015, 38, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.H.; Lee, J.H.; Jung, S.J. Low pathogenicity of FLIV (flounder iridovirus) and the absence of cross-protection between FLIV and RBIV (rock bream iridovirus). J Fish Dis. 2016, 39, 1325–1333. [Google Scholar] [CrossRef]
- Jung, M.H.; Lee, J.H.; Ortega-Villaizan, M.; Perez, L.; Jung, S.J. Protective immunity against Megalocytivirus infection in rock bream (Oplegnathus fasciatus) following CpG ODN administration. Vaccine. 2017, 35, 3691–3699. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.H.; Jung, S.J. Protective immunity against rock bream iridovirus (RBIV) infection and TLR3-mediated type I interferon signaling pathway in rock bream (Oplegnathus fasciatus) following poly (I:C) administration. Fish Shellfish Immunol. 2017, 67, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.H.; Nikapitiya, C.; Vinay, T.N.; Lee, J.; Jung, S.J. Rock bream iridovirus (RBIV) replication in rock bream (Oplegnathus fasciatus) exposed for different time periods to susceptible water temperatures. Fish Shellfish Immunol. 2017, 70, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Gui, J.F. Molecular regulation of interferon antiviral response in fish. Dev Comp Immunol. 2012, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Bergan, V.; Kileng, Ø.; Sun, B. Robertsen B. Regulation and function of interferon regulatory factors of Atlantic salmon. Mol Immunol. 2010, 47, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Mamane, Y.; Heylbroeck, C.; Génin, P.; Algarté, M.; Servant, M.J.; LePage, C.; DeLuca, C.; Kwon, H.; Lin, R. , Hiscott, J. Interferon regulatory factors: the next generation. Gene. 1999, 237, 1–14. [Google Scholar] [CrossRef]
- Yasuike, M.; Kondo, H.; Hirono, I.; Aoki, T. Identification and characterization of Japanese flounder, Paralichthys olivaceus interferon-stimulated gene 15 (Jf-ISG15). Comp Immunol Microb. 2011, 34, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hirono, I.; Aoki, T. Cloning and analysis of expression of Mx cDNA in Japanese flounder, Paralichthys olivaceus. Dev Comp Immunol. 2000, 24, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Antiviral actions of interferons. Clin Microbiol Rev. 2001, 14, 778–809. [Google Scholar] [CrossRef] [PubMed]
- Hallen, L.C.; Burki, Y.; Ebeling, M.; Broger, C.; Siegrist, F.; Oroszlan-Szovik, K.; Bohrmann, U.; Certa, U.; Foser, S. Antiproliferative activity of the human IFN-α-inducible protein IFI44. J Interferon Cytokine Res. 2007, 27, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, B.; Phan, U.T.; Geuze, H.J.; Cresswell, P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT). Proc Natl Acad Sci. 2000, 97, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Landolfo, S.; Gribaudo, G.; Angeretti, A.; Gariglio, M. Mechanisms of viral inhibition by interferons. Pharmacol Therapeut. 1995, 65, 415–442. [Google Scholar] [CrossRef]
- Chen, Y.M.; Su, Y.L.; Lin, J.H.Y.; Yang, H.L.; Chen, T.Y. Cloning of an orange-spotted grouper (Epinephelus coioides) Mx cDNA and characterisation of its expression in response to nodavirus. Fish Shellfish Immunol. 2006, 20, 58–71. [Google Scholar] [CrossRef]
- Larsen, R.; Røkenes, T.P.; Robertsen, B. Inhibition of infectious pancreatic necrosis virus replication by Atlantic salmon Mx1 protein. J Virol. 2004, 78, 7938–7944. [Google Scholar] [CrossRef]
- Lin, C.H.; John, J.A.C.; Lin, C.H.; Chang, C.Y. Inhibition of nervous necrosis virus propagation by fish Mx proteins. Biochem Bioph Res Co. 2006, 351, 534–539. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Hirono, I.; Aoki, T. In vitro inhibition of fish rhabdoviruses by Japanese flounder, Paralichthys olivaceus Mx. Virology. 2003, 317, 373–382. [Google Scholar] [CrossRef]
- Avunje, S.; Kim, W.S.; Park, C.S.; Oh, M.J.; Jung, S.J. Toll-like receptors and interferon associated immune factors in viral haemorrhagic septicaemia virus-infected olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2011, 31, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Thanasaksiri, K.; Sakai, N.; Yamashita, H.; Hirono, I.; Kondo, H. Influence of temperature on Mx gene expression profiles and the protection of sevenband grouper, Epinephelus septemfasciatus, against red-spotted grouper nervous necrosis virus (RGNNV) infection after poly (I:C) injection. Fish Shellfish Immunol. 2014, 40, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. The influence of temperature on viral replication and antiviral-related genes response in hirame rhabdovirus-infected flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2017, 68, 260–265. [Google Scholar] [CrossRef]
- Jung, M.H.; Jung, S.J. Gene expression regulation of the TLR9 and MyD88-dependent pathway in rock bream against rock bream iridovirus (RBIV) infection. Fish Shellfish Immunol. 2017, 70, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.H.; Nikapitiya, C.; Song, J.Y.; Lee, J.H.; Lee, J.H.; Oh, M.J.; Jung, S.J. Gene expression of pro- and anti-apoptotic proteins in rock bream (Oplegnathus fasciatus) infected with Megalocytivirus (family Iridoviridae). Fish Shellfish Immunol. 2014, 37, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Nikapitiya, C.; Jung, S.J.; Jung, M.H.; Song, J.Y.; Lee, J.H.; Lee, J.H.; Oh, M.J. Identification and Molecular Characterization of Z/ZE Lineage MHC Class I Heavy Chain Homologue and β2-microglobulin from Rock Bream Oplegnathus fasciatus. Fish Pathol. 2014, 49, 93–112. [Google Scholar] [CrossRef]
- Jung, M.H.; Jung, S.J. CpG ODN 1668 induce innate and adaptive immune responses in rock bream (Oplegnathus fasciatus) against rock bream iridovirus (RBIV) infection. Fish Shellfish Immunol. 2017, 69, 247–257. [Google Scholar] [CrossRef]
- Jung, M.H.; Jung, S.J. Innate immune responses against rock bream iridovirus (RBIV) infection in rock bream (Oplegnathus fasciatus) following poly (I:C) administration. Fish Shellfish Immunol. 2017, 71, 171–176. [Google Scholar] [CrossRef]
- Jung, M.H.; Nikapitiya, C.; Jung, S.J. DNA vaccine encoding myristoylated membrane protein (MMP) of rock bream iridovirus (RBIV) induces protective immunity in rock bream (Oplegnathus fasciatus). Vaccine. 2018, 6, 802–810. [Google Scholar] [CrossRef]
- Zenke, K.; Nam, Y.K.; Kim, K.H. Molecular cloning and expression analysis of double-stranded RNA-dependent protein kinase (PKR) in rock bream (Oplegnathus fasciatus). Veterinary Immunol Immunopathol. 2010, 133, 290–295. [Google Scholar] [CrossRef]
- Wan, Q.; Wicramaarachchi, W.N.; Whang, I.; Lim, B.S.; Oh, M.J.; Jung, S.J.; Kim, H.C.; Yeo, S.Y. , Lee, J.H. Molecular cloning and functional characterization of two duplicated two-cysteine containing type I interferon genes in rock bream Oplegnathus fasciatus. Fish Shellfish Immunol. 2012, 33, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Bathige, S.D.N.K.; Whang, I.; Umasuthan, N.; Lim, B.S.; Park, M.A.; Kim, E.; Park, H.C.; Lee, J.H. Interferon regulatory factors 4 and 8 in rock bream, Oplegnathus fasciatus: structural and expressional evidence for their antimicrobial role in teleosts. Fish Shellfish Immunol. 2012, 33, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Wickramaarachchi, W.N.; Wan, Q.; Lim, B,S. ; Jung, H.B.; De Zoysa, M.; Park, M.A.; Lee, J.H.; Hwang, I.S. Genomic characterization of interferon regulatory factor 5 from rock bream (Oplegnathus fasciatus) and its role in antiviral defense. Fish Shellfish Immunol. 2014, 37, 256–267. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jung, M.H.; Jung, S.J. Correlation of virus replication and spleen index in rock bream iridovirus infected rock bream Oplegnathus fasciatus. J Fish Pathol. 2019, 32, 1–8. [Google Scholar] [CrossRef]
- Kim, H.J.; Oseko, N.; Nishizawa, T.; Yoshimizu, M. Protection of rainbow trout from infectious hematopoietic necrosis (IHN) by injection of infectious pancreatic necrosis virus (IPNV) or poly (I:C). Dis Aquat Organ. 2009, 83, 105–113. [Google Scholar] [CrossRef]
- Oh, M.J.; Takami, I.; Nishizawa, T.; Kim, W.S.; Kim, C.S.; Kim, S.R.; Park, M.A. Field tests of Poly (I:C) immunization with nervous necrosis virus (NNV) in sevenband grouper, Epinephelus septemfasciatus (Thunberg). J Fish Dis. 2012, 35, 187–191. [Google Scholar] [CrossRef]
- Avunje. S.; Jung, S.J. Poly (I:C) and imiquimod induced immune responses and their effects on the survival of olive flounder (Paralichthys olivaceus) from viral haemorrhagic septicaemia. Fish Shellfish Immunol. 2017, 71, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y,J,; Kim, K,H. Effect of CpG-ODNs belonging to different classes on resistance of olive flounder (Paralichthys olivaceus) against viral hemorrhagic septicemia virus (VHSV) and Miamiensis avidus (Ciliata; Scuticociliatia) infections. Aquaculture. 2012, 324, 39–43. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Y.; Cai, J.; Wei, S.; Ouyang, Z.; Qin, Q. Molecular cloning, expression and functional analysis of ISG15 in orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol. 2013, 34, 1094–102. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.H.; Chico, V.; Ciordia, S.; Mena, M.C.; Jung, S.J.; Ortega-Villaizan, M.D.M. The Megalocytivirus RBIV Induces Apoptosis and MHC Class I Presentation in Rock Bream (Oplegnathus fasciatus) Red Blood Cells. Front Immunol. 2019, 10, 160. [Google Scholar] [CrossRef]
- Avunje, S.; Kim, W.S.; Oh, M.J.; Choi, I.; Jung, S.J. Temperature-dependent viral replication and antiviral apoptotic response in viral haemorrhagic septicaemia virus (VHSV)-infected olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2012, 32, 1162–1170. [Google Scholar] [CrossRef]
| Group | Infection dose per fish |
Number of fish | Sampling times (dpi) | Mortality (%) |
| A (26 °C) | 1.1 × 107 | 50 | 1, 4, 8, and 10 | 100 |
| B (23 °C reduced to 17 °C at 7 dpi) | 1.1 × 107 | 60 | 2, 4, 7, 10, 15, 20, 22, 25, 28, and 30 | 28 |
| C (17 °C) | 1.1 × 107 | 50 | 2, 4, 7, 10, 15, 20, 25, 30, and 40 | 0 |
| Name | Sequence (Forward) | Sequence (Reverse) | Accession number |
| β-actin | CAGGGAGAAGATGACCCAGA | CATAGATGGGCACTGTGTGG | FJ975145 |
| MCP | TGCACAATCTAGTTGAGGAGGTG | AGGCGTTCCAAAAGTCAAGG | AY849394 |
| IRF1 | CTGTGACCCAAAGACGTGGA | CTTGGTGGCCTTTGTTGACG | GQ903769.1 |
| IRF3 | GTGTCTAAAGGGACTGAACATCG | CCCTCAAACGTTACTGGATACTG | KF267453.1 |
| IRF4 | ATGGCTTATACGCTAAGCGCCTCT | GTTTGCATGGCTGTTCCTTCTCCA | JQ388475 |
| IRF8 | GGAGCTCTTCAGCGTGTACTAGA | CCTCCTTTATGTCTCCACTGTGA | JQ388478 |
| STAT1 | AGCTCAACATGCTGGCTGACAAAC | AAAGCTTTCTCGTTGGCGCTCTTG | KX714722 |
| Mx | GATCGCCTCTCCTGATGTTC | ACCACCAAGCTGATGGTTTC | FJ155359.1 |
| ISG15 | CTTTAAGACCAAGGTCCAATGC | GCCTCCACATTGTAGTCTGAAAG | BAJ16365.1 |
| PKR | GCTCAACAAATAGTCAGTGGAGT | CTCTGGTGACCAGACCAAAGTCC | FJ179396.1 |
| Viperin | TCAGCAGTACAAAGTGGCGT | TCCCCGTCTATCAGCAGACA | AB665349.1 |
| GVIN1 | TGCAGCTGGTCAAAGTGTCA | CGCTACCTGCCAACTCAAGA | MK674614 |
| IFI44 | GCAGTTCGAGCTCAGAAGGT | CGAGCCTGAGTCACATCCTC | MK674612 |
| ISG56 | GTGAGCGCCTTTAAGCACAG | AGGTACAGAGCAGCGAGGTA | MK674613 |
| IFI30 | GCTGCAGAGGGTTTCTCACT | ATTCCTGTTCCCCATGCTGG | GQ903766.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




