In Acute lymphocytic leukemia (ALL) patients, intrathecal (IT) chemotherapy is known to reduce the risk of central nervous system (CNS) leukemia[
1]. However, rare but serious complications can arise after methotrexate (MTX)-based chemotherapy, such as Methotrexate-induced myelopathy, which can lead to significant long-term sequelae[
2,
3].
Guillain-Barre syndrome (GBS) is an acute polyneuropathy that typically progresses from the lower limbs to the upper limbs following an infection[
4]. Nerve conduction studies have played a crucial role in diagnosing and distinguishing subtypes of GBS, aiding in long-term prognosis prediction[
5]. Although most cases start in the lower limbs and progress to the upper limbs, some cases may present initially as lower limb weakness, potentially leading to initial misdiagnosis as myelopathy[
6]. The association between GBS and ALL is not definitively established. In patients with ALL, GBS can arise due to immunosuppression following intensive chemotherapy[
7], and immune system neoplasms can induce AIDP in a manner akin to certain viral infections[
8]. In some rare cases, GBS can occur after a stem cell transplantation due to various factors.
Here we report the case of MTX-induced myelopathy initially misdiagnosed as GBS.
A 39-year-old male patient, diagnosed with chronic myeloid leukemia (CML) in June 2010 and later with B-cell acute lymphoblastic leukemia (B-ALL) in August 2017, presented with bilateral sole paresthesia. He received intrathecal MTX and blinatumomab treatment for six months from September 2021 to March 2022 due to relapsed B-ALL. However, B-ALL relapsed again after six months, resulting in the administration of inotuzumab, intrathecal MTX, and cytarabine for three months from September 2022 to December 2022.
In December 2022, the patient visited our clinic with bilateral foot paresthesia and gradually progressive weakness in the lower extremities that persisted for a week following intrathecal MTX treatment. Neurological examination revealed Manual muscle testing (MMT) grade 4 in both lower limbs. Nerve conduction study (NCS) conducted at the time revealed Prolonged F-wave latency in bilateral peroneal nerves and the left tibial nerve, slightly slow motor nerve conduction velocity in in the right sural and superficial peroneal nerves and low compound motor action potential (CMAP) in bilateral peroneal nerves, and low sensory compound nerve action potential (CNAP) in bilateral peroneal nerves. These findings indicate a diffuse sensory-motor peripheral neuropathy with a predominant axonopathy. A follow-up evaluation of muscle strength conducted seven days later showed MMT grade of 2- at bilateral lower limb. And Nerve conduction study results no significant changed compared to the previous study. Considering the clinical presentation and electromyographic findings, Acute Motor and Sensory Axonal Neuropathy (AMSAN) variant of GBS was suspected.
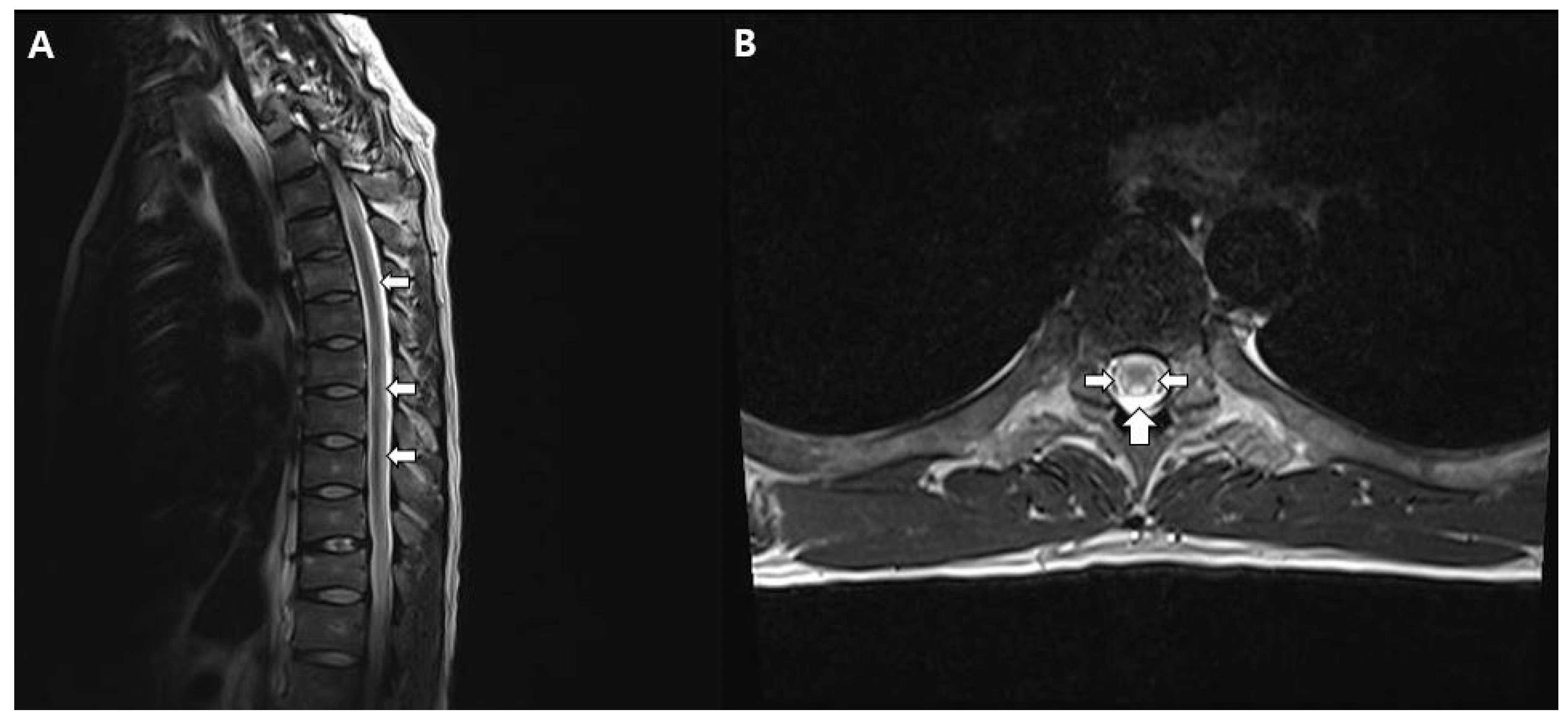
Consequently, intravenous immunoglobulin (IVIG) treatment was administered for five days, but muscle strength evaluation eight days later indicated no improvement, but more progressed at bilateral lower limb (MMT 0). Additionally, the patient complained decreased pain, temperature sensation, vibratory sensation, and proprioception below the T6 level, so spine MRI was taken. T2-weighted images of the thoracic spinal cord revealed high signal intensity in both lateral and posterior columns. (
Figure 1).
In laboratory test, Vitamin B12 levels were elevated (1183), as were folate levels (>40 ng/mL). The MRI findings of Subacute Combined Degeneration (SCD) typically display a distinct symmetrically increased signal intensity in the posterior column on T2-weighted images, as seen in this patient. This condition arises from vitamin B12 deficiency; however, in this case, SCD could be excluded as the patient had elevated vitamin B12 levels. Unfortunately, a cerebrospinal fluid (CSF) study could not be performed at the time. Considering all of the above findings, a diagnosis of intrathecal methotrexate-induced myelopathy was established. Therefore, intravenous immunoglobulin (IVIG) treatment was stopped. Intravenous Vitamin B12 supplementation was administered and continued rehabilitation. Five months later, there was no improvement in symptoms and motor recovery. The NCS follow-up examination revealed absence of CMAP responses for both the peroneal and tibial nerves, as well as absence of SNAP responses for both superficial peroneal nerves. The remaining conduction velocities were within normal limits. Ultimately, the patient progressed to paraplegia.
The exact mechanism by which MTX induces neurotoxicity remains unclear, but several hypotheses exist. First, MTX functions as an antimetabolite by inhibiting dihydrofolate reductase(DHFR), preventing the conversion of folate to tetrahydrofolic acid (THF) and causing intracellular folate deficiency [
9]. Additionally, vitamin B12 and 5-methyltetrahydrofolate are necessary for the conversion of homocysteine to S-adenosylmethionine (SAM), but DHFR inhibition leads to SAM deficiency [
9,
10]. SAM plays a crucial role in the formation and maintenance of myelin sheaths; its deficiency can lead to demyelination.
MRI findings in patients with MTX-induced myelopathy typically exhibit high signal intensity on T2-weighted images in the posterior column, closely resembling the characteristics of subacute combined degeneration [
11]. Similarly, our case displayed predominantly high signal intensity on T2-weighted images in the posterior and lateral columns, consistent with previous reports. Subacute combined degeneration (SCD) is a progressive spinal cord disorder characterized by lower limb weakness, sensory disturbances, and motor deficits due to vitamin B12 deficiency. Symmetrically increased signal intensity in the posterior column on T2-weighted MRI images is a hallmark of SCD. A condition resembling subacute combined degeneration was also taken into consideration, considering the findings from spinal MRIs. However, these patients exhibited elevated levels of vitamin B12, and the administration of vitamin B12 supplementation did not result in significant improvement. Therefore, the diagnosis of IT methotrexate-induced myelopathy seemed to be a more appropriate consideration than subacute combined degeneration.
Recent reports suggest rapid recovery of lower limb paralysis in MTX-induced myelopathy patients with the administration of various folate metabolism compounds, such as SAM, folic acid, and cyanocobalamin. While cyanocobalamin administration did not lead to improvement in our case, considering other cases, prompt diagnosis and aggressive folate therapy might have potentially mitigated neurological sequelae. Ultimately, when patients who have undergone Intrathecal MTX chemotherapy present with complaints of progressive paralysis, it is important to promptly differentiate spinal cord pathology.
Author Contributions
Conceptualization, E,L. and D.L.; methodology, D.L.; formal analysis, D.L.; investigation, E,L. and D.; writing—original draft preparation, D.L.; writing—review and editing, D.L.; visualization, E.L.; funding acquisition, D.L.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Science Research Program though the National Research Funding of Korea (NRF) and funded by the Ministry of Education (2022R1I1A3071887).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Yeungnam University (YUMC 2023-09-027 and date of approval; 25 SEP 2023 ).
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Data are available on a reasonable request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paul, S.; Kantarjian, H.; Jabbour, E.J. Adult Acute Lymphoblastic Leukemia. Mayo Clin Proc 2016, 91, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Luddy, R.E.; Gilman, P.A. Paraplegia following intrathecal methotrexate. J Pediatr 1973, 83, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Bay, A.; Oner, A.F.; Etlik, O.; Yilmaz, C.; Caksen, H. Myelopathy due to intrathecal chemotherapy: report of six cases. J Pediatr Hematol Oncol 2005, 27, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Asbury, A.K.; Cornblath, D.R. Assessment of current diagnostic criteria for Guillain-Barre syndrome. Ann Neurol 1990, 27, S21–S24. [Google Scholar] [CrossRef] [PubMed]
- Cornblath, D.R.; Mellits, E.D.; Griffin, J.W.; McKhann, G.M.; Albers, J.W.; Miller, R.G.; Feasby, T.E.; Quaskey, S.A. Motor conduction studies in Guillain-Barre syndrome: description and prognostic value. Ann Neurol 1988, 23, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Kimachi, T.; Yuki, N.; Kokubun, N.; Yamaguchi, S.; Wakerley, B.R. Paraparetic Guillain-Barre syndrome: Nondemyelinating reversible conduction failure restricted to the lower limbs. Muscle Nerve 2017, 55, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Aral, Y.Z.; Gursel, T.; Ozturk, G.; Serdaroglu, A. Guillain-Barre syndrome in a child with acute lymphoblastic leukemia. Pediatr Hematol Oncol 2001, 18, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Brigo, F.; Balter, R.; Marradi, P.; Ferlisi, M.; Zaccaron, A.; Fiaschi, A.; Frasson, E.; Bertolasi, L. Vincristine-related neuropathy versus acute inflammatory demyelinating polyradiculoneuropathy in children with acute lymphoblastic leukemia. J Child Neurol 2012, 27, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Ziereisen, F.; Dan, B.; Azzi, N.; Ferster, A.; Damry, N.; Christophe, C. Reversible acute methotrexate leukoencephalopathy: atypical brain MR imaging features. Pediatr Radiol 2006, 36, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, R.; Semmler, A.; Maurer, G.D.; Hattingen, E.; Fornoff, F.; Steinbach, J.P.; Linnebank, M. Methotrexate-induced myelopathy responsive to substitution of multiple folate metabolites. J Neurooncol 2010, 97, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Yao, M.; Liu, H.M.; Chen, Y.F. MR findings of intrathecal chemotherapy-related myelopathy in two cases: mimicker of subacute combined degeneration. J Neuroimaging 2007, 17, 184–187. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).