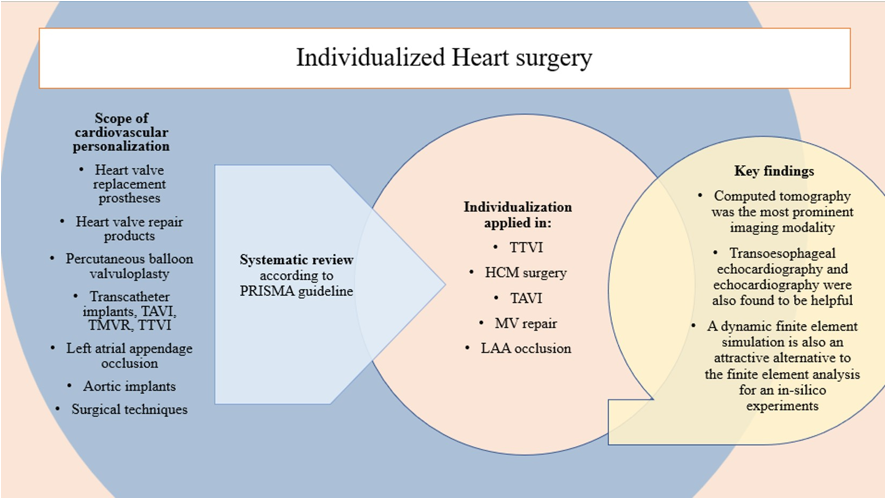
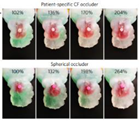
1. Introduction
Precision medicine, with a specific focus on personalized surgery, has been an evolving field of practice since the late 1990s [
1]. Over the past decade, significant progress has been made, largely attributed to advancements in technology, notably the cost reduction of three-dimensional computational modeling. These technological breakthroughs have ignited growing interest and applications in various surgical sub-specialties [
2]. Personalized surgery has gained immense importance across a wide spectrum of surgical disciplines by facilitating comprehensive preoperative assessments of individual patients. This process involves the meticulous consideration of predefined variables, allowing surgical procedures to be tailored precisely to the unique needs and characteristics of each patient [
3,
4,
5]. This transformative shift in the approach to surgery has had a profound impact on the field of cardiovascular surgery, extending its reach and capabilities. By embracing the principles of personalized surgery, cardiovascular surgeons can now offer highly customized treatment strategies that account for the specific anatomical, physiological, and genetic traits of their patients. This not only enhances the precision and effectiveness of surgical interventions but also contributes to improved patient outcomes and overall healthcare quality.
Personalized cardiac surgery represents a meticulous approach where surgical techniques are tailored to suit the specific needs and attributes of an individual patient's heart [
6]. This method acknowledges the inherent uniqueness in the anatomy, functionality, and pathological conditions of each patient's heart. Consequently, relying on a standardized or universally applicable surgical approach may not lead to the most favorable outcomes. Interestingly, it's important to note that the application of individualized surgical methods in the context of cardiovascular replacement surgery has not been comprehensively examined or assessed in previous research endeavors. Rather than adhering to a one-size-fits-all or ready-made surgical strategy, this approach underscores the importance of tailoring surgical techniques to optimize results and address the specific needs of the patient's heart. Remarkably, despite the potential benefits, the systematic examination of personalized surgical techniques in the realm of cardiovascular replacement surgery has been somewhat lacking in prior research.
Traditionally, cardiovascular implant surgery has predominantly focused on replacement procedures aimed at addressing diseased heart valves [
7,
8,
9]. While aortic and mitral valve diseases are the most prevalent and severe, necessitating invasive interventions, valve-related issues can affect any single valve or a combination of the four [
10,
11,
12,
13]. Treatment modalities for heart valve disease encompass various approaches, ranging from invasive methods such as tissue or mechanical heart valve replacement and repair products to less invasive techniques like percutaneous balloon valvuloplasty and minimally invasive transcatheter procedures [
14,
15,
16]. However, the current prosthetic solutions exhibit limitations. They often involve the insertion of a rigid, circular implant at the level of the patient's native valve annulus, which may not fully account for the intricacies of individual anatomy. Furthermore, commercially available prostheses, characterized by their inflexible and symmetrical designs, are highly dependent on medications with significant side effects, potentially resulting in serious health complications [
17,
18,
19].
One of the current challenges that warrants attention is the matter of Patient Prosthesis Mismatch (PPM), which has received significant attention, particularly in the context of heart valve replacement therapy. PPM is a term frequently discussed in clinical circles, referring to a scenario wherein a surgically implanted medical device is, in essence, too small in proportion to the patient's needs, thus giving rise to a condition reminiscent of "valve stenosis." This predicament has provoked criticism towards surgeons, as it seems that they are effectively replacing one medical ailment with another. The inadequacies inherent in the design of commercially available prosthetic devices only serve to underscore the urgent necessity for a concrete solution, one that can be achieved through the widespread adoption of personalized prosthetic devices.
The primary goal of personalized prostheses is to create a device or implant that is tailored specifically to suit the unique needs of an individual recipient. This approach has found considerable success in various fields such as orthodontics, orthopedics, and reconstructive surgery. Despite these successes, it is noteworthy that the concept of personalization has not yet been widely embraced in the realm of cardiovascular replacement therapy. As of now, there are no commercially available personalized devices designed for this particular subspecialty, although some are currently undergoing evaluation and development. Given this situation, it becomes imperative to consider and explore the potential benefits and applications of personalized prostheses in the context of cardiovascular replacement therapy. In essence, the focus is on creating prosthetic solutions that are not one-size-fits-all but rather precisely customized to meet the distinct requirements and anatomical characteristics of individual patients. While this approach has demonstrated its effectiveness in other medical disciplines, it is an exciting frontier that holds significant promise for enhancing the outcomes and quality of life for individuals in need of cardiovascular replacement therapy.
Furthermore, they come in a few pre-determined sizes and are offered off-the-shelf during the surgical procedure itself, based on rough eye-balling or subjective assessments [
20,
21,
22,
23]. Similarly, Aortic surgery and concomitant cardiac procedures like left atrial appendage occlusion are not addressed in precision but rather largely dominated by the standardized prosthesis and subjective assessment [
24,
25]. Hence, this review of the application of personalization in cardiovascular replacement surgery will help identify opportunities for expanded surgical adaptation and future research directions that can address the rational challenges in this field.
2. Materials and Methods
2.1. Search Strategy
A comprehensive search of existing literature was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [
26]. This search was carried out electronically and encompassed multiple databases, including Medline (via PubMed), Scopus, Embase, and Web of Science, spanning from their inception up to July 29, 2023. To ensure a thorough and exhaustive retrieval of pertinent literature, a meticulous search strategy involving a combination of Medical Subject Headings (MeSH) terms was employed. These MeSH terms included 'Precision Medicine,' 'Personalized,' 'Individualized,' 'Heart Valve,' 'Aortic,' 'Cardiovascular Surgical Procedures,' 'Mitral Valve Replacement,' 'Mitral Valve Implantation,' 'Transcatheter,' 'Transcutaneous,' and 'Transcatheter Mitral Valve Replacement.' The extracted published papers resulting from this search underwent a rigorous and systematic screening and assessment process.
2.2. Inclusion Criteria and Exclusion Criteria
This analysis encompassed a range of research methodologies, specifically focusing on studies related to the utilization of personalized prosthetic devices in cardiovascular replacement surgery. The eligible types of studies considered in this review comprised randomized controlled trials, prospective observational investigations, interventional studies, experimental research, retrospective cohort studies, and cross-sectional studies. These studies were selected because they provided valuable insights into the application of personalized prostheses in the context of cardiovascular surgery.
However, certain types of research were deliberately omitted from this analysis. Articles that involved concomitant medical procedures, surgical repairs, cases related to congenital or adult congenital heart disease surgery, survey-based investigations, and studies not written in the English language were excluded from our examination. These exclusions were made with the intention of maintaining the concentration of our analysis on the particular field of individualized prosthesis in cardiovascular replacement surgery and omitting irrelevant information or studies that would have presented language hurdles.
2.3. Study Selection and Outcome of Interest
Three authors conducted a thorough and systematic review of the available studies independently in order to determine their eligibility for inclusion in their research. The initial screening of published articles involved an examination of their titles and abstracts, with intentionally broad criteria employed to ensure that all potentially relevant studies were considered. To manage the vast array of extracted citations, the authors utilized reference software called EndNote X9 [
27]. In the subsequent stage of the review process, studies that had successfully passed the initial screening were subjected to a more in-depth evaluation.
In cases where a definitive decision could not be reached during the initial screening, a full-text review of the articles was carried out to confirm their relevance to the research. During this stage, each author independently abstracted key details from the selected studies, including information about the study's design, applied research methods, the model of personalization utilized, the intended purpose of the study, and the observed outcomes. In addition to the information extracted from the selected articles, the authors conducted a manual search to identify any additional relevant data using a backward snowballing method. This comprehensive approach ensured that the research was based on a thorough examination of all pertinent literature, providing a solid foundation for their study.
2.4. Quality of Evidence and Risk of Bias Assessment
The research encompassed a collection of studies, all of which fell into the categories of observational or experimental investigations, with the majority of these studies primarily focusing on the initial outcomes of their respective experiments. To gauge the quality of evidence within these studies, the GradePro tool was employed. Additionally, in accordance with the guidance provided in Chapter 11 of the Cochrane Handbook of Reviews, the ROBINS-I tool (Risk of Bias in Non-randomized Studies-of Interventions) was utilized to assess the potential for bias in studies that lacked randomization [
28]. In order to comprehensively evaluate the articles included in this research endeavor, an assessment of their risk of bias and the overall quality of evidence was conducted. This evaluation was facilitated through the use of statistical software known as Revmen 5.4 [
29]. To ascertain the risk of bias in the studies that formed part of this investigation, the assessment was carried out in accordance with the guidelines set forth in Chapter 8 of the Cochrane Handbook of Reviews [
28].
3. Results
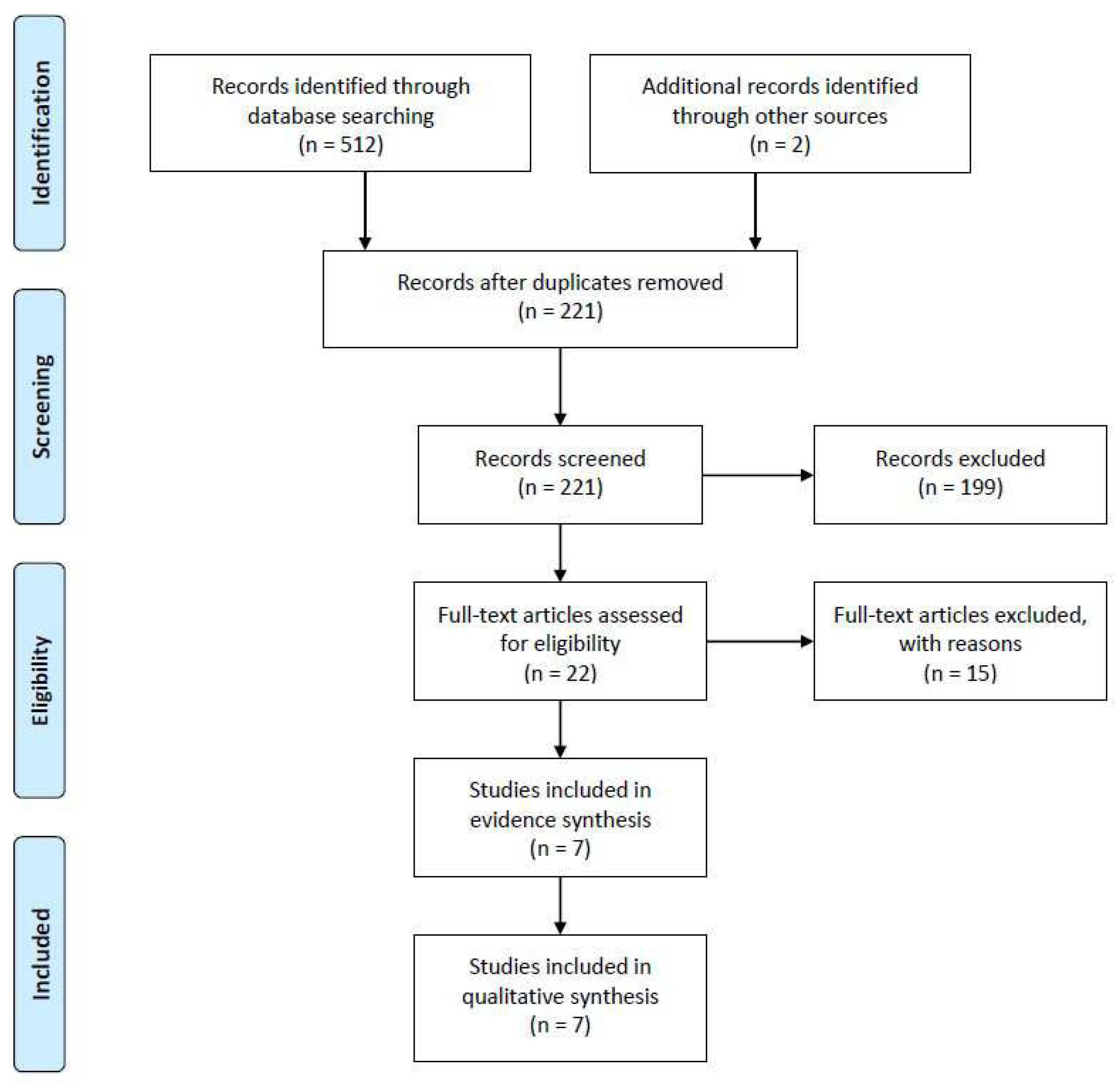
A comprehensive search across multiple databases, including Medline (via PubMed), Scopus, Embase, and Web of Science, yielded a total of 514 potential articles. These articles were identified electronically as potential sources of information. After rigorous screening based on predefined inclusion criteria, seven studies were chosen for further analysis in order to synthesize evidence. These criteria were established in advance to ensure the selection of relevant and high-quality studies. In these seven studies, a total of 229 individuals who had received personalized prostheses for cardiovascular replacement therapy were included as participants. For a visual representation of this selection process, please refer to
Figure 1.
3.1. Quantity of Evidence
The preliminary systematic scrutiny of our study involved a meticulous and systematic examination of electronic sources, which yielded a total of 512 published articles. To ensure comprehensiveness, we conducted an additional, iterative search on platforms such as Google Scholar, Research Gate, and the Ubiquity Partner Network, which led us to discover two more articles of relevance. Subsequently, employing citation management software, specifically Endnote X9 [
27], we meticulously removed duplicate entries, resulting in a refined collection of 221 published articles for further scrutiny and evaluation.
To efficiently narrow down our selection, we initially scrutinized the titles and abstracts of these 221 papers using Endnote X9. Articles that did not align with our predefined inclusion and exclusion criteria were excluded from the subsequent stages of our review process. As a result, we retained 22 articles for in-depth examination, while 199 records were excluded from further consideration. Moving forward, a comprehensive assessment of the 22 selected articles was conducted by performing a full-text review. This phase of our evaluation process led to the exclusion of studies that did not fit the specific focus areas of our research, including those centered on congenital heart surgery (n = 7), immunotherapy after heart transplantation (n = 5), as well as review articles (n = 2) and editorial/commentary pieces (n = 1). Consequently, a final set of seven articles, spanning references [
30,
31,
32,
33,
34,
35,
36], were deemed suitable for evidence synthesis and, ultimately, were included in our study for analysis (as detailed in
Supplementary Table S1).
3.2. Included Studies Characteristics
The seven studies encompassed in this analysis [
30,
31,
32,
33,
34,
35,
36] were conducted at single medical centers, with the majority of them being situated in the United States and various European countries. One notable exception was a study carried out in China [
36], as indicated in
Table 1. These studies exhibited significant heterogeneity in both the choice of medical procedures employed and the selection criteria for study participants. It is worth noting that the majority of personalized approaches were focused on human subjects, although two studies, namely Amerini et al. [
30] and Robinson et al. [
35], employed experimental large animals for the purposes of testing and evaluating medical devices.
Among the studies included in this review, only one was a clinical trial, conducted by Collis et al. [
31], which assessed personalized surgical strategies rather than the assessment of a newly designed prosthetic device. It is noteworthy that computed tomography imaging was the prevailing modality employed for preoperative assessments in most of the studies included in this review. However, some investigators opted for alternative modalities, such as echocardiography [
31], transesophageal echocardiography [
34], and 3D transesophageal echocardiography [
35] for their evaluations.
3.3. Summary of the Intervention
The comprehensive overview of interventions conducted across all the studies included in our analysis can be found in
Table 1. In the pursuit of crafting tailored prosthetic solutions, the majority of the cases opted for an “in-silico” approach, relying on Finite Element Analysis. It is noteworthy that Collis et al. [
31] deviated from this trend by employing a clinical assessment methodology, specifically based on preoperative echocardiography. The utilization of software tools for the prototyping process exhibited a degree of diversity among the studies we reviewed. However, a common thread across these investigations was the utilization of computer-aided design in conjunction with 3D rendering techniques for the reconstruction of anatomical models. Notably, there were variations observed in the final design of personalized prosthetic devices in the studies, reflecting a degree of customization to cater to individual patient needs. There was variation in the final model or the personalized devices under the study, but most of the prostheses were modeled by three-dimensional printing using various artificial printing materials. None of the studies used 3D bioprinting materials.
3.4. Outcomes and Interest Variables
With the data summarized in
Table 1, which has been gathered from seven different research studies involving a total of 229 recipients, reveals a consistent trend of utilizing either surgical procedures or catheter-based interventions, with one exception in the study by Rim et al. [
34], which employed a virtual dynamic finite element simulation. Following these interventions, the majority of the devices underwent assessment through CT scans and echocardiography, encompassing both 2D and 3D imaging techniques. However, it's important to note that Rim et al. [
34] relied on finite element analysis (FEA), while Yuan et al. [
36] utilized angiographic evaluation instead. Notably, Amerini et al. [
30] and Robinson et al. [
35] took a different approach by testing the devices in large animals, specifically porcine and canine subjects, respectively. Subsequently, these studies conducted postoperative autopsies after a predetermined period in accordance with their respective study designs.
4. Discussion
This comprehensive systematic review explores the field of personalized therapy in cardiovascular replacement surgery and highlights the idea of personalizing and the concept of tailoring surgical procedures to meet the unique needs of each patient. It offers a meticulous synthesis of evidence supporting the notion of "personalized surgery" and its pivotal role within the cardiovascular surgery domain. The review not only explores the significance of personalization but also delves into the intricacies of the personalization process, the various interventional methods employed, and a qualitative assessment of its efficacy. Presently, the landscape of cardiovascular intervention devices available in the commercial market remains devoid of individualization [
25,
37]. Specifically, the vast majority of prosthetic heart valves are offered in a limited range of three or fewer predefined sizes, further compounded by the constraints imposed by the hospital's inventory management system. This predicament leaves cardiovascular surgeons with only premanufactured options when embarking on the implantation procedure. Consequently, surgeons are left with no choice but to utilize these rigid, circular designs when inserting them into a human heart, all the while harboring unrealistic hopes for long-term success. This challenge is further exacerbated by concerns related to PPM.
Although there is an obvious absence of specialized equipment created especially for tailored surgical operations, it is important to note that researchers and medical professionals have acknowledged the relevance and necessity of filling this gap. Furthermore, it is noteworthy that a broad range of target regions have adopted individualized methods and prostheses in cardiac surgery. Many researchers have explored various techniques and treatment plans in this area. An important finding from the literature at hand is the wide range of preoperative imaging techniques used to collect the crucial recipient information needed to develop customized surgical models. These imaging modalities cover a variety of procedures, such as computed tomography scans, magnetic resonance imaging, preoperative echocardiography, and three-dimensional transesophageal echocardiography. These instruments frequently provide comprehensive anatomical information to assist in surgical planning. The lack of a unified opinion on the "optimal imaging modality" for the construction of three-dimensional representations and the collecting of preoperative scan data is also an important topic to underline. This lack of agreement emphasizes the continual investigation and assessment of diverse imaging techniques in the pursuit of accuracy and efficacy in individualized surgical operations.
However, various researchers used diverse diagnostic imaging techniques in their individual studies. The principal imaging technique used by Amerini et al. [
30], Pasta et al. [
33], and Yuan et al. [
36] was CT scanning. Collis et al. [
31] shown a preference for echocardiography in contrast. Transesophageal echocardiography was successfully used by Rim et al. [
34], but it was interesting to see that Robinson et al. [
35] took it a step further by including 3D transesophageal echocardiography into their strategy. Before any planned procedure, each patient underwent at least one echocardiography scan, an essential step in gathering pre-operative data for personalized care. Additionally, some patients also underwent preoperative CT scans as a routine practice, which proved valuable in tailoring imaging to collect individualized data. It's worth highlighting that over the past decade, there has been remarkable improvement in the quality of CT scans, making them more patient-centric [
38]. More individualized and accurate diagnostics are becoming more common in the world of transcatheter valves. Multidetector computed tomography (MDCT) scans and sophisticated 3D reconstructions are used to take exact measurements. This method improves decision-making, especially when choosing the right prosthesis type and size for each patient's particular requirements. Yet that does not impact manufacture and prosthesis design. A combination of both modalities can be a greater approach to generate a larger preoperative data pool.
In the field of current practice, it is customary to employ the data obtained from preoperative imaging of patients for this purpose, followed by subsequent "in-silico" processing using finite element analysis and/or three-dimensional personalized modeling techniques [
39,
40,
41]. The utilization of three-dimensional computer-aided design (3D CAD) and finite element analysis has proven to be highly effective in creating in-silico models. These models are constructed with the assistance of advanced geometry and mesh generation software, including but not limited to SolidWorks, Rhino CAD, and MIMIC 16.0 [
33,
35,
36]. In the majority of studies reviewed, a consistent methodology was employed, commencing with the development of a virtual 3D model. This model was then subjected to a 3D rendering process and subsequently personalized 3D printing [
39,
40]. It's worth noting, though, that the specific form and functionality of the resultant devices varied significantly, contingent on the targeted organ or the therapeutic objectives of the procedure.
The personalized prostheses generated in these studies were crafted from materials such as artificial alumide mould, nitinol, and silicone [
30,
32,
35]. Notably, none of these devices were created using 3D bioprinting techniques. This exclusion can be attributed to the inherent limitations and challenges associated with 3D bioprinting in the context of cardiac tissues [
42,
43]. Postoperative verification of the precision of the surgical prostheses was routinely carried out, primarily relying on computed tomography (CT) scans and echocardiography as the standard modalities for these assessments. However, a promising avenue emerged in the form of virtual dynamic finite element simulations, which demonstrated their potential for accurately estimating and quantifying the motion of mitral valve leaflets [
34,
44]. It's interesting to note that in the cases we evaluated, individualized prostheses were positioned consistently for both surgical and transcatheter procedures. This finding is consistent with the growing preference for catheter-based therapies, indicating the growing importance of these procedures in the field of cardiovascular replacement therapy.
4.1. Limitations
Firstly, it is essential to emphasize that this systematic review is exclusively centered on research concerning personalized cardiovascular replacement surgery, which represents a highly specialized and focused area of investigation. Secondly, the findings of this study rely on experimental research with preliminary data, which has limitations concerning procedural responses and the reproducibility of trend data. This element imposes some limitations on the replicability of trend data as well as procedural replies. The trials examined in this review also show notable differences in the cardiovascular interventions they focus on, a feature that is probably due to the dearth of empirical data in this specialized field. Furthermore, the studies included in this review display significant variations in the specific cardiovascular interventions they address, a phenomenon likely stemming from the limited availability of scientific evidence in this niche area. The included studies also exhibit significant heterogeneity in terms of the specific cardiovascular interventions, likely due to the limited pool of available scientific evidence. A notable limitation of this study is the restricted utilization of large animal models in the conducted experiments, a factor that impacts the statistical significance of the obtained results. Lastly, it becomes evident that there is a pressing need for additional randomized clinical trials and expansive clinical investigations to tackle outstanding inquiries and offer a more comprehensive assessment of personalized prosthetic applications in actual clinical scenarios.
4.2. Future Directions
The scope of future directions in this research in this field is multifaceted and encompasses a range of crucial areas. Firstly, there is a need for a comprehensive systematic review that goes beyond the confines of personalized cardiovascular replacement surgery. This expanded review should encompass the entire spectrum of cardiovascular diseases and the various treatment methods that fall under the umbrella of personalized cardiovascular medicine. Moreover, it is essential to explore and delve into the application of individualization methodologies in diverse surgical subspecialties, including but not limited to orthopedics, neurosurgery, and plastic surgery. By adopting this interdisciplinary approach, we can contribute significantly to gaining a more profound and holistic comprehension of personalized prostheses throughout the medical field.
Another avenue for further investigation involves conducting specific comparative studies focusing on personalized prostheses in distinct cardiovascular replacement surgeries, such as heart valve replacement. These comparative studies are pivotal as they can provide insights into the advantages and limitations of personalized approaches in various cardiac procedures. This comparative analysis will shed light on the relevance and effectiveness of tailoring treatments to individual patients in the domain of cardiovascular care. Lastly, there is a pressing need for rigorous clinical trials and large-scale studies. These studies should be designed to address lingering questions regarding the practical utilization of personalized prostheses in real-world clinical settings. The accumulation of robust clinical evidence is of paramount importance as it serves to establish the safety and efficacy of personalized approaches in the realm of patient care. This empirical foundation is essential for ensuring that personalized medicine can be confidently integrated into the standard practices of healthcare, ultimately benefiting patients across the board.
5. Conclusions
The concept of a universal "one size fits all" approach continues to persist in practical application, especially within the cardiovascular domain of what is often considered the "Gold standard therapy." However, it's crucial to acknowledge the limitations of such an approach, as it essentially caters to only a select few, failing to adequately address the needs of many. This limitation becomes even more apparent when we consider that for some individuals, the conventional approach hardly fits at all. This situation is particularly evident in the context of healthcare, given the increasing prevalence of comorbidities and the intricate and diverse ways in which diseases manifest in patients. Nevertheless, there is hope on the horizon. The adoption of personalized prosthetic solutions has the potential to yield significant improvements in patient-specific clinical outcomes.
We envision and believe that cutting-edge technological advancements will be crucial in ushering in a new era of highly individualized healthcare. This change will involve a variety of medical procedures, including tablets, tests, and implants that are customized to each patient's particular anatomical characteristics and metabolic or biological profile. This marks a shift from the prevalent model of mass-producing generic medical products and places more emphasis on the significance of individualized treatment as the future's gold standard.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Supplementary Table S1.
Author Contributions
Conceptualization, F.S., and T.K.; methodology, I.M., F.S.; software, A.G. F.S. I.M., validation, A.G., I.M., J.S., F.S., R.K.; formal analysis, F.S., I.M., resources, A.G., F.S., T.K.; data curation, I.M., F.S., writing—original draft preparation, I.M., F.S., writing—review and editing, I.M., A.G., J.S., F.S., R.K., T.K.; visualization, I.M., F.S., supervision, R.K., T.K.; project administration, F.S.; funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors disclosed receipt of the following financial support for the research and publication of this article. This research was supported by The National Research Foundation (NRF), Singapore, Central Gap Fund [NRF2020NRF-CG001-018].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Derived data supporting the findings of this study are available from the corresponding author on request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study's design, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Boland, G. M., & Meric-Bernstam, F. (2015). The role of surgeons in building a personalized medicine program. Journal of surgical oncology, 111(1), 3–8. [CrossRef]
- Lopez-Perez, A., Sebastian, R., & Ferrero, J. M. (2015). Three-dimensional cardiac computational modelling: methods, features and applications. Biomedical engineering online, 14, 35. [CrossRef]
- Wang, D. D., Qian, Z., Vukicevic, M., Engelhardt, S., Kheradvar, A., Zhang, C., Little, S. H., Verjans, J., Comaniciu, D., O'Neill, W. W., & Vannan, M. A. (2021). 3D Printing, Computational Modeling, and Artificial Intelligence for Structural Heart Disease. JACC. Cardiovascular imaging, 14(1), 41–60. [CrossRef]
- Nan, J., Rezaei, M., Mazhar, R., Jaber, F., Musharavati, F., Zalnezhad, E., & Chowdhury, M. E. H. (2020). Finite Element Analysis of the Mechanism of Traumatic Aortic Rupture (TAR). Computational and mathematical methods in medicine, 2020, 6718495. [CrossRef]
- Duraiswamy, N., Weaver, J. D., Ekrami, Y., Retta, S. M., & Wu, C. (2016). A Parametric Computational Study of the Impact of Non-circular Configurations on Bioprosthetic Heart Valve Leaflet Deformations and Stresses: Possible Implications for Transcatheter Heart Valves. Cardiovascular engineering and technology, 7(2), 126–138. [CrossRef]
- Archbold, A., Akowuah, E., Banning, A. P., Baumbach, A., Braidley, P., Cooper, G., Kendall, S., MacCarthy, P., O'Kane, P., O'Keeffe, N., Shah, B. N., Watt, V., & Ray, S. (2022). Getting the best from the Heart Team: guidance for cardiac multidisciplinary meetings. Heart (British Cardiac Society), 108(11), e2. [CrossRef]
- Pragt, H., Pieper, P. G., van Slooten, Y. J., Freling, H. G., van Dijk, A. P. J., Sieswerda, G. T. J., Bouma, B. J., Post, M. C., Jongbloed, M. R. M., Willems, T. P., Ebels, T., & van Melle, J. P. (2019). Quality of Life Among Patients With Congenital Heart Disease After Valve Replacement. Seminars in thoracic and cardiovascular surgery, 31(3), 549–558. [CrossRef]
- Matsumoto, K., Hisashi, Y., & Imoto, Y. (2015). Replacement of the heavily calcified ascending aorta in aortic valve replacement. Asian cardiovascular & thoracic annals, 23(3), 349–352. [CrossRef]
- Mahesh, B., Wells, F., Nashef, S., & Nair, S. (2013). Role of concomitant tricuspid surgery in moderate functional tricuspid regurgitation in patients undergoing left heart valve surgery. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery, 43(1), 2–8. [CrossRef]
- Mrsic, Z., Hopkins, S. P., Antevil, J. L., & Mullenix, P. S. (2018). Valvular Heart Disease. Primary care, 45(1), 81–94. [CrossRef]
- Hollenberg S. M. (2017). Valvular Heart Disease in Adults: Etiologies, Classification, and Diagnosis. FP essentials, 457, 11–16.
- Gopal S, Hauser JM, Mahboobi SK. Mechanical Aortic Valve Replacement. 2021 Jun 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID: 33232009.
- Bykowski A, Perez OA, Kanmanthareddy A. Balloon Valvuloplasty. 2020 Dec 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID: 30137817.
- Bykowski A, Perez OA, Kanmanthareddy A. Balloon Valvuloplasty. 2020 Dec 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID: 30137817.
- Nickenig, G., Weber, M., Schüler, R., Hausleiter, J., Nabauer, M., von Bardeleben, R. S., Sotiriou, E., Schäfer, U., Deuschl, F., Alessandrini, H., Kreidel, F., Juliard, J. M., Brochet, E., Latib, A., Montorfano, M., Agricola, E., Baldus, S., Friedrichs, K. P., Deo, S. H., Gilmore, S. Y., … Maisano, F. (2021). Tricuspid valve repair with the Cardioband system: two-year outcomes of the multicentre, prospective TRI-REPAIR study. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology, 16(15), e1264–e1271. [CrossRef]
- Price M. J. (2019). Transcatheter Mitral Valve Repair and Replacement: New Standards of Care and New Horizons for Therapy. Interventional cardiology clinics, 8(3), xi–xii. [CrossRef]
- Grégoire C, Nellessen E, Defraigne JO, Radermecker MA. La prothèse valvulaire idéale n'existe toujours pas. quels facteurs entrent en compte pour orienter les choix d'une valve mécanique ou biologique? [The ideal valvular prosthesis is still to come. Which factors can help decide between mechanical and bioprosthetic heart valve replacement?]. Rev Med Liege. 2014 Nov;69(11):600-4. French. PMID: 25796772.
- Damle, S., & Thourani, V. H. (2021). Surgical Valve Prosthesis Choices: Choose for the Now, But Don't Forget About the Future. The Annals of thoracic surgery, 111(4), 1206. [CrossRef]
- Kim, W., Choi, H., Kweon, J., Yang, D. H., & Kim, Y. H. (2020). Effects of pannus formation on the flow around a bileaflet mechanical heart valve. PloS one, 15(6), e0234341. [CrossRef]
- Luthra, S., Malvindi, P. G., Olevano, C., Zingale, A., Salem, H., & Ohri, S. K. (2021). Impact of valve size, predicted effective and indexed effective orifice area after aortic valve replacement. Journal of cardiac surgery, 36(3), 961–968. [CrossRef]
- Dumesnil, J. G., & Pibarot, P. (2011). Prosthesis-patient mismatch: an update. Current cardiology reports, 13(3), 250–257. [CrossRef]
- Salna, M., Khalique, O. K., Chiuzan, C., Kurlansky, P., Borger, M. A., Hahn, R. T., Leon, M. B., Smith, C. R., Kodali, S. K., & George, I. (2018). Impact of small prosthesis size on transcatheter or surgical aortic valve replacement outcomes. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions, 91(4), 765–773. [CrossRef]
- Ali, N. F., Mahadevan, V. S., Muir, A., Maguire, C., Young, D., Campalani, G., Campbell, N. P., & Danton, M. H. (2006). The influence of prosthesis size and design on exercise dynamics after aortic valve replacement. The Journal of heart valve disease, 15(6), 755–762.
- Hagenah, J., Werrmann, E., Scharfschwerdt, M., Ernst, F., & Metzner, C. (2016). Prediction of individual prosthesis size for valve-sparing aortic root reconstruction based on geometric features. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, 2016, 3273–3276. [CrossRef]
- Hori, D., Kusadokoro, S., Shimizu, T., Kimura, N., & Yamaguchi, A. (2020). Prosthetic Graft Dilation at the Aortic Arch in the Era of Hybrid Aortic Surgery. Annals of vascular diseases, 13(2), 163–169. [CrossRef]
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine, 6(7), e1000097. [CrossRef]
- Reference management software, Endnote X9. Available from: https://endnote.com/product-details.
- Schünemann HJ, Higgins JPT, Vist GE, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from: https://training.cochrane.org/handbook/current/chapter-14.
- Review Manager (RevMan) [Computer program]. Version 5.4. Copenhagen: the Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Amerini, A., Hatam, N., Malasa, M., Pott, D., Tewarie, L., Isfort, P., Goetzenich, A., Hildinger, M., Autschbach, R., & Spillner, J. (2014). A personalized approach to interventional treatment of tricuspid regurgitation: experiences from an acute animal study. Interactive cardiovascular and thoracic surgery, 19(3), 414–418. [CrossRef]
- Collis, R., Tsang, V., Pantazis, A., Tome-Esteban, M., Elliott, P. M., & McGregor, C. G. A. (2018). Individualized surgical strategies for left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery, 53(6), 1237–1243. [CrossRef]
- Ovcharenko, E. A., Klyshnikov, K. U., Yuzhalin, A. E., Savrasov, G. V., Kokov, A. N., Batranin, A. V., Ganyukov, V. I., & Kudryavtseva, Y. A. (2016). Modeling of transcatheter aortic valve replacement: Patient specific vs general approaches based on finite element analysis. Computers in biology and medicine, 69, 29–36. [CrossRef]
- Pasta, S., Cannata, S., Gentile, G., Di Giuseppe, M., Cosentino, F., Pasta, F., Agnese, V., Bellavia, D., Raffa, G. M., Pilato, M., & Gandolfo, C. (2020). Simulation study of transcatheter heart valve implantation in patients with stenotic bicuspid aortic valve. Medical & biological engineering & computing, 58(4), 815–829. [CrossRef]
- Rim, Y., Choi, A., McPherson, D. D., & Kim, H. (2015). Personalized Computational Modeling of Mitral Valve Prolapse: Virtual Leaflet Resection. PloS one, 10(6), e0130906. [CrossRef]
- Robinson, S. S., Alaie, S., Sidoti, H., Auge, J., Baskaran, L., Avilés-Fernández, K., Hollenberg, S. D., Shepherd, R. F., Min, J. K., Dunham, S. N., & Mosadegh, B. (2018). Patient-specific design of a soft occluder for the left atrial appendage. Nature biomedical engineering, 2(1), 8–16. [CrossRef]
- Yuan, D., Luo, H., Yang, H., Huang, B., Zhu, J., & Zhao, J. (2017). Precise treatment of aortic aneurysm by three-dimensional printing and simulation before endovascular intervention. Scientific reports, 7(1), 795. [CrossRef]
- Mathew P, Kanmanthareddy A. Prosthetic Heart Valve. [Updated 2021 Jul 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536987/.
- Alkadhi, H., & Euler, A. (2020). The Future of Computed Tomography: Personalized, Functional, and Precise. Investigative radiology, 55(9), 545–555. [CrossRef]
- Kong, F., Caballero, A., McKay, R., & Sun, W. (2020). Finite element analysis of MitraClip procedure on a patient-specific model with functional mitral regurgitation. Journal of biomechanics, 104, 109730. [CrossRef]
- Pham, T., Kong, F., Martin, C., Wang, Q., Primiano, C., McKay, R., Elefteriades, J., & Sun, W. (2017). Finite Element Analysis of Patient-Specific Mitral Valve with Mitral Regurgitation. Cardiovascular engineering and technology, 8(1), 3–16. [CrossRef]
- Kong, F., Pham, T., Martin, C., Elefteriades, J., McKay, R., Primiano, C., & Sun, W. (2018). Finite element analysis of annuloplasty and papillary muscle relocation on a patient-specific mitral regurgitation model. PloS one, 13(6), e0198331. [CrossRef]
- Kato, B., Wisser, G., Agrawal, D. K., Wood, T., & Thankam, F. G. (2021). 3D bioprinting of cardiac tissue: current challenges and perspectives. Journal of materials science. Materials in medicine, 32(5), 54. [CrossRef]
- Sazzad, F., Goh, J. H., Ong, Z. X., Almsherqi, Z. A. M., Lakshminarasappa, S. R., Ramanathan, K. R., & Kofidis, T. (2022). Three dimensional modeling of atrioventricular valves provides predictive guides for optimal choice of prosthesis. Scientific reports, 12(1), 7432. [CrossRef]
- Nappi, F., Spadaccio, C., Mihos, C. G., & Fraldi, M. (2017). Biomechanics raises solution to avoid geometric mitral valve configuration abnormalities in ischemic mitral regurgitation. Journal of thoracic disease, 9(Suppl 7), S624–S628. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).