1. Introduction
The concept of green synthesis has garnered significant attention among nanotechnology researchers. Typically, the synthesis of nanoparticles, such as silver (Ag), gold (Au), and platinum (Pt), involves chemical and physical approaches [
1,
2,
3]. However, the biological method has emerged as a more economically viable, eco-friendly, and non-toxic alternative for nanoparticle synthesis. Various biological entities, including microorganisms, algae, bacteria, viruses, and plants, have been explored for their potential in green biological nanoparticle synthesis [
4,
5,
6]. Among these biological sources, food-plants’ origin have gained prominence due to their ability to produce a wide range of phytochemicals that are non-pathogenic and facilitate a faster synthesis process compared to microorganisms. Consequently, plants are considered highly advantageous for nanoparticle production. Extensive research has been conducted on synthesizing a diverse spectrum of nanoparticles using various plant species. The stabilization and production of silver nanoparticles, for instance, have been found to rely on the presence of phenolic chemicals, flavonoids, alkaloids, proteins, and reducing sugars found within plants. These compounds serve as crucial reduction and capping agents, contributing to the efficient synthesis of silver nanoparticles [
7]. This green approach to nanoparticle synthesis holds promise for both its environmental benefits and its potential for a wide array of applications.
Aerodramus fuciphagus, commonly known as the “white-nest swiftlet”, was created by a small bird belonging to the swift family. It is typically found in Southeast Asia, primarily inhabiting regions in Southeast Asia and South China. These birds construct nests primarily using their saliva, which solidifies to form edible bird nests (EBN). EBN is categorized into two types, namely
Aerodramus fuciphagus (white-nest) and
Aerodramus maximus (black-nest). These nests contain various nutritional components, including amino acids, essential trace elements, proteins, carbohydrates, and heavy metals [
8]. A previous study compared the nutritional value of white-nest swiftlets from Pahang and Terengganu, from Malaysia, highlighting the significance of calcium, sodium glycoprotein, carbohydrates, and potassium in EBN [
9,
10].
Traditionally, EBN has been a part of Chinese cuisine and is often prepared as a soup by double boiling, typically sweetened with rock sugar. Chinese people consume EBN for its numerous health benefits, such as promoting the growth of new skin cells and maintaining smooth skin. EBN is also known for its antiviral and antimicrobial properties against viruses and bacteria, with sialic acid being a key component contributing to its antiviral effects. Experimental studies in mice have shown that maternal administration of EBN during the pregnancy and lactation periods can improve the spatial learning performances in the offspring [
11]. Moreover, there is evidence indicating that EBN extract consumption can increase bone mass and slow the skin aging in postmenopausal women [
12]. Research conducted by Khalid et al. suggests that to enhance the cell repair from damage through higher cell proliferation rate and proper functioning maintenance in the wound healing of corneal tissues [
13].
To the best of our knowledge, there have been no prior studies investigating the intercorrelation between silver nanoparticles and bioactive compounds of edible bird nests. Given the increasing potential of EBN extracts to serve as effective reducing and capping agents for nanoparticles, we introduce a novel biological extract for the synthesis of stable silver nanoparticles (AgNPs) operating via consumption of EBN product. In this method, we successfully synthesized nanoparticles without the need for a stabilizing agent, employing amount of silver nitrate (AgNO3) while adjusting the EBN concentration accordingly. The characterization of the produced silver nanoparticles was conducted through UV-Visible spectrophotometry, FTIR, FESEM, and EDX analysis.
2. Materials and Methods
2.1.Equipment and Chemicals
The AgNPs were characterised using a UV-Visible spectrophotometer (Thermo Scientific GENESYS 10S UV-Visible spectrophotometer), Fourier transforms Infrared Spectroscopy (FTIR, PerkinElmer, USA), Fourier scanning electron microscope (FESEM, JSM-7800F) equipped with energy dispersive X-ray diffraction (EDX) analyser. Silver nitrate solution (0.05 M AgNO3) in deionised distilled water purchased from (Sigma-Aldrich, Saint Louis, MO, United States).
2.2. Preparation of EBN Extract and Stock Solution
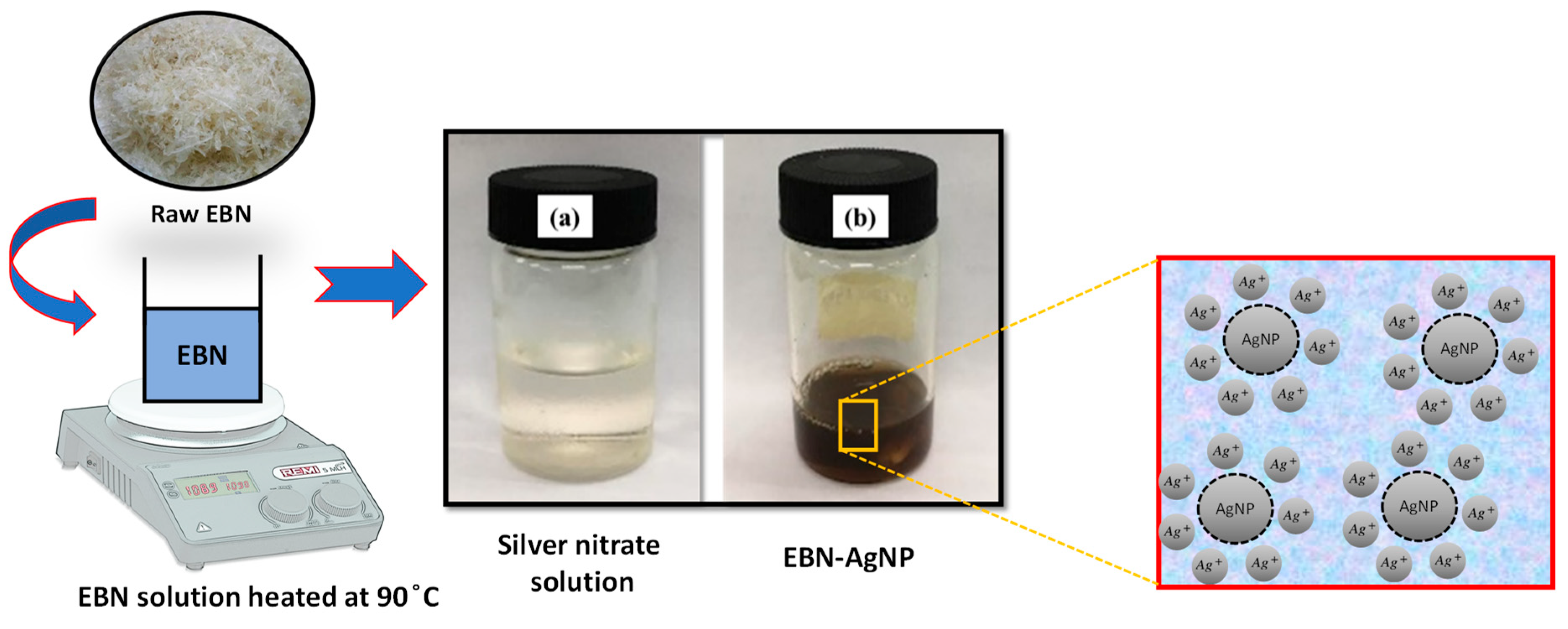
The edible bird nests (EBN) in a chips form were bought from Prosper Food & Beverages Sdn. Bhd. in Kuala Lumpur in their raw, cleaned form. Edible bird nests were weighed for 6 % (w/v in 100 mL deionised water) by heated and stirred at 90 °C for 1 hour until clear solutions formed. After the solution was fully cooled, EBN solution were filtered by filter paper WhatmanTM. The extract was kept at 4 °C until it was needed.
2.3. Biosynthesis of Silver Nanoparticles
The solid crystalline AgNO3 was prepared at 0.05 M solution concentration. An amount of 10 mL EBN extract was prepared at room temperature, thereafter, mixed with 800 µl of 0.05M AgNO3 aqueous solution. The mixed solution was kept stirred for 15 minutes and the colour transformation was observed and recorded.
2.4. Characterization of EBN-AgNP Nanoparticles
The observation of colour changes was made with a UV-Visible spectrophotometer (Shimadzu UV-1800) in a spatial range of 200-800 nm to determine the presence of AgNP. The absorbance reading was taken on a fully completed reduction of AgNO3, - colour changed from colourless to dark brown after 15 minutes.
The FTIR (Perkin Elmer Spectrum 100) characterisation was used to determine the functional groups in white-nest swiftlets (Aerodramus fuciphagus) responsible for reducing and capping AgNP. The prepared sample was placed on an ATR sample cell and run in the wavelength range of 4000-650 cm-1.
Particle size and morphological structures of AgNP were measured using the FESEM (JSM-7800F). A film of nanoparticle sample was made by dropping it on carbon-coated copper grids and was allowed to dry in the chamber for 2 hours. The morphology images of AgNP were produced at 1.3 nm resolution in the high vacuum of 30 kV. Element identification in silver nanoparticles was identified using EDX analysis attached to the FESEM analyser. The same sample on FESEM has been used in EDX.
Chromatographic analyses were carried out by LC-QTOF mass spectrophotometer (Vion IMS LC-QTOF-MS) in positive ion mode to screen non-volatile compounds in EBN extract. A 5-minute linear gradient at flow rates of 0.6mL/min between solvent A (water + 0.1 % Formic acid) and solvent B (Acetonitrile) was used. The mass spectra were recorded between the range of m/z (50-100). High-purity nitrogen was used for cone gas (50 L/h), desolvation gas (800 L/h) and a capillary voltage of 2.50 kV. The source temperature was 120 C and the desolvation temperature was 550 °C.
2.5. Antibacterial Activity of AgNPs
Biosynthesized antimicrobial activity of EBN-AgNP nanofibers was tested against bacteria gram-positive: Staphylococcus aureus (S. aureus) and gram-negative: Escherichia coli (E.coli) using agar diffusion assay. Nutrient agar (NA) was used to keep bacterial cultures alive at 4°C. To create the bacterial suspension, a single bacterial colony was taken from NA and added to 10 mL of sterile saline water with a sterile loop. The resulting microbial culture suspension's optical densities were modified and compared to the 0.5 McFarland standards. 100 µL of bacterial suspension was then spread over sterile potato dextrose agar (PDA) plate. The sterilise nanofiber disc was transferred to the middle surface of the plate. The zone of inhibition was then determined after the plates had been incubated at 37 °C for 24 hours.
3. Results and Discussion
3.1. UV-Visible Analysis of AgNPs
In this experiment, AgNPs were synthesised by reducing silver ions using edible bird nest (EBN) extract. The effect of EBN concentration was studied. Initial research was done under the lighting of a laboratory. 15 minutes after the addition of AgNO
3 to the EBN solution, the reaction mixture's colour changed from colourless to reddish-brown,
Figure 1. The colour change and its stabilisation after 15 minutes indicated the green synthesis of silver nanoparticles (biotransformation from Ag
+ to Ag
0). According to a prior study, proteinase-rich spiderweb and paper wasp nests may be responsible for the production of silver nanoparticles. This appearance of colour in the reaction mixture is due to the strong absorption of electromagnetic waves called surface plasmon resonance (SPR). The existence of AgNPs was detected through UV-Visible spectroscopy between 300 nm to 800 nm after a 15-minute reaction. The SPR phenomenon started when nanoparticles were enforced by an electromagnetic field. The excitation peaks of the UV-visible spectrum stated the creation of AgNPs.The SPR bands showed depend upon the nanoparticle size. This study thus demonstrates that the eco-friendly synthesis of silver nanoparticles can also be implied by the metabolites of edible bird nests.
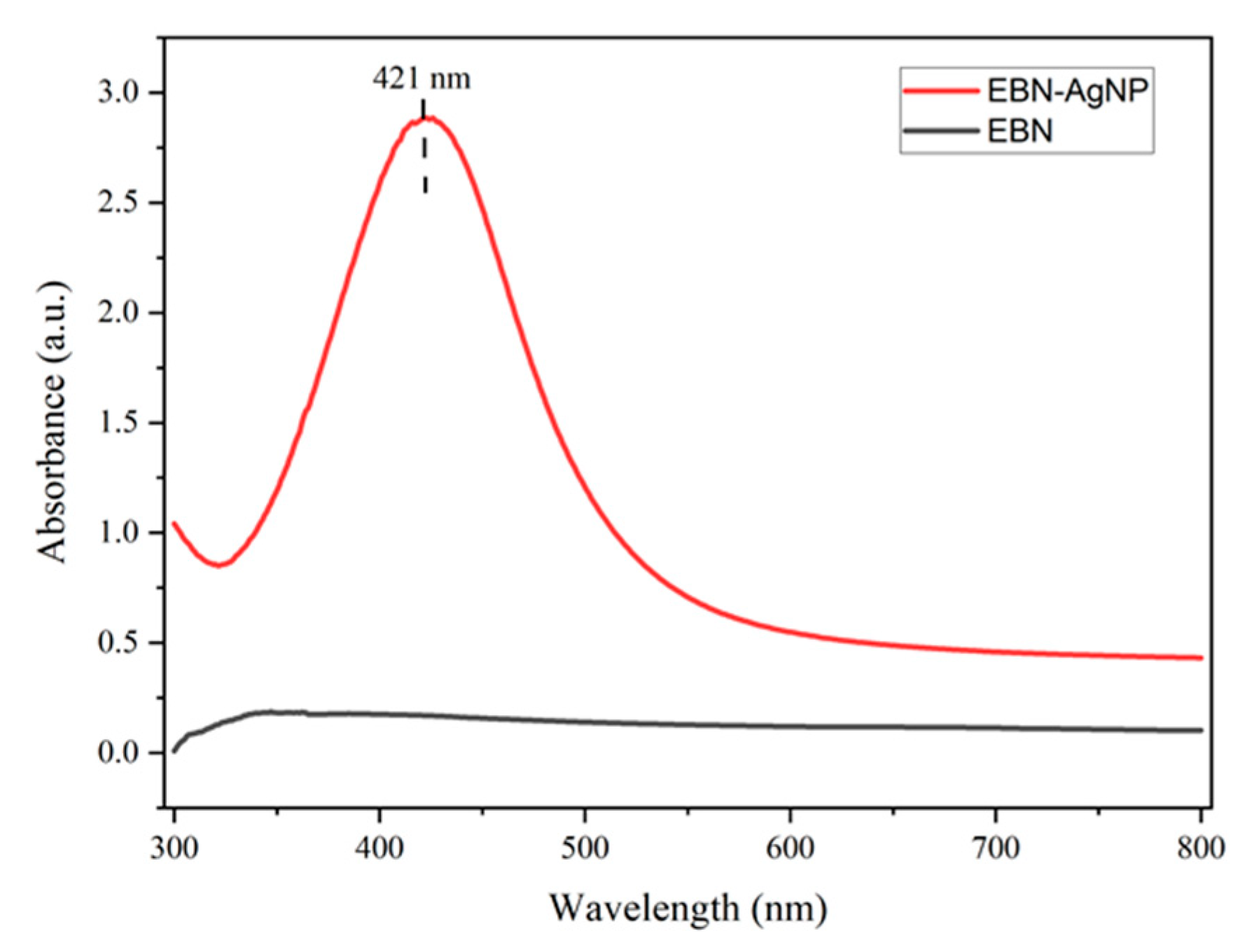
The SPR bands in the UV-Vis spectra of AgNPs samples were formed in
Figure 2. The absorption spectra were performed at wavelength 300 to 800 nm. The excitation of surface plasmon vibration showed absorbance at 421 nm after 15 minutes synthesis of EBN added with silver nitrate, whereas no peak was observed in this region with EBN alone
Figure 2. This indicated that EBN contains phenolic agents and other compounds responsible for capping and stabilizing silver synthesis. As reported by earlier research, the observed broadening of the maximum peak within the range of 400 to 450 nm might be attributed to spherical nanoparticles. Several other authors have stated that AgNPs were observed on the absorption spectrum between 425 to 460 nm. In a study, green tea extracts synthesized silver showed a strong peak at 217 nm. The stabilizing action of the extract was impacted by the production of metal nanoparticles.
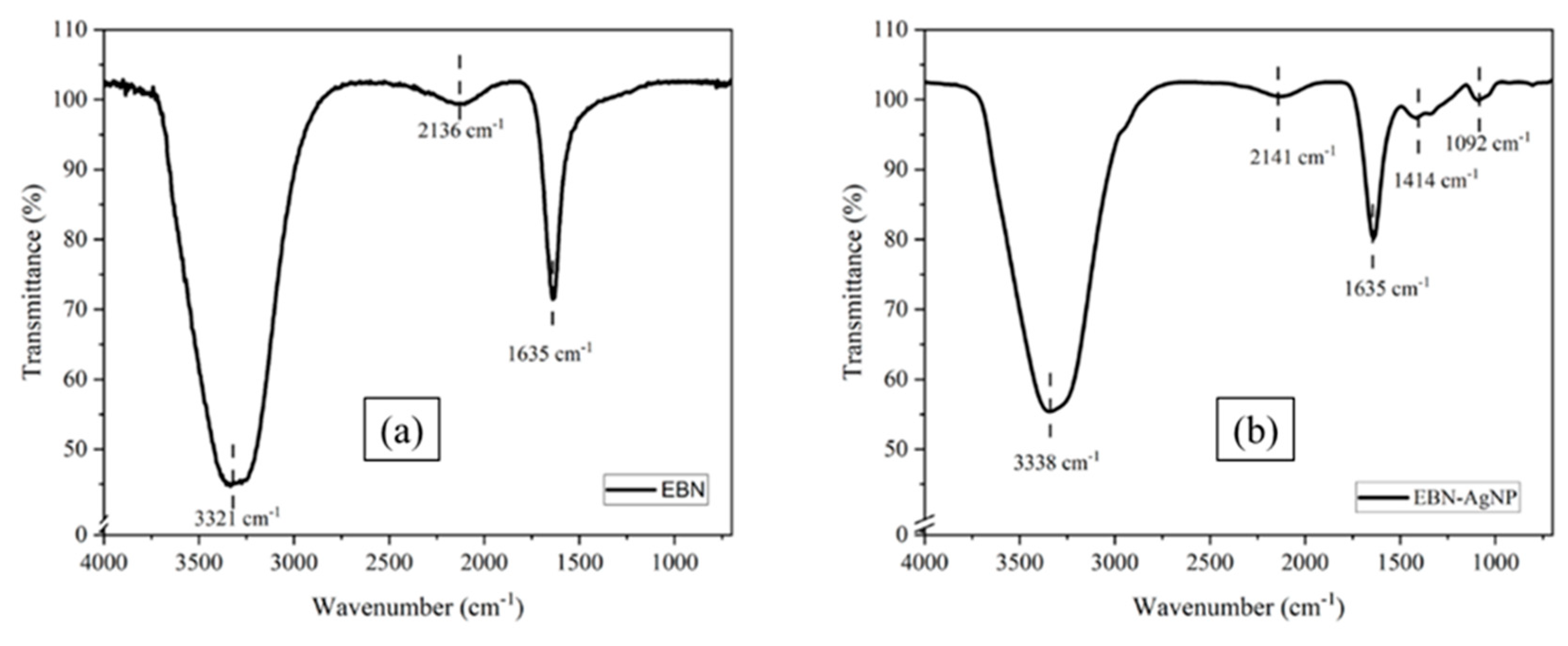
3.2. Evaluation of FT-IR Analysis Data
The surface composition of the solutions involved in the reducing and stabilising process for synthesised silver nanoparticles was described using FTIR analysis data on both EBN and AgNPs. The results showed three absorbance bands occurred at 3321 cm
-1, 2136 cm-1, and 1635 cm
-1 for edible bird nests extract
Figure 3a. 3321 cm
-1 suggests O-H stretching in alcohol and phenolic metabolites, 2136 cm
-1 represents C≡N stretching in the alkyne group, and 1635 cm
-1 refers to C=O stretching in the carbonyl group. The carbonyl group from the amino acid proved the presence of a chelating agent that can absorb and bind on the metal nano-sized particles of the silver ions.
Figure 3b showed the peaks shifted increases and the addition of some weak peaks indicating that the compound in EBN contributed to the biosynthesis reduction of AgNPs. The peaks at 3321 cm
-1 and 2136 cm
-1 shifted to 3338 cm
-1 and 2141 cm
-1, while 1635 cm
-1 remained the same as a strong and sharp peak after the synthesis process occurred. The new peak at 1414 cm
-1 and 1092 cm
-1 also appeared as O-H bending, and C-N stretching in the amine group might also be responsible for capping AgNPs.
3.3. Evaluation of FESEM and EDX Analysis Data
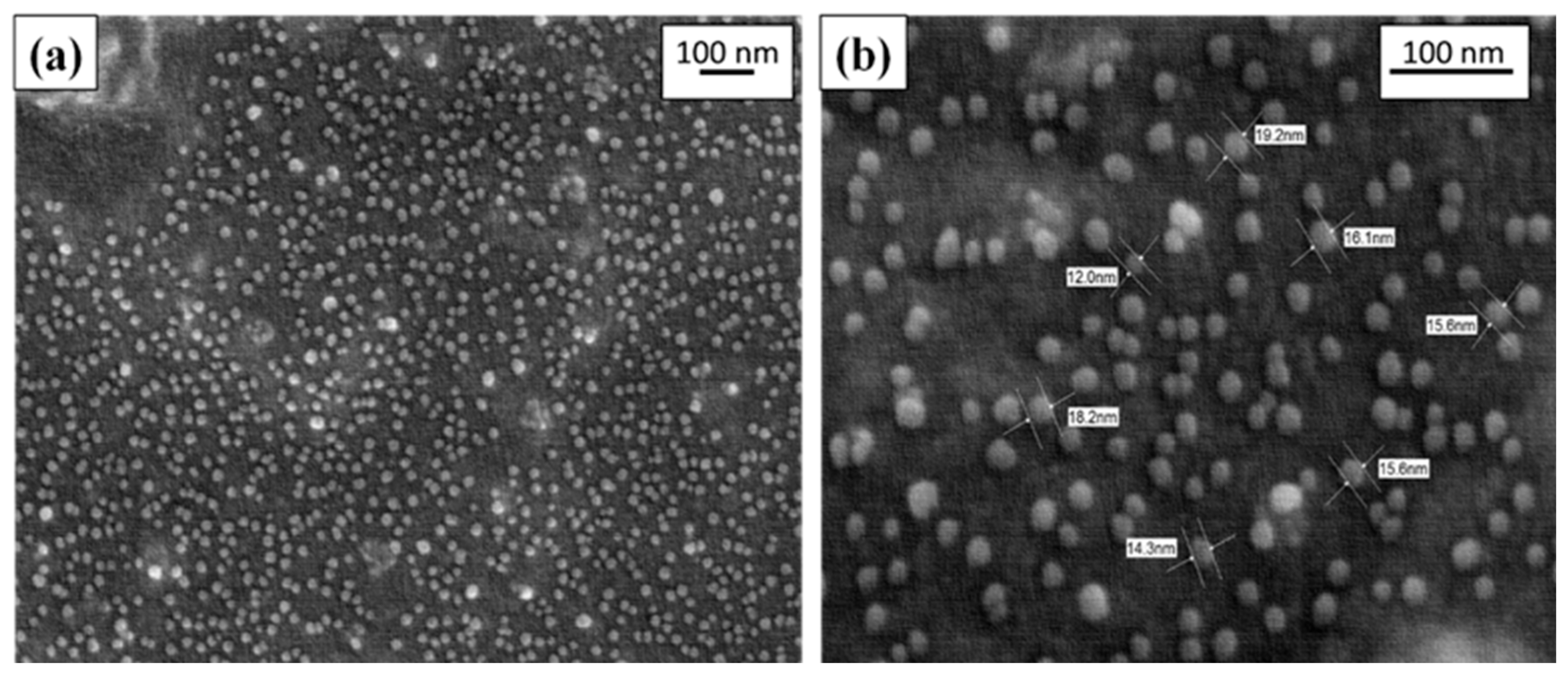
AgNPs synthesised were further characterised through FESEM and EDX analysis to know the size and shape of nanometals. For the FESEM analysis, a drop of synthesised AgNPs solution was pipetted out over the carbon-coated grids disc and dried in the chamber to form a thin smear. The surface morphology and elemental composition of EBN-AgNPs were analysed through FESEM build with EDX analysis. In
Figure 4b, FESEM images clearly showed the distribution of spherical-shaped silver nanoparticles, ranging from 10 to 20 nm. The average diameter of silver nanoparticles spherically shaped was found in a range from 10 to 35 nm. According to the results, nanomaterials were almost spherical, nano-sized and in clusters that were not attached. Thus, it can be stated that the AgNPs were in stable condition.
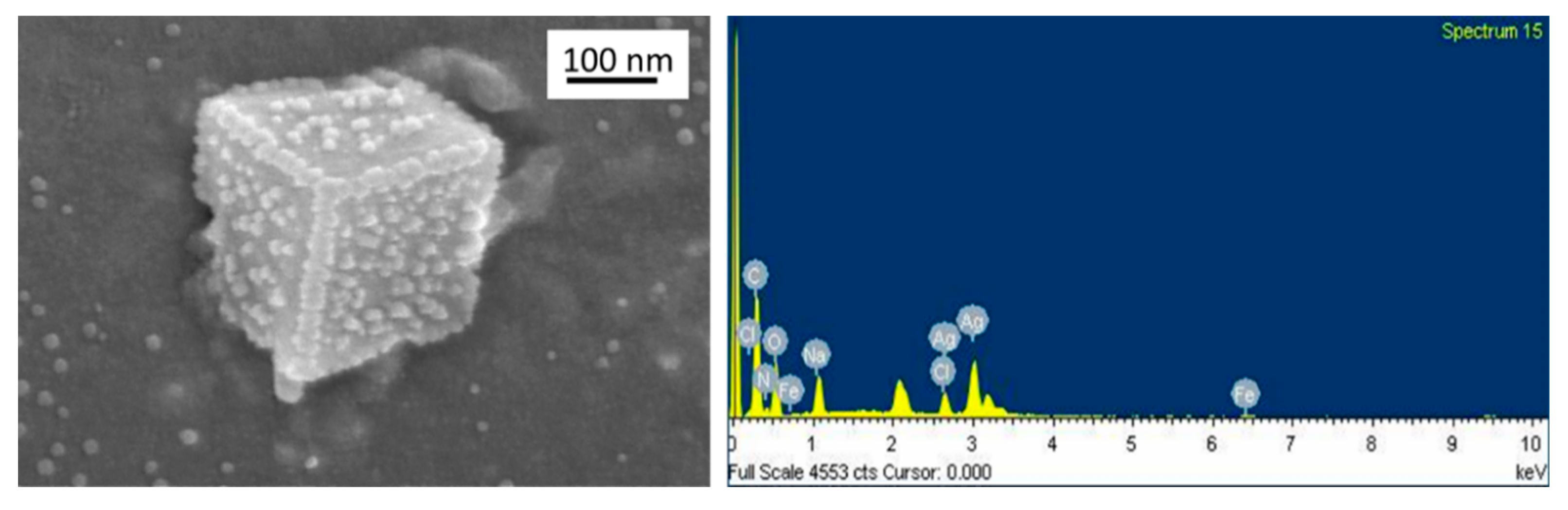
By using EDX analysis in conjunction with FESEM EDX profile photos, which revealed a peak at 3 keV, the elemental composition of the AgNPs was verified, confirming the presence of silver (
Figure 5). The other component also detected, such as C, O and N. The phytochemical elements on the surface of AgNPs reveal emerging peaks on the spectrum. Carbon and oxygen peaks on spectral images were recorded due to the presence of biomolecules on AgNPs. All elements showed different peaks on the X-ray spectrum due to different atomic structures. The EDX analysis confirmed the signal of silver nanoparticles approximately at 3 keV due to surface plasmon resonance. Spectral images revealed the silver peaks at 2.98 keV to establish the silver element's presence.
Table 1 shows the atomic percentage of the silver ions for five concentrations of AgNPs. The concentration of AgNPs was directly proportional to atomic percentage. The weight of silver element affects the amount of phenolic agent in reducing and stabilising nanoparticles.
3.4. Liquid Chromatography Quadrupole Time of Flight Mass Spectrometry (LC-QTOF-MS) Analysis
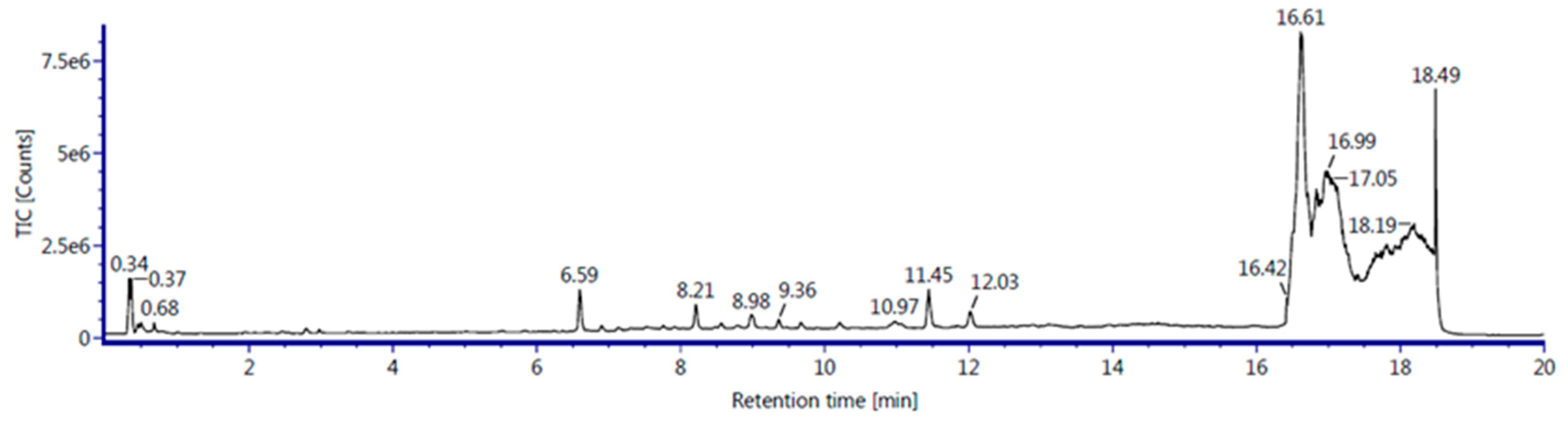
The analysis of EBN with LC-QTOF-MS revealed that metabolites group were identified in 3% and 4% of EBN extract. Solution EBN was test using this equipment to screen the non-volatile compound contain in EBN.
Table 2 presents identified compounds with their retention time (min), compound name, retention drift (ms), compound nature and response. The identified metabolites in this analysis belong to different chemical classes. It is important to identify the metabolites composition in EBN to know the composition that can give health benefit towards EBN.
Figure 6.
Total ion chromatogram of the EBN extract with LCQTOF MS.
Figure 6.
Total ion chromatogram of the EBN extract with LCQTOF MS.
This study reported metabolite compositions in EBN and each of that has their own functions. Firstly, for the compound group of lipids, cortisone is a steroid drug was found that used to treat allergic reaction, treatment of autoimmune disease and helps to decrease on inflammation and immune response. This composition will stop the release of molecules that cause inflammation. Other than that, sulfatide also found that usually major in myelin sheath. It has important function includes regulation of cell growth and cell adhesion. Tricosanoic acid can be categorized in fatty acids with alternative parents of carboxylic acids. This compounds reported to have antibacterial effect against E.coli and S.aureus. Next, cholesteryl ester also found to be used in intestinal cholesterol absorption. Peptides group in protein was found as Thymidine in EBN extract. Thymidine used in DNA replication and synthesized of cells. Moreover, Citrusin C was belonged to the class of organic compound known as phenolic glycosides. This was proven that EBN contain phenolic structure that help in this study to naturally reducing the silver ions for synthesis of silver nanoparticles. Polysaccharide of mannotriose also suggested in enhancing the cell viability.
3.5. Effect of AgNPs against Bacteria
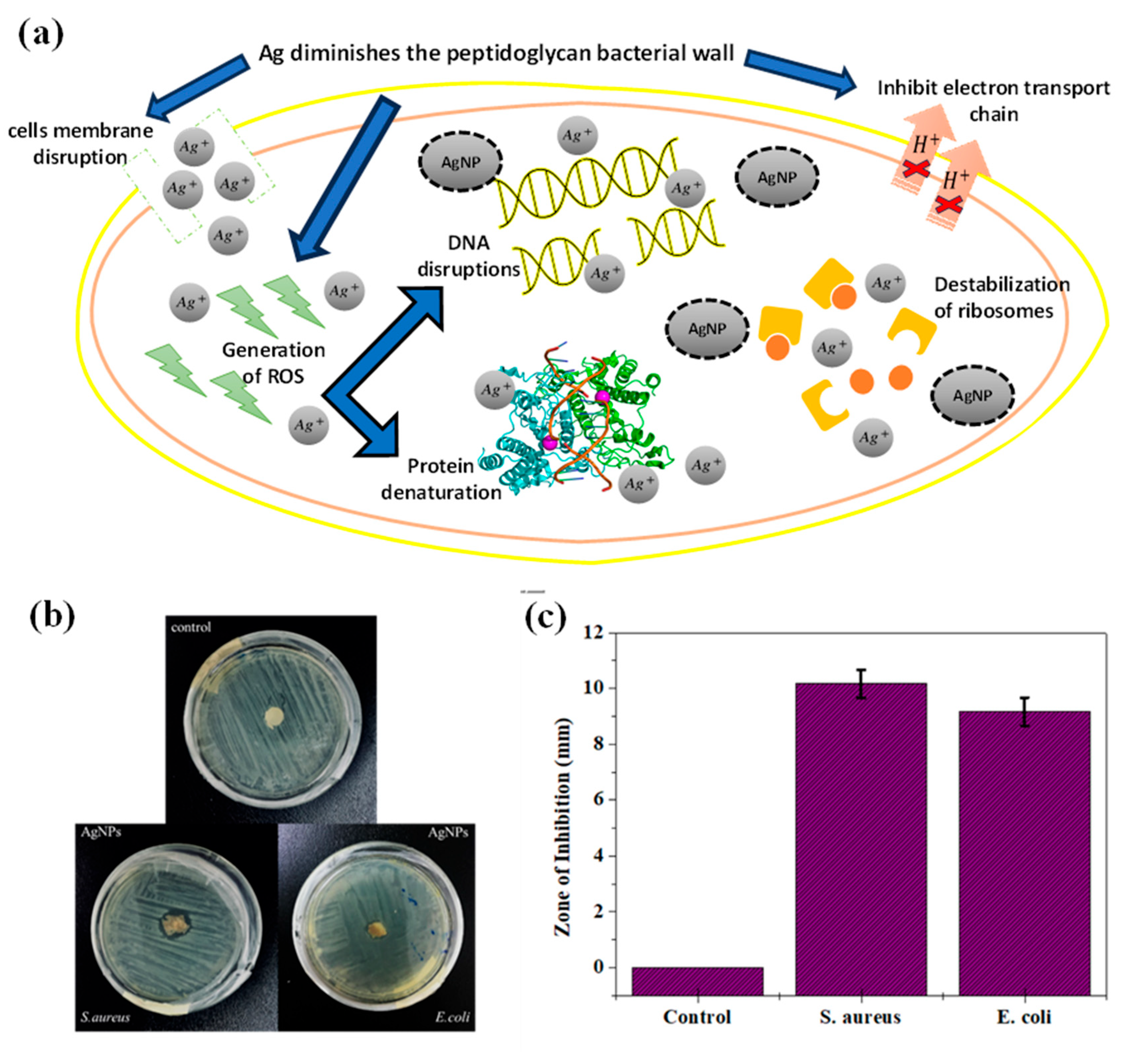
The antimicrobial effects were evaluated against Escherichia coli and Staphylococcus aureus due to the most common pathogen that affects the wound. The agar diffusion method evaluated the antibacterial effects between the control sample (pure EBN extract) and the 6% nanoparticles (EBN-AgNPs). For the two pathogens, an inhibitory zone formed on the nanometals sample, whereas there was none for the control, meant that the silver had slowed the growth of the microbe. In Figure, the zone of inhibition on S.aureus is (10 ± 0.2 mm) (a) bigger compared to the E.coli (9 ± 0.1 mm) (b). AgNPs were found to be more effective on Gram-positive than Gram-negative. The concentration of AgNO3 gives a greater susceptibility of positive bacteria for AgNPs even though the cell wall of positive bacteria is stronger than negative bacteria due to high peptidoglycan content. The synthesis of AgNPs releases the silver ions as antimicrobial effect by disrupting bacteria cell walls and intervention with DNA replication.
Figure 7.
(a) Theoretical framework on mechanism of AgNPs antibacterial applications. During Ag-cell membrane interactions, nanoparticles perturb the integrity of the cell membrane, inducing changes in permeability and cytoplasmic leakage. It also disrupts bacterial cell walls by interfering with peptidoglycan, leading to bacterial structural compromise. Silver ions facilitate the generation of reactive oxygen species (ROS) intracellularly, thus inciting oxidative stress which lead to i) DNA damage; inducing genetic lesions that impede replication and transcription processes, and ii) interrupts protein denaturation; affecting vital bacterial metabolic pathways. In addition, by perturbing the electron transport chain, they impede energy production, and destabilize ribosomal function, resulting in erroneous protein synthesis. (b) Micrograph of antibacterial activity, and (c) zone of inhibition diagram of pure PVA as negative control, and EBN/AgNPs, against S.aureus and E.coli.
Figure 7.
(a) Theoretical framework on mechanism of AgNPs antibacterial applications. During Ag-cell membrane interactions, nanoparticles perturb the integrity of the cell membrane, inducing changes in permeability and cytoplasmic leakage. It also disrupts bacterial cell walls by interfering with peptidoglycan, leading to bacterial structural compromise. Silver ions facilitate the generation of reactive oxygen species (ROS) intracellularly, thus inciting oxidative stress which lead to i) DNA damage; inducing genetic lesions that impede replication and transcription processes, and ii) interrupts protein denaturation; affecting vital bacterial metabolic pathways. In addition, by perturbing the electron transport chain, they impede energy production, and destabilize ribosomal function, resulting in erroneous protein synthesis. (b) Micrograph of antibacterial activity, and (c) zone of inhibition diagram of pure PVA as negative control, and EBN/AgNPs, against S.aureus and E.coli.
4. Conclusions
The white-nest swiftlet (Aerodramus fuciphagus) extract was used in this study to synthesise silver nanoparticles in an environmentally benign, non-toxic, and straightforward manner. The efficient green synthesis of stable AgNPs was completed within 15 minutes by adding 0.05 M of AgNO3. UV-Visible spectrophotometer, FTIR technique, FESEM and EDX analysis characterised the synthesised AgNPs. The compound in EBN revealed helps in reducing and stabilising of AgNPs. Moreover, the surface morphology conducted by FESEM mostly showed spherical shapes ranging from 10 to 20 nm. EDX data analysis confirmed that Ag was formed in the EBN-AgNPs solution. Gram-positive bacteria were more effectively combated by AgNPs than Gram-negative bacteria, according to studies on bacteria. Additionally, the studies aimed to comprehend AgNPs' mechanism of action and synthesis from the EBN extract, which is cost-effective and environmentally favourable for creating nanometals with green technology.
Author Contributions
Conceptualization, F.H.Z. and N.F.I.M.J.; methodology, N.F.I.M.J; investigation, N.F.I.M.J.; writing—original draft preparation, N.F.I.M.J.; writing—review and editing, F.H.Z., H.A.H, F.S.J.H.; visualization, F.H.Z. and N.F.I.M.J.; supervision, F.H.Z.; project administration, F.H.Z; funding acquisition, F.H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Ministry of Higher Education for providing financial support under Fundamental Research Grant Scheme (FRGS) No. FRGS/1/2019/WAB13/UMP/02/1 (University reference RDU1901117) and Universiti Malaysia Pahang Al-Sultan Abdullah for laboratory facilities as well as additional financial support under Internal Research grant RDU210331.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Horne, J.; De Bleye, C.; Lebrun, P.; Kemik, K.; Van Laethem, T.; Sacré, P.Y.; Hubert, P.; Hubert, C.; Ziemons, E. Optimization of Silver Nanoparticles Synthesis by Chemical Reduction to Enhance SERS Quantitative Performances: Early Characterization Using the Quality by Design Approach. J. Pharm. Biomed. Anal. 2023, 233, 115475. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Koirala, P.; Stair, P.; Marks, L. ALD Synthesis of Platinum Nanoparticles on Single-Crystal SrTiO3 Pretreated with Wet Chemical Etching. Appl. Surf. Sci. 2017, 422, 661–665. [Google Scholar] [CrossRef]
- Ghazy, O.A.; Saleh, H.H.; Shehata, M.M.; Hosni, H.M.; Ali, Z.I. Electron Beam Radiation Induced Solid-State Synthesis of Gold Nanoparticles in Polyvinyl Alcohol Films and Their Physico-Chemical Properties. Radiat. Phys. Chem. 2022, 191, 109848. [Google Scholar] [CrossRef]
- Hassan, K.T.; Ibraheem, I.J.; Hassan, O.M.; Obaid, A.S.; Ali, H.H.; Salih, T.A.; Kadhim, M.S. Facile Green Synthesis of Ag/AgCl Nanoparticles Derived from Chara Algae Extract and Evaluating Their Antibacterial Activity and Synergistic Effect with Antibiotics. J. Environ. Chem. Eng. 2021, 9, 105359. [Google Scholar] [CrossRef]
- Chandhru, M.; Logesh, R.; Rani, S.K.; Ahmed, N.; Vasimalai, N. One-Pot Green Route Synthesis of Silver Nanoparticles from Jack Fruit Seeds and Their Antibacterial Activities with Escherichia Coli and Salmonella Bacteria. Biocatal. Agric. Biotechnol. 2019, 20, 101241. [Google Scholar] [CrossRef]
- Singh, N.A.; Narang, J.; Garg, D.; Jain, V.; Payasi, D.; Suleman, S.; Swami, R.K. Nanoparticles Synthesis via Microorganisms and Their Prospective Applications in Agriculture. Plant Nano Biol. 2023, 100047. [Google Scholar] [CrossRef]
- Khan, M.R.; Urmi, M.A.; Kamaraj, C.; Malafaia, G.; Ragavendran, C.; Rahman, M.M. Green Synthesis of Silver Nanoparticles with Its Bioactivity, Toxicity and Environmental Applications: A Comprehensive Literature Review. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100872. [Google Scholar] [CrossRef]
- Quek, M.C.; Chin, N.L.; Yusof, Y.A.; Law, C.L.; Tan, S.W. Characterization of Edible Bird’s Nest of Different Production, Species and Geographical Origins Using Nutritional Composition, Physicochemical Properties and Antioxidant Activities. Food Res. Int. 2018, 109, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cao, J.; Wang, Y.; Chen, Y.; Jiang, L. A Comprehensive Review of Edible Bird’s Nest. Food Res. Int. 2021, 140, 109875. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, D.A.; Mansor, R.; Md Ajat, M.M.; Abas, F.; Ideris, A.; Abu, J. Differentiation of Malaysian Farmed and Commercialised Edible Bird’s Nests through Nutritional Composition Analysis. Pertanika J. Trop. Agric. Sci. 2019, 42, 871–881. [Google Scholar]
- Oliveros, E.; Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Buck, R.; Rueda, R.; Martín, M.J. Sialic Acid and Sialylated Oligosaccharide Supplementation during Lactation Improves Learning and Memory in Rats. Nutrients 2018, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.P.; Tang, S.Y.; Ji, H.R.; He, P.Y.; Li, Y.H.; Dong, X.L.; Du, M.N.; Maznah, I.; He, W.J. Edible Bird’s Nest Attenuates Menopause-Related Bone Degeneration in Rats via Increaing Bone Estrogen-Receptor Expression. Chin. J. Integr. Med. 2021, 27, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalid, S.; Rashed, A.; Aziz, S.; Ahmad, H. Effects of Sialic Acid from Edible Bird Nest on Cell Viability Associated with Brain Cognitive Performance in Mice. World J. Tradit. Chin. Med. 2019, 5, 214. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).