Submitted:
09 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Methylglyoxal in (patho)physiology
1.1. Endogenous sources of MGO
1.2. Exogenous sources of MGO
1.3. MGO-modification of macromolecules
1.3.1. MGO-derived AGEs (MAGEs)
1.3.2. MGO-derived DNA modifications
1.4. MGO scavenging system
2. MGO and MAGEs in metabolic syndrome and diabetes
2.1. Metabolic syndrome
2.2. MGO and MAGEs in metabolic syndrome and diabetes in animal models and cell cultures
| Experimental model | MGO/MAGEs and associated major findings | Ref./year |
|---|---|---|
| Spontaneously hypertensive rats (SHR) and Wistar Kyoto rats (WKY) | In comparison with normal WKY, in SHR: higher MGO level in blood plasma and kidney (increasing with age), higher CML and CEL staining in the kidney, decreased GSH and GSH/GSSG ratio in the kidney of the oldest 20-week rats. |
[93]/2004 |
| Spontaneously hypertensive rats (SHR) and Wistar Kyoto rats (WKY) | In comparison with normal WKY, in SHR: higher MGO level in blood plasma and aorta (increasing with age), higher MGO level in the liver and kidney (but not in the heart) in 13-week rats, higher CML and CEL staining in the aorta (mostly in endothelial cells, lower in smooth muscle cells), increased oxidative stress (superoxide anion and hydrogen peroxide) in 13-week rats aortas decreased GSH in 13-week rats’ aortas, decreased activities of glutathione peroxidase and reductase in 13-week rats’ aortas, increased activity of SSAO in blood plasma, no difference in blood plasma GSH. |
[94]/2005 |
| aminoguanidine treated SHR, untreated SHR, and WKY rats | In comparison with untreated SHR, in aminoguanidine treated SHR: Attenuation of systolic blood pressure, Correction of MGO level in blood plasma and aorta (raised in untreated SHR) to the level comparable with WKY, Correction of AP and CEL levels in the aorta and mesenteric artery (raised in untreated SHR) to the level comparable with WKY, Attenuation of oxidative stress in aortic tissue (decreased superoxide anion and nitric oxide, increased GSH), Correction of nitric oxide synthases expression in aortic tissue (decrease in iNOS and increase in eNOS) to the level comparable with WKY, Improvement of morphologic changes and endothelium-dependent relaxation in mesenteric artery. |
[95]/2007 |
| Fructose-fed SD rats, Wistar–Kyoto (WKY) rats, SHR rats, and lean, obese, and diabetic Zucker rats |
In aortas of fructose-fed SD rats: increase in MGO and fructose; upregulation of GLUT-5, fructokinase and aldolase B (at mRNA levels). In SHR rats (as compared to WKY control): in the serum: similar Glc, increase in MGO, fructose and insulin; in aortas: increase in MGO and fructose; upregulation of GLUT-5, fructokinase and aldolase B (at mRNA levels). In obese and/or diabetic Zucker rats (as compared to lean Zucker rats control): in the serum: increase in Glc, MGO and fructose increase in insulin in obese rats, but decrease in insulin in diabetic rats. in aortas: increase in MGO and fructose; upregulation of GLUT-5, fructokinase and aldolase B (at mRNA levels); increase in aldose reductase and sorbitol in diabetic rats. |
[88]/2011 |
| aminoguanidine treated SHR, untreated SHR, and WKY rats | In comparison with untreated SHR, in aminoguanidine treated SHR: Attenuation of blood pressure, Correction of AP and CEL levels in the mesenteric artery (raised in untreated SHR) to the level comparable with WKY, Correction of Ang II-induced contraction of the mesenteric artery, Normalization of endothelium-dependent (ACh-induced) relaxation impaired in SHR mesenteric artery, Attenuation of oxidative stress in mesenteric artery, downregulation of NOX1 (but not NOX2) and AT2R expression (upregulated in SHR) in mesenteric artery, no changes in eNOS and SOD-(1-3) Effect of NOX inhibitor on mesenteric artery from SHR: enhanced ACh-induced relaxation, but no effect on Ang II-induced contraction |
[96]/2012 |
| Mesenteric artery isolated from Wistar rats | Upon long-term MGO treatment (42 µM for 3 days): impairment of endothelium-dependent (ACh-induced) relaxation, no effect on endothelium-independent (SNP-induced) relaxation, decrease in ACh-induced: NO production and VASP phosphorylation, increase in apoptosis of endothelial cells associated with superoxide radical elevation, Upon long-term MGO treatment (42 µM for 3 days) and a NOX inhibitor: reversal of MGO-impaired endothelium-dependent (ACh-induced) relaxation, reversal of MGO-caused eNOS downregulation. |
[97]/2013 |
| Endothelium-denuded thoracic aorta and superior mesenteric artery isolated from Wistar rats | Upon MGO treatment (420 µM for 30 min): Inhibition of noradrenaline-induced contraction of aorta and mesenteric artery, in aorta prevented by a large conductance Ca2+-activated K+ (BKCa)-channel inhibitor. |
[98]/2009 |
| Wistar rats infused with MGO, or treated with MGO and retinoic acid (RA). | Upon MGO treatment: increase in CML in blood plasma; In heart tissue: decrease in catalase, SOD and GSH; increase in cardiac fibrosis; up-regulated expression of RAGE (3.5 fold), TGF-β (4.4 fold), SMAD2 (3.7 fold), SMAD3 (6.0 fold), IL-6 (4.3 fold) and TNF-α (5.5 fold). Attenuation of the above effects by RA co-treatment. |
[99]/2017 |
| Lactating Wistar rats treated by gavage with MGO and they adult male offspring | Upon MGO treatment in mother rats: In blood plasma: increase in Glc and fructosamine; decrease in insulin and the functionality of pancreatic β-cells; increase in total cholesterol, triglycerides, cholesterol (LDL and VLDL); decrease in HDL cholesterol. In breast milk: increase in Glc, TAG, cholesterol, fructosamine, decrease in insulin. In the offspring: increase in body weight and adipose tissue; increase in Glc, insulin and fructosamine; decrease in the functionality of pancreatic β-cells; increase in total plasma cholesterol and LDL and VLDL cholesterol, TAG; decrease in HDL cholesterol. |
[87]/2018 |
| Sprague Dawley (SD) rats: untreated, fructose treated, N-acetyl cysteine (NAC)-treated, fructose+ NAC treated. | In comparison with untreated SD, in Fru-treated SD rats: Increase in blood pressure, increase in blood serum TAG (attenuated by NAC co-treatment), increase in blood serum insulin (attenuated by NAC co-treatment),, decrease in insulin-induced Glc uptake by visceral adipose tissue (improved by NAC co-treatment), increase in MGO in the adipose tissue and serum (attenuated by NAC co-treatment), increase in PI3K protein in the adipose tissue (counteracted by NAC co-treatment), decreased PI3K recruitment to phosphorylated IRS-1 (restored by NAC co-treatment), no changes in IR, IRS-1 expression and phosphorylation in the adipose tissue, no changes in total cholesterol, HDL-cholesterol, HbA1c, Glc in the blood serum |
[100]/2007 |
| Sprague Dawley (SD) rats: untreated, fructose treated, metformin treated, fructose+metformin treated. | In comparison with untreated SD, in fructose treated SD rats: increase in systolic blood pressure (attenuated by metformin co-treatment), increase in MGO in the serum and aorta (reduced by metformin co-treatment), increased CEL staining in the aorta (normalized by metformin co-treatment), decreased eNOS staining in endothelial cells of the aorta (increased by metformin co-treatment), increased hydrogen peroxide in the aorta, and decreased GSH in the serum (corrected by metformin co-treatment), no difference in aorta GSH levels. damaged mesenteric artery (increased wall thickness, decreased lumen) (corrected by metformin co-treatment), increased CEL and CML staining in the mesenteric artery (corrected by metformin co-treatment). |
[31]/2008 |
| Leprdb/db murine model of metabolic syndrome | Higher CEdG (238.4±112.8 pmol/24 h) in urine of hyperglycemic mice (FPG, ≥200 mg/dL) than normoglycemic mice (16.1±11.8 pmol/24 h). Enhanced CEL in urine of hyperglycemic mice |
[12]/2017 |
| STZ-treated rats (T1DM) | Around 4-fold increase in CEdG in urine of diabetic rats. | [13]/2008 |
| STZ-treated rats (T1DM) | Increased MGO and D-lactate in the lens and blood. Increased MGO in the kidney of diabetic rats. |
[14]/1993 |
| Glo1 KO mice | In comparison with Glo1+/+ mice; MG-H1 elevation in murine liver; but not in the brain. |
[101]/2017 |
| STZ-treated Glo1 KO mice | Neither MGO nor MG-H1 elevation in hyperglycemic mice. | [80]/2018 |
| STZ-treated Glo1 overexpressing rats | Reduction of hyperglycemia-elevated MGO/AGEs. | [102]/2011 |
| STZ-treated normal and Glo1 overexpressing rats | Effects of diabetes: increase in 3DG and CML, but not MGO and CEL in the heart. Effects of Glo1 overexpression: decrease in diabetes-enhanced cardiac mRNA profile associated with oxidative stress and fibrosis, partial attenuation of diabetes-upregulated cardiac RAGE |
[103]/2013 |
| STZ-treated normal and Glo1 overexpressing Wistar rats | Endothelium-dependent NO-mediated relaxation in mesenteric arteries isolated from the rats: impaired in diabetic normal rats, improved in diabetic Glo1 overexpressing rats. MGO-exposed mesenteric arteries: increase in MG-H1 (in adventitia and endothelium) in arteries from diabetic normal rats, but not from diabetic Glo1 overexpressing rats, impaired vasoreactivity corrected by Glo1 overexpression, increase in nitrotyrosine (a peroxynitrite marker). |
[104]/2010 |
| STZ-treated normal and Glo1 overexpressing Wistar rats | Effects of Glo1 overexpression: prevention of diabetes-stimulated MG-H1 and CML increase in mesenteric arteries isolated from the rats; correction of diabetes-impaired endothelium-dependent relaxation of mesenteric arteries; prevention of diabetes-increased VCAM-1 and ICAM-1 in mesenteric arteries isolated from the rats; attenuation of diabetes-cause markers of early damage in the kidney; no impact on diabetes-increased ICAM-1 in blood plasma no impact on eNOS expression, as well as mesenteric arteries morphology and collagen between groups. |
[105]/2014 |
| STZ-treated Glo1 overexpressing ApoE KO mice |
Effects of Glo1 overexpression: prevention of diabetes-stimulated MG-H1 modification of proteins in aortas and kidneys, no impact on diabetes-stimulated aortal collagen glycation (FL, CML, 3DG-H, MG-H1), no impact on diabetes-induced serum fasting glucose level, no impact on diabetes-induced serum cholesterol and TAG levels, no impact on diabetes-induced atherosclerotic lesions in aortas. |
[106]/2014 |
| Glo1 underexpressing ApoE KO mice | Effects of Glo1 underexpression (around 75% activity decrease): increase in MG-H1 protein modifications in aortas and kidneys, no impact on aortal collagen glycation (e.g. FL, CML, 3DG-H, MG-H1), no impact on atherosclerotic lesions in aortas, no impact on serum fasting glucose, cholesterol and TAG levels |
[106]/2014 |
| STZ-treated ApoE KO and RAGE/ApoE DKO mice; MGO-fed ApoE KO and RAGE/ApoE DKO mice; Glo1 inhibited (BBGC-treated) apoE KO mice |
Increase in MGO level in diabetic mice comparably to MGO-fed and BBGC-treated mice. Increase in atherosclerotic plaques in aortas from MGO-fed apoE KO and RAGE/apoE DKO mice, and BBGC-treated apoE KO mice (similarly to diabetic mice). Upregulation of adhesive and proinflammatory molecules (ICAM-1, tetherin, MCP-1, mac-1,2) in aortas of MGO- and BBGC-treated ApoE KO. Upregulation of ICAM-1, tetherin, and mac-1 in aortas of MGO-fed RAGE/ApoE DKO mice. |
[107]/2014 |
| STZ-treated ApoE KO mice (DM); STZ-treated ApoE KO mice fed with high-lipid diet (DM + HLD); STZ-treated ApoE KO mice fed with high-lipid diet and NAC (DM + HLD + NAC) |
All of the below effects were attenuated by NAC (in DM + HLD + NAC mice). In the serum from the mice: increase in MDA in DM mice, and more enhanced increase in DM + HLD mice; decrease in NO in DM mice, and more enhanced decrease in DM + HLD mice; In aortas extracted from the mice: increase in atherosclerotic plaque lesion in the aortic root from DM mice and more enhanced increase in DM + HLD; increase in MGO and protein carbonyls in DM and DM + HLD; decrease in SOD-1 and GPX-1 protein expression in DM and more enhanced decrease in DM + HLD; decrease in phosphorylated forms of Akt and eNOS in DM and more enhanced decrease in DM + HLD; decrease in GSH in DM and DM + HLD. |
[108]/2021 |
| MGO-treated C57/BL6 male mice | Increase in systemic insulin resistance. Reduction in insulin-induced activation of Akt and eNOS in murine aortas, reflected by reduction of insulin-stimulated increase in serum NO. Induction of ERK ½ phosphorylation in murine aortas and endothelin-1 release (comparable to insulin effect). |
[109]/2014 |
| Glo1 overexpressing ApoE KO mice | No effect of Glo1 overexpression on the size and severity of atherosclerotic plaques in the murine aortas. No effect of Glo1 overexpression on inflammatory markers (e.g. MCP-1, IL-6) in murine aortas, neither systemic inflammation (blood plasma lymphocytes T and B, cytokines - MCP-1, IL-1β,6,10, IFN-γ). No improvement of oxidative stress markers worsened in apoE KO mice by Glo1 overexpression. No differences in MGO and AGE markers (CML, CEL, MG-H1) in blood plasma and aortas between the murine groups. |
[110]/2014 |
| STZ-treated ApoE KO and Glo1 overexpressing ApoE KO-mice | No effect of Glo1 overexpression on: diabetes-induced increase in AGE markers (CML, GO), diabetes-enhanced atherosclerotic plaque lesions in murine aortas, inflammatory phenotype (MCP-1, monocytes), diabetes-induced plasma fasting glucose level, diabetes-induced plasma fasting cholesterol level. |
[[110]/2014 |
| MGO-fed normal Wistar rats and (non-obese) T2DM Goto-Kakizaki (GK) rats | In both Wistar and diabetic rats upon MGO treatment: decline in NO-dependent vascular relaxation, increase in superoxide and nitrotyrosine, upregulation of aortal MCP-1, AGEs and RAGE, increase in plasma MGO and urinary 8-OHdG levels. |
[111]/2012 |
| MGO-fed normal Wistar rats and (non-obese) T2DM Goto-Kakizaki rats | In MGO-fed normal Wistar rats (in comparison with normal Wistar rats): increase in plasma free fatty acids, decrease in serum adiponectin, increase in plasma and tissue MGO and urinary 8-OHdG levels. In the adipose tissue from MGO-fed Wistar rats: increase in AGEs, glycoconjugates and fibrosis, higher expression of TGF-β (but not its cleaved form), increase in proapoptotic factors (decreased Bcl2/Bax ratio and upregulation of caspase 3), decrease in VEGF level, but unchanged angiopoietin 2, increase in MCP-1 and F4/80 |
[112]/2012 |
| MGO-fed Wistar rats | In MGO-fed Wistar rats (in comparison with control Wistar rats): increase in plasma free fatty acids; no impact on glycemia (fasting and 2 h after glucose administration), glycated haemoglobin, insulinemia and serum total cholesterol, triglycerides and adiponectin levels. In the adipose tissue from MGO-fed Wistar rats: increase in CEL and fibrosis. In the adipose tissue of MGO-fed Wistar rats after blood supply reduction: increase of ERK1/2 phosphorylation (p-ERK1 plus p-ERK2); increase in perilipin A degradation (due to MGO-induced glycation); decrease in IkBa; decrease in PPARγ expression; decrease in Akt activation. |
[113]/2013 |
| Wistar rats: control (C), MGO-fed (MGO), high-fat diet-fed (HFD), high-fat diet group with MG supplementation (HFDMGO), and T2DM (non-obese) Goto-Kakizaki (GK) rats |
In the circulation of HFDMGO rats (as compared with control): increase in FFAs, insulin, Glc intolerance development. In the adipose tissue of HFDMGO and GK rats (as compared with control): increase in CEL; no change in GLO1 levels; increase in hypoxia; no change in HIF-1α, but decrease in HIF-2α expression; decrease in IR phosphorylation; no changes in phosphorylated Akt, PGC1α and the differentiation factors PPAR-γ and C/EBPα. In the adipose tissue of HFDMG and MGO rats (as compared with control): increase in (proinflammatory) M1 macrophages and CD31 (endothelial cell marker) In the adipose tissue of HFDMG, MGO and GK rats (as compared with control): decrease in blood flow; decrease in VEGF/Ang-2 ratio. In the adipose tissue of HFDMGO rats (as compared with control): decrease in perilipin A. Upon MGO exposition (50-1000 µM) and/or Glo1 inhibition in adipose tissue explants: inhibition of capillarization. In the skeletal muscles of HFDMGO rats (as compared with control): decrease in IR protein (but not phosphorylated IR), active Akt (phosphorylated Akt) and GLUT-4. |
[114]/2017 |
| MGO-treated (intragastrically) hereditary hypertriglyceridaemic rats (HHTg) | Upon MGO treatment: increase in non-fasting Glc and insulin in blood serum; increase in proinflammatory MCP-1 and TNFα in the serum; decrease in the conversion of Glc into lipids upon insulin-stimulation in white adipose tissue (WAT); increase in adrenaline-stimulated lipolysis in WAT; shift in components of phospholipids: increase in saturated fatty acids (e.g. palmitic and myristic) and decrease in polyunsaturated fatty acids (especially ω-3; e.g. α-linelenic and docosahexaenoic acids) in WAT; no effect on Glo1 expression in WAT; decrease in Nrf2 expression in WAT; increase in MCP-1 and TNFα expression in WAT; no effect on HIF-1 expression in WAT |
[115]/2020 |
| MGO-fed normal Wistar rats | Upon MGO treatment: no change in serum glucose, increase in serum cholesterol, creatinine and fructosamine, proinflammatory and profibrotic response (increased IL-1β, TNF-α, CTGF, TGF-β; disturbances in wound healing), upregulation of AGEs and RAGE in skin vasculature, progressive thickening of skin blood vessel wall followed by its detachment from matrix, luminal occlusion and endothelial cells death ending up with vessel destruction, no vasodilation upon nitroglycerine treatment. |
[116]/2005 |
| MGO-fed normotensive Sprague-Dawley rats | Upon MGO treatment: Decrease in insulin sensitivity (improved by NAC and TM2002 (AGEs inhibitor). Increase in CEL and nitrotyrosine in the kidney from the rats. |
[117]/2009 |
| Sprague-Dawley (SD) rats | Thoracic aortic rings isolated from SD rats upon MGO treatment: inhibition of ACh-induced endothelium-dependent relaxation (prevented by aminoguanidine (AG) and N-acetyl cysteine (NAC), but not restored by NOX inhibitor), no effect on endothelium-independent relaxation |
[118]/2010 |
| MGO-treated Sprague-Dawley (SD) rats | Upon MGO treatment (and attenuated by alagebrium) in SD rats: impairment in Glc tolerance, increase in plasma insulin, decrease in plasma glutathione. In the visceral adipose tissue isolated from the studied rats: decrease in insulin-stimulated glucose uptake, reduced plasma membrane GLUT-4 and IRS-1 tyrosine phosphorylation, no change in insulin receptor and IRS-1 protein expression. |
[119]/2010 |
| Fru-fed, and continuously MGO-treated Sprague-Dawley (SD) rats | In Fru-fed SD rats (as compared to SD control): increase in blood pressure and vascular remodeling; increase in MGO in plasma and aorta tissue (attenuated by metformin); increase in Akt1 phosphorylation at Ser-473 in aorta (attenuated by metformin). In MGO-treated SD rats (as compared to SD control): increase in Akt1 phosphorylation at Ser-473 in aorta (attenuated by alagebrium). |
[120]/2011 |
| Continuously MGO-treated Sprague-Dawley (SD) rats | Upon constant MGO treatment (and attenuated by alagebrium) in SD rats: increase in fasting plasma glucose, total cholesterol, TAG and free fatty acids decrease in fasting plasma insulin and HDL, and plasma and tissue glutathione, enhanced formation of CML and increased apoptosis of pancreatic β-cells. In the adipose tissue isolated from the studied rats: decrease in insulin-stimulated glucose uptake, reduced plasma membrane GLUT-4, IRS-1 phosphorylation and PI3K activity, no change in insulin receptor and IRS-1 protein expression, In the pancreatic islets (β-cells) isolated from the studied rats: reduced GLUT-2 (= decreased Glc uptake) and glucokinase, lowered insulin secretion – down-regulation of factors promoting insulin expression (PDX-1 and MafA), and up-regulation of the factor inhibiting insulin expression (C/EBPβ), upregulation of NF-kB and RAGE. |
[121]/2011 |
| Continuously MGO-treated Sprague-Dawley (SD) rats | Upon constant MGO treatment (and attenuated by alagebrium) in SD rats: increase in blood pressure; increase in plasma norepinephrine, epinephrine, dopamine, angiotensin, renin, and aldosterone. In aortas from the rats: elevated adrenergic α1D receptor, angiotensin AT1 receptor, and angiotensin protein and mRNA. In the kidney from the rats: Increase in angiotensin AT1 receptor, renin, and angiotensin protein and mRNA. In aortas and kidney from the rats: increase in phosphorylated Erk 1/2 (p-Erk 1/2) and NFATc expression. |
[122]/2014 |
| C57BL/6J mice, diabetic Akita and Leprdb/db mice |
Reduced aortic endothelial outgrowth in both diabetic mice clones, normalized by inhibitors of lysosomal enzymes/autophagy. Decreased VEGFR-2 in diabetic mice aortas. |
[123]/2012 |
| STZ-treated Glo1 overexpressing C57BL/6 mice |
Preventive effect of Glo1 overexpression on: diabetes-upregulated circulating inflammatory markers in mice, diabetes-reduced endothelial cell number in murine hearts, diabetes-caused deterioration in cardiomyocytes function associated with their enhanced death (via the stabilization of neuregulin, NOS, Bcl-2), diabetes-induced RAGEs and TNF-α in the murine hearts, diabetes-caused cardiac functions loss. |
[124]/2016 |
| STZ-treated Glo1 overexpressing C57BL/6 mice |
Effects of Glo1 overexpression: increased survival of BMCs (extracted from Glo1 diabetic mice) cultured in hyperglycemic (20 mM Glc) and proapoptotic conditions, associated with upregulation of anti-apoptotic Bcl-2 and Bcl-XL, and decrease in oxidative stress markers (protein carbonyls), maintenance of migratory potential of diabetic BMCs, recovery of neovascularization and blood flow in diabetic mice. |
[125]/2014 |
| Glo1 knockdown mice (C57BL/6J mice treated with Glo1 inhibitor - BBGC) | Observations in aortas extracted from the mice: increase in MG-H1, decrease in aortas sprouting (impaired angiogenesis). |
[126]/2019 |
| High-fat diet fed C57BL/6 mice | Increase in body weight, glycaemia, glucose intolerance and insulin resistance. Increased expression of NF-κB-p65 and HoxA5 in aortas. |
[127]/2019 |
| MGO and/or metformin (MET) treated C57BL/6 mice | Upon MGO treatment in murine blood/serum (partially restored with MET pretreatment): decrease in the levels of SOD, CAT, and GPX; increase in MDA; increase in proinflammatory cytokines (IL-1β and IL-6) and the anti-inflammatory cytokine IL-10. Upon MGO treatment in murine aortas (partially restored with MET co-treatment): increase in aortas thickness, apoptosis; decrease in Nrf2 expression and Akt phosphorylation. |
[128]/2022 |
| Mouse aortic tissue isolated from non-diabetic Glo1KD mice after insulin injection | Decrease in miR-190a expression and insulin sensitivity |
[129]/2017 |
| Mouse aortic tissue isolated from non-diabetic Glo1KD mice | Decrease in miR-214 expression |
[130]/2018 |
| Experimental model | MGO/MAGEs and associated major findings | Ref./year |
|---|---|---|
| Single aortic VSMCs from spontaneously hypertensive rats (SHR) and Wistar Kyoto rats (WKY) | In comparison with normal WKY, in VSMCs from SHR: higher MGO and AGEs levels, increased AGEs formation upon MGO exposition, increased oxidative stress (enhanced further by MGO exposition), decreased GSH/GSSG ratio, greater activation of NF-κB and expression of ICAM-1 (both enhanced further by MGO exposition). Increase in GSSG content in both WKY and SHR VSMCs upon MGO exposition. |
[131]/2002 |
| Rat L6 myoblasts | Upon MGO treatment (the myoblasts exposed to 0.5-2.5, 2.5 or 5 mM MGO for 10-30 min. – with 3% of MGO entering the cells) and insulin stimulation: reduced Glc uptake not mediated by ROS generation (improved by aminoguanidine), no changes in insulin receptor autophosphorylation, reduced IRS-1 tyrosine phosphorylation, no changes in serine/threonine IRS-1 phosphorylation, abolished IRS-1–associated PI3K activity (reversed by aminoguanidine), decreased PKB Ser/Thr phosphorylation (attenuated by aminoguanidine). Upon MGO treatment (the myoblasts exposed to 2.5 mM MGO for 30 min): increased p-ERK (partially mediated by ROS generation; prevented by aminoguanidine). Upon MGO treatment (the myoblasts exposed to 5 mM MGO up to 3h): ROS generation (reversed by aminoguanidine). |
[132]/2006 |
| L6 GLUT4 myc -tagged myoblasts | Upon MGO treatment (the myoblasts exposed to 100-400 µM for 24 h) (and previous insulin stimulation): increase in GLUT-4 on the plasma membrane Upon MGO treatment (the myoblasts exposed to 400 µM for 24 h) (without previous insulin stimulation): increase in MG-H1 (prevented by NAC but not by tiron); increase in GLUT-4 on the plasma membrane (prevented by NAC but not by tiron); increase in MG-H1 on GLUT-4; increase in ROS (prevented by NAC and tiron); decrease in Akt1 protein expression and increase in apoptosis; no effect on Akt2 and total Akt phosphorylation. |
[133]/2014 |
| 3T3-L1 cells (cell line from mouse adipose tissue); L8 cells (rat skeletal muscle cell line), H4-II-E cells (rat hepatocyte cell line), cloned INS-1E cells (derived from rat insulinoma) | Upon MGO-modified insulin treatment (3T3-L1 and L8 cells) in comparison with unmodified insulin stimulation: reduced Glc uptake. Upon insulin and MGO co-treatment (3T3-L1 cells exposed to 3-300 µM MGO) or MGO pretreatment (3T3-L1 cells exposed to 1-30 µM MGO for 24/48h) and insulin stimulation: no effect on Glc uptake. Upon MGO treatment (3T3-L1 cells exposed to 3 or 30 µM MGO for 24h): no effect on insulin receptor expression (at mRNA level). Upon MGO-modified insulin treatment (H4-II-E and INS-1E cells) in comparison with unmodified insulin: abolishing of C-peptide release by INS-1E cells and decrease in modified insulin clearance by H4-II-E cells. |
[68]/2006 |
| insulin-secreting INS-1E rat beta cells | Upon MGO treatment (the cells exposed to 0.25-1.0 mM MGO for up to 60 min – with 12.5% of MGO entering the cells): no impact on ROS generation. Upon MGO treatment (the cells exposed to different MGO levels within 0.25-1.0 mM MGO for 30 min, and insulin stimulation): no effect on IR Tyr phosphorylation; decrease in insulin-dependent IRS phosphorylation (prevented by aminoguanidine (AG)); decrease in insulin-dependent complex formation between IRS and PI3K p85 subunit (prevented by AG); decrease in insulin-dependent PKB phosphorylation at Thr 308 (prevented by AG); decrease in insulin-dependent (and PKB-catalyzed) GSK-3 phosphorylation at Ser (prevented by AG). Upon MGO treatment (the cells exposed to 0.5 mM MGO for 30 min, and 0.05 or 0.1 mM for 24 h): formation of CEL and argpyrimidine (AP) on IRS (prevented by AG); decrease in insulin-induced Pdx1, Ins1 and Gck mRNA expression (restored by AG). Upon Glc and MGO treatment (the cells exposed to 0.5 mM MGO for 30 min. and 0.05 or 0.1 mM for 24 h): decrease in Glc-stimulated insulin secretion (prevented by AG); decrease in Glc-stimulated PKB phosphorylation at Thr 308 (prevented by AG); decrease in Glc-induced Pdx1, Ins1 and Gck mRNA expression |
[134]/2011 |
| mouse insulinoma cells (MIN6) and rat insulinoma cells (INS-1) | Upon MGO treatment (0.05 mM or 0.1 mM for 3 h) in both cell lines: decrease in Glc-stimulated insulin secretion (prevented by NAC); increase in ROS (prevented by NAC). Upon MGO treatment (0.05 or 0.1 mM for 3 h) in MIN6 cells: increase in apoptosis (prevented by NAC); decrease in mitochondrial membrane potential (prevented by NAC); decrease in ATP synthesis (prevented by NAC); up-regulation of uncoupling protein 2 (UCP-2) (mRNA and protein) (prevented by NAC); increased expression of p-JNK, JNK, p-P38, and P-38. |
[135]/2016 |
| 3T3-L1 cells (cell line from mouse adipose tissue) | Upon MGO treatment (20 µM): reduced Glc uptake (improved by NAC), no changes in IR, IRS-1 and PI3K expression, reduced IRS-1 tyrosine phosphorylation and PI3K kinase activity (reversed by NAC). |
[100]/2007 |
| A-10 cells: rat thoracic aortic SMC line (VSMC) | Upon MGO treatment (3-300 µM for 45min.-18h): increase in hydrogen peroxide, nitric oxide, superoxide anion and peroxynitrite |
[89]/2005 |
| A-10 cells: rat thoracic aortic SMC line (VSMC) | Upon MGO treatment (30 µM for 18h) (and attenuated by alagebrium): increase in CEL, nitric oxide, nitrotyrosine in the cells; In the cells’ mitochondria: increase in ROS and superoxide anion; decrease in MnSOD activity; decrease in complex III activity of the respiratory chain, and decrease in ATP production. |
[90]/2009 |
| A-10 cells: rat thoracic aortic SMC line (VSMC) | VSMC upon MGO treatment (10, 30, 50 µM for 24 and/or 72 h): increase in DNA synthesis and cells proliferation (abolished by Akt inhibitor and in Akt1 knock-down VSMC); increase in Akt1 phosphorylation at Ser-473, and GSK-3α/β phosphorylation; decrease in total p21; increase in phosphorylated p21 and p27; increase in CDK2 activity. VSMC upon MGO treatment (100 µM for 24h): increase in cells apoptosis. |
[120]/2011 |
| A-10 cells: rat thoracic aortic SMC line (VSMC) | Upon fructose or Glc treatment (25 mM for 6, 12 or 24h): increase in MGO; upregulation of Glo1 and Glo2 (at the protein level); Upon fructose treatment (25 mM for 6, 12 or 24h): upregulation of GLUT-5 and fructokinase (at mRNA levels), and aldolase B (at mRNA and protein levels) – further enhanced by fructose + insulin co-treatment Inhibition of fructose-induced MGO increase by aldolase B knock-down. Upon Glc treatment (25 mM for 12h): downregulation of aldolase A and upregulation of aldolase B. Inhibition of Glc-induced MGO increase by aldolase B knock-down and inhibitors of polyol pathway. |
[88]/2011 |
| A-10 cells: rat thoracic aortic SMC line (VSMC) | Upon MGO (30 µM for 24 h) or Glc treatment (25 mM for 24 h): increase in adrenergic α1D receptor, angiotensin AT1 receptor, and angiotensin protein and mRNA (attenuated by alagebrium); Upon MGO (30 µM for 24 h): increase in phosphorylated Erk 1/2 (p-Erk 1/2) and NFATc expression (attenuated by alagebrium); increase in the protein and mRNA for NF-κB, angiotensin, AT1 receptor, and adrenergic α1D receptor (attenuated by alagebrium); Upon RAGE siRNA: attenuation of the increase in RAGE and NF-kB p65 protein expression (induced by MGO). Upon angiotensinogen siRNA: attenuation of the increase in NF-kB p65, angiotensin, AT1 receptor, and adrenergic α1D receptor protein expression (induced by MGO). |
[122]/2014 |
| A-10 cells: rat thoracic aortic SMC line (VSMC) | Upon Fru treatment (15 and/or 30 mM): increase in MGO, peroxynitrite, nitric oxide and superoxide anion. Upon MGO treatment (10 µM, 6h): increase in nitric oxide and superoxide anion, increase in iNOS staining |
[136]/2006 |
| VSMCs isolated from the thoracic aorta of male Wistar rats | Upon MGO treatment (10 µM, 3 or 9 h): upregulation of ER stress markers (induction of PERK phosphorylation, increase in IRE1α and ATF6 expression); no effect on apoptosis |
[137]/2022 |
| Human immortalised endothelial cells (ECRF-24) | Effects of Glo1 silencing in ECRF-24: decrease in the cells viability; upregulation of pro-inflammatory factors (MCP-1, IL-6, TNF); upregulation of vascular activating factors (VCAM-1 and ICAM-1). |
[105]/2014 |
| Mouse aortic endothelial cells (MAECs) | Upon 0.5 Mm MGO (up to 16 h) or Glo1 inhibitor (BBGC) treatment, followed by insulin stimulation): suppression of insulin-stimulated IRS-1 tyrosine phosphorylation and Akt activation; suppression of insulin-stimulated eNOS activation (reduction of Ser-1177 phosphorylation and threonine-497 dephosphorylation) and NO production. Upon 0.5 Mm MGO treatment (up to 16 h): Induction of ERK ½ phosphorylation and endothelin-1 release (comparable to insulin effect). Upon Glo1 inhibitor (BBGC) treatment: Induction of ERK½ phosphorylation(comparable to insulin effect). Reversal of the above MGO effects by ERK½ inhibitors. |
[109]/2014 |
| Human umbilical vein endothelial cells (HUVECs) treated with 0.56 mM (1-24 h) MGO or 0.56 mM MGO and NAC | The below effect was attenuated by Telmisartan. Upon MGO treatment: increase in the cells apoptosis |
[138]/2008 |
| Human umbilical vein endothelial cells (HUVECs) treated with 1 mM MGO or 1 mM MGO and NAC | All of the below effects were attenuated by NAC. Upon MGO treatments: increase in ROS, decrease in SOD-1 and GPX-1 protein expression; decrease in phosphorylated forms of Akt and eNOS. |
[108]/2021 |
| Human umbilical vein endothelial cells (HUVECs) | Upon MGO treatment (200 μM for 24 h) (reversed by metformin pretreatment): increase in apoptosis (increase in cleaved caspase-3 and increase in the Bax/Bcl-2 ratio); decrease in Akt phosphorylation, and Nrf2 and HO-1 expression; Upon MGO treatment (200 μM for 1 h) (reversed by metformin pretreatment): increase in ROS and MDA, decrease in SOD, CAT, and GPX-1 activities; decrease in mitochondrial membrane potential and structural damage to the mitochondrial membranes. |
[128]/2022 |
| Primary aortic endothelial cells isolated from C57BL/6 mice, cultured with Glo1 inhibitor (BBGC) | Increased expression of adhesion molecules (VCAM, ICAM-1, tetherin, MCP-1) followed by increased adhesion of monocytes to endothelium. |
[107]/2014 |
| Primary aortic endothelial cells isolated from RAGE KO mice, cultured with Glo1 inhibitor (BBGC) | Increased expression of adhesion molecules (VCAM, ICAM-1, tetherin) followed by increased adhesion of monocytes to endothelium |
[107]/2014 |
| Rat aortic endothelial cells (RAECs) from SD rats, Human umbilical vein endothelial cells (HUVECs) |
Upon 25 mM Glc exposition for 24 h: increase in MGO level in both RAECs and HUVECs. Upon MGO exposition (30 µM for 3 or 24 h): increase in NOX activity, ROS generation in both cell lines, decrease in NO production by eNOS and decrease in GSH in both cell lines, decrease in Ser-1177 phosphorylation in eNOS in HUVECs (prevented by AG) |
[118]/2010 |
| MGO-exposed bone marrow-derived EPCs (from C57BL/6 mice) | MGO effect: decrease in EPCs viability, downregulation of VEGFR-2 (mRNA and protein decrease), impairment in blood vessel tube formation in EPCs, restoration of MGO-induced EPCs dysfunctions by RAGE antagonist. |
[139]/2018 |
| MGO-exposed bovine aortic endothelial cells (BAEC) and human umbilical cord vein endothelial cells (HUVEC) | MGO effect: downregulation of VEGFR-2 (protein decrease) in both cell lines in a dose- and time-dependent manner (enhanced in BAEC by Glo1 downregulation, and abolished by Glo1 up-regulation), around 80% reversal of VEGFR-2 reduction in RAGE-knock-down cells, decrease in tube formation and BAEC cell migration. |
[123]/2012 |
| Human U937 monocytes |
Hypoxia increased MGO level. TNF decreased Glo1 activity and increased MGO, MG-H1 and CML levels. TNF increased IL-8, MCP-1 and MMP-9. Hydrogen peroxide increased CML. High Glc (30 mM) had no effect on Glo1, MGO, AGEs. Exposition of the cells on MGO/CML had no effect on IL-8, MCP-1 and MMP-9. MGO exposition increased MG-H1 and CML. MGO exposition induced apoptosis. Glo1 attenuation (with siRNA) had no effect on MGO production. |
[140]/2014 |
| Human vascular endothelial cells (HVECs) obtained from patients with coronary heart disease | Upon MGO, GO or combined MGO + GO exposition (150 – 300 µM for 72 h): induction of senescence by increasing ROS production and p21 expression; increase in CML and MG-H1; arrest of the cells in the G2 cell cycle phase. The above MGO/GO induced effects were prevented by aminoguanidine. Reduction of Glo1 expression. |
[141]/2017 |
| Human aortic endothelial cells (HAECs) | Upon MGO (1-100 μmol/L MGO for 20 min) treatment: increase in superoxide production from mitochondria; partial stimulation of NOS. |
[142]/2010 |
| Human aortic endothelial cells (HAECs) | Upon MGO (1 or 5 mM for 2-8 h) treatment: decrease in cell viability; decrease in thioredoxin protein and mRNA levels (abolished by metformin); oxidative damage of peroxiredoxin; increase in ROS and mitochondrial-dependent apoptosis. |
[143]/2012 |
| Human aortic endothelial cells (HAECs) | Upon Glc treatment (20 mM for 2-6 days): increase in MGO, D-lactate and MG-H1 in the cells and cell culture; down-regulation of Glo1 (activity and protein, but not mRNA); up-regulation of (among others) UPR pathways (e.g. heat shock proteins); down-regulation of a mediator of EC migration (annexin-A1), an anti-coagulatory factor (annexin-A5); an anti-inflammatory factor (chromobox protein homolog-5; (CBX5); increase in hexokinase-2 (protein but not mRNA) and glycogen. |
[144]/2019 |
| Human aortic endothelial cells (HAECs) from healthy (H-HAECs) and T2DM (D-HAECs) donors |
D-HAECs – impaired network formation, proliferation, increased apoptosis (in comparison with H-HAECs). MGO (10µM)-treated H-HAECs – impaired network formation, proliferation, increased apoptosis – reversed by KATP channel inhibition. D-HAECs – upregulation of three MAPK pathways (p-JNK, p-p38, and p-ERK) (in comparison with H-HAECs). MGO (10µM)-treated H-HAECs – upregulation of three MAPK pathways (p-JNK, p-p38, and p-ERK) – reversal of JNK activation by KATP channel inhibition. |
[126]/2019 |
| Mouse aortic endothelial cells (MAECs) isolated from Glo1KD and WT mice; MGO or Glo1 inhibitor treated mouse coronary artery endothelial cells (MCECs) |
Glo1KD MAECs (in comparison with WT MAECs): decreased cell growth, proliferation, migration and Matrigel invasion; 2-fold upregulation of NF-κB-p65 and antiangiogenic HoxA5 (prevented by AG) associated with downregulation of VEGFR-2 (no change in VEGF expression); improvement of migration and invasion by HoxA5 silencing. Upon 0.5 Mm MGO (for 16 h) or Glo1 inhibitor (BBGC) treatment of MCECs: decreased migration; upregulation of NF-κB-p65 and HoxA5. |
[127]/2019 |
| MGO-treated mouse aortic endothelial cells (MAECs); MGO-treated human endothelial cells (HUVECs) | Upon 0.5 Mm MGO (for 16 h) treatment of MAECs and HUVECs: downregulation of miR-190a. Upon miR-190a inhibition: decrease in insulin-induced Tyr phosphorylation of IRS1, and AKT phosphorylation at Ser473 and Thr308 (no effect on IR); impairment of insulin-dependent eNOS activation/NO release; increase in ERK1/2 phosphorylation. Upon miR-190a over-expression: prevention of MGO impairment of insulin stimulated IRS1/Akt/eNOS/NO release pathway. Upon MGO exposure and miR-190 inhibition: Upregulation of GTPase Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS). |
[129]/2017 |
| MGO-treated mouse aortic endothelial cells (MAECs) | Upon 0.5 Mm MGO (for 16 h): downregulation of miR-214. Upon MGO exposure and miR-214 inhibition: 4-fold upregulation of PH domain leucine-rich repeat protein phosphatase 2 (PHLPP2). Upon miR-214 overexpression: downregulation of PHLPP2. Upon miR-214 overexpression in MGO treated and insulin-stimulated MAECs: Reversal of MGO-impaired Ser473 phosphorylation on Akt |
[130]/2018 |
| Human endothelial cells (HUVECs and microvascular endothelial cells from human foreskin) | No binding neither activation of endothelial cells by MGO-modified albumin and CML-modified albumin (no increase in adhesion molecules – VCAM-I, ICAM-I and E-selectin) | [56]/2006 |
| Human endothelial cells (HUVECs) | Hyperglycemic conditions (30 mM Glc) caused: increase in MGO and argpyrimidine (AP) in the cells (no impact on 2-deoxyglucosone, glyoxal, CML and CEL), Hsp-27 was the major AP-modified protein. decrease in cells proliferation. |
[145]/2006 |
| Human endothelial cells (HUVECs) | Upon 0.5 Mm MGO (for 24 h) treatment (reversed by phosphocreatine and NAC): increase in apoptosis (caspases upregulation and Bcl-2/Bax decrease); increase in ROS and calcium; upregulation of NOX4; decrease in mitochondrial membrane potential; decrease in Akt and eNOS phosphorylation; decrease in cGMP and NO; induction of NF-κB. |
[146]/2017 |
| Human endothelial cells (HUVECs) | Upon TNF-α (12.5ng/ml) and MGO (800 µM for 24 h) treatment: down-regulation of genes mainly associated with cell cycle (topoisomerase (DNA) II alpha, marker of proliferation Ki-67, cyclin A2, etc.); up-regulation of heme oxygenase-1, insulin like growth factor binding protein 3, plasminogen activator inhibitor 2, and others; decrease of VCAM-1. Some MGO-induced effects prevented by L-carnosine (20 mM). |
[147]/2019 |
| Human endothelial cells (HUVECs) | Upon MGO (800 µM for 5 h) treatment: increase in DNA damage and p53 phosphorylation (at Ser15); decrease in mTORC1 targets phosphorylation (4EBP1 and p70S6K); increase in autophagy; increase in protein carbonylation; no effect on GSH/ GSSG. |
[148]/2021 |
| Human endothelial cells (HUVECs) |
Upon MGO (500 µM) and MGO-modified Hb: increase in HUVECs apoptosis; decrease in HUVECs proliferation and migration; Upon MGO-modified Hb: increase in ROS, decrease in mitochondrial membrane potential; decrease in phosphorylated JNK and p-38; Upon MGO (500 µM): increase in phosphorylated JNK. |
[66]/2021 |
| eNOS overexpressing human endothelial cells (HUVECs) | No inhibition of eNOS activity by MG-H1 and AP | [149]/2008 |
| Human endothelial cells (HUVECs and EA.hy926) | MGO exposition induced apoptosis via ROS generation and c-FLIPL downregulation (c-FLIPL downregulation was probably mediated by inactivation of NF-κB pathway through p65 downregulation). | [150]/2017 |
| Human endothelial cells (EA.hy926) | Upon MGO treatment (50 – 200 μM for 2-8 h): increase in superoxide radical (probably produced by eNOS through MGO-induced eNOS uncoupling); Upon MGO treatment (100 μM for 8 h): decrease in eNOS phosphorylation (at Ser-1177) |
[151]/2013 |
| Glo1 KO human cell line (Glo1-/- HEK293 cells) | No elevation of MGO. |
[54]/2018 |
| Glo1 knockdown human cell line (GLO1-siRNA-transfected HAECs) incubated at high Glc concentration (25 mM) | MGO increase. No increase in MG-H1 of cellular proteins. Increase in MG-H1 free adduct in the culture medium. No impact on eNOS Downregulation of some structural proteins and enzymes metabolizing collagen. Increase in endothelin-1, collagen 1 and 5 expression. Induction of apoptosis. Increase in markers of inflammation and endothelial dysfunction (IL-6, RAGE, MCP-1, sVCAM-1, sICAM-1). |
[152]/2016 |
| Glo1 overexpressing human cell line (GLO1-transfected human dermal microvascular ECs (HMEC-1 cells) incubated at high Glc concentration (20 mM) | 4-fold increased GLO1 activity improved hyperglycemia diminished tube formation. | [153]/2008 |
| Glo1 overexpressing human cell line (GLO1-transfected human cardiac ECs (HCEC cells) exposed to MGO (5 µM), high glucose (30 mM), TNF-α (10 ng/mL) | 9-fold increased GLO1 expression protected from cell death induced by MGO, high glucose, TNF-α. | [124]/2016 |
2.2.1. Enzymatic mechanisms compensating for deficient glyoxalases
2.2.2. MGO/MAGEs in insulin resistance development
2.3. MGO, its metabolic products and MAGEs in patients with metabolic syndrome and diabetes
3. MGO and MAGEs in cardiovascular disorders
3.1. Pathological routes linking metabolic syndrome and diabetes with cardiovascular complications
3.2. MGO/MAGEs contribution to blood vessels wall impairment, hypertension, dyslipidemia and atherosclerosis
3.2.1. Blood vessels focusing on endothelium – impairment of angiogenesis
3.2.2. Cardiovascular system in animal models
3.2.3. Cardiovascular disorders in patients
3.2.4. Atherosclerosis
3.2.5. Endoplasmic reticulum stress (ER stress) followed by unfolded protein response (UPR) in blood vessels
3.2.6. Hypertensive and pro-coagulatory properties of MGO/MAGE
3.2.7. Dyslipidemia
4. Potential glycation inhibitors and MGO scavengers – therapeutic strategies
4.1. Overview of the potential glycation inhibitors and MGO scavengers
4.1.1. Oral antihyperglycemic agents
4.1.2. Angiotensin II receptor antagonists, and angiotensin-converting enzyme inhibitors
4.1.3. Calcium channel blockers
4.1.4. Arterial smooth muscle agents
4.1.5. Lipid modifying agents (statins)
4.1.6. Peripheral vasodilators and vasoprotectives
4.1.7. Anti-inflammatory, analgesic and antipyretic agents
4.1.8. Selected B vitamins
4. Conclusions and remarks for future research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| AG | aminoguanidine |
| AGEs | advanced glycation end products |
| AKRs | Aldoketo reductases |
| Akt | PKB = protein kinase B (serine/threonine kinase) |
| ALDHs | Aldehyde dehydrogenases |
| AMPK | AMP-activated kinase |
| Ang II (Ang-2) | angiotensin II |
| AP | argpyrimidine |
| apoA1 | apolipoprotein A1 |
| apoB100 | apolipoprotein B100 |
| ApoE KO | apolipoprotein E knockout |
| ATF6 | activating transcription factor 6 |
| AT2R | angiotensin II receptor type 2 |
| BBGC | bromobenzyl-glutathione cyclopentyl diester (glyoxalase-1 inhibitor) |
| BMCs | bone marrow cells |
| CAD | coronary artery disease |
| CAT | catalase |
| CEA | N7 -carboxyethyl arginine |
| C/EBP | transcription factor C/EBP |
| CEdG | N2 -carboxyethyl-20 –deoxyguanosine |
| CEL | Nε -(1-carboxyethyl)lysineB |
| C. elegans | Caenorhabditis elegans |
| CETP | cholesteryl ester transfer protein |
| CKD | chronic kidney disease |
| CML | Nε-(1-carboxymethyl)lysine |
| CTGF | connective tissue growth factor |
| CHD | coronary heart diseases |
| CVD | cardiovascular diseases |
| DAG | diacylglycerol |
| DJ-1 (PARK7) | Parkinson’s disease protein 7 |
| DKO | double knock-out |
| 3-DG | 3-deoxyglucosone |
| 3DG-H | 3-DG-derived hydroimidazolones |
| EA.hy926 | hybrid human umbilical vein endothelial cell line |
| ECM | extracellular matrix |
| eNOS | endothelial nitric oxide synthase |
| EPCs | endothelial progenitor cells |
| ER | endoplasmic reticulum |
| esRAGE | endogenous secretory RAGE proteolytically exfoliated by metalloproteinases |
| FFAs | free fatty acids |
| FL | Nε-fructosyl-lysine |
| FPG | fasting plasma glucose |
| Fru | fructose |
| Glc | glucose |
| GlcNAc | N-acetylglucosamine |
| Glo1 | Glyoxalase 1 |
| Glo1 KO | Glo1 knockout |
| Glo2 | Glyoxalase 2 |
| GLUT | glucose transporter |
| GO | glyoxal |
| GPX | glutathione peroxidase |
| GSH | reduced glutathione |
| GSK-3 | Glycogen synthase kinase-3 |
| GSSG | oxidized glutathione |
| HAECs | human aortic endothelial cells |
| HbA1c | hemoglobin A1c |
| HEK293 | human embryonic kidney cells |
| HIF | hypoxia-inducible factor |
| HoxA5 | homeobox A5 transcription factor |
| HO-1 | heme oxygenase 1 |
| HSPG | heparan sulfate proteoglycan |
| HUVECs | human umbilical cord vein endothelial cells |
| ICAM-1 | intercellular adhesion molecule 1 |
| IFN-γ | interferon gamma |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| IL-1β | interleukin-1 β |
| IR | insulin receptor |
| IRE1 | inositol-requiring enzyme-1 |
| IRS-1 | insulin receptor substrate 1 |
| KATP channel | ATP-sensitive potassium channel |
| KRAS | GTPase Kirsten Rat Sarcoma Viral Oncogene Homolog |
| LCAT | lecithin-cholesterol acyltransferase |
| Mac-1 | macrophage-1 antigen |
| Mac-2 | macrophage-2 antigen |
| MAECs | mouse aortic endothelial cells |
| MafA | musculoaponeurotic fibrosarcoma oncogene family A |
| MAGEs | MGO-derived AGEs |
| MCP-1 | monocyte chemoattractant peptide-1 |
| MDA | malondialdehyde |
| MG-dG | 3-(20–deoxyribosyl)-6,7-dihydro-6,7-dihydroxy-6/7-methylimidazo-[2,3-b]purin-9(8)one |
| MG-H1-3 | MGO-derived hydroimidazolones 1-3 |
| MG-H1 | [Nδ -(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine] |
| MG-H2 | 2-amino-5-(2-amino-5-hydro-5-methyl-4- imidazolon-1-yl)-pentanoic acid |
| MG-H3 | 2-amino-5-(2-amino-4-hydro-4-methyl-5-imidazolon-1-yl)-pentanoic acid |
| MGO | methylglyoxal |
| MMP-9 | matrix metalloproteinase 9 |
| MnSOD | manganese superoxide dismutase |
| MODIC | 2-ammonio-6-((2-[(4- ammonio-5-oxido-5-oxopentyl)amino]-4-methyl-4,5- dihydro-1H-imidazol-5-ylidene)amino)hexanoate |
| MOLD | 1,3-di(Nε-lysino)-4-methyl-imidazolium |
| mTORC1 | mammalian target of rapamycin complex 1 |
| NAC | N-acetyl cysteine |
| NFATc | Nuclear factor of activated T-cells, cytoplasmic |
| NO | nitric oxide |
| NOX | NADPH oxidase |
| Nrf2 | nuclear factor erythroid 2 related factor 2 |
| OGTT | oral glucose tolerance test |
| p38 MAPK | p38 mitogen-activated protein kinase |
| PAI-1 | plasminogen activator inhibitor 1 |
| PARP | poly(ADP-ribose) polymerase |
| Pdx1 | gene coding for pancreatic duodenal homeobox-1 |
| PDX-1 | homeodomain (HD)-containing transcription factor (syn: IPF-1 (insulin promoter factor 1) |
| PERK | double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase |
| PGC1α | transcriptional coactivator PGC1-α |
| PI3K | phosphatidylinositol (PI) 3-kinase |
| PKB/Akt | protein kinase B (serine/threonine kinase) |
| PKC | protein kinase C |
| p-JNK | phosphorylated c-Jun NH2 - terminal kinase |
| p-p38 | phosphorylated p38 kinase |
| p-ERK | phosphorylated extracellular signal-regulated kinase |
| PHLPP2 | PH domain leucine-rich repeat protein phosphatase 2 |
| PON1 | paraoxonase 1 |
| PPAR | peroxisome proliferation-activated receptor |
| RAGE | AGEs receptor |
| RAAS | renin-angiotensin-aldosterone system |
| RCS | Reactive carbonyl species |
| RONS | Reactive oxygen and nitrogen species |
| sdLDL | small dense low density lipoproteins |
| SD rats | Sprague Dawley rats |
| SHR | Spontaneously hypertensive rats |
| SNP | sodium nitroprusside |
| SSAO | semicarbazide-sensitive amine oxidase |
| STZ | streptozotocin |
| sICAM-1 | soluble intercellular adhesion molecule 1 |
| SOD-(1-3) | superoxide dismutase (1-3) |
| sPLA2 | secreted phospholipase A 2 |
| sRAGE | soluble RAGE produced by alternative splicing |
| sVCAM-1 | soluble vascular cell adhesion molecule 1 |
| TAG | triacylglycerol |
| TAK1 | transforming growth factor-β-activated kinase 1 |
| T1DM | type 1 diabetes |
| T2DM | type 2 diabetes |
| TGF-β | transforming growth factor β |
| THP | tetrahydropyrimidine |
| TNF-α | tumor necrosis factor α |
| UCP-2 | uncoupling protein 2 |
| UDPGlcNAc | uridine diphosphate N-acetylglucosamine |
| UPR | unfolded protein response |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
| VEGFR-2 | vascular endothelial growth factor receptor 2 |
| VSMCs | vascular smooth muscle cells |
| 8-OHdG | 8-hydroxy-2-deoxyguanosine |
| WKY | Wistar Kyoto rats |
References
- Nigro C, Leone A, Fiory F, Prevenzano I, Nicolò A, Mirra P, Beguinot F, Miele C. Dicarbonyl Stress at the Crossroads of Healthy and Unhealthy Aging. Cells. 2019, 8, 749. [CrossRef]
- Stratmann, B. Dicarbonyl Stress in Diabetic Vascular Disease. Int J Mol Sci. 2022 May 31;23(11):6186. [CrossRef]
- Kosmachevskaya, O.V.; Novikova, N.N.; Topunov, A.F. Carbonyl Stress in Red Blood Cells and Hemoglobin. Antioxidants (Basel). 2021, 10, 253. [CrossRef] [PubMed]
- Hoffmann, G.R. A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response. 2009, 7, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Zemva, J.; Fink, C.A.; Fleming, T.H.; Schmidt, L.; Loft, A.; Herzig, S.; Knieß, R.A.; Mayer, M.; Bukau, B.; Nawroth, P.P. Hormesis enables cells to handle accumulating toxic metabolites during increased energy flux. Redox Biol. 2017, 13, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, M.; Priebe, S.; Grigolon, G.; Rozanov, L.; Groth, M.; Laube, B.; Guthke, R.; Platzer, M.; Zarse, K.; Ristow, M. Impairing L-Threonine Catabolism Promotes Healthspan through Methylglyoxal-Mediated Proteohormesis. Cell Metab. 2018, 27, 914–925. [Google Scholar] [CrossRef]
- Masania J, Malczewska-Malec M, Razny U, Goralska J, Zdzienicka A, Kiec-Wilk B, Gruca A, Stancel-Mozwillo J, Dembinska-Kiec A, Rabbani N, Thornalley PJ. Dicarbonyl stress in clinical obesity. Glycoconj J. 2016, 33, 581–9. [CrossRef]
- Bierhaus, A.; Fleming, T.; Stoyanov, S.; Leffler, A.; Babes, A.; Neacsu, C.; Sauerj, S.K.; Eberhardt, M.; Schnölzer, M.; et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat. Med. 2012, 18, 926–933, Erratum in: Nat Med. 2012 Sep;18(9):1445. Lasischka, Felix [corrected to Lasitschka, Felix]. [Google Scholar] [CrossRef]
- McLellan, A.C.; Thornalley, P.J.; Benn, J.; Sonksen, P.H. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin. Sci. (Lond). 1994, 87, 21–29. [Google Scholar] [CrossRef]
- Fleming, T.; Cuny, J.; Nawroth, G.; Djuric, Z.; Humpert, P.M.; Zeier, M.; Bierhaus, A.; Nawroth, P.P. Is diabetes an acquired disorder of reactive glucose metabolites and their intermediates?. Diabetologia. 2012, 55, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, R.; Shuck, S.C.; Chan, Y.S.; Liu, X.; Bates, S.E.; Lim, P.P.; Tamae, D.; Lacoste, S.; O'Connor, T.R.; Termini, J. DNA Advanced Glycation End Products (DNA-AGEs) Are Elevated in Urine and Tissue in an Animal Model of Type 2 Diabetes. Chem. Res. Toxicol. 2017, 30, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Synold, T.; Xi, B.; Wuenschell, G.E.; Tamae, D.; Figarola, J.L.; Rahbar, S.; Termini, J. Advanced glycation end products of DNA: quantification of N2-(1-Carboxyethyl)-2'-deoxyguanosine in biological samples by liquid chromatography electrospray ionization tandem mass spectrometry. Chem. Res. Toxicol. 2008, 21, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.A.; Mirrlees, D.; Thornalley, P.J. Modification of the glyoxalase system in streptozotocin-induced diabetic rats. Effect of the aldose reductase inhibitor Statil. Biochem. Pharmacol. 1993, 46, 805–811. [Google Scholar] [CrossRef]
- Chou, C.K.; Lee, Y.T.; Chen, S.M.; Hsieh, C.W.; Huang, T.C.; Li, Y.C.; Lee, J.A. Elevated urinary D-lactate levels in patients with diabetes and microalbuminuria. J. Pharm. Biomed. Anal. 2015, 116, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.L.; Hanssen, N.M.; van de Waarenburg, M.P.; Jonkers, D.M.; Stehouwer, C.D.; Schalkwijk, C.G. L(+) and D(-) lactate are increased in plasma and urine samples of type 2 diabetes as measured by a simultaneous quantification of L(+) and D(-) lactate by reversed-phase liquid chromatography tandem mass spectrometry. Exp. Diabetes Res. 2012, 2012, 234812. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.K.; Touchette, A.D.; Lal, S.; Szwergold, B.S. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes. 1999, 48, 198–202. [Google Scholar] [CrossRef]
- McLellan, A.C.; Phillips, S.A.; Thornalley, P.J. The assay of methylglyoxal in biological systems by derivatization with 1,2-diamino-4,5-dimethoxybenzene. Anal. Biochem. 1992, 206, 17–23. [Google Scholar] [CrossRef]
- McLellan, A.C.; Phillips, S.A.; Thornalley, P.J. Fluorimetric assay of D-lactate. Anal. Biochem. 1992, 206, 12–16. [Google Scholar] [CrossRef]
- Christopher, M.M.; Broussard, J.D.; Fallin, C.W.; Drost, N.J.; Peterson, M.E. Increased serum D-lactate associated with diabetic ketoacidosis. Metabolism. 1995, 44, 287–290. [Google Scholar] [CrossRef]
- van Eupen MG, Schram MT, Colhoun HM, Hanssen NM, Niessen HW, Tarnow L, Parving HH, Rossing P, Stehouwer CD, Schalkwijk CG. The methylglyoxal-derived AGE tetrahydropyrimidine is increased in plasma of individuals with type 1 diabetes mellitus and in atherosclerotic lesions and is associated with sVCAM-1.. Diabetologia. 2013, 56, 1845–55. [CrossRef]
- Kilhovd BK, Giardino I, Torjesen PA, Birkeland KI, Berg TJ, Thornalley PJ, Brownlee M, Hanssen KF. Increased serum levels of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism. 2003, 52, 163–7. [CrossRef]
- Hanssen, N.M.; Beulens, J.W.; van Dieren, S.; Scheijen, J.L.; van der A, D.L.; Spijkerman, A.M.; van der Schouw, Y.T.; Stehouwer, C.D.; Schalkwijk, C.G. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: a case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes. 2015, 64, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Fosmark DS, Torjesen PA, Kilhovd BK, Berg TJ, Sandvik L, Hanssen KF, Agardh CD, Agardh E. Increased serum levels of the specific advanced glycation end product methylglyoxal-derived hydroimidazolone are associated with retinopathy in patients with type 2 diabetes mellitus. Metabolism. 2006, 55, 232–6. [CrossRef]
- Phillips, S.A.; Thornalley, P.J. The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur. J. Biochem. 1993, 212, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sousa Silva, M.; Gomes, R.A.; Ferreira, A.E.; Ponces Freire, A.; Cordeiro, C. The glyoxalase pathway: the first hundred years... and beyond. Biochem. J. 2013, 453, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, J.; Campos Campos, M.; Nawroth, P.; Fleming, T. The Glyoxalase System-New Insights into an Ancient Metabolism. Antioxidants (Basel). 2020, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem. J. 1988, 254, 751–755. [Google Scholar] [CrossRef]
- Martins, A.M.; Cordeiro, C.A.; Ponces Freire, A.M. In situ analysis of methylglyoxal metabolism in Saccharomyces cerevisiae. FEBS Lett. 2001, 499, 41–44. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Wang X, Jia X, Chang T, Desai K, Wu L.. Attenuation of hypertension development by scavenging methylglyoxal in fructose-treated rats. J Hypertens. 2008, 26, 765–72. [CrossRef]
- Zhang X, Scheijen JLJM, Stehouwer CDA, Wouters K, Schalkwijk CG. Increased methylglyoxal formation in plasma and tissues during a glucose tolerance test is derived from exogenous glucose. Clin Sci (Lond). 2023, 137, 697–706. [CrossRef]
- Mortera, R.R.; Bains, Y.; Gugliucci, A. Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front. Biosci. (Landmark Ed). 2019, 24, 186–211. [Google Scholar] [CrossRef]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef]
- Vasdev S, Ford CA, Longerich L, Gadag V, Wadhawan S. Role of aldehydes in fructose induced hypertension. Mol Cell Biochem. 1998, 181, 1–9. [CrossRef]
- Naomi, N.D.; Ngo, J.; Brouwer-Brolsma, E.M.; Buso, M.E.C.; Soedamah-Muthu, S.S.; Pérez-Rodrigo, C.; Harrold, J.A.; Halford, J.C.G.; Raben, A.; Geleijnse, J.M.; et al. Sugar-sweetened beverages, low/no-calorie beverages, fruit juice and non-alcoholic fatty liver disease defined by fatty liver index: the SWEET project. Nutr. Diabetes. 2023, 13, 6. [Google Scholar] [CrossRef]
- Gugliucci, A. Fructose surges damage hepatic adenosyl-monophosphate-dependent kinase and lead to increased lipogenesis and hepatic insulin resistance. Med Hypotheses. 2016, 93, 87–92. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids. 2012, 42, 1133–1142. [Google Scholar] [CrossRef]
- Hernandez-Castillo, C.; Shuck, S.C. Diet and Obesity-Induced Methylglyoxal Production and Links to Metabolic Disease. Chem. Res. Toxicol. 2021, 34, 2424–2440. [Google Scholar] [CrossRef]
- Kalapos, M.P. Where does plasma methylglyoxal originate from?. Diabetes Res. Clin. Pract. 2013, 99, 260–271. [Google Scholar] [CrossRef]
- Lyles, G.A.; Chalmers, J. The metabolism of aminoacetone to methylglyoxal by semicarbazide-sensitive amine oxidase in human umbilical artery. Biochem. Pharmacol. 1992, 43, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, Jafari, S.M.; Zou, L.; et al.; et al. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-dicarbonyl compounds in commonly consumed foods. J. Agric. Food. Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-H.; Shin, H.-S. In-solution derivatization and detection of glyoxal and methylglyoxal in alcoholic beverages and fermented foods by headspace solid-phase microextraction and gas chromatography−mass spectrometry. J. Food Compos. Anal. 2020, 92, 103584. [Google Scholar] [CrossRef]
- Papetti, A.; Mascherpa, D.; Gazzani, G. Free α-dicarbonyl compounds in coffee, barley coffee and soy sauce and effects of in vitro digestion. Food Chem. 2014, 164, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Lorenzo, G.; Morales, F.J. Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J. Agric. Food Chem. 2010, 58, 2966–2972. [Google Scholar] [CrossRef]
- Degen, J.; Vogel, M.; Richter, D.; Hellwig, M.; Henle, T. Metabolic transit of dietary methylglyoxal. J. Agric. Food. Chem. 2013, 61, 10253–10260. [Google Scholar] [CrossRef]
- Lo, T.W.; Westwood, M.E.; McLellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar] [CrossRef]
- Ahmed, N.; Argirov, O.K.; Minhas, H.S.; Cordeiro, C.A.; Thornalley, P.J. Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem. J. 2002, 364, 1–14. [Google Scholar] [CrossRef]
- Klöpfer, A.; Spanneberg, R.; Glomb, M.A. Formation of arginine modifications in a model system of Nα-tert-butoxycarbonyl (Boc)-arginine with methylglyoxal. J. Agric. Food Chem. 2011, 59, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Dobler, D.; Dean, M.; Thornalley, P.J. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J. Biol. Chem. 2005, 280, 5724–5732. [Google Scholar] [CrossRef]
- Ahmed, N.; Thornalley, P.J.; Dawczynski, J.; Franke, S.; Strobel, J.; Stein, G.; Haik, G.M. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest. Ophthalmol. Vis. Sci. 2003, 44, 5287–5292. [Google Scholar] [CrossRef]
- Wang, T.; Streeter, M.D.; Spiegel, D.A. Generation and characterization of antibodies against arginine-derived advanced glycation endproducts. Bioorg. Med. Chem. Lett. 2015, 25, 4881–4886. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J.J; Wepy, J.A.; Streeter, M.D.; Kingsley, P.J.; Mitchener, M.M.; Wauchope, O.R.; Beavers, W.N.; Rose, K.L.; Wang, T.; Spiegel, D.A.; Marnett, L.J. Methylglyoxal-derived posttranslational arginine modifications are abundant histone marks. Proc. Natl. Acad. Sci. U S A. 2018, 115, 9228–9233. [Google Scholar] [CrossRef]
- Oya T, Hattori N, Mizuno Y, Miyata S, Maeda S, Osawa T, Uchida K. Methylglyoxal modification of protein. Chemical and immunochemical characterization of methylglyoxal-arginine adducts. J Biol Chem. 1999, 274, 18492–502. [CrossRef]
- Lieuw-a-Fa ML, Schalkwijk CG, Engelse M, van Hinsbergh VW. Interaction of Nepsilon(carboxymethyl)lysine- and methylglyoxal-modified albumin with endothelial cells and macrophages. Splice variants of RAGE may limit the responsiveness of human endothelial cells to AGEs. Thromb Haemost. 2006, 95, 320–8. [CrossRef]
- Ahmed, N.; Thornalley, P.J. Peptide mapping of human serum albumin modified minimally by methylglyoxal in vitro and in vivo. Ann. N. Y. Acad. Sci. 2005, 1043, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Tanase, S.; Nakajou, K.; Maruyama, T.; Kragh-Hansen, U.; Otagiri, M. Role of arg-410 and tyr-411 in human serum albumin for ligand binding and esterase-like activity. Biochem. J. 2000, 349, 813–819. [Google Scholar] [CrossRef]
- Faure, P.; Troncy, L.; Lecomte, M.; Wiernsperger, N.; Lagarde, M.; Ruggiero, D.; Halimi, S. Albumin antioxidant capacity is modified by methylglyoxal. Diabetes Metab. 2005, 31, 169–177. [Google Scholar] [CrossRef]
- Abordo, E.A.; Thornalley, P.J. Synthesis and secretion of tumour necrosis factor-alpha by human monocytic THP-1 cells and chemotaxis induced by human serum albumin derivatives modified with methylglyoxal and glucose-derived advanced glycation endproducts. Immunol. Lett. 1997, 58, 139–147. [Google Scholar] [CrossRef]
- Fan, X.; Subramaniam, R.; Weiss, M.F.; Monnier, V.M. Methylglyoxal-bovine serum albumin stimulates tumor necrosis factor alpha secretion in RAW 264.7 cells through activation of mitogen-activating protein kinase, nuclear factor kappaB and intracellular reactive oxygen species formation. Arch. Biochem. Biophys. 2003, 409, 274–286. [Google Scholar] [CrossRef]
- Westwood, M.E.; Thornalley, P.J. Induction of synthesis and secretion of interleukin 1 beta in the human monocytic THP-1 cells by human serum albumins modified with methylglyoxal and advanced glycation endproducts. Immunol. Lett. 1996, 50, 17–21. [Google Scholar] [CrossRef]
- Dobler, D.; Ahmed, N.; Song, L.; Eboigbodin, K.E.; Thornalley, P.J. Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes. 2006, 55, 1961–1969. [Google Scholar] [CrossRef]
- Monnier, V.M.; Sell., D.R.; Strauch, C.; Sun, W.; Lachin, J.M.; Cleary, P.A.; Genuth, S.; DCCT Research Group. The association between skin collagen glucosepane and past progression of microvascular and neuropathic complications in type 1 diabetes. J. Diabetes Complications. 2013, 27, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bose, T.; Bhattacherjee, A.; Banerjee, S.; Chakraborti, A.S. Methylglyoxal-induced modifications of hemoglobin: structural and functional characteristics. Arch. Biochem. Biophys. 2013, 529, 99–104. [Google Scholar] [CrossRef]
- Lee JH, Samsuzzaman M, Park MG, Park SJ, Kim SY. Methylglyoxal-derived hemoglobin advanced glycation end products induce apoptosis and oxidative stress in human umbilical vein endothelial cells. Int J Biol Macromol. 2021, 187, 409–421. [CrossRef] [PubMed]
- Chen HJ, Chen YC, Hsiao CF, Chen PF. Mass Spectrometric Analysis of Glyoxal and Methylglyoxal-Induced Modifications in Human Hemoglobin from Poorly Controlled Type 2 Diabetes Mellitus Patients. Chem Res Toxicol. 2015, 28, 2377–89. [CrossRef]
- Jia X, Olson DJ, Ross AR, Wu L. Structural and functional changes in human insulin induced by methylglyoxal. FASEB J. 2006, 20, 1555–7. [CrossRef]
- Morcos, M.; Du, X.; Pfisterer, F.; Hutter, H.; Sayed, A.A.; Thornalley, P.; Ahmed, N.; Baynes, J.; Thorpe, S.; Kukudov, G.; et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell. 2008, 7, 260–269. [Google Scholar] [CrossRef]
- Queisser, M.A.; Yao, D.; Geisler, S.; Hammes, H.P.; Lochnit, G.; Schleicher, E.D.; Brownlee, M.; Preissner, K.T. Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes. 2010, 59, 670–678. [Google Scholar] [CrossRef]
- Folz, R.; Laiteerapong, N. The legacy effect in diabetes: are there long-term benefits?. Diabetologia. 2021, 64, 2131–2137. [Google Scholar] [CrossRef]
- Li, H.; Nakamura, S.; Miyazaki, S.; Morita, T.; Suzuki, M.; Pischetsrieder, M.; Niwa, T. N2-carboxyethyl-2'-deoxyguanosine, a DNA glycation marker, in kidneys and aortas of diabetic and uremic patients. Kidney Int. 2006, 69, 388–392. [Google Scholar] [CrossRef]
- Lee, J.Y.; Song, J.; Kwon, K.; Jang, S.; Kim, C.; Baek, K.; Kim, J.; Park, C. Human DJ-1 and its homologs are novel glyoxalases. Hum. Mol. Genet. 2012, 21, 3215–3225. [Google Scholar] [CrossRef]
- Smolders, S.; Van Broeckhoven, C. Genetic perspective on the synergistic connection between vesicular transport, lysosomal and mitochondrial pathways associated with Parkinson's disease pathogenesis. Acta Neuropathol. Commun. 2020, 8, 63. [Google Scholar] [CrossRef]
- Dolgacheva, L.P.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Abramov, A.Y. Role of DJ-1 in the mechanism of pathogenesis of Parkinson's disease. J. Bioenerg. Biomembr. 2019, 51, 175–188. [Google Scholar] [CrossRef]
- Richarme, G.; Mihoub, M.; Dairou, J.; Bui, L.C.; Leger, T.; Lamouri, A. Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem. 2015, 290, 1885–1897. [Google Scholar] [CrossRef]
- Richarme, G.; Liu, C.; Mihoub, M.; Abdallah, J.; Leger, T.; Joly, N.; Liebart, J.C.; Jurkunas, U.V.; Nadal, M.; Bouloc, P. et al. Guanine glycation repair by DJ-1/Park7 and its bacterial homologs. Science. 2017, 357, 208–211. [CrossRef]
- Pfaff, D.H.; Fleming, T.; Nawroth, P.; Teleman, A.A. Evidence Against a Role for the Parkinsonism-associated Protein DJ-1 in Methylglyoxal Detoxification. J. Biol. Chem. 2017, 292, 685–690. [Google Scholar] [CrossRef]
- Baba, S.P.; Barski, O.A.; Ahmed, Y.; O'Toole, T.E.; Conklin, D.J.; Bhatnagar, A.; Srivastava, S. Reductive metabolism of AGE precursors: a metabolic route for preventing AGE accumulation in cardiovascular tissue. Diabetes. 2009, 58, 2486–2497. [Google Scholar] [CrossRef]
- Schumacher, D.; Morgenstern, J.; Oguchi, Y.; Volk, N.; Kopf, S.; Groener, J.B.; Nawroth, P.P.; Fleming, T.; Freichel, M. Compensatory mechanisms for methylglyoxal detoxification in experimental & clinical diabetes. Mol. Metab. 2018, 18, 143–152. [Google Scholar] [CrossRef]
- Morgenstern, J.; Fleming, T.; Schumacher, D.; Eckstein, V.; Freichel, M.; Herzig, S.; Nawroth, P. Loss of Glyoxalase 1 Induces Compensatory Mechanism to Achieve Dicarbonyl Detoxification in Mammalian Schwann Cells. J. Biol. Chem. 2017, 292, 3224–3238. [Google Scholar] [CrossRef]
- Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, Assi HI. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int J Mol Sci. 2022, 23, 786. [CrossRef]
- Lemieux, I.; Després, J.P. Metabolic Syndrome: Past, Present and Future. Nutrients. 2020, 12, 3501. [Google Scholar] [CrossRef]
- Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects), Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.B.; Danaei, G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet. 2014, 383, 970–983. [CrossRef]
- Lee, G.H.; Proenca, R.; Montez, J.M.; Carroll, K.M.; Darvishzadeh, J.G.; Lee, J.I.; Friedman, J.M. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996, 379, 632–635. [Google Scholar] [CrossRef]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Francisco FA, Barella LF, Silveira SDS, Saavedra LPJ, Prates KV, Alves VS, Franco CCDS, Miranda RA, Ribeiro TA, Tófolo LP, Malta A, Vieira E, Palma-Rigo K, Pavanello A, Martins IP, Moreira VM, de Oliveira JC, Mathias PCF, Gomes RM. Methylglyoxal treatment in lactating mothers leads to type 2 diabetes phenotype in male rat offspring at adulthood. Eur J Nutr. 2018, 57, 477–486. [CrossRef]
- Liu J, Wang R, Desai K, Wu L. Upregulation of aldolase B and overproduction of methylglyoxal in vascular tissues from rats with metabolic syndrome. Cardiovasc Res. 2011, 92, 494–503. [CrossRef] [PubMed]
- Chang T, Wang R, Wu L. Methylglyoxal-induced nitric oxide and peroxynitrite production in vascular smooth muscle cells. Free Radic Biol Med. 2005, 38, 286–93. [CrossRef]
- Wang H, Liu J, Wu L. Methylglyoxal-induced mitochondrial dysfunction in vascular smooth muscle cells. Biochem Pharmacol. 2009, 77, 1709–16. [CrossRef] [PubMed]
- Tsokanos FF, Muley C, Khani S, Hass D, Fleming T, Wolff G, Bartelt A, Nawroth P, Herzig S. Methylglyoxal Drives a Distinct, Nonclassical Macrophage Activation Status. Thromb Haemost. 2021, 121, 1464–1475. [CrossRef]
- Prantner D, Nallar S, Richard K, Spiegel D, Collins KD, Vogel SN.Classically activated mouse macrophages produce methylglyoxal that induces a TLR4- and RAGE-independent proinflammatory response. J Leukoc Biol. 2021, 109, 605–619. [CrossRef]
- Wang X, Desai K, Clausen JT, Wu L. Increased methylglyoxal and advanced glycation end products in kidney from spontaneously hypertensive rats. Kidney Int. 2004, 66, 2315–21. [CrossRef]
- Wang X, Desai K, Chang T, Wu L. Vascular methylglyoxal metabolism and the development of hypertension. J Hypertens. 2005, 23, 1565–73. [CrossRef]
- Wang X, Chang T, Jiang B, Desai K, Wu L. Attenuation of hypertension development by aminoguanidine in spontaneously hypertensive rats: role of methylglyoxal. Am J Hypertens. 2007, 20, 629–36. [CrossRef]
- Mukohda M, Okada M, Hara Y, Yamawaki H. Methylglyoxal accumulation in arterial walls causes vascular contractile dysfunction in spontaneously hypertensive rats. J Pharmacol Sci. 2012, 120, 26–35. [CrossRef]
- Mukohda M, Morita T, Okada M, Hara Y, Yamawaki H. Long-term methylglyoxal treatment causes endothelial dysfunction of rat isolated mesenteric artery. J Vet Med Sci. 2013, 75, 151–7. [CrossRef]
- Mukohda M, Yamawaki H, Nomura H, Okada M, Hara Y. Methylglyoxal inhibits smooth muscle contraction in isolated blood vessels. J Pharmacol Sci. 2009, 109, 305–10. [CrossRef]
- Subramanian U, Nagarajan D. All-Trans Retinoic Acid supplementation prevents cardiac fibrosis and cytokines induced by Methylglyoxal. Glycoconj J. 2017, 34, 255–265. [CrossRef]
- Jia X, Wu L. Accumulation of endogenous methylglyoxal impaired insulin signaling in adipose tissue of fructose-fed rats. Mol Cell Biochem. 2007, 306, 133–9. [CrossRef]
- Jang, S.; Kwon, D.M.; Kwon, K.; Park, C. Generation and characterization of mouse knockout for glyoxalase 1. Biochem. Biophys. Res. Commun. 2017, 490, 460–465. [Google Scholar] [CrossRef]
- Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J. Biol. Chem. 2011, 286, 1374–1380. [Google Scholar] [CrossRef]
- Brouwers, O.; de Vos-Houben, J.M.; Niessen, P.M.; Miyata, T.; van Nieuwenhoven, F.; Janssen, B.J.; Hageman, G.; Stehouwer, C.D.; Schalkwijk, C.G. Mild oxidative damage in the diabetic rat heart is attenuated by glyoxalase-1 overexpression. Int. J. Mol. Sci. 2013, 14, 15724–15739. [Google Scholar] [CrossRef]
- Brouwers O, Niessen PM, Haenen G, Miyata T, Brownlee M, Stehouwer CD, De Mey JG, Schalkwijk CG. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010, 53, 989–1000. [CrossRef]
- Brouwers O, Niessen PM, Miyata T, Østergaard JA, Flyvbjerg A, Peutz-Kootstra CJ, Sieber J, Mundel PH, Brownlee M, Janssen BJ, De Mey JG, Stehouwer CD, Schalkwijk CG. Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia. 2014, 57, 224–35. [CrossRef]
- Geoffrion, M.; Du, X.; Irshad, Z.; Vanderhyden, B.C.; Courville, K.; Sui, G.; D'Agati, V.D.; Ott-Braschi, S.; Rabbani, N.; Thornalley, P.J.; et al. Differential effects of glyoxalase 1 overexpression on diabetic atherosclerosis and renal dysfunction in streptozotocin-treated, apolipoprotein E-deficient mice. Physiol. Rep. 2014, 2, e12043. [Google Scholar] [CrossRef]
- Tikellis, C.; Pickering, R.J.; Tsorotes, D.; Huet, O.; Cooper, M.E.; Jandeleit-Dahm, K.; Thomas, M.C. Dicarbonyl stress in the absence of hyperglycemia increases endothelial inflammation and atherogenesis similar to that observed in diabetes. Diabetes. 2014, 63, 3915–3925. [Google Scholar] [CrossRef]
- Fang X, Liu L, Zhou S, Zhu M, Wang B. N-acetylcysteine inhibits atherosclerosis by correcting glutathione-dependent methylglyoxal elimination and dicarbonyl/oxidative stress in the aorta of diabetic mice. Mol Med Rep. 2021, 23, 201. [CrossRef]
- Nigro C, Raciti GA, Leone A, Fleming TH, Longo M, Prevenzano I, Fiory F, Mirra P, D'Esposito V, Ulianich L, Nawroth PP, Formisano P, Beguinot F, Miele C. Methylglyoxal impairs endothelial insulin sensitivity both in vitro and in vivo. Diabetologia. 2014, 57, 1485–94. [CrossRef]
- Hanssen, N.M.; Brouwers, O.; Gijbels, M.J.; Wouters, K.; Wijnands, E.; Cleutjens, J.P.; De Mey, J.G.; Miyata, T.; Biessen, E.A.; Stehouwer, C.D.; et al. Glyoxalase 1 overexpression does not affect atherosclerotic lesion size and severity in ApoE-/- mice with or without diabetes. Cardiovasc. Res. 2014, 104, 160–170. [Google Scholar] [CrossRef]
- Sena, C.M.; Matafome, P.; Crisóstomo, J.; Rodrigues, L.; Fernandes, R.; Pereira, P.; Seiça, R.M. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol. Res. 2012, 65, 497–506. [Google Scholar] [CrossRef]
- Matafome P, Santos-Silva D, Crisóstomo J, Rodrigues T, Rodrigues L, Sena CM, Pereira P, Seiça R. Methylglyoxal causes structural and functional alterations in adipose tissue independently of obesity. Arch Physiol Biochem. 2012, 118, 58–68. [CrossRef]
- Rodrigues T, Matafome P, Seiça R. Methylglyoxal further impairs adipose tissue metabolism after partial decrease of blood supply. Arch Physiol Biochem. 2013, 119, 209–18. [CrossRef]
- Rodrigues T, Matafome P, Sereno J, Almeida J, Castelhano J, Gamas L, Neves C, Gonçalves S, Carvalho C, Arslanagic A, Wilcken E, Fonseca R, Simões I, Conde SV, Castelo-Branco M, Seiça R. Methylglyoxal-induced glycation changes adipose tissue vascular architecture, flow and expansion, leading to insulin resistance. Sci Rep. 2017, 7, 1698. [CrossRef]
- Hüttl M, Markova I, Miklankova D, Makovicky P, Pelikanova T, Šeda O, Šedová L, Malinska H. Adverse Effects of Methylglyoxal on Transcriptome and Metabolic Changes in Visceral Adipose Tissue in a Prediabetic Rat Model. Antioxidants (Basel). 2020, 9, 803. [CrossRef]
- Berlanga, J.; Cibrian, D.; Guillén, I.; Freyre, F.; Alba, J.S.; Lopez-Saura, P.; Merino, N.; Aldama, A.; Quintela, A.M.; Triana, M.E.; et al. Methylglyoxal administration induces diabetes-like microvascular changes and perturbs the healing process of cutaneous wounds. Clin. Sci. (Lond). 2005, 109, 83–95. [Google Scholar] [CrossRef]
- Guo Q, Mori T, Jiang Y, Hu C, Osaki Y, Yoneki Y, Sun Y, Hosoya T, Kawamata A, Ogawa S, Nakayama M, Miyata T, Ito S. Methylglyoxal contributes to the development of insulin resistance and salt sensitivity in Sprague-Dawley rats. J Hypertens. 2009, 27, 1664–71. [CrossRef]
- Dhar A, Dhar I, Desai KM, Wu L.. Methylglyoxal scavengers attenuate endothelial dysfunction induced by methylglyoxal and high concentrations of glucose. Br J Pharmacol. 2010, 161, 1843–56. [CrossRef]
- Dhar A, Desai KM, Wu L.. Alagebrium attenuates acute methylglyoxal-induced glucose intolerance in Sprague-Dawley rats. Br J Pharmacol. 2010, 159, 166–75. [CrossRef]
- Chang T, Wang R, Olson DJ, Mousseau DD, Ross AR, Wu L.. Modification of Akt1 by methylglyoxal promotes the proliferation of vascular smooth muscle cells. FASEB J. 2011, 25, 1746–57. [CrossRef]
- Dhar A, Dhar I, Jiang B, Desai KM, Wu L.. Chronic methylglyoxal infusion by minipump causes pancreatic beta-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes. 2011, 60, 899–908. [CrossRef]
- Dhar I, Dhar A, Wu L, Desai KM. Methylglyoxal, a reactive glucose metabolite, increases renin angiotensin aldosterone and blood pressure in male Sprague-Dawley rats. Am J Hypertens. 2014, 27, 308–16. [CrossRef]
- Liu, H.; Yu, S.; Zhang, H.; Xu, J. Angiogenesis impairment in diabetes: role of methylglyoxal-induced receptor for advanced glycation endproducts, autophagy and vascular endothelial growth factor receptor 2. PLoS One. 2012, 7, e46720. [Google Scholar] [CrossRef]
- Vulesevic, B.; McNeill, B.; Giacco, F.; Maeda, K.; Blackburn, N.J.; Brownlee, M.; Milne, R.W.; Suuronen, E.J. Methylglyoxal-Induced Endothelial Cell Loss and Inflammation Contribute to the Development of Diabetic Cardiomyopathy. Diabetes. 2016, 65, 1699–1713. [Google Scholar] [CrossRef]
- Vulesevic, B.; McNeill, B.; Geoffrion, M.; Kuraitis, D.; McBane, J.E.; Lochhead, M.; Vanderhyden, B.C.; Korbutt, G.S.; Milne, R.W.; Suuronen, E.J. Glyoxalase-1 overexpression in bone marrow cells reverses defective neovascularization in STZ-induced diabetic mice. Cardiovasc. Res. 2014, 101, 306–316. [Google Scholar] [CrossRef]
- Wang, Y.; Hall, L.M.; Kujawa, M.; Li, H.; Zhang, X.; O'Meara, M.; Ichinose, T.; Wang, J.M. Methylglyoxal triggers human aortic endothelial cell dysfunction via modulation of the KATP/MAPK pathway. Am. J. Physiol. Cell. Physiol. 2019, 317, C68–C81. [Google Scholar] [CrossRef]
- Nigro C, Leone A, Longo M, Prevenzano I, Fleming TH, Nicolò A, Parrillo L, Spinelli R, Formisano P, Nawroth PP, Beguinot F, Miele C. Methylglyoxal accumulation de-regulates HoxA5 expression, thereby impairing angiogenesis in glyoxalase 1 knock-down mouse aortic endothelial cells. Biochim Biophys Acta Mol Basis Dis. 2019, 1865, 73–85. [CrossRef]
- Wang G, Wang Y, Yang Q, Xu C, Zheng Y, Wang L, Wu J, Zeng M, Luo M. Metformin prevents methylglyoxal-induced apoptosis by suppressing oxidative stress in vitro and in vivo. Cell Death Dis. 2022, 13, 29. [CrossRef]
- Mirra P, Nigro C, Prevenzano I, Procopio T, Leone A, Raciti GA, Andreozzi F, Longo M, Fiory F, Beguinot F, Miele C. The role of miR-190a in methylglyoxal-induced insulin resistance in endothelial cells. Biochim Biophys Acta Mol Basis Dis. 2017, 1863, 440–449. [CrossRef]
- Nigro C, Mirra P, Prevenzano I, Leone A, Fiory F, Longo M, Cabaro S, Oriente F, Beguinot F, Miele C. miR-214-Dependent Increase of PHLPP2 Levels Mediates the Impairment of Insulin-Stimulated Akt Activation in Mouse Aortic Endothelial Cells Exposed to Methylglyoxal. Int J Mol Sci. 2018, 19, 522. [CrossRef]
- Wu L, Juurlink BH. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension. 2002, 39, 809–14. [CrossRef]
- Riboulet-Chavey A, Pierron A, Durand I, Murdaca J, Giudicelli J, Van Obberghen E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes. 2006, 55, 1289–99. [CrossRef]
- Engelbrecht B, Mattern Y, Scheibler S, Tschoepe D, Gawlowski T, Stratmann B. Methylglyoxal impairs GLUT4 trafficking and leads to increased glucose uptake in L6 myoblasts. Horm Metab Res. 2014, 46, 77–84. [CrossRef]
- Fiory F, Lombardi A, Miele C, Giudicelli J, Beguinot F, Van Obberghen E. Methylglyoxal impairs insulin signalling and insulin action on glucose-induced insulin secretion in the pancreatic beta cell line INS-1E. Diabetologia. 2011, 54, 2941–52, Erratum in: Diabetologia. 2012, 55, 272. [CrossRef]
- Bo J, Xie S, Guo Y, Zhang C, Guan Y, Li C, Lu J, Meng QH. Methylglyoxal Impairs Insulin Secretion of Pancreatic β-Cells through Increased Production of ROS and Mitochondrial Dysfunction Mediated by Upregulation of UCP2 and MAPKs. J Diabetes Res. 2016;2016:2029854. [CrossRef]
- Wang H, Meng QH, Chang T, Wu L.. Fructose-induced peroxynitrite production is mediated by methylglyoxal in vascular smooth muscle cells. Life Sci. 2006, 79, 2448–54. [CrossRef]
- Kırça M, Yeşilkaya A. Methylglyoxal stimulates endoplasmic reticulum stress in vascular smooth muscle cells. J Recept Signal Transduct Res. 2022, 42, 279–284. [CrossRef]
- Baden T, Yamawaki H, Saito K, Mukohda M, Okada M, Hara Y. Telmisartan inhibits methylglyoxal-mediated cell death in human vascular endothelium. Biochem Biophys Res Commun. 2008, 373, 253–7. [CrossRef]
- Kim, J.H.; Kim, K.A.; Shin, Y.J.; Kim, H.; Majid, A.; Bae, O.N. Methylglyoxal induced advanced glycation end products (AGE)/receptor for AGE (RAGE)-mediated angiogenic impairment in bone marrow-derived endothelial progenitor cells. J. Toxicol. Environ. Health A. 2018, 81, 266–277. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Wouters, K.; Huijberts, M.S.; Gijbels, M.J.; Sluimer, J.C.; Scheijen, J.L.; Heeneman, S.; Biessen, E.A.; Daemen, M.J.; Brownlee, M.; et al. Higher levels of advanced glycation endproducts in human carotid atherosclerotic plaques are associated with a rupture-prone phenotype. Eur. Heart J. 2014, 35, 1137–1146. [Google Scholar] [CrossRef]
- Navarrete Santos A, Jacobs K, Simm A, Glaubitz N, Horstkorte R, Hofmann B. Dicarbonyls induce senescence of human vascular endothelial cells. Mech Ageing Dev. 2017 Sep;166:24-32. [CrossRef]
- Miyazawa N, Abe M, Souma T, Tanemoto M, Abe T, Nakayama M, Ito S. Methylglyoxal augments intracellular oxidative stress in human aortic endothelial cells. Free Radic Res. 2010, 44, 101–7. [CrossRef]
- Oba T, Tatsunami R, Sato K, Takahashi K, Hao Z, Tampo Y. Methylglyoxal has deleterious effects on thioredoxin in human aortic endothelial cells. Environ Toxicol Pharmacol. 2012, 34, 117–126. [CrossRef]
- Irshad Z, Xue M, Ashour A, Larkin JR, Thornalley PJ, Rabbani N. Activation of the unfolded protein response in high glucose treated endothelial cells is mediated by methylglyoxal. Sci Rep. 2019, 9, 7889. [CrossRef]
- Schalkwijk CG, van Bezu J, van der Schors RC, Uchida K, Stehouwer CD, van Hinsbergh VW. Heat-shock protein 27 is a major methylglyoxal-modified protein in endothelial cells. FEBS Lett. 2006, 580, 1565–70, Erratum in: FEBS Lett. 2006, 580, 2400. [CrossRef]
- Chu P, Han G, Ahsan A, Sun Z, Liu S, Zhang Z, Sun B, Song Y, Lin Y, Peng J, Tang Z. Phosphocreatine protects endothelial cells from Methylglyoxal induced oxidative stress and apoptosis via the regulation of PI3K/Akt/eNOS and NF-κB pathway. Vascul Pharmacol. 2017 Apr;91:26-35. [CrossRef]
- Braun JD, Pastene DO, Breedijk A, Rodriguez A, Hofmann BB, Sticht C, von Ochsenstein E, Allgayer H, van den Born J, Bakker S, Hauske SJ, Krämer BK, Yard BA, Albrecht T. Methylglyoxal down-regulates the expression of cell cycle associated genes and activates the p53 pathway in human umbilical vein endothelial cells. Sci Rep. 2019, 9, 1152. [CrossRef]
- Zhang X, Rodriguez-Niño A, Pastene DO, Pallavi P, van den Born J, Bakker SJL, Krämer BK, Yard BA. Methylglyoxal induces p53 activation and inhibits mTORC1 in human umbilical vein endothelial cells. Sci Rep. 2021, 11, 8004. [CrossRef]
- Brouwers O, Teerlink T, van Bezu J, Barto R, Stehouwer CD, Schalkwijk CG. Methylglyoxal and methylglyoxal-arginine adducts do not directly inhibit endothelial nitric oxide synthase. Ann N Y Acad Sci. 2008 Apr;1126:231-4. [CrossRef]
- Jang, J.H.; Kim, E.A.; Park, H.J.; Sung, E.G.; Song, I.H.; Kim, J.Y.; Woo, C.H.; Doh, K.O.; Kim, K.H.; Lee, T.J. Methylglyoxal-induced apoptosis is dependent on the suppression of c-FLIPL expression via down-regulation of p65 in endothelial cells. J. Cell. Mol. Med. 2017, 21, 2720–2731. [Google Scholar] [CrossRef]
- Su Y, Qadri SM, Wu L, Liu L. Methylglyoxal modulates endothelial nitric oxide synthase-associated functions in EA.hy926 endothelial cells. Cardiovasc Diabetol. 2013 Sep 19;12:134. [CrossRef]
- Stratmann, B.; Engelbrecht, B.; Espelage, B.C.; Klusmeier, N.; Tiemann, J. Gawlowski, T.; Mattern, Y.; Eisenacher, M.; Meyer, H.E.; Rabbani, N. et al. Glyoxalase 1-knockdown in human aortic endothelial cells - effect on the proteome and endothelial function estimates. Sci. Rep. 2016, 6, 37737. [Google Scholar] [CrossRef]
- Ahmed, U.; Dobler, D.; Larkin, S.; Rabbani, N.; Thornalley, P.J. Reversal of hyperglycemia-induced angiogenesis deficit of human endothelial cells by overexpression of glyoxalase 1 in vitro. Ann. N. Y. Acad. Sci. 2008, 1126, 262–264. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bose, N.; Gong, J.; Hall, D.; Rifkind, A.; Bhaumik, D.; Peiris, T.H.; Chamoli, M.; Le, C.H.; Liu, J.; et al. A Caenorhabditis elegans Model Elucidates a Conserved Role for TRPA1-Nrf Signaling in Reactive α-Dicarbonyl Detoxification. Curr. Biol. 2016, 26, 3014–3025. [Google Scholar] [CrossRef]
- Moraru, A.; Wiederstein, J.; Pfaff, D.; Fleming, T.; Miller, A.K.; Nawroth, P.; Teleman, A.A. Elevated Levels of the Reactive Metabolite Methylglyoxal Recapitulate Progression of Type 2 Diabetes. Cell Metab. 2018, 27, 926–934.e8. [Google Scholar] [CrossRef]
- Lodd, E.; Wiggenhauser, L.M.; Morgenstern, J.; Fleming, T.H.; Poschet, G.; Büttner, M.; Tabler, C.T.; Wohlfart, D.P.; Nawroth, P.P.; Kroll, J. The combination of loss of glyoxalase1 and obesity results in hyperglycemia. JCI Insight. 2019, 4, e126154. [Google Scholar] [CrossRef]
- Nigro C, Leone A, Raciti GA, Longo M, Mirra P, Formisano P, Beguinot F, Miele C. Methylglyoxal-Glyoxalase 1 Balance: The Root of Vascular Damage. Int J Mol Sci. 2017, 18, 188. [CrossRef]
- Shamsaldeen YA, Mackenzie LS, Lione LA, Benham CD. Methylglyoxal, A Metabolite Increased in Diabetes is Associated with Insulin Resistance, Vascular Dysfunction and Neuropathies. Curr Drug Metab. 2016, 17, 359–67. [CrossRef]
- Mey JT, Haus JM. Dicarbonyl Stress and Glyoxalase-1 in Skeletal Muscle: Implications for Insulin Resistance and Type 2 Diabetes. Front Cardiovasc Med. 2018 Sep 10;5:117. [CrossRef]
- Sztalryd C, Brasaemle DL. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Oct;1862(10 Pt B):1221-1232. [CrossRef]
- Mirra P, Nigro C, Prevenzano I, Leone A, Raciti GA, Formisano P, Beguinot F, Miele C. The Destiny of Glucose from a MicroRNA Perspective. Front Endocrinol (Lausanne). 2018 Feb 26;9:46. [CrossRef]
- Minjares M, Wu W, Wang JM. Oxidative Stress and MicroRNAs in Endothelial Cells under Metabolic Disorders. Cells. 2023, 12, 1341. [CrossRef]
- Matafome P, Rodrigues T, Sena C, Seiça R. Methylglyoxal in Metabolic Disorders: Facts, Myths, and Promises. Med Res Rev. 2017, 37, 368–403. [CrossRef]
- Ozdemir AM, Hopfer U, Rosca MV, Fan XJ, Monnier VM, Weiss MF. Effects of advanced glycation end product modification on proximal tubule epithelial cell processing of albumin. Am J Nephrol. 2008, 28, 14–24. [CrossRef]
- Bierhaus A, Nawroth PP. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia. 2009, 52, 2251–63. [CrossRef]
- Jack MM, Ryals JM, Wright DE. Characterisation of glyoxalase I in a streptozocin-induced mouse model of diabetes with painful and insensate neuropathy. Diabetologia. 2011, 54, 2174–82. [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Eringa, E.C.; Serne, E.H.; Meijer, R.I.; Schalkwijk, C.G.; Houben, A.J.; Stehouwer, C.D.; Smulders, Y.M.; van Hinsbergh, V.W. Endothelial dysfunction in (pre)diabetes: characteristics, causative mechanisms and pathogenic role in type 2 diabetes. Rev. Endocr. Metab. Disord. 2013, 14, 39–48. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin. Sci. (Lond). 2005, 109, 143–159. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Micali, L.R.; Wouters, K. Advanced glycation endproducts in diabetes-related macrovascular complications: focus on methylglyoxal. Trends Endocrinol. Metab. 2023, 34, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature. 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Ebara, T.; Conde, K.; Kako, Y.; Liu, Y.; Xu, Y.; Ramakrishnan, R.; Goldberg, I.J.; Shachter, N.S. Delayed catabolism of apoB-48 lipoproteins due to decreased heparan sulfate proteoglycan production in diabetic mice. J. Clin. Invest. 2000, 105, 1807–1818. [Google Scholar] [CrossRef]
- Koya, D.; Jirousek, M.R.; Lin, Y.W; Ishii, H.; Kuboki, K.; King, G.L. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J. Clin. Invest. 1997, 100, 115–126. [Google Scholar] [CrossRef]
- Giacco, F. ; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Du, X.; Edelstein, D.; Obici, S.; Higham, N.; Zou, M.H.; Brownlee, M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J. Clin. Invest. 2006, 116, 1071–1080. [Google Scholar] [CrossRef]
- Ren, X.; Ren, L.; Wei, Q.; Shao, H.; Chen, L.; Liu, N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc. Diabetol. 2017, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Ravi, R.; Ragavachetty Nagaraj, N.; Subramaniam Rajesh, B. Effect of advanced glycation end product on paraoxonase 2 expression: Its impact on endoplasmic reticulum stress and inflammation in HUVECs. Life Sci. 2020, 246, 117397. [Google Scholar] [CrossRef]
- Shinohara, M.; Thornalley, P.J.; Giardino, I.; Beisswenger, P.; Thorpe, S.R.; Onorato, J.; Brownlee, M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J. Clin. Invest. 1998, 101, 1142–1147. [Google Scholar] [CrossRef]
- Wang H, Guo M, Wei H, Chen Y. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal Transduct Target Ther. 2023, 8, 92. [CrossRef]
- Brouwers O, Yu L, Niessen P, Slenter J, Jaspers K, Wagenaar A, Post M, Miyata T, Backes W, Stehouwer C, Huijberts M, Schalkwijk C. Glyoxalase-1 overexpression partially prevents diabetes-induced impaired arteriogenesis in a rat hindlimb ligation model. Glycoconj J. 2016, 33, 627–30. [CrossRef]
- Nin JW, Jorsal A, Ferreira I, Schalkwijk CG, Prins MH, Parving HH, Tarnow L, Rossing P, Stehouwer CD. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes Care. 2011, 34, 442–7. [CrossRef]
- Hanssen NM, Engelen L, Ferreira I, Scheijen JL, Huijberts MS, van Greevenbroek MM, van der Kallen CJ, Dekker JM, Nijpels G, Stehouwer CD, Schalkwijk CG. Plasma levels of advanced glycation endproducts Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine, and pentosidine are not independently associated with cardiovascular disease in individuals with or without type 2 diabetes: the Hoorn and CODAM studies. J Clin Endocrinol Metab. 2013, 98, E1369–E1373. [CrossRef]
- Semba RD, Bandinelli S, Sun K, Guralnik JM, Ferrucci L.. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J Am Geriatr Soc. 2009, 57, 1874–80. [CrossRef]
- Semba RD, Ferrucci L, Sun K, Beck J, Dalal M, Varadhan R, Walston J, Guralnik JM, Fried LP. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin Exp Res. 2009, 21, 182–90. [CrossRef]
- Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Hanssen KF, Laakso M. Increased serum levels of methylglyoxal-derived hydroimidazolone-AGE are associated with increased cardiovascular disease mortality in nondiabetic women. Atherosclerosis. 2009, 205, 590–4. [CrossRef]
- Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Birkeland KI, Berg TJ, Hanssen KF, Laakso M. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: a population-based 18-year follow-up study. Arterioscler Thromb Vasc Biol. 2005, 25, 815–20. [CrossRef]
- Busch M, Franke S, Wolf G, Brandstädt A, Ott U, Gerth J, Hunsicker LG, Stein G; Collaborative Study Group. The advanced glycation end product N(epsilon)-carboxymethyllysine is not a predictor of cardiovascular events and renal outcomes in patients with type 2 diabetic kidney disease and hypertension. Am J Kidney Dis. 2006, 48, 571–9. [CrossRef]
- Schwedler SB, Metzger T, Schinzel R, Wanner C. Advanced glycation end products and mortality in hemodialysis patients. Kidney Int. 2002, 62, 301–10. [CrossRef]
- Hanssen NMJ, Scheijen JLJM, Jorsal A, Parving HH, Tarnow L, Rossing P, Stehouwer CDA, Schalkwijk CG. Higher Plasma Methylglyoxal Levels Are Associated With Incident Cardiovascular Disease in Individuals With Type 1 Diabetes: A 12-Year Follow-up Study. Diabetes. 2017, 66, 2278–2283. [CrossRef]
- Hanssen NMJ, Westerink J, Scheijen JLJM, van der Graaf Y, Stehouwer CDA, Schalkwijk CG; SMART Study Group. Higher Plasma Methylglyoxal Levels Are Associated With Incident Cardiovascular Disease and Mortality in Individuals With Type 2 Diabetes. Diabetes Care. 2018, 41, 1689–1695. [CrossRef]
- Ogawa S, Nakayama K, Nakayama M, Mori T, Matsushima M, Okamura M, Senda M, Nako K, Miyata T, Ito S. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension. 2010, 56, 471–6. [CrossRef]
- Hanssen NMJ, Scheijen JLJM, Houben AJHM, van de Waarenburg M, Berendschot TTJM, Webers CAB, Reesink KD, van Greevenbroek MMJ, van der Kallen C, Schaper NC, Schram MT, Henry RMA, Stehouwer CDA, Schalkwijk CG. Fasting and post-oral-glucose-load levels of methylglyoxal are associated with microvascular, but not macrovascular, disease in individuals with and without (pre)diabetes: The Maastricht Study. Diabetes Metab. 2021, 47, 101148. [CrossRef]
- Baidoshvili A, Niessen HW, Stooker W, Huybregts RA, Hack CE, Rauwerda JA, Meijer CJ, Eijsman L, van Hinsbergh VW, Schalkwijk CG. N(omega)-(carboxymethyl)lysine depositions in human aortic heart valves: similarities with atherosclerotic blood vessels. Atherosclerosis. 2004, 174, 287–92. [CrossRef]
- Varona JF, Ortiz-Regalón R, Sánchez-Vera I, López-Melgar B, García-Durango C, Castellano Vázquez JM, Solís J, Fernández-Friera L, Vidal-Vanaclocha F. Soluble ICAM 1 and VCAM 1 Blood Levels Alert on Subclinical Atherosclerosis in Non Smokers with Asymptomatic Metabolic Syndrome. Arch Med Res. 2019, 50, 20–28. [CrossRef]
- Boekholdt SM, Keller TT, Wareham NJ, Luben R, Bingham SA, Day NE, Sandhu MS, Jukema JW, Kastelein JJ, Hack CE, Khaw KT. Serum levels of type II secretory phospholipase A2 and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. Arterioscler Thromb Vasc Biol. 2005, 25, 839–46. [CrossRef]
- Berge K, Aengevaeren VL, Mosterd A, Velthuis BK, Lyngbakken MN, Omland T, Schalkwijk CG, Eijsvogels TMH. Plasma Advanced Glycation End Products and Dicarbonyl Compounds Are Not Associated with Coronary Atherosclerosis in Athletes. Med Sci Sports Exerc. 2023, 55, 1143–1150. [CrossRef]
- Kerkeni M, Weiss IS, Jaisson S, Dandana A, Addad F, Gillery P, Hammami M. Increased serum concentrations of pentosidine are related to presence and severity of coronary artery disease. Thromb Res. 2014, 134, 633–8. [CrossRef]
- Mäkinen VP, Civelek M, Meng Q, Zhang B, Zhu J, Levian C, Huan T, Segrè AV, Ghosh S, Vivar J, Nikpay M, Stewart AF, Nelson CP, Willenborg C, Erdmann J, Blakenberg S, O'Donnell CJ, März W, Laaksonen R, Epstein SE, Kathiresan S, Shah SH, Hazen SL, Reilly MP; Coronary ARtery DIsease Genome-Wide Replication And Meta-Analysis (CARDIoGRAM) Consortium, Lusis AJ, Samani NJ, Schunkert H, Quertermous T, McPherson R, Yang X, Assimes TL.. Integrative genomics reveals novel molecular pathways and gene networks for coronary artery disease. PLoS Genet. 2014, 10, e1004502. [CrossRef]
- Ren J, Bi Y, Sowers JR, Hetz C, Zhang Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol. 2021, 18, 499–521. [CrossRef]
- Amponsah-Offeh M, Diaba-Nuhoho P, Speier S, Morawietz H. Oxidative Stress, Antioxidants and Hypertension. Antioxidants (Basel). 2023, 12, 281. [CrossRef]
- Natalucci S, Ruggeri P, Cogo CE, Picchio V, Burattini R. Insulin sensitivity and glucose effectiveness estimated by the minimal model technique in spontaneously hypertensive and normal rats. Exp Physiol. 2000, 85, 775–81. [CrossRef]
- Natalucci S, Ruggeri P, Cogo CE, Picchio V, Brunori A, Burattini R. Age-related analysis of glucose metabolism in spontaneously hypertensive and normotensive rats. Exp Physiol. 2003, 88, 399–404. [CrossRef]
- Jacobson R, Mignemi N, Rose K, O'Rear L, Sarilla S, Hamm HE, Barnett JV, Verhamme IM, Schoenecker J. The hyperglycemic byproduct methylglyoxal impairs anticoagulant activity through covalent adduction of antithrombin III. Thromb Res. 2014, 134, 1350–7. [CrossRef]
- Qiao YN, Zou YL, Guo SD. Low-density lipoprotein particles in atherosclerosis. Front Physiol. 2022 Aug 30;13:931931. [CrossRef]
- Rabbani N, Godfrey L, Xue M, Shaheen F, Geoffrion M, Milne R, Thornalley PJ. Glycation of LDL by methylglyoxal increases arterial atherogenicity: a possible contributor to increased risk of cardiovascular disease in diabetes. Diabetes. 2011, 60, 1973–80. [CrossRef]
- Rabbani N, Chittari MV, Bodmer CW, Zehnder D, Ceriello A, Thornalley PJ. Increased glycation and oxidative damage to apolipoprotein B100 of LDL cholesterol in patients with type 2 diabetes and effect of metformin. Diabetes. 2010, 59, 1038–45. [CrossRef]
- Lankin, V.; Konovalova, G.; Tikhaze, A.; Shumaev, K.; Kumskova, E.; Viigimaa, M. The initiation of free radical peroxidation of low-density lipoproteins by glucose and its metabolite methylglyoxal: a common molecular mechanism of vascular wall injure in atherosclerosis and diabetes. Mol. Cell. Biochem. 2014, 395, 241–252. [Google Scholar] [CrossRef]
- Godfrey L, Yamada-Fowler N, Smith J, Thornalley PJ, Rabbani N. Arginine-directed glycation and decreased HDL plasma concentration and functionality. Nutr Diabetes. 2014, 4, e134. [CrossRef]
- Kurosaki Y, Tsukushi T, Munekata S, Akahoshi T, Moriya T, Ogawa Z. Semiquantitative analysis of apolipoprotein A-I modified by advanced glycation end products in diabetes mellitus. J Clin Lab Anal. 2013, 27, 231–6. [CrossRef]
- Schalkwijk, C.G.; Micali, L.R.; Wouters, K. Advanced glycation endproducts in diabetes-related macrovascular complications: focus on methylglyoxal. Trends Endocrinol. Metab. 2023, 34, 49–60. [Google Scholar] [CrossRef]
- Miller, A. G. , Tan, G., Nagaraj, R. H., Cooper, M. E., & Wilkinson-Berka, J. L. (2009). Candesartan Attenuates Vascular Injury in Diabetic Retinopathy by Restoring Glyoxalase I Function. Investigative Ophthalmology & Visual Science, 50(13), 4806-4806.
- Miller AG, Tan G, Binger KJ, Pickering RJ, Thomas MC, Nagaraj RH, Cooper ME, Wilkinson-Berka JL.. Candesartan attenuates diabetic retinal vascular pathology by restoring glyoxalase-I function. Diabetes. 2010, 59, 3208–15. [CrossRef]
- Oh S, Ahn H, Park H, Lee JI, Park KY, Hwang D, Lee S, Son KH, Byun K. The attenuating effects of pyridoxamine on adipocyte hypertrophy and inflammation differ by adipocyte location. J Nutr Biochem. 2019 Oct;72:108173. [CrossRef]
- Rabbani N, Xue M, Weickert MO, Thornalley PJ. Reversal of Insulin Resistance in Overweight and Obese Subjects by trans-Resveratrol and Hesperetin Combination-Link to Dysglycemia, Blood Pressure, Dyslipidemia, and Low-Grade Inflammation. Nutrients. 2021, 13, 2374. [CrossRef]
- Salazar J, Navarro C, Ortega Á, Nava M, Morillo D, Torres W, Hernández M, Cabrera M, Angarita L, Ortiz R, Chacín M, D'Marco L, Bermúdez V. Advanced Glycation End Products: New Clinical and Molecular Perspectives. Int J Environ Res Public Health. 2021, 18, 7236. [CrossRef]
- Van den Eynde MDG, Geleijnse JM, Scheijen JLJM, Hanssen NMJ, Dower JI, Afman LA, Stehouwer CDA, Hollman PCH, Schalkwijk CG. Quercetin, but Not Epicatechin, Decreases Plasma Concentrations of Methylglyoxal in Adults in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial with Pure Flavonoids. J Nutr. 2018, 148, 1911–1916. [CrossRef]
- Yoon SJ, Yoon YW, Lee BK, Kwon HM, Hwang KC, Kim M, Chang W, Hong BK, Lee YH, Park SJ, Min PK, Rim SJ. Potential role of HMG CoA reductase inhibitor on oxidative stress induced by advanced glycation endproducts in vascular smooth muscle cells of diabetic vasculopathy. Exp Mol Med. 2009, 41, 802–11. [CrossRef]
- Recabarren-Leiva D, Burgos CF, Hernández B, Garcïa-García FJ, Castro RI, Guzman L, Fuentes E, Palomo I, Alarcón M. Effects of the age/rage axis in the platelet activation. Int J Biol Macromol. 2021 Jan 1;166:1149-1161. [CrossRef]
- Wang F, Yuan Q, Chen F, Pang J, Pan C, Xu F, Chen Y. Fundamental Mechanisms of the Cell Death Caused by Nitrosative Stress. Front Cell Dev Biol. 2021 Sep 20;9:742483. [CrossRef]
- Yamagishi S, Nakamura K, Matsui T, Ueda S, Fukami K, Okuda S. Agents that block advanced glycation end product (AGE)-RAGE (receptor for AGEs)-oxidative stress system: a novel therapeutic strategy for diabetic vascular complications. Expert Opin Investig Drugs. 2008, 17, 983–96. [CrossRef]
- Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L.. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med. 2018, 24, 59. [CrossRef]
- Quade-Lyssy P, Kanarek AM, Baiersdörfer M, Postina R, Kojro E. Statins stimulate the production of a soluble form of the receptor for advanced glycation end products. J Lipid Res. 2013, 54, 3052–61. [CrossRef]
- Toprak C, Yigitaslan S. Alagebrium and Complications of Diabetes Mellitus. Eurasian J Med. 2019, 51, 285–292. [CrossRef]
- Hanssen NMJ, Tikellis C, Pickering RJ, Dragoljevic D, Lee MKS, Block T, Scheijen JL, Wouters K, Miyata T, Cooper ME, Murphy AJ, Thomas MC, Schalkwijk CG. Pyridoxamine prevents increased atherosclerosis by intermittent methylglyoxal spikes in the aortic arches of ApoE-/- mice. Biomed Pharmacother. 2023 Feb;158:114211. [CrossRef]
- Thornalley, PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, Foiles PG, Freedman BI, Raskin P, Ratner RE, Spinowitz BS, Whittier FC, Wuerth JP; ACTION I Investigator Group. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004, 24, 32–40. [CrossRef]
- Kinsky OR, Hargraves TL, Anumol T, Jacobsen NE, Dai J, Snyder SA, Monks TJ, Lau SS. Metformin Scavenges Methylglyoxal To Form a Novel Imidazolinone Metabolite in Humans. Chem Res Toxicol. 2016, 29, 227–34. [CrossRef]
- Jinnouchi Y, Yamagishi S, Takeuchi M, Ishida S, Jinnouchi Y, Jinnouchi J, Imaizumi T. Atorvastatin decreases serum levels of advanced glycation end products (AGEs) in patients with type 2 diabetes. Clin Exp Med. 2006, 6, 191–3. [CrossRef]
- Scharnagl H, Stojakovic T, Winkler K, Rosinger S, März W, Boehm BO. The HMG-CoA reductase inhibitor cerivastatin lowers advanced glycation end products in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2007, 115, 372–5. [CrossRef]
- Contreras, C. L. Guzman-Rosiles, I., Del Castillo, D., Gomez-Ojeda, A., & Garay-Sevilla, M. E. (2017). Advanced glycation end products (AGEs) and sRAGE levels after benfotiamine treatment in diabetes mellitus type 2. The FASEB Journal, 2017, 31, 646–32. [Google Scholar] [CrossRef]
- Van den Eynde MDG, Houben AJHM, Scheijen JLJM, Linkens AMA, Niessen PM, Simons N, Hanssen NMJ, Kusters YHAM, Eussen SJMP, Miyata T, Stehouwer CDA, Schalkwijk CG. Pyridoxamine reduces methylglyoxal and markers of glycation and endothelial dysfunction, but does not improve insulin sensitivity or vascular function in abdominally obese individuals: A randomized double-blind placebo-controlled trial. Diabetes Obes Metab. 2023, 25, 1280–1291. [CrossRef]
- Bednarska K, Fecka I, Scheijen JLJM, Ahles S, Vangrieken P, Schalkwijk CG. A Citrus and Pomegranate Complex Reduces Methylglyoxal in Healthy Elderly Subjects: Secondary Analysis of a Double-Blind Randomized Cross-Over Clinical Trial. Int J Mol Sci. 2023, 24, 13168. [CrossRef]
- Bhuiyan MN, Mitsuhashi S, Sigetomi K, Ubukata M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci Biotechnol Biochem. 2017, 81, 882–890. [CrossRef]
- Colzani M, De Maddis D, Casali G, Carini M, Vistoli G, Aldini G. Reactivity, Selectivity, and Reaction Mechanisms of Aminoguanidine, Hydralazine, Pyridoxamine, and Carnosine as Sequestering Agents of Reactive Carbonyl Species: A Comparative Study. ChemMedChem. 2016, 11, 1778–89. [CrossRef]
- Bednarska K, Fecka I. Potential of Vasoprotectives to Inhibit Non-Enzymatic Protein Glycation, and Reactive Carbonyl and Oxygen Species Uptake. Int J Mol Sci. 2021, 22, 10026. [CrossRef]
- Voziyan PA, Metz TO, Baynes JW, Hudson BG. A post-Amadori inhibitor pyridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation. J Biol Chem. 2002, 277, 3397–403. [CrossRef]
- Ruggiero-Lopez D, Lecomte M, Moinet G, Patereau G, Lagarde M, Wiernsperger N. Reaction of metformin with dicarbonyl compounds. Possible implication in the inhibition of advanced glycation end product formation. Biochem Pharmacol. 1999, 58, 1765–73. [CrossRef]
- Rahbar S, Natarajan R, Yerneni K, Scott S, Gonzales N, Nadler JL. Evidence that pioglitazone, metformin and pentoxifylline are inhibitors of glycation. Clin Chim Acta. 2000 Nov;301(1-2):65-77. [CrossRef]
- Kiho T, Kato M, Usui S, Hirano K. Effect of buformin and metformin on formation of advanced glycation end products by methylglyoxal. Clin Chim Acta. 2005 Aug;358(1-2):139-45. [CrossRef]
- Adeshara K, Tupe R. Antiglycation and cell protective actions of metformin and glipizide in erythrocytes and monocytes. Mol Biol Rep. 2016, 43, 195–205. [CrossRef]
- Peters AS, Wortmann M, Fleming TH, Nawroth PP, Bruckner T, Böckler D, Hakimi M. Effect of metformin treatment in patients with type 2 diabetes with respect to glyoxalase 1 activity in atherosclerotic lesions. Vasa. 2019, 48, 186–192. [CrossRef]
- Qais FA, Sarwar T, Ahmad I, Khan RA, Shahzad SA, Husain FM. Glyburide inhibits non-enzymatic glycation of HSA: An approach for the management of AGEs associated diabetic complications. Int J Biol Macromol. 2021 Feb 1;169:143-152. [CrossRef]
- Li W, Ota K, Nakamura J, Naruse K, Nakashima E, Oiso Y, Hamada Y. Antiglycation effect of gliclazide on in vitro AGE formation from glucose and methylglyoxal. Exp Biol Med (Maywood). 2008, 233, 176–9. [CrossRef]
- Lee KY, Kim JR, Choi HC. Gliclazide, a KATP channel blocker, inhibits vascular smooth muscle cell proliferation through the CaMKKβ-AMPK pathway. Vascul Pharmacol. 2018 Mar;102:21-28. [CrossRef]
- Koyama H, Tanaka S, Monden M, Shoji T, Morioka T, Fukumoto S, Mori K, Emoto M, Shoji T, Fukui M, Fujii H, Nishizawa Y, Inaba M. Comparison of effects of pioglitazone and glimepiride on plasma soluble RAGE and RAGE expression in peripheral mononuclear cells in type 2 diabetes: randomized controlled trial (PioRAGE). Atherosclerosis. 2014, 234, 329–34. [CrossRef]
- Marx N, Walcher D, Ivanova N, Rautzenberg K, Jung A, Friedl R, Hombach V, de Caterina R, Basta G, Wautier MP, Wautiers JL.. Thiazolidinediones reduce endothelial expression of receptors for advanced glycation end products. Diabetes. 2004, 53, 2662–8. [CrossRef]
- Liu Y, Park JM, Chang KH, Huh HJ, Lee K, Lee MY. AMP-Activated Protein Kinase Mediates the Antiplatelet Effects of the Thiazolidinediones Rosiglitazone and Pioglitazone. Mol Pharmacol. 2016, 89, 313–21. [CrossRef]
- Adeshara KA, Agrawal SB, Gaikwad SM, Tupe RS. Pioglitazone inhibits advanced glycation induced protein modifications and down-regulates expression of RAGE and NF-κB in renal cells. Int J Biol Macromol. 2018 Nov;119:1154-1163. [CrossRef]
- Oz Gul O, Tuncel E, Yilmaz Y, Ulukaya E, Gul CB, Kiyici S, Oral AY, Guclu M, Ersoy C, Imamoglu S. Comparative effects of pioglitazone and rosiglitazone on plasma levels of soluble receptor for advanced glycation end products in type 2 diabetes mellitus patients. Metabolism. 2010, 59, 64–9. [CrossRef]
- Jakuš V, Hrnciarová M, Cársky J, Krahulec B, Rietbrock N. Inhibition of nonenzymatic protein glycation and lipid peroxidation by drugs with antioxidant activity. Life Sci. 1999;65(18-19):1991-3. [CrossRef]
- Miyata T, van Ypersele de Strihou C, Ueda Y, Ichimori K, Inagi R, Onogi H, Ishikawa N, Nangaku M, Kurokawa K. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol. 2002, 13, 2478–87. [CrossRef]
- Nangaku M, Miyata T, Sada T, Mizuno M, Inagi R, Ueda Y, Ishikawa N, Yuzawa H, Koike H, van Ypersele de Strihou C, Kurokawa K. Anti-hypertensive agents inhibit in vivo the formation of advanced glycation end products and improve renal damage in a type 2 diabetic nephropathy rat model. J Am Soc Nephrol. 2003, 14, 1212–22. [CrossRef]
- Sobal G, Menzel EJ, Sinzinger H. Calcium antagonists as inhibitors of in vitro low density lipoprotein oxidation and glycation. Biochem Pharmacol. 2001, 61, 373–9. [CrossRef]
- Brown BE, Mahroof FM, Cook NL, van Reyk DM, Davies MJ. Hydrazine compounds inhibit glycation of low-density lipoproteins and prevent the in vitro formation of model foam cells from glycolaldehyde-modified low-density lipoproteins. Diabetologia. 2006, 49, 775–83. [CrossRef]
- Aviram M, Rosenblat M, Bisgaier CL, Newton RS. Atorvastatin and gemfibrozil metabolites, but not the parent drugs, are potent antioxidants against lipoprotein oxidation. Atherosclerosis. 1998, 138, 271–80. [CrossRef]
- Lu L, Peng WH, Wang W, Wang LJ, Chen QJ, Shen WF. Effects of atorvastatin on progression of diabetic nephropathy and local RAGE and soluble RAGE expressions in rats. J Zhejiang Univ Sci B. 2011, 12, 652–9. [CrossRef]
- Hwang AR, Nam JO, Kang YJ. Fluvastatin inhibits advanced glycation end products-induced proliferation, migration, and extracellular matrix accumulation in vascular smooth muscle cells by targeting connective tissue growth factor. Korean J Physiol Pharmacol. 2018, 22, 193–201. [CrossRef]
- Fukuda K, Matsumura T, Senokuchi T, Ishii N, Kinoshita H, Yamada S, Murakami S, Nakao S, Motoshima H, Kondo T, Kukidome D, Kawasaki S, Kawada T, Nishikawa T, Araki E. Statins meditate anti-atherosclerotic action in smooth muscle cells by peroxisome proliferator-activated receptor-γ activation. Biochem Biophys Res Commun. 2015, 457, 23–30. [CrossRef]
- Ishibashi Y, Yamagishi S, Matsui T, Ohta K, Tanoue R, Takeuchi M, Ueda S, Nakamura K, Okuda S. Pravastatin inhibits advanced glycation end products (AGEs)-induced proximal tubular cell apoptosis and injury by reducing receptor for AGEs (RAGE) level. Metabolism. 2012, 61, 1067–72. [CrossRef]
- Spadaccio C, De Marco F, Di Domenico F, Coccia R, Lusini M, Barbato R, Covino E, Chello M. Simvastatin attenuates the endothelial pro-thrombotic shift in saphenous vein grafts induced by Advanced glycation endproducts. Thromb Res. 2014, 133, 418–25. [CrossRef]
- Deng J, Cai X, Hao M, Liu X, Chen Z, Li H, Liu J, Liao Y, Fu H, Chen H, Qin G, Yan D. Calcium Dobesilate (CaD) Attenuates High Glucose and High Lipid-Induced Impairment of Sarcoplasmic Reticulum Calcium Handling in Cardiomyocytes. Front Cardiovasc Med. 2021 Feb 2;8:637021. [CrossRef]
- Ribeiro ML, Seres AI, Carneiro AM, Stur M, Zourdani A, Caillon P, Cunha-Vaz JG; DX-Retinopathy Study Group. Effect of calcium dobesilate on progression of early diabetic retinopathy: a randomised double-blind study. Graefes Arch Clin Exp Ophthalmol. 2006, 244, 1591–600. [CrossRef]
- Liu G, Xia Q, Lu Y, Zheng T, Sang S, Lv L.. Influence of Quercetin and Its Methylglyoxal Adducts on the Formation of α-Dicarbonyl Compounds in a Lysine/Glucose Model System. J Agric Food Chem. 2017, 65, 2233–2239. [CrossRef]
- Liu XM, Liu YJ, Huang Y, Yu HJ, Yuan S, Tang BW, Wang PG, He QQ. Dietary total flavonoids intake and risk of mortality from all causes and cardiovascular disease in the general population: A systematic review and meta-analysis of cohort studies. Mol Nutr Food Res. 2017 Jun;61(6). [CrossRef]
- van Boekel MA, van den Bergh PJ, Hoenders HJ. Glycation of human serum albumin: inhibition by Diclofenac. Biochim Biophys Acta. 1992, 1120, 201–4. [CrossRef]
- Rendell, M., Nierenberg, J., Brannan, C., Valentine, J. L., Stephen, P. M., Dodds, S., Mercer P.; Smith P.K.; Walder, J. (1986). Inhibition of glycation of albumin and hemoglobin by acetylation in vitro and in vivo. The Journal of Laboratory and Clinical medicine. 1986, 108(4), 277–285. [CrossRef]
- Harding JJ, Egerton M, Harding RS. Protection against cataract by aspirin, paracetamol and ibuprofen. Acta Ophthalmol (Copenh). 1989, 67, 518–24. [CrossRef]
- Roberts KA, Harding JJ. Ibuprofen, a putative anti-cataract drug, protects the lens against cyanate and galactose. Exp Eye Res. 1990, 50, 157–64. [CrossRef]
- Rasheed S, Sánchez SS, Yousuf S, Honoré SM, Choudhary MI. Drug repurposing: In-vitro anti-glycation properties of 18 common drugs. PLoS One. 2018, 13, e0190509. [CrossRef]
- Booth AA, Khalifah RG, Hudson BG. Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation end-products: comparison with aminoguanidine. Biochem Biophys Res Commun. 1996, 220, 113–9. [CrossRef]
- Ahmed LA, Hassan OF, Galal O, Mansour DF, El-Khatib A. Beneficial effects of benfotiamine, a NADPH oxidase inhibitor, in isoproterenol-induced myocardial infarction in rats. PLoS One. 2020, 15, e0232413. [CrossRef]
- Ma H, Li SY, Xu P, Babcock SA, Dolence EK, Brownlee M, Li J, Ren J. Advanced glycation endproduct (AGE) accumulation and AGE receptor (RAGE) up-regulation contribute to the onset of diabetic cardiomyopathy. J Cell Mol Med. 2009 Aug;13(8B):1751-1764. Retraction in: J Cell Mol Med. 2023 May 7. Retraction in: J Cell Mol Med. 2023 May 7. [CrossRef]
- Metz TO, Alderson NL, Chachich ME, Thorpe SR, Baynes JW. Pyridoxamine traps intermediates in lipid peroxidation reactions in vivo: evidence on the role of lipids in chemical modification of protein and development of diabetic complications. J Biol Chem. 2003, 278, 42012–9. [CrossRef]
- Hagiwara S, Gohda T, Tanimoto M, Ito T, Murakoshi M, Ohara I, Yamazaki T, Matsumoto M, Horikoshi S, Funabiki K, Tomino Y. Effects of pyridoxamine (K-163) on glucose intolerance and obesity in high-fat diet C57BL/6J mice. Metabolism. 2009, 58, 934–45. [CrossRef]
- Unoki-Kubota H, Yamagishi S, Takeuchi M, Bujo H, Saito Y. Pyridoxamine, an inhibitor of advanced glycation end product (AGE) formation ameliorates insulin resistance in obese, type 2 diabetic mice. Protein Pept Lett. 2010, 17, 1177–81. [CrossRef]
- Takahashi H, Shibasaki T, Park JH, Hidaka S, Takahashi T, Ono A, Song DK, Seino S. Role of Epac2A/Rap1 signaling in interplay between incretin and sulfonylurea in insulin secretion. Diabetes. 2015, 64, 1262–72. [CrossRef]
- Lu Q, Li X, Liu J, Sun X, Rousselle T, Ren D, Tong N, Li J. AMPK is associated with the beneficial effects of antidiabetic agents on cardiovascular diseases. Biosci Rep. 2019, 39, BSR20181995. [CrossRef]
- Marx N, Walcher D, Ivanova N, Rautzenberg K, Jung A, Friedl R, Hombach V, de Caterina R, Basta G, Wautier MP, Wautiers JL.. Thiazolidinediones reduce endothelial expression of receptors for advanced glycation end products. Diabetes. 2004, 53, 2662–8. [CrossRef]
- Liu Y, Park JM, Chang KH, Huh HJ, Lee K, Lee MY. AMP-Activated Protein Kinase Mediates the Antiplatelet Effects of the Thiazolidinediones Rosiglitazone and Pioglitazone. Mol Pharmacol. 2016, 89, 313–21. [CrossRef]
- Reyaz A, Alam S, Chandra K, Kohli S, Agarwal S. Methylglyoxal and soluble RAGE in type 2 diabetes mellitus: Association with oxidative stress. J Diabetes Metab Disord. 2020, 19, 515–521. [CrossRef]
- Semo D, Obergassel J, Dorenkamp M, Hemling P, Strutz J, Hiden U, Müller N, Müller UA, Zulfikar SA, Godfrey R, Waltenberger J. The Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitor Empagliflozin Reverses Hyperglycemia-Induced Monocyte and Endothelial Dysfunction Primarily through Glucose Transport-Independent but Redox-Dependent Mechanisms. J Clin Med. 2023, 12, 1356. [CrossRef]
- Hwang AR, Han JH, Lim JH, Kang YJ, Woo CH. Fluvastatin inhibits AGE-induced cell proliferation and migration via an ERK5-dependent Nrf2 pathway in vascular smooth muscle cells. PLoS One. 2017, 12, e0178278. [CrossRef]
- Tusa I, Menconi A, Tubita A, Rovida E. Pathophysiological Impact of the MEK5/ERK5 Pathway in Oxidative Stress. Cells. 2023, 12, 1154. [CrossRef]
- Niedzielski M, Broncel M, Gorzelak-Pabiś P, Woźniak E. New possible pharmacological targets for statins and ezetimibe. Biomed Pharmacother. 2020 Sep;129:110388. [CrossRef]
- McCarty MF, O'Keefe JH, DiNicolantonio JJ. Pentoxifylline for vascular health: a brief review of the literature. Open Heart. 2016, 3, e000365. [CrossRef]
- Zhang X, Liu W, Wu S, Jin J, Li W, Wang N. Calcium dobesilate for diabetic retinopathy: a systematic review and meta-analysis. Sci China Life Sci. 2015, 58, 101–7. [CrossRef]
- Deng, J. , Feng, J., Sun, L., Suo, X., Yao, L., LI, Z., Wang Y., Zhou J., Wang, L. (2009). Aristolochic acid-induced endothelial cell injury and the mechanism of calcium dobesilate antagonism. Journal of Chinese Physician, 913-916.
- Haller H, Ji L, Stahl K, Bertram A, Menne J. Molecular Mechanisms and Treatment Strategies in Diabetic Nephropathy: New Avenues for Calcium Dobesilate-Free Radical Scavenger and Growth Factor Inhibition. Biomed Res Int. 2017;2017:1909258. [CrossRef]
- Zhou Y, Qi C, Li S, Shao X, Mou S, Ni Z. Diabetic Nephropathy Can Be Treated with Calcium Dobesilate by Alleviating the Chronic Inflammatory State and Improving Endothelial Cell Function. Cell Physiol Biochem. 2018, 51, 1119–1133. [CrossRef]
- Holovko, O. O. , Dmytrenko, O. P., Lesiuk, A. I., Kulish, M. P., Pavlenko, O. L., Naumenko, A. P., Pinchuk-Rugal, T. M.; Kaniuk M. I.; Veklich, T. O. (2023). Mechanisms of the interaction of bovine serum albumin with quercetin. -15. [CrossRef]
- Wang X, Ouyang YY, Liu J, Zhao G. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014, 111, 1–11. [CrossRef]
- Kim Y, Je Y. Flavonoid intake and mortality from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies. Clin Nutr ESPEN. 2017 Aug;20:68-77. [CrossRef]
- Grosso G, Micek A, Godos J, Pajak A, Sciacca S, Galvano F, Giovannucci EL.. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am J Epidemiol. 2017, 185, 1304–1316. [CrossRef]
- Micek A, Godos J, Del Rio D, Galvano F, Grosso G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose-Response Meta-Analysis. Mol Nutr Food Res. 2021, 65, e2001019. [CrossRef]
- Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2003, 57, 904–8. [CrossRef]
- Jiang W, Wei H, He B. Dietary flavonoids intake and the risk of coronary heart disease: a dose-response meta-analysis of 15 prospective studies. Thromb Res. 2015, 135, 459–63. [CrossRef]
- Indurthi VS, Leclerc E, Vetter SW. Calorimetric investigation of diclofenac drug binding to a panel of moderately glycated serum albumins. Eur J Pharm Sci. 2014 Aug 1;59:58-68. [CrossRef]
- Raval AD, Thakker D, Rangoonwala AN, Gor D, Walia R. Vitamin B and its derivatives for diabetic kidney disease. Cochrane Database Syst Rev. 2015 Jan 12;1:CD009403. [CrossRef]
- Ramis R, Ortega-Castro J, Caballero C, Casasnovas R, Cerrillo A, Vilanova B, Adrover M, Frau J. How Does Pyridoxamine Inhibit the Formation of Advanced Glycation End Products? The Role of Its Primary Antioxidant Activity. Antioxidants (Basel). 2019, 8, 344. [CrossRef]
- Maessen DE, Brouwers O, Gaens KH, Wouters K, Cleutjens JP, Janssen BJ, Miyata T, Stehouwer CD, Schalkwijk CG. Delayed Intervention With Pyridoxamine Improves Metabolic Function and Prevents Adipose Tissue Inflammation and Insulin Resistance in High-Fat Diet-Induced Obese Mice. Diabetes. 2016, 65, 956–66. [CrossRef]
- Borg DJ, Forbes JM. Targeting advanced glycation with pharmaceutical agents: where are we now? Glycoconj J. 2016, 33, 653–70. [CrossRef]
- Sambon M, Wins P, Bettendorff L.. Neuroprotective Effects of Thiamine and Precursors with Higher Bioavailability: Focus on Benfotiamine and Dibenzoylthiamine. Int J Mol Sci. 2021, 22, 5418. [CrossRef]
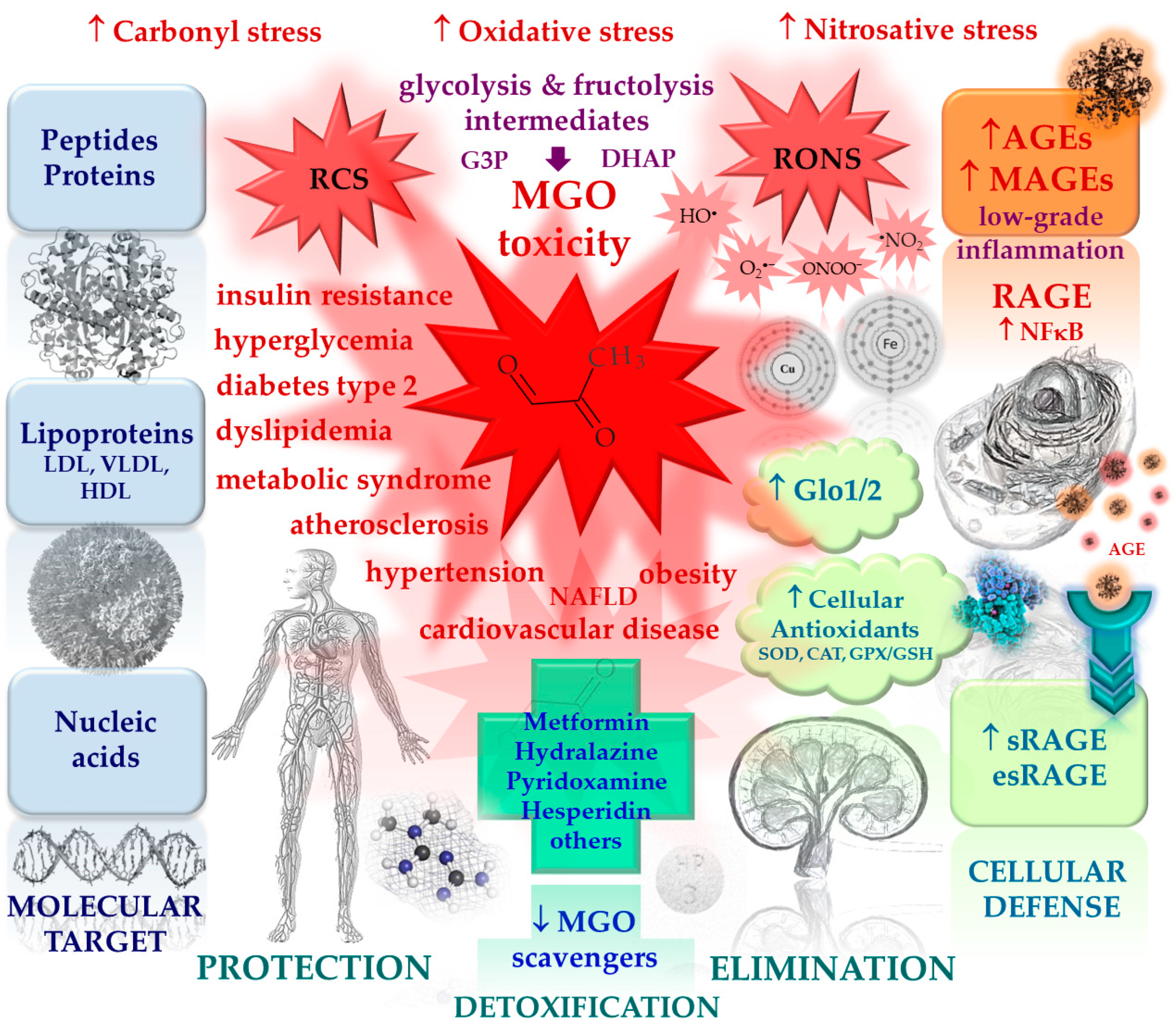
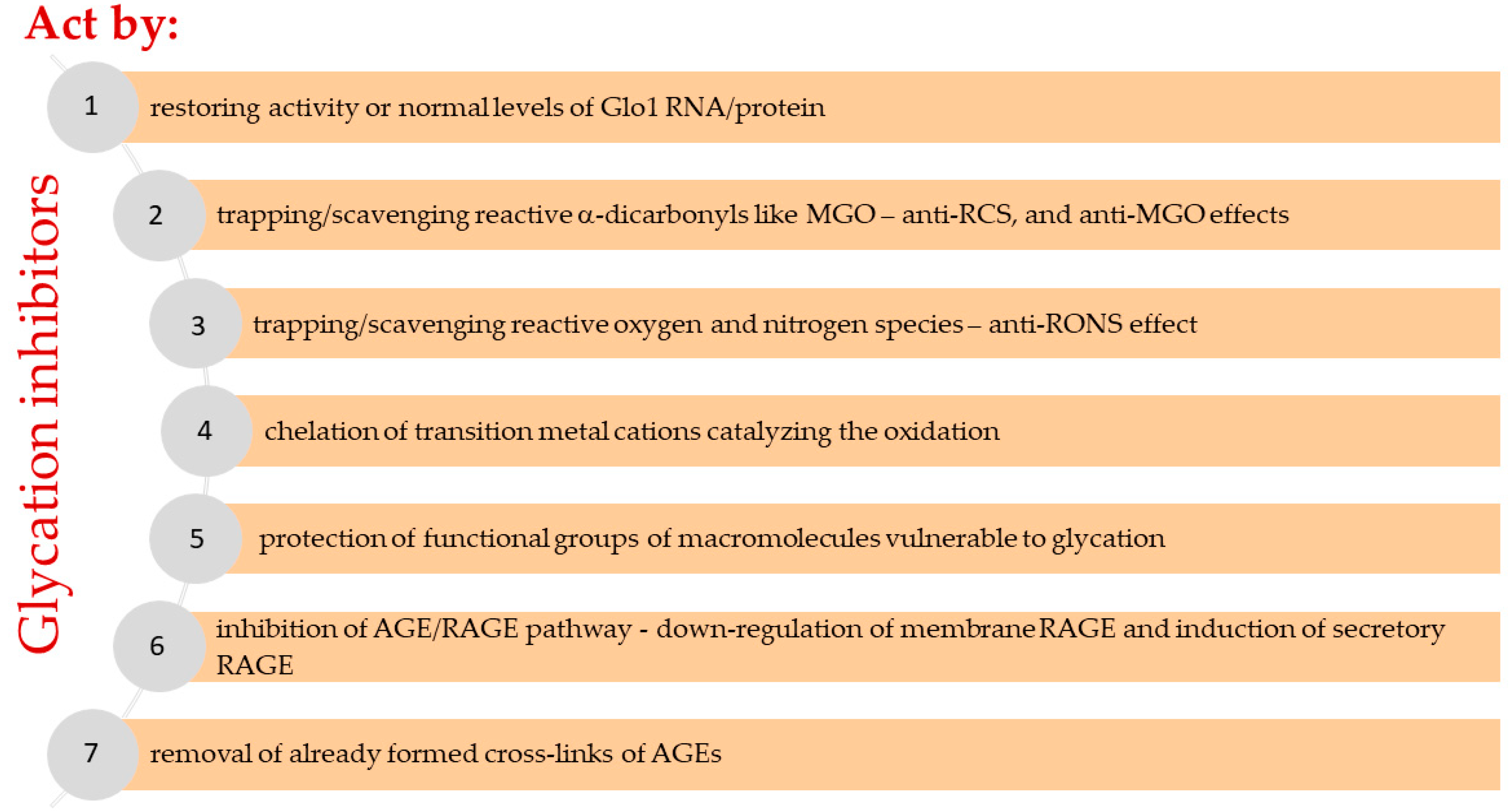
| Medication | AGE-RAGE/MAGE-RAGE axis grip points, and biochemical and physiological effects | Research models and methods |
|---|---|---|
| 1. Antihyperglycemic agents used in the management of type 2 diabetes (blood glucose lowering agents) | ||
| 1.1. Biguanides | ||
|
Metformin |
In vitro: MGO scavenger, inhibits carbonyl stress; reduces ↓ cross-linking, ↓ AGE, and ↓ HbA1c formation; restores the level of antioxidants in THP-1 cells and erythrocytes; diminishes mitochondrial complex I activity; activates ↑ AMPK; Human studies: reduces ↓ MGO in plasma in a dose-dependent manner; enhances ↑ Glo1 activity in peripheral blood cells and atherosclerotic lesions; scavenges MGO to form imidazolinone metabolite excreted in the urine |
BSA-MGO; LC-MS, and spectroscopic analysis of MGO adducts [238]; BSA-glucose, models of the early stage of glycation - Amadori products, and late glycation products - AGEs, ELISA [239]; BSA-MGO, RNase-MGO; ELISA, and western blot with the anti-AGE antibody [240]; BSA-MGO; glycation markers (fructosamine, carbonyls, free thiols, β-amyloid aggregation), and protein structural markers (absorption spectroscopy, gel electrophoresis) were examined; monocytes (THP-1 cells) and erythrocytes were treated with medication; and anti-oxidant indices (CAT, SOD, GSH, NO), cell viability, lipid peroxidation, and others were determined [241]; Case-control study: two groups of subjects with T2DM who were either treated with metformin (500 to 2500 mg/day for ≥ 3 months) or not treated with metformin and nondiabetic control subjects; HPLC, GC-MS [17]; patients with T2DM and carotid artery disease were included into the study prospectively; Glo1-activity and protein expression was measured by a spectrophotometric assay and western blot [242]; Proteomic and Metabolomic Biomarker Investigation of T2DM (project no. 07-0812-01), subjects recruited into the study included nondiabetic, pre-type-2 diabetic, and diabetic patients; NMR, LC-MS [228] |
| Buformin | In vitro: reduces ↓ AGE formation (more potent inhibitor than metformin) and ↓ cross-linking | BSA-MGO, RNase-MGO; ELISA, and western blot with the anti-AGE antibody; LC-MS analysis of MGO adducts [240] |
| 1.2. Sulfonylureas | ||
|
Glibenclamide (= glyburide) |
In vitro and Animal studies: reduces ↓ AGE formation; KATP channel antagonist; activates the JNK/p38 MAPK pathway; this effect arises partly through activation of KATP | HSA-glucose, HSA-MGO; AGEs, fructosamine, carbonyl groups, free lysine, and free thiol groups were determined; interaction studies, molecular docking [243]; male C57BL/6J mice; HAECs from healthy and T2DM donors; PCR and western blot analyses; Glo1 activity; immunofluorescence staining of MGO-AGEs in mouse aorta [126] |
| Gliclazide | In vitro and Animal studies: reduces ↓ AGE formation; KATP channel antagonist; inhibits vascular smooth muscle cell proliferation through the CaMKKβ–AMPK pathway; effects of KATP on AMPK activity is mediated by the regulation of intracellular Ca2+ levels | BSA-MGO, BSA-glucose; AGEs were assessed by fluorescence, ELISA, and western blot [244]; male C57BL/6 mice; LKB1-deficient A549 cells; VSMCs were isolated from the Sprague–Dawley rat thoracic aortas; cell proliferation assays using MTT; intracellular Ca2+ concentrations in VSMCs were assessed by a Ca2+ indicator dye; RT-PCR and western blot [245] |
| Glipizide | In vitro: reduces ↓ AGE formation; restores the level of antioxidants in THP-1 cells and erythrocytes | BSA-MGO; glycation markers (fructosamine, carbonyls, free thiols, β-amyloid aggregation) and protein structural markers (absorption spectroscopy, gel electrophoresis) were studied; THP-1 cells (monocytes) and erythrocytes were treated with medication; and antioxidant indicators (CAT, SOD, GSH, NO) were determined; cell viability, lipid peroxidation, and erythrocyte hemolysis were determined [241] |
| Glimepiride | Human studies: increases plasma ↑ esRAGE and reduces ↓ RAGE expression in peripheral mononuclear cells less than pioglitazone | RCT: a single-center randomized trial of pioglitazone versus glimepiride (clinical trial no. UMIN000002055); participants naive to glucose-lowering therapy at screening or taking 2 mg/day or less glimepiride or the equivalent dosage of another sulfonylurea; levels in the plasma of sRAGE and esRAGE, and RAGE expression in peripheral mononuclear cells were determined [246] |
| 1.3. Thiazolidinediones | ||
| Pioglitazone |
In vitro: reduces ↓ AGE and ↓ HbA1c formation; reduces ↓ RAGE and ↓ RAGE mRNA expression in human endothelial cells, thus limiting the EC susceptibility toward proinflammatory AGE effects; suppresses RAGE and NF-κB levels, hence alleviating cellular oxidative stress and inflammation; preferentially binds to protein and relieving protein structural changes; pioglitazone restores cellular antioxidants and reduces levels of IL-6 and TNF-α by declining expression of membrane RAGE and NF-κB; pioglitazone and rosiglitazone inhibit platelet aggregation by activating ↑ AMPK; Human studies: there is a significant increase in circulating ↑ sRAGE or sRAGE/esRAGE in the pioglitazone group; this effect is not observed in the rosiglitazone and medical nutrition therapy groups; pioglitazone suppresses RAGE expression and increases circulating sRAGE/esRAGE, and those activities are not necessarily dependent on plasma glucose or insulin resistance levels |
BSA-glucose; Amadori products, and late glycation products – AGEs; ELISA [239]; HUVECs, AGE-BSA, flow cytometry, western and northern blot, RT-PCR [247]; rat platelets; AMPK was examined by western blot with a specific antibody; PPAR-γ DNA binding with a PPAR-γ transcription factor assay kit; cGMP using a cGMP assay kit [248]; BSA-MGO; human embryonic kidney – 293 (HEK-293) cells; ROS was measured by fluorophotometric method; structural modification of albumins was analyzed by electrophoresis (native-PAGE) and HPLC; proteins were visualized with a chemiluminescence kit, and densitometry analysis of scanned western blot images; cytokines by ELISA; fructosamine, protein carbonyls, β-amyloid, free thiols in glycated albumin and AOPP were determined [249]; RCT: T2DM subjects were randomly assigned to receive pioglitazone (30 mg/day), rosiglitazone (4 mg/day), or placebo (medical nutrition therapy) for 12 weeks; changes in plasma glucose, HbA1c, insulin resistance (homeostasis model assessment), total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and sRAGE were evaluated at baseline and after 12 weeks [250]; a single-center randomized trial of pioglitazone versus glimepiride (clinical trial no. UMIN000002055); participants naive to glucose-lowering therapy at screening or taking 2 mg/day or less glimepiride or the equivalent dosage of another sulfonylurea; levels in the plasma of sRAGE and esRAGE, and RAGE expression in peripheral mononuclear cells were determined [246] |
| Rosiglitazone | ||
| 2.Agents for the treatment of cardiovascular conditions | ||
| 2.1. Angiotensin II receptor antagonists (blockers, ARBs) and angiotensin-converting enzyme inhibitors | ||
|
Candesartan Irbesartan Losartan Olmesartan Telmisartan Valsartan (ARBs) |
In vitroand Animal studies: reduces ↓ AGE (argpyrimidine, pentosidine and CML) formation; chelates transition metal cations, acts as an antioxidant, and inhibits the formation of ↓ ROS and ↓ RCS; the effect on AGE formation is common to all tested ARBs; a similar but milder effect is observed with ACE inhibitors (IC50 of pentosidine formation in BSA-arabinose model: valsartan > candesartan > olmesartan > temocaprilat > enalaprilat > irbesartan = losartan = telmisartan > captopril > perindoprilat); candesartan attenuates vascular injury in diabetic retinopathy by restoring Glox1 function and reducing ↓ ∙NO; reinstates both ↑ Glo1 activity and ↑ Glo1 mRNA level; reduces ↓ mRNA levels of ICAM-1, VEGF, TNF-α and iNOS; decreases ↓ total AGEs, MAGEs, and argpyrimidine in retina and plasma; olmesartan, in rats with type 2 like diabetes, reduces in a dose-dependent manner the development of diabetic nephropathy as evidenced by a decrease in proteinuria and pathologic evidence of diabetic glomerulosclerosis | HSA-glucose; Amadori products, and fluorescent AGEs were determined [251]; BSA-arabinose; pentosidine measurement by LC; CML by GC-MS; inhibition on leukocyte-derived superoxide production; RCS trapping; LC analysis of GO adducts; metal chelating activity test [252]; male subline of spontaneously hypertensive/NIH-corpulent rat - SHR/NDmc-cp (fat/fat); biochemical measurements in blood and urine; morphologic analysis; immunohistochemistry; pentosidine and CML were determined by LC and GC/MS, respectively [253]; bovine retinal endothelial cells (BREC), and bovine retinal pericytes (BRP); diabetic Sprague-Dawley rats; Ren-2 rats with an enhanced renin-angiotensin system; Glo1 activity was assessed by measuring the formation of S-lactoylglutathione; •NO with using 2,3-diaminonaphthalene; mRNA for Glo1, intercellular adhesion molecule (ICAM-1), vascular endothelial growth factor (VEGF), TNF-α, iNOS, and RAS component were quantitated by RT-PCR; argpyrimidine, MAGEs, and total AGEs (all AGEs including MAGEs) by ELISA [212,213] |
|
Captopril Enalaprilat (active metabolite of enalapril) Perindoprilat (active metabolite of perindopril) Temocaprilat (active metabolite of temocapril) (ACE inhibitors) | ||
| 2.2. Calcium channel antagonists (blockers) | ||
|
Amlodipine Isradipine Lacidipine Nifedipine (with vascular effects) |
In vitro: acts as an antioxidant (lacidipine > semotiadil > amlodipine > nifedipine > diltiazem), inhibits ↓ glycation and ↓ glycoxidation; inhibits the copper-mediated oxidation of non-glycated and glycated LDL | LDL-glucose; AGEs by ELISA; early glycation products were determined with the fructosamine test, and lipid peroxidation by TBARS quantitation [254] |
|
Diltiazem (with direct cardiac effects) | ||
| Semotiadil (experimental) | ||
| 2.3.Arterial smooth muscle agents, hydrazinophthalazine derivatives | ||
| Hydralazine |
In vitro: MGO scavenger, inhibits carbonyl stress; inhibits the formation of AGEs (pentosidine and CML); chelates transition metal cations, acts as an antioxidant, and inhibits the formation of ↓ ROS; inhibits the glycation of LDL and prevents the formation of model foam cells from RCS-modified low-density lipoproteins; Animal studies: the effect of hydralazine (5 mg) is similar to that of olmesartan (1 mg) but reached statistical significance only for kidney pentosidine content |
Ubiquitin-RCS; reaction products of MGO were generated in vitro and characterized by ESI-MS [235]; LDL-MGO; LDL modification was characterized by changes in mobility (agarose gel electrophoresis), cross-linking (SDS-PAGE) and loss of amino acid residues (LC); accumulation of cholesterol and cholesteryl esters in murine macrophages was assessed by LC [255]; male subline of spontaneously hypertensive/NIH-corpulent rat - SHR/NDmc-cp (fat/fat); biochemical measurements in blood and urine; morphologic analysis; immunohistochemistry; pentosidine and CML by LC and GC/MS, respectively [253] |
| 2.4. Statins (lipid modifying agents, HMG-CoA reductase inhibitors) | ||
| Atorvastatin |
In vitro: atorvastatin o- and p-OH metabolites are potent antioxidants and protect LDL, VLDL, and HDL from oxidation; the inhibitory effects of these metabolites on HDL oxidation are associated with the protection of paraoxonase activity; Animal studies: a serum and renal ↑ sRAGE level was up-regulated and associated with a reduction of AGEs, though renal esRAGE mRNA expression was not significantly increased; Human studies: decreases serum levels of ↓ AGEs in hypercholesterolemic T2DM patients without CVD but do not lower fasting glucose or HbA1c levels; AGEs changes do not correlate with lipid parameters; atorvastatin tends to decrease the serum level of 8-OHdG, but not significantly |
VLDL, LDL, and HDL were isolated from fasted normolipidemic volunteers; lipoprotein oxidation was measured by TBARS assay and photometrically by the lipid peroxidation test; LDL oxidation by macrophages; lipoprotein electrophoresis; DPPH assay; paraoxonase activity measurements [256]; Sprague-Dawley rats after streptozotocin-induced diabetes with or without atorvastatin treatment (10 mg/kg for 24 weeks); sRAGE and glycated albumin levels were measured by ELISA and bromocresol purple methods; renal AGEs, RAGE, sRAGE, and esRAGE were determined with PCR and western blot [257]; RTC: patients were treated with 10 mg atorvastatin by 4 weeks, control subjects were treated with diet therapy by 4 weeks; blood pressure, serum AGEs, serum 8-hydroxydeoxy-guanosine (8-OHdG), oxidative stress markers and others were measured before and after the treatment; AGEs by ELISA; others with commercially laboratory tests [229] |
| Lovastatin | In vitro: significantly increases the level of ↑ sRAGE by enhancement of full-length RAGE shedding but did not influence the secretion of esRAGE | Cell lines HEK/RAGE (stably expressing full-length RAGE) and HEK/esRAGE (stably expressing esRAGE being a recombinant version of the splice variant esRAGE); mouse alveolar epithelial (MLE-12) cells; immunoblot analysis; RT-PCR, and others [223] |
| Cerivastatin | Human studies: significantly lowered the concentration of ↓ CML-derived AGEs (compared to the placebo group); the effect on CML-AGEs is correlated with the reduction of LDL cholesterol and LDL apolipoprotein B; HbA1c is not changed | RCT: a multicenter, double-blind, randomized, parallel-group comparison of cerivastatin 0.4 mg daily for 12 weeks vs. placebo; the primary objective of the study was the effect of cerivastatin on dense LDL subfractions; CML-AGEs was assessed in patients with elevated fasting glucose, impaired glucose tolerance, or diabetes [230] |
| Fluvastatin |
In vitro: inhibits mitogen-activated protein kinase kinase ↓ MEK (MAPK/ERK kinase also known as MAP2K, MAPKK), which downregulated the transcription of EGR-1 (early growth response protein 1) and leads to decreased levels of CTGF (connective tissue growth factor), and consequently reduces proliferation, migration, and ECM (extracellular matrix) accumulation in AGE-induced VSMCs; activates ↑ PPAR-γ in HASMCs, but not in HUVECs; induces COX-2 expression in HASMCs, but not in HUVECs; suppresses migration and proliferation of HASMCs, and inhibited lipopolysaccharide-induced expression of MCP-1 (monocyte chemoattractant protein-1) and TNF-α in HASMCs; Animal studies: statins suppress atherosclerotic lesion formation in Apoe−/− mice; transcriptional activity of ↑ PPAR-γ was increased; and the expression of ↓ MCP-1 and ↓ TNF-α was decreased in the aorta of statin-treated Apoe−/− mice |
Primary cell culture of rat VSMCs; mRNA for CTGF as amplified cDNA was analyzed by qPCR; protein levels by western blot; cell proliferation by MTT assay; cell cycle by flow cytometry [258]; human aortic smooth muscle cell (HASMC) and human umbilical vein endothelial cell (HUVECs) cultures; ELISA assay kits of human MCP-1 and TNF-α; thymidine incorporation assay; cell migration assay; in an animal model (Apoe−/− mice) plasma total cholesterol, LDL cholesterol, HDL cholesterol, and triglyceride concentrations were measured [259] |
| Pitavastatin | ||
| Pravastatin | In vitro: inhibits ↓ AGE-induced up-regulation of RAGE mRNA level; inhibits ROS generation, and apoptosis in human renal proximal tubular cells | AGE-BSA in cultures of human renal proximal tubule epithelial cells ex vivo; gene expression was evaluated by qRT-PCR; ROS with dihydroethidium staining; apoptosis by ELISA; and others [260] |
| Rosuvastatin | ||
| Simvastatin |
In vitro: significantly reduces ↓ AGE-induced oxidative stress (ROS overproduction) in ECs and decreases neutrophil adhesion to endothelium; decreases ↓ RAGE mRNA expression, and non-statistically increases ↑ PPAR-γ mRNA expression (PPAR -γ has a protective effect on ECs by inhibiting endothelin-1 release and attenuating/preventing the endothelial inflammatory response); Animal studies: 12-week administration attenuates AGE-induced proliferation of aortic smooth muscle cells in Sprague-Dawley rats and reduces ↓ NF-κβ and ↓ MAPK activation in those cells |
AGE-BSA in cultures of endothelial cells (EC) ex vivo; EC were isolated from healthy subjects and diabetic patients (Hb1Ac< 6%, AGE > 7 μg/mL); fluorescently labeled neutrophils were counted manually under a microscope; ROS accumulation was estimated by spectrofluorimetric analysis; mRNA for RAGE and PPAR-γ as amplified cDNA was analyzed by RT-PCR [261]; male Sprague-Dawley rats; streptozocin-induced hyperglycemia; rat aortic smooth muscle cells (RASMCs); cultured RASMCs were co-incubated with AGE-BSA; measurement of intracellular ROS generation [218] |
| 2.5. Peripheral vasodilators (purine derivatives) | ||
| Pentoxifylline | In vitro: reduces ↓ AGE and ↓ HbA1C formation | BSA-glucose; models of the early stage of glycation - Amadori products, and late glycation products – AGEs; ELISA [239] |
| 2.6. Vasoprotectives (e.g. for the treatment of peripheral vascular disease) | ||
| 2.6.1. Antivaricose agents | ||
| Calcium dobesylate |
In vitro: reduces ↓ AGE formation; ROS scavenger; inhibits the formation of ↓ ROS, acts as an antioxidant; protects glycation reaction substrates from ROS and MGO-induced modifications; reduces the impairment of calcium handling in the sarcoplasmic reticulum and ↓ ROS formation in rat cardiomyocytes caused by high glucose and high lipid levels; Human studies: significantly reduces the permeability of the blood-retinal barrier (BRB) as measured by the posterior vitreous penetration ratio (PVPR); the effect is manifest regardless of the degree of metabolic control and the use of anti-hypertensive and lipid-lowering agents; has a significant beneficial effect in controlling the hemorrhages and the global evolution of diabetic retinopathy |
BSA-MGO; dicarbonyl trapping; LC-MS analysis of MGO and GO adducts [236]; neonatal rat ventricular myocytes (NRVMs); measurement of Action Potential (AP)-Elicited Ca2+ transient and SR Ca2+ content; confocal microscopy [262]; RCT: a double-blind placebo-controlled study, subjects with T2DM and early diabetic retinopathy, treatment was 2 g daily for 24 months, the primary parameter - posterior vitreous penetration ratio (PVPR), was measured every 6-months by fluorophotometry; secondary parameters were fundus photography, fluorescein angiography, and safety assessments [263] |
| 2.6.2. Capillary stabilizing agents (bioflavonoids) | ||
|
Diosmin (diosmetin – aglycone) |
In vitro: MGO scavenger (but not diosmin and troxerutin), ROS scavenger, inhibits the formation of ↓ ROS and ↓ RCS, acts as an antioxidant, chelates transition metal cations; reduces ↓ AGE formation; Human studies: quercetin-3-O-glucoside and hesperidin in (pre)hypertensive and healthy subjects decreased plasma ↓ MGO concentration (by ~10-11%); there was no significant change in Glo1 expression; the hesperetin and trans-resveratrol combination (tRES-HESP) induces expression of Glo1 countering the accumulation of MGO in overweight and obese subjects; produces a reversal of insulin resistance, improving dysglycemia, and low-grade inflammation; MGO metabolism-related variables correlated with BMI, dysglycemia, vascular inflammation, blood pressure, and dyslipidemia; in the meta-analysis: dose-response analysis showed that those consuming 200 mg/day of total flavonoids had the lowest risk of all-cause mortality |
BSA-glucose, BSA-ribose, BSA-MGO, lysine-glucose; LC-MS, ESI-MS and NMR analyses of MGO adducts, effect of metal cations on AGE formation [234,236,264]; RCT (clinica trial No. NCT01691404): a randomized, double-blind, placebo-controlled crossover study of quercetin-3-O-glucoside 160 mg/d for 4 weeks separated by 4-week washout periods; RNA was isolated from the PBMCs; MGO was evaluated by LC-MS; the gene expression of Glo1 by qRT-PCR [217]; RCT (clinical trial no. NCT03781999 ): a randomized, double-blind, placebo-controlled crossover study of hesperidin 450 mg/d (and punicalagin 60 mg/d) for 4 weeks, with a 4 weeks washout; MGO was evaluated by LC-MS [233]; RCT (clinicaltrial no. NCT02095873): a randomized, double-blind, placebo-controlled crossover study of 120 mg hesperetin + 90 mg trans-resveratrol/d, for 8 weeks, with 6 weeks washout; the primary endpoint was insulin sensitivity measured by oral glucose insulin sensitivity index (OGIS) in oral glucose tolerance tests (OGTTs) at the start and end of each treatment period; secondary endpoints were brachial artery flow-mediated dilatation response, also including brachial artery dilatation response to a sub-therapeutic dose of glyceryl trinitrate (FMD-GTN); Glo1 activity and mRNA of PBMCs were assayed by spectrophotometric and Nanostring methods, respectively; plasma MGO, plasma protein and urine MGO-derived AGE (MG-H1), were measured by LC-MS; plasma D-lactate was assayed by endpoint enzymatic assay by microplate fluorimetry; urinary metabolites by LC-MS [215]; meta-analysis of cohort studies; the random-effect model was used to calculate the summary risk estimates and dose-response analysis was performed; ten studies were included in the meta-analysis [265] |
|
Hesperidin (hesperetin – aglycone) | ||
|
Rutin Troxerutin Isoquercitrin (= quercetin-3-O-glucoside) (quercetin – aglycone) | ||
| 3. Anti-inflammatory, analgesic and antipyretic agents (including non-steroidal anti-inflammatory drugs NSAIDs) | ||
|
Acetylsalicylic acid (= aspirin) |
In vitro: inhibits ↓ albumin and hemoglobin glycation (but not salicylic acid), blocks at least one of the main glycation sites of HSA; Animal studies: decreases glycohemoglobin and glycoalbumin levels in diabetic rats; Human studies: low doses protect against cataracts |
HSA-glucose 6-phosphate [266]; albumin and hemoglobin glycation, diabetic rats in a long-term experiment, the affinity aminophenyl boronic acid procedure for determination of glycosylated protein was used [267]; a case-control study involving 423 cataract patients and 608 control subjects on the protective effect against cataracts associated with the consumption of painkillers (aspirin, paracetamol, and ibuprofen family) [268] |
| Diclofenac | In vitro: reduces ↓ albumin and hemoglobin glycation, blocks at least one of the main glycation sites of HSA | HSA-glucose 6-phosphate [266] |
| Ibuprofen |
In vitro: prevents modification of lens proteins by carbonylation and non-enzymatic glycation; reduces cyanate and galactose binding but not glucose 6-phosphate; protects against opacities; it appears to have a different mechanism of action from that of aspirin; Human studies: low doses protect against cataracts |
Animal lenses were incubated with 14C-labelled galactose, 14C-labelled glucose 6-phosphate and 14C-labelled potassium cyanate [269]; A case-control study involving 423 cataract patients and 608 control subjects on the protective effect against cataracts associated with the consumption of analgesics (aspirin, paracetamol, and ibuprofen family) [268] |
| Nimesulide | In vitro: reduces ↓ AGE formation; acts as an antioxidant, chelates transition metal cations | BSA-MGO, BSA-glucose, DPPH test; Fe+2 chelation test [270] |
| Mefenamic acid | ||
| Meloxicam | ||
| Piroxicam | ||
| Paracetamol | Human studies: low doses protect against cataracts | A case-control study involving 423 cataract patients and 608 control subjects on the protective effect against cataracts associated with the consumption of analgesics (aspirin, paracetamol, ibuprofen) [268] |
| 4. Selected B vitamins | ||
| Thiamine pyrophosphate (B1) | In vitro: reduces ↓ AGE formation (thiamine and thiamine monophosphate are not inhibitors); is essential for sustaining cellular defenses against oxidative stress | BSA-glucose, ribonuclease A-glucose, human hemoglobin-glucose; AGEs by ELISA [271] |
|
Benfotiamine (a lipid soluble thiamine derivative) |
Animal studies: reduces ↓ AGE formation; activates antioxidant defense mechanisms, an ↓ NADPH oxidase inhibitor (this enzyme plays an essential role in ROS production and myocardial cytotoxicity); improves markers of oxidative stress, inflammation, and apoptosis; inhibits ↓ NF-κB by activating transketolase in diabetic animals, prevents experimental diabetic retinopathy; significantly attenuates or ablates diabetes-induced elevation in cardiac levels of ↓ MGO, ↓ AGEs (MAGEs), ↓ RAGE, and ↓ cross-linked collagen without affecting hypertriglyceridemia and hypercholesterolemia; Human studies: significantly reduces ↓ CML-derived AGE levels in the subject group and sRAGE in the placebo group |
Wistar rats; a myocardial injury was induced by isoproterenol hydrochloride; the NADPH oxidase activity in cardiac tissue was measured by lucigenin chemiluminescence method; oxidative stress markers were assessed calorimetrically; inflammatory markers (PKC, NF-κB and metalloproteinase-9), and apoptotic markers (p53 and caspase-8) by ELISA; histopathologic assessment of myocardial damage [272]; STZ-induced diabetic mice; cardiomyocytes with MAGEs; MGO was measured by o-phenylenediamine-based assay; AGEs by immunohistochemical staining and ELISA; western blot was applied to measure of RAGE, GSK-3β, phospho-GSK-3β; qPCR quantification of RAGE, immunofluorescence detection of MAGEs and RAGE in isolated cardiomyocytes; myocardial collagen cross-linking; measurement of mitochondrial membrane potential [273]; RCT: a randomized, controlled, double-blind, clinical trial (clinical trial no. NCT02772926); T2DM subjects with no complications; benfotiamine treatment (900 mg/day) or placebo for 12 weeks; basal and final anthropometric data, blood pressure, glucose, HbA1c, and lipid profile were measured; CML-AGEs and sRAGE were measured by ELISA kits [231] |
| Pyridoxamine, pyridoxal, pyridoxal phosphate, pyridoxine (B6) |
In vitro: GO and MDA scavenger, reduces ↓ AGE formation; reduces ↓ ALE formation (but pyridoxine is slightly effective at the highest concentrations); Animal studies: increases ↑ Glo1 expression in visceral and perivascular adipose tissue; inhibits the ↓ AGE/RAGE pathway; pyridoxamine reduces atherosclerosis and inflammation induced by MGO; in obese mice improves glucose tolerance and insulin metabolism; prevents adipose tissue inflammation and vascular dysfunction; decreases fasting insulin levels and improved insulin sensitivity in obese and type 2 diabetic mice, most likely by trapping MGO and inhibiting AGE formation; Human studies: reduces ↓ MGO (9%), ↓AGEs (MG-H1), ↓ sVCAM-1, and SOCOM-1, but does not affect insulin sensitivity and vascular function in abdominally obese individuals; the reduction in adhesion markers is promising because these are important in the pathogenesis of endothelial damage and atherosclerosis |
BSA-glucose, ribonuclease A-glucose, human hemoglobin-glucose; ubiquitin-RCS; AGEs were assessed by ELISA; adducts with MGO, GO and MDA were analyzed by ESI-MS, 1H and 13C NMR [235,237,271,274]; Sprague-Dawley rats; murine macrophage cultures (Raw 264.7); Mus musculus pre-adipocyte cells (3T3-L1-MBX); interactions between AGEs and RAGE were investigated by ELISA; qPCR, western blot, histological assessment, and others [214]; Male C57BL/6J mice; serum insulin, hydrogen peroxide, MDA, AGEs, and urinary 8-hydroxy-2′-deoxyguanosine were measured; antioxidant enzymes and adipocytokine messenger RNA expressions in the adipose tissues, and Akt/protein kinase B activity and glucose transporter 4 translocation in skeletal muscle were also measured [275]; KK-Ay mice, a model animal of obese, type 2 diabetes; fasting blood glucose, serum levels of insulin and AGEs were elevated [276]; Wild-type C57Bl6 mice; AGEs and AGE precursors were assessed by LC-MS; metabolic, urinary and atherosclerotic parameters were analyzed; and flow cytometry was performed to identify circulating immune cells [225]; RCT (clinical trial no. NCT02954588): a randomized, double-blind, placebo-controlled crossover study pyridoxamine of 25 or 200 mg per day; insulin sensitivity, β-cell function, insulin-mediated microvascular recruitment, skin microvascular function, flow-mediated dilation, plasma inflammation and endothelial function markers were assessed; pyridoxamine metabolites, RCS and AGEs were measured using LC-MS [232] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





