Submitted:
18 October 2023
Posted:
19 October 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Locations
River Brede
River Køge-Lellinge
2.2. Sampling, Ageing and Growth
River Brede
Age and Growth
2.3. Natural Mortality and Number of Glass eel
River Brede
River Køge-Lellinge
2.4. Biological Production
River Brede
River Køge-Lellinge
2.5. Data Treatment
3. Results
4. Discussion
4.1. Linear Growth of Yellow eel
4.2. Mortality
4.3. The Production of Silver eel and Yellow eel
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Als, T.D., Hansen, M.M., Maes, G.E., Castonguay, M., Riemann, L., Aarestrup, K., Munk, P., All roads lead to home: panmixia of European eel in the Sargasso Sea. Molecular Ecology, 20: 1333-1346. [CrossRef]
- Sinha, V., R., P. & Jones, J.W., 1967. On the food of the freshwater eels and their feeding relationship with the salmonids. Journal of Zoology, London, 153: 119-137. [CrossRef]
- Rasmussen G. & Therkildsen B., 1979. Food, Growth and Production of Anguilla anguilla L. in a small Danish stream. Rapports et Proces-Verbaux des Reunions, Conseil internationale pour l’Exploitation de la Mer, 174: 32–40.
- ICES (2018). European eel (Anguilla anguilla) throughout its natural range. ICES Advice: Recurrent Advice. Report. [CrossRef]
- Castonguay, M., Hudson, P.V., Moriarty, C., Drinkwater, K.F. & Jessop, B.M. 1994. Is there a role of ocean environment in American and European eel decline? Fisheries Oceanography 3: 197–203. [CrossRef]
- Friedland, K.D., Miller, M.J. & Knights, B. 2007. Oceanic changes in the Sargasso Sea and declines in recruitment of the European eel. ICES Journal of Marine Science 64: 519–530. [CrossRef]
- ICES. 2005. Report of the ICES ⁄ EIFAC Working Group on Eels. No. ICES CM 2005 ⁄I:01. Galway, Ireland, 184 pp.
- Pedersen, M.I., Rasmussen, G.H., 2018. Fisheries regulation on European Eel (Anguilla anguilla) for 2018: how big is the effect? Journal of Fish Research, volume 2, Issue 1, 17-18.
- EU, 2007. Council regulation (EC) No 1100/2007 of 18. September 2007 establishing measures for the recovery of the stock of European eel. Official Journal of the European Union. L 248, 17-23.
- Pedersen, M.I., Rasmussen, G.H., 2013. Background material for preparation of eel management plan in Denmark. DTU Aqua-rapport nr. 271-2013. (In Danish).
- Aprahamian, M.W., Derek, W.E, Briand, C., Walker, A.M., McElarney, Y., Allen, M., 2021. The changing times of Europe´s largest remaining commercial harvested population of Eel Anguilla anguilla L. Journal of Fish Biology; 99:1201-1221. [CrossRef]
- Ivlev VS (1966). The biological productivity of waters (English version). Journal of Fisheries Research Board of Can, 23:1727–1759. [CrossRef]
- Chapman, D.W. 1967. Production in fish populations, 3-29. The Biological Basis of Fish Production, Edited by Shelby D. Gerking. Blackwell Scientific Publications, Oxford and Edinburg.
- Nielsen. G., 1981. River Brede system, the silver eel production. DTU Aqua. (In Danish).
- Larsen, K., 1972. Studies on the biology of Danish stream fishes. III. On seasonal fluctuations in the stock density of yellow eel in shallow stream biotopes, and their causes. Meddelelser i Danmarks Fiskeri og Havundersøgelser. N. S. 7 (2): 23-46.
- Sinha, V., R., P. & Jones, J.W., 196. On the sex and distribution of the freshwater eel (Anguilla anguilla). Journal of Zoology, London, 150: 371-385. [CrossRef]
- Christensen, J.M., 1964. Burning of otoliths, a technique for age determination of soles and other fish. Journal Conseil Permanent Internationale pour l’Exploration de la Mer, 29, 73-81. [CrossRef]
- Moriarty C. & Steinmetz B, 1979. On age determination of eel. Rapport et Proces-verbaux des Reunions. Conseil international pour l’Exploration de la Mer, 174, 70-74.
- ICES. 2009. Workshop on Age Reading of European and American Eel (WKAREA). Bordeaux, France: ICES CM 2009\ACOM: 48, 66 pp.
- Durif, C.M.F., Diserud, O., Sandlund, O., Thorstad, E.B., Poole, R., Bergesen, K., Escobar-Lux, R.H., Shema S., 2020. Age of European silver eels during a period of declining abundance in Norway. Ecology and Evolution, 10, 4801-4815. [CrossRef]
- Pedersen, M.I., Rasmussen, G.H., Jepsen, N., 2023. Density-dependent growth, survival, and biomass production of stocked glass eels (Anguilla anguilla) in semi natural ponds. Fisheries and Management Ecology. [CrossRef]
- Rasmussen G., 1983. Recent investigations on the population dynamics of eel (Anguila anguilla L.) in some Danish streams. Proceeding of the 3rd British Freshwater Fish Conference, 71-77.
- Bevacqua, S., Melia, P., De Leo, G.A., Gatto, M., 2011. Intra-specific scaling of natural mortality in fish: paradigmatic case of the European eel. Oecologia (2011) 165:333–339. [CrossRef]
- Ursin, E., 1967. A mathematical model of some aspects of fish growth, respiration and mortality. Journal of Fisheries Research Board of Canada, 24, 2355–2391. [CrossRef]
- Lester, N.P., Shuter, B.J., Abrams, P.A., 2004. Interpreting the von Bertalanffy model of somatic growth in fishes: the cost of reproduction. Proceeding of the Royal Society. M 271, 1625:1631. [CrossRef]
- Day, T. & Taylor, P.D. 1997. Von Bertalanffy¨s growth equation should not be used to model age and size at maturity. The American Naturalist, vol. 149, no. 2:381-392. [CrossRef]
- Rasmussen, C.J., 1952. Size and age of the silver eel (Anguilla anguilla L) in Esrum Lake. Rep. Dansk Biolologisk Station, LIV (I).
- Dahl, J., 1967. Some recent observations on the age and growth of eel. Proceeding of the 3rd British Coarse Fish Conference, 48-52.
- Sinha, V., R., P. & Jones, J.W., 1967. On the age and growth of the freshwater eel (Anguilla anguilla). Journal of Zoolology, London, 153: 99-117. [CrossRef]
- Vøllestad, L.A., 1992. Geographic Variation in Age and Length at Metamorphosis of Maturing European Eel: Environmental Effects and Phenotypic Plasticity. Journal of Animal Ecology , Feb., 1992, Vol. 61, No. 1, 41-48. [CrossRef]
- Vøllestad, L.A., Jonsson, B., 1988. A 13-year study of the population dynamics and growth of the European eel Anguilla anguilla in a Norwegian river: evidence for density-dependent mortality, and development of a model for predicting yield. Journal of Animal Ecology, 57, 983-997. [CrossRef]
- Berg, S., Jørgensen, J., 1994. Stocking experiments with O+ eel (Anguilla anguilla L.) in Danish streams: post stocking movements, densities and mortality: In Rehabilitation of Freshwater Fisheries, 314 - 325. Edited by I.G. Cowx. Fishing News Books. University of Hull, U.K. ISBN 0-85238-195-6.
- De Leo, G. A., Gatto, M., 1996. Trends in vital rates of the European eel: Evidence for density dependence? Ecological Applications Volume: 6, 1281-1294. [CrossRef]
- Lorenzen, K., 1996. The relationship between body weight and natural mortality in juvenile and adult fish: a comparison of natural ecosystems and aquaculture. Journal of Fish Biology, 49, 627–647. [CrossRef]
- Lobon-Cervia, J., Utrilla. C.G., Rincon, P.A. 1995. Variations in the population dynamics of the Europeaneel Anguilla anguilla (L.) along course of a Catabrian river. Ecology of Freshwater Fish, 4: 17-27. [CrossRef]
- Lobon-Cervia, J., Rasmussen, G., 2023. Determinants and Dynamics of Production Rates of Stream-Dwelling Salmonids: The importance of intrinsic factors. Advances in the Ecology of Stream-Dwelling Salmonids. Springer. In press.
- Anonymous 2008. Danish Eel Management Plan. Ministry of Food, Agriculture and Fisheries.
- Anon 2021. Fourth Danish progress report (2021) on implementation of the Eel Regulation and Eel Management Plan (EMP) in Denmark.
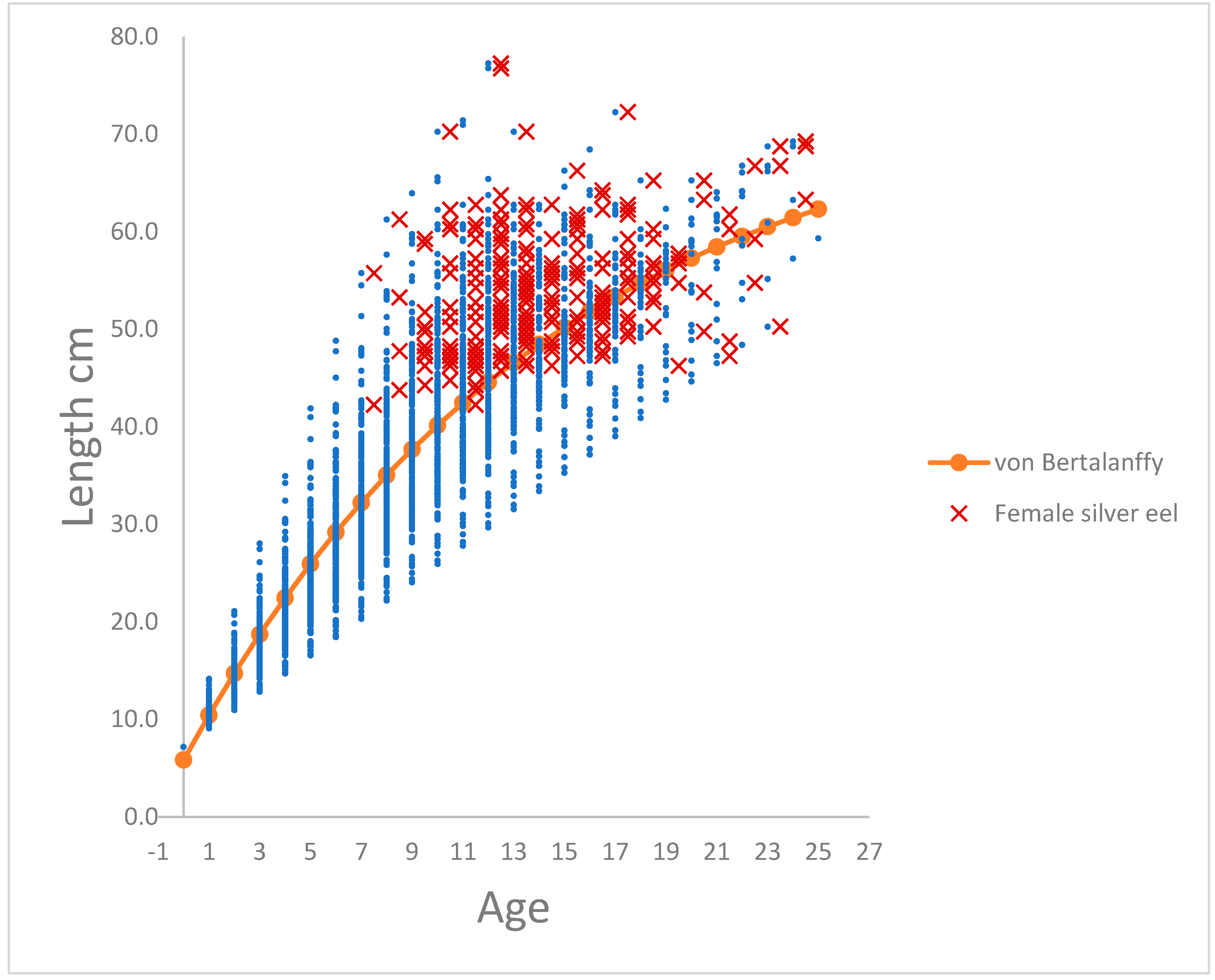
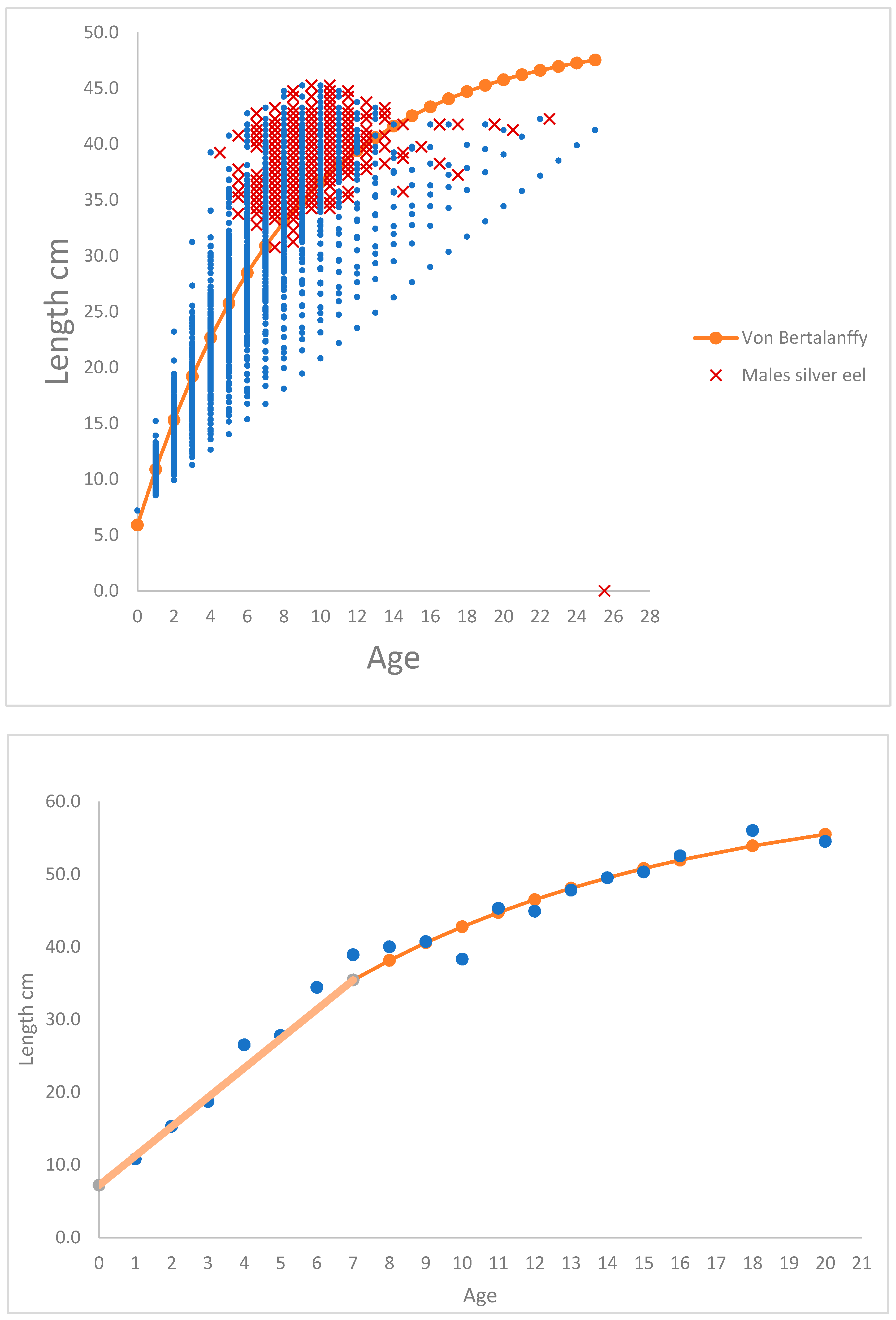
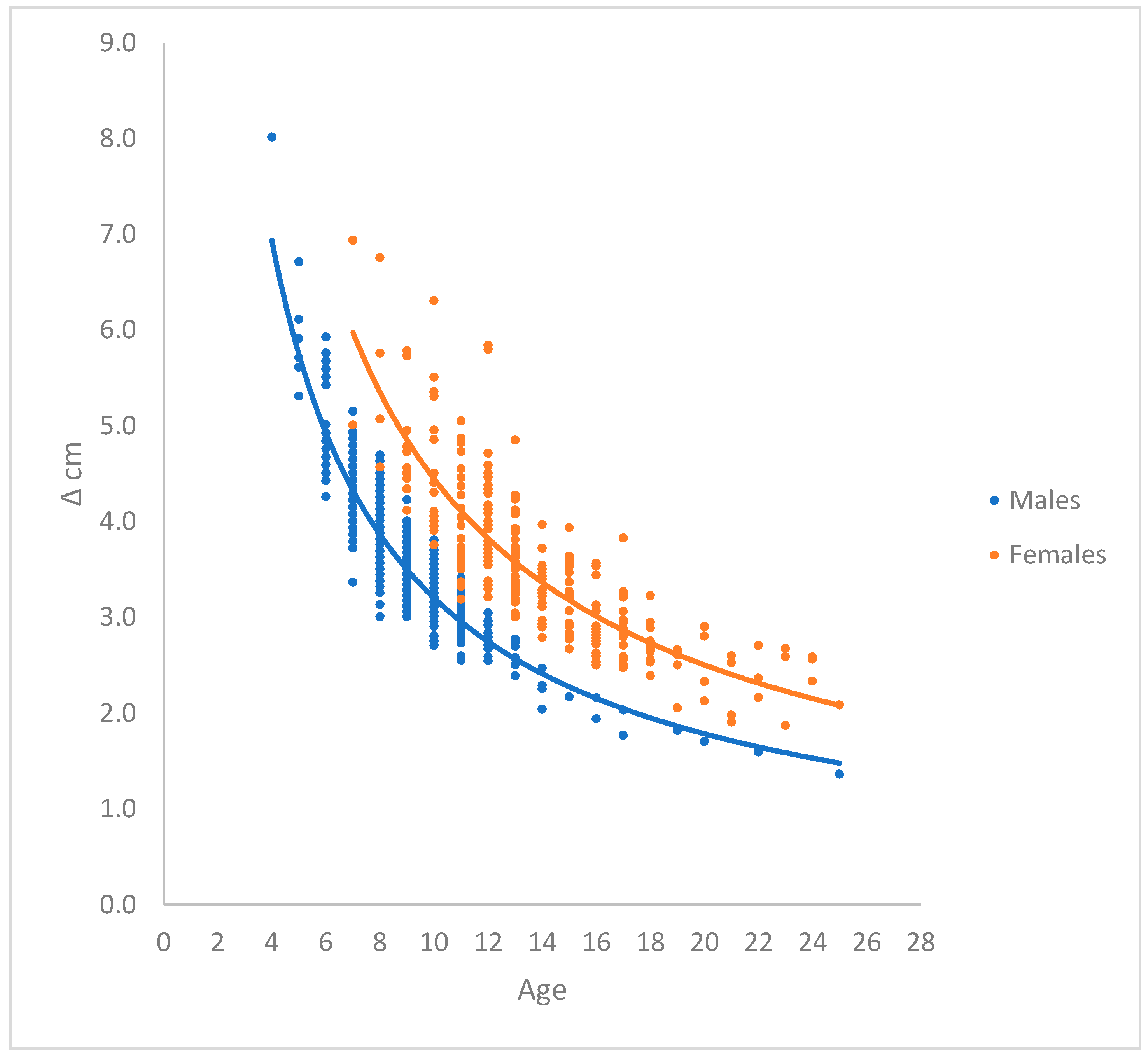
| Males | 1981 | Mean | Annual growth | Silver eel | Total nr. | Glass eel | Biological | ||
| Silver Age | Number | Number% | Accumulated% | Length cm | ∆ cm | Total number | Glass Eel | per Silver Eel | Production g |
| 4 | 3 | 0.2 | 0.2 | 34.6 | 6.9 | 47 | 103 | 2.2 | 3,753 |
| 5 | 18 | 1.3 | 1.6 | 35.7 | 5.7 | 277 | 722 | 2.6 | 24,795 |
| 6 | 51 | 3.9 | 5.5 | 36.6 | 4.9 | 801 | 2,487 | 3.1 | 79,309 |
| 7 | 158 | 12.1 | 17.5 | 37.3 | 4.3 | 2,497 | 9,193 | 3.7 | 269,982 |
| 8 | 325 | 24.8 | 42.3 | 38.0 | 3.8 | 5,119 | 22,292 | 4.4 | 599,607 |
| 9 | 377 | 28.8 | 71.1 | 38.6 | 3.5 | 5,946 | 30,549 | 5.1 | 749,677 |
| 10 | 226 | 17.2 | 88.3 | 39.1 | 3.2 | 3,563 | 21,548 | 6.0 | 481,155 |
| 11 | 81 | 6.2 | 94.6 | 39.5 | 2.9 | 1,284 | 9,124 | 7.1 | 185,036 |
| 12 | 29 | 2.2 | 96.8 | 40.0 | 2.7 | 459 | 3,821 | 8.3 | 70,292 |
| 13 | 13 | 1.0 | 97.8 | 40.4 | 2.6 | 208 | 2,030 | 9.8 | 33,850 |
| 14 | 10 | 0.8 | 98.6 | 40.7 | 2.4 | 163 | 1,857 | 11.4 | 28,052 |
| 15 | 3 | 0.2 | 98.8 | 41.1 | 2.3 | 47 | 652 | 13.8 | 8,610 |
| 16 | 5 | 0.4 | 99.2 | 41.4 | 2.1 | 77 | 1,199 | 15.6 | 14,868 |
| 17 | 6 | 0.4 | 99.6 | 41.7 | 2.0 | 93 | 1,679 | 18.1 | 18,859 |
| 18 | 99.6 | ||||||||
| 19 | 1 | 0.1 | 99.7 | 42.2 | 1.8 | 21 | 510 | 24.6 | 4,707 |
| 20 | 1 | 0.1 | 99.8 | 42.5 | 1.8 | 21 | 593 | 28.6 | 4,967 |
| 21 | 99.8 | ||||||||
| 22 | 1 | 0.1 | 99.9 | 42.9 | 1.6 | 17 | 544 | 31.8 | 4,559 |
| 23 | 99.9 | ||||||||
| 24 | 99.9 | ||||||||
| 25 | 1 | 0.1 | 100.0 | 43.6 | 1.5 | 21 | 1,037 | 50.0 | 6,525 |
| Total | 1,310 | 100.00 | 20,660 | 109,940 | 5.3 | 2,588,601 | |||
| Females | 1981 | Mean | Annual growth | Silver eel | Total nr. | Glass eel | Biological | ||
| Silver Age | Number | Number% | Accumulated% | Length cm | ∆ cm | Total number | Glass Eel | per Silver Eel | Production g |
| 7 | 2 | 0.8 | 0.8 | 48.9 | 6.0 | 36 | 68 | 1.9 | 4,741 |
| 8 | 4 | 1.7 | 2.5 | 50.0 | 5.3 | 76 | 342 | 4.5 | 20,320 |
| 9 | 11 | 4.7 | 7.1 | 50.9 | 4.9 | 211 | 1,120 | 5.3 | 11,520 |
| 10 | 16 | 7.0 | 14.1 | 51.8 | 4.5 | 318 | 2,213 | 7.0 | 98,104 |
| 11 | 21 | 9.0 | 23.1 | 52.5 | 4.1 | 408 | 3,370 | 8.3 | 134,899 |
| 12 | 32 | 13.7 | 36.9 | 53.2 | 3.8 | 623 | 6,093 | 9.8 | 219,829 |
| 13 | 28 | 12.1 | 49.0 | 53.8 | 3.6 | 551 | 6,361 | 11.5 | 206,582 |
| 14 | 15 | 6.7 | 55.7 | 54.4 | 3.4 | 304 | 4,142 | 13.6 | 120,970 |
| 15 | 18 | 7.9 | 63.7 | 55.0 | 3.2 | 360 | 5,782 | 16.0 | 146,996 |
| 16 | 26 | 11.2 | 74.9 | 55.5 | 3.0 | 507 | 9,569 | 18.9 | 211,796 |
| 17 | 19 | 8.3 | 83.1 | 56.0 | 2.9 | 375 | 8,306 | 22.2 | 159,375 |
| 18 | 10 | 4.2 | 87.3 | 56.4 | 2.7 | 191 | 1,937 | 10.1 | 80,429 |
| 19 | 4 | 1.8 | 89.1 | 56.9 | 2.6 | 80 | 2,358 | 29.3 | 35,421 |
| 20 | 4 | 1.9 | 91.0 | 57.3 | 2.5 | 84 | 3,001 | 35.7 | 37,522 |
| 21 | 5 | 2.2 | 93.2 | 57.7 | 2.4 | 101 | 4,229 | 41.7 | 45,570 |
| 22 | 3 | 1.2 | 94.4 | 58.0 | 2.3 | 53 | 2,483 | 46.6 | 26,624 |
| 23 | 3 | 1.2 | 95.5 | 58.4 | 2.2 | 52 | 2,982 | 56.9 | 24,834 |
| 24 | 7 | 3.2 | 98.7 | 58.7 | 2.1 | 143 | 9,524 | 66.4 | 65,223 |
| 25 | 3 | 1.3 | 100.0 | 59.1 | 2.1 | 59 | 4,563 | 77.4 | 28,507 |
| Total | 231 | 100.0 | 4,535 | 78,442 | 17.3 | 1,679,262 |
| Instantaneous | Biological | ||||||||||
| Age | Number | Natural M | Total Z | Mean | Mean | Growth rate | Biomass | Production | Emigration E | Silver eel | Silver eel |
| Yellow eel | Mortality | Mortality | Length cm | Body mass g | G | g | g | Mortality | Number | g | |
| 0 | 55.70 | 0.755 | 7.2 | 0.3 | |||||||
| 1 | 26.17 | 0.376 | 11.2 | 2.3 | 2.089 | 38.4 | 80.27 | ||||
| 2 | 17.96 | 0.276 | 15.3 | 5.9 | 0.926 | 84.1 | 77.96 | ||||
| 3 | 13.62 | 0.218 | 19.3 | 12.0 | 0.708 | 135.1 | 95.61 | ||||
| 4 | 10.95 | 0.180 | 23.3 | 21.3 | 0.573 | 198.4 | 113.69 | ||||
| 5 | 9.14 | 0.154 | 27.4 | 34.5 | 0.481 | 274.1 | 131.93 | ||||
| 6 | 7.84 | 0.134 | 31.4 | 52.2 | 0.415 | 362.1 | 150.25 | ||||
| 7 | 6.86 | 0.118 | 35.4 | 75.2 | 0.365 | 462.3 | 168.60 | ||||
| 8 | 3.78 | 0.110 | 0.595 | 38.1 | 93.9 | 0.222 | 544.4 | 120.91 | 0.485 | 1.83 | 172.22 |
| 9 | 2.09 | 0.103 | 0.595 | 40.6 | 113.1 | 0.187 | 369.7 | 68.95 | 0.492 | 1.03 | 116.03 |
| 10 | 1.15 | 0.098 | 0.595 | 42.8 | 132.5 | 0.158 | 243.0 | 38.46 | 0.497 | 0.57 | 75.78 |
| 11 | 0.63 | 0.094 | 0.595 | 44.7 | 151.7 | 0.135 | 155.6 | 21.08 | 0.501 | 0.32 | 48.28 |
| 12 | 0.35 | 0.090 | 0.595 | 46.5 | 170.5 | 0.117 | 97.5 | 11.39 | 0.505 | 0.18 | 30.14 |
| 13 | 0.19 | 0.087 | 0.595 | 48.1 | 188.7 | 0.101 | 60.1 | 6.08 | 0.508 | 0.10 | 18.51 |
| 14 | 0.11 | 0.085 | 0.595 | 49.5 | 206.1 | 0.088 | 36.5 | 3.22 | 0.510 | 0.05 | 11.20 |
| 15 | 0.06 | 0.083 | 0.595 | 50.8 | 222.6 | 0.077 | 21.9 | 1.69 | 0.512 | 0.03 | 6.70 |
| 16 | 0.03 | 0.081 | 0.595 | 51.9 | 238.2 | 0.068 | 13.0 | 0.88 | 0.514 | 0.02 | 3.97 |
| 17 | 0.02 | 0.079 | 0.595 | 53.0 | 252.8 | 0.059 | 7.6 | 0.45 | 0.516 | 0.01 | 2.33 |
| 18 | 0.01 | 0.078 | 0.595 | 53.9 | 266.4 | 0.052 | 4.5 | 0.23 | 0.517 | 0.01 | 1.36 |
| 19 | 0.01 | 0.077 | 0.595 | 54.7 | 279.0 | 0.046 | 2.6 | 0.12 | 0.518 | 0.00 | 0.79 |
| 20 | 0.00 | 0.075 | 0.595 | 55.5 | 290.7 | 0.041 | 1.5 | 0.06 | 0.520 | 0.00 | 0.45 |
| Total | 100.98 | 3.352 | 9.696 | 3112.3 | 1091.8 | 6.596 | 4.15 | 487.77 |
| River Køge-Lellinge | |
| Number of glass eel ha-1 | 5,570 |
| Number of silver eel ha-1 | 415 |
| Production Silver eel kg ha-1 | 48.8 |
| Biological production yellow eel kg ha-1 | 108.9 |
| No.glass eel pr kg produced silver eel | 114 |
| No. glass eel pr biological production yellow eel kg | 51 |
| River Brede | |
| Number of glass eel ha-1 | 2,894 |
| Number of silver eel ha-1 | 387 |
| Production Silver eel kg ha-1 | 49.2 |
| Biological production yellow eel kg ha-1 | 65.7 |
| No. glass eel pr kg produced silver eel | 59 |
| No. glass eel pr biological production yellow eel kg | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





