Submitted:
14 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Peripheral Nerve Injury
2a. Chromatolysis and Gene Expression in Axotomized Neurons
2b. Neuronal Molecular Signaling of Nerve Injury
2c. Wallerian Degeneration
2d. Schwann Cell Response to Nerve Injury
2e. Axonal Regeneration
3. Poor Recovery of Function after Peripheral Nerve Injury and Repair
3a. Time- and Distance-Related Decline in Nerve Regenerative Capacity
3b. Transient Expression of Regeneration Associated Genes
4. Neurotrophic Factors to Sustain Their Levels
4a. Exogenous Application of Neurotrophic Factors
4b. Endogenous Neurotrophic Factors
5. Neuronal Activity and Nerve Regeneration
5a. Reduced Activity and Synapse Withdrawal
5b. Staggered Nerve Regeneration
5c. Low Frequency Electrical Stimulation Accelerates Axon Outgrowth
5d. Preferential Reinnervation and Growth Factors
5e. ES Promotes Axon Outgrowth after Delayed Surgery
5f. Exercise and Axonal Regeneration
5g. Conditioning Lesion and Conditioning ES
5g. Drug-Induced cAMP Elevation Mimics the Effect of Electrical Stimulation (ES) on Nerve Regeneration
5h. ES Promotes Sensory Nerve Outgrowth in the Central Nervous System
5i. ES Signalling Pathways
5j. ES Accelerates Human Nerve Regeneration and Muscle Reinnervation
6. Conclusions
Acknowledgments
References
- Cajal, S.R. Degeneration and Regeneration of the Nervous System (translated by R.M. May). 1928, Oxford University Press, New York, Oxford.
- Sunderland, S. Nerve and Nerve Injuries. 1978, Livingstone, Edinburgh.
- Kline, D.G.; Hudson, A.R. Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments and Tumors. 1995, W.B. Saunders, Philadelphia, PA.
- Fenrich, K.; Gordon, T. Canadian Association of Neuroscience review: axonal regeneration in the peripheral and central nervous systems – current issues and advances. Can. J. Neurol. Sci. 2004, 31, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M. Nerve Repair. 2011, Oxford University Press, Oxford.
- Sulaiman, O.A.R.; Midha, R.; Gordon, T. Pathophysiology of surgical nerve disorders. In Youmans’ Neurological Surgery 6th ed., Part 2, 2011, 2368-Ed Winn HR. Philadelphia, PA.
- Ruijs, A.C.J.; Jaquet, J.-B.; Kalmign, S.; Giele, H.; Hovius, S.E.R. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast. Reconstr. Surg. 2005, 116, 484–94. [Google Scholar] [CrossRef] [PubMed]
- Scholz, T.; Krichevsky, A.; Sumarito, A.; Jaffurs, D.; Wirth, G.A.; Paydar, K.; Evans, G.R.D. Peripheral nerve injuries: An international survey of current treatments and future perspectives. J. Reconstr. Microsurg 2009, 25, 339–344. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, G.S.; Kim, I.S.; Chang, J.-C. Brachial plexus injury in adults. The Nerve 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Smania, N.; Berto, G.; La Marchina, E.; Melotti, C.; Midiri, A.; Roncori, L.; Zenorini, A.; Ianes, P.; Picelli, A.; Waldner, A.; Faccioli, S.; Gandolfi, M. Rehabilitation of brachial plexus injuries in adults and children. Eur. J. Phys. Rehabil. Med. 2012, 48, 483–506. [Google Scholar]
- Tian, A.; Sachar, R.; Ray, W.Z.; Brogan, D.M.; Dy, C.J. Indirect cost of traumatic brachial plexus injuries in the United States. J. Bone Joint Surg. Am. 2019, 101, e80. [Google Scholar]
- Rosberg, H.E.; Carlsson, K.S.; Hojgard, S.; Lindgren, B.; Lundborg, G.; Dahlin, L.B. Injury to the human median and ulnar nerves in the forearm- analysis of costs for treatment and rehabilitation of 69 patients in southern Sweden. J Hand Surg (Edinburgh, Scotland) 2005, 30, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Bergmeister, K.D.; Groβe-Hartlage, L.; Daeschler, S.C.; et al. Acute and long-term costs of 268 peripheral nerve injuries in the upper extremity. Plos One 2020, 15, e0229530. [Google Scholar] [CrossRef]
- Jaquet, J.B.; Luijsterburg, A.J.; Kalmijn, S.; Kuypers, P.D.; Hofman, A.; Hovius, S.E. Median, ulnar, and combined medium-ulnar nerve injuries: Functional outcome and return to productivity. J. Trauma. 2001, 51, 687–692. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997, 14, 67–116. [Google Scholar] [CrossRef]
- Boyd, J.G.; Gordon, T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury Mol. Neurobiol. 2003, 27, 277–324. [Google Scholar]
- Chan, K.M.; Gordon, T.; Zochodne, D.W.; Power, H.A. Improving peripheral nerve regeneration: From molecular mechanisms to potential therapeutic targets. Exp. Neurol. 2014, 261, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Farraj, P.J.; Coleman, M.P. Lessons from injury: How nerve injury studies reveal basic biological mechanisms and therapeutic opportunities for peripheral nerve diseases. Neurotherapeutics 2021, 2200–2221. [Google Scholar] [CrossRef] [PubMed]
- Daeschler, S.C.; Feinberg, K.; Harhaus, L.; Kneser, U.; Gordon, T.; Borschel, G.H. Advancing nerve regeneration: Translational perspectives of tacrolimus (FK506). Int. J. Mol. Sci. 2023, 24, 12771. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, E.L; Houghton, P.; Anthony, J.; Rennie, S.; Shay, B.L; Hoens, A.M. Neuromuscular electrical stimulation of muscle impairment: Critical review and recommendations for clinical practice. Physiother. Can. 2017, 69, 1–78. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Hsiao, H.-F.; Li, L.-F.; Chen, N.-H.; Huang, C.-C. Effects of electrical muscle stimulation in subjects undergoing prolonged mechanical ventilation. Respir. CRE 2019, 64, 262–271. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, Y.; Liu, Y.; Lu, J. Global trends and hot topics in electrical stimulation of skeletal muscle research over the past decade: A bibliometric analysis. Frontiers Neurol. 2022, 13, 991099. [Google Scholar] [CrossRef]
- Nix, W.A.; Hopf, H.C. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res. 1983, 272, 21–25. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Neumann, C.M.; Brushart, T.M.; Gordon, T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci. 2000, 20, 2602–2608. [Google Scholar] [CrossRef]
- Brushart, T.M.; Hoffman, P.N.; Royall, R.M.; Murinson, B.B.; Witzel, C.; Gordon, T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J. Neurosci. 2002, 22, 6631–6638. [Google Scholar] [CrossRef]
- Brushart, T.M.; Jari, R.; Verge, V.; Rohde, C.; Gordon, T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp. Neurol. 2005, 194, 221–229. [Google Scholar] [CrossRef]
- Geremia, N.M.; Gordon, T.; Brushart, T.M.; Al-Majed, A.A.; Verge, V.M. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp. Neurol. 2007, 205, 347–359. [Google Scholar] [CrossRef]
- Elzinga, K.; Tyreman, N.; Ladak, A.; Savaryn, B.; Olson, J.; Gordon, T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp. Neurol. 2015, 269, 142–153. [Google Scholar] [CrossRef]
- Pockett, S.; Gavin, R.M. Acceleration of peripheral nerve regeneration after crush injury in rat. Neurosci. Lett. 1985, 59, 221–224. [Google Scholar] [CrossRef]
- Marqueste, T.; Alliez, J.R.; Alluin, O.; Jammes, Y.; Decherchi, P. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J. Appl. Physiol. 2004, 96, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- English, A.W. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J. Comp. Neurol. 2005, 490, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Rouabhia, M.; Wang, Z.; Roberge, C.; Shi, G.; Roche, P.; Li, J.; Dao, L.H. Electrically conductive biodegradable polymer composite for nerve regeneration: electricity-stimulated neurite outgrowth and axon regeneration. Artificial Organs 2006, 31, 13–22. [Google Scholar] [CrossRef]
- Ahlborn, P.; Schachner, M.; Irintchev, A. One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp. Neurol. 2007, 208, 137–144. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Schwartz, G.; Meador, W.; Sabatier, M.J.; Mulligan, A. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev. Neurobiol. 2007, 67, 18–172. [Google Scholar] [CrossRef] [PubMed]
- Hetzler, L.E.; Sharma, N.; Tanzer, L.; Wurter, R.D.; Leonetti, J.; Marco, S.J.; Jones, K.J.; Foecking, E.M. Accelerating functional recovery after rat facial nerve injury: effects of gonadal steroids and electrical stimulation. Otolaryng Head. Neck 2008, 139, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Lal, D.; Hetzler, L.T.; Sharma, N.; Wurster, R.D.; Marzo, S.J. Jones, K.J.; Foecking, E.M. Electrical stimulation facilitates rat facial nerve recovery from a crush injury. Otolaryng Head. Neck 2008, 139, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Vivó, M.; Puigdemasa, A.; Casals, L.; Asensio, E.; Udina, E.; Navarro, X. Immediate electrical stimulation enhances regeneration and reinnervation and modulates spinal plastic changes after sciatic nerve injury and repair. Exp. Neurol. 2008, 211, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Pinilla, E.; Udina, E.; Jaramillo, J.; Navarro, X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 2009, 219, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Coughlin, L.; Porter, R.G.; Tanzer, L.; Wurster, R.D.; Marzo, S.J.; Jones, K.J.; Foecking, E.M. Effects of electrical stimulation and gonadal steroids on rat facial nerve regenerative properties. Restor. Neurol. Neurosci. 2009, 27, 633–644. [Google Scholar] [CrossRef]
- Alrashdan, M.S.; Park, J.C.; Sung, M.A.; Yoo, S.B.; Jahng, J.W.; Lee, T.H.; Kim, S.J; Lee, J.H. Thirty minutes of low intensity electrical stimulation promotes nerve regeneration after sciatic nerve crush injury in a rat model. Acta Neurol. Belg. 2010, 110, 168–179. [Google Scholar]
- Huang, J.; Lu, L.; Hu, X.; Ye, Z.; Peng, Y.; Yan, X.; Geng, D.; Luo, Z. Electrical stimulation accelerates motor functional recovery in the rat model of 15-mm sciatic nerve gap bridged by scaffolds with longitudinally oriented microchannels. Neurorehabil Neural Repair. 2010, 24, 736–745. [Google Scholar] [CrossRef]
- Sharma, N.; Marzo, S.J.; Jones, K.J.; Foecking, E.M. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp. Neurol. 2010, 223, 183–191. [Google Scholar] [CrossRef]
- Wan, L.; Xia, R.; Ding, W. Short-term low-frequency electrical stimulation enhanced remyelination of injured peripheral nerves by inducing the promyelination effect of brain-derived neuro- trophic factor on Schwann cell polarization. J. Neurosci. Res. 2010, 88, 2578–2587. [Google Scholar] [CrossRef]
- Yeh, C.C.; Lin, Y.C.; Tsai, F.J.; Huang, C.Y.; Yao, C.H.; Chen, Y.S. Timing of applying electrical stimulation is an important factor deciding the success rate and maturity of regenerating rat sciatic nerves. Neurorehabil Neural Repair. 2010, 24, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Alrashdan, M.S.; Sung, M.A.; Kwon, Y.K.; Chung, H.J.; Kim, S.J.; Lee, J.H. Effects of combining electrical stimulation with BDNF gene transfer on the regeneration of crushed rat sciatic nerve. Acta Neurol (Wien) 2011, 153, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Han, S.J.; Shin, D.H.; Lee, W.S.; Choi, J.Y. Subthreshold continuous electrical stimulation facilitates functional recovery of facial nerve after crush injury in rabbit. Muscle Nerve 2011, 43, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Foecking, E.M.; Fargo, K.N.; Coughlin, L.M.; Kim, J.T.; Marzo, S.J.; Jones, K.J. Single session of brief electrical stimulation immediately following crush injury enhances functional recovery of rat facial nerve. J. Rehabil. Res. Dev. 2012, 49, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Xu, Q. G.; Franz, C. K.; Zhang, R.; Dalton, C.; Gordon, T.; Verge, V.M.K.; Midha, R.; Zochodne, D.W. Accelerated axon outgrowth, guidance, and target reinnervation across nerve transection gaps following a brief electrical stimulation paradigm. J. Neurosurg. 2012, 116, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.H.; Chang, R.L.; Chang, S.L.; Tsai, C.C.; Tsai, F.J.; Chen, Y.S. Electrical stimulation improves peripheral nerve regeneration in streptozotocin-induced diabetic rats. J. Trauma. Acute Care Surg. 2012, 72, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Lu, L.; Hu, X.; Luo, Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats Europ J. Neurosci. 2013, 38, 3691–3701. [Google Scholar]
- Koppes, A.N.; Nordberg, A.L.; Paolillo, G.M.; Goodsell, N.M.; Darwish, H.A.; Zhang, L.; Thompson, D.M. Electrical stimulation of Schwann cells promotes sustained increases in neurite outgrowth. Tiss. Eng. Pt. A 2014, 20, 494–506. [Google Scholar] [CrossRef]
- Keane, G.C.; Pan, D.; Roh, J.; Larson, E.L.; Schellhardt, L.; Hunter, D.A.; Snyder-Warwick, A.K.; Moore, A.M.; Mackinnon, S.E.; Wood, M.D. The effects of intraoperative electrical stimulation on regeneration and recovery after nerve isograft repair in a rat model. Hand 2020, 17, 540–548. [Google Scholar] [CrossRef]
- Zuo, K.J.; Shafa, G.; Antonyshyn, K.; Chan, K.; Gordon, T.; Borschel, G.H. A single session of brief electrical stimulation enhances axon regeneration through nerve autografts. Exp. Neurol. 2020, 323, 113074. [Google Scholar] [CrossRef]
- Javeed, S.; Faraji, A.J.; Dy, C.; Ray, W.Z.; MacEwan, M.R. Application of electrical stimulation for peripheral nerve regeneration: Stimulation parameters and future horizons. Interdiscipl Neurosurg. 2021, 24, 101117. [Google Scholar] [CrossRef]
- Birenbaum, N.K.; Yan, Y.; Odabas, A.; Chandra, N.S.; Ray, W.Z.; MacEwan, M.R. Multiple sessions of therapeutic electrical stimulation using implantable thin-film wireless nerve stimulators improve functional recovery after sciatic nerve isograft repair. Muscle & Nerve 2023, 67, 244–251. [Google Scholar]
- Keane, G.C.; Marsh, E.B.; Hunter, D.A.; Schelhardt, L.; Walker, E.R.; Wood, M.D. Lidocaine nerve block diminishes the effects of therapeutic electrical stimulation to enhance nerve regeneration in rats. Hand 2023, 18 (1_suppl), 119S–125S. [Google Scholar] [CrossRef]
- Sulaiman, O.A.R.; Voda, J.; Gold, B.G.; Gordon, T. FK506 increases peripheral nerve regeneration after chronic axotomy but not after chronic Schwann cell denervation. Exp. Neurol. 2002, 175, 127–137. [Google Scholar] [CrossRef]
- Tajdaran, K.; Shoichet, M.S.; Gordon, T.; Borschel, G.H. A novel polymeric drug delivery system for localized and sustained release of tacrolimus (FK506). Biotech. and Bioeng. 2015, 112, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Tajdaran, K.; Chan, K.; Zhang, J.; Gordon, T.; Borschel, G.H. Local FK506 dose-dependent study using a novel three-dimensional organotypic assay. Biotech. Bioengin 2019, 116, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Tajdaran, K.; Chan, K.; Shoichet, M.; Gordon, T.; Borschel, G.H. Local delivery of FK506 to injured peripheral nerve enhances axon regeneration after surgical nerve repair in rats. Acta Biomater 2019, 96, 211–221. [Google Scholar] [CrossRef]
- Zuo, K.J.; Shafa, G.; Chan, K.; Zhang, J.; Hawkins, C.; Tajdaran, K.; Gordon, T.; Borschel, G.H. Local FK506 drug delivery enhances nerve regeneration through fresh, unprocessed peripheral nerve allografts. Exp. Neurol. 2021, 341, 113680. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Sulaiman, O.A.R.; Boyd, J.G. Experimental strategies to promote functional recovery after peripheral nerve injuries. J. Periph Nerv. System 2003, 236–250. [Google Scholar] [CrossRef]
- Sulaiman, O.A.R.; Gordon, T. Cellular and molecular interactions after peripheral and central nerve injury. Biomed. Rev. 2003, 14, 51–62. [Google Scholar] [CrossRef]
- Sulaiman, O.A.R.; Gordon, T. Neurobiology of peripheral nerve regeneration and functional recovery: from bench top research to bedside application. Ochsner J. 2013, 13, 100–180. [Google Scholar]
- Gordon, T. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics 2016, 13, 295–310. [Google Scholar] [CrossRef]
- Gordon, T.; English, A.W. Strategies to promote functional recovery after nerve injury: Electrical stimulation and/or exercise. Eur. J. Neurosci. 2016, 43, 336–350. [Google Scholar] [CrossRef]
- Gordon, T. The neurotrophic factor rationale for using brief electrical stimulation to promote peripheral nerve regeneration in animal models and human patients. In “Conductive Polymers: Electrical interactions in Cell Biology and Medicine”: Eds Zhang, Z.; Rouabhia, M.; Moulton, S.E. CRC Press, New York, 2017, Ch 11, pp 231-249.
- Tajdaran, K.; Chan, K.; Gordon, T.; Borschel, G.H. Matrices, scaffolds, and carriers for protein and molecule delivery peripheral nerve regeneration. Exp. Neurol. 2019, 319, 112817. [Google Scholar] [CrossRef]
- Gordon, T. Peripheral nerve regeneration and muscle reinnervation Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef]
- Zuo, K.J.; Gordon, T.; Chan, K.M.; Borschel, G.H. Electrical stimulation to enhance peripheral nerve regeneration: Update in molecular investigations and clinical translation. Exp. Neurol. 2020, 332, 113397. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J. Neurosci. 1995, 15, 3886–3895. [Google Scholar] [CrossRef]
- Lieberman, A.R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int. Rev. Neurobiol. 1971, 14, 49–124. [Google Scholar] [PubMed]
- Grafstein, B. The nerve cell body response to axotomy. Exp. Neurol. 1975, 48, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.E. Cellular responses to axotomy and to related procedures. Br. Med. Bull. 1974, 30, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T. Dependence of peripheral nerves on their target organs, in Somatic and Autonomic Nerve-Muscle Interactions (Bumstock G., Vrbova G., and O'Brien R. A., eds.), Elsevier, New York, 1993, pp. 289–323.
- Patodia, S.; Raivich, G. Role of transcription factors in peripheral nerve regeneration. Frontiers Mol. Neurosci. 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, W.; Bisby, M.A.; Kreutzberg, G.W. Changes in cytoskeletal proteins in the rat facial nucleus following axotomy. J. Neurosci. 1988, 8, 3181–3189. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.; Tetzlaff, W.; Bisby, M.; Fawcett, J.W.; Milner, R. Rapid induction of the major embryonic alpha-tubulin mRNA, T alpha 1, during nerve regeneration in adult rats. J. Neurosci. 1989, 9, 1452–1463. [Google Scholar] [CrossRef]
- Kato, S.; Ide, C. Axonal sprouting at the node of Ranvier of the peripheral nerve disconnected with the cell body. Restor. Neurol. Neurosci. 1994, 6, 181–187. [Google Scholar] [CrossRef]
- Hoffman, P.N.; Thompson, G.W.; Griffen, J.W.; Price, D.L. Changes in neurofilament transport coincide temporally with alterations in the caliber of axons in regenerating motor fibers. J. Cell Biol. 1985, 101, 1332–1340. [Google Scholar] [CrossRef]
- Hoffman, P.N.; Cleveland, D.W.; Griffin, J.W.; Landes, R.W.; Cowan, N.J.; Price, D.L. Neurofilament gene expression: a major determinant of axon caliber. Proc. Natl. Acad. Sci. USA 1997, 84, 3472–3476. [Google Scholar] [CrossRef]
- Gordon, T.; Stein, R.B. Time course and extent of recovery of reinnervated motor units of cat triceps surae muscles. J. Physiol. 1982, 323, 307–323. [Google Scholar] [CrossRef]
- Gordon, T.; Gillespie, J.; Orozco, R.; Davis, L. Axotomy-induced changes in rabbit hindlimb nerves and the effects of chronic electrical stimulation. J. Neurosci. 1991, 11, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Kerns, J.M.; Danielsen, N.; Holmquist, B.; Kanje, M.; Lundborg, G. The influence of predegeneration on regeneration through peripheral nerve grafts in the rat. Exp. Neurol. 1993, 122, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.R.; Bedard, A.M.; Hincke, M.T.; Tetzlaff, W. Increased expression of BDNF and trkB mRNA in rat facial motoneurons after axotomy. Eur. J. Neurosci. 1996, 8, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Brushart, T.M.; Gordon, T. Electrical stimulation accelerates and increases expression of BDNF and TrkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 2000, 12, 4381–4390. [Google Scholar] [CrossRef] [PubMed]
- Renthal, W.; Tochitsky, I.; Yang, L.; Cheng, Y.C.; Li, E.; Kawaguchi, R.; Gerschwind, D.H.; Woolf, C.J. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron 2020, 108, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Hanz, S.; Perlson, E.; Willis, D.; Zheng, J.-Q.Q.; Massarwa, R.; Huerta, J.J.; Koltzenburg, M.; Kohler, M.; van-Minnen, J.; Twiss, J.L.; Fainzilber, M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 2003, 40, 1095–1104. [Google Scholar] [CrossRef]
- Rishal, I.; Fainziber, M. Axon-soma communication in neuronal injury. Nat. Rev. Neurosci. 2014, 15, 32–42. [Google Scholar] [CrossRef]
- Saito, A.; Cavelli, V. Signaling over distances. Mol. Cell Proteomics 2016, 15, 382–393. [Google Scholar] [CrossRef]
- Spira, M. E.; Benbassat, D.; Dormann, A. Resealing of the proximal and distal cut ends of transected axons: electrophysiological and ultrastructural analysis. J Neurobiol. 1993, 24, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, G.; Madeddu, F.; Bozzi, Y.; Maffei, L.; Ratto, G.M. Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. FASEB J. 2004, 18, 1934–1936. [Google Scholar] [CrossRef]
- Kershensteiner, M.; Schwab, M.E.; Lichtman, J.W.; Misgeld, T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med. 2005, 11, c572–c557. [Google Scholar] [CrossRef]
- Kamber, D.; Erez, H.; Spira, M.E. Local calcium-dependent mechanisms determine whether a cut axonal end assembles a retarded endbulb or competent growth cone. Exp. Neurol. 2009, 219, 112–125. [Google Scholar] [CrossRef]
- Bradke, F.; Fawcett, J.W.; Spira, M.E. Assembly of a new growth cone after axotomy: The precursor to axon regeneration. Nat. Rev. Neurosci. 2012, 13, 183–193. [Google Scholar] [CrossRef]
- Ambron, R. T.; Walters, E. T. Priming events and retrograde injury signals. A new perspective on the cellular and molecular biology of nerve regeneration. Mol. Neurobiol. 1996, 13, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Chierzi, S.; Codd, A.M.; Campbell, D.S.; Meyer, R.L; Holt, C.E.; Fawcett, J.W. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J. Neurosci. 2005, 25, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Vogelaar, C. F.; Gervasi, N.M.; Gumy, L.F.; Story, D.J.; Raja-Chowdhury, R.; Leung, K.-M.; Hold, C.E.; Fawcett, J.W. Axonal mRNAs: characterisation and role in the growth and regeneration of dorsal root ganglion axons and growth cones. Mol. Cell Neurosci. 2009, 42, 102–115. [Google Scholar] [CrossRef]
- Yan, D.; Wu, Z.; Chisholm, A. D.; Jin, Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell 2009, 138, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Omotade, O.F.; Pollitte, S.F.; Zheng, J.Q. Actin-based growth cone motility and guidance. Mol. Cell Neurosci. 2017, 84, 4–10. [Google Scholar] [CrossRef]
- Liu, M.; Li, P.; Jia, Y.; Cui, Q.; Zhang, K.; Jiang, J. Role of non-coding RNAs in axon regeneration after peripheral nerve injury. In J. Biol. Sci. 2022, 18, 3435–3446. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.C.; Willis, D.E. What makes a RAG regeneration associated? Front. Mol. Neurosci. 2015, 8, 43. [Google Scholar] [CrossRef]
- Hao, Y.; Frey, E.; Yoon, C.; Wong, H.; Nestorovski, D.; Holtzman, L.B.; Giger, R.J.; DiAntonio, A.; Collins, C. An evolutionarily conserved mechanism for cAMP elicited axonal regeneration involves direct activation of the dual leucine zipper kinase DLK. eLife 2016, 5, e14048. [Google Scholar] [CrossRef]
- Shin, J.E.; Cho, Y.; Beirowski, B.; Milbrandt, J.; Cavalli, V.; DiAntonio, A. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 2012, 74, 1015–1022. [Google Scholar] [CrossRef]
- Kiryu-Seo, S.; Kato, R.; Ogawa, T.; Nakagomi, S.; Nagata, K.; Kiyama, H. Neuronal injury-inducible gene is synergistically regulated by ATF3, c-Jun, and STAT3 through the interaction with Sp1 in damaged neurons. J. Biol. Chem. 2008, 283, 6988–6996. [Google Scholar] [CrossRef]
- Valakh, V.; Frey, E.; Babetto, E.; Walker, L.J.; DiAntonio, A. Cytoskeletal disruption activates the DLK/JNK pathway, which promotes axonal regeneration and mimics a preconditioning injury. Neurobiol. Dis. 2015, 77, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Ha, H.; Kim, Y.K.; Cho, Y.; DiAntonio, A. DLK regulates a distinctive transcriptional regeneration program after peripheral nerve injury. Neurobiol. Dis. 2019, 127, 178. [Google Scholar] [CrossRef] [PubMed]
- George, E.B.; Glass, J.D.; Griffen, J.W. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J. Neurosci. 1995, 15, 6445–6452. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflamm 2001, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Sievers, C.; Platt, N.; Perry, V.H.; Coleman, M.P.; Conforti, L. Neurites undergoing Wallerian degeneration show an apoptotic-like process with annexin V positive staining and loss of mitochondrial membrane potential. Neurosci. Res. 2003, 46, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kershensteiner, M.; Schwab, M.E.; Lichtman, J.W.; Misgeld, T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med. 2005, 11, c572–557. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Nascimento-Ferreira, I.; Orsomando, G.; Mori, V.; Gilley, J.; Brown, R. Janeckova, L; Vargas, M.E.; Worrell, L..A.; Loreto, A.; Tickle, J.; Patrick, J.; Webster, J.R.M.; Marangoni, M.; Carpi,, F.M.; Pucciarelli, S.; Rossi, ;Meng, W.; Sagasti, A.; Ribchester, R.R.; Magni, G.; Coleman, M.P.; Conforti, L. A rise in NAD precursor nicotinamide mono-nucleoride (NMN) after injury promotes axon degeneration. Cell Death Differen 2015, 22, 731–742. [Google Scholar]
- Loreto, A.; Hill, C.S.; Hewitt, V.L.; Orsomando, G.; Angeletti, C.; Gilley, J.; Lucci, C.; Sanchez-Martines, A.; Whitworth, A.J.; Conforti, L.; Dajas-Bailador, F.; Coleman, M.P. Mitochondrial impairment activates the Wallerian pathway through depletion of NMNAT2 leading to SARM1-dependent axon degeneration. Neurobiol. Dis. 2020, 134, 104678. [Google Scholar] [CrossRef]
- Waller, A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Phil Trans. Royal Soc. Lond. 1850, 140, 423–429. [Google Scholar]
- Küry, P.; Stoll, G.; Müller, H.W. Molecular mechanisms of cellular interactions in peripheral nerve regeneration. Curr. Opin. Neurol. 2001, 14, 635–639. [Google Scholar] [CrossRef]
- Stoll, G.; Jander, S.; Myers, R.R. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller’s observations to neuroinflammation. J. Peripher. Nerv. Syst. 2002, 7, 13–27. [Google Scholar] [CrossRef]
- Ziv, N.E.; Spira, M.E. Axotomy induces a transient and localized elevation of the free intracellular calcium concentration to the millimolar range. J. Neurophysiol. 1995, 74, 2625–2537. [Google Scholar] [CrossRef] [PubMed]
- Ziv, N.E.; Spira, M.E. Localized and Transient Elevations of Intracellular Ca21 Induce the Dedifferentiation of Axonal Segments into Growth Cones. J. Neurosci. 1997, 3568–3579. [Google Scholar] [CrossRef] [PubMed]
- Spira, M.E.; Oren, R.; Dormann, A.; Gitler, D. Critical calpain-dependent ultrastructural alterations underlie the transformation of an axonal segment into a growth cone after axotomy of cultured Aplysia neurons. J. Comp. Neurol. 2003, 457, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Lonze, B.E.; Ginty, D.D. Function and regulation: Review of CREB family transcription factors in the nervous system. Neuron 2002, 605–623. [Google Scholar] [CrossRef] [PubMed]
- Ghosh-Roy, A.; Wu, Z.; Concharov, A.; Jin, Y.; Chisholm, A.D. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J. Neurosci. 2010, 3175–3183. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Deng, K.; Hou, J.; Bryson, J.B.; Barco, A.; Nikulina, E.; Spencer, T.; Mellado, W.; Kandel, E.R.; Filbin, M.T. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron 2004, 44, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shin, J.E.; Ewan, E.E.; Oh, Y.M.; Pita-Thomas, W.; Cavalli, V. Activating injury-responsive genes with hypoxia enhances axon regeneration through neuronal HIF-1alpa. Neuron 2015, 88, 720–734. [Google Scholar] [CrossRef]
- Song, W.; Cho, Y.; Watt, D.; Cavalli, V. Tubulin-tyrosine ligase (TTL) mediated increase in tyrosinated α-tubulin in injured axons is required for retrograde injury signaling and axon regeneration. J. Biol. Chem. 2015, 290, 14765–14775. [Google Scholar] [CrossRef]
- Hanz, S.; Perlson, E.; Willis, D.; Zheng, J.Q.; Massarwa, R.; Huerta, J.J.; Koltzenburg, M.; Kohler, M.; van-Minnen, J.; Twiss, J.F.; Fainzilber, M. Axoplasmic importins enable retrograde injury blot analysis. Signaling in lesioned nerve. Neuron 2003, 40, 1095–1104. [Google Scholar] [CrossRef]
- Perlson, E.; Hanz, S.; Ben-Yaakov, K.; Segal-Ruder, Y.; Seger, R.; Fainzilber, M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 2005, 45, 715–726. [Google Scholar] [CrossRef]
- Lezana, J.P.; Dagan, S.Y.; Robinson, A.; Goldstein, R.S.; Fainzilber, M.; Bronfman, F.C.; B1onfman, M. Axonal PPARγ promotes neuronal regeneration after injury. Dev. Neurobiol. 2016, 76, 688–701. [Google Scholar] [CrossRef]
- Terenzio, M.; Koley, S.; Samra, N.; Rishal, I.; Zhao, Q.; Sahoo, P.K.; Uirsman, A.; Marvaldi, L.; Oses-Priesto, J.A.; Forester, C.; Goes, C.; Kliniski, A.L.; Di Pizio, A.; Doron-Mandel, E.D.; Perry, R.B-T.; Koppel, I.; Twiss, J.L.; Burlingame, A.L.; Fainzilber, M. Locally translated mTOR controls axonal local translation in nerve injury. Science 2018, 359, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Mahar, M.; Cavelli, V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018, 19, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Misra, V.; Verge, V.M.K. Sensing nerve injury at the axonal ER: activated Luman/CREB3 serves as a novel axonally synthesized retrograde regeneration signal. PNAS 2014, 16142–16147. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Zhai, R.; McLean, N.A.; Johnson, J.M.; Misra, V.; Verge, V.M.K. The unfolded protein response and cholesterol biosynthesis link Lumen/CREB3 to regenerative axon growth in sensory neurons. J. Neurosci. 2015, 35, 4557–14570. [Google Scholar] [CrossRef] [PubMed]
- Hasmatali, J.C.D.; De Guzman, J.; Zhai, R.; Yang, L.; McLean, N.A.; Hutchinson, C. ; Johnston.; Misra, V.; Verge, V.M.K. Axotomy induces phasic alterations in Luman/CREB3 expression and nuclear localization in injured and contralateral uninjured sensory neurons: correlation with intrinsic axon growth capacity. Neuropathol. Exp. Neurol. 2019, 78, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Wingender, E.; Schoeps, T.; Haubrock, M.; Dönitz, J. TFClass: a classification of human transcription factors and their rodent orthologs. Nucleic Acids Res 2015, 43, D97–D102. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Hunt, S.P. (1991) Long-term increase in the levels of c-jun mRNA and jun protein-like immunoreactivity in motor and sensory neurons following axon damage. Neurosci. Lett. 1991, 129, 107–110. [Google Scholar] [CrossRef]
- Van Kesteren, R.E.; Mason, M.R.; Macgillavry, H.D.; Smit, A.B.; Verhaagen, J. A gene network perspective on axonal regeneration. Front. Mol. Neurosci. 2011, 4, 46. [Google Scholar] [CrossRef]
- Song, J.; Singh, M. From hub proteins to hub modules: the relationship between essentiality and centrality in the yeast interactome at different scales of organization. PLoS Comput. Biol. 2013, 9, 1002910. [Google Scholar] [CrossRef]
- Fontana, X.; Hristova, M.; Costa, C.D.; Patodia, S.; Thei, L.; Makwana, M.; Spencer-Dene, B.; Latouche, M.; Mirsky, R.; Jessen, K.R.; Klein, R.; Raivich, G.; Behrens, A. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J. Cell Biol. 2012, 198, 127–141. [Google Scholar] [CrossRef]
- Jessen, K. R.; Mirsky, R.; Arthur-Farraj, P. The role of cell plasticity in tissue repair: adaptive cellular reprogramming. Devel Cell 2005, 34, 613–620. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia 2008, 56, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Herdegen, T.; Skene, P.; Bähr, M. The c-Jun transcription factor–bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci. 1997, 20, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Michaeleviski, I.; Segal-Ruder, Y.; Rozenbaum, M.; Medzihradszky, K.F.; Shalem, O.; Coppola, G.; Horn-Saban, S.; Ben-Yaakov, K.; Dagan, S.Y.; Rishal, I.; Geschwind, D.H.; Pilpel, Y.l.; Burlingame, A.L.; Fainzilber, M. Signaling to transcription networks in the neuronal retrograde injury response. Sci. Signal. 2010, 3, ra53. [Google Scholar] [CrossRef] [PubMed]
- Blesch, A.; Lu, P.; Tsukada, S.; Alto, L.T.; Roet, K.; Coppola, G.; Geschwind, D.; Tuszynski, M.H. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to cAMP-mediated effects. Exp. Neurol. 2012, 235, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, F. W.; Hager, G.; Schmitt, A. B.; Horvat, A.; Streif, R.; Spitzer, C.; Gamal, S.; Breuer, S.; Brook, G.A.; Nacimiento, W.; Kreutzberg, G.W. Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT). Eur. J. Neurosci. 2000, 12, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, H.; Kondo, E.; Fukuoka, T.; Dai, Y. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol. Cell Neurosci. 2000, 15, 170–182. [Google Scholar] [CrossRef]
- Seijffers, R.; Allchorne, A.J.; Woolf, C.J. The transcription factor ATF3 promotes neurite outgrowth. Mol. Cell Neurosci. 2006, 143–154. [Google Scholar] [CrossRef]
- Seijffers, R.; Mills, C.D.; Woolf, C.J. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J. Neurosci. 2007, 27, 7911–7920. [Google Scholar] [CrossRef]
- Lindwall, C.; Kanje, M. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol. Cell Neurosci. 2005, 29, 269–282. [Google Scholar] [CrossRef]
- Schweizer, U.; Gunnersen, J.; Karch, C.; Wiese, S.; Holtmann, B.; Takeda, K.; Akira, S.; Sendtner, M. Conditional gene ablation of Stat3 reveals differential signaling requirements for survival of motoneurons during development and after nerve injury in the adult. J. Cell Biol. 2002, 156, 287–297. [Google Scholar] [CrossRef]
- Bareyre, F.M.; Garzorz, N.; Lang, C.; Misgeld, T.; Buning, H.; Kerschensteiner, M. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc. Natl. Acad. Sci. U S A 2011, 108, 6282–6287. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.P.; Cornuet, P.K.; McIlwrath, S.; Koerber, H.R.; Albers, K.M. SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neurosci. 2006, 143, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.P.; McIlwrath, S.L.; Jing, X.; Cornuet, P.K.; Salerno, K.M.; Koerber, H.R.; Albers, K.M. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009, 1256, 43–54. [Google Scholar] [CrossRef]
- Jankowski, M.P.; Miller, L.; Koerber, H.R. Increased expression of transcription factor SRY-box-containing gene 11 (Sox11) enhances neurite growth by regulating neurotrophic factor responsiveness. Neurosci. 2018, 382, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wang, T.; Huang, S.; Glorioso, J.C.; Albers, K.M. The transcription factor Sox11 promotes nerve regeneration through activation of the regeneration-associated gene Sprr1a. Exp. Neurol. 2012, 233, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Ho, C.; Wong, K.; Tessier-Lavigne, M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J. Neurosci. 2009, 29, 7116–7123. [Google Scholar] [CrossRef] [PubMed]
- Finelli, M.G.; Wong, J.K.; Zou, H. Epigenetic regulation of sensory axon regeneration after spinal cord injury. J. Neurosci. 2013, 33, 19664–19676. [Google Scholar] [CrossRef] [PubMed]
- Saijilafu, E.-M.H.; Hur, C.-M.; Liu, C.-M.; Jiao, Z.; Xu, W.-L; Zhou, F.-Q. P13K-GSK3 signalling regulates mammalian axon regeneration by inducing expression of Smad. Nat Commun. 2013, 4, 2690. [Google Scholar] [CrossRef]
- Saijilafu, Z.B.Y.; Zhou, F.Q. Signaling pathways that regulate axon regeneration. Neurosci. Bull. 2013, 29, 411–420. [Google Scholar] [CrossRef]
- Okuyama, N.; Kiryu-Seo, S.; Kiyama, H. Altered expression of Smad family members in injured motor neurons of rat. Brain Res. 2007, 1132, 36–41. [Google Scholar] [CrossRef]
- Jiang, J.J.; Liu, C.M.; Zhang, B.Y.; Wang, W.X.; Zhang, M.; Saijilafu, E.-M.H. MicroRNA-26a supports mammalian axon regeneration in vivo by suppressing GSK3beta expression. Cell Death Dis. 2015, 6, e1865. [Google Scholar] [CrossRef]
- Nadeau, S.; Hein, P.; Fernandes, K.J.; Peterson, A.C.; Miller, F.D. A transcriptional role for C/EBP beta in the neuronal response to axonal injury. Mol. Cell Neurosci. 2005, 29, 525–535. [Google Scholar] [CrossRef]
- Ma, J.J.; Ju, X.; Xu, R.J.; Wang, W.H.; Luo, Z.-P.; Liu, C.-M.; Yang, L.; Li, B.; Chen, J.-Q.; Meng, B.; Yang, H.-L.; Zhou, F.-Q. ; Saijilafu. Telomerase reverse transcriptase and p53 regulate mammalian peripheral nervous system and CNS axon regeneration downstream of c-Myc. J Neurosci 2019, 39, 9107–9118. [Google Scholar] [CrossRef]
- Di Giovanni, S.; Knights, C.D.; Rao, M.; Yakovlev, A.; Beers, J.; Catania, J.; Avantaggiati, M. L.; Faden, A. I. The tumor suppressor protein p53 is required for neurite outgrowth and axon regeneration. EMBO J. 2006, 25, 4084–4096. [Google Scholar] [CrossRef]
- Moore, D. L.; Blackmore, M. G.; Hu, Y.; Kaestner, K. H.; et al. KLF family members regulate intrinsic axon regeneration ability. Science 2009, 326, 298–301. [Google Scholar] [CrossRef]
- Xu, J.H.; Qin, X.Z.; Zhang, H.N.; Ma, Y.X.; Qi, S.-B.; Zhang, H.-C.; Ma, J.-J.; Fu, X.-Y.; Xie, J.-L. Saijilafu. Deletion of Kruppel-like factor-4 promotes axonal regeneration in mammals. Neural Regen. Res. 2021, 16, 166–171. [Google Scholar]
- Palmisano, I.; Di Giovanni, S. Advances and limitations of current epigenetic studies investigating mammalian axonal regeneration. Neurotherapeutics 2018, 15, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J.; Handy, D.E. Epigenetic modifications: basic mechanisms and role in cardiovascular disease (2013 Grover Conference series. Pulm. Circ. 2014, 169–174. [Google Scholar] [CrossRef]
- Zhou, Y.; Garcia, B.A. Comprehensive catalog of currently documented histone modifications. Spring Harb. Perspect. Biol. 2015, 7, a025064. [Google Scholar] [CrossRef]
- Puttagunta, R.; Tedeschi, A.; Soria, M.G.; Hervera, A.; Lindner, R.; Rathore, K.I.; Gaub, P.; Joshi, Y.; Nguyen, T.; Schmandke, A.; Laskowski, C.J.; Boutillier, A.-L.; Bradke, F.; Giovanni, S.D. PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nature Comm. 2014, 5, 3527. [Google Scholar] [CrossRef]
- Karpranov, P.; Willingham, A.T.; Gingeras, T.R. Genome-wide transcription and the implications for genomic organization. Nat. Rev. Genet. 2007, 8, 413–423. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; He, Y.; Wang, L. Long noncoding RNA nuclear enriched abundant transcript 1 promotes the proliferation and migration of Schwann cells by regulating the miR-34a/Satb1 axis. Glia 2021, 234, 15357–16366. [Google Scholar] [CrossRef]
- Strickland, I.T; Richards, L.; Holes, F.E.; Wynick, D.; Uney, J.B.; Wong, L.F. Axotomy-induced miR-21 promotes axon growth in adult dorsal root ganglion neurons. PLoS One 2011, 6, e23423. [Google Scholar] [CrossRef]
- Zhou, S.; Ding, F.; Gu, X. Non-coding RNAs as emerging regulators of neural injury response and regeneration. Neurosci. Bull. 2016, 32, 253–264. [Google Scholar] [CrossRef]
- Dorrier, C.E.; Jones, H.E.; Pintarić, L.; Siegenthaler, J.A.; Daneman, R. Emerging roles for CNS fibroblasts in health, injury and disease. Nature Rev. Neurosci. 2022, 23, 23–34. [Google Scholar] [CrossRef]
- Knöferle, J.; Koch, J.C.; Ostendorf, T.; Michel, V.; Planchamp, V.; Vutova, P.; Tönges, L.; Stadelmann, C.; Brück, W.; Bähr, M.; Lingor, P. Mechanisms of acute axonal degeneration in the optic nerve in vivo. PNAS 2010, 107, 6064–6069. [Google Scholar] [CrossRef]
- Mårtensson, L.B.; Blom, C.L.; Dahlin, L.B. Ca2+ involvement in activation of extracellular-signal-regulated-kinase 1/2 and m-calpain after axotomy of the sciatic nerve. Neural Reg Res B 2017, 12, 623–628. [Google Scholar]
- Miledi, R.; Slater, C.R. On the degeneration of rat neuromuscular junctions after nerve section. J. Physiol. 1970, 207, 507–528. [Google Scholar] [CrossRef]
- Gilliatt, R. W.; Horth, R.J. Nerve conduction during Wallerian degeneration in the baboon. J. Neurol. Neurosurg. Psychiatry 1972, 35, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Lubinska, L. Early course of Wallerian degeneration in myelinated fibres of the rat phrenic nerve. Brain Res. 1977, 130, 47–63. [Google Scholar] [CrossRef]
- Mackenzie, S.J.; Smirnov, I.; Calancie, B. Cauda equina repair in the rat: part Time course of ventral root conduction failure. J. Neurotrauma 2012, 29, 1683–1690. [Google Scholar] [CrossRef]
- Harrisingh, M.C.; Perez-Nadales, E.; Parkinson, D.B.; Malcolm, D.S.; Mudge, A.W.; Lloyd, A.C. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004, 23, 3061–3071. [Google Scholar] [CrossRef]
- Napoli, I.; Noon, L.A.; Ribeiro, S.; Kerai, A.P.; Parrinello, S.; Rosenberg, L.H.; Collins, M.J.; Harrisingh, M.C.; White, I.J.; Woodhoo, A.; Lloyd, A.C. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration. Neuron 2012, 73, 729–742. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J. A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M..; Griffith, M.; Hantke, J.; Maciias-Camara, N.; Azkargorta, M.; Aurrekoetxea, I.; De Juan, V.G.; Jefferies, H.B.; Aspichueta, P.; Elortza, F.; Aransay, A.M.; Martínez-Chantar, M.L.; Baas, F.; Mato, J.M.; Mirsky, R.; Woodhoo, A.; Jessen, K.R. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol. 2015, 210, 153–168. [Google Scholar] [CrossRef]
- Jang, S. Y.; Shin, Y. K.; Park, S. Y.; Park, J. Y.; Lee, H. J.; Yoo, Y. H.; Kim, J.K.; Park, H.W. Autophagic myelin destruction by Schwann cells during Wallerian degeneration and segmental demyelination. Glia 2016, 64, 730–742. [Google Scholar] [CrossRef]
- Brosius Lutz, A.; Chung, W. S.; Sloan, S. A.; Carson, G. A.; Zhou, L.; Lovelett, E.; Posada, S.; Zuchero, J.B.; Barres, B.A. Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury. Proc. Natl. Acad. Sci. USA 2017, 114, E8072–E8080. [Google Scholar] [CrossRef] [PubMed]
- Perry, V. H.; Tsao, J. W.; Fearn, S.; Brown, M. C. Radiation-induced reductions in macrophage recruitment have only slight effects on myelin degeneration in sectioned peripheral nerves of mice. Eur. J. Neurosci. 1995, 7, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Gregson, N.A.; Hall, S.M. A quantitative analysis of the effects of the intraneural injection of lysophosphatidyl choline. J. Cell Sci. 1973, 13, 257–277. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Tigueros, M.A.; Kalvas, A.; David, S. Phospholipase A2 plays an important role in myelin breakdown and phagocytosis during Wallerian degeneration. Mol. Cell Neurosci. 2003, 24, 753–765. [Google Scholar] [CrossRef]
- Banner, L.R.; Patterson, P.H. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc Natl Acad Sci U S A 1994, 91, 7109–7113. [Google Scholar] [CrossRef]
- Bolin, M.; Verity, A.N.; Silver, J.E.; Shooter, E.M.; Abrams, J.S. Interleukin-6 production by Schwann cells and induction in sciatic nerve injury. J Neurochem 1995, 64, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Bourde, O.; Kiefer, R.; Toyka, K.V.; Hartung, H.P. Quantification of interleukin-6 mRNA in Wallerian degeneration by competitive reverse transcription polymerase chain reaction. J. Neuroimmunol. 1996, 69, 135–140. [Google Scholar] [PubMed]
- Kurek, J.B.; Austin, L.; Cheema, S.S.; Bartlett, P.F.; Murphy, M. Up-regulation of leukaemia inhibitory factor and interleukin-6 in transected sciatic nerve and muscle following denervation. Neuromuscul. Disorders 1996, 6, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, H.S.; Roytta, M. Increased expression of chemokines (MCP-1, MIP-1alpha, RANTES) after peripheral nerve transection. J Periph Nerv Syst 2000, 5, 75–81. [Google Scholar] [CrossRef]
- Subang, M.C.; Richardson, P.M. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 mRNA in peripheral nervous tissue. Eur. J. Neurosci. 2001, 13, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.E.; Lacroix, S.; Aviles-Trigueros, M.; David, S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain 2005, 128, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Boivin, A.; Pineau, I.; Barrette, B.; Filali, M.; Vallieres, N.; Rivest, S.; Lacroix, S. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J. Neurosci. 2007, 27, 12565–12576. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Angert., M.; Dolkas, J.; Campana, W.M.; Palenscar, K.; Myers, R.R. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci 2006, 31, 407–415. [Google Scholar] [CrossRef]
- Cattopadhyay, S.; Meyers, R.R.; Janes, J. Shubayev, V. Cytokine regulation of MMP-9 in peripheral glia: implications for pathological processes and pain in injured nerve. Brain Behav Immun 2007, 21, 561–568. [Google Scholar] [CrossRef]
- Beuche, W.; Friede, R.L. The role of non-resident cells in Wallerian degeneration. J. Neurocytol. 1984, 13, 767–796. [Google Scholar] [CrossRef]
- Avellino, A.M.; Hart, D.; Dailey, A.T.; MacKinnon, M.; Ellegala, D.; Kliot, M. Differential macrophage responses in the peripheral and central nervous system during allerian degeneration of axons. Exp. Neurol. 1995, 136, 183–198. [Google Scholar] [CrossRef]
- Brück, W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997, 7, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Griffin, J.W.; Li, C.Y.; Trapp, B.D. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J. Neurocytol. 1989, 18, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Kubes, P.; Zochodne, D.W. Delayed peripheral nerve degeneration, regeneration, and pain in mice lacking inducible nitric oxide synthase. J. Neuropathol. Exp. Neurol. 2001, 60, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Bruck, W.; Friede, R.L. The role of complement in myelin phagocytosis during PNS wallerian degeneration. J. Neurol. Sci. 1991, 103, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E. Phagocytosis of myelin in demyelinative disease: a review. Neurochem. Res. 1999, 24, 261–268. [Google Scholar] [CrossRef] [PubMed]
- da Costa, C.C.; van der Laan, L.J.; Dijkstra, C.D.; Bruck, W. The role of the mouse macrophage scavenger receptor in myelin phagocytosis. Eur. J. Neurosci. 1997, 9, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, R.E.; Echevarria, F.D. Macrophage biology in the peripheral nervous system after injury Prog. Neurobiol. 2019, 102–121. [Google Scholar]
- Nadeau, S.; Filali, M.; Zhang, J.; Kerr, B.J.; Rivest, S.; Soulet, D.; Iwakura, Y.; de Rivero Vaccari, J.; Keane, R.W.; Lacroix, S. Functional recovery after peripheral nerve injury in dependent n the proinflammatory cytokines IL-1beta and TNF: implications for neuropathic pain. J. Neurosci. 2011, 31, 12533–12542. [Google Scholar] [CrossRef]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef]
- Liu, P.; Peng, J.; Han, G.H.; Ding, X.; Wei, S.; Gao, G.; Huang, K.; Chang, F.; Wang, Y. Role of macrophages in peripheral nerve injury and repair. Neural Regen. Res. 2019, 14, 1335–1342. [Google Scholar]
- Hall, S.M. The response of the (myelinating) Schwann cell population to multiple episodes of demyelination. J. Neurocytol. 1983.12, 1–12. [CrossRef]
- Doree, M.; Hunt, T. From Cdc3 to Cdk1: when did the cell cycle kinase join its cyclin partner? Cell Sci. 2002, 2451–2464. [Google Scholar]
- Han, S.; Seo, T.B.; Kim, K.-H.; Yoon, J.-H.; Yoon, S.-J.; Namgung, U.K. Cdc2-mediated Schwann cell migration during peripheral nerve regeneration. J. Cell Sci. 2007, 120, 246–255. [Google Scholar] [CrossRef]
- Namgung, U.K. The role of Schwann cell-axon interaction in peripheral nerve regeneration. Cell Tiss. Organs 2014, 4, 6–12. [Google Scholar] [CrossRef]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; Wicher, G.K.; Mitter, R.; Greensmith, L.; Behrens, A.; Raivich, G.; Mirsky, R.; Jessen, K.R. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Arthur-Farraj, M.P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef] [PubMed]
- De Leon, M.; Welcher, A.A.; Suter, U.; Shooter, E.M. Identification of transcriptionally regulated genes after sciatic nerve injury. J. Neurosci. Res. 1991, 29, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wu, J.; Zhou, S.; Wang, Y.; Qin, J.; Yu, B.; Gu, X.; Yao, C. Global analysis of transcriptome in dorsal root ganglia following peripheral nerve injury in rats. Biochem. Biophys. Res. Commun. 2016, 478, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Shamash, S.; Reichert, F.; Rotshenker, S. The cytokine network of Wallerian degeneration: tumor necrosis factor-α, Interleukin-1α, and Interleukin-1β. J. Neurosci. 2002, 22, 3052–3060. [Google Scholar] [CrossRef]
- Tofaris, G.K.; Patterson, P.H.; Jessen, K.R.; Mirsky, R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci 2002, 2002 22, 6696–6703. [Google Scholar] [CrossRef]
- Marchionni, M.A.; Goodearl, A.D.; Chen, M.S.; Bermingham-McDonogh, O.; Kirk, C.; Hendricks, M.; Danehy, F.; Misumi, D.; Sudhalta, J.; Kobayashi, K.; Wroblewski, D.; Lynch, C.; Baldassare, M.; Hiles, I.; Davis, J.B.; Hsuan, J.J.; Totty, N.F.; Otsu, M.l.; McBurney, R.N.; Waterfield, M.D.; Strooband, P.; Gwynne, D. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature 1993, 362, 312–318. [Google Scholar] [CrossRef]
- Falls, D.L. Neuregulins and the neuromuscular system: 10 years of answers and questions. J. Neurocytol. 2003, 32, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Falls, D.L. Neuregulins: functions, forms, and signaling strategies. Exp. Cell Res. 2003, 284, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.M. The biology of chronically denervated Schwann cells. Ann. NY Acad. Sci. 1999, 883, 215–233. [Google Scholar] [CrossRef]
- Carroll, S.L.; Miller, M.L.; Frohnert, P.W.; Kim, S.S.; Corbett, J.A. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J. Neurosci. 1997, 17, 1642–1659. [Google Scholar] [CrossRef]
- Stassart, R. M.; Fledrich, R.; Velanac, V.; Brinkmann, B. G.; Schwab, M. H.; Meijer, D.; Swerenda, M.W.; Nave, K.-A. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat. Neurosci. 2013, 16, 48–54. [Google Scholar] [CrossRef]
- Ronchi, G.; Haastert-Talini, K.; Fornasari, B. E.; Perroteau, I.; Geuna, S.; Gambarotta, G. The Neuregulin1/ErbB system is selectively regulated during peripheral nerve degeneration and regeneration. Eur. J. Neurosci. 2016, 43, 351–364. [Google Scholar] [CrossRef]
- El Soury, M.; Fornasari, B.; Morano, M.; Grazio, E.; Ronchi, G.; Incarnato, D.; Giacobini, M.; Geuna, S. ; Provero. Gambarotta, G. Soluble Neuregulin1 down-regulates myelination genes. Frontiers Cell Neurosci. 2018, 11, 157. [Google Scholar]
- Salzer, J.L; Bunge, R.P.; Glaser, L. Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J. Cell Biol. 1980, 84, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Spencer, P.S. Schwann cell mitosis in response to regenerating peripheral axons in vivo. Brain Res. 1985, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Clemence, A.; Mirsky, R.; Jessen, K.R. Non-myelin-forming Schwann cells proliferate rapidly during Wallerian degeneration in the rat sciatic nerve. J. Neurocytol. 1989, 18, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Rahmatullah, M.; Schroering, A.; Rothblum, K.; Stahl, R.C.; Urban, B.; Carey, D.J. Synergistic regulation of Schwann cell proliferation by heregulin and forskolin. Mol. Cell Biol. 1998, 18, 6245–6252. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.J.; Eccleston, P.A.; Jessen, K.R.; Mirsky, R. Interaction between cAMP elevation, identified growth factors, and serum components in regulating Schwann cell growth. J. Neurosci. Res. 1991, 30, 346–352. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Lin, P.X.; Chang, Y.; Webster, H.D. Effects of nerve segment supernatants on cultured Schwann cell proliferation and laminin production. J. Neurosci. Res. 1994, 37, 612–622. [Google Scholar] [CrossRef]
- Morrissey, T.K.; Levi, A.D.; Nuijens, A.; Sliwkowski, M.X.; Bunge, R.P. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbBProc Natl. Acad. Sci. USA 1995, 92, 1431–1435. [Google Scholar] [CrossRef]
- Shin, Y.K.; Jang, S.Y.; Yun, S.H.; Choi, Y.Y.; Yoon, B.-A.; Jo, Y.R.; Park, S.Y.; Pak, M.G.; Park, J.I.; Park, H.T. Cooperative interaction of hepatocyte growth factor and neuregulin regulates Schwann cell migration and proliferation through Grb2-associated binder-2 in peripheral nerve repair. Glia 2017, 65, 794–1808. [Google Scholar] [CrossRef]
- Adams, S. J.; Aydin, I. T.; Celebi, J. T. GAB2-A scaffolding protein in cancer. Mol. Cancer Res. 2012, 10, 1265–1270. [Google Scholar] [CrossRef]
- Wu, G.; Li, X.; Li, M.; Zhang, Z. Long non-coding RNA MALAT1 promotes the proliferation and migration of Schwann cells by elevating BDNF through sponging miR-129-5p. Exp. Cell Res. 2020, 390, 111937. [Google Scholar] [CrossRef]
- Yao, C.; Wang, Q.; Wang, Y.; Wu, J.; Cao, X.; Lu, Y.; Chen, Y.; Feng, W.; Gu, X.; Dun, X.-P.; Yu, B. Loc6802454 regulates Schwann cell proliferation through Psrc1 and Ska1-a as a microRNA sponge following sciatic nerve injury. Glia 2021, 69, 2391–2403. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Zhang, X.; Ding, L.; Zeng, Q. Microanalysis of the expression profile of IncRNA BC088327 as an agonist to heregulin-1β-induced cell proliferation in the peripheral nervous system. Int. J. Mol. Med. 2018, 41, 3477–3484. [Google Scholar]
- Yao, C.; Chen, Y.; Wang, J.; Wu, J.; Cao, X.; Lu, Y.; Chen, Y.; Feng, W.; Gu, X.; Dun, X.-P.; Yu, B. LncRNA BC088259 promotes Schwann cell migration through vimentin following peripheral nerve injury. Glia 2020, 68, 670–679. [Google Scholar] [CrossRef]
- Ma, Y.; Zhai, D.; Zhang, W.; Zhang, H.; Dong, I.; Zhou, Y.; Feng, D.; Zheng, W.; Wang, T.; Mao, C.; Wang, W. Down-regulation of long non-coding RNA MEG3 promotes Schwann cell proliferation and migration and repairs sciatic nerve injury in rats. J. Cell Mol. Med. 2020, 24, 7460–7469. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Huang, T.; Chen, Y.; Shen, L.; Zhou, S.; Zhang, S.; Yu, B. Circ-Spidr enhances axon regeneration after peripheral nerve injury. Cell Death Dis. 2019, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, H.; Frisen, J.; Barbany, G.; Timmusk, T.; Zachrisson, O.; Verge, V.M.; Persson, H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J. Cell Biol. 1993, 123, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Hoke, A.; Redett, R.; Hameed, H.; Jari, R.; Zhou, C.; Li, Z.B.; Griffin, J.W.; Brushart, T.M. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J. Neurosci. 2006, 26, 9646–9655. [Google Scholar] [CrossRef] [PubMed]
- Mi, R.; Chen, W.; Hoke, A. Pleiotrophin is a neurotrophic factor for spinal motor neurons. PNAS 2007, 104, 4664–4669. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M.; Aspalter, M.; Griffin, J.W.; Redett, R.; Hameed, H.; Zhou, C.; Wright, M.; Vyas, A.; Hoke, A. Schwann cell phenotype is regulated by axon modality and central–peripheral location, and persists in vitro. Exp. Neurol. 2013, 247, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Sulaiman, A.R. Nerve regeneration in the peripheral nervous system. Neuroglia (3 edn), Ch 55, 2012, 701-714.
- Wang, X. Pleiotrophin: activity and mechanism. Adv. Clin. Chem. 2020, 98, 1–89. [Google Scholar]
- Heumann, R.; Lindholm, D.; Bandtlow, C.; Meyer, M.; Radeke, M.J.; Misko, T.P.; Shooter, E.; Thoenen, H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerves during development, degeneration and regeneration: role of macrophages. Proc. Natl. Acad. Sci. USA 1987, 84, 8735–8739. [Google Scholar] [CrossRef]
- Meyer, M.; Matsuoka, I.; Wetmore, C.; Olson, L.; Thoenen, H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J. Cell Biol. 1992, 119, 45–54. [Google Scholar] [CrossRef]
- Naveilhan, P.; ElShamy, W.E.; Ernsfors, P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDFRα after sciatic nerve lesion in the mouse. Eur. J. Neurosci. 1997, 9, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Petrov, T.; Chung, P.H.; Gordon, T. The expression of the low affinity nerve growth factor receptor in long-term denervated Schwann cells. Glia 1997, 20, 87–100. [Google Scholar] [CrossRef]
- Seo, T.B.; Oh, M.J.; You, B.G.; Kwon, K.B.; Chang, I.A.; Yoon, J.H.; Lee, C.-Y; Namgung, U.K. Erk1/2-mediated Schwann cell proliferation in the regenerating sciatic nerve by treadmill training. J. Neurotrauma 2009, 26, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Jang, S.Y.; Park, J.Y.; Park, S.Y.; Lee, H.J.; Suh, D.J.; Park, H.T. The Neuregullin-RacMKK7 pathway regulates antagonistic c-jun/Krox20 expression in Schwann cell dedifferentiation. Glia 2013, 61, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Blom, C.L.; Martensson, L.B.; Dahlin, L.B. Nerve injury-induced c-activation in Schwann cells is JNK independent. Biomed. Res. Int. 2014, 2014, 392971. [Google Scholar]
- Friede, R.L.; Bischhausen, R. The fine structure of stumps of transected nerve fibers in subserial sections. J NeuroI Sci. 1980, 44, 181–203. [Google Scholar] [CrossRef]
- McQuarrie, I.G. Effect of a conditioning lesion on axonal sprout formation at nodes of Ranvier I. Comp. Neurol. 1985, 231, 239–249. [Google Scholar] [CrossRef]
- McQuarrie, I.G.; Lasek, R.J. Transport of cytoskeletal elements from parent axons into regenerating daughter axons. J. Neurosci. 1989, 9, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Ranson, S.W. Degeneration and regeneration of nerve fibres J. Comp. Neurol. 1912, 22, 487–546. [Google Scholar]
- Aitken J., T.; Sherman, M.; Young J., Z. Maturation of peripheral nerve fibres with various peripheral connections. J. Anat. 1947, 81, 1–22. [Google Scholar]
- Mackinnon, S.E.; Dellon, A.L.; O-Brien, J.P. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve 2001, 14, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Toft, P.B.; Fugleholm, K.; Schmalbruch, H. Axonal branching following crush lesions of peripheral nerves of rats. Muscle Nerve 1988, 11, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, S.; Napoli, I.; Ribeiro, S.; Digby, P.W.; Fedorova, M.; Parkinson, D.B.; Doddrell, R.D.; Nakayama, M.; Adams, R.H.; Lloyd, A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 2010, 143, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Bixby, J. l.; Lilien, J.; Reichardt, l. F. Identification of the major proteins that promote neuronal process outgrowth on Schwann cells in vitro. J. Cell Biol. 1988, 107, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Schwarzbauer, J.E.; DeSimone, D.W. Fibronectins, their firbillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 2011, 3, a005041. [Google Scholar] [CrossRef]
- Gonzalez Perez, F.; Udina, E.; Navarro, X. Chapter 10 Extracellular matrix components in peripheral nerve regeneration. Int. Rev. Neurobiol. 2013, 108, 257–275. [Google Scholar]
- Lefcort, F.; Venstrom, K.; McDonald, J.A.; Reichardt, L.F. Regulation of expression of fibronectin and its receptor, α5β1, during development and regeneration of peripheral nerve. Development 1992, 116, 767–782. [Google Scholar] [CrossRef]
- Mathews, G.A.; Ffrench-Constant, C. Embryonic fibronectins are up-regulated following peripheral nerve injury in rats J. Neurobiol. 1995, 26, 171–188. [Google Scholar] [CrossRef]
- Gardiner, N. Integrins and the extracellular matrix: key mediators of development and regeneration of the sensory nervous system. Dev Neurobiol 2011, 71, 1054–1072. [Google Scholar] [CrossRef]
- Gonzalez Perez, F.; Alé, A.; Santos, D.; Barwig, C.; Freier, T.; Navarro, X.; Udina, E. Substratum preferences of motor and sensory neurons in postnatal and adult rats. Europ J. Neurosci. 2016, 43, 431–442. [Google Scholar] [CrossRef]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of fibronectin extracellular matrix. Ann Rev Cell Dev Biol 2010, 26, 397–419. [Google Scholar] [CrossRef]
- Kloss, C. U. A.; Werner, A.; Klein, M.A.; Shen, J.; Menuz, K.; Probst, J.C.; Kreutzberg, G.W.; Raivich, G. Integrin family of cell adhesion molecules in the injured brain: regulation and cellular localization in the normal and regenerating mouse facial motor nucleus. J Comp Neurol 1999, 411, 162–178. [Google Scholar] [CrossRef]
- Yanagida, H.; Tanaka, J.; Maruo, S. Immunocytochemical localization of a cell adhesion molecule, integrin α5β1, in nerve growth cones. J. Orthop. Sci. 1999, 4, 353–360. [Google Scholar] [CrossRef]
- Hammarberg, H.; Wallquist, W.; Piehl, F.; Risling, M.; Cullheim, S. Regulation of laminin-associated integrin subunit mRNAs in rat spinal motoneurons during postnatal development and after axonal injury. J. Comp. Neurol. 2000, 428, 294–304. [Google Scholar] [CrossRef]
- Vogelezang, M.G.; Liu, Z.; Relvas, J.B.; Raivich, G.; Scherer, S.S.; Charles ffrench-Constant, C. α4 Integrin Is expressed during peripheral nerve regeneration and enhances neurite outgrowth. J. Neurosci. 2001, 21, 6732–6744. [Google Scholar] [CrossRef] [PubMed]
- Wallquist, W.; Zelano, J.; Plantman, S.; Kaufman, S.J.; Cullheim, S.; Hammarberg, H. Dorsal root ganglion neurons up-regulate the expression of laminin-associated integrins after peripheral but not central axotomy. J. Comp. Neurol. 2004, 480, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, N.J.; Fernyhough, P.; Tomlinson, D.R.; Mayer, U.; von der, M.H.; Streuli, C.H. Alpha7 integrin mediates neurite outgrowth of distinct populations of adult sensory neurons. Mol. Cell Neurosci. 2005, 28, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Strittmatter, S.M. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J. Neurosci. 2008, 28, 1262–1269. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Haenzi, B.; Andrews, M.R.; Verhaagen, J.; Fawcett, J.W. Integrins promote axonal regeneration after injury of the nervous system. Biol. Rev. Camb. Philos. Soc. 2018, 1339–1362. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, C.; Bow, C.M.; Remahl, I.N. Myelination and myelin sheath remodeling in normal and pathological PNS nerve fibres. Prog. Neurobiol. 1994, 43, 85–141. [Google Scholar] [CrossRef] [PubMed]
- Fricker, F.R.; Bennett, D.A. The role of neuregulin-1 in the response to nerve injury. Future Neurol. 2011, 6, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Fricker, F.R.; Antunes-Martins, A.; Jorge Galino, J.; Paramsothy, R.; La Russa, F.; Perkins, J.; Goldberg, R.; Brelstaff, J.; Zhu, N.; McMahan, S.B.; Orengo, C.; Garratt, A.N.; Birchmeier, C.; Bennett, D.L.H. Axonal neuregulin 1 is a rate limiting but not essential factor for nerve remyelination. Brain 2013, 136, 2279–2297. [Google Scholar] [CrossRef]
- Haftek, J.; Thomas, P. K. Electron-microscope observation on the effects of localized crush injuries on the connective tissues of the peripheral nerve. J. Anat. 1968, 103, 233–243. [Google Scholar]
- Devor, M.; Govrin-Lippman, R. Maturation of axonal sprouts after nerve crush. Exp. Neurol. 1979, 64, 260–270. [Google Scholar] [CrossRef]
- Gordon, T.; Stein, R.B. Time course and extent of recovery in reinnervated motor units of cat triceps surae muscles. J. Physiol. 1982, 323, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Shin, A.Y. Management and complications of traumatic peripheral nerve injuries. Hand Clin. 2015, 31, 151–163. [Google Scholar] [CrossRef]
- Ransom, S.C.; Shahrestani, S.; Lien, B.V.; Tafreshi, A.R.; Brown, N.J.; Hanst, B.; Lehrich, B.M.; Ransom, R.C.; Sahyouni, R. Translational approaches to electrical stimulation for peripheral nerve regeneration. Neurorehabil Neural Repair. 2020, 34, 979–985. [Google Scholar] [CrossRef]
- Rasulić, L.; Simić, V.; Savić, A.; Lepić, M.; Kovačević, V.; Puzović, V’.; Vitošević, F.; Novaković, N.; Samardžić, M.; Rotim, K. Management of brachial plexus missile injuries. Acta Clin. Croat. 2018, 57, 487–496. [Google Scholar] [CrossRef]
- Sunderland, S. Rate of regeneration of motor fibers in the ulnar and sciatic nerves. Arch. Neurol. Psych. 1947, 58, 7–13. [Google Scholar] [CrossRef]
- Sunderland, S. Rate of regeneration of sensory nerve fibers. Arch. Neurol. Psychiat 1947, 58, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, H.J.; Spencer, P.S. The fate of Schwann cells isolated from axonal contact J. Neurocytol. 1978, 7, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Röyttä, M.; Salonen, V. Long-term endoneurial changes after nerve transection. Acta Neuropathol. 1988, 76, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Salonen, V.; Peltonen, J.; Röyttä, M.; Virtanen, I. Laminin in traumatized peripheral nerve: basement membrane changes during degeneration and regeneration. J. Neurocytol. 1987, 16, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Salonen, V.; Röyttä, M.; Peltonen, J. The effects of nerve transection on the endoneurial collagen fibril sheaths. Acta Neuropathol. 1987, 74, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.L.; Abernethy, D.A.; King, R.H.M.; Muddle, J.R.; Thomas, P.K. Neural architecture I transected rabbit sciatic nerve after prolonged nonreinnervation. J. Anat. 1998, 192, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Dedkov, E.I.; Kostrominova, T. Y.; Borisov, A. B.; Carlson, B. M. Survival of Schwann cells in chronically denervated skeletal muscles. Acta Neuropath 2002, 103, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Wood, P.; Sulaiman, O.A.R. Long-term denervated rat Schwann cells retain their capacity to proliferate and to myelinate axons in vitro. Frontiers Cell Neurosci. 2019, 12, 511. [Google Scholar] [CrossRef]
- Qian, T.; Wang, P.; Chen, Q.; Yi, S.; Liu, Q.; Wang, H.; Wang, s.; Geng, W.; Liu, Z.; Li, S. The dynamic changes of main cell types in the microenvironment of sciatic nerves following sciatic nerve injury and the influence of let-7 on their distribution. RSC Adv. 2018, 8, 41181–41191. [Google Scholar] [CrossRef]
- Sulaiman, O.A.R.; Gordon, T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia 2000, 32, 234–246. [Google Scholar] [CrossRef]
- Gordon, T. The biology, limits, and promotion of peripheral nerve regeneration in rats and human. Nerves and Nerve Injuries. Elsevier, New York, 2015, pp. 903–1019.
- Benito, C.; Davis, C.M.; Gomez-Sanchez, J.A.; Turmain, M.; Meijer, D.; Poli, V.; Mirsky, R.; Jessen, K.R. STAT3 controls the long-term survival and phenotype of repair Schwann cells during nerve regeneration. J. Neurosci. 2017, 37, 4255–4269. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, D.B.; Dong, Z.; Bunting, H.; Whitfield, J.; Meier, C.; Marie, H.; Mirsky, R.; Jessen, K.R. Transforming growth factor beta (TGFbeta) mediates Schwann cell death in vitro and in vivo: examination of c-Jun activation, interactions with survival signals, and the relationship of TGFbeta-mediated death to Schwann cell differentiation. J. Neurosci. 2001, 21, 8572–8585. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, D.B.; Bhaskaran, A.; Droggiti, A.; Dickinson, S.; D-Antonio, M.; Mirsky, R.; Jessen, K.R. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J. Cell Biol. 2004, 164, 385–394. [Google Scholar] [CrossRef]
- Parkinson, D.B.; Bhaskaran, A.; Arthur-Farraj, P.; Noon, L.A. c-Jun is a negative regulator of myelination. J. Cell Biol. 2008, 181, 625–637. [Google Scholar] [CrossRef]
- Lundborg, G. Nerve Injury and Repair. 2004, Churchill Livingstone, New York.
- Adhihetty, PJ.; O’Leary, M.F.N.; Chabi, B.; Wicks, K.L.; Hood, D.A. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J. Appl. Physiol. 2007, 102, 1143–1151. [Google Scholar] [CrossRef]
- O′Leary, M.F.N.; Vainshtein, A.; Carter, H.N.; Zhang, Y.; Hood, D.A. Denervation-induced mitochondrial dysfunction and autophagy in skeletal muscle of apoptosis-deficient animals. Am. J. Physiol. Physiol. 2012, 303, 447–454. [Google Scholar] [CrossRef]
- Yang, X.; Xue, P.; Chen, H.; Yuan, M.; Kang, Y.; Duscher, D.; Machens, H.-G.; Chen, Z. Denervation drives skeletal muscle atrophy and induces mitochondrial dysfunction, mitophagy and apoptosis via miR-142a-5p/MFN1 axis. Theranostics 2020, 10, 1415–1432. [Google Scholar] [CrossRef] [PubMed]
- Warwick, D.; Srinivasan, H.; Solomon, L. Peripheral Nerve Disorder. In: Arnold, H., editor. Apley’s system of Orthopaedics and Fracture. 9th ed. 2010. pp. 269–United Kingdom.
- Holmes, W.; Young J., Z. Nerve regeneration after immediate and delayed suture. J. Anat. 1942, 77, 63–I06. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J. Neurosci. 1995, 15, 3876–3885. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.G.; Gordon, T. The neurotrophin receptors, trkB and p75, differentially regulate motor axonal regeneration. J. Neurobiol. 2001, 49, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.G.; Gordon, T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain derived neurotrophic factor. Eur. J. Neurosci. 2002, 15, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.G.; Gordon, T. Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp. Neurol. 2003, 183, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, O.A.R.; Gordon, T. Transforming growth factor-β and forskolin attenuate the adverse effects of long-term Schwann cell denervation on peripheral nerve regeneration in vivo. Glia 2002, 37, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, O.A.R.; Midha, R.; Munro, C.A.; Matusyama, T.; Al-Majed, A.A.; Gordon, T. Chronic Schwann cell denervation and the presence of a sensory nerve reduce motor axonal denervation. Exp. Neurol. 2002, 176, 342–354. [Google Scholar] [CrossRef]
- Gordon, T.; Tyreman, N.; Raji, M.A. The basis for diminished functional recovery after delayed peripheral nerve repair. J. Neurosci. 2011, 31, 5325–5334. [Google Scholar] [CrossRef]
- Gordon, T.; Brushart, T.M.; Chan, M. Augmenting nerve regeneration with electrical stimulation. Neurol. Res. 2008, 30, 1012–1022. [Google Scholar] [CrossRef]
- Gordon, T. The role of neurotrophic factors in nerve regeneration. Invited review. Neurosurg. Focus. 2009, 26, E3. [Google Scholar] [CrossRef]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Mackinnon, S.E.; Cullen, D.K. Biomedical engineering strategies for peripheral nerve repair: Surgical applications, state of the art, and future challenges. Crit. Rev. Biomed. Eng. 2011, 39, 5–48. [Google Scholar] [CrossRef]
- Gordon, T. The Biology, Limits, and Promotion of Peripheral Nerve Regeneration in Rats and Humans. In Nerves and Nerve Injuries. Eds: Tubbs RS, Rizk E, Shoja M, Loukas M, and Spinner RJ. Elsevier, Vol. 2 Ch 61, 2015, pp. 993–1019.
- Rafuse, V.R.; Gordon, T. Self-reinnervated cat medial gastrocnemius muscles. I. Comparisons of the capacity for regenerating nerves to form enlarged motor units after extensive peripheral nerve Injuries. J. Neurophysiol. 1996, 75, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Rafuse, V.R.; Gordon, T. Self-reinnervated cat medial gastrocnemius muscles. II. Analysis of the mechanisms and significance of fiber type grouping in reinnervated muscles. J. Neurophysiol. 1996, 75, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Rafuse, V.F.; Gordon, T.; Orozco, R. Proportional enlargement of motor units after partial denervation of cat triceps surae muscles. J. Neurophysiol. 1992, 68, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Borschel, G.H. The use of the rat as a model for studying peripheral nerve regeneration and sprouting after complete and partial nerve injuries. Invited review for a special issue on in vivo models of neural regeneration. Exp. Neurol. 2017, 287, 331–347. [Google Scholar] [CrossRef]
- Dyck, P.J.; Boes, C.J.; Mulder, D.; Millikan, C.; Windebank, A.J.; Dyck, J.B.; Espinosa, R. History of standard scoring, notation, and summation of neuromuscular signs. A current survey and recommendation. J. Peripher. Nerv. Syst. 2005, 10, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, E.K.; Faber, C.G.; van Nes, S.I.; Jacobs, B.C.; van Doorn, P.A.; van Doorn, P.A; van Koningsveld, R.; Cornblath, D.R.; van der Kooi, A.J.; Cats, E.A.; van den Berg, L.H.; Notermans, N.C.; van der Pol, W.L.; Hermans, M.C.E.; van der Beek, N.A.M.E.; Gorson, K.C.; Eurelings, M.; Jeroen Engelsman, J.; Hendrik Boot, H.; Meijer, R.J.; Lauria, G.; Tennant, A.; Merkies, I.A.J.; PeriNomS Study Group. Modifying the Medical Research Council grading system through Rasch analyses. Brain 2012, 135, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Rafuse, V.R.; Gordon, T. Size of myelinated nerve fibres is not increased by expansion of the peripheral field in cats. J. Physiol. 1998, 532, 835–849. [Google Scholar]
- Dedkov, E.L.; Kostrominova, T.Y.; Borisov, A.B.; Carlson, B.M. Reparative myogenesis in long-term denervated skeletal muscles of adult rats results in a reduction of the satellite cell population. Anat. Rec. 2001, 263, 139–154. [Google Scholar] [CrossRef]
- Schmalbruch, H.; Lewis, D.M. A comparison of the morphology of denervated with aneurally regenerated soleus muscle of rat. Muscle Res. Cell Motil. 1994, 15, 256–266. [Google Scholar] [CrossRef]
- Jejurikar, S.S.; Marcelo, C.L.; Kuzon, W.M., Jr. Skeletal muscle denervation increases satellite cell susceptibility to apoptosis. Plast. Reconstr. Surg. 2002, 110, 160–168. [Google Scholar] [CrossRef]
- Charge, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Le Grand, F.; Rudnicki, M.A. Skeletal muscle satellite cells and adult myogenesis. Curr. Opin. Cell Biol. 2007, 19, 628–633. [Google Scholar] [CrossRef]
- Singh, K.; Dilworth, F.J. Differential modulation of cell cycle progression distinguishes members of the myogenic regulatory factor family of transcription factors. FEBS 2013, 280, 3991–4003. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Suroto, H.; Wardana, G.R.; Sugianto, J.A.; Aprilya, D.; Samijo, S. Time to surgery and myo-D expression in biceps muscle of adult brachial plexus injury: a preliminary study. BMC Res. Notes 2023, 16, 51. [Google Scholar] [CrossRef]
- Anzil, A.P.; Wernig, A. Muscle fibre loss and reinnervation after long-term denervation. J. Neurocytol. 1989, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Schmalbruch, H.; Lewis, D.M. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and degenerated rat muscles. Muscle Nerve 2000, 23, 617–626. [Google Scholar] [CrossRef]
- Gutmann, E.; Zelena, J. Morphological changes in the denervated muscle. In The Denervated Muscle (edited by Gutmann, E.), 1962, pp. 57–Prague: Publishing House of the Czechoslovak Academy of Sciences.
- Gordon, T.; Tetzlaff, W. Regeneration associated genes decline in injured rat axotomized sciatic motoneurons. Eur. J. Neurosci. 2015, 42, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; You, S.; Cassar, S.L.; Tetzlaff, W. Reduced expression of regeneration associated genes in chronically axotomized facial motoneurons. Exp. Neurol. 2015, 264, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hoke, A.; Gordon, T.; Zochodne, D.W.; Sulaiman, O.A.R. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp. Neurol. 2002, 173, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, L.J.; Gomez-Sanchez, J.A.; Fazal, S.V.; Otto, G.W.; et al. Failures of nerve regeneration caused by aging or chronic denervation are rescued by restoring Schwann cell c-Jun. eLife 2021, 10e. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ishii, K.; Nakamura, Y.; Watabe, K.; Kohsaka, S.; Akazawa, C. Neuroprotective effect of sonic hedgehog up-regulated in Schwann cells following sciatic nerve injury. J. Neurochem. 2008, 24, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.R.; Yuk, D.; Alberta, J.A.; Zhu, Z.; Pawlitzky, I.; Chan, J.; McMahon, C.D.; Stiles, C.D.; Rowitch, D.H. Sonic hedgehog–regulated oligodendrocyte lineage genes encoding BHLH proteins in the mammalian central nervous system. Neuron 2000, 25, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Trakanant, S.; Nihara, J.; Kudo, T.; Seo, K.l.; Saeki, M.; Kurose, M.; Matsumaru, D.; Maeda, T.; Ohazama, A. Gli3 is a key factor in the Schwann cells from both intact and injured peripheral nerves. Neurosci. 2020, 432, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Pepinsky, R.B.; Shapiro, R.I.; Wang, S.; Chakraborty, A.; Gill, A.; Lepage, D.J.; Wen, D.; Rayhorn, R.; Horan, G.S.B.; Taylor, F.R.; Garber, E.A.; Galdes, A.; Engber, T.M. Long-acting forms of sonic hedgehog with improved pharmacokinetic and pharmacodynamic properties are efficacious in a nerve injury model. J Pharm Sci 2002, 91, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.W.; Angeloni, N.; Harrington, D.; Stupp, S.; Podlasek, C.A. Sonic hedgehog regulates brain-derived neurotrophic factor in normal and regenerating cavernous nerves. J. Sex. Med. 2013, 10, 730–737. [Google Scholar] [CrossRef]
- Martinez, J.A.; Kobayashi, M.; Krishnan, A.; Webber, C.; Christie, K.; Guo, GF.; Singh, V.; Zouchodne, D.W. Intrinsic facilitation of adult peripheral nerve regeneration by the sonic hedgehog morphogen. Exp. Neurol. 2015, 271, 493–505. [Google Scholar] [CrossRef]
- Ma, K.H.; Hung, H.A.; Srinivasan, R.; Xie, H.; Orkin, S.H.; Svaren, J. Regulation of peripheral nerve myelin maintenance by gene repression through polycomb repressive complex J. Neurosci. 2015, 35, 8640–8652. [Google Scholar] [CrossRef]
- Boerboom, A.; Dion, V.; Chariot, A.; Franzen, R. Molecular mechanisms involved in Schwann cell plasticity. Frontiers Mol. Neurosci. 2017, 10, 38. [Google Scholar] [CrossRef]
- Meyer, R.C.; Giddens, M.M.; Schaefer, S.A.; Hall, R.A. GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin. PNAS 2013, 110, 9529–9534. [Google Scholar] [CrossRef]
- Hiraiwa, M.; Campana, W.M.; Mizisin, A.P.; Mohiuddin, L.; O’Brien, J.S. Prosaposin: A myelinotrophic protein that promotes expression of myelin constituents and is secreted after nerve injury. Glia 1999, 26, 353–360. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, R.; Wang, Z.; Lin, G.; Lue, T.F.; Lin, C. Adipose tissue-derived stem cells secrete CXCL5 cytokine with neurotrophic effects on cavernous nerve regeneration. J. Sex. Med. 2011, 8, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Eskin, A.; Chareyre, F.; Jessen, W.J.; Manent, J.; Niwa-Kawakita, M.; Chen, R.; White, C.M.; Jaffer, Z.M.; Nelson, S.F.; Rubenstein, A.E.; Giovannini, M. Therapeutic potential of HSP90 inhibition for neurofibromatosis type Clin. Cancer Res. 2013, 19, 3856–3870. [Google Scholar]
- Weiss, T.; Taschner-Mandl, S.; Bileck, A.; Slany, A.; Kromp, F.; Rifatbegovic, D.; Frech, C.; Windhager, R.; Kitzinger, H.; Tzou, C.-H.; Ambros, P.F.; Gerner, C.; Ambros, I.M. Proteomics and transcriptomics of peripheral nerve tissue and cells unravel new aspects of the human Schwann cell repair phenotype. Glia 2016, 64, 2133–2153. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.H.; Hung, H.A.; Svaren, J. Epigenomic regulation of Schwann cell reprogramming in peripheral nerve injury. J. Neurosci. 2016, 36, 9135–9147. [Google Scholar] [CrossRef] [PubMed]
- Brosius Lutz, A.; Chung, W.S.; Sloan, S.A.; Carson, G.A.; et al. Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury. PNAS 2017, 114, E8072–E8080. [Google Scholar] [CrossRef]
- Wolter, P.; Schmitt, K.; Fackler, M.; Kremling, H.; Probst, L.; Hauser, S.; Gruss, O.J.; Gaubatz, S. GAS2L3, a target gene of the DREAM complex, is required for proper cytokinesis and genomic stability. J. Cell Sci. 2012, 125, 2393–2406. [Google Scholar] [CrossRef]
- Chung, W.S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; Smith, S.J.; Barres, B.A. A. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 2013, 504, 394–400. [Google Scholar] [CrossRef]
- Levi-Montalcini, R.; Calissano, P. The nerve-growth factor. Sci. Am. 1979, 240, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Medawar, P. B.; Young, J. Z. Fibrin suture of peripheral nerves. Lancet 1940, 239, 126–128. [Google Scholar]
- Carlsen, R.C. Delayed induction of the cell body response and enhancement of regeneration following a condition/test lesion of frog peripheral nerve at 15 °C. Brain Res. 1983, 279, 9–18. [Google Scholar] [CrossRef]
- Kilmer, S.L.; Carlsen, R.C. Forskolin activation of adenylate cyclase in vivo stimulates nerve regeneration. Nature 1984, 307, 455–457. [Google Scholar] [CrossRef]
- Sjoberg, J.; Kanje, M.; Edstrom, A. Influence of non-neuronal cells on regeneration of the rat sciatic nerve. Brain Res. 1988, 453, 221–226. [Google Scholar] [CrossRef]
- Kanje, M.; Skottner, A.; Sjöberg, J.; Lundborg, G. Insulin-like growth factor I (IGF-I) stimulates regeneration of the rat sciatic nerve. Brain Res. 1989, 486, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, J.; Kanje, M. The initial period of peripheral nerve regeneration and the importance of the local environment for the conditioning lesion effect. Brain Res. 1990, 529, 79–84. [Google Scholar] [CrossRef]
- Holmquist, B.; Kanje, M.; Kerns, J.M.; Danielsen, N. A mathematical model for regeneration rate and initial delay following surgical repair of peripheral nerves. J. Neurosci. Meth 1993, 48, 27–33. [Google Scholar] [CrossRef]
- Wood, M.D.; Kemp, S.W.P; Weber, C.; Borschel, G.H.; Gordon, T. Outcome measures of peripheral nerve regeneration. Ann. Anat. 2011, 193, 321–333. [Google Scholar] [PubMed]
- Fouad, K.; Pearson, K. Restoring walking after spinal cord injury. Prog. Neurobiol. 2004, 73, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Vrbova, G.; Gordon, T.; Jones, R. Nerve-Muscle Interaction 1978, Chapman and Hall, Ltd, Ch 6, p 113.
- Tajdaran, K.; Gordon, T.; Wood, M.D.; Shoichet, M.S.; Borschel, G.H. A glial cell line-derived neurotrophic factor delivery system enhances nerve regeneration across acellular nerve allografts. Acta Biomat 2016, 29, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Hoffer, J.A.; Jhamandas, J.; Stein, R.B. Long-term effects of axotomy on neural activity during cat locomotion. J. Physiol. 1980, 303, 243–263. [Google Scholar] [CrossRef]
- Mendell, L.M.; Munson, J.B.; Scott, J.G. Alterations of synapses on axotomized motoneurons. J. Physiol. 1976, 255, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Titmus, M.; Faber, D. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog. Neurobiol. 1990, 35, 1–51. [Google Scholar] [CrossRef]
- Moran, L.B.; Graeber, M.B. The facial nerve axotomy model. Brain Res. Rev. 2004, 44, 154. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.J.; Bullinger, K.L.; Titus, H.E.; Nardelli, P.; Cope, T.C. Permanent reorganization of Ia afferent synapses on motoneurons after peripheral nerve injuries. Ann. NY Acad. Sci. 2010, 1198, 231–241. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Titus-Mitchel, H.E.; Bullinger, K.L.; Kraszpulski, M.; Nardelli, P.; Cope, T.C. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J. Neurophysiol. 2011, 106, 2450–2470. [Google Scholar] [CrossRef]
- Sumner, B.E. A quantitative analysis of boutons with different types of synapse in normal and injured hypoglossal nuclei. Exp. Neurol. 1975, 49, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Chen, D. H. Qualitative and quantitative study of synaptic displacement in chromatolyzed spinal motoneurons of the cat. J. Comp. Neurol. 1978, 177, 635–664. [Google Scholar] [CrossRef]
- Blinzinger, K.; Kreutzberg, G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z. Zellforsch. Mikrosk. Anat. 1968, 85, 145–157. [Google Scholar] [CrossRef]
- Brannstrom, T.; Kellerth, J. O. Changes in synaptology of adult cat spinal alpha-motoneurons after axotomy. Exp. Brain Res. 1998, 118, 1–13. [Google Scholar]
- Lindå, H.; Shupliakov, O.; Ornung, G.; Ottersen, O. P.; Storm-Mathisen, J.; Risling, M.; Cullheim, S. Ultrastructural evidence for a preferential elimination of glutamate-immunoreactive synaptic terminals from spinal motoneurons after intramedullary axotomy. J. Comp. Neurol. 2000, 425, 10–23. [Google Scholar] [CrossRef]
- Oliveira, A. L. R.; Thams, S.; Lidman, O.; Piehl, F.; Hökfelt, T.; Kärre, K.; Lindå, H.; Cullheim, S.A. role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc. Natl. Acad. Sci. USA 2004, 101, 17843–17848. [Google Scholar] [CrossRef]
- Rotterman, T.M.; Nardelli, P.; Cope, T.C.; Alvarez, F.J. Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury. J. Neurosci. 2014, 34, 3475–3492. [Google Scholar] [CrossRef]
- Liu, C.; Ward, P.J.; English, A.W. The effects of exercise on synaptic stripping require androgen receptor signaling. PLoS One 2014, 9, e98633. [Google Scholar] [CrossRef]
- Brandt, J.; Evans, J.T.; Mildenhall, T.; Mulligan, A.; Konieczny, A.; Rose, S.J; English, A.W. Delaying the onset of treadmill exercise following peripheral nerve injury has different effects on axon regeneration and motoneuron synaptic plasticity. J. Neurophysiol. 2015, 113, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Aldskogius, H.; Liu, L.; Svensson, M. Glial responses to synaptic damage and plasticity. J. Neurosci. Res. 1999, 58, 33–41. [Google Scholar] [CrossRef]
- Krakowiak, J.; Liu, C.; Papudesu, C.; Ward, P.J.; Wilhelm, J.C.; English, A.W. Neuronal BDNF signalling is necessary for the effects of treadmill exercise on synaptic stripping of axotomized motoneurons. Neural Plast. 2015, 11, 392591. [Google Scholar]
- Brushart, T.M.; Seiler, W.A. Selective reinnervation of distal motor stumps by peripheral motor axons. Exp. Neurol. 1987, 97, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M. Motor axons preferentially reinnervate motor pathways. J. Neurosci. 1993, 13, 2730–2738. [Google Scholar] [CrossRef]
- Gutmann, E.; Gutmann, L.; Medawar, P.K.; Young, J. Z. The rate of regeneration. J. Exp. Biol. 1942, 19, 14–44. [Google Scholar] [CrossRef]
- Witzel, C.; Rohde, C.; Brushart, T.M. Pathway sampling by regenerating peripheral axons. J. Comp. Neurol. 2005, 485, 183–190. [Google Scholar] [CrossRef]
- Burke, R.E. Motor units: anatomy, physiology and functional organization. In Handbook of physiology. Sect. I. Vol. II. The Nervous System: Motor Control. Part I. Edited by V.B. Brooks. American Physiological Society, Washington, DC. 1981, pp. 345–422.
- Zhang, J.Y.; Luo, X.G.; Xian, C.J.; Liu, Z.H.; Zhou, X.F. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents, Eur. J. Neurosci. 2000, 12, 4171–4180. [Google Scholar]
- Griesbeck, O.; Parsadanian, A.S.; Sendtner, M.; Thoenen, H. Expression of neurotrophins in skeletal muscle: Quantitative comparison and significance for motoneuron survival and maintenance of function. J. Neurosci. Res. 1995, 42, 21–33. [Google Scholar] [CrossRef]
- Juckett, L.; Saffari, T.M.; Ormseth, B.; Senger, J.-L.; Moore, A.M. The effect of electrical stimulation on nerve regeneration following peripheral nerve injury. Biomolec 2022, 12, 1856. [Google Scholar] [CrossRef]
- Gordon, T.; Amirjani, N.; Edwards, D.C.; Chan, K. M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 2010, 223, 192–202. [Google Scholar] [CrossRef]
- Wong, J.N.; Olson, J.L.; Morhart, M.J.; Chan, K.M. Electrical stimulation enhances sensory recovery: a randomized controlled trial. Ann. Neurol. 2015, 77, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Power, H.A.; Morhart, M.J.; Olson, J.L.; Chan, K.M. Postsurgical electrical stimulation enhances recovery following surgery for severe Cubital Tunnel Syndrome: A Double-blind randomized controlled trial. Neurosurg. 2020, 86, 769–777. [Google Scholar] [CrossRef] [PubMed]
- McLean, N.A.; Verge, V. Dynamic impact of brief electrical stimulation on the neural immune axis-polarization of macrophages toward a pro-repair phenotype in demyelinated peripheral nerve. Glia 2016, 64, 1546–1561. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, T.; Li, C.; Xu, W.; Guan, Y.; Li, X.; Cheng, H.; Chen, S.; Yang, B.; Liu, Y.; Ren, Z.; Song, X.; Jia, Z.; Wang, Y.; Tang, J. Electrical stimulation accelerates Wallerian degeneration and promotes nerve regeneration after sciatic nerve injury. GLIA 2023, 71, 758–774. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; McEwan, S.-K.; Kang, S.M.; Won, S.M.; Stephen, M.; Gamble, P.; Xie, Z.; Yan, Y.; Chen, Y.-Y.; Shin, J.; Birenbaum, N.; Chung, S.; Kim, S.B.; Khalifeh, J.; Harburg, D.V.; Bean, K.; Paskett, M.; Kim, J.; Zohny, Z.S.; Lee, S.M.; Zhang, R.; Luo, K.; Ji, B.; Banks, A.; Lee, H.M.; Huang, Y.; Ray, W.Z.; Rogers, J.A. Wireless bioresorbable electronic system enables sustained non-pharmacological neuroregenerative therapy. Nature Med. 2018, 24, 1830–1836. [Google Scholar] [CrossRef]
- Ju, C.; Park, E.; Kim, T.; Kim, T.; Kang, M.; Lee, K.; Park, S.-M. Effectiveness of electrical stimulation on nerve regeneration after crush injury: comparison between invasive and non-invasive stimulation. Plos One 2020, 15, e0233531. [Google Scholar] [CrossRef]
- Yan, X.; Ye, Z.; Huang, J.; He, F.; Xiao, W.; Hu, X.; Luo, Z. CaMKI-mediated CREB phosphorylation is involved in Ca2+-induced BDNF mRNA transcription and neurite outgrowth mediated by electrical stimulation. Plos One 2016, 11, e0162784. [Google Scholar] [CrossRef] [PubMed]
- Sayanagi, J.; Jesus, A.; Acevedo-Cintrog, B.S; Pan, D.; Schellhardt, L.; Snyder-Warwick, A.K.; Mackinnon, S.E.; Wood, M.D. Brief electrical stimulation accelerates axon regeneration and promotes recovery following nerve transection and repair in mice. J. Bone Joint Surg. Am. 2021, 103, e80. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Schellhardt, L.; Keane, G.C.; Hunter, D.A.; Moore, A.M.; Snyder-Warwick, A.K.; Mackinnon, S.E.; Wood, M.D. Short-duration, pulsatile, electrical stimulation therapy accelerates axon regeneration and recovery following tibial nerve injury and repair in rats. Plast. Reconstr. Surg. 2022, 149, 681e–690e. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.P.C.; Lanzinger, W.; Willits, R.K. Effect of intraoperative electrical stimulation on recovery after rat sciatic nerve isograft repair. Neurotrauma Rep 2020, 1, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Ecanow, A.; Berglund, K.; Carrasco, D.; Isaacson, R.; English, A.W. Enhancing motor and sensory axon regeneration after peripheral nerve injury using bioluminescent optogenetics. Int. J. Mol. Sci. 2022, 23, 16084. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.J.; Sengelaub, D.R.; English, A.W. Enhancement of peripheral nerve regeneration due to treadmill training and electrical stimulation is dependent on androgen receptor signaling. Dev. Neurobiol. 2014, 74, 531–540. [Google Scholar] [CrossRef]
- Hosli, E.; Jurasin, K.; Ruhl, W.; Luthy, R.; Hosli, L. Colocalization of androgen, estrogen and cholinergic receptors on cultured astrocytes of rat central nervous system. Int. J. Dev. Neurosci. 2001, 19, 11–19. [Google Scholar] [CrossRef]
- Garcia-Ovejero, D.; Veiga, S.; Garcia-Segura, L.; Doncarlo, S.L. Glial expression of estrogen and androgen receptors after rat brain injury. J. Comp. Neurol. 2002, 450, 256–271. [Google Scholar] [CrossRef]
- English, A.W.; Wilhelm, J.C.; Ward, P.J. Exercise, neurotrophins, and axon regeneration in the PNS. Physiol. 2014, 29, 437–445. [Google Scholar] [CrossRef]
- 415 Gordon, T. Neurotrophic factor expression in denervated motor and sensory Schwann cells: Relevance to specificity of peripheral nerve regeneration. Exp. Neurol. 2014, 25, 499–108. [Google Scholar] [CrossRef]
- Franz, C.K.; Rutishauser, U.; Rafuse, V.F. Polysialylated neural cell adhesion molecule is necessary for selective targeting of regenerating motor neurons. J. Neurosci. 2005, 25, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Ying, Z.; Roy, R.R.; Molteni, R.; Edgerton, V.R. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 2002, 88, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J. Neurosci. Res. 2004, 76, 356–362. [Google Scholar] [CrossRef]
- Vaynman, S.; Gomez-Pinilla, F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehab Neural Res. 2005, 19, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, M.J.; Redmon, N.; Schwartz, G.; English, A.W. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp. Neurol. 2008, 211, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Pinilla, E.; Udina, E.; Jaramillo, J.; Navarro, X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 2009, 219, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, J.C.; Xu, M.; Cucoranu, D.; Chmielewski, S.; Holmes, T.; Lau, S.L.; Bassell, G.J.; English, A.W. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J. Neurosci. 2012, 32, 5002–5009. [Google Scholar] [CrossRef]
- Wood, K.; Wilhelm, J.C.; Sabatier, M.J.; Liu, K.; Gu, J.; English, A.W. Sex differences in the effectiveness of treadmill training in enhancing axon regeneration in injured peripheral nerves. Dev. Neurobiol. 2012, 72, 688–698. [Google Scholar] [CrossRef]
- English, A.W.; Mulligan, A.; Cucoranu, D.; Rodriguez, J.A.; Sabatier, M.J. Neurotrophin 4/5 is implicated in the enhancement of axon regeneration produced by treadmill training following peripheral nerve injury. Erup J. Neurosci. 2011 33, 2265–2271. [CrossRef]
- Gutmann, E.; Jakoubek, B. Effect of increased motor activity on regeneration of the peripheral nerve in young rats. Physiol. Bohemoslov. 1963, 12, 463–468. [Google Scholar]
- Herbison, G.J.; Jaweed, M.M.; Ditunno, J.F.; Scott, C.M. Effect of overwork during reinnervation of rat muscle. Exp. Neurol. 1973, 41, 1–14. [Google Scholar] [CrossRef]
- Van Meeteren, N.L.; Brakkee, J.H.; Hamers, F.P.; Helders, P.J.; Gispen, W.H. Exercise training improves functional recovery and motor nerve conduction velocity after sciatic nerve crush lesion in the rat. Arch. Phys. Med. Rehab 1997, 78, 70–77. [Google Scholar] [CrossRef]
- Van Meeteren, N.L.; Brakkee, J.H.; Helders, P.J.; Gispen, W.H. The effect of exercise training on functional recovery after sciatic nerve crush in the rat. J. Peripher. Nerv. Syst. 1998, 3, 277–282. [Google Scholar]
- Hutchinson, K.J.; Gomez-Pinilla, F.; Crowe, M.J.; Ying, Z.; Basso, D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 2004, 127, 1403–1414. [Google Scholar] [CrossRef]
- Molteni, R.; Zheng, J.Q.; Ying, Z.; Gomez-Pinilla, F.; Twiss, J.L. Voluntary exercise increases axonal regeneration from sensory neurons. Proc. Natl. Acad. Sci. USA 2004, 101, 8473–8478. [Google Scholar] [CrossRef]
- Teodori, R.M.; Betini, J.; de Oliveira, L.S.; Sobral, L.L.; Takeda, S.Y.; de Lima Montebelo, M.I. Swimming exercise in the acute or late phase after sciatic nerve crush accelerates nerve regeneration. Neural Plast. 2011, 783901. [Google Scholar] [CrossRef]
- Udina, E.; Puigdemasa, A.; Navarro, X. Passive and active exercise improve regeneration and muscle reinnervation after peripheral nerve injury in the rat. Muscle Nerve 2011, 43, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Ottem, E.N.; Poort, J.E.; Wang, H.; Jordan, C.L; Breedlove, S.M. Differential expression and regulation of brain-derived neurotrophic factor (BDNF) mRNA isoforms in androgen-sensitive motoneurons of the rat lumbar spinal cord. Mol. Cell Endocrinol. 2010, 328, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M. Preferential reinnervation of motor nerves by regenerating motor axons. J. Neurosci. 1988, 8, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M.; Mesulam, M.M. Alteration in connections between muscle and anterior horn motoneurons after peripheral nerve repair. Science 1980, 208, 603–605. [Google Scholar] [CrossRef] [PubMed]
- English, A.W. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. Exp. Neurol. 2005, 490, 421–441. [Google Scholar] [CrossRef]
- English, A.W.; Cucoranu, D.; Mulligan, M.; Sabatier, M. Treadmill training enhances axon regeneration in injured mouse peripheral nerves without increased loss of topographic specificity. J. Comp. Neurol. 2009, 517, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.K.; Hinkle, M.L.; Nicolini, J.; Rambo, L.N.; Rexwinkle, A.M.; Rose, S.J.; Sabatier, M.J.; Backus, D.; English, A.W. Misdirection of regenerating axons and functional recovery following sciatic nerve injury in rats. J. Comp. Neurol. 2011, 519, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, M.J.; To, B.N.; Nicolini, J.; English, A.W. Effect of axon misdirection on recovery of electromyographic activity and kinematics after peripheral nerve injury. Cells Tiss. Organs 2011, 193, 298–309. [Google Scholar] [CrossRef]
- Sabatier, M.J.; To, B.N.; Nicolini, J.; English, A.W. Effect of slope and sciatic nerve injury on ankle muscle recruitment and hindlimb kinematics during walking in the rat. J. Exp. Biol. 2011, 214, 1007–1016. [Google Scholar] [CrossRef]
- de Ruiter, G.C.W.; Malessy, M.J.A.; Alaid, A.O.; Spinner, R.J.; Englestad, J.K.; Sorenson, E.J.; Kaufman, K.R.; Dyck, P.J.; Windebank, A.J. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp. Neurol. 2008, 211, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Senger, J.L.B.; Verge, W.M.K.; Olson, J.L.; Chan, K.M.; Webber, C.A. Electrical stimulation as a conditioning strategy for promoting and accelerating peripheral nerve regeneration. Exp. Neurol. 2017, 302, 75–84. [Google Scholar] [CrossRef]
- Senger, J.L.B.; Verge, W.M.K.; Chan, K.M.; Webber, C.A. The nerve conditioning lesion – a strategy to enhance nerve regeneration. Annals Neurol. 2018, 83, 691–702. [Google Scholar] [CrossRef]
- Senger, J.-L.; Chan, K.M.; Macandili, H.; Chan, A.W.M.; Verge, V.M.K.; Jones, K.E.; Webber, C.A. Conditioning electrical stimulation promotes functional nerve regeneration. Exp. Neurol. 2019, 315, 60–71. [Google Scholar] [CrossRef]
- Senger, J.B.; Chan, A.W.M.; Chan, K.M.; Kwon-Wong, T.; Acton, L.; Olson, J.; Webber, C.A. Conditioning electrical stimulation is superior to postoperative electrical stimulation in enhanced nerve regeneration and functional recovery following nerve graft repair. Neurorehabil Neural Repair. 2020, 34, 299–308. [Google Scholar] [CrossRef]
- Senger, J.B.; Chan, K.M.; Webber, C.A. Conditioning electrical stimulation is superior to postoperative electrical stimulation, resulting in enhanced nerve regeneration and functional recovery. Exp. Neurol. 2020, 325, 113147. [Google Scholar] [CrossRef]
- Senger, J.B.; Rabey, K.N.; Morhart, M.J.; Chan, K.M.; Webber, C.A. Conditioning electrical stimulation accelerates regeneration in nerve transfers. Ann. Neurol. 2020, 88, 363–374. [Google Scholar] [CrossRef]
- Senger, J.-L.B.; Rabey, K.N.; Acton, L.; Lin, Y.-H.; Lingrell, S.; Chan, K.M.; Webber, C.A. Recovering the regenerative potential in chronically injured nerves by using conditioning electrical stimulation. J. Neurosurg. 2021, 136, 1442–1454. [Google Scholar] [CrossRef]
- Richardson, P.M.; Verge, V.M. Axonal regeneration in dorsal spinal roots is accelerated by peripheral axonal transection. Brain Res. 1987, 411, 406–408. [Google Scholar] [CrossRef]
- Arntz, C.; Kanje, M.; Lundborg, G. Regeneration of the rat sciatic nerve after different conditioning lesions: effects of the conditioning interval. Microsurg. 1989, 10, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.M.; McQuarrie, I.G. Acceleration of axonal outgrowth in rat sciatic nerve at one week after axotomy. J. Neurobiol. 1993, 24, 356–367. [Google Scholar] [CrossRef] [PubMed]
- McQuarrie, I.G.; Grafstein, B. Axon outgrowth enhanced by a previous nerve injury. Arch. Neurol. 1973, 29, 53–55. [Google Scholar] [CrossRef] [PubMed]
- McQuarrie, I.G. Structural protein transport in elongation of motor axons after sciatic nerve crush: Effect of a conditioning lesion. Neurochem. Pathol. 1986, 5, 5,153–164. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.E.; McQuarrie, I.G. Increased slow transport in axons of regenerating Newt limbs after a nerve conditioning lesion. Dev. Biol. 1990, 140, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Torigoe, K.; Hashimoto, K.; Lundborg, G. A role of migratory Schwann cells in a conditioning effect of peripheral nerve regeneration Exp. Neurol. 1999, 160, 99–108. [Google Scholar]
- Hoffman, P.N. A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp. Neurol. 2010, 223, 11–18. [Google Scholar] [CrossRef]
- Neumann, S.; Bradke, F.; Tessier-Lavigne, M.; Basbaum, A.I. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 2002, 34, 885–893. [Google Scholar] [CrossRef]
- Udina, E.; Furey, M.; Busch, S.; Silver, J.; Gordon, T.; Fouad, K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp. Neurol. 2008, 210, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, C.; Beal, D.; Leechavengvongs, S.; Salon, A.; Dauge, M.C.; Sarcy, J.J. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. J. Hand Surg. Am. 1994, 19, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Teboul, F.; Kakkar, R.; Ameur, N.; Beaulieu, J.-Y.; Oberlin, C. Transfer of fascicles from the ulnar nerve to the nerve to the biceps in the treatment of upper brachial plexus palsy. J. Bone J. Surg. Am. 2004, 86, 1485–1590. [Google Scholar] [CrossRef] [PubMed]
- Midha, R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg. Focus. 2004, 16, 1–10. [Google Scholar] [CrossRef]
- Midha, R. Techniques and options in nerve reconstruction and repair. in Youmans Neurological Surgery, 6th Ed, 2011, Ch 239, 2456-3464.
- Mackinnon, S.E. Donor distal, recipient proximal and other personal perspectives on nerve transfers. Hand Clin. 2016, 32, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Wagner, I.J.; Fox, I.K. Principles of nerve repair in complex wounds of the upper extremity. Semin. Plastic Surg. 2015, 29, 40–47. [Google Scholar] [CrossRef]
- Peters, B.R.; Van Handel, A.C.; Russo, S.A.; Moore, A.M. five reliable nerve transfers for the treatment of isolated upper extremity nerve injuries. Plast. Reconstr. Surg. 2021, 147, 830e. [Google Scholar] [CrossRef]
- Viterbo, F.; Trindade, J.C.; Hoshino, K.; Mazzoni, A. Two end-to-side neurorrhaphies and nerve graft with removal of the epineural sheath: experimental study in rats. Br. J. Plast. Surg. 1994, 47, 75–80. [Google Scholar] [CrossRef]
- Viterbo, F.; Amr, A.H.; Stipp, E.J.; Reis, F.J. End-to-side neurorrhaphy: past, present, and future. Plast. Reconstr. Surg. 2009, 124, e351–e358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Fischer, K.A. End-to-side neurorrhaphy. Microsurgery 2002, 22, 122–127, 109. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, Y.; Abe, H. Hypoglossal-facial nerve side-to-end anastomosis for preservation of hypoglossal function: results of delayed treatment with a new technique. J. Neurosurg. 1997, 86, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Soucacos, P.N.; Bo, J.; Beris, A.E. Evaluation of collateral sprouting after end-to-side nerve coaptation using a fluorescent double-labeling technique. Microsurg. 1999, 19, 281–286. [Google Scholar] [CrossRef]
- Placheta, E.; Wood, M.D.; Lafontaine, C.; Liu, E.H.; Hendry, J.M.; Angelov, D.N.; Frey, M.; Gordon, T.; Borschel, G.H. Enhancement of facial nerve motoneuron regeneration through cross-face nerve grafts by adding end-to-side sensory axons. Plast. Reconstr. Surg. 2015, 135, 460–71. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, O.; Gordon, T. A rat study of the use of an end-to-side repair as a ‘baby-sitting’ technique to reduce the deleterious effect of chronic denervation. J. Neurosurg. 2018, 131, 6221–632. [Google Scholar]
- Placheta, E.; Wood, M.D.; Lafontaine, C.; Frey, M.; Gordon, T.; Borschel, G.H. Macroscopic in vivo imaging of facial nerve regeneration in Thy1-GFP rats. JAMA Facial Plast. Surg. 2015, 17, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Placheta, E.; Borschel, G.H. Delayed peripheral nerve repair: methods, including surgical ‘cross-bridging’, to promote nerve regeneration. Neural Regen. Res. 2015, 10, 1540–1544. [Google Scholar] [CrossRef]
- Yuksel, F.; Karacaoglu, E.; Guler, M.M. Nerve regeneration through side-to-side neurorrhaphy sites in a rat model: a new concept in peripheral nerve surgery. Plast. Reconstr. Surg. 1999, 104, 2092–2099. [Google Scholar] [CrossRef]
- Ladak, A.l.; Schembri, P.; Olson, J.; Udina, E.; Tyreman, N.; Gordon, T. Side-to-side nerve grafts sustain chronically denervated peripheral nerve pathways during axon regeneration and result in improved functional reinnervation. Neurosurg. 2011, 68, 1654–65. [Google Scholar] [CrossRef]
- Gordon, T.; Hendry, M.; Lafontaine, C.A.; Cartar, H.; Zhang, J.J.; Borschel, G.H. Nerve cross-bridging to enhance nerve regeneration in a rat model of delayed nerve repair. PLoS One 2015, 10, e0127397. [Google Scholar] [CrossRef]
- Hendry, J.M.; Alvarez-Veronesi, C.; Snyder-Warwick, A.; Gordon, T.; Borschel, G.H. Side-to-side nerve bridges support donor axon regeneration Into chronically denervated nerves and are associated with characteristic changes in Schwann cell phenotype. Neurosurg. 2015, 77, 803–813. [Google Scholar] [CrossRef]
- Pearse, D.D.; Pereira, F.C.; Marcillo, A.E.; Bates, M.L.; Berrocal, Y.A.; Filbin, M.T; Bunge, M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004, 10, 610–616. [Google Scholar] [CrossRef]
- Krause, W.; Kulne, G. Pharmacokinetics of rolipram in rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica 1988, 18, 561–571. [Google Scholar] [CrossRef]
- Kilmer, S.L.; Carlsen, R.C. Forskolin activation of adenylate cyclase in vivo stimulates nerve regeneration. Nature 1984, 307, 455–457. [Google Scholar] [CrossRef]
- Cai, D.; Cao, Z.; McAtee, M.; Bregman, B.S.; Filbin, M.T. Neuronal cyclic AMP controls the developmental loss of ability of axons to regenerate. J. Neurosci. 2001, 21, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Hannila, S.S.; Filbin, M.T. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp. Neurol. 2008, 209, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Cai, D.; Dai, H.; McAtee, M.; Hoffman, P.N.; Bregman, B.S.; Filbin, M.T. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 2002, 34, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Nikulina, E.; Mellado, W.; Filbin, M.T. Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase. J. Neurosci. 2003, 23, 11770–11777. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Woolf, C.J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 1999, 23, 83–91. [Google Scholar] [CrossRef]
- Oudega, M.; Varon, S.; Hagg, T. Distribution of corticospinal motor neurons in the postnatal rat: quantitative evidence for massive collateral elimination and modest cell death. J. Comp. Neurol. 1994, 347, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, Y.; Bo, X.; Huang, W.; Fang, X.; Zhang, X.; Miao, T.; Subang, M.C.; Richardson, P.M. Actions of neuropoietic cytokines and cyclic AMP in regenerative conditioning of rat primary sensory neurons. Exp. Neurol. 2007, 204, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Han, P.J.; Shukla, S.; Subramanian, P.S.; Hoffman, P.N. Cyclic AMP elevates tubulin expression without increasing intrinsic axon growth capacity. Exp. Neurol. 2004, 189, 293–302. [Google Scholar] [CrossRef]
- Dusart, I.; Schwab, M.E. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur. J. Neurosci. 1994, 6, 712–724. [Google Scholar] [CrossRef]
- Popovich, P.G.; Longbrake, E.E. Can the immune system be harnessed to repair the CNS? Nat. Rev. Neurosci. 2008, 9, 481–493. [Google Scholar] [CrossRef]
- Kurimoto, T.; Yin, Y.; Habboub, G.; Gilbert, H.Y.; Li, Y.; Nakao, S.; Hafezi-Moghadam, A.; Benowitz, L.I. Neutrophils express oncomodulin and promote optic nerve regeneration. J. Neurosci. 2013, 33, 14816–14824. [Google Scholar] [CrossRef]
- Yin, Y.; Henzl, M.T.; Lorber, B.; Nakazawa, T.; Thomas, T.T.; Jiang, F.; Langer, R.; Benowitz, L.I. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 2006, 9, 843–852. [Google Scholar] [CrossRef] [PubMed]
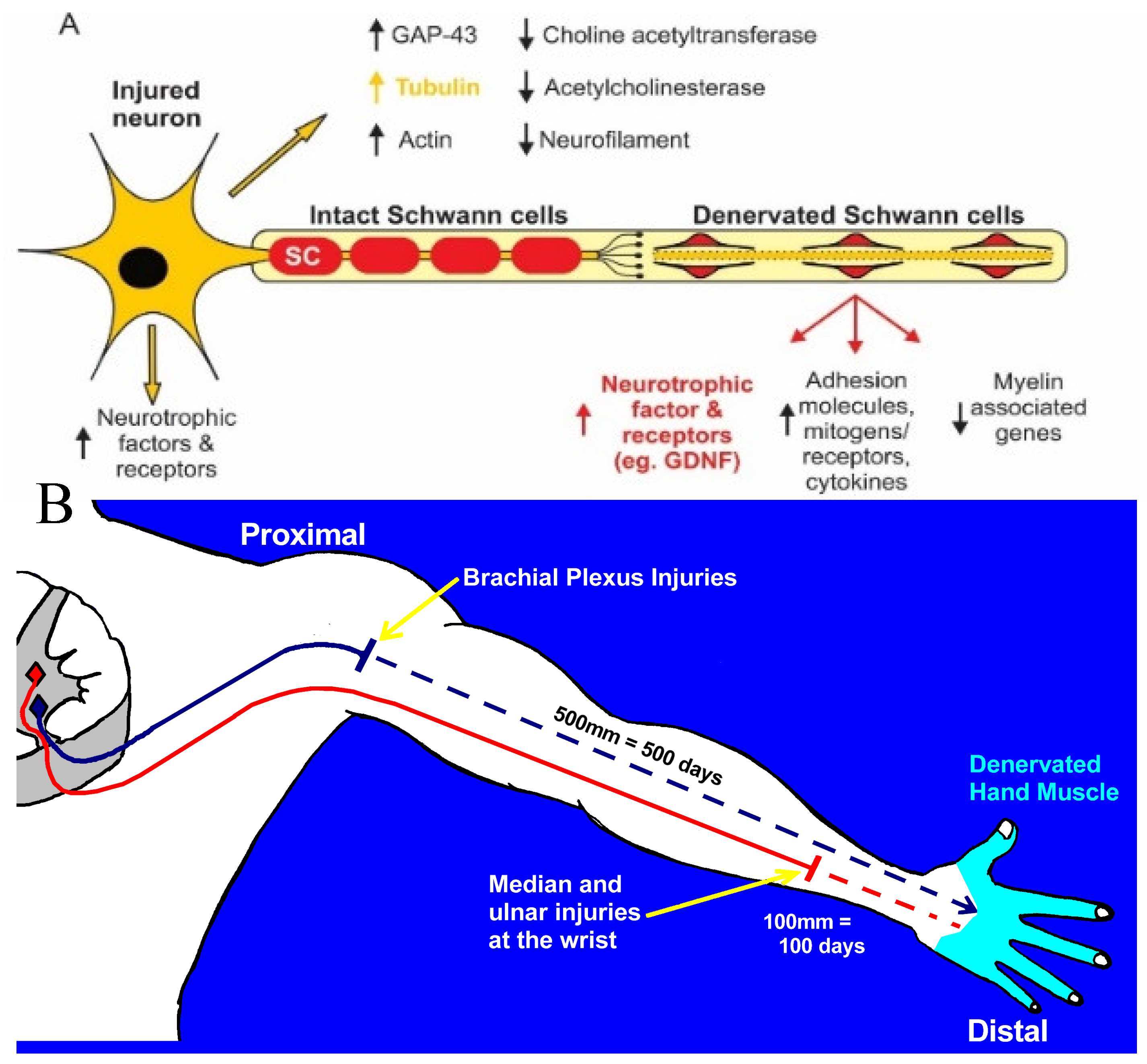
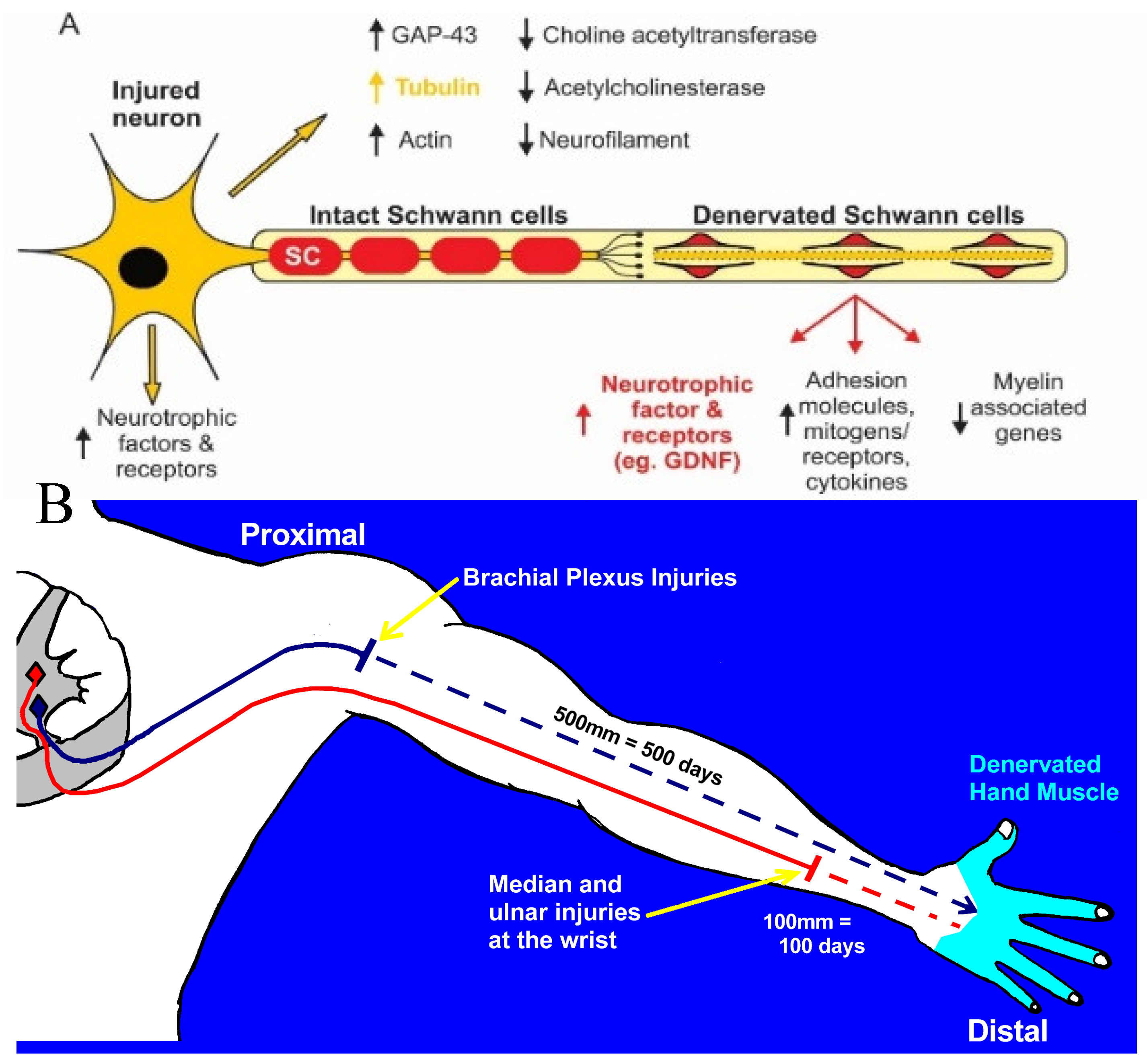


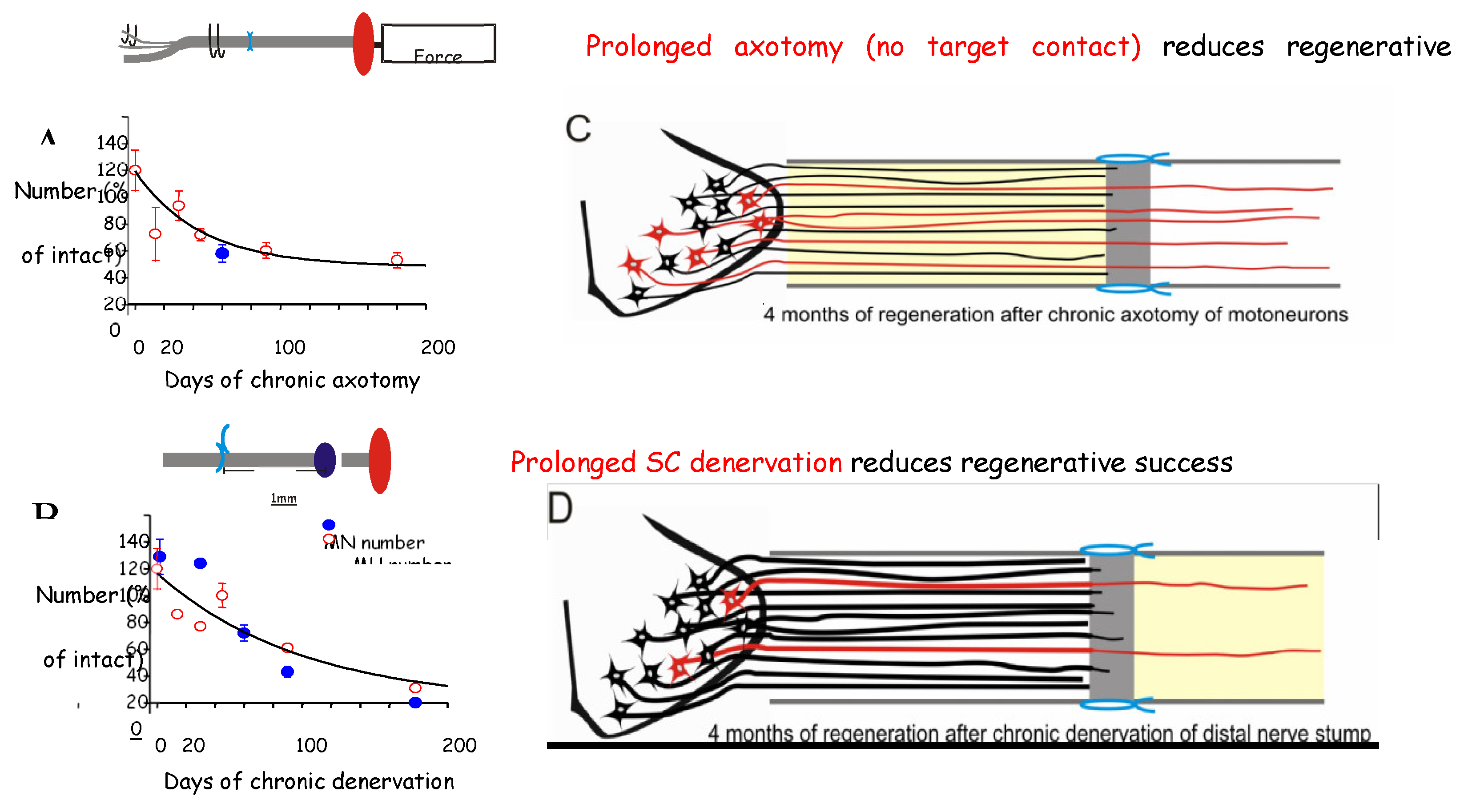
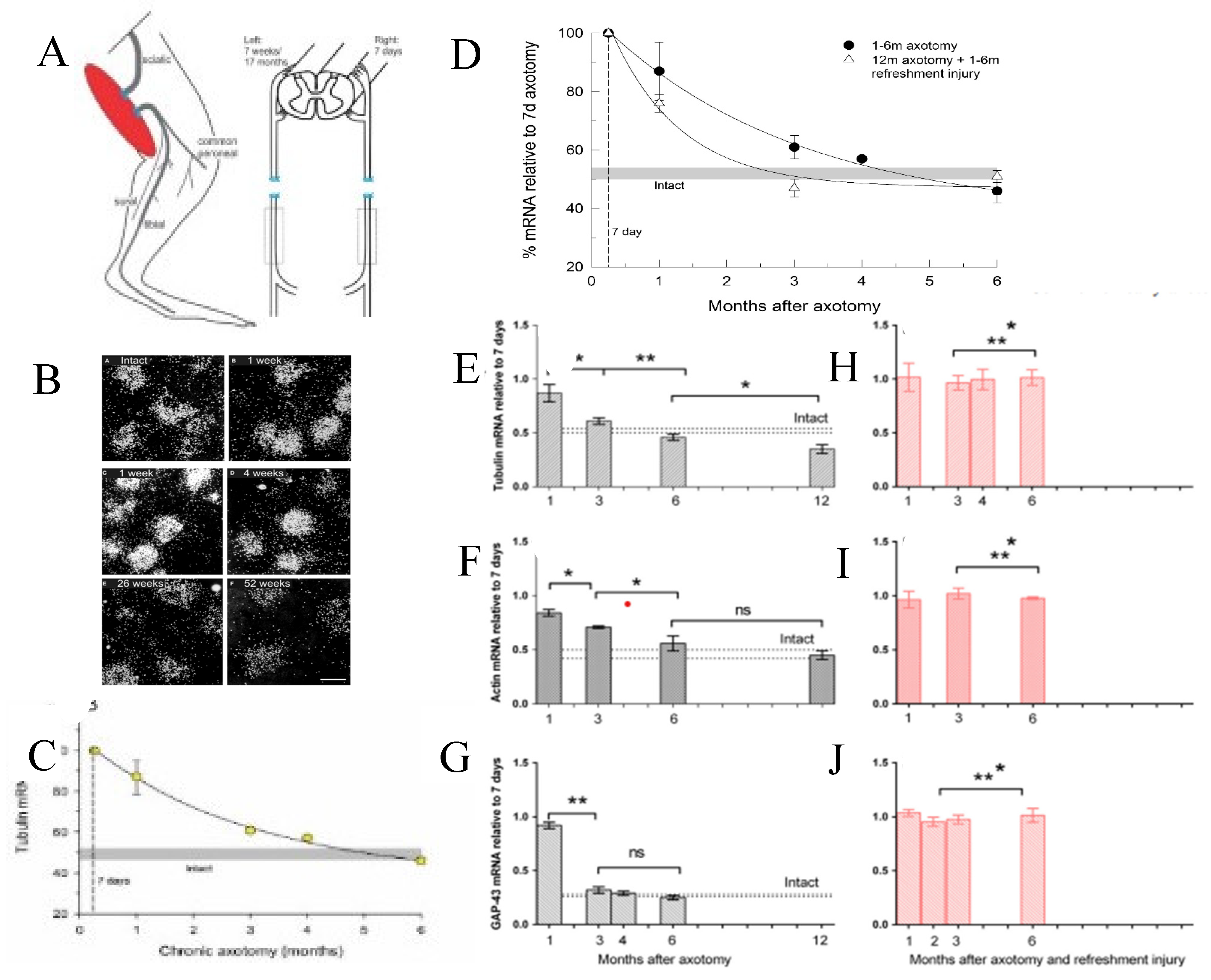
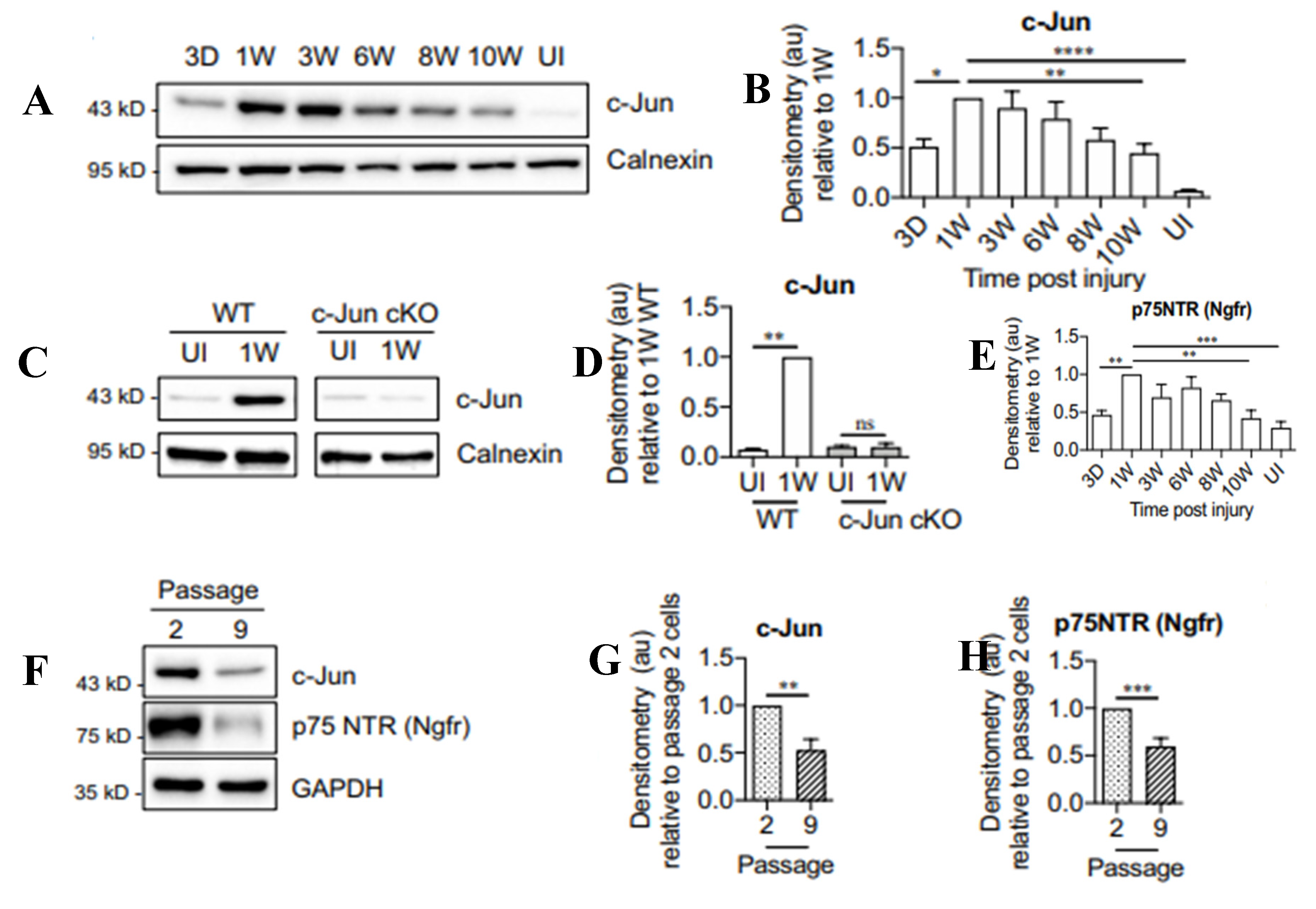
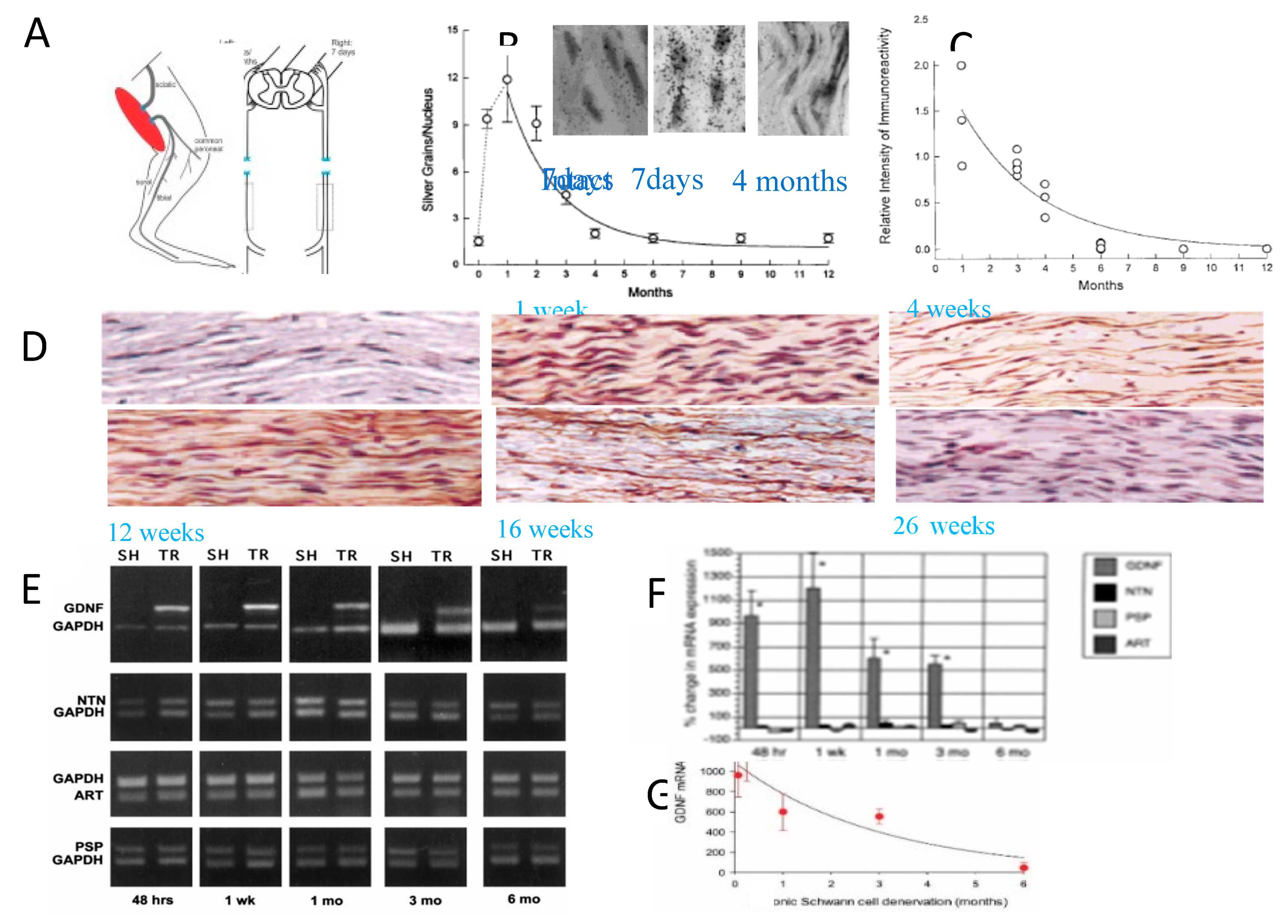
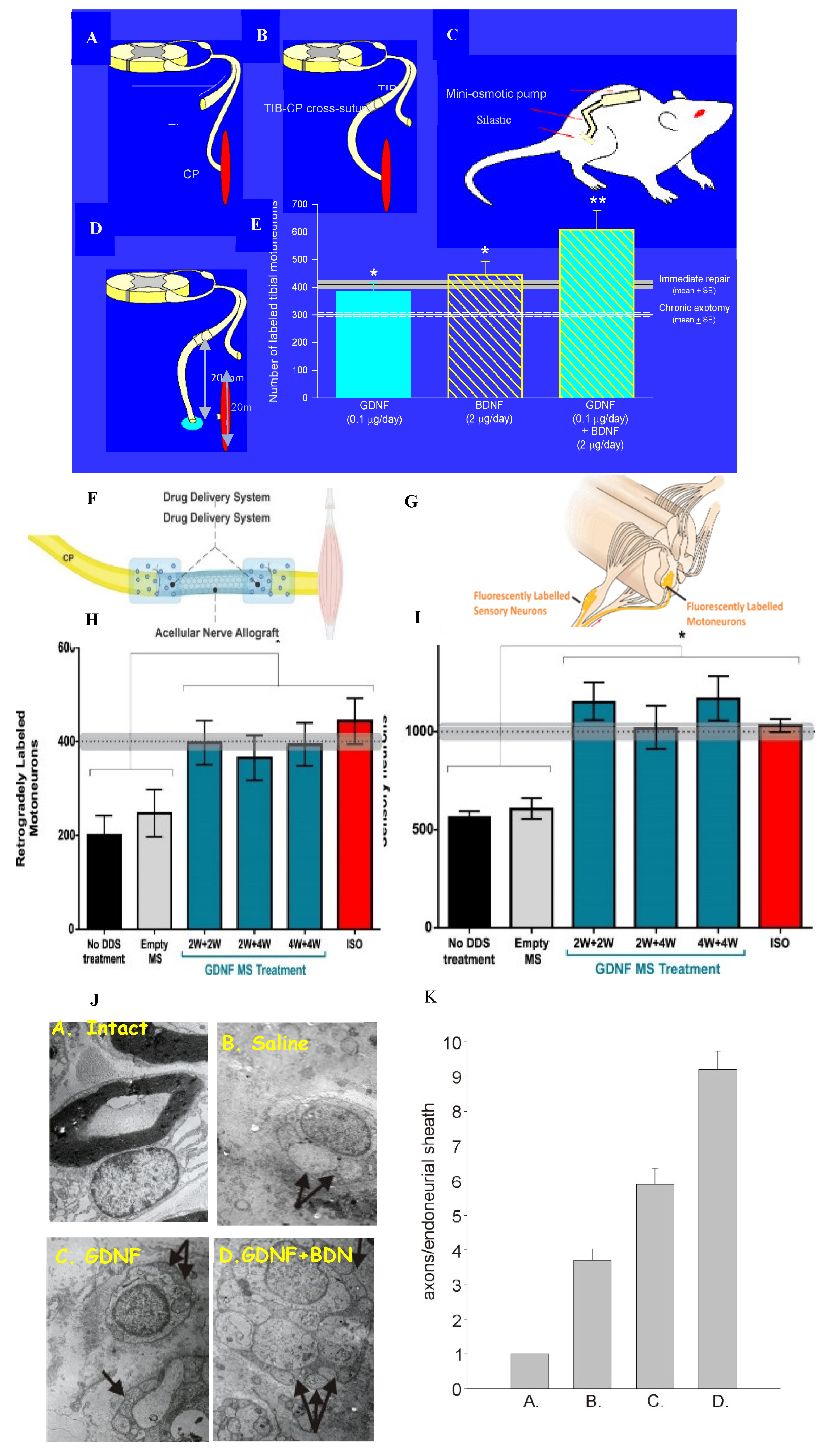
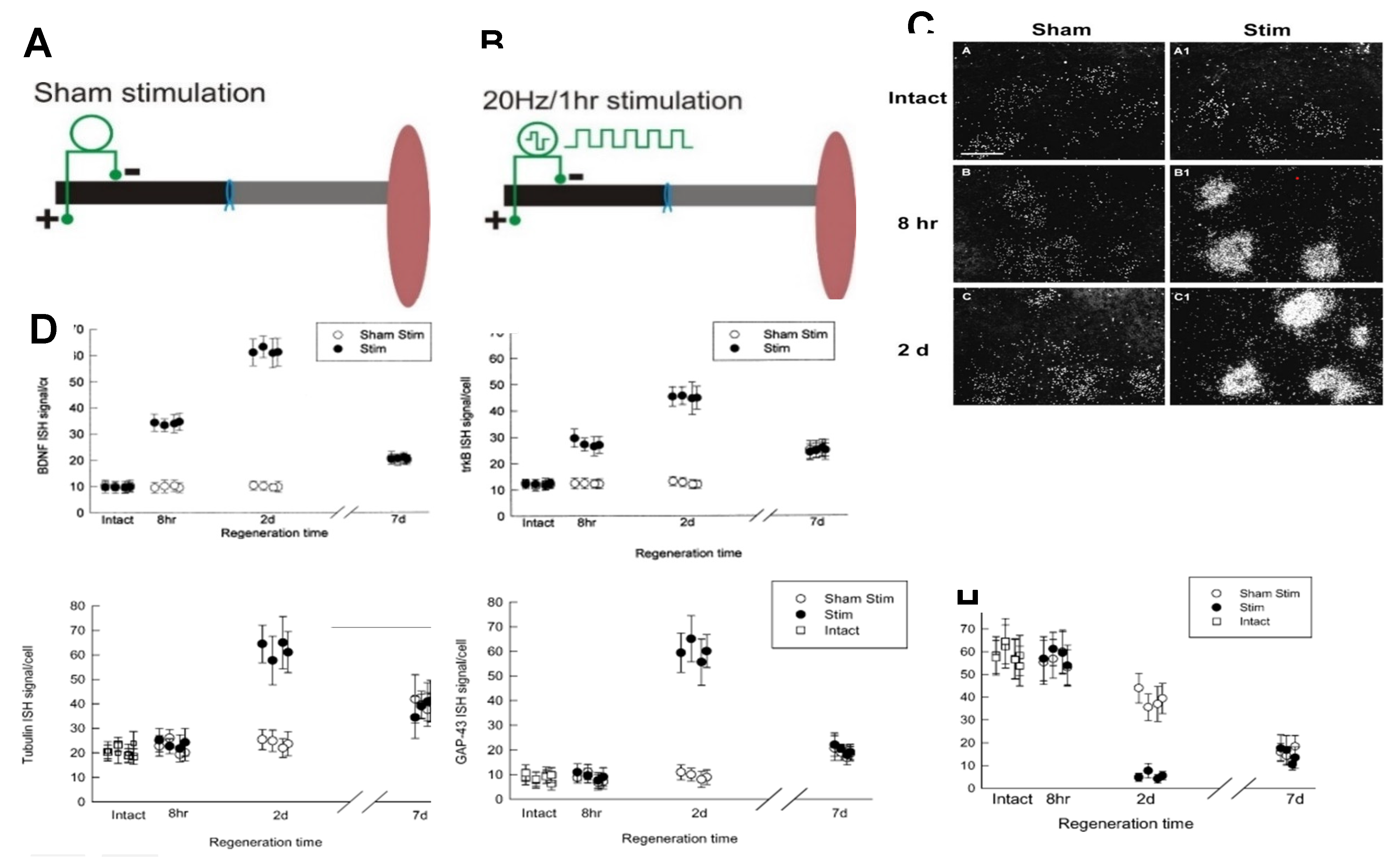
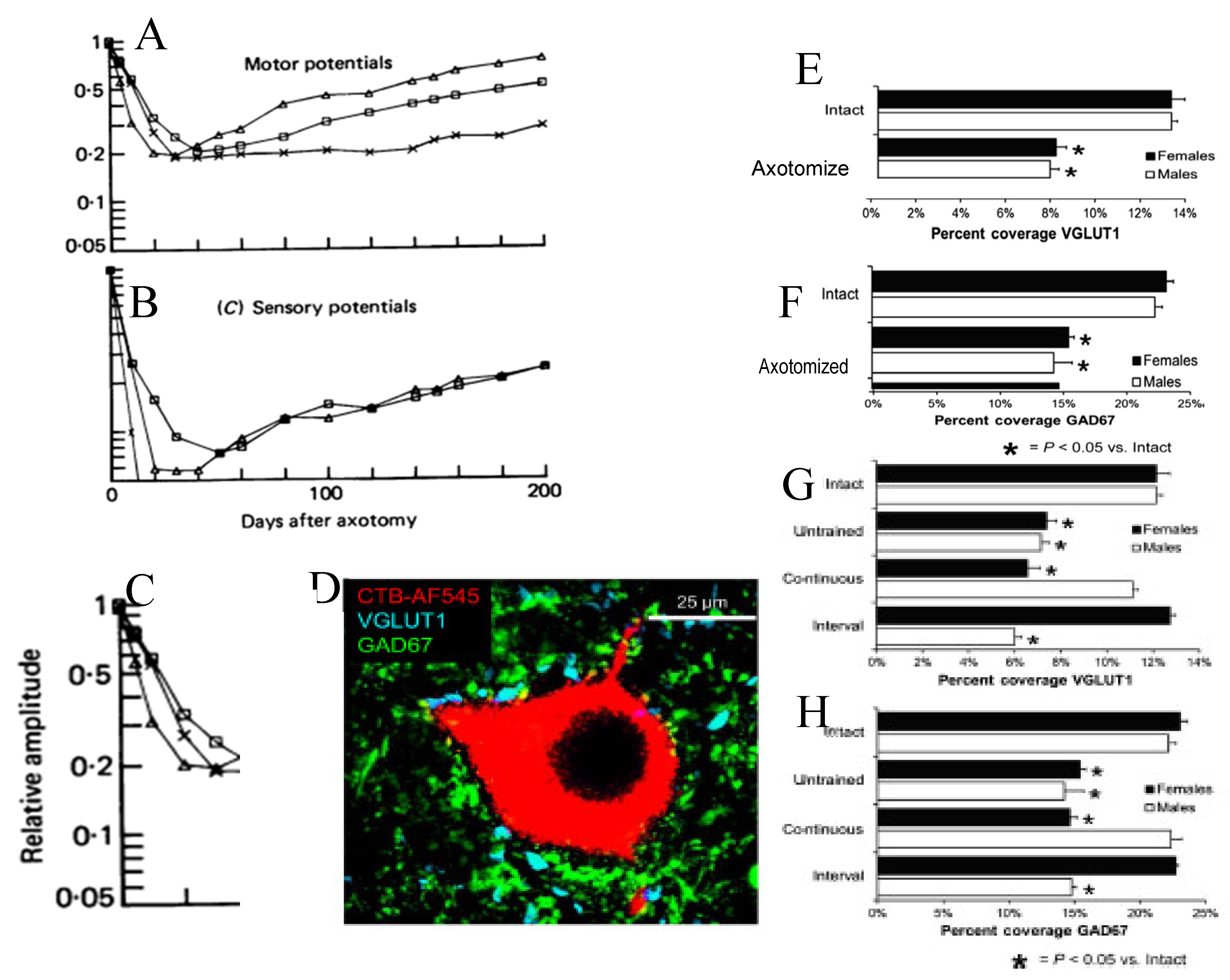
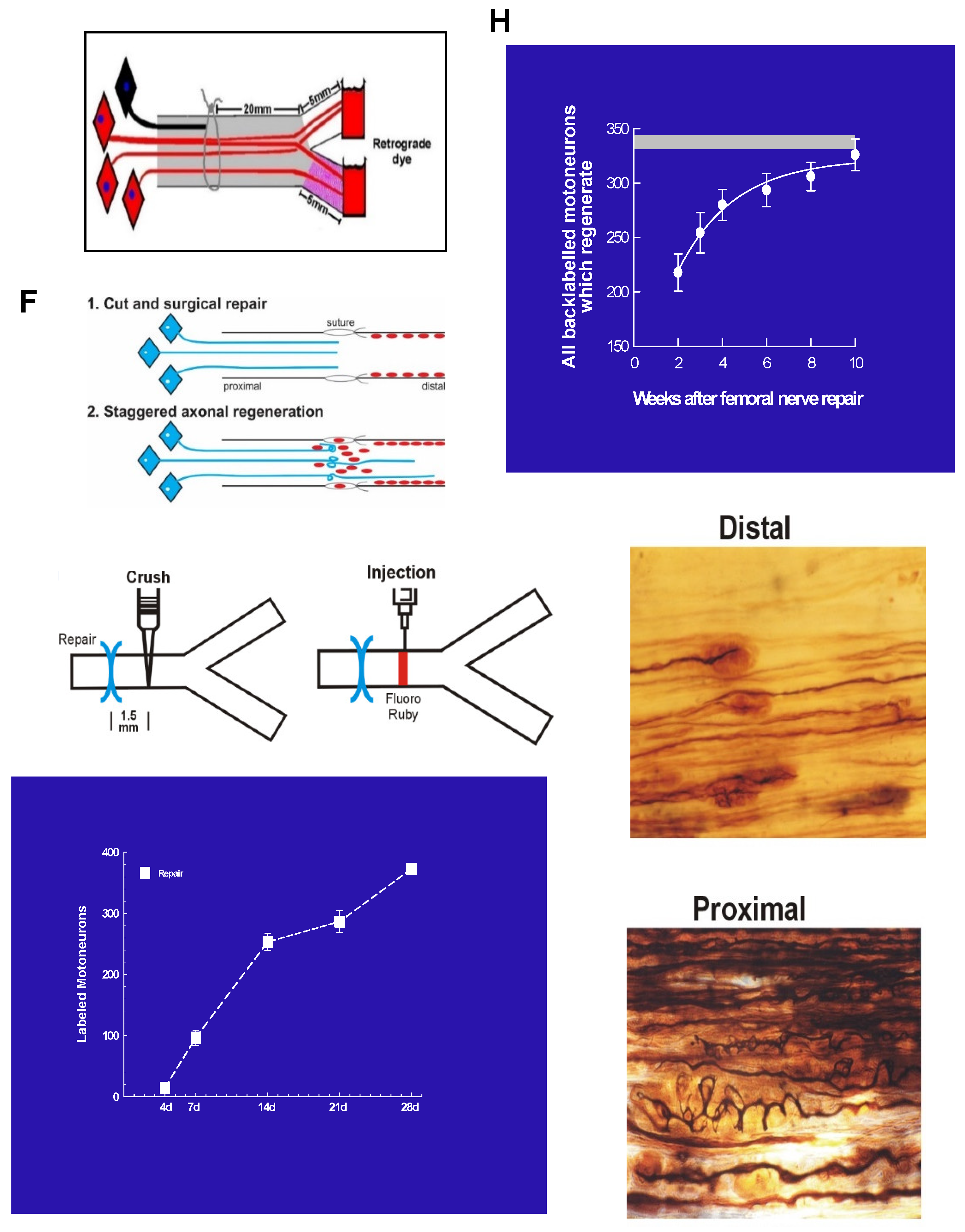
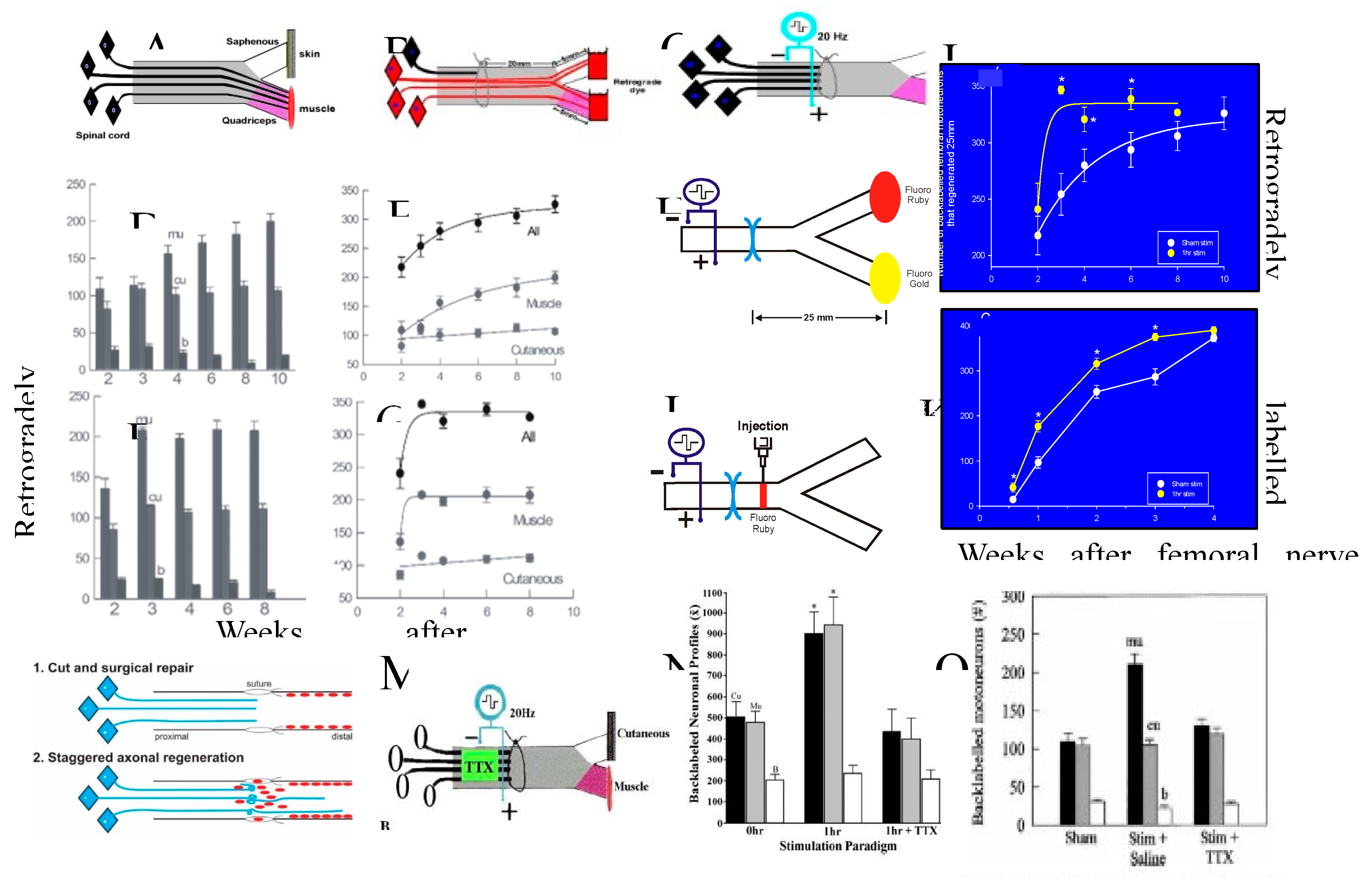
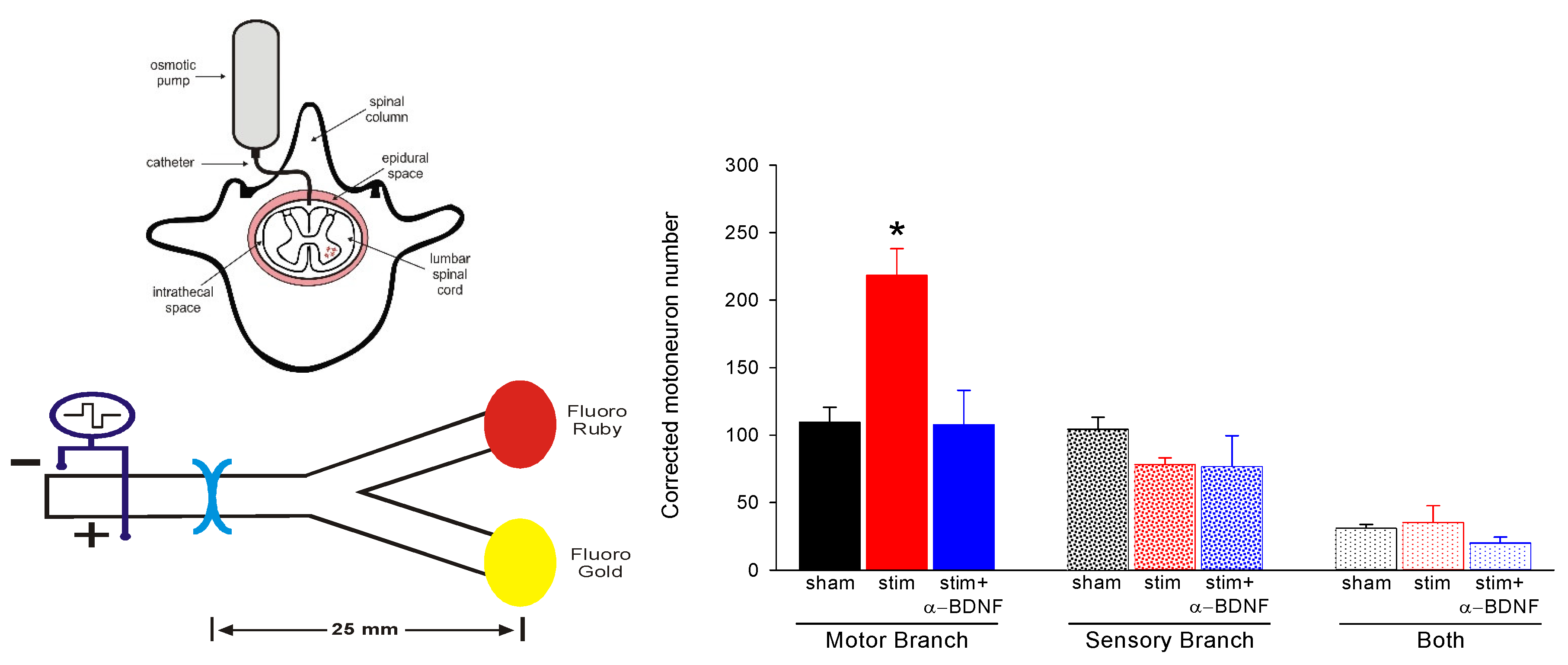
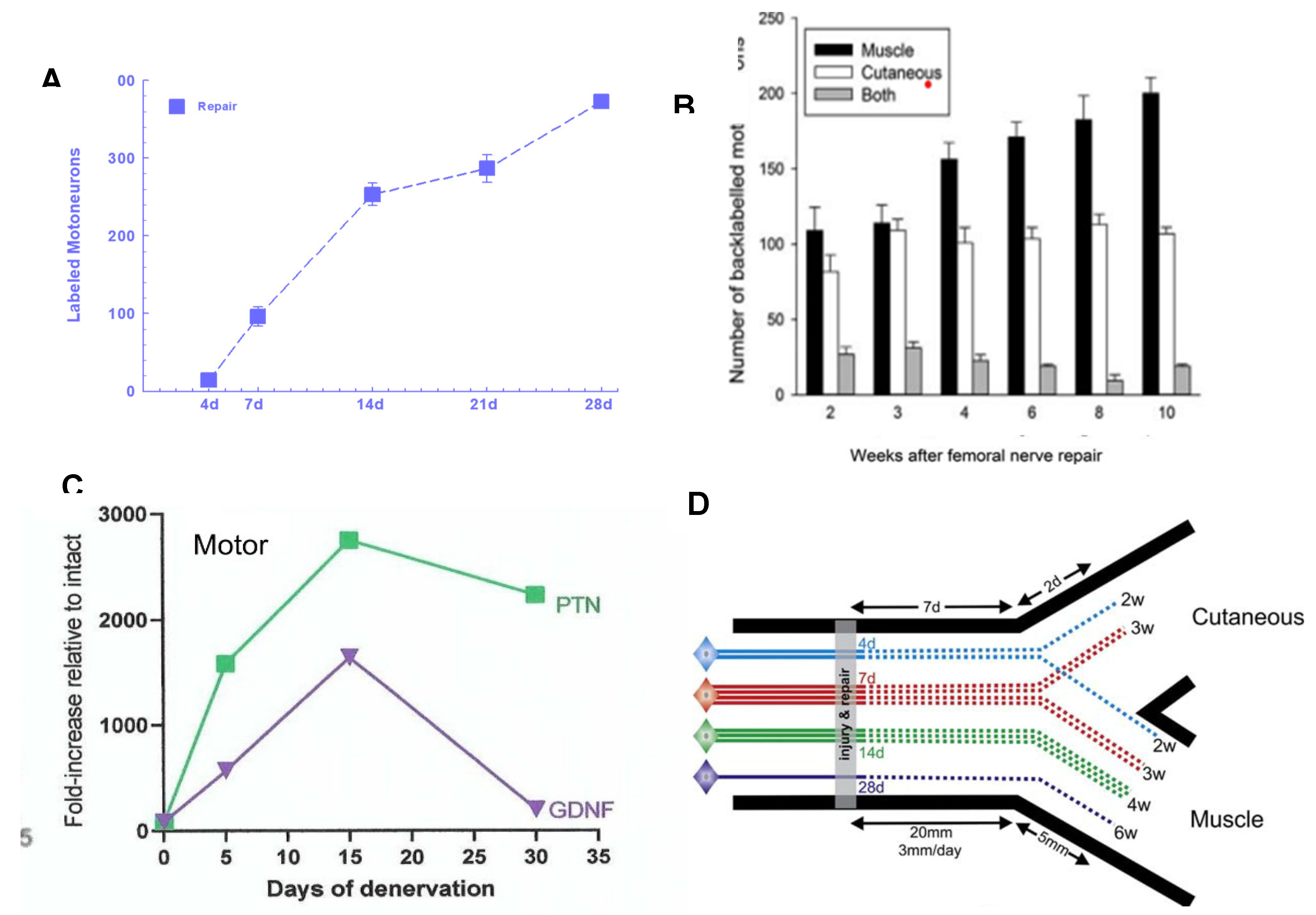
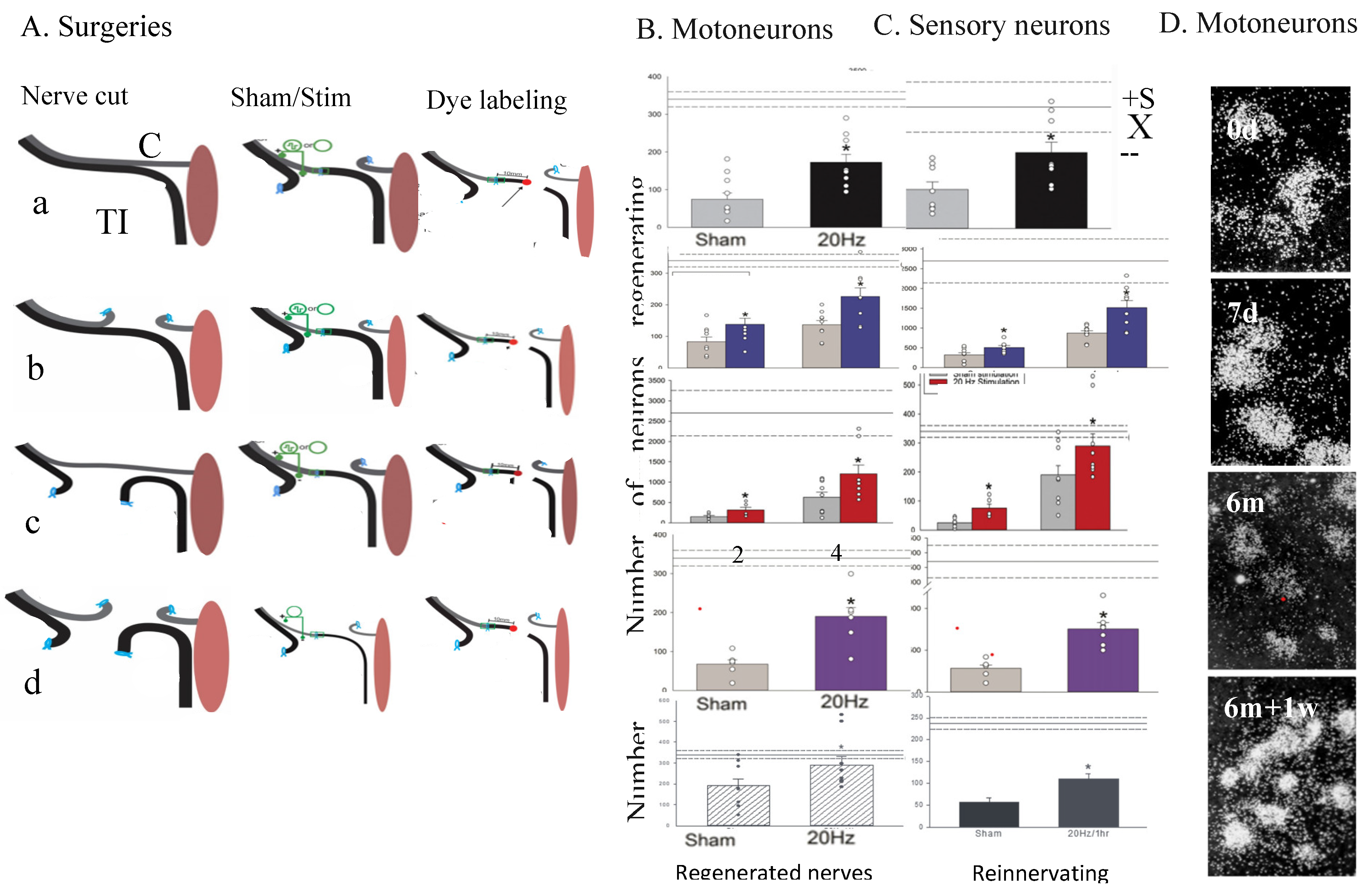
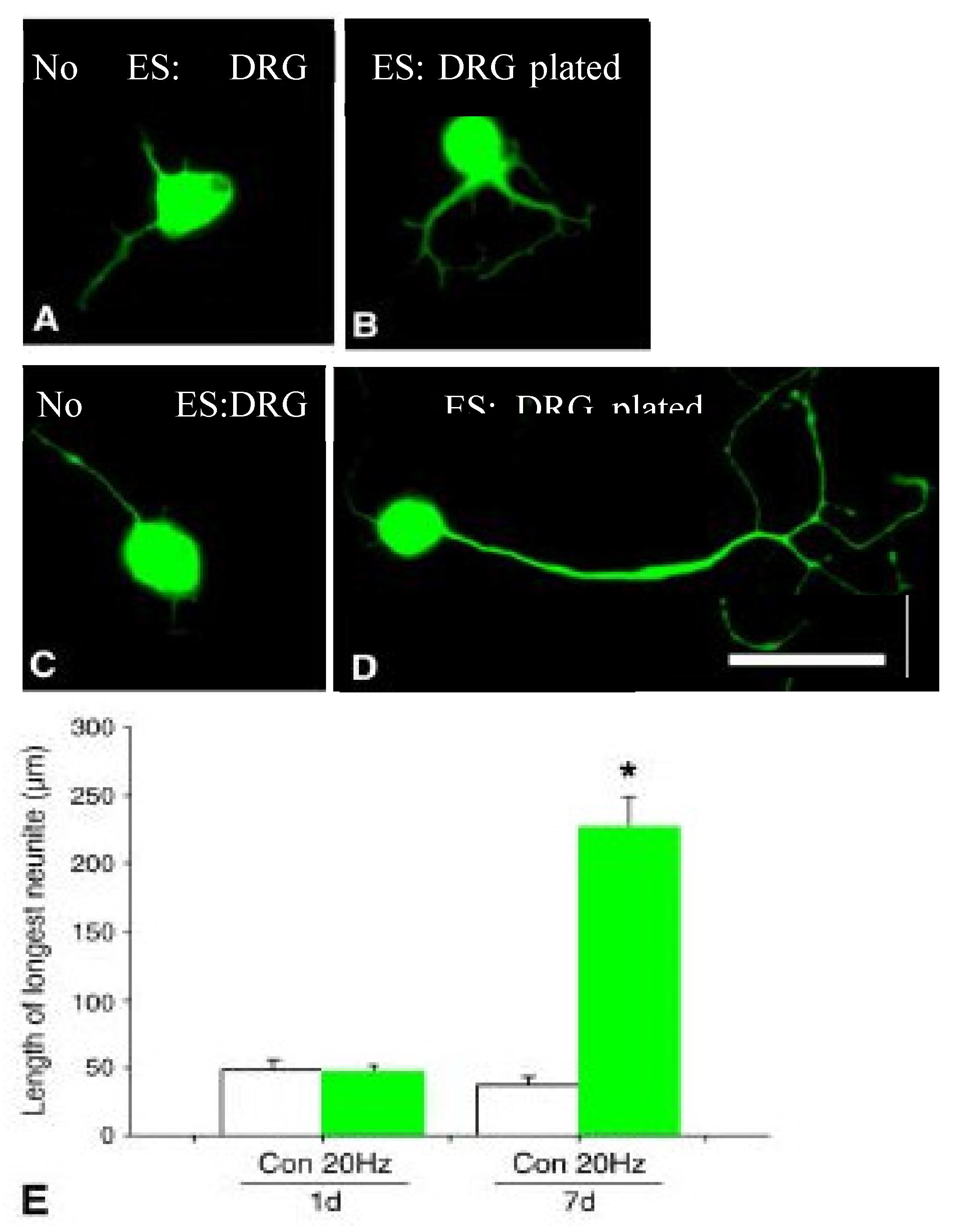
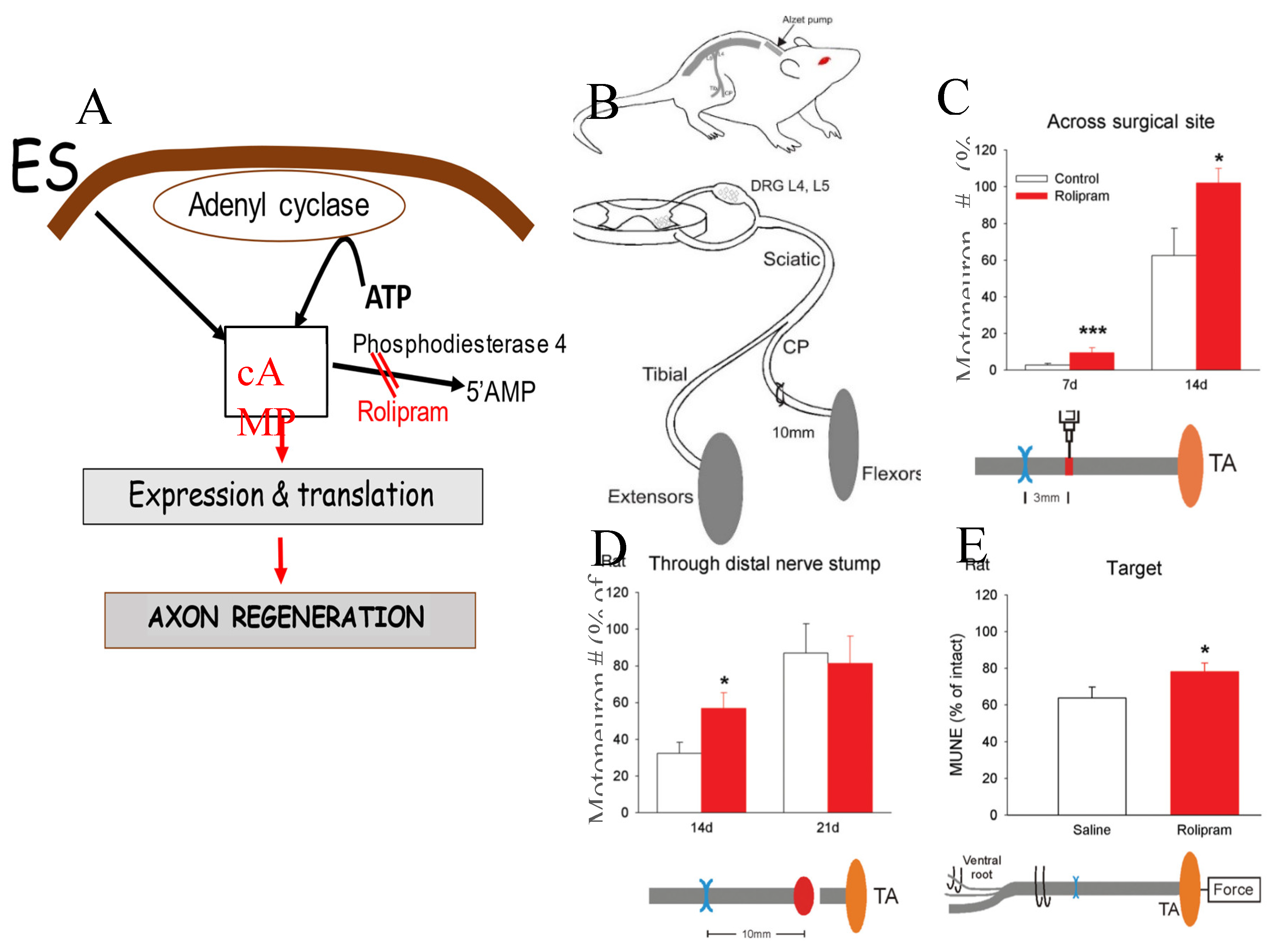
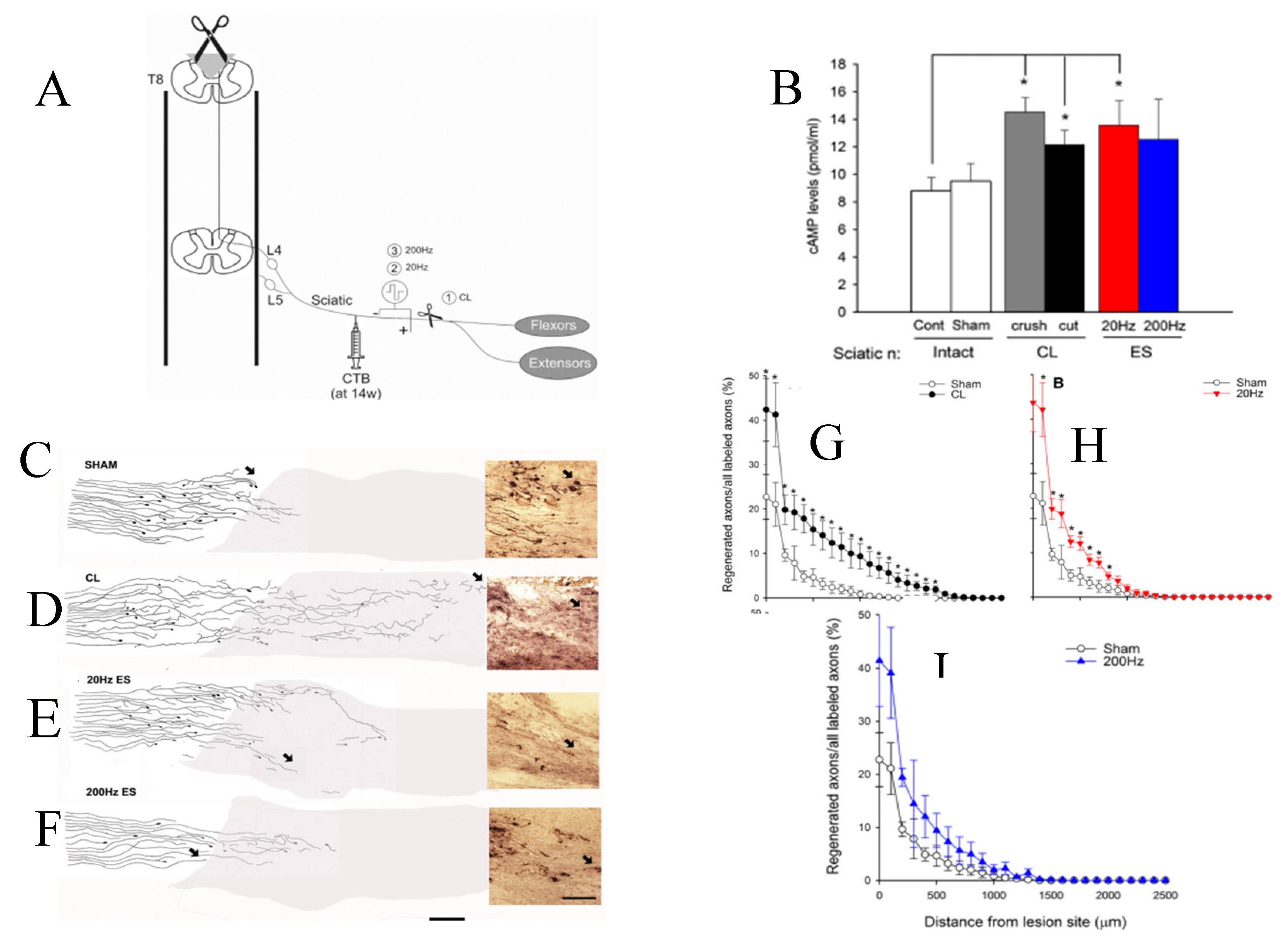
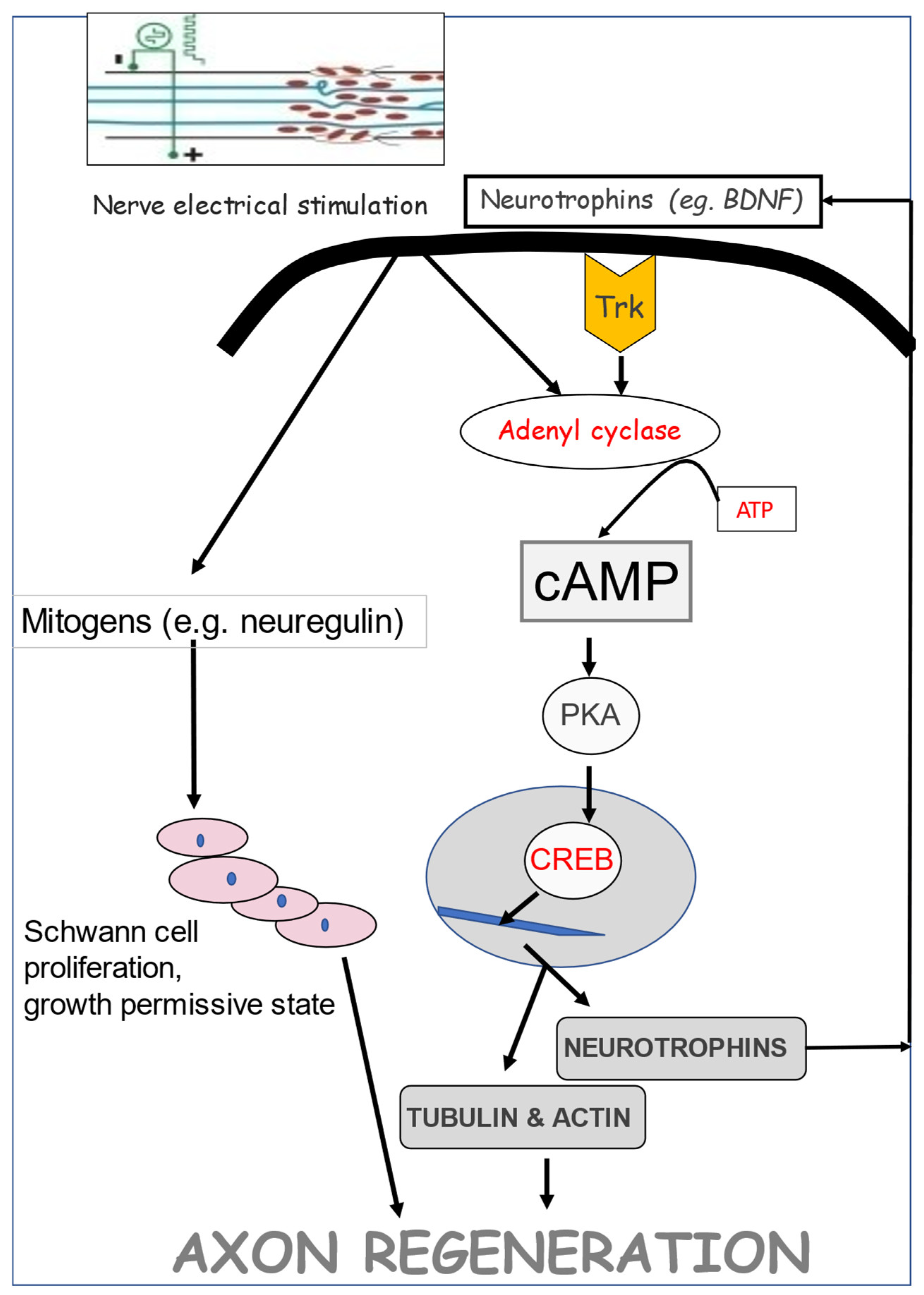
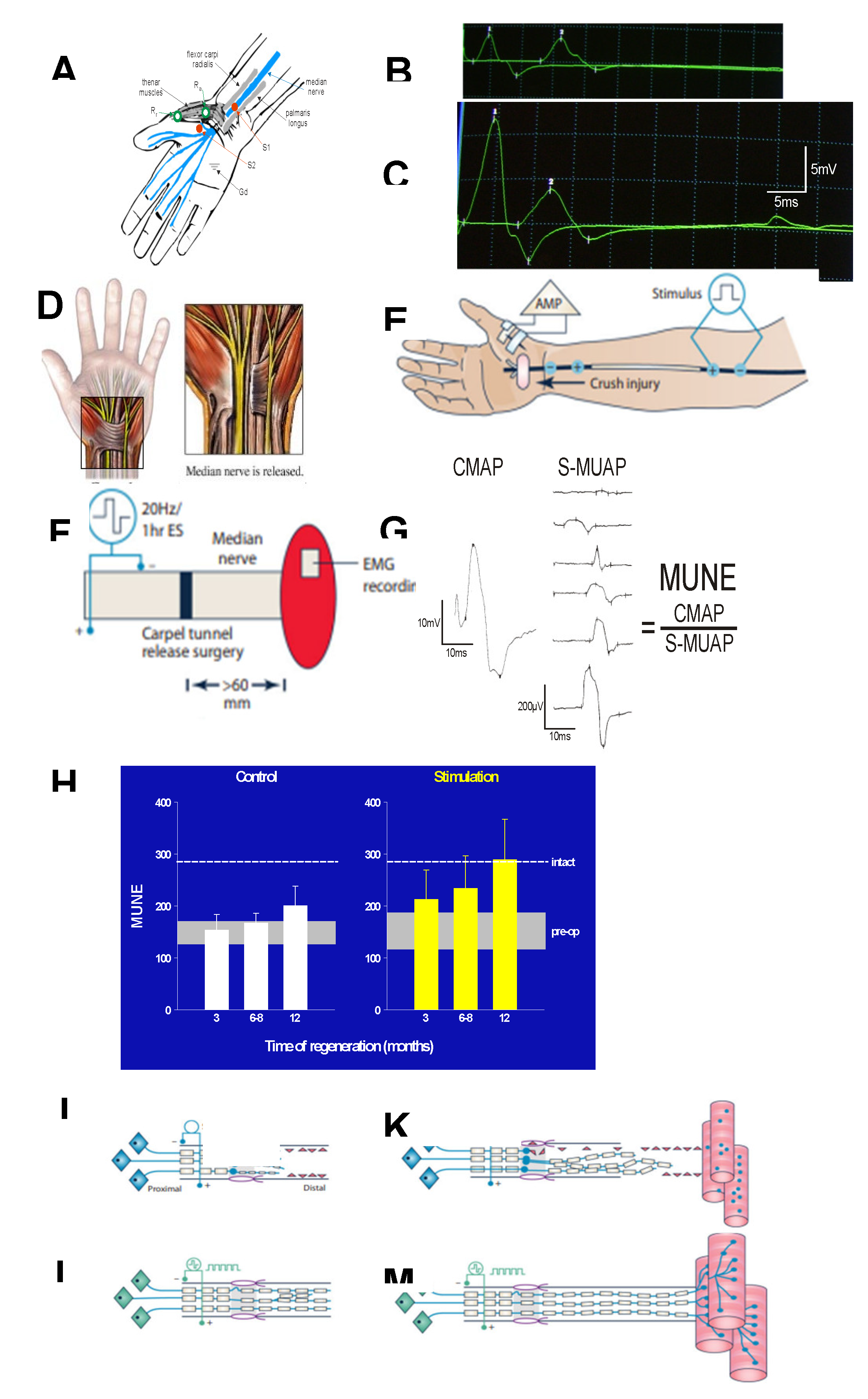
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



