Submitted:
09 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
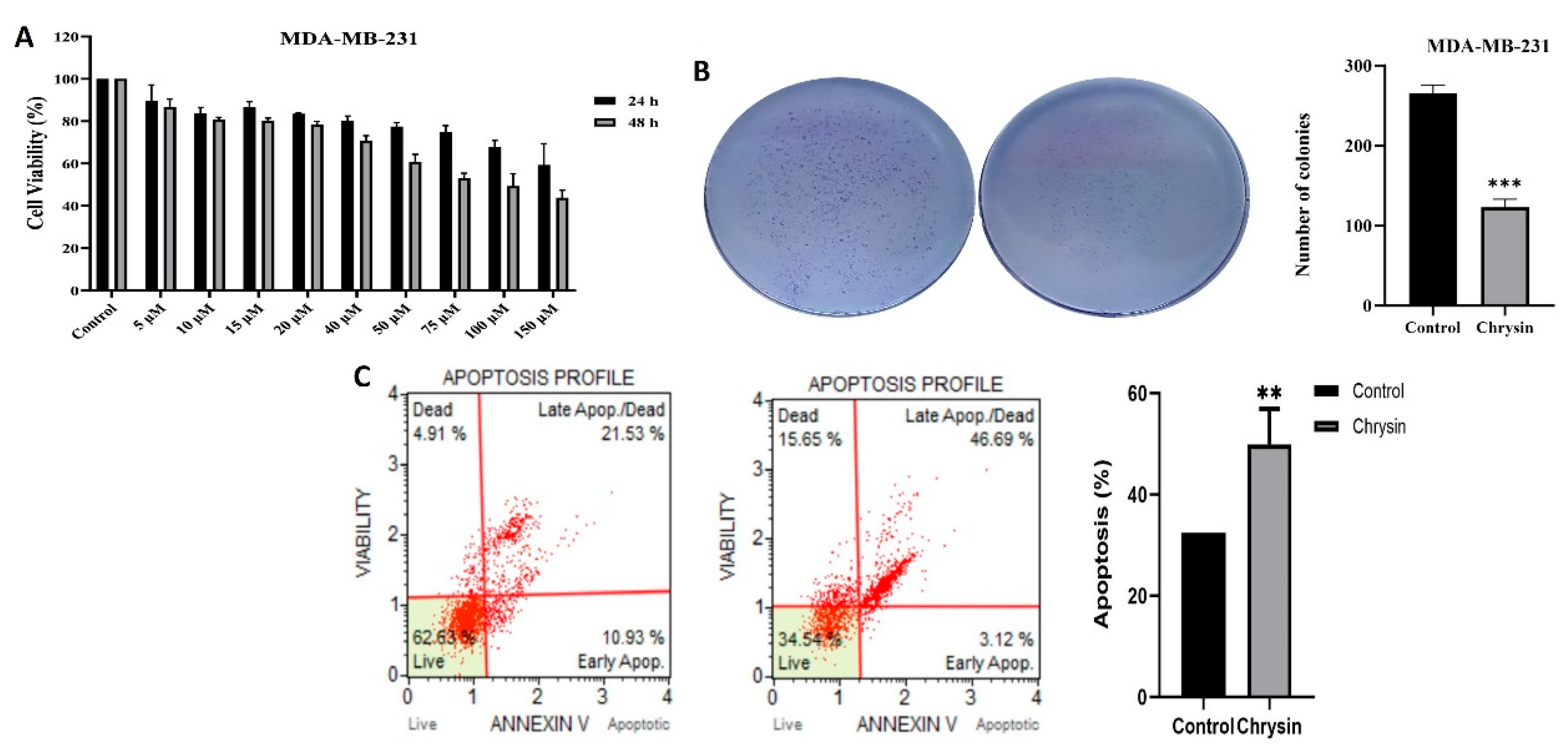
2.1. Chrysin inhibits the proliferation of MDA-MB-231 cells.
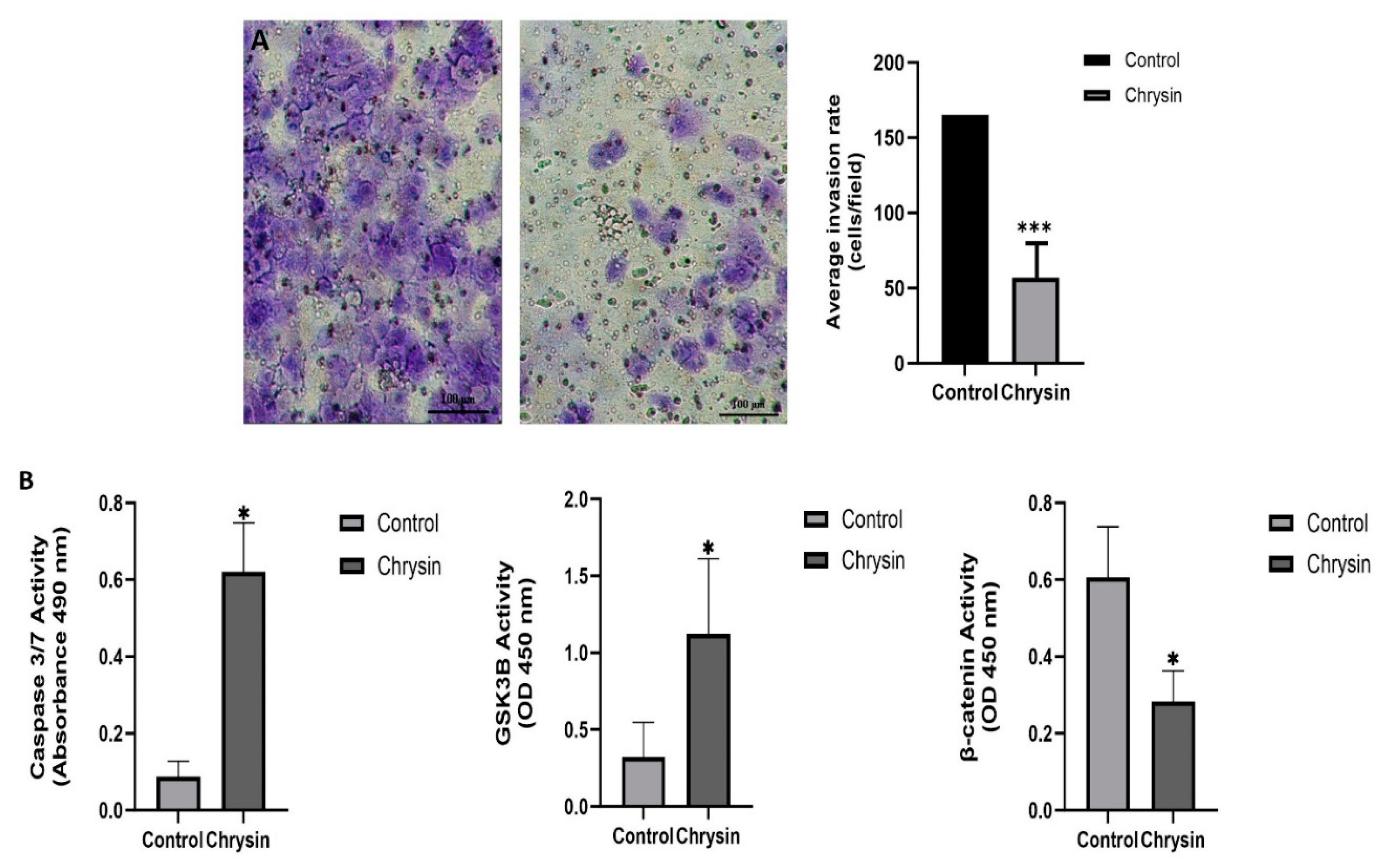
2.2. Chrysin reduced the invasive potential of MDA-MB-231 cells.
2.3. Chrysin induced apoptotic cell death by WNT/β-catenin pathway in MDA-MB-231 cells.
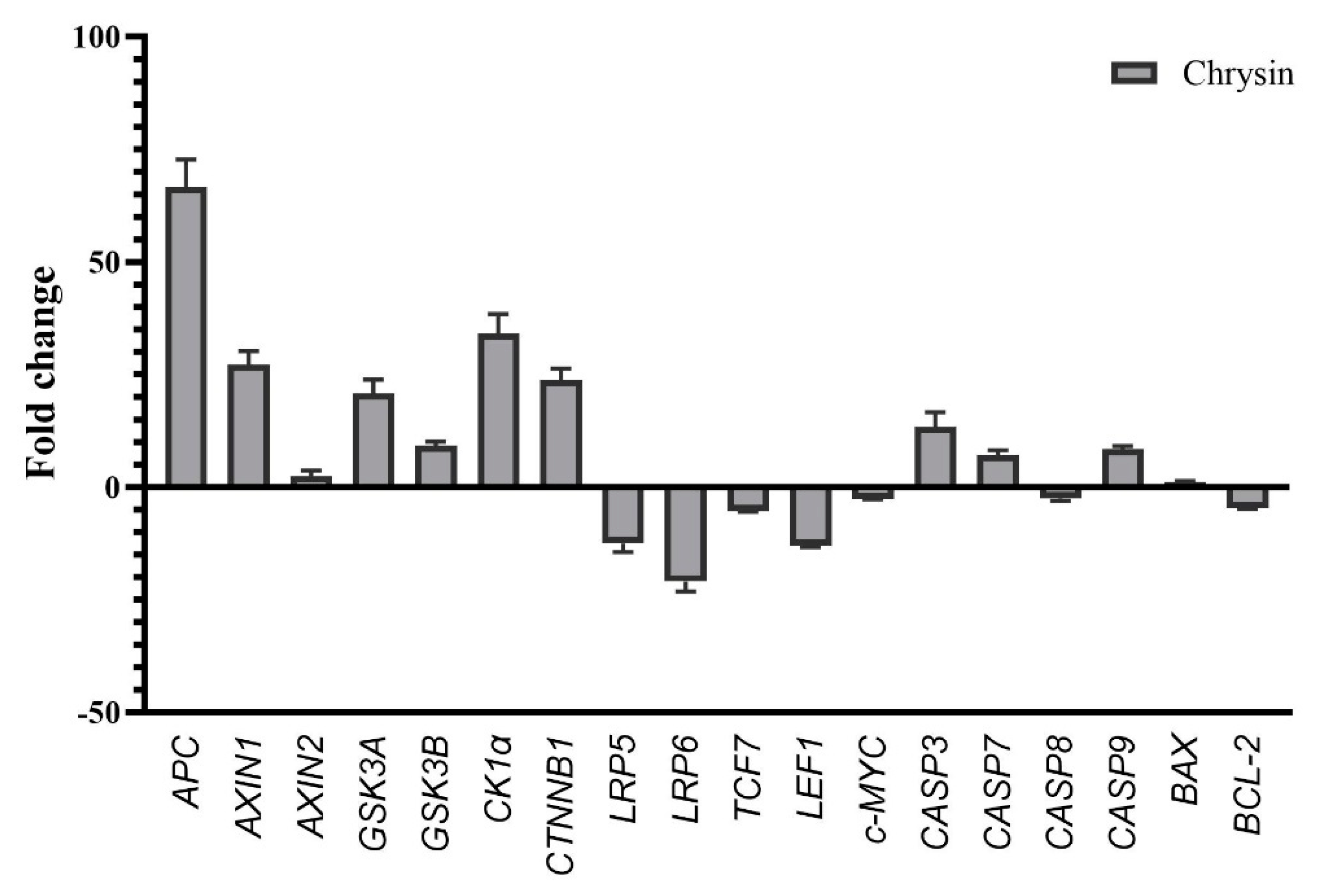
2.4. Chrysin effects the expression profiles of genes associated with apoptosis and the WNT/β-catenin pathway in MDA-MB-231 cells.
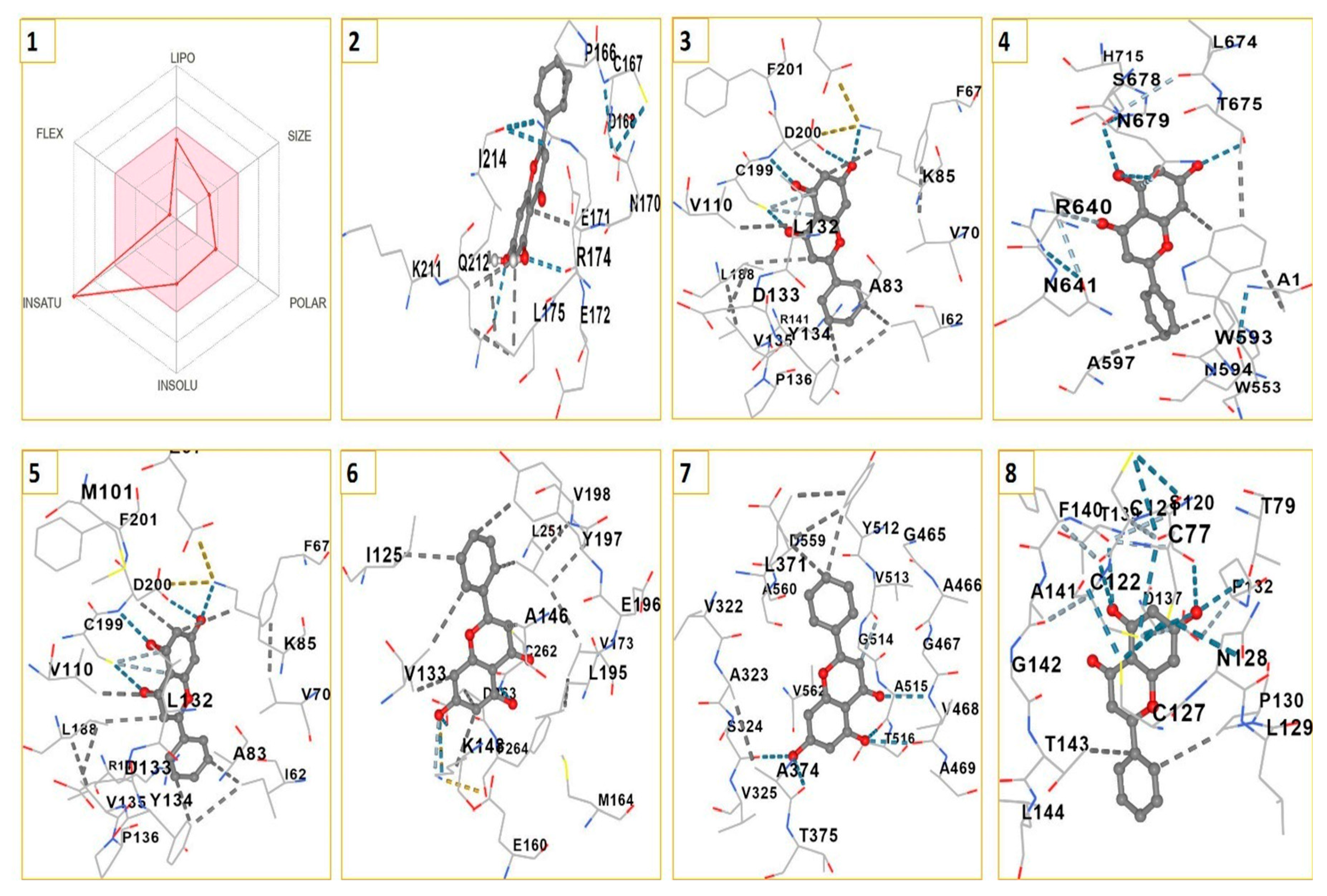
2.5. Computational ADME and molecular docking evaluations suggest a significant role for chrysin in the WNT/β-catenin signaling pathway.
3. Discussion
4. Materials and Methods
4.1. Cell culture and reagents
4.2. Cell proliferation assay
4.3. RNA isolation and real time quantitative PCR analysis (RT-qPCR)
4.4. Caspase 3/7 activation
4.5. Flow cytometry analysis
4.6. Cell colony formation assay
4.7. Cell invasion assay
4.8. GSK3B and β-katenin activity assay
4.8. Computational ADME and molecular docking evaluation of chrysin's role in the WNT/β-catenin signaling pathway
4.9. Statistical Analysis
5. Conclusion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. (2020). Breast cancer: Prevention and control.
- National Cancer Institute. (2021). Physician Data Query (PDQ). Breast Cancer Treatment – Health Professional Version. Accessed at https://www.cancer.gov/types/breast/hp/breast-treatment-pdq on August 10, 2021. 10 August.
- Shahbaz, M.; Naeem, H.; Imran, M.; Hassan, H.U.; Alsagaby, S.A.; Al Abdulmonem, W.; Waqar, A.B.; Ghorab, A.H.; Abdelgawad, M.A.; Ghoneim, M.M.; et al. Chrysin a promising anticancer agent: recent perspectives. Int. J. Food Prop. 2023, 26, 2294–2337. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Zanoli, P.; Avallone, R.; Baraldi, M. Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia 2000, 71, S117–S123. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Ali, N.; Rashid, S.; Jain, T.; Nafees, S.; Tahir, M.; Khan, A.Q.; Lateef, A.; Khan, R.; Hamiza, O.O.; et al. Alleviation of hepatic injury by chrysin in cisplatin administered rats: Probable role of oxidative and inflammatory markers. Pharmacol. Rep. 2014, 66, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; Antunes, M.S.; Filho, C.B.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.R.; Donato, F.; Prigol, M.; Boeira, S.P.; Jesse, C.R. Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav. 2015, 134, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Bharti, S.; Bhatia, J.; Nag, T.; Ray, R.; Arya, D.S. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem.-Biol. Interact. 2016, 250, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wadibhasme, P.G.; Ghaisas, M.M.; Thakurdesai, P.A. Anti-asthmatic potential of chrysin on ovalbumin-induced bronchoalveolar hyperresponsiveness in rats. Pharm. Biol. 2011, 49, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Filho, C.; Jesse, C.; Donato, F.; Giacomeli, R.; Del Fabbro, L.; Antunes, M.d.S.; de Gomes, M.; Goes, A.; Boeira, S.; Prigol, M.; et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+,K+-ATPase activity in the hippocampus and prefrontal cortex of mice: Antidepressant effect of chrysin. Neuroscience 2015, 289, 367–380. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Madana, R.M.; V, A.K.; Gogoi, R.; Barua, C.C. Chemopreventive and therapeutic potential of chrysin in cancer: mechanistic perspectives. Toxicol. Lett. 2015, 233, 214–225. [Google Scholar] [CrossRef]
- Garg, A.; Chaturvedi, S. A Comprehensive Review on Chrysin: Emphasis on Molecular Targets, Pharmacological Actions and Bio-pharmaceutical Aspects. Curr. Drug Targets 2022, 23, 420–436. [Google Scholar] [CrossRef]
- Khoo, B.Y.; Chua, S.L.; Balaram, P. Apoptotic Effects of Chrysin in Human Cancer Cell Lines. Int. J. Mol. Sci. 2010, 11, 2188–2199. [Google Scholar] [CrossRef]
- Yang, B.; Huang, J.; Xiang, T.; Yin, X.; Luo, X.; Huang, J.; Luo, F.; Li, H.; Li, H.; Ren, G. Chrysin inhibits metastatic potential of human triple-negative breast cancer cells by modulating matrix metalloproteinase-10, epithelial to mesenchymal transition, and PI3K/Akt signaling pathway. J. Appl. Toxicol. 2014, 34, 105–112. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Simal-Gandara, J.; Kopustinskiene, D.M.; Bernatoniene, J.; Samarghandian, S. Emerging cellular and molecular mechanisms underlying anticancer indications of chrysin. Cancer Cell Int. 2021, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- Sassi, A.; Fredon, M.; Cotte, A.K.; Fuselier, C.; Schneider, C.; Martiny, L.; Monchaud, D.; Chekir-Ghedira, L.; Aires, V.; Delmas, D. Chrysin-Induced Regression of Angiogenesis via an Induction of DNA Damage Response and Oxidative Stress in In Vitro and In Vivo Models of Melanoma. Cells 2023, 12, 1561. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Jung, J. Upregulation of G Protein-Coupled Estrogen Receptor by Chrysin-Nanoparticles Inhibits Tumor Proliferation and Metastasis in Triple Negative Breast Cancer Xenograft Model. Front. Endocrinol. 2020, 11, 560605. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Sun, K.; Wang, X.; Pan, H.; Zhu, J.; Ji, X.; Li, X. Chrysin suppresses proliferation, migration, and invasion in glioblastoma cell lines via mediating the ERK/Nrf2 signaling pathway. Drug Des. Dev. Ther. 2018, ume 12, 721–733. [Google Scholar] [CrossRef]
- Saleh, D.O.; El-Nasr, N.M.A.; Fayez, A.M.; Ahmed, K.A.; Mohamed, R.A. Uro-protective role of chrysin against cyclophosphamide-induced hemorrhagic cystitis in rats involving the turning-off NF-κB/P38-MAPK, NO/PARP-1 and STAT-3 signaling cascades. Chem. Interactions 2023, 382, 110585. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, E.R.; Ang, H.L.; Asnaf, S.E.; Zabolian, A.; Saleki, H.; Yavari, M.; Esmaeili, H.; Zarrabi, A.; Ashrafizadeh, M.; Kumar, A.P. Broad-Spectrum Preclinical Antitumor Activity of Chrysin: Current Trends and Future Perspectives. Biomolecules 2020, 10, 1374. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Guan, H.; Fang, L.; Yang, Y.; Zhu, X.; Yuan, J.; Wu, J.; Li, M. MicroRNA-374a activates Wnt/β-catenin signaling to promote breast cancer metastasis. J. Clin. Investig. 2013, 123, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fu, Q.; Li, S.; Li, J.; Liu, S.; Wang, Z.; Su, Z.; Song, J.; Lu, D. Emetine exhibits anticancer activity in breast cancer cells as an antagonist of Wnt/β-catenin signaling. Oncol. Rep. 2019, 42, 1735–1744. [Google Scholar] [CrossRef]
- Hong, T.B.; Rahumatullah, A.; Yogarajah, T.; Ahmad, M.; Yin, K.B. Potential Effects of Chrysin on MDA-MB-231 Cells. Int. J. Mol. Sci. 2010, 11, 1057–1069. [Google Scholar] [CrossRef]
- Parajuli, P.; Joshee, N.; Rimando, A.M.; Mittal, S.; Yadav, A.K. In vitroAntitumor Mechanisms of VariousScutellariaExtracts and Constituent Flavonoids. Planta Medica 2009, 75, 41–48. [Google Scholar] [CrossRef]
- Sun, L.-P.; Chen, A.-L.; Hung, H.-C.; Chien, Y.-H.; Huang, J.-S.; Huang, C.-Y.; Chen, Y.-W.; Chen, C.-N. Chrysin: A Histone Deacetylase 8 Inhibitor with Anticancer Activity and a Suitable Candidate for the Standardization of Chinese Propolis. J. Agric. Food Chem. 2012, 60, 11748–11758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, X.; Qu, L.; Wu, J.; Cui, R.; Zhao, Y. Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells. Bioorganic Med. Chem. 2004, 12, 6097–6105. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics 2011, 66, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tong, Y.; Ying, J.; Lei, Z.; Wan, L.; Zhu, X.; Ye, F.; Mao, P.; Wu, X.; Pan, R.; et al. Chrysin induces cell growth arrest, apoptosis, and ER stress and inhibits the activation of STAT3 through the generation of ROS in bladder cancer cells. Oncol. Lett. 2018, 15, 9117–9125. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.-H.; Lee, J.-H.; Han, S.-H.; Woo, J.-S.; Choi, E.-Y.; Jeon, S.-J.; Han, E.-J.; Jung, S.-H.; Park, Y.-S.; Park, B.-K.; et al. Chrysin Induces Apoptosis via the MAPK Pathway and Regulates ERK/mTOR-Mediated Autophagy in MC-3 Cells. Int. J. Mol. Sci. 2022, 23, 15747. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.-H.; Wang, Z.-J. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol. Vitr. 2009, 23, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.H.; Zafaryab; Nafees, S. ; Khan, A.; Ahmad, I.; Bin Hafeez, Z.; Alam Rizvi, M. Chrysin Sensitizes Human Lung Cancer Cells to Tumour Necrosis Factor Related Apoptosis-Inducing Ligand (TRAIL) Mediated Apoptosis. Asian Pac. J. Cancer Biol. 2019, 4, 27–33. [Google Scholar] [CrossRef]
- Al-Oudat, B.A.; Alqudah, M.A.; Audat, S.A.; Al-Balas, Q.A.; El-Elimat, T.; Hassan, M.A.; Frhat, I.N.; Azaizeh, M.M. Design, synthesis, and biologic evaluation of novel chrysin derivatives as cytotoxic agents and caspase-3/7 activators. Drug Des. Dev. Ther. 2019, ume 13, 423–433. [Google Scholar] [CrossRef]
- King, T.D.; Suto, M.J.; Li, Y. The wnt/β-catenin signaling pathway: A potential therapeutic target in the treatment of triple negative breast cancer. J. Cell. Biochem. 2012, 113, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gangrade, A.; Pathak, V.; Augelli-Szafran, C.E.; Wei, H.-X.; Oliver, P.; Suto, M.; Buchsbaum, D.J. Preferential Inhibition of Wnt/β-Catenin Signaling by Novel Benzimidazole Compounds in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2018, 19, 1524. [Google Scholar] [CrossRef]
- Khan, M.S.; Halagowder, D.; Devaraj, S.N. Methylated chrysin induces co-ordinated attenuation of the canonical Wnt and NF-kB signaling pathway and upregulates apoptotic gene expression in the early hepatocarcinogenesis rat model. Chem. Interactions 2011, 193, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ayan, I. .; Güçlü, E.; Vural, H.; Dursun, H.G. Piceatannol induces apoptotic cell death through activation of caspase-dependent pathway and upregulation of ROS-mediated mitochondrial dysfunction in pancreatic cancer cells. Mol. Biol. Rep. 2022, 49, 11947–11957. [Google Scholar] [CrossRef] [PubMed]
| Gene | Gene Primer sequence | |
|---|---|---|
| CASP3 | F | GAGCCATGGTGAAGAAGGAATA |
| R | TCAATGCCACAGTCCAGTTC | |
| CASP7 | F | CGAAACGGAACAGACAAAGATG |
| R | TTAAGAGGATGCAGGCGAAG | |
| CASP8 | F | GCCCAAACTTCACAGCATTAG |
| R | GTGGTCCATGAGTTGGTAGATT | |
| CASP9 | F | CGACCTGACTGCCAAGAAA |
| R | CATCCATCTGTGCCGTAGAC | |
| BAX | F | GGAGCTGCAGAGGATGATTG |
| R | GGCCTTGAGCACCAGTTT | |
| BCL2 | F | GTGGATGACTGAGTACCTGAAC |
| R | GAGACAGCCAGGAGAAATCAA | |
| APC | F | TGGTTGGCACTCTTACTTACC |
| R | GCCTGTGGTCCTCATTTGTA | |
| AXIN1 | F | GAGGTATGTGCAGGAGGTTATG |
| R | TCCTCTGCGATCTTGTCTCT | |
| AXIN2 | F | CTTATCGTGTGGGCAGTAAGA |
| R | GTTCTCGGGAAATGAGGTAGAG | |
| GSK3A | F | CCTGGACAAAGGTGTTCAAATC |
| R | CAGACATCGCAGTTCATCAAAG | |
| GSK3B | F | GAGAGCTCCAGATCATGAGAAAG |
| R | GAACATAGTCCAGCACCAGATTA | |
| CK1α | F | GGTATTGGGCGTCACTGTAATA |
| R | GAGAAAGATGGGTCCTGAGAAG | |
| CTNNB1 | F | CTTCACCTGACAGATCCAAGTC |
| R | CCTTCCATCCCTTCCTGTTTAG | |
| LRP5 | F | CGTACAGGCCCTACATCATTC |
| R | GTCCGAGTTCAAATCCAGGTAG | |
| LRP6 | F | CCGAGTCAGAACCTGGAAATAC |
| R | CTCCAACTGATCTCCCATCTAATC | |
| DVL1 | F | GACTCATCCGGAAGCACAAA |
| R | GACATGGTGGAGTCGGTTATG | |
| DVL2 | F | CCTTCTCTGAGCAGTGCTATTAC |
| R | GCAGGGTATTGGTAGGAGAAAG | |
| TCF7 | F | ACTCTTCCCGGACAAACTTC |
| R | CAGATTGAAGGCGGAGTAGAC | |
| LEF1 | F | GATCACACCCGTCACACATC |
| R | ACCCGGAGACAAGGGATAAA | |
| GAPDH | F | TGAACGGGAAGCTCACTGG |
| R | TCCACCACCCTGTTGCTGTA | |
| ACTN | F | TGTGAACAAAGCGCTGGA |
| R | ATGGCGAACCTAAGGATGATG | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




