Submitted:
16 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design, setting and ethical issues
2.2. Subjects
2.3. Data collection
2.4. MRI and MRE protocols
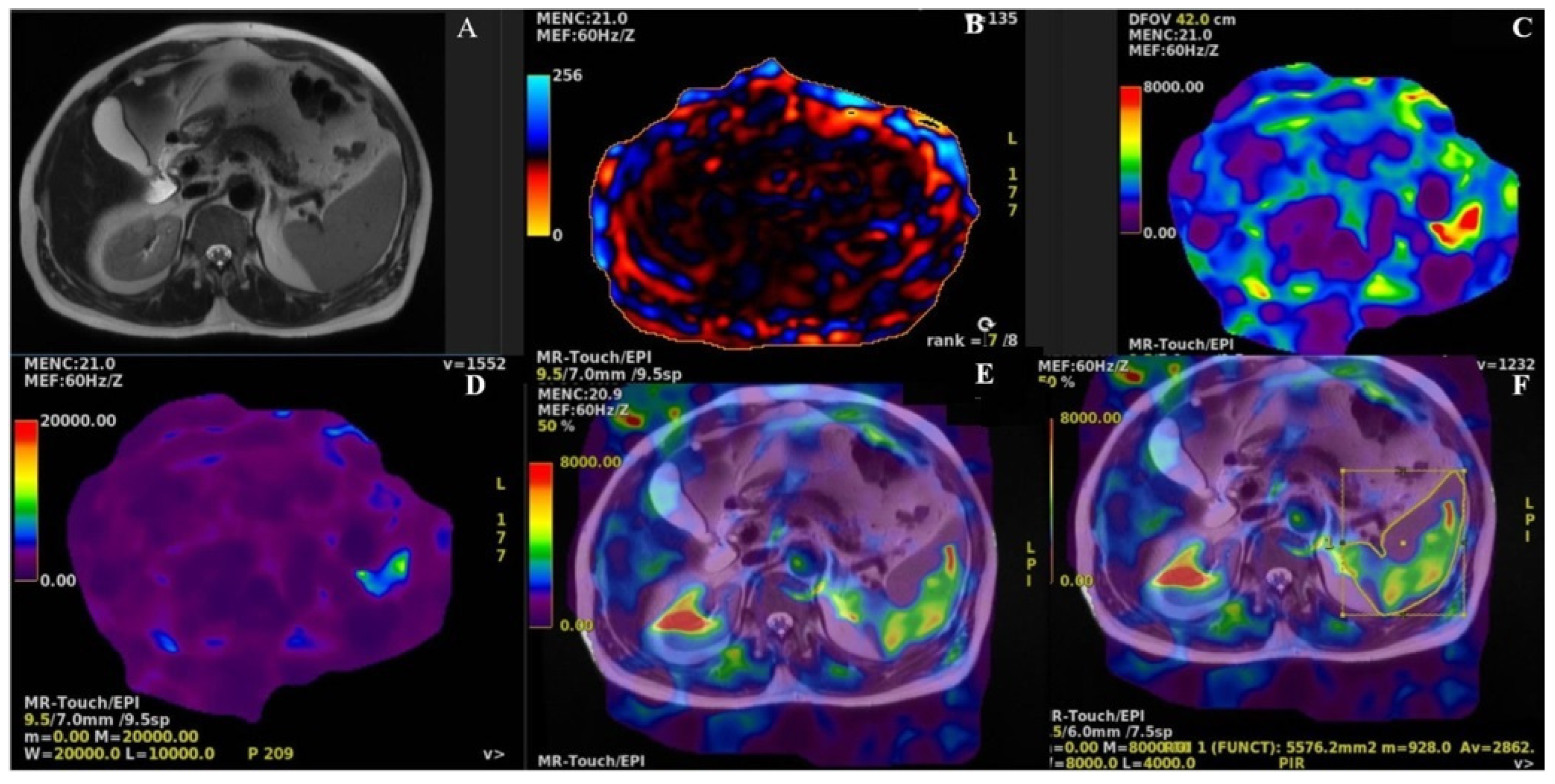
2.5. Outcomes
2.6. Statistical Analysis
3. Results
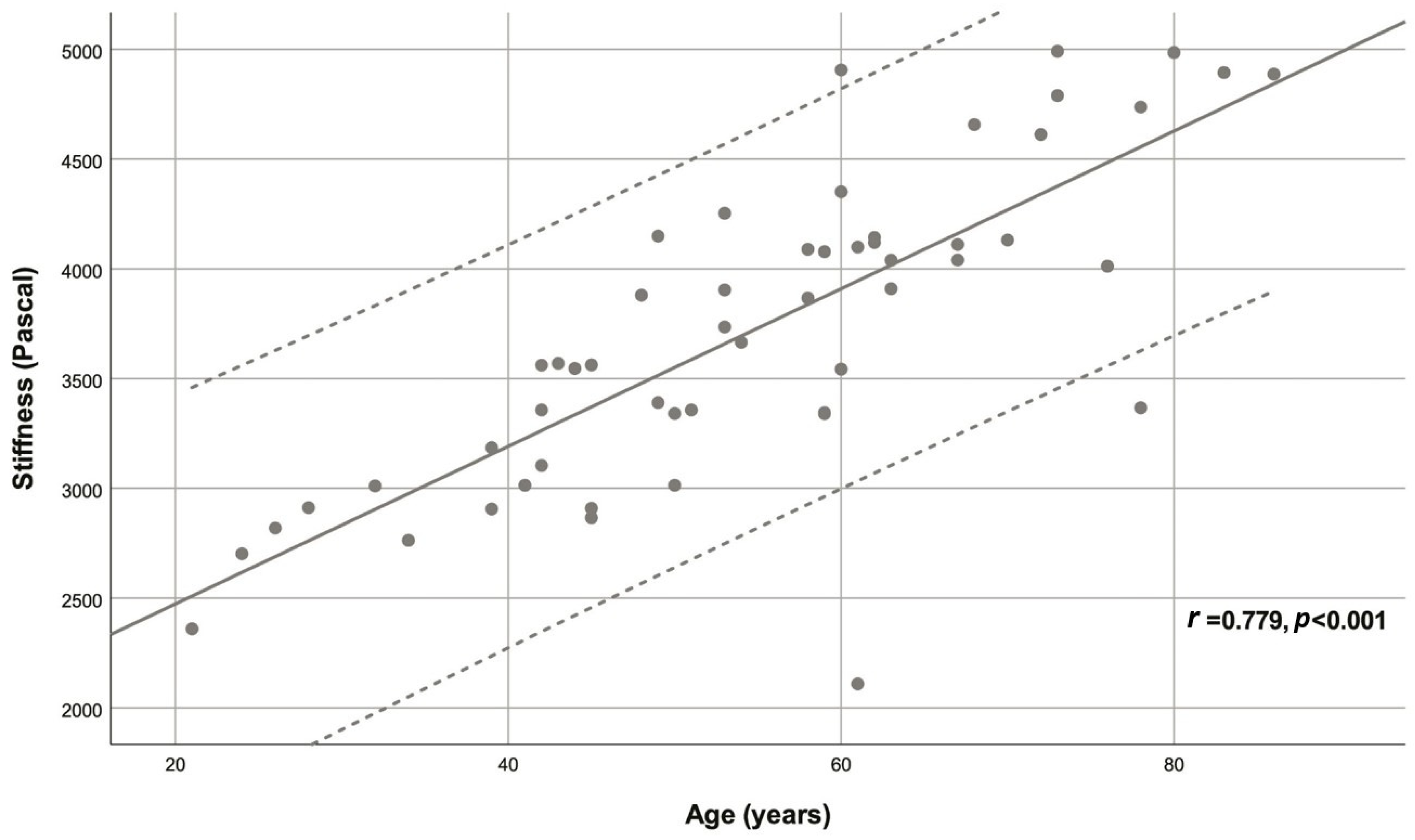
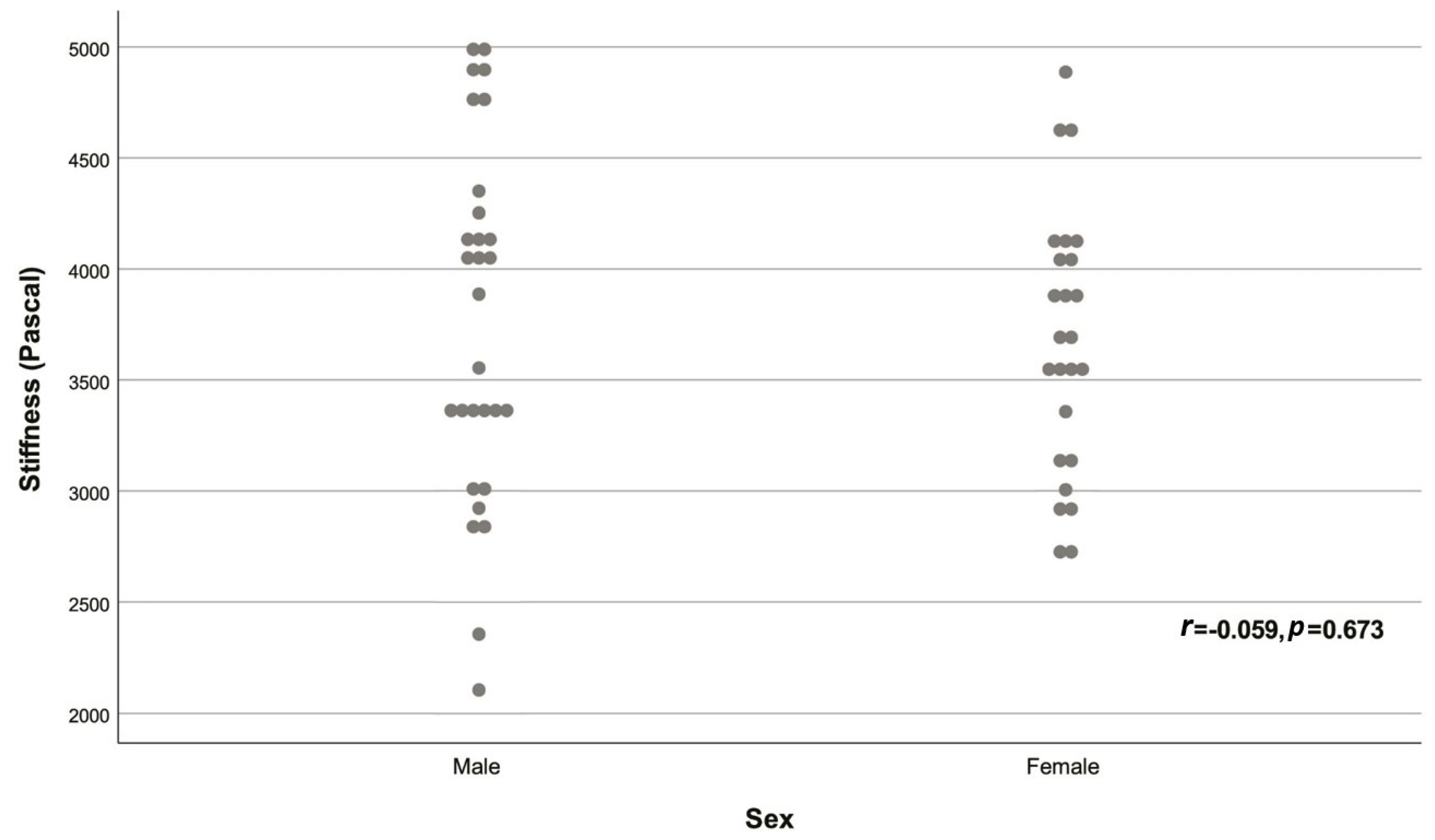
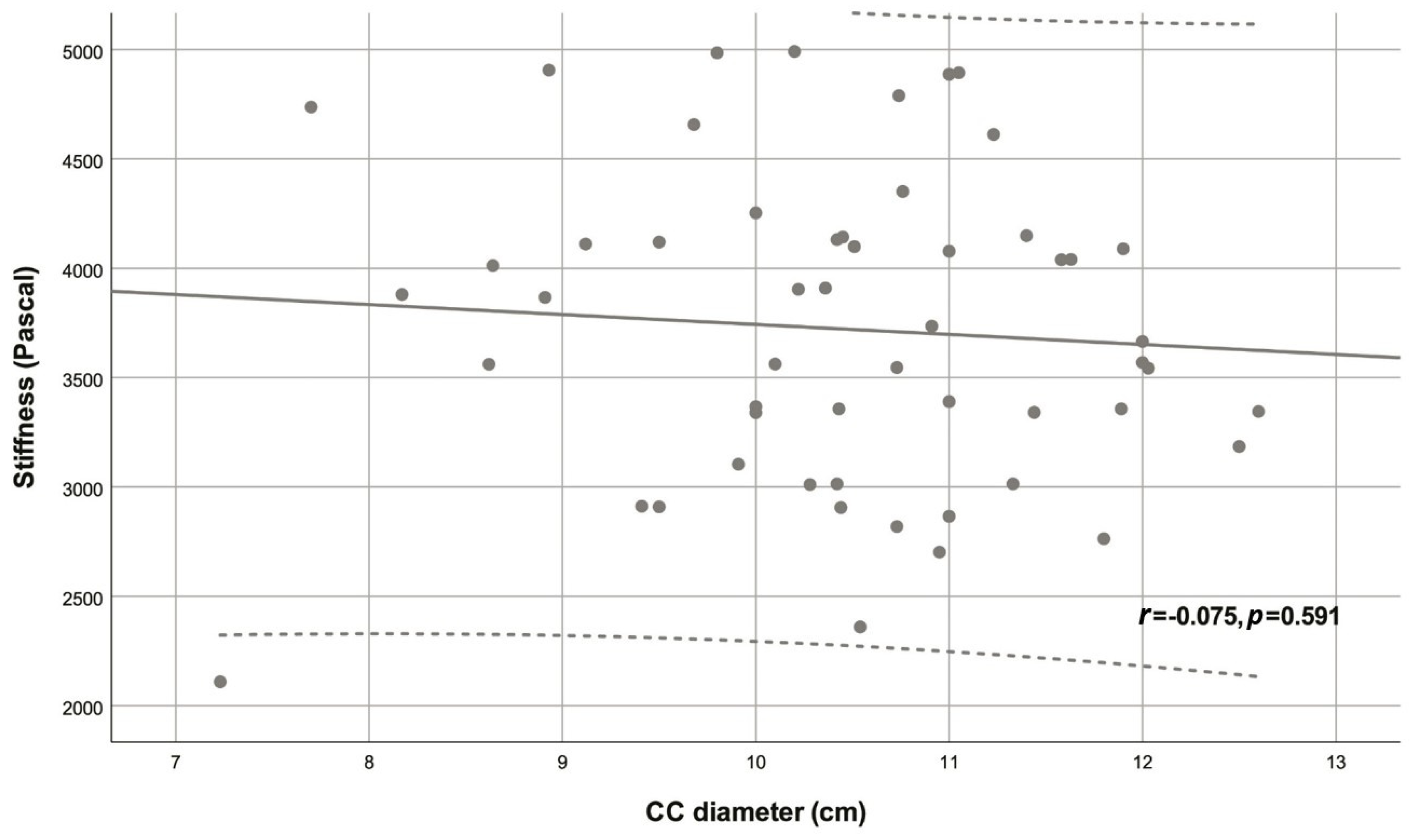
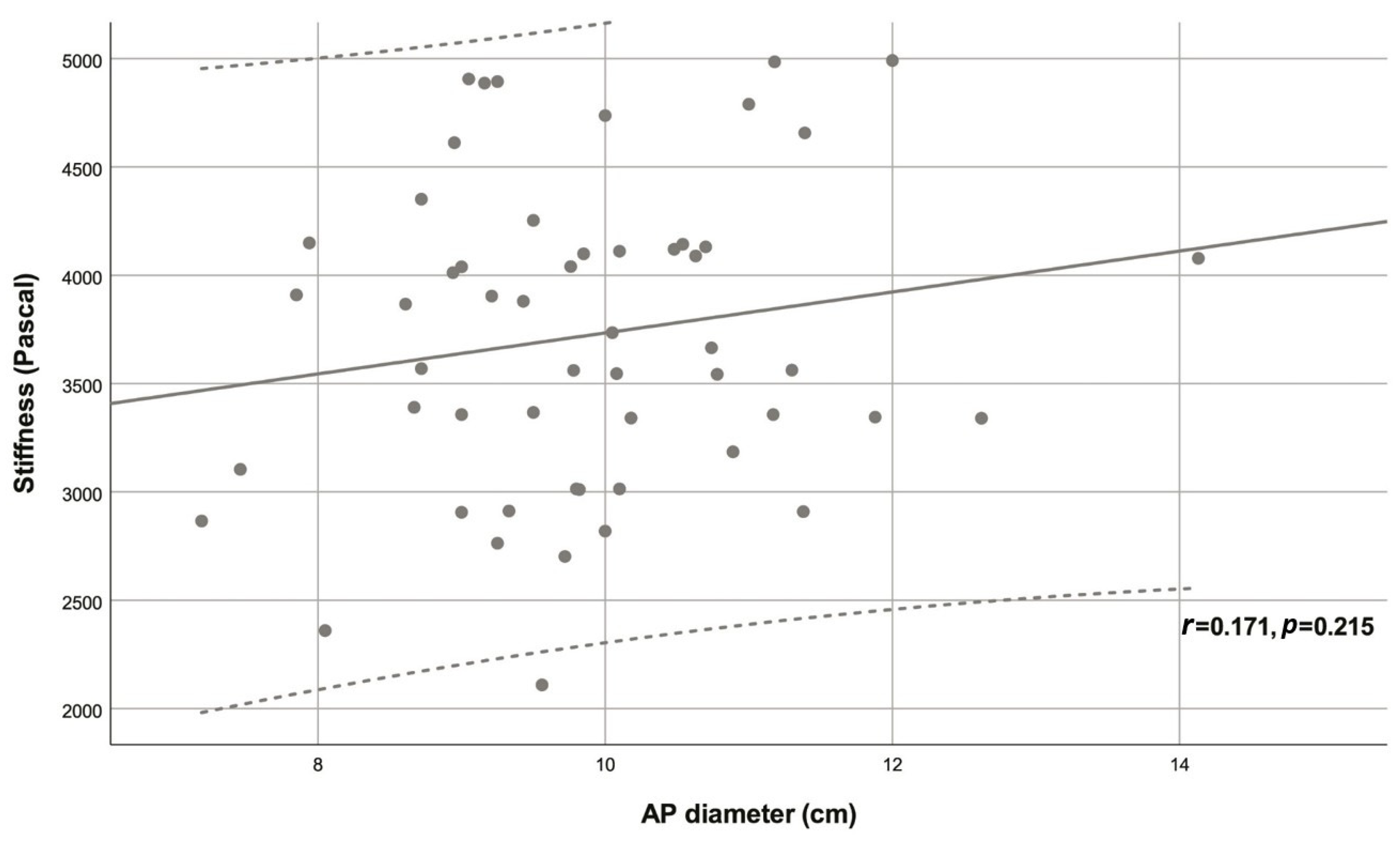
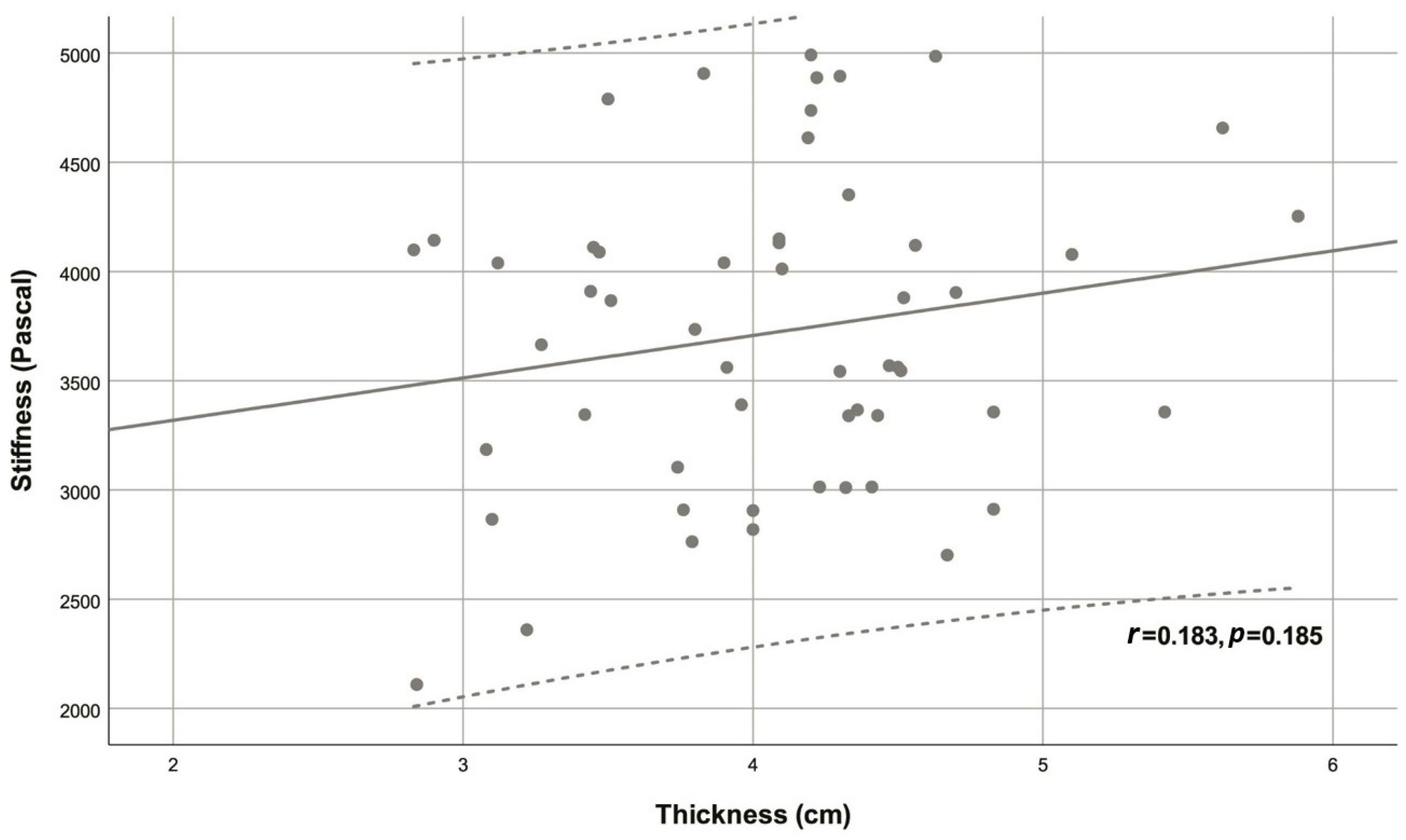
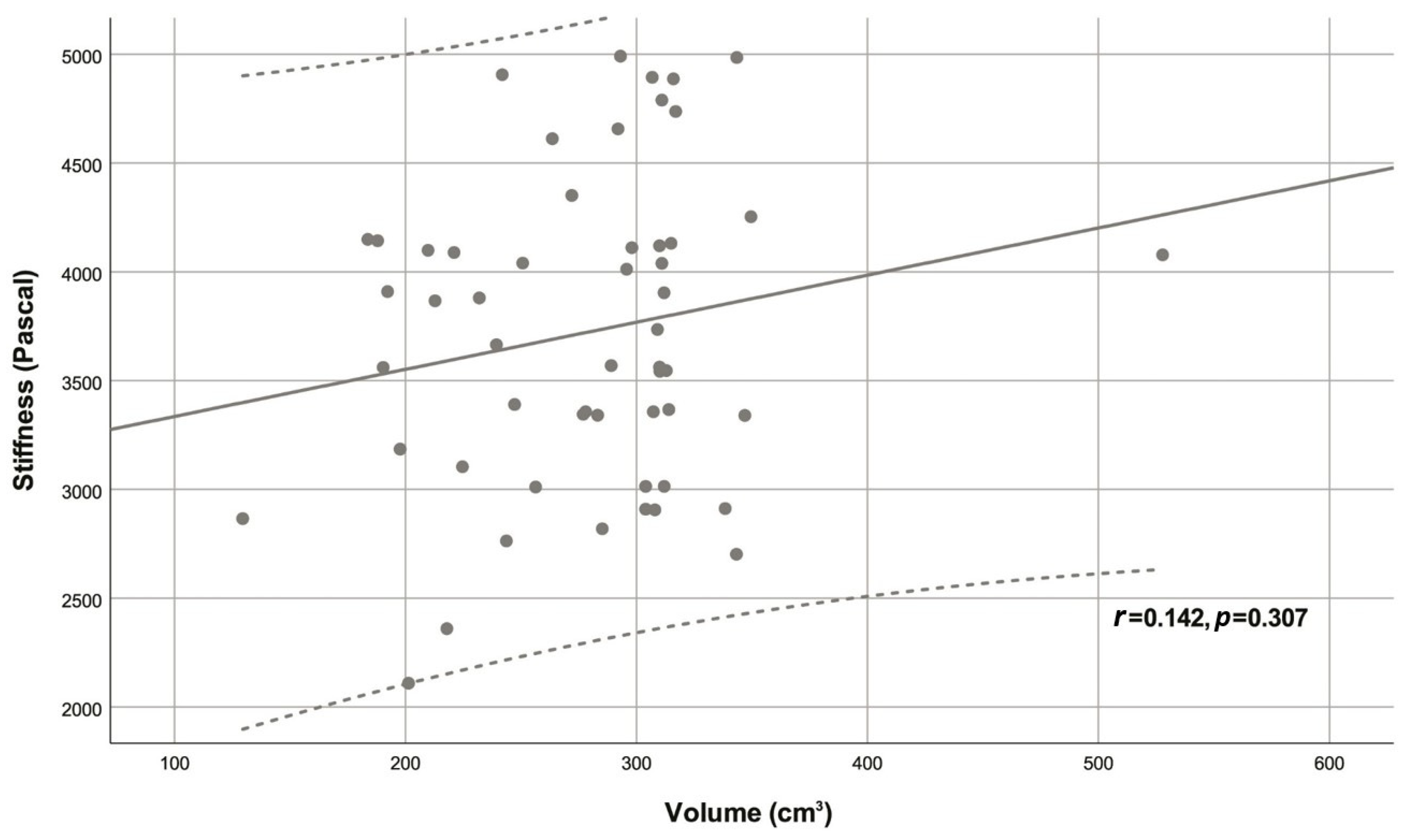
| Variable | Descriptive statistic | r | p |
|---|---|---|---|
| Age (years) | 54.78 ± 15.40 | 0.779 | <0.001 |
| Sex | |||
| Male | 29 (53.70%) | -0.059 | 0.673 |
| Female | 25 (46.30%) | ||
| CC diameter (cm) | 10.46 ± 1.16 | -0.075 | 0.591 |
| AP diameter (cm) | 9.88 ± 1.29 | 0.171 | 0.215 |
| Thickness (cm) | 4.08 ± 0.67 | 0.183 | 0.185 |
| Volume (cm3) | 292.54 (239.40 - 311.00) | 0.142 | 0.307 |
| Stiffness (Pascal) | 3721.94 ± 709.69 | - | - |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis S., M.; Williams, A.; Eisenbarth S., C. Structure and function of the immune system in the spleen. Sci Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Pawluś, A.; Inglot M., S.; Szymańska, K.; Kaczorowski, K.; Markiewicz B., D.; Kaczorowska, A.; Gąsiorowski, J.; Szymczak, A.; Inglot, M.; Bladowska, J.; et al. Shear wave elastography of the spleen: evaluation of spleen stiffness in healthy volunteers. Abdom Radiol (NY). 2016, 41, 2169–2174. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Wu, H.; Feng, Y.; Han, X.; Bu, H.; Zhu, Q. Spleen Stiffness Is Superior to Liver Stiffness for Predicting Esophageal Varices in Chronic Liver Disease: A Meta-Analysis. PLoS One. 2016, 11, e0165786. [Google Scholar] [CrossRef]
- Manatsathit, W.; Samant, H.; Kapur, S.; Ingviya, T.; Esmadi, M.; Wijarnpreecha, K.; Mccashland, T. Accuracy of liver stiffness, spleen stiffness, and LS-spleen diameter to platelet ratio score in detection of esophageal varices: Systemic review and meta-analysis. J Gastroenterol Hepatol. 2018, 33, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Dajti, E.; Ravaioli, F.; Alemanni L., V.; Capuano, F.; Gjini, K.; Colecchia, L.; Puppini, G.; Cusumano, C.; Renzulli, M.; et al. Spleen stiffness measurement for assessing the response to β-blockers therapy for high-risk esophageal varices patients. Hepatol Int. 2020, 14, 850–857. [Google Scholar] [CrossRef]
- Colecchia, A.; Colli, A.; Casazza, G.; Mandolesi, D.; Schiumerini, R.; Reggiani L., B.; Marasco, G.; Taddia, M.; Lisotti, A.; Mazzella, G.; et al. Spleen stiffness measurement can predict clinical complications in compensated HCV-related cirrhosis: a prospective study. J Hepatol. 2014, 60, 1158–1164. [Google Scholar] [CrossRef]
- Buechter, M.; Manka, P.; Theysohn J., M.; Reinboldt, M.; Canbay, A.; Kahraman, A. Spleen stiffness is positively correlated with HVPG and decreases significantly after TIPS implantation. Dig Liver Dis. 2018, 50, 54–60. [Google Scholar] [CrossRef]
- Ekinci, O.; Ozgokce, M.; Turko, E.; Merter, M. Spleen Stiffness Measurement by Using Shear-Wave Elastography as a Predictor of Progression to Secondary Myelofibrosis. Ultrasound Q. 2021, 37, 149–154. [Google Scholar] [CrossRef]
- Abraldes J., G.; Reverter, E.; Berzigotti, A. Spleen stiffness: toward a noninvasive portal sphygmomanometer? Hepatology. 2013, 57, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, E.; Server, S. The relationship of spleen stiffness value measured by shear wave elastography with age, gender, and spleen size in healthy volunteers. J Med Ultrason (2001). 2019, 46, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.; Merkel, C.; Sacerdoti, D.; Nava, V.; Gatta, A. Role of spleen enlargement in cirrhosis with portal hypertension. Dig Liver Dis. 2002, 34, 144–150. [Google Scholar] [CrossRef]
- Fraquelli, M.; Giunta, M.; Pozzi, R.; Rigamonti, C.; Della Valle, S.; Massironi, S.; Conti C., B.; Aghemo, A.; Ronchi, G.; Iurlo, A.; et al. Feasibility and reproducibility of spleen transient elastography and its role in combination with liver transient elastography for predicting the severity of chronic viral hepatitis. J Viral Hepat. 2014, 21, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Takuma, Y.; Nouso, K.; Morimoto, Y.; Tomokuni, J.; Sahara, A.; Toshikuni, N.; Takabatake, H.; Shimomura, H.; Doi, A.; Sakakibara, I.; et al. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013, 144, 92–101.e102. [Google Scholar] [CrossRef]
- Singh, R.; Wilson M., P.; Katlariwala, P.; Murad M., H.; Mcinnes M. D., F.; Low, G. Accuracy of liver and spleen stiffness on magnetic resonance elastography for detecting portal hypertension: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021, 32, 237–245. [Google Scholar] [CrossRef]
- Zykus, R.; Jonaitis, L.; Petrenkienė, V.; Pranculis, A.; Kupčinskas, L. Liver and spleen transient elastography predicts portal hypertension in patients with chronic liver disease: a prospective cohort study. BMC Gastroenterol. 2015, 15, 183. [Google Scholar] [CrossRef]
- Procopet, B.; Berzigotti, A.; Abraldes J., G.; Turon, F.; Hernandez-Gea, V.; García-Pagán J., C.; Bosch, J. Real-time shear-wave elastography: applicability, reliability and accuracy for clinically significant portal hypertension. J Hepatol. 2015, 62, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Calvaruso, V.; Di Marco, V.; Bronte, F.; Licata, G.; Simone, F.; Butera, G.; Pecoraro, G.; Cabibbi, D.; Alessi, N.; Cammà C. J. J. O., H. Spleen stiffness correlates with portal hypertension and increases the accuracy of detection of esophageal varices in HCV cirrhosis. J Hepatol. 2010, S159–S160. [Google Scholar] [CrossRef]
- Mannelli, L.; Godfrey, E.; Joubert, I.; Patterson A., J.; Graves M., J.; Gallagher F., A.; Lomas D., J. MR elastography: Spleen stiffness measurements in healthy volunteers--preliminary experience. AJR Am J Roentgenol. 2010, 195, 387–392. [Google Scholar] [CrossRef]
- Serai S., D.; Elsingergy M., M.; Hartung E., A.; Otero H., J. Liver and spleen volume and stiffness in patients post-Fontan procedure and patients with ARPKD compared to normal controls. Clin Imaging. 2022, 89, 147–154. [Google Scholar] [CrossRef]
- Talwalkar J., A.; Yin, M.; Venkatesh, S.; Rossman P., J.; Grimm R., C.; Manduca, A.; Romano, A.; Kamath P., S.; Ehman R., L. Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. AJR Am J Roentgenol. 2009, 193, 122–127. [Google Scholar] [CrossRef]
- Kassym, L.; Nounou M., A.; Zhumadilova, Z.; Dajani A., I.; Barkibayeva, N.; Myssayev, A.; Rakhypbekov, T.; Abuhammour A., M. New combined parameter of liver and splenic stiffness as determined by elastography in healthy volunteers. Saudi J Gastroenterol. 2016, 22, 324–330. [Google Scholar] [CrossRef]
- Bhatia, A.; Bhatia, H.; Saxena A., K.; Lal S., B.; Sodhi K., S. Shear wave elastography of the spleen using elastography point quantification: stiffness values in healthy children. Abdom Radiol (NY). 2022, 47, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, M.; Ochi, H.; Koizumi, Y.; Kisaka, Y.; Abe, M.; Ikeda, Y.; Matsuura, B.; Hiasa, Y.; Onji, M. Splenic elasticity measured with real-time tissue elastography is a marker of portal hypertension. Radiology. 2011, 261, 960–968. [Google Scholar] [CrossRef]
- Tanaka, H.; Iijima, H.; Nishimura, J.; Takashima, T.; Ishii, A.; Sakai, Y.; Iwata, K.; Ikeda, N.; Iwata, Y.; Enomoto H., editors. Could spleen stiffness measurement using Virtual Touch tissue quantification be a good predictor of the presence of varices, including large esophageal varices? Hepatology. NJ USA: Wiley-Blackwell, 2012.
- Yasar T., K.; Wagner, M.; Bane, O.; Besa, C.; Babb J., S.; Kannengiesser, S.; Fung, M.; Ehman R., L.; Taouli, B. Interplatform reproducibility of liver and spleen stiffness measured with MR elastography. J Magn Reson Imaging. 2016, 43, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Hines C., D.; Bley T., A.; Lindstrom M., J.; Reeder S., B. Repeatability of magnetic resonance elastography for quantification of hepatic stiffness. J Magn Reson Imaging. 2010, 31, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Bota, S.; Herkner, H.; Sporea, I.; Salzl, P.; Sirli, R.; Neghina A., M.; Peck-Radosavljevic, M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013, 33, 1138–1147. [Google Scholar] [CrossRef]
- Ferraioli, G.; Tinelli, C.; Lissandrin, R.; Zicchetti, M.; Bernuzzi, S.; Salvaneschi, L.; Filice, C. Ultrasound point shear wave elastography assessment of liver and spleen stiffness: effect of training on repeatability of measurements. Eur Radiol. 2014, 24, 1283–1289. [Google Scholar] [CrossRef]
- Singh, S.; Eaton J., E.; Murad M., H.; Tanaka, H.; Iijima, H.; Talwalkar J., A. Accuracy of spleen stiffness measurement in detection of esophageal varices in patients with chronic liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014, 12, 935–945.e934. [Google Scholar] [CrossRef]
- Shire N., J.; Yin, M.; Chen, J.; Railkar R., A.; Fox-Bosetti, S.; Johnson S., M.; Beals C., R.; Dardzinski B., J.; Sanderson S., O.; Talwalkar J., A.; et al. Test-retest repeatability of MR elastography for noninvasive liver fibrosis assessment in hepatitis C. J Magn Reson Imaging. 2011, 34, 947–955. [Google Scholar] [CrossRef]
- Morisaka, H.; Motosugi, U.; Ichikawa, S.; Sano, K.; Ichikawa, T.; Enomoto, N. Association of splenic MR elastographic findings with gastroesophageal varices in patients with chronic liver disease. J Magn Reson Imaging. 2015, 41, 117–124. [Google Scholar] [CrossRef]
- Reiter, R.; Tzschätzsch, H.; Schwahofer, F.; Haas, M.; Bayerl, C.; Muche, M.; Klatt, D.; Majumdar, S.; Uyanik, M.; Hamm, B.; et al. Diagnostic performance of tomoelastography of the liver and spleen for staging hepatic fibrosis. Eur Radiol. 2020, 30, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Hectors, S.; Bane, O.; Gordic, S.; Kennedy, P.; Besa, C.; Schiano T., D.; Thung, S.; Fischman, A.; Taouli, B. Noninvasive prediction of portal pressure with MR elastography and DCE-MRI of the liver and spleen: Preliminary results. J Magn Reson Imaging. 2018, 48, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Cho Y., S.; Lim, S.; Kim, Y.; Sohn J., H.; Jeong J., Y. Spleen Stiffness Measurement Using 2-Dimensional Shear Wave Elastography: The Predictors of Measurability and the Normal Spleen Stiffness Value. J Ultrasound Med. 2019, 38, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Lee M., J.; Kim M., J.; Han K., H.; Yoon C., S. Age-related changes in liver, kidney, and spleen stiffness in healthy children measured with acoustic radiation force impulse imaging. Eur J Radiol. 2013, 82, e290–294. [Google Scholar] [CrossRef] [PubMed]
- Arda, K.; Ciledag, N.; Aktas, E.; Aribas B., K.; Köse, K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol. 2011, 197, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, F.; Schmidberger, J.; Schlingeloff, P.; Binzberger, A.; Kratzer, W. Comparison of point and two-dimensional shear wave elastography of the spleen in healthy subjects. World J Radiol. 2021, 13, 137–148. [Google Scholar] [CrossRef]
- Amin, B.; Bowser B., L.; Robinson R. a., S. Quantitative proteomics to study aging in rabbit spleen tissues. Exp Gerontol. 2022, 167, 111908. [Google Scholar] [CrossRef]
- Cesta M., F. Normal Structure, Function, and Histology of the Spleen. Toxicol Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef]
- Alex, L.; Rajan M., L.; Xavier, B.; Jacob, P.; Rani K., D.; Lakshmi G., V. Microscopic study of human spleen in different age groups. Int J Res Med Sci. 2015, 1701–1706. [Google Scholar] [CrossRef]
- Losco, P. Normal development, growth, and aging of the spleen. Pathology of the Aging Rat. 1992, 75–94. [Google Scholar]
- Hogenesch, H.; Hahn, F. The lymphoid organs: anatomy, development, and age-related changes. Pathobiology of the Aging Dog. 2001, 1, 127e135. [Google Scholar]
- Madden K., S.; Bellinger D., L.; Felten S., Y.; Snyder, E.; Maida M., E.; Felten D., L. Alterations in sympathetic innervation of thymus and spleen in aged mice. Mech Ageing Dev. 1997, 94, 165–175. [Google Scholar] [CrossRef]
- Slagboom P., E.; De Leeuw W., J.; Vijg, J. Messenger RNA levels and methylation patterns of GAPDH and beta-actin genes in rat liver, spleen and brain in relation to aging. Mech Ageing Dev. 1990, 53, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Cheung H., T.; Nadakavukaren M., J. Age-dependent changes in the cellularity and ultrastructure of the spleen of Fischer F344 rats. Mech Ageing Dev. 1983, 22, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Rifai, K.; Sebagh, M.; Karam, V.; Saliba, F.; Azoulay, D.; Adam, R.; Castaing, D.; Bismuth, H.; Reynès, M.; Samuel, D.; et al. Donor age influences 10-year liver graft histology independently of hepatitis C virus infection. J Hepatol. 2004, 41, 446–453. [Google Scholar] [CrossRef]
- Stefanescu, H.; Grigorescu, M.; Lupsor, M.; Procopet, B.; Maniu, A.; Badea, R. Spleen stiffness measurement using Fibroscan for the noninvasive assessment of esophageal varices in liver cirrhosis patients. J Gastroenterol Hepatol. 2011, 26, 164–170. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Souza, F.; Muñoz, C.; Augustin, S.; Loo, N.; Deng, Y.; Ciarleglio, M.; Garcia-Tsao, G. Liver and Spleen Stiffness Measurements by Point Shear Wave Elastography via Acoustic Radiation Force Impulse: Intraobserver and Interobserver Variability and Predictors of Variability in a US Population. J Ultrasound Med. 2016, 35, 2373–2380. [Google Scholar] [CrossRef]
- Mannelli, L.; Godfrey, E.; Graves M., J.; Patterson A., J.; Beddy, P.; Bowden, D.; Joubert, I.; Priest A., N.; Lomas D., J. Magnetic resonance elastography: feasibility of liver stiffness measurements in healthy volunteers at 3T. Clin Radiol. 2012, 67, 258–262. [Google Scholar] [CrossRef]
- Dittmann, F.; Tzschätzsch, H.; Hirsch, S.; Barnhill, E.; Braun, J.; Sack, I.; Guo, J. Tomoelastography of the abdomen: Tissue mechanical properties of the liver, spleen, kidney, and pancreas from single MR elastography scans at different hydration states. Magn Reson Med. 2017, 78, 976–983. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




