1. Introduction
Systemic lupus erythematosus (SLE), a systemic autoimmune disease affects all organ systems [
1]. It affects the skin, the joints, the vascular system, the heart, and may have hematological manifestations [
2,
3]. The disease may be associated with various autoimmune manifestations [
4,
5,
6]. It may also be associated with autoimmune thyroid disease [
7,
8]. Autoimmune Hashimoto’s thyroiditis has been observed in patients with SLE [8). In the context of autoimmune Hashimoto’s thyroiditis hypothyroidism may be detected [
9]. Hyperthyroidism has also been observed in patients with SLE [
9,
10]. However, the exact association between thyroid autoimmunity and SLE needs investigation. The aim was to investigate the relationship between thyroid disease and more specifically autoimmune thyroid disease with SLE.
Thyroid disease has been associated with systemic autoimmune diseases [
11] such as rheumatoid arthritis (RA) [
12], Sjogren’s syndrome and systemic sclerosis. In particular, RA a frequent progressive, systemic autoimmune disease characterized by chronic inflammation affecting multiple joints with associated systemic manifestations and a worldwide prevalence of 0,5-1% has been associated with thyroid autoimmunity [
13]. Various studies have estimated the presence of thyroid hormone dysfunction and autoimmune thyroid disease in RA between 6 and 33% [
14]. In a hospital based observational, descriptive study performed in RA patients in India thyroid dysfunction was observed in 20% [
14]. The most common thyroid disorder was overt hypothyroidism followed by subclinical hypothyroidism and subclinical hyperthyroidism. Thyroid peroxidase antibodies were present in most of the patients with RA and overt hypothyroidism [
14]. Sjogren’s syndrome has also been strongly associated with thyroid autoimmunity [
15,
16]. Autoimmune thyroid disease has been found in systemic sclerosis [
17]. The aim was to evaluate the relationship between SLE and thyroid autoimmunity in a cohort of lupus patients.
2. Materials and Methods
A cohort of 45 patients with SLE were consecutively and prospectively studied as they came for evaluation (table 1). All patients fulfilled the 2019 EULAR/ACR criteria for the diagnosis of SLE. Serum was collected for thyroid function determination and for the measurement of thyroid peroxidase (TPOab) and thyroglobulin (Tgab) antibodies. A group of 45 control patients was evaluated as well. None of the female patients or the controls was pregnant when entering the study. The study was approved by the ethical committee of Asclepeion Hospital (approval number 2377, 2022). All the patients and the controls gave their informed consent before entering the study.
TSH levels were measured in serum by the ARCHITECT TSH immunoassay (Abbott Park IL) which is a chemiluminescent microparticle immunoassay with an analytical sensitivity of <0.0025 μIU/ml, a precision of <10% and an interassay coefficient of variation of <20%. The ARCHITECT TSH assay is a two-step immunoassy which utilizes chemiluminescent microparticel immunoassay technology with flexible assay protocols, that are referred to as Chemiflex. In the first step, sample, anti-β TSH antibody coated paramagnetic microparticles and TSH Assay Diluent were combined. TSH present in the sample was bound to the anti-TSH antibody coated microparticles. After washing, anti-α TSH acridinium labeled conjugate was added in the second step. Pre-Trigger and Trigger Solutions were then added to the reaction mixture; the resulting chemiluminescent reaction was measured as relative light units. A direct relationship exists between the amount of TSH in the sample and the relative light units detected by the ARCHITECT i optical system.
Free T3 (FT3) levels were measured by the ARCHITECT FT3 assay which is a chemiluminescent microparticle immunoassay (Abbott Park IL), with an analytical sensitivity of <1.0 pg/ml and an analytical specificity of <0.001%. The ARCHITECT Free T3 assay is a two-step immunoassay for the determination of free T3 in human serum and plasma using Chemiluminescent Microparticle Immunoassay (CMIA) technology with flexible assay protocols. In the first step, sample and anti-T3 coated paramagnetic microparticles were combined. Free T3 (unbound) in the sample bonded to the anti-T3 coated microparticles. After washing, T3 acridinium labeled conjugate was added in the second step. Pre-Trigger and Trigger Solutions were then added to the reaction mixture; the resulting chemiluminescent reaction was measured as relative light units. An inverse relationship existed between the amount of FT3 in the sample and the relative light units detected by the ARCHITECT i optical system.
Free T4 (FT4) levels were measured by the ARCHITECT FT4 assay which is a chemiluminescent microparticle immunoassay (Abbott Park IL) with an analytical sensitivity of <0.4 ng/dl and a precision of <10%. The ARCHITECT Free T4 assay is a two-step immunoassay to determine the presence of free thyroxine (Free T4) in human serum and plasma using Chemiluminescent Microparticle Immunoassay (CMIA) technology with flexible assay protocols. In the first step, sample and anti-T4 coated paramagnetic microparticles were combined. FT4 (unbound) present in the sample bonded to the anti-T4 coated microparticles. After washing, T4 acridinium labeled conjugate was added in the second step. Pre-Trigger and Trigger Solutions were then added to the reaction mixture; the resulting chemiluminescent reaction was measured as relative light units. An inverse relationship exists between the amount of Free T4 in the sample and the relative light units detected by the ARCHITECT i optical system.
Determination of TPOab was performed by the ARCHITECT Anti-TPO assay, with a precision of <10% for samples >5.61 IU/ml and a within run CV of 3.9% at a concentration of 1.56 IU/ml. The ARCHITECT anti-TPO assay is a two-step immunoassay for the quantitative determination of anti-TPO in human serum and plasma using CMIA technology with flexible assay protocols, referred to as Chemiflex®. In the first step, sample, assay diluent and TPO coated paramagnetic microparticles were combined and incubated. After washing, anti-human IgG acridinium labeled conjugate was added in the second step. Following another incubation and wash, pre-trigger and trigger solutions were added to the reaction mixture. The resulting chemiluminescent reaction was measured as relative light units. A direct relationship is observed between the amount of TPOab in the sample and the relative light units detected by the ARCHITECT i* system optics.
TgAb were assayed by the ARCHITECT Anti-Tg assay. The ARCHITECT Anti-Tg assay is a two-step immunoassay for the quantitative determination of the IgG class of thyroglobulin autoantibodies (anti-Tg) in human serum and plasma using CMIA technology with flexible assay protocols, referred to as Chemiflex®, with a In the first step, sample, assay diluent and Tg coated paramagnetic microparticles were combined and incubated. Anti-Tg present in the sample bonded to the Tg coated microparticles. After washing, anti-human IgG acridinium labeled conjugate was added in the second step. Following another incubation and wash, pre-trigger and trigger solutions were added to the reaction mixture. The resulting chemiluminescent reaction was measured as relative light units. A direct relationship exists between the amount of anti-Tg in the sample and the relative light units detected by the ARCHITECT i* system optics.
All the patients underwent a complete hematological, biochemical and immunological laboratory evaluation. All of them had a chest x-ray done and in all of them the thyroid was examined by palpation. Clinical criteria were used for the evaluation of thyroid status as well as the thyroid hormone profile. Clinical criteria were applied for the diagnosis of hyperthyroidism (weight loss, heat intolerance, tachycardia) and hypothyroidism (weight gain, cold intolerance, tachycardia) and thyroid hormone measurement. Normal FT3 and FT4 values but elevated TSH levels were consistent with subclinical hypothyroidism.
Statistical evaluation of the results was performed by the SPSS statistical package (IBM SPSS v27).
Table 1.
Characteristics of SLE patients and controls, age years (mean±SEM), disease duration years (mean±SEM). Leucopenia was defined as white blood cells <4000/μL.
Table 1.
Characteristics of SLE patients and controls, age years (mean±SEM), disease duration years (mean±SEM). Leucopenia was defined as white blood cells <4000/μL.
| |
SLE patients |
| Age |
47.97±2.17 |
| Sex |
41 F/4 M |
| SLE disease duration |
5.71±0.49 |
| SLEDAI-2K |
9.24±0.65 |
| Leucopenia |
35% |
| Cutaneous involvement |
32% |
| Renal involvement |
28.9% |
| |
|
3. Results
3.1. Clinical thyroid disease in the SLE and control groups
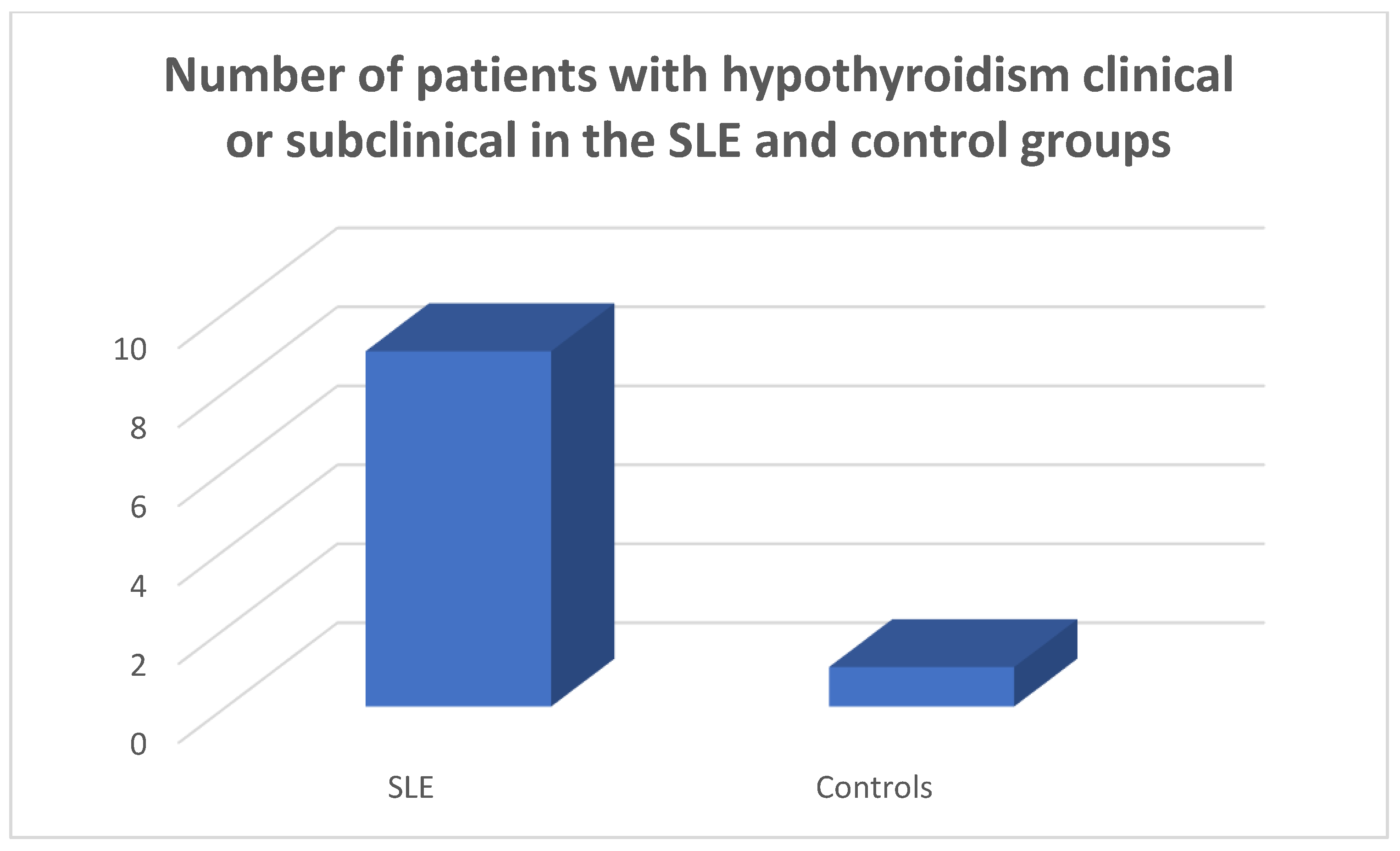
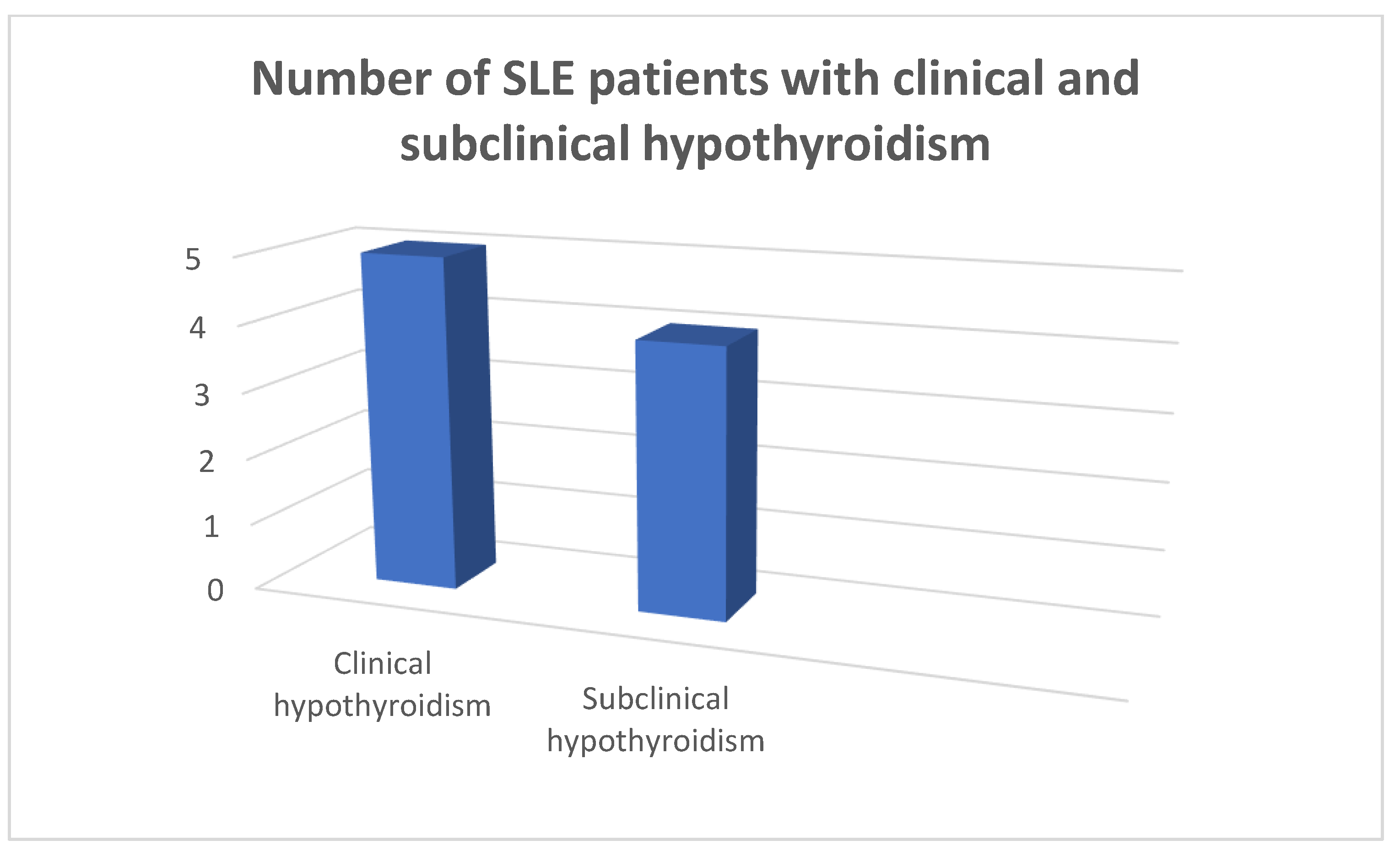
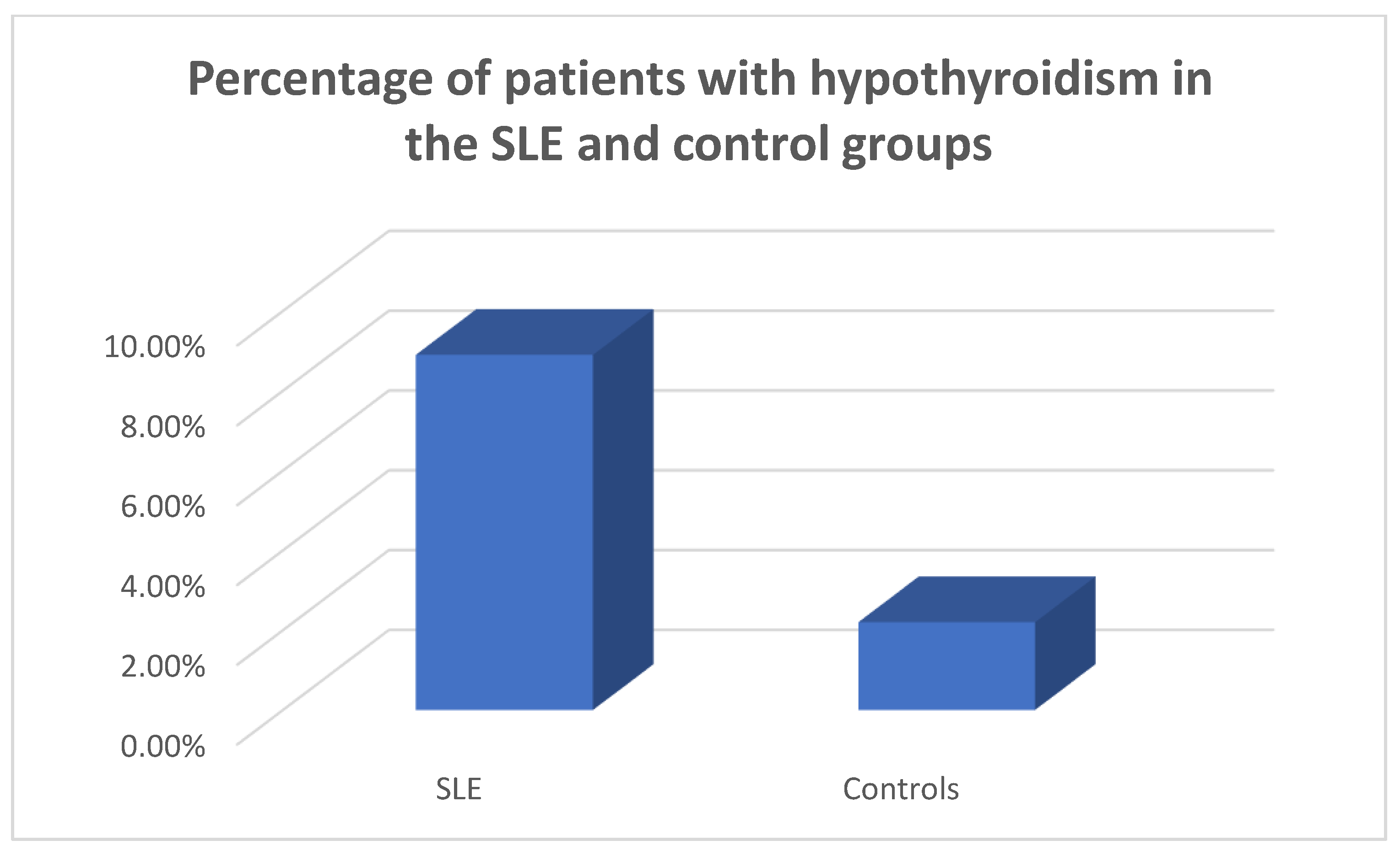
In the patients and the controls mean FT3, FT4 values and TSH values were within the normal range. Four patients (8.9%) were found to suffer from primary hypothyroidism, 5 (11.11%) from subclinical hypothyroidism (
Figure 1,
Figure 2 and
Figure 3) as opposed to 1 (2.22%) with hypothyroidism in the control group, chi square test p=0.0073, Fisher’s exact test=0.015. Within the SLE group 1 patient had hyperthyroidism (2.22%) and 1 of the controls (2.22%) had hyperthyroidism, chi square test p>0.05, Fisher’s exact test=1, p>0.05. Five patients (11.11%) had a thyroid hormone profile compatible with the presence of euthyroid sick syndrome.
3.2. Antithytoid antibodies in the SLE and control groups
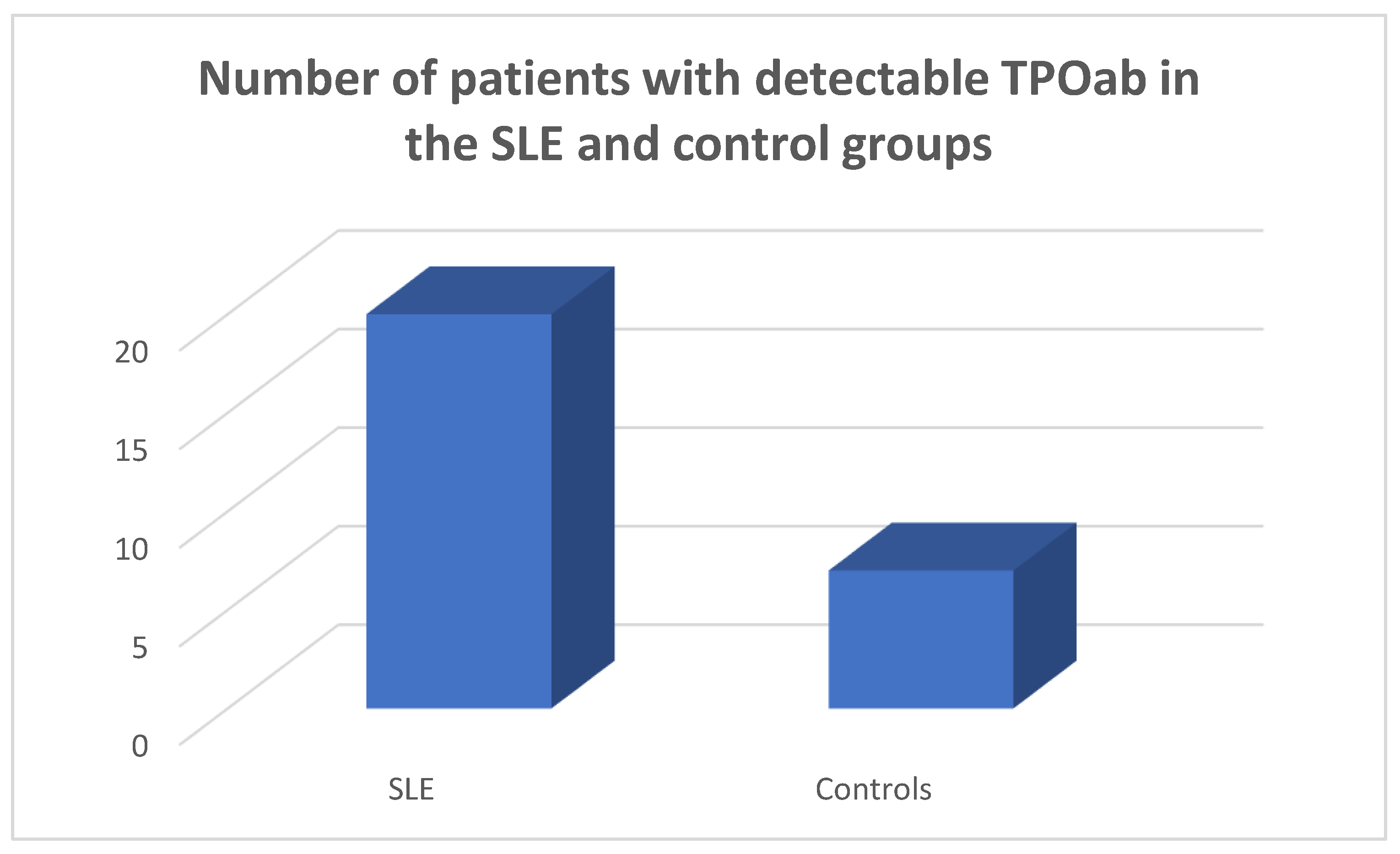
TPOab were detected in 20/45 of the SLE population and in 7/45 of the controls, chi square test p=0.0028, Fisher’s exact test=0.0052 (
Figure 4).
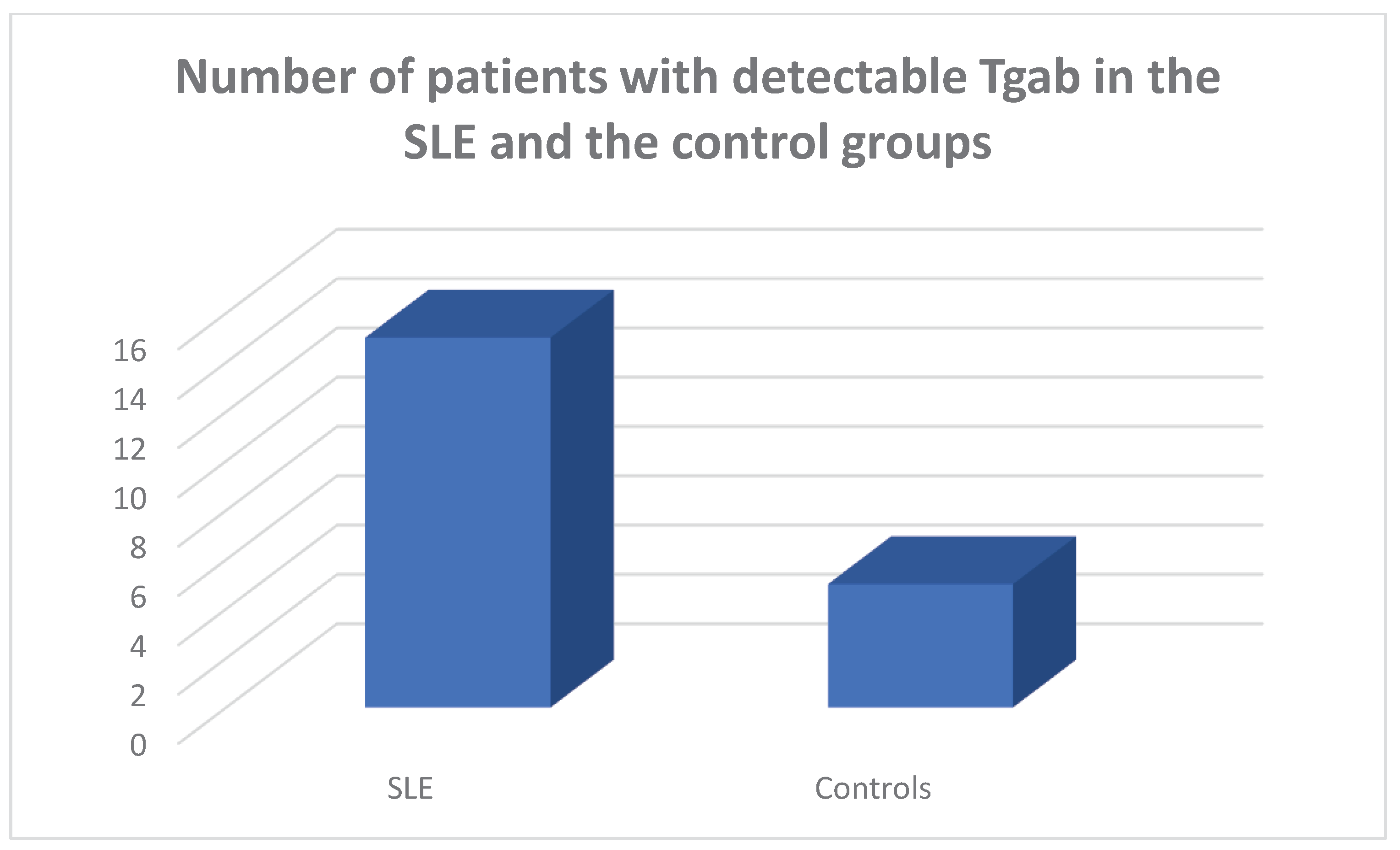
Tgab antibodies were detected in 15/45 of the SLE population and in 5/45 of the controls, respectively, chi square test p=0.011, Fisher’s exact test=0.021.
Amongst the Tgab positive SLE patients 3 were hypothyroid, 2 had subclinical hypothyroidism and 10 were euthyroid. Amongst the TPOab positive SLE patients 3 had hypothyroidism and 2 had subclinical hypothyroidism. Amongst the SLE patients who had both types of antithyroid antibodies detectable, 2 were hypothyroid and 2 had subclinical hypothyroidism.
4. Discussion
In a prospective 12-month study of 45 Greek SLE patients a significantly higher prevalence of Tgab and TPOab was observed as opposed to a control population. Amongst the SLE patients 4 had overt primary hypothyroidism, 5 subclinical primary hypothyroidism and 1 patient had hyperthyroidism. Five patients had low FT3 levels, low FT4 and normal TSH levels, a hormone profile compatible with the presence of euthyroid sick syndrome.
There are previous reports showing that patients with SLE have a higher prevalence of thyroid disease than a control population. In a very early study published in 1961 Hijmans et al [
18] detected thyroid antibodies in SLE patients at about 3 times the frequency observed in an age and sex matched controls. In an earlier study including 332 SLE patients Miller et al [
19] found a prevalence of hypothyroidism of 6.6% and increased antimicrosomal antibodies in 18%. In study of 150 patients with SLE Kausman and Isenberg [
20] found 21% positive for thyroid autoantibodies. They observed fluctuation of thyroid autoantibody levels over time. They also observed clinical thyroid disease in some of the patients with positive thyroid autoantibodies. In a study performed in Singapore involving 129 SLE patients Boey et al [
21] found increased thyroid antibodies in 32.2% and clinical thyroid disease in some of the patients. In a study performed in Korea involving 63 lupus patients Park et al (22) found positive thyroid antibodies in 27%. In an early study increased TPO antibodies were observed in sera of SLE patients [
23]. In a seminal study performed in India [
24] involving a cohort of 100 SLE patients and 100 controls 30% of the lupus patients displayed positive thyroid antibodies as opposed to 10% of the controls. Increased disease activity as demonstrated by the SLEDAI index was associated with the presence of euthyroid sick syndrome. Mulhern et al [
25] in a retrospective study found no correlation between SLE and Hashimoto’s thyroiditis. Goh and Wang [
26] in a study of 319 SLE Asian patients found a higher incidence of Graves’ disease than in the general population. Byron and Mowat [
27] in a study in Oxford found that amongst 64 patients, 61 female and 3 male with SLE, 10 female had thyroid disease. Amongst these 10 SLE patients had hyperthyroidism (11.5%) and 3 had hypothyroidism (4.9%), whereas in the British population as a whole the incidence of hyperthyroidism and hypothyroidism was 1.9% and 1%, respectively. In a more recent study performed in Brazil Posselt et al [
7] studied a cohort of 301 SLE patients and 140 controls for the presence of antithyroid antibodies and Hashimoto thyroiditis. They observed a prevalence of Hashimoto’s thyroiditis of 12.6% in lupus patients as compared to 5.6% in the control population. They also observed that lupus patients with Hashimoto’s thyroiditis were characterized by the presence of less facial rash. They additionally found that lupus patients with Hashimoto’s were characterized by the detection of more anti-Sm antibodies. In this cohort anti-Sm antibodies were more common in the group of lupus patients with both thyroid antibodies detectable. They also observed an absence of an association between Hashimoto’s thyroiditis and lupus disease activity or cumulative lupus damage. They concluded that there is a two-fold augmented risk of Hashimoto’s thyroiditis in lupus. In a study performed in China Liu et al [
28] collected clinical, laboratory and immunologic data related to 63 patients with lupus and Hashimoto’s thyroiditis. They observed a negative correlation between FT3 levels and lupus disease activity and a negative correlation between Tgab and the complement component C4. In a study performed in Hungary Szanto et al [
29] studied o group of 56 SLE patients who also had Sjogren’s syndrome and compared them with 50 patients with SLE and 50 patients with Sjogren’s syndrome and found an increased prevalence of thyroiditis in the group of patients with both disorders. In a study performed in China Liu et al [
30] investigated the relationship between SLE and hypothyroidism using complementary genetic approaches, such as genetic correlation and colocalization analysis. The linkage disequilibrium score found a shared genetic structure between SLE and primary hypothyroidism. In a Mendelian randomization study using data from genome-wide association studies of SLE and thyroid disease in people with European ancestry the causal link between SLE and thyroid disease was assessed [
9]. The Mendelian randomization analysis showed a relationship between SLE and an increased incidence of hypothyroidism and hyperthyroidism. The sensitivity analysis of the study did not reveal any pleiotropy or heterogeneity. By contrast, Qin et al [
31] in a study using Mendelian randomization analysis found an association between SLE and hypothyroidism but did not find an association between SLE and hyperthyroidism. The authors performed a two-step analysis using univariable and multivariable Mendelian randomization analysis in three genome-wide association studies datasets. The authors concluded based on this analysis that SLE is associated with hypothyroidism but not with hyperthyroidism.
Thyroid disease has been associated with systemic autoimmunity [
32]. In particular, thyroid disease has been associated with RA, progressive systemic sclerosis and other connective tissue disorders [
33,
34]. In a study performed in Russia Odin et al [
35] studied 53 patients, 92% female, with both RA and autoimmune thyroiditis and described some subsets of RA, such as RA occurring during the active reproductive period and late-onset RA affected by autoimmune thyroiditis. Various studies have confirmed an augmented prevalence of autoimmune thyroid disease in RA patients. This finding is in accordance with the idea that autoimmune conditions may emerge in the same patient and in families, a finding which may be related to a defect in immune tolerance [
36]. Both the prevalence of autoimmune thyroid disease in RA and that of RA in autoimmune thyroiditis is increased by 1-6-fold and 1-3-fold, respectively [
34]. Various early cross-sectional and observational studies have observed a prevalence of thyroiditis in RA patients of 12% [
37]. RA is a common systemic autoimmune disease associated with autoimmune thyroid disease [
12]. An increased prevalence of antithyroid antibodies, both TPOab and Tgab has been observed in RA patients ranging from 5% - 37% and 5%-31%, respectively, while both types of antithyroid antibodies have a recorded prevalence of 4% to 32% [
38,
39,
40]. In a study performed in China a higher prevalence of antithyroid antibodies was observed in seropositive as opposed to seronegative RA [
41]. It is interesting noting that a study from China, found a significantly higher prevalence of positive tests for aTPO and aTg in patients with RF as compared to those seronegative for RF [
38]. The association between systemic sclerosis and autoimmune thyroid diseases has been evaluated by Fallahi et al [
17]. Systemic sclerosis is a connective tissue disorder characterized by microvascular involvement, immune activation and fibrosis [
42]. In systemic sclerosis autoimmune thyroiditis and hypothyroidism have been noted with an increased incidence and prevalence, especially in female patients [
17]. Graves’ disease has also been noted in patients with systemic sclerosis [
43].
The pathogenesis of Hashimoto’s thyroiditis is related to the production of antithyroid antibodies, namely TPOab and Tgab, with attendant lymphocytic infiltration by B and T lymphocytes. It is theorized that amongst the first events in the pathogenesis of Hashimoto’s thyroiditis is a functional alteration of B lymphocytes leading to the production of autoantibodies [
44,
45]. Subsequently, T cell dysfunction is associated with the breakdown of immune homeostasis against thyroid tissue. Serum TPOab are considered the most important feature of Hashimoto’s thyroiditis and are detectable in more than 95% of the cases [
46]. By contrast, Tgab are observed in 60-80% of the cases and are less reliable for diagnosis [
47]. It is thought that Tgab represent an index of an initial immune response, whereas TPOab represent an immune escalation [
48]. However, both types of antithyroid antibodies are not entirely specific for Hashimoto’s thyroiditis and are present in other autoimmune conditions as well [
49,
50,
51].
In conclusion, in a cohort of SLE patients antithyroid antibodies were detected and thyroid disease was diagnosed. It appears that SLE patients should be screened and followed-up for the presence of thyroid autoimmunity and thyroid disease.
5. Conclusions
It appears that SLE patients may develop antithyroid antibodies and may present with thyroid disease. Thus, lupus patients should be screened and followed-up for the presence of thyroid autoimmunity and thyroid disease.
Author Contributions
Conceptualization, L.A. and I.K.A.; methodology, L.A, P.A.; software, I.K.A, G.K., P.T.; validation, N.K., S.M., P.T, and C.S.; formal analysis, L.A, I.K.A.; investigation, N.K.; resources, P.T., S.M..; data curation, I.K.A..; writing—original draft preparation, L.A., I.K.A.; writing—review and editing, G.K., S.C. and P.A.; visualization, L.A.; supervision, C.S, P.A..; project administration, P..A.; funding acquisition, P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Asclepeion Hospital (approval number 2377, 2022). All the patients and the controls gave their informed consent before entering the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsokos, GC. Systemic lupus erythematosus. N Engl J Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef]
- Yu H, Nagafuchi Y, Fujio K. Clinical and Immunological Biomarkers for Systemic Lupus Erythematosus. Biomolecules. 2021, 11. [Google Scholar]
- Kiriakidou M, Ching CL. Systemic Lupus Erythematosus. Ann Intern Med. 2020, 172, Itc81–itc96. [Google Scholar] [CrossRef] [PubMed]
- Prabhat N, Chakravarty K, Pattnaik SN, Takkar A, Ray S, Lal V. Systemic lupus erythematosus with autoimmune neurological manifestations in a carrier of chronic granulomatous disease - a rare presentation. J Neuroimmunol. 2020, 343, 577229. [Google Scholar] [CrossRef]
- Fortuna G, Brennan MT. Systemic lupus erythematosus: epidemiology, pathophysiology, manifestations, and management. Dent Clin North Am. 2013, 57, 631–655. [Google Scholar] [CrossRef]
- Zucchi D, Elefante E, Schilirò D, Signorini V, Trentin F, Bortoluzzi A, et al. One year in review 2022: systemic lupus erythematosus. Clin Exp Rheumatol. 2022, 40, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Posselt RT, Coelho VN, Skare TL. Hashimoto thyroiditis, anti-thyroid antibodies and systemic lupus erythematosus. Int J Rheum Dis. 2018, 21, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Garber JJ, Worthington JW, Randall RV, Kierland RR. Lupus erythematosus and hashimoto's thyroiditis. Postgrad Med. 1969, 46, 100–105. [Google Scholar] [CrossRef]
- Duan L, Shi Y, Feng Y. Systemic lupus erythematosus and thyroid disease: a Mendelian randomization study. Clin Rheumatol. 2023, 42, 2029–2035. [Google Scholar] [CrossRef]
- Saettini F, Cattoni A, Redaelli M, Silvestri D, Ferrari GM, Biondi A, et al. Primary immunodeficiencies, autoimmune hyperthyroidism, coeliac disease and systemic lupus erythematosus in childhood immune thrombocytopenia. Acta Paediatr. 2021, 110, 643–651. [Google Scholar] [CrossRef]
- Boutzios G, Koukoulioti E, Goules AV, Kalliakmanis I, Giovannopoulos I, Vlachoyiannopoulos P, et al. Hashimoto Thyroiditis, Anti-Parietal Cell Antibodies: Associations With Autoimmune Diseases and Malignancies. Front Endocrinol (Lausanne). 2022, 13, 860880. [Google Scholar] [CrossRef]
- Conigliaro P, D'Antonio A, Pinto S, Chimenti MS, Triggianese P, Rotondi M, et al. Autoimmune thyroid disorders and rheumatoid arthritis: A bidirectional interplay. Autoimmun Rev. 2020, 19, 102529. [Google Scholar] [CrossRef]
- Andonopoulos AP, Siambi V, Makri M, Christofidou M, Markou C, Vagenakis AG. Thyroid function and immune profile in rheumatoid arthritis. A controlled study. Clin Rheumatol. 1996, 15, 599–603. [Google Scholar] [CrossRef]
- Meena L, Chejara R, Meena PD, Nawal CL, Vedwal A. A Study to Evaluate the Thyroid Function in Sero Positive Rheumatoid Arthritis. J Assoc Physicians India. 2022, 70, 11–12. [Google Scholar]
- Girón-Pïllado M, Cruz-Bautista I, Saavedra-González V, Atisha-Fregoso Y, Barraza G, Aguilar-Salinas CA, et al. Autoimmune Thyroid Disease in Primary Sjögren's Syndrome: Real-life Screening Practice and Clinical Outcomes. Curr Rheumatol Rev. 2022, 18, 272–277. [Google Scholar] [CrossRef]
- Anaya JM, Restrepo-Jiménez P, Rodríguez Y, Rodríguez-Jiménez M, Acosta-Ampudia Y, Monsalve DM, et al. Sjögren's Syndrome and Autoimmune Thyroid Disease: Two Sides of the Same Coin. Clin Rev Allergy Immunol. 2019, 56, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Fallahi P, Ruffilli I, Giuggioli D, Colaci M, Ferrari SM, Antonelli A, et al. Associations between Systemic Sclerosis and Thyroid Diseases. Front Endocrinol (Lausanne). 2017, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Hijmans W, Doniach D, Roitt IM, Holborow EJ. Serological overlap between lupus erythematosus, rheumatoid arthritis, and thyroid auto-immune disease. Br Med J. 1961, 2, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Miller FW, Moore GF, Weintraub BD, Steinberg AD. Prevalence of thyroid disease and abnormal thyroid function test results in patients with systemic lupus erythematosus. Arthritis Rheum. 1987, 30, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Kausman D, Isenberg DA. Thyroid autoimmunity in systemic lupus erythematosus: the clinical significance of a fluctuating course. Br J Rheumatol. 1995, 34, 361–364. [Google Scholar] [CrossRef]
- Boey ML, Fong PH, Lee JS, Ng WY, Thai AC. Autoimmune thyroid disorders in SLE in Singapore. Lupus. 1993, 2, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Park DJ, Cho CS, Lee SH, Park SH, Kim HY. Thyroid disorders in Korean patients with systemic lupus erythematosus. Scand J Rheumatol. 1995, 24, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kohno Y, Naito N, Saito K, Hoshioka A, Niimi H, Nakajima H, et al. Anti-thyroid peroxidase antibody activity in sera of patients with systemic lupus erythematosus. Clin Exp Immunol. 1989, 75, 217–221. [Google Scholar]
- Kumar K, Kole AK, Karmakar PS, Ghosh A. The spectrum of thyroid disorders in systemic lupus erythematosus. Rheumatol Int. 2012, 32, 73–78. [Google Scholar] [CrossRef]
- Mulhern LM, Masi AT, Shulman LE. Hashimoto's disease. A search for associated disorders in 170 clinically detected cases. Lancet. 1966, 2, 508–511. [Google Scholar]
- Goh KL, Wang F. Thyroid disorders in systemic lupus erythematosus. Ann Rheum Dis. 1986, 45, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Byron MA, Mowat AG. Thyroid disorders in systemic lupus erythematosus. Ann Rheum Dis. 1987, 46, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Liu H, Yang LH, Yin G, Xie QB. [Correlation of Thyroid Autoantibodies,System Lupus Erythematosus Immunologic Indicators and Disease Activity in SLE with HT]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2018, 49, 179–182. [Google Scholar]
- Szanto A, Szodoray P, Kiss E, Kapitany A, Szegedi G, Zeher M. Clinical, serologic, and genetic profiles of patients with associated Sjögren's syndrome and systemic lupus erythematosus. Hum Immunol. 2006, 67, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Liu X, Yuan J, Zhou H, Wang Y, Tian G, Wang X, et al. Association Between Systemic Lupus Erythematosus and Primary Hypothyroidism: Evidence from Complementary Genetic Methods. J Clin Endocrinol Metab. 2023, 108, 941–949. [Google Scholar] [CrossRef]
- Qin Q, Zhao L, Ren A, Li W, Ma R, Peng Q, et al. Systemic lupus erythematosus is causally associated with hypothyroidism, but not hyperthyroidism: A Mendelian randomization study. Front Immunol. 2023, 14, 1125415. [Google Scholar] [CrossRef] [PubMed]
- Khizroeva J, Nalli C, Bitsadze V, Lojacono A, Zatti S, Andreoli L, et al. Infertility in women with systemic autoimmune diseases. Best Pract Res Clin Endocrinol Metab. 2019, 33, 101369. [Google Scholar] [CrossRef] [PubMed]
- Lazúrová I, Benhatchi K, Rovenský J, Kozáková D, Wagnerová H, Tajtáková M, et al. Autoimmune thyroid disease and autoimmune rheumatic disorders: a two-sided analysis. Ann N Y Acad Sci. 2009, 1173, 211–216. [Google Scholar] [CrossRef]
- Weetman, AP. Diseases associated with thyroid autoimmunity: explanations for the expanding spectrum. Clin Endocrinol (Oxf). 2011, 74, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Odin VI, Dvorovkin AE, Inamova OV, Tyrenko VV, Gumilevskaya OP. [Features of the ontogenetic forms of rheumatoid arthritis associated with autoimmune thyroiditis.]. Adv Gerontol. 2018, 31, 125–131. [Google Scholar]
- Lazúrová I, Jochmanová I, Benhatchi K, Sotak S. Autoimmune thyroid disease and rheumatoid arthritis: relationship and the role of genetics. Immunol Res. 2014, 60, 193–200. [Google Scholar] [CrossRef]
- Hart, FD. Rheumatoid arthritis: extra-articular manifestations. II. Br Med J. 1970, 2, 747–752. [Google Scholar] [CrossRef]
- Cárdenas Roldán J, Amaya-Amaya J, Castellanos-de la Hoz J, Giraldo-Villamil J, Montoya-Ortiz G, Cruz-Tapias P, et al. Autoimmune thyroid disease in rheumatoid arthritis: a global perspective. Arthritis. 2012, 2012, 864907. [Google Scholar]
- El-Sherif WT, El Gendi SS, Ashmawy MM, Ahmed HM, Salama MM. Thyroid disorders and autoantibodies in systemic lupus erythematosus and rheumatoid arthritis patients. Egypt J Immunol. 2004, 11, 81–90. [Google Scholar]
- Nakamura H, Usa T, Motomura M, Ichikawa T, Nakao K, Kawasaki E, et al. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J Endocrinol Invest. 2008, 31, 861–865. [Google Scholar] [CrossRef]
- Chen YL, Lin JZ, Mo YQ, Liang JJ, Li QH, Zhou CJ, et al. Joint damage is amplified in rheumatoid arthritis patients with positive thyroid autoantibodies. PeerJ. 2018, 6, e4216. [Google Scholar] [CrossRef]
- Barsotti S, Stagnaro C, d'Ascanio A, Della Rossa A. One year in review 2016: systemic sclerosis. Clin Exp Rheumatol. 2016, 34 (Suppl. 100), 3–13. [Google Scholar]
- Wimmersberger Y, Zuercher D. Graves' disease associated with primary systemic sclerosis. Orbit. 2009, 28, 262–263. [Google Scholar]
- McLachlan SM, Rapoport B. Breaking tolerance to thyroid antigens: changing concepts in thyroid autoimmunity. Endocr Rev. 2014, 35, 59–105. [Google Scholar] [CrossRef]
- Smith TJ, Hegedüs L. Graves' Disease. N Engl J Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- McLachlan SM, Rapoport B. Why measure thyroglobulin autoantibodies rather than thyroid peroxidase autoantibodies? Thyroid. 2004, 14, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Rose, NR. Prediction and prevention of autoimmune disease: a personal perspective. Ann N Y Acad Sci. 2007, 1109, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Antonelli A, Fallahi P, Mosca M, Ferrari SM, Ruffilli I, Corti A, et al. Prevalence of thyroid dysfunctions in systemic lupus erythematosus. Metabolism. 2010, 59, 896–900. [Google Scholar] [CrossRef]
- Annunziata P, Lore F, Venturini E, Morana P, Guarino E, Borghi S, et al. Early synthesis and correlation of serum anti-thyroid antibodies with clinical parameters in multiple sclerosis. J Neurol Sci. 1999, 168, 32–36. [Google Scholar] [CrossRef]
- Scappaticcio L, Castellana M, Virili C, Bellastella G, Centanni M, Cannavò S, et al. Alemtuzumab-induced thyroid events in multiple sclerosis: a systematic review and meta-analysis. J Endocrinol Invest. 2020, 43, 219–229. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).