1. Introduction
Laparotomy is an invasive procedure frequently performed in horses. It is associated with substantial somatic pain and visceral hyperalgesia necessitating early and aggressive treatment. Early treatment of pain results in improved recovery and reduced hospitalization time [
1]. Discomfort in those patients can increase the risk of development of postoperative colic. Persistent pain after surgery can result in sympathetic overstimulation which can lead to altered gastrointestinal motility [
2,
3,
4,
5]. Systemic analgesics like opioids and alpha-2 adrenoceptor agonists are commonly employed to treat pain, although they can cause side effects like cardiovascular depression or gastrointestinal transit delay and ileus [
6]. In this context, the inclusion of locoregional techniques could be beneficial to provide effective analgesia while reducing systemic side effects.
Locoregional techniques have gained popularity in both human and veterinary medicine to treat perioperative pain [
7]. The rectus sheath block was first described in people by Schleich in 1899 [
8] and it was initially used for abdominal wall muscle relaxation and analgesia during midline laparotomy by blocking the terminal branches of the thoracolumbar nerves. It is indicated for laparoscopic surgery, peri-umbilical incisions, umbilical and paraumbilical hernia repair, abdominoplasty, and open radical cystectomy [
9]. In the equine neonate, pathologies involving the umbilical structures are an important cause of morbidity and surgical removal of the urachal remnants is a common procedure in clinical practice [
10]. The immaturity of the different organ systems of the neonatal foal makes them more susceptible to the pharmacologic side effects of systemic drugs [
11]. This makes the inclusion of locoregional technics into balanced anaesthetic protocols interesting in order to reduce the consumption of opioids and inhalant anaesthetic agents as reported in human medicine [
12].
In horses, the lateral and ventral abdominal wall is innervated by the ventral branches of the 10th to 18th thoracic (Th) spinal nerves. They run within the fascial plane between the
m. transversus abdominis and the
m. obliquus internus abdominis. In the most ventral aspect of the abdomen at the level of the internal rectus sheath, they pierce the internal face of the
m. rectus abdominis and innervate the muscle and skin in the vicinity of the
linea alba [
13]. They carry motor fibers for the
m. rectus abdominis, sensory fibers for the parietal peritoneum, and close to the
linea alba give rise to the short ventral cutaneous branches [
14]. Interfascial blocks to provide abdominal wall analgesia have been described in cadaveric studies in horses [
15]. The rectus sheath block has been described in cadaveric studies in dogs, pigs and calves with promising results [
7,
16,
17,
18]. A study evaluated a two-point rectus sheath block in the middle point between the xyphoid process and the umbilicus. The authors reported a significant increase in mechanical nociceptive threshold in adult horses [
19]. To the author´s knowledge, the literature evaluating rectus sheath block in the equines is scarce and there is no cadaveric study describing the distribution after periumbilical injection in foals.
The aims of this study were to describe a two-point bilateral ultrasound guided rectus sheath block at the level of the umbilicus and to evaluate the extension of the dye distribution in foal carcasses.
2. Materials and Methods
Foal carcasses were obtained from the Clinic for horses of the University of Veterinary Medicine Hannover Foundation and were euthanized for reasons unrelated to the study. Informed written consent from the owners was obtained for all animals. No animal use protocol or ethical approval was required for the use of foal cadavers according to the University policies.
The study was divided into two parts. Part I involved an examination of the anatomy of the abdominal wall, with a specific focus on the ventral aspect and structures closely related to the rectus sheath muscle and the terminal branches of the intercostal nerves (nn. intercostales, rr. cutanei laterales). For this purpose, one foal cadaver weighing 40 kg was used. Part II consisted of performance and evaluation of the ultrasound-guided rectus sheath block in fresh foal cadavers.
The foal was positioned in lateral recumbency, and a meticulous dissection of the lateral and ventral abdominal wall was conducted. The skin, mm. cutaneous trunci, mm obliquus externus, and mm. obliquus internus were retracted to expose the surface of the mm. transversus abdominis, mm. rectus abdominis, and the ventral branches of the last thoracic and first lumbar spinal nerves. The anatomical evaluation and dissection were performed by a veterinary anatomist (RK), with the veterinary anaesthetist (AG) who performed the blocks in Part II.
Ten fresh foal cadavers with an average weight of 37 ± 5 kg (mean ± SD) were included in the study. The number of animals included was based on a previous study performed in foal cadavers [
15]. These animals were euthanized for causes unrelated to the study. The study was conducted on the same day of the euthanasia or withing a maximum of 24 hours thereafter. In cases where the study was delayed, the cadaver was stored in a cold room. Inclusion criteria included foals with no history of trauma and/or abdominal surgery and immediate availability for the study after euthanasia.
Four injections were performed on each abdomen (one cranial and one caudal to the umbilicus, on each hemiabdomen), with a volume of injectate of 0.25 mL kg-1 in each one (total of 1 mL kg-1 for animal). The injectate consisted of a mixture of 1/5 iodate contrast medium (Imeron® 300 mg mL-1, Bracco Imaging Deutschland GmbH) + 4/5 of blue dye (Tissue Marking Dyes, Blue. Cancer Diagnostics, Inc.). The original dye was diluted (1 mL of dye in 250 mL of NaCl 0,9%) to cause no marked changes in viscosity of the injectate. The determination of the injectate volume was based on preliminary data from a study (unpublished) conducted on cadavers not included in the results.
The cadavers were placed in dorsal recumbency, and their limbs were secured with ropes to stabilize them. The area around the umbilicus was clipped, leaving a 15 cm margin in the cranial, caudal, and bilateral directions. A linear 12 MHz ultrasound probe (Ultrasound Transducer 12L-RS, GE Medical Systems, China Co., Ltd.) connected to an ultrasound machine (Logic V2; GE Medical Systems, China Co., Ltd.) was used. Initially, the linear array ultrasound transducer was positioned in a transverse orientation just cranial to the umbilicus to identify the rest of the urachus, linea alba, and transversal window of the mm. rectus abdominis. Subsequently, the transducer was advanced 5 cm cranially and then laterally until the lateral border of the mm. rectus abdominis, which had a triangular shape, was located. Two hyperechoic railway-like lines, deep within it, were identified as the posterior rectus sheath and fascia transversalis (6). The injection point was determined to be between the rectus abdominis and the posterior sheath, positioned at the lateral third of the muscle (immediately before the nerves puncture and enter the muscle, as described in Part I). A 22-gauge, 75 mm Quincke needle (Spinal needle Quincke type point, BD S.A., Madrid, Spain), attached to an extension and a syringe pre-filled with the solution, was inserted in a medio-ventral to latero-dorsal direction with a 30-degree angle to the probe, using an "in-plane" approach. When the needle tip was believed to be in the target point, a small aliquot of the solution was injected to create a "pocket" in the interfascial plane, and subsequently, the rest of the volume was administered to cause hydro dissection of the plane. If the aliquot was not in the correct place, repositioning of the needle was performed. This process was repeated as described 5 cm caudal to the umbilicus and in the contralateral hemiabdomen (Pictures 1 and 2 in Supplementary material).
The classification below described in a similar study in dog’s cadavers [
7] was employed:
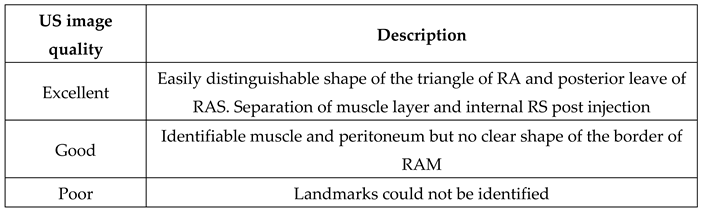
Table 1.
Evaluation of the ultrasound image quality during the realization of the block.
Table 1.
Evaluation of the ultrasound image quality during the realization of the block.
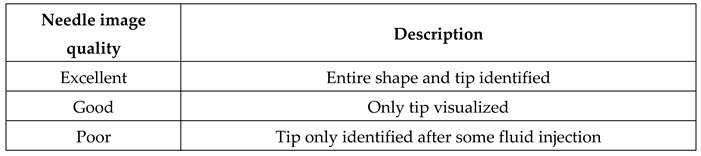
Table 2.
Evaluation of the needle visualization during the realization of the block.
Table 2.
Evaluation of the needle visualization during the realization of the block.
After the injection, a computed tomography (CT) scan of the abdomen was performed and evaluated by a different operator (FJS). Image acquisition for all the cadavers was conducted using a 16-slice multiple-detector CT scanner (BrillianceTM CT, Philips Medical Systems, Best, The Netherlands). The foals were placed in dorsal recumbency. The CT images were acquired using helical acquisition, with a slice thickness of 0.9 mm, a helical pitch of 0.567, a tube current of 354 mAs, a tube potential of 140 kVp, a matrix size of 1024x1024, and a reconstruction algorithm for both soft tissue and bone. The images were reviewed using a bone window (window level 300, window width 2800). The following CT features were documented: intraperitoneal and intramuscular contrast contamination, the relative cranio-caudal distribution of the contrast (in centimeters per milliliter of injectate), and its relationship with the last thoracic, lumbar, and sacral vertebrae.
Subsequently, the foals were transported to a dissection room and positioned in lateral recumbency. To maintain the anatomical reference, the umbilicus was marked with a metal clamp. A skin incision was made with a semicircular shape from the xiphoid, along the costal arch to the iliac crest. The incision was then extended directly to the ventral abdominal midline. A meticulous dissection followed, involving the layers of the abdominal wall in the following sequence: cutis, mm. cutaneus, mm. obliquus externus, and mm. obliquus internus. The mm. rectus abdominis was then isolated from mm. transversus abdominis, exposing the thoracic nerves as they descended over mm. transversus abdominis and identifying the point where they entered the internal face of mm. rectus abdominis. The first nerve caudal to the umbilicus was traced to its origin in the intervertebral foramen for accurate identification. Nerve staining was classified using a previously employed scale (Table 3) [7], and the intraabdominal location of the dye was also documented. Results are presented as frequency (n/n) and percentage (%) of nerves stained from total of nerves identified.
Table 3.
Evaluation of the nerve staining.
Table 3.
Evaluation of the nerve staining.
3. Results
3.1. Ultrasound injection and CT scan
The quality of the ultrasound image was excellent in all cases, with clear visibility of anatomy and reference points. Needle visualization was excellent in all cases except one, where it was calssified as good. The CT scan results are detailed in
Table 4.
Figure 1 illustrates the distribution of the contrast in relation to the lumbar and sacral vertebrae. Unfortunately, the CT scan results for the second animal (RS2) had to be excluded due to technical issues with the scanner.
3.2. Dissection
The
Table 5 describes the degree and frequency of staining of the nerves of interest examined in the dissection. Picture 3 in supplementary material shows the dissection and identification of the stained nerves in one foal.
The location of the umbilicus in relation to spinal nerves is shown in Table 3
4. Discussion
This is the first study that describes a four-points periumbilical ultrasound guided rectus sheath block in foals with a total volume of 1 mL kg
-1. The aim was to evaluate the extend of dye and to quantify the number of spinal nerves reached. The high percentage of stained nerves around the area of interest suggests that the technique could provide effective pain relief for umbilical procedures. Clear sonographic images of the anatomy and the needle were obtained during the injection. The hydro-dissection was observed during the administration of the stain, confirming the right placement of the needle, similar to the results obtained by other authors [
17,
19].
In a systematic review and meta-analysis in 2014, epidural analgesia was considered gold-standard technic in human medicine for abdominal surgery, due to the strong analgesic effect on the corresponding dermatomes and to the reduction in mortality and morbidity outcomes[
20]. In veterinary medicine, epidural or intrathecal anaesthesia is also performed for abdominal procedures especially in small animals [
21]. In equine patients epidural anaesthesia can be performed similarly, however potential important complications like hypotension, postoperative ataxia or muscle weakness can be fatal in equine patients. This makes the quest for alternative peripheral locoregional analgesic methods even more important [
22].
There are several cadaveric studies evaluating locoregional techniques for the abdominal wall in horses. Baldo et al 2018 [
23] performed a one point injection transversus abdominis plane (TAP) block in the midpoint between iliac crest and the most caudal extent of the last rib, injecting 0.5 mL kg
-1 per hemiabdomen. Although the technique successfully reached some of the ventral branches of the nerves involved in the innervation of the abdominal wall (mostly Th16, Th17 and Th18), a dorsal spread of the dye was observed with involvement of major psoas and quadratus lumborum muscles. This could lead to femoral nerve blockade and complications during recovery from general anaesthesia [
23]. This complication has already been reported in human medicine [
24] and it should be noted that the consequences in equine medicine can be much more serious. Approaching the spinal nerves in a more distal position, like in the rectus sheath, eliminate this risk as shown in our results. The authors suggested to add an additional injection site as described in other species to improve the spreading and reach additional cranial nerves [
25]. To achieve this, we decided to use a two points approach, but our results regarding the spread of the injectate are comparable to the ones obtained by Baldo et al. 2018. On the other hand, the aim of this study was to reach the nerves involved in procedures involving the umbilical structures. The nerves more frequently stained were those involved in the innervation of such structures (Th16, Th17 and Th18). Similarly, Küls et al. in 2020 published a modified three-point TAP block in cadavers and in life ponies, successfully desensitizing dermatomes of the lateral and ventral abdominal wall from Th8 to Th18 with a total volume of 0,3 mL kg
-1 per hemiabdomen [
6]. Even though with this approach the authors managed to desensitize the area involved in an exploratory laparotomy, we decided to apply a two-point technique to provide analgesia for umbilical surgeries, with a smaller area desensitized to avoid a third injection with its potential complications.
Foals are commonly presented for surgery of the umbilical area due to pathologies like umbilical remnant infection or umbilical hernia repair [
10,
26]. Administration of analgesic drugs before application of a nociceptive stimulus reduces postoperative pain and analgesic requirements, and may decrease the incidence of peripheral and central sensitisation [
27]. In humans, superior pain relief and faster postoperative recovery is achieved when pre-emptive regional anaesthetics are employed in comparison with opioid therapy [
28]. Infiltration of local anaesthetics around the surgical area is a commonly employed technique for perioperative pain management [
29]. However, the use of US-guided techniques presents several advantages when compared to blind techniques: visualization of targeted area (nerve or fascial plane), need for smaller volumes of local anaesthetics because of proximity to the nerve, avoiding puncture of neural and vascular structures and organ damage [
33]. Rectus sheath block is used to provide effective pain relief for umbilical hernia repair in human paediatric surgery [
11] and it has been shown to be superior compared to local anaesthetic infiltration for postoperative analgesia in children undergoing umbilical hernia repair [
12].
There are some studies evaluating rectus sheath block in veterinary medicine. Ienello et al in 2022, compared two volumes of injectate (low volume 0.5 mL kg
-1, high volume 0.8 mL kg
-1) administered cranially to the umbilicus in miniature swine [
13]. The results showed that the injectate had spread mostly cranially, leaving the nerves caudal to the umbilicus free of stain. In contrast to our study, that used two injection points (cranial und caudal to the umbilicus), where we could see nerves staining in both directions. A study performed in dogs showed greater nerve staining when using a high volume (0.5 mL kg
-1) compared to a low volume (0.25 mL kg
-1) [
14]. According to these results and due to a lack of a cadaveric study in foals evaluating volumes of spread, we decided to use 0,5 mL kg
-1 per hemiabdomen divided in two injection points.
Ishikawa et al in 2023 published a study evaluating a rectus sheath block first in two cadavers and afterwards in six healthy standing horses [
19]. Although they used the same volume as in our study, they reported a higher proportion of nerves stained in the two cadavers (Th9 to L2) as opposed to our results (Th13 to L1). They also noticed that the dispersion of the injectate was limited to the rectus sheath, while in our study it reached the transversus abdominis muscle. This could be explained by several reasons. The injection point they employed was much more cranially that in our study, so more ventral branches of the thoracic spinal nerves could be reached. Second, the injectate in their study remained in the rectus sheath, therefore a greater cranio-caudally dispersion of the same volume of injectate could be achieved. Third, they included only adult horses in their study, which differ substantially in soft tissues composition, stiffness, tension, and anatomical conformation from neonatal foals investigated in our study.
Freitag et al in 2021 evaluated the influence of recumbency (lateral or dorsal) in an US-guided subcostal transversus abdominis plane block [
15]. Analyzing the materials and methods in depth, the authors performed the injection of the contrast between the rectus abdominis muscle and the transversus abdominis. This approach is very similar to that performed in our study unless that both injection points were done cranially to the umbilicus instead of cranially and caudally. The number of nerves stained in the dorsal recumbency group was very similar to our results, with the difference that they did not stain the most caudal nerves (Th18 and L1) as frequent as in the approach of the present study. That could be explained, as they mentioned in the discussion, because most of the nerves of the abdominal wall are situated in the area between the xyphoid cartilage and the umbilicus. Nevertheless, to provide complete analgesia of the periumbilical area, the nerves involved in the innervation of the most caudal part of the abdominal wall need to be reached (TH18 and L1) as shown in our results. The volume of the injectate used by Freitag and colleagues was much smaller compared to our study; nevertheless, the total number of nerves reached was similar. The explanation we have found is, as mentioned earlier, that they performed the block where most of the ventral branches of the thoracic spinal nerves are closely situated to each other.
As the internal layer of the rectus sheath is very thin in neonates, intraperitoneal injection can be possible. In our study intraperitoneal dye was founded in 4/10 animals. As soon as we realised the high incidence of peritoneal injection, the injection technique was slightly modified. Administration of the injectate was started before the desired point was reached while advancing the needle, so that a “pocket” was created, and intraperitoneal injection was prevented. Nevertheless, under direct visualization, the probability of accidental organ puncture is low. There is a study comparing blind versus US-guided locoregional anaesthesia in cows that reported organ (renal and spleen) contamination with contrast media in the blind technique[
16]. For this reason, and as previously mentioned, the authors prefer the US-guided overthe blind technique [
17]. It has to be considered that cadavers may suffer from autolytic process. The anatomy could be altered, affecting the distribution of the injectate, and facilitating the contamination of the abdominal cavity.
One limitation of this study is the small number of cadavers employed. The number of animals included depended on the cadavers available during the time of the study. On the other hand, descriptive studies about locoregional techniques have usually employed a similar sample size[
7,
34]. The evaluation of only one volume of spread is also a limitation of the current study. A higher volume could result in a wider spread and consequently, a greater number of nerves stained. We used this volume based on preliminary result of pre-trials and considering that this volume could be used in the clinical setting, bearing in mind the total dose of local anaesthetics and not to alter the anatomy of the surgical area. Another limitation is that injectate distribution in cadavers can differ from in vivo due to changes in muscle tension, temperature, absorption, motion, and blood and lymphatic flow. Finally, local anesthetics have a lower viscosity than the contrast agent used in the study, which may result in reduced distribution of the latter.
5. Conclusions
The described US-guided RSB in foals is an easy and reliable “in plane” technique. The distribution of contrast in this study suggest that the block can provide analgesia for surgical procedures concerning the periumbilical area. Additionally, this approach avoids dorsal distribution of injectate to the proximity of the femoral nerve, which could cause severe complications. However, further studies are necessary to evaluate different volumes of injectate, the extent of the block in living animals as well as investigation of clinical efficacy.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Picture 1, 2 and 3.
Author Contributions
Conceptualization, A.J.G.B., F.J.S., R.K., methodology, A.J.G.B., F.J.S.,S.K., investigation, A.J.G.B., F.J.S., data curation A.J.G.B., writing-original draft preparation, A.J.G.B., writing review F.J.S., S.K., J.V.M., supervision S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive external fundings.
Institutional Review Board Statement
Foal carcasses included in the study were euthanised/dead for causes unrelated to the study. No ethical approval was required for this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to acknowledge the stuff of the anatomy department of the Veterinary University of Hannover for the support and the help in the dissection and the preliminary anatomic evaluation. We also thank the team of the Horse Hospital of the University of Hannover for their help in recruitment of cadavers and handling.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Graubner, C.; Gerber, V.; Doherr, M.; Spadavecchia, C. Clinical Application and Reliability of a Post Abdominal Surgery Pain Assessment Scale (PASPAS) in Horses. The Veterinary Journal 2011, 188, 178–183. [CrossRef]
- Muir, W.W. Pain: Mechanisms and Management in Horses. Veterinary Clinics of North America: Equine Practice 2010, 26, 467–480. [CrossRef]
- Robertson, S.A.; Sanchez, L.C. Treatment of Visceral Pain in Horses. Veterinary Clinics of North America: Equine Practice 2010, 26, 603–617. [CrossRef]
- Koenig, J.; Cote, N. Equine Gastrointestinal Motility — Ileus and Pharmacological Modification. Can Vet J 2006, 47, 551–559.
- Lisowski, Z.M.; Pirie, R.S.; Blikslager, A.T.; Lefebvre, D.; Hume, D.A.; Hudson, N.P.H. An Update on Equine Post-Operative Ileus: Definitions, Pathophysiology and Management. Equine Veterinary Journal 2018, 50, 292–303. [CrossRef]
- Küls, N.; Trujanovic, R.; Otero, P.E.; Larenza-Menzies, M.P. Ultrasound-Guided Transversus Abdominis Plane Block in Shetland Ponies: A Description of a Three-Point Injection Technique and Evaluation of Potential Analgesic Effects. Journal of Equine Veterinary Science 2020, 90, 102994. [CrossRef]
- St James, M.; Ferreira, T.H.; Schroeder, C.A.; Hershberger-Braker, K.L.; Schroeder, K.M. Ultrasound-Guided Rectus Sheath Block: An Anatomic Study in Dog Cadavers. Vet Anaesth Analg 2020, 47, 95–102. [CrossRef]
- Schleich, C.L. Schmerzlose Operationen; 5th ed.; Springer, Berlin, Heidelberg, 1906; ISBN 978-3-642-50470-9.
- Ferguson, S.; Thomas, V.; Lewis, I. The Rectus Sheath Block in Paediatric Anaesthesia: New Indications for an Old Technique? Pediatric Anesthesia 1996, 6, 463–466. [CrossRef]
- Reig Codina, L.; Werre, S.R.; Brown, J.A. Short-Term Outcome and Risk Factors for Post-Operative Complications Following Umbilical Resection in 82 Foals (2004-2016). Equine Vet J 2019, 51, 323–328. [CrossRef]
- Fischer, B.; Clark-Price, S. Anesthesia of the Equine Neonate in Health and Disease. Veterinary Clinics of North America: Equine Practice 2015, 31, 567–585. [CrossRef]
- Jakobsson, J.; Wickerts, L.; Forsberg, S.; Ledin, G. Transversus Abdominal Plane (TAP) Block for Postoperative Pain Management: A Review. F1000Res 2015, 4, F1000 Faculty Rev-1359. [CrossRef]
- Ellenberger; Baum Handbuch der vergleichenden Anatomie der Haustiere; Zietzschmann, O., Ackerknecht, E., Grau, H., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 1974; ISBN 978-3-642-80833-3.
- Budras, K.D.; Sack, W.O.; Rock, S.; Berg, R. Anatomy of the Horse; 6th ed.; Schluetersche: London, 2012; ISBN 978-0-429-06836-2.
- Freitag, F.A.V.; Amora, D.D.S.; Muehlbauer, E.; Dornbusch, P.T.; Machado, M.; Montiani-Ferreira, F.; Prisco Farias, E.L.; Valverde, A.; Duque Moreno, J.C. Ultrasound-Guided Modified Subcostal Transversus Abdominis Plane Block and Influence of Recumbency Position on Dye Spread in Equine Cadavers. Vet Anaesth Analg 2021, 48, 596–602. [CrossRef]
- Calice, I.; Kau, S.; Knecht, C.; Trujanovic, R.; Auer, U. Ultrasound-Guided Rectus Sheath Injection in Pig Cadavers – a Pilot Study. Veterinary Anaesthesia and Analgesia 2021, 48, S985–S986. [CrossRef]
- Ferreira, T.H.; Schroeder, C.A.; James, M.S.; Hershberger-Braker, K.L. Description of an Ultrasound-Guided Rectus Sheath Block Injection Technique and the Spread of Dye in Calf Cadavers. Veterinary Anaesthesia and Analgesia 2021, 0. [CrossRef]
- Ienello, L.; Kennedy, M.; Wendt-Hornickle, E.; Baldo, C.; Moshnikova, V.; Guedes, A. Ultrasound-Guided Rectus Sheath Block Injections in Miniature Swine Cadavers: Technique Description and Distribution of Two Injectate Volumes. Veterinary Anaesthesia and Analgesia 2022, 49, 210–218. [CrossRef]
- Ishikawa, Y.; Sakai, D.M.; Im, J.S.; Zhang, S.; Reed, R.A.; Quandt, J.E.; Baldo, C.F.; Walters, B.; Barletta, M. Antinociceptive Effects of Bupivacaine Injected within the Internal Abdominis Rectus Sheath in Standing Healthy Horses. Veterinary Anaesthesia and Analgesia 2023, 50, 294–301. [CrossRef]
- Pöpping, D.M.; Elia, N.; Van Aken, H.K.; Marret, E.; Schug, S.A.; Kranke, P.; Wenk, M.; Tramèr, M.R. Impact of Epidural Analgesia on Mortality and Morbidity After Surgery: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Annals of Surgery 2014, 259, 1056. [CrossRef]
- Lardone, E.; Sarotti, D.; Giacobino, D.; Ferraris, E.; Franci, P. Thoracic Epidural Anaesthesia vs Intrathecal Morphine in Dogs Undergoing Major Thoracic and Abdominal Surgery: Clinical Study. BMC Vet Res 2022, 18, 200. [CrossRef]
- Doherty, T.; Valverde, A. Epidural Analgesia and Anesthesiea. In Manual of Equine Anesthesia & Analgesia; Blackwell Publishing, 2006; pp. 275–281.
- Baldo, C.F.; Almeida, D.; Wendt-Hornickle, E.; Guedes, A. Transversus Abdominis Plane Block in Ponies: A Preliminary Anatomical Study. Vet Anaesth Analg 2018, 45, 392–396. [CrossRef]
- Salaria, O.N.; Kannan, M.; Kerner, B.; Goldman, H. A Rare Complication of a TAP Block Performed after Caesarean Delivery. Case Rep Anesthesiol 2017, 2017, 1072576. [CrossRef]
- Otero, P.E.; Romano, M.; Zaccagnini, A.S.; Fuensalida, S.E.; Verdier, N.; Sanchez, F.; Portela, D.A. Transversus Abdominis Plane Block in Cat Cadavers: Anatomical Description and Comparison of Injectate Spread Using Two- and Three-Point Approaches. Vet Anaesth Analg 2021.
- Enzerink, E.; van Weeren, P.R.; van der Velden, M.A. Closure of the Abdominal Wall at the Umbilicus and the Development of Umbilical Hernias in a Group of Foals from Birth to 11 Months of Age. Vet Rec 2000, 147, 37–39. [CrossRef]
- Woolf, C.J.; Chong, M.-S. Preemptive Analgesia—Treating Postoperative Pain by Preventing the Establishment of Central Sensitization. Anesthesia & Analgesia 1993, 77, 362–379.
- Capdevila, X.; Barthelet, Y.; Biboulet, P.; Ryckwaert, Y.; Rubenovitch, J.; d’Athis, F. Effects of Perioperative Analgesic Technique on the Surgical Outcome and Duration of Rehabilitation after Major Knee Surgery. Anesthesiology 1999, 91, 8–15. [CrossRef]
- Stamenkovic, D.M.; Bezmarevic, M.; Bojic, S.; Unic-Stojanovic, D.; Stojkovic, D.; Slavkovic, D.Z.; Bancevic, V.; Maric, N.; Karanikolas, M. Updates on Wound Infiltration Use for Postoperative Pain Management: A Narrative Review. J Clin Med 2021, 10, 4659. [CrossRef]
- Willschke, H.; Bösenberg, A.; Marhofer, P.; Johnston, S.; Kettner, S.C.; Wanzel, O.; Kapral, S. Ultrasonography-Guided Rectus Sheath Block in Paediatric Anaesthesia–a New Approach to an Old Technique. Br J Anaesth 2006, 97, 244–249. [CrossRef]
- Dingeman, R.S.; Barus, L.M.; Chung, H.K.; Clendenin, D.J.; Lee, C.S.; Tracy, S.; Johnson, V.M.; Dennett, K.V.; Zurakowski, D.; Chen, C. Ultrasonography-Guided Bilateral Rectus Sheath Block vs Local Anesthetic Infiltration After Pediatric Umbilical Hernia Repair: A Prospective Randomized Clinical Trial. JAMA Surgery 2013, 148, 707–713. [CrossRef]
- D’Anselme, O.; Hartnack, A.; Andrade, J.S.S.; Alfaro Rojas, C.; Ringer, S.K.; de Carvalho Papa, P. Description of an Ultrasound-Guided Erector Spinae Plane Block and Comparison to a Blind Proximal Paravertebral Nerve Block in Cows: A Cadaveric Study. Animals (Basel) 2022, 12, 2191. [CrossRef]
- Sites, B.D.; Brull, R. Ultrasound Guidance in Peripheral Regional Anesthesia: Philosophy, Evidence-Based Medicine, and Techniques. Current Opinion in Anesthesiology 2006, 19, 630. [CrossRef]
- Serra, R.M.; Jimenez, C.P.; Monticelli, P.; Plested, M.; Viscasillas, J. Assessment of an Ultrasound-Guided Technique for Catheterization of the Caudal Thoracic Paravertebral Space in Dog Cadavers. Open Vet J 2019, 9, 230–237. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).