Submitted:
12 October 2023
Posted:
13 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
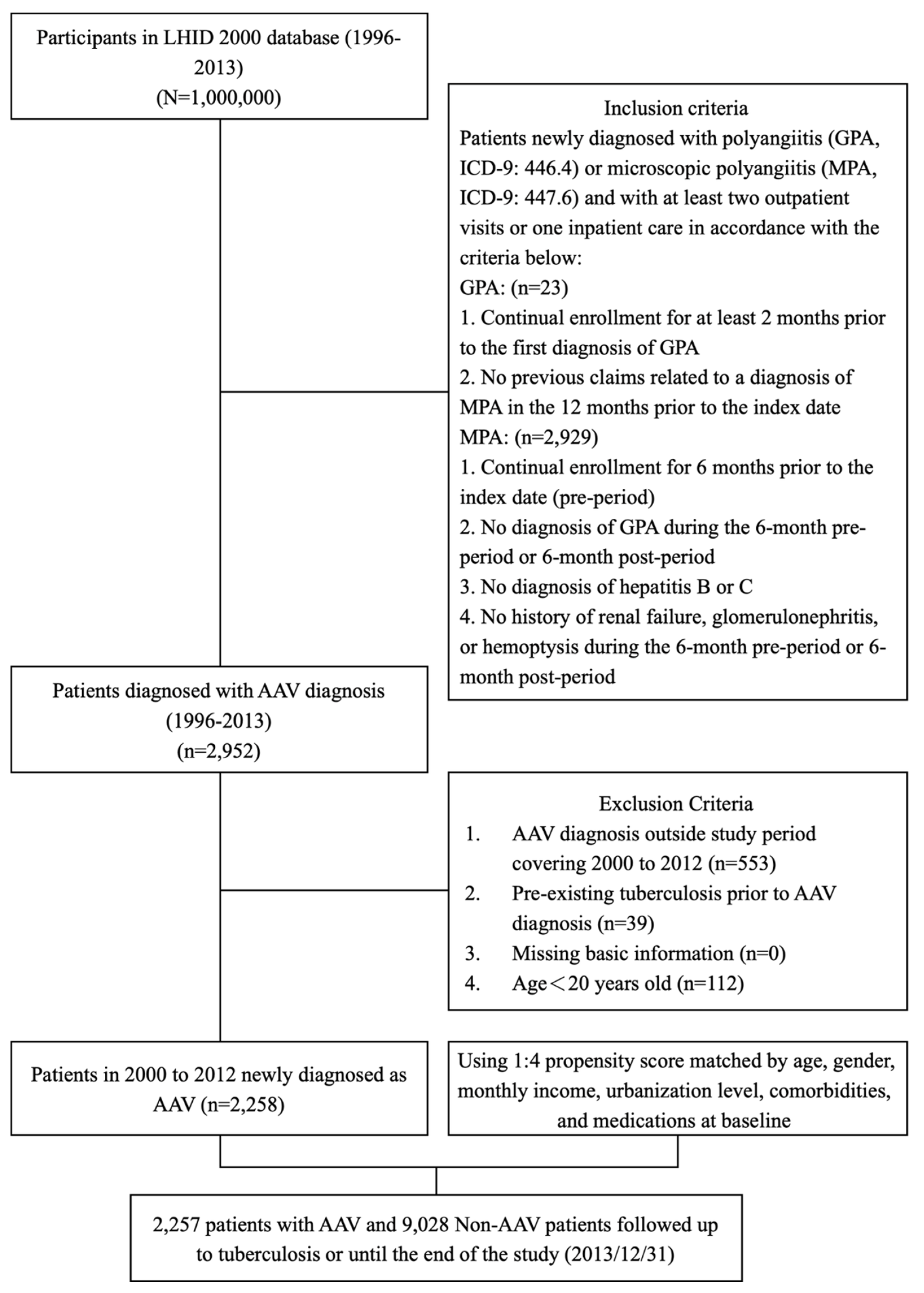
2. Materials and Methods
2.1. Data source
2.2. Study design
2.3. Study Population
2.4. Selection of patients with granulomatous polyangiitis
2.5. Selection of patients with microscopic polyangiitis
2.6. Selection of patients with incidental ANCA-associated vasculitis
2.7. Matched cohort selection
2.8. Variables and comorbidity
2.9. Outcome measures
2.10. Statistical analysis
3. Results
3.1. AAV cohort
3.2. Baseline demographic data
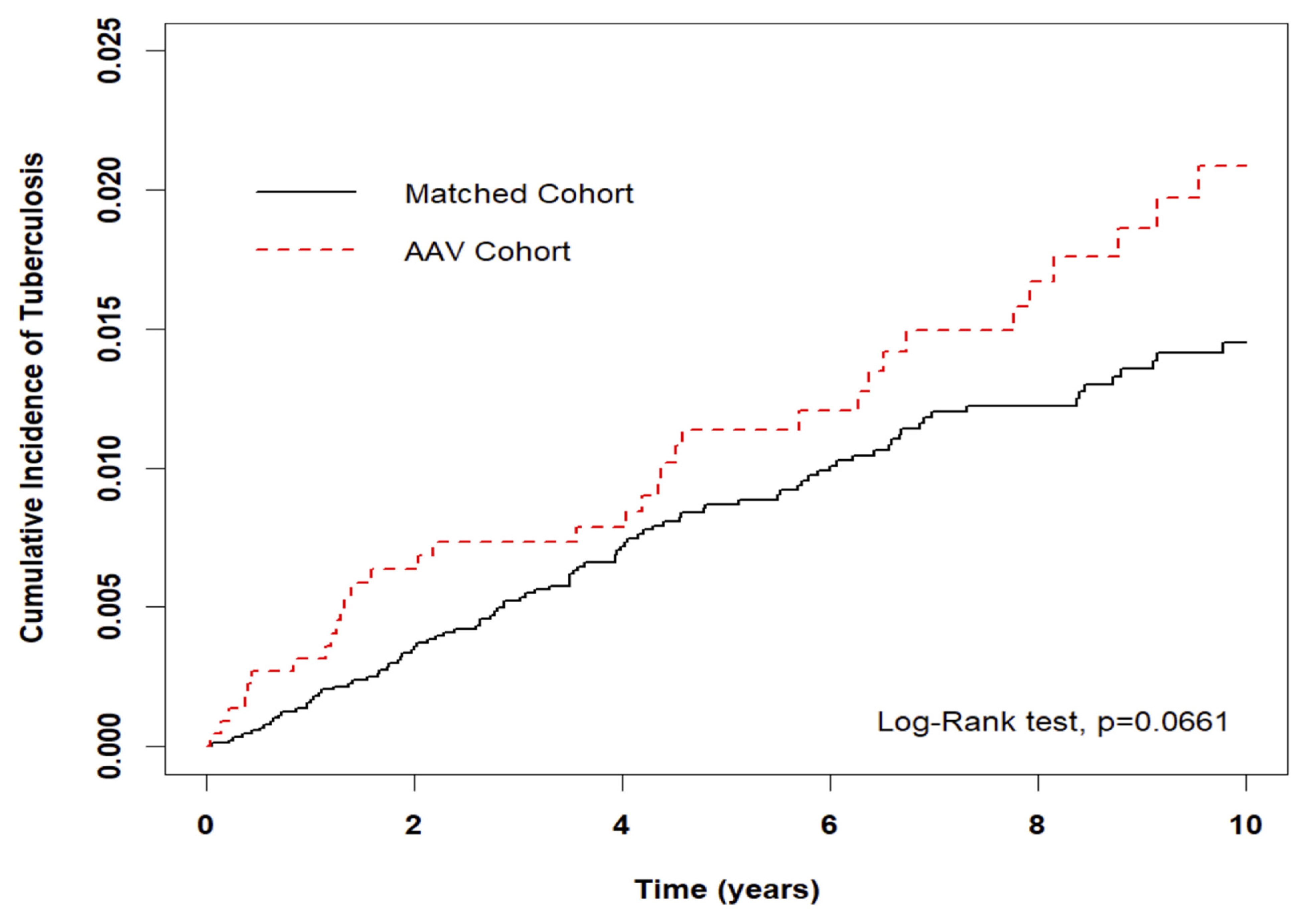
3.3. Risk of incidental TB in entire cohort
3.4. Risk of incidental TB: Stratification and interaction tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frieden TR, Brudney KF, Harries AD. Global tuberculosis: perspectives, prospects, and priorities. JAMA 2014;312:1393-4. 10.1001/jama.2014.11450.
- World Health Organization. Global tuberculosis report, 2013. WHO/HTM/TB/2013.11. Geneva, Switzerland: WHO, 2013.
- Taiwan Centers for Disease Control. Tuberculosis monitor data. [Internet. Accessed November 18, 2020.] Available from: https://daily.cdc.gov.tw/stoptb/Indicator.aspx.
- Flossmann O, Berden A, de Groot K, Hagen C, Harper L, Heijl C, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011;70:488-94. 10.1136/ard.2010.137778.
- McGregor JG, Negrete-Lopez R, Poulton CJ, Kidd JM, Katsanos SL, Goetz L, et al. Adverse events and infectious burden, microbes and temporal outline from immunosuppressive therapy in antineutrophil cytoplasmic antibody-associated vasculitis with native renal function. Nephrol Dial Transplant 2015;30 Suppl 1:i171-81. [CrossRef]
- Raimundo K, M. Farr A, Kim G, Duna G. Clinical and Economic Burden of Antineutrophil Cytoplasmic Antibody–associated Vasculitis in the United States. The Journal of Rheumatology 2015;42:2382-91. [CrossRef]
- D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-81. [CrossRef]
- Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-61. [CrossRef]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [CrossRef]
- Yang Y, Thumboo J, Tan BH, Tan TT, Fong CHJ, Ng HS, et al. The risk of tuberculosis in SLE patients from an Asian tertiary hospital. Rheumatol Int 2017;37:1027-33. [CrossRef]
- Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol 2011;4:288-93. [CrossRef]
- Xu Y, Xu H, Zhen Y, Sang X, Wu H, Hu C, et al. Imbalance of Circulatory T Follicular Helper and T Follicular Regulatory Cells in Patients with ANCA-Associated Vasculitis. Mediators Inflamm 2019;8421479. [CrossRef]
- Lai CC, Lee M-T G, Lee S-H, Lee S-H, Chang S-S, Lee C-C. Risk of incidental active tuberculosis and use of corticosteroids. Int J Tuberc Lung Dis 2015;19:936-42. [CrossRef]
- Brode SK, Jamieson FB, Ng R, Campitelli MA, Kwong JC, Paterson JM, et al. Increased risk of mycobacterial infections associated with anti-rheumatic medications. Thorax 2015;70:677-82. [CrossRef]
- Mohammad AJ, Segelmark M, Smith R, Englund M, Nilsson JA, Westman K, et al. Severe Infection in Antineutrophil Cytoplasmic Antibody-associated Vasculitis. The Journal of Rheumatology 2017;44:1468-75. [CrossRef]
- Nachman PH, Hogan SL, Jennette JC, Falk RJ. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996;7:33. [CrossRef]
- Reinhold-Keller E, Herlyn K, Wagner-Bastmeyer R, Gross WL. Stable incidence of primary systemic vasculitides over five years: results from the German vasculitis register. Arthritis Rheum 2005;53:93-99. [CrossRef]
- Gonzalez-Gay MA, Garcia-Porrua C. Systemic vasculitis in adults in northwestern Spain, 1988-1997. Clinical and epidemiologic aspects. Medicine 1999;78:292-308. [CrossRef]
- Watts RA, Lane SE, Bentham G, Scott DG. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum 2000;43:414-9. [CrossRef]
- Takala JH, Kautiainen H, Malmberg H, Leirisalo- Repo M. Incidence of Wegener’s granulomatosis in Finland 1981-2000. Clin Exp Rheumatol 2008;26:S81-5.
- Berti A, Cornec D, Crowson CS, Specks U, Matteson EL. The epidemiology of antineutrophil cytoplasmic autoantibody-associated vasculitis in Olmsted County, Minnesota: a twenty-year US population-based study. Arthritis Rheumatol 2017;69:2338-50. [CrossRef]
- Pierini FS, Scolnik M, Scaglioni V, Mollerach F, Soriano ER. Incidence and prevalence of granulomatosis with polyangiitis and microscopic polyangiitis in health management organization in Argentina: a 15-year study. Clin Rheumatol 2019;38: 1935-40. [CrossRef]
- National Health Research Institutes. 2019 Annual Report on Kidney Disease in Taiwan. Taiwan, June 2020. Page 198-203. ISBN 9789865439439, GPN 1010900805.
| Variable | AAV | Standardize Mean Difference (SMD)§ | ||
|---|---|---|---|---|
| Total | No | Yes | ||
| N=11,285 | n=9,028 | n=2,257 | ||
| n | n (%) / mean (SD) | n (%) / mean (SD) | ||
| Sex | 0.030 | |||
| Female | 6,028 | 4,795 (53.1) | 1,233 (54.6) | |
| Male | 5,257 | 4,233 (46.9) | 1,024 (45.4) | |
| Age at baseline (years) | 0.081 | |||
| <40 | 2,631 | 2,055 (22.8) | 576 (25.5) | |
| 40-65 | 5,261 | 4,202 (46.5) | 1,059 (46.9) | |
| ≧65 | 3,393 | 2,771 (30.7) | 622 (27.6) | |
| Mean (SD) ‡ | 54.6 (17.5) | 53.1 (17.0) | 0.086 | |
| Monthly Income (NT$) | 0.072 | |||
| 0-15,840 | 4,450 | 3,540 (39.2) | 910 (40.3) | |
| 15,841-28800 | 4,939 | 3,937 (43.6) | 1,002 (44.4) | |
| 28,801-45800 | 1,389 | 1,121 (12.4) | 268 (11.9) | |
| >45,800 | 507 | 430 (4.8) | 77 (3.4) | |
| Urbanization | 0.081 | |||
| 1 (highest) | 3,540 | 2,808 (31.1) | 732 (32.4) | |
| 2 | 3,315 | 2,693 (29.8) | 622 (27.6) | |
| 3 | 1,954 | 1,520 (16.8) | 434 (19.2) | |
| 4 | 2,476 | 2,007 (22.2) | 469 (20.8) | |
| CCI score | 0.058 | |||
| 0 | 8,840 | 7,074 (78.4) | 1,766 (78.2) | |
| 1 | 1,258 | 981 (10.9) | 277 (12.3) | |
| ≧2 | 1,187 | 973 (10.8) | 214 (9.5) | |
| Baseline comorbidity | ||||
| Diabetes | 2,396 | 1,948 (21.6) | 448 (19.8) | 0.043 |
| Hypertension | 5,031 | 4,072 (45.1) | 959 (42.5) | 0.053 |
| Hyperlipidemia | 2,882 | 2,351 (26) | 531 (23.5) | 0.058 |
| Atrial Fibrillation | 230 | 189 (2.1) | 41 (1.8) | 0.020 |
| Valvular heart disease | 720 | 580 (6.4) | 140 (6.2) | 0.009 |
| Parkinsonism | 139 | 110 (1.2) | 29 (1.3) | 0.006 |
| Autoimmune disease | 239 | 179 (2) | 60 (2.7) | 0.045 |
| Characteristics | No. of Events | Crude | Adjusted | |||
|---|---|---|---|---|---|---|
| (n=142) | HR (95% CI) | P value | HR (95% CI) | P value | ||
| AAV | ||||||
| No | 104 | Ref. | Ref. | |||
| Yes | 38 | 1.41(0.98-2.05) | 0.067 | 1.48(1.02-2.15) | 0.041 | |
| Sex | ||||||
| Female | 51 | Ref. | Ref. | |||
| Male | 91 | 2.11(1.50-2.98) | <0.001 | 2.49(1.70-3.63) | <0.001 | |
| Age at baseline (years) | ||||||
| <40 | 8 | Ref. | Ref. | |||
| 40-65 | 41 | 2.57(1.20-5.47) | 0.015 | 2.75(1.26-6.02) | 0.012 | |
| ≧65 | 93 | 11.26(5.47-23.19) | <0.001 | 9.10(4.12-20.06) | <0.001 | |
| Monthly Income (NT$) | ||||||
| 0-15,840 | 65 | Ref. | Ref. | |||
| 15,841-28,800 | 64 | 0.82(0.58-1.16) | 0.256 | 0.82(0.57-1.20) | 0.308 | |
| 28,801-45,800 | 11 | 0.49(0.26-0.93) | 0.029 | 0.67(0.35-1.31) | 0.243 | |
| >45,800 | 2 | 0.24(0.06-0.98) | 0.047 | 0.34(0.08-1.43) | 0.141 | |
| Urbanization | ||||||
| 1 (highest) | 40 | Ref. | Ref. | |||
| 2 | 34 | 0.92(0.58-1.46) | 0.728 | 0.86(0.55-1.37) | 0.536 | |
| 3 | 27 | 1.23(0.75-2.00) | 0.410 | 1.14(0.69-1.87) | 0.610 | |
| 4 | 41 | 1.49(0.97-2.31) | 0.072 | 1.07(0.67-1.71) | 0.763 | |
| CCI score | ||||||
| 0 | 87 | Ref. | Ref. | |||
| 1 | 24 | 2.27(1.45-3.58) | <0.001 | 1.24(0.77-2.01) | 0.377 | |
| ≧2 | 31 | 3.96(2.62-5.98) | <0.001 | 1.92(1.19-3.10) | 0.007 | |
| Baseline comorbidity | ||||||
| Diabetes | 40 | 1.68(1.16-2.42) | 0.006 | 1.14(0.70-1.86) | 0.600 | |
| Hypertension | 88 | 2.37(1.69-3.32) | <0.001 | 0.91(0.54-1.55) | 0.735 | |
| Hyperlipidemia | 31 | 0.97(0.65-1.45) | 0.901 | 0.68(0.42-1.11) | 0.122 | |
| Atrial Fibrillation | 1 | 0.53(0.07-3.82) | 0.531 | 0.21(0.03-1.57) | 0.129 | |
| Valvular heart disease | 8 | 1.16(0.57-2.37) | 0.684 | 0.74(0.35-1.54) | 0.420 | |
| Parkinsonism | 2 | 1.67(0.41-6.77) | 0.470 | 0.65(0.16-2.64) | 0.543 | |
| Autoimmune disease | 2 | 0.80(0.20-3.22) | 0.752 | 1.02(0.25-4.17) | 0.981 | |
| Variables | Matched Cohort | AAV Cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=9,028 | n=2,257 | Crude HR | P-value | Adjusted HR | P-value | P for interaction | |||||||
| Event | Person years | IR | Event | Person years | IR | (95% CI) | (95% CI) | ||||||
| Overall | 104 | 66,914 | 15.54 | 38 | 17,385 | 21.86 | 1.41(0.98-2.05) | 0.067 | 1.48(1.02-2.15) | 0.041 | |||
| Sex | 0.001 | ||||||||||||
| Female | 28 | 36,191 | 7.74 | 23 | 9,579 | 24.01 | 3.10(1.79-5.39) | <0.001 | 3.24(1.85-5.67) | <0.001 | |||
| Male | 76 | 30,722 | 24.74 | 15 | 7,806 | 19.22 | 0.78(0.45-1.36) | 0.385 | 0.78(0.45-1.36) | 0.384 | |||
| Age at baseline (years) | 0.707 | ||||||||||||
| <40 | 4 | 16,434 | 2.43 | 4 | 4,539 | 8.81 | 3.60(0.90-14.40) | 0.070 | 4.21(1.00-17.79) | 0.050 | |||
| 40-65 | 31 | 33,110 | 9.36 | 10 | 8,745 | 11.44 | 1.23(0.60-2.50) | 0.573 | 1.18(0.58-2.42) | 0.643 | |||
| ≧65 | 69 | 17,370 | 39.72 | 24 | 4,101 | 58.52 | 1.47(0.92-2.34) | 0.103 | 1.44(0.90-2.30) | 0.128 | |||
| CCI score | 0.597 | ||||||||||||
| 0 | 65 | 55,435 | 11.73 | 22 | 14,321 | 15.36 | 1.31(0.81-2.13) | 0.272 | 1.43(0.88-2.33) | 0.149 | |||
| 1 | 16 | 6,400 | 25.00 | 8 | 1,979 | 40.42 | 1.62(0.69-3.79) | 0.266 | 1.74(0.72-4.23) | 0.220 | |||
| ≧2 | 23 | 5,079 | 45.29 | 8 | 1,085 | 73.76 | 1.62(0.73-3.63) | 0.237 | 1.53(0.67-3.53) | 0.316 | |||
| Baseline comorbidity | |||||||||||||
| Diabetes | 0.514 | ||||||||||||
| No | 76 | 54,278 | 14.00 | 26 | 14,202 | 18.31 | 1.31(0.84-2.05) | 0.234 | 1.37(0.87-2.14) | 0.172 | |||
| Yes | 28 | 12,636 | 22.16 | 12 | 3,183 | 37.70 | 1.77(0.90-3.47) | 0.100 | 1.94(0.97-3.90) | 0.062 | |||
| Hypertension | 0.435 | ||||||||||||
| No | 41 | 39,553 | 10.37 | 13 | 10,602 | 12.26 | 1.18(0.63-2.21) | 0.599 | 1.22(0.65-2.29) | 0.538 | |||
| Yes | 63 | 27,361 | 23.03 | 25 | 6,783 | 36.86 | 1.62(1.02-2.58) | 0.041 | 1.75(1.10-2.79) | 0.019 | |||
| Hyperlipidemia | 0.286 | ||||||||||||
| No | 83 | 51,970 | 15.97 | 28 | 13,870 | 20.19 | 1.27(0.83-1.95) | 0.276 | 1.33(0.86-2.04) | 0.200 | |||
| Yes | 21 | 14,943 | 14.05 | 10 | 3,515 | 28.45 | 2.06(0.97-4.37) | 0.061 | 2.59(1.19-5.60) | 0.016 | |||
| Follow-up period, year | Matched Cohort | AAV Cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=9,076 | n=2,269 | Crude HR | P-value | Adjusted HR | P-value | |||||||
| Event | Person years | IR | Event | Person years | IR | (95% CI) | (95% CI) | |||||
| Years of follow-up | ||||||||||||
| <2 | 31 | 17,415 | 17.80 | 14 | 4,355 | 32.15 | 1.81(0.96-3.39) | 0.067 | 1.91(1.01-3.60) | 0.046 | ||
| 2-5 | 37 | 21,473 | 17.23 | 9 | 5,467 | 16.46 | 0.96(0.46-1.98) | 0.900 | 1.03(0.49-2.14) | 0.941 | ||
| >5 | 36 | 28,027 | 12.84 | 15 | 7,564 | 19.83 | 1.55(0.85-2.83) | 0.153 | 1.63(0.88-2.99) | 0.118 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





