Submitted:
05 October 2023
Posted:
05 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the chitosan-activated charcoal composite
2.3. Characterizations of material
2.4. Batch adsorption experiments
3. Results and Discussion
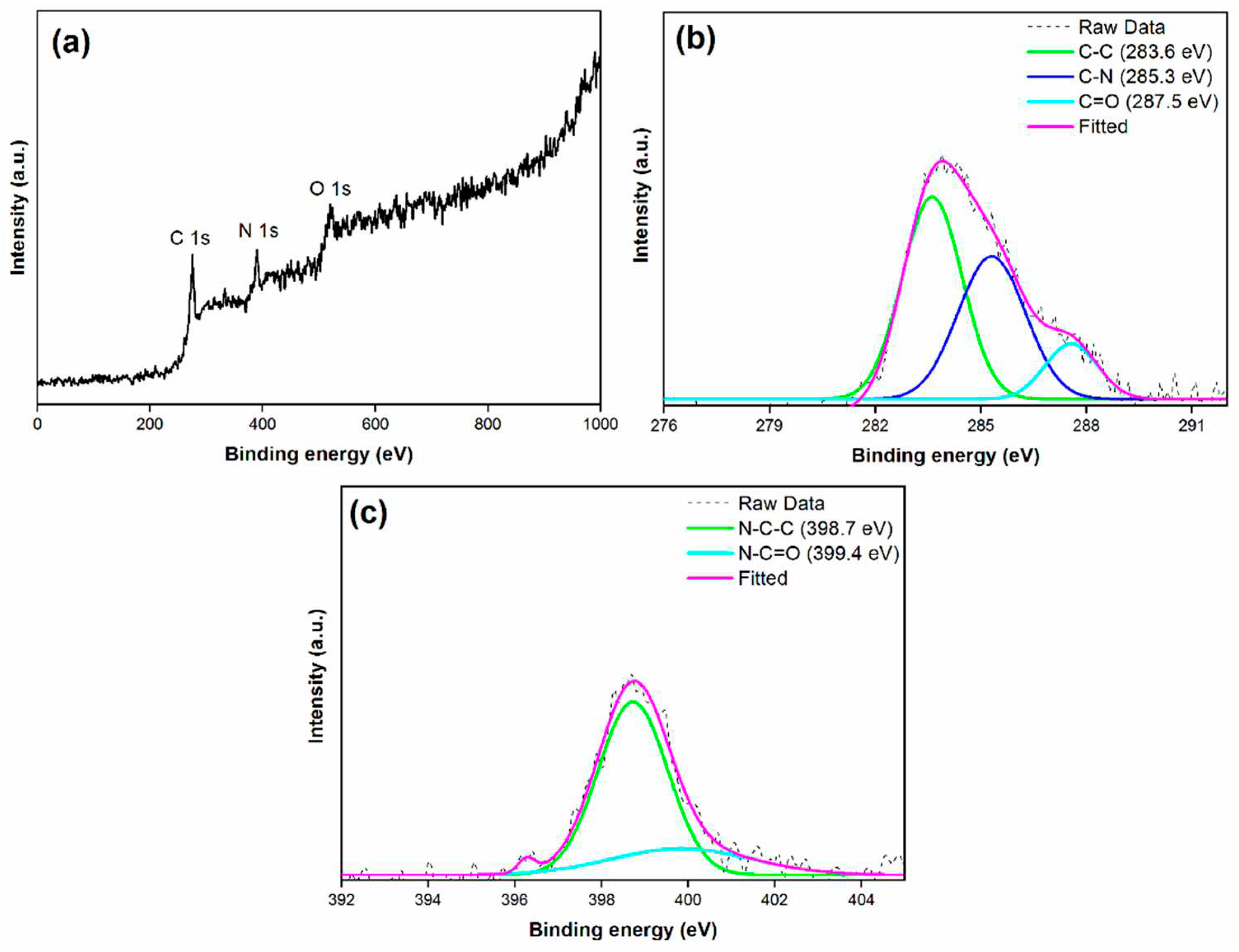
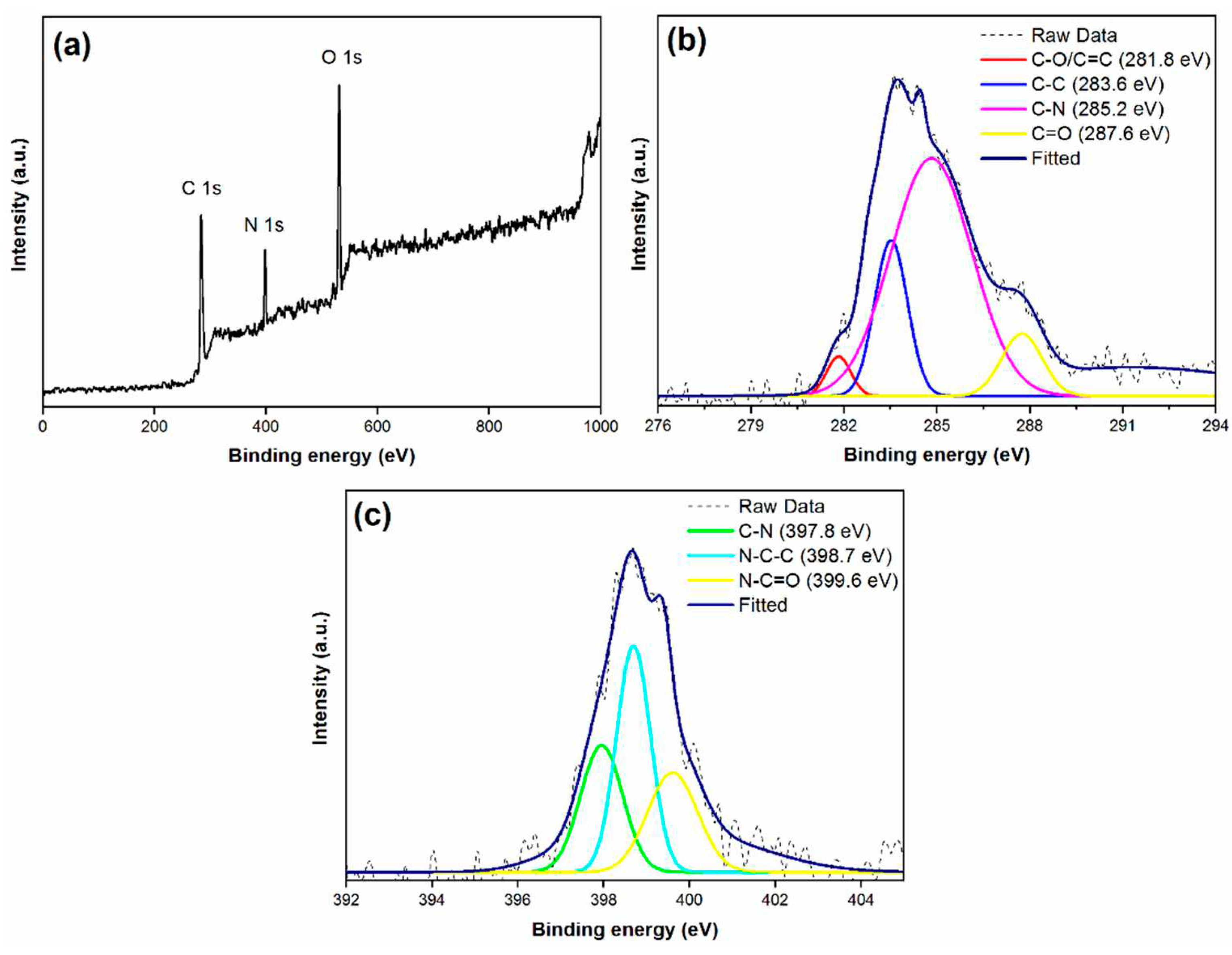
3.1. X-ray Photoelectron Spectroscopy (XPS) analysis of synthesised material
3.2. FTIR analysis of synthesised material
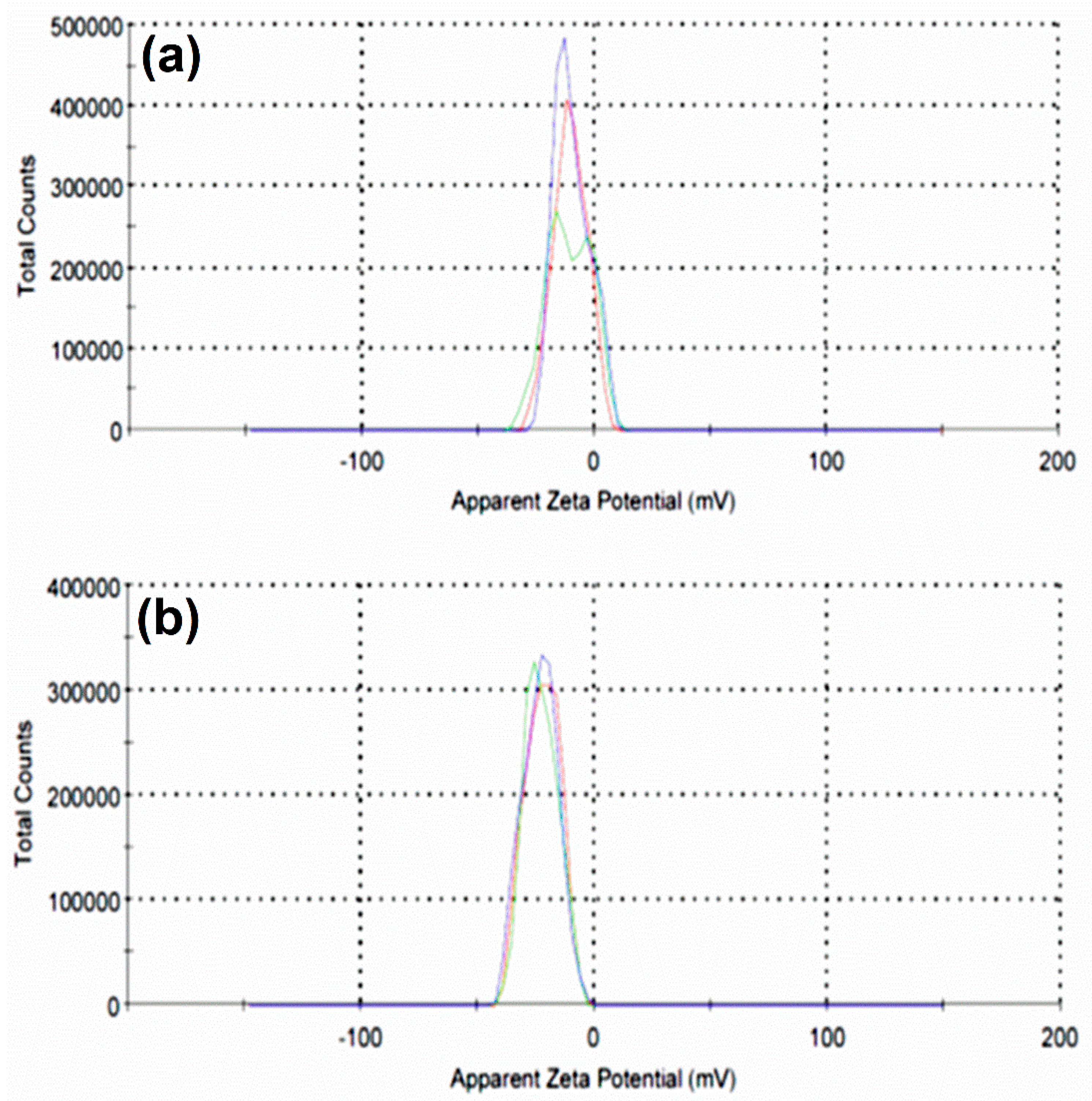
3.3. Zeta-potential analysis of synthesised material
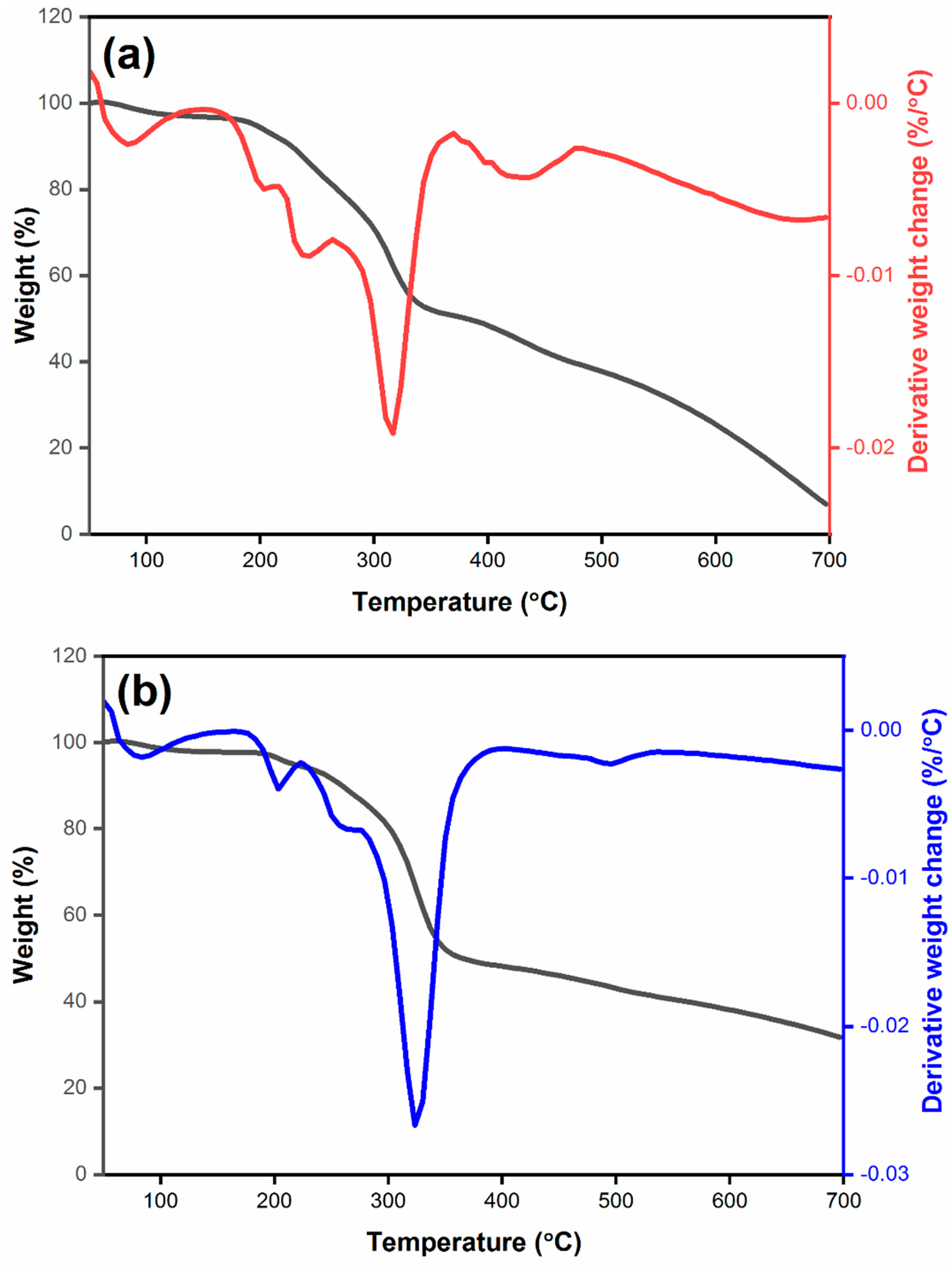
3.4. Thermogravimetric analysis (TGA) of synthesised materials
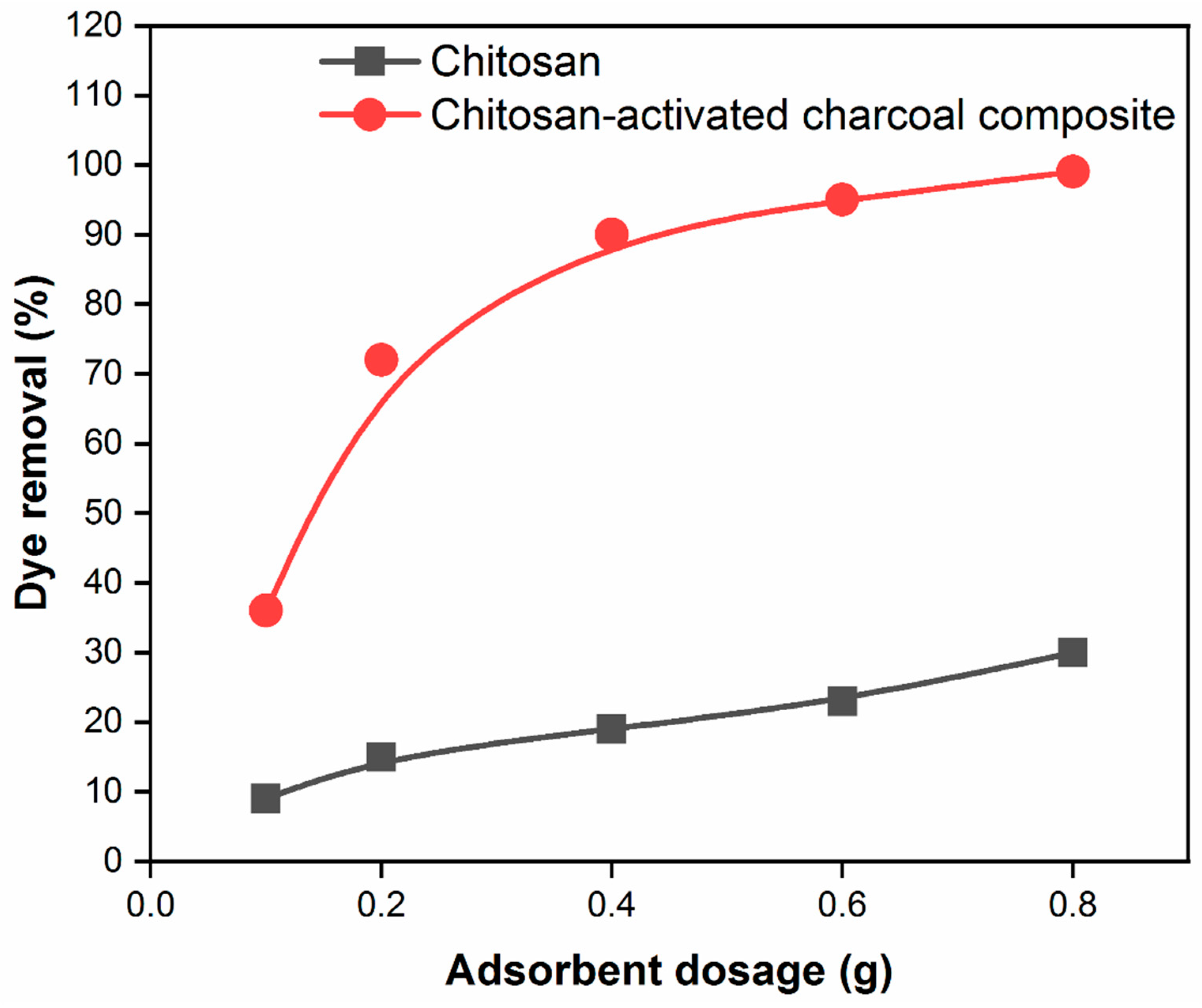
3.5. Effect of adsorbent dosage on the adsorption capacity
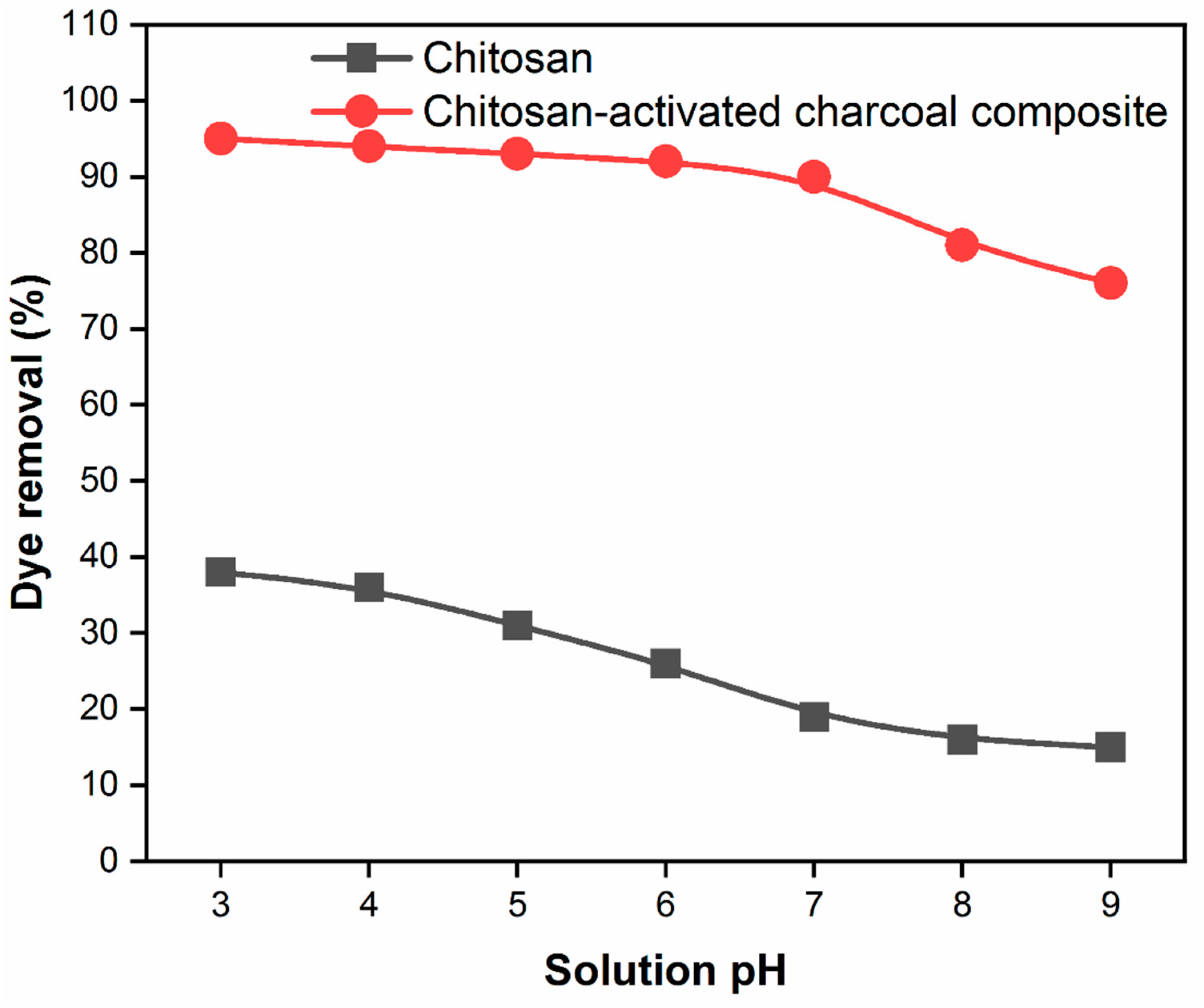
3.6. The effect of pH on the adsorption capacity
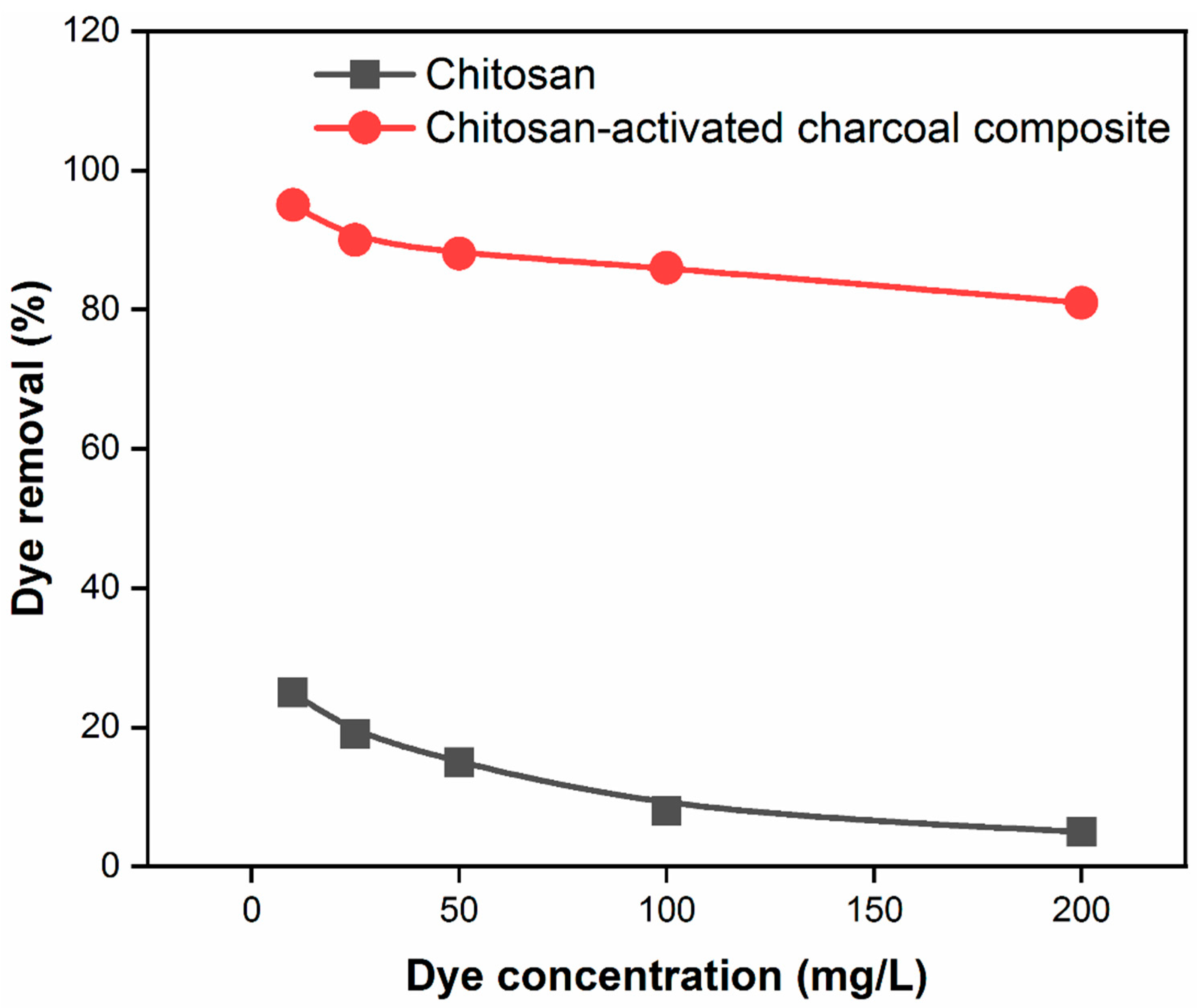
3.7. Effect of dye concentration on the adsorption capacity
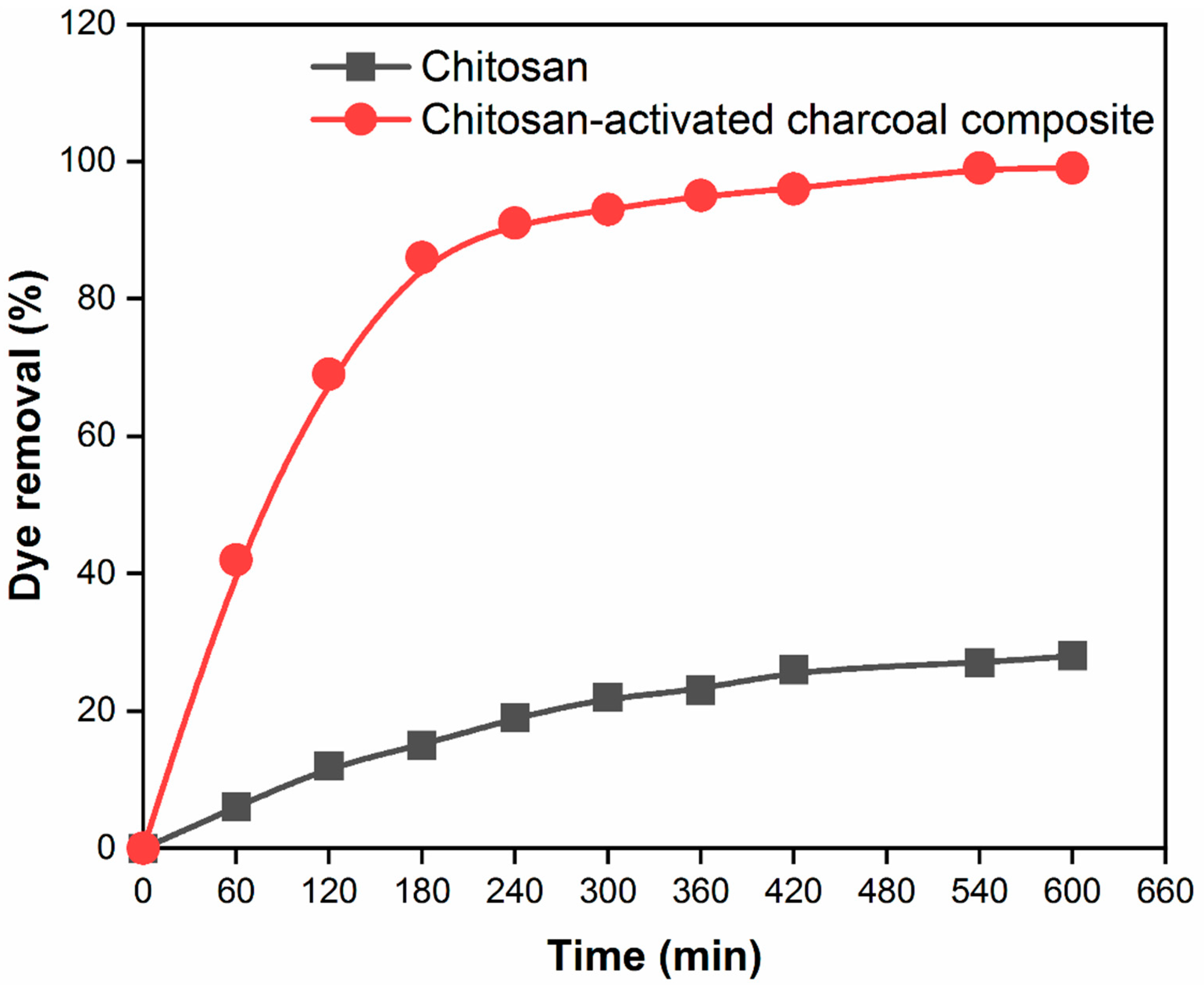
3.8. Effect of reaction time for dye adsorption capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, N.; Jiang, B.; Zhang, L.; Huang, Z.; Sun, Y.; Zong, Y.; Zhang, H. Low-pressure electroneutral loose nanofiltration membranes with polyphenol-inspired coatings for effective dye/divalent salt separation. Chem. Eng. J. 2019, 359, 1442–1452. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Adsorption removal of Methylene Blue (MB) dye from aqueous solution by bio-char prepared from Eucalyptus sheathiana bark: kinetic, equilibrium, mechanism, thermodynamic and process design. Desalin. Water Treat. 2016, 57, 28964–28980. [Google Scholar] [CrossRef]
- Harrou, A.; Gharibi, E.; Nasri, H.; El Ouahabi, M. Thermodynamics and kinetics of the removal of methylene blue from aqueous solution by raw kaolin. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Rajoriya, S.; Saharan, V.K.; Pundir, A.S.; Nigam, M.; Roy, K. Adsorption of methyl red dye from aqueous solution onto eggshell waste material: Kinetics, isotherms and thermodynamic studies. Curr. Res. Green Sustain. Chem. 2021, 4, 100180. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Alene, A.N. A comparative study of acidic, basic, and reactive dyes adsorption from aqueous solution onto kaolin adsorbent: Effect of operating parameters, isotherms, kinetics, and thermodynamics. Emerg. Contam. 2022, 8, 59–74. [Google Scholar] [CrossRef]
- Gürses, A.; Hassani, A.; Kranşan, M.; Açşl, Ö.; Karaca, S. Removal of methylene blue from aqueous solution using by untreated lignite as potential low-cost adsorbent: Kinetic, thermodynamic and equilibrium approach. J. Water Process Eng. 2014, 2, 10–21. [Google Scholar] [CrossRef]
- Brahma, D.; Saikia, H. Synthesis of ZrO2/MgAl-LDH composites and evaluation of its isotherm, kinetics and thermodynamic properties in the adsorption of congo red dye. Chem. Thermodyn. Therm. Anal. 2022, 7, 100067. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Kumar, V.B.; Luong, J.H.T.; Gedanken, A. Kinetics, Isotherm, and Thermodynamic Studies of Methylene Blue Adsorption on Polyaniline and Polypyrrole Macro-Nanoparticles Synthesized by C-Dot-Initiated Polymerization. ACS Omega 2018, 3, 7196–7203. [Google Scholar] [CrossRef]
- Salunkhe, B.; Schuman, T.P. Super-Adsorbent Hydrogels for Removal of Methylene Blue from Aqueous Solution: Dye Adsorption Isotherms, Kinetics, and Thermodynamic Properties. Macromol 2021, 1, 256–275. [Google Scholar] [CrossRef]
- Tan, Y.; Sun, Z.; Meng, H.; Han, Y.; Wu, J.; Xu, J.; Xu, Y.; Zhang, X. A new MOFs/polymer hybrid membrane: MIL-68(Al)/PVDF, fabrication and application in high-efficient removal of p-nitrophenol and methylene blue. Sep. Purif. Technol. 2019, 215, 217–226. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Badrinezhad, L.; Ghasemi, S.; Azizian-Kalandaragh, Y.; Nematollahzadeh, A. Preparation and characterization of polysulfone/graphene oxide nanocomposite membranes for the separation of methylene blue from water. Polym. Bull. 2018, 75, 469–484. [Google Scholar] [CrossRef]
- Alam, J.; Shukla, A.K.; Ansari, M.A.; Abdulraqeb, F.; Ali, A.; Alhoshan, M. Dye Separation and Antibacterial Activities of Polyaniline Thin Film-Coated Poly ( phenyl sulfone ) Membranes. Membranes (Basel). 2021, 11, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Khademian, E.; Salehi, E.; Sanaeepur, H.; Galiano, F.; Figoli, A. A systematic review on carbohydrate biopolymers for adsorptive remediation of copper ions from aqueous environments-part A: Classification and modification strategies. Sci. Total Environ. 2020, 738, 139829. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.N. Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater. In; Rashed, M.N., Ed.; IntechOpen: Rijeka, 2013; p. Ch. 7. [Google Scholar]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Ohale, P.E.; Igwegbe, C.A.; Iwuozor, K.O.; Emenike, E.C.; Obi, C.C.; Białowiec, A. A review of the adsorption method for norfloxacin reduction from aqueous media. MethodsX 2023, 10, 102180. [Google Scholar] [CrossRef]
- Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. C 2021, 7, 39. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, C.; Zhang, S.; Wang, C. Cellulose Acetate/Activated Carbon Composite Membrane with Effective Dye Adsorption Performance. J. Macromol. Sci. Part B Phys. 2019, 58, 909–920. [Google Scholar] [CrossRef]
- Elmaghraby, N.A.; Omer, A.M.; Kenawy, E.R.; Gaber, M.; Ragab, S.; Nemr, A. El Composite nanofiber formation using a mixture of cellulose acetate and activated carbon for oil spill treatment. Environ. Sci. Pollut. Res. 2023, 30, 38683–38699. [Google Scholar] [CrossRef]
- Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology. C 2021, 7, 19. [Google Scholar] [CrossRef]
- Rendón-Villalobos, R.; Ortíz-Sánchez, A.; Sánchez, E.T.; Flores-Huicochea, E. The Role of Biopolymers in Obtaining Environmentally Friendly Materials. In; Poletto, M., Ed.; IntechOpen: Rijeka, 2016; p. Ch. 8. ISBN 978-953-51-2794-9. [Google Scholar]
- S, H.; U Chandran, G.; P R, J.; Sambhudevan, S. Biomedical Applications of Chitin BT - Handbook of Biopolymers. In; Thomas, S., AR, A., Jose Chirayil, C., Thomas, B., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 1–28. ISBN 978-981-16-6603-2. [Google Scholar]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers (Basel). 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of chitosan for the generation of functional derivatives. Appl. Sci. 2019, 9. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications; 2015; Vol. 13; ISBN 8523943803.
- Grzybek, P.; Jakubski, Ł.; Dudek, G. Neat Chitosan Porous Materials: A Review of Preparation, Structure Characterization and Application. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Saheed, I.O.; Oh, W. Da; Suah, F.B.M. Chitosan modifications for adsorption of pollutants – A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Q.; Huang, C.; Wei, H.; Wang, R.; Wang, C. Highly efficient separation membrane based on cellulose acetate/chitosan fibrous composite substrate with activated carbon functional adsorption layer. J. Chem. Technol. Biotechnol. 2021, 96, 672–679. [Google Scholar] [CrossRef]
- Salama, A.; Mohamed, A.; Aboamera, N.M.; Osman, T.; Khattab, A. Characterization and mechanical properties of cellulose acetate/carbon nanotube composite nanofibers. Adv. Polym. Technol. 2018, 37, 2446–2451. [Google Scholar] [CrossRef]
- Nandanwar, P.; Jugade, R.; Gomase, V.; Shekhawat, A.; Bambal, A.; Saravanan, D.; Pandey, S. Chitosan-Biopolymer-Entrapped Activated Charcoal for Adsorption of Reactive Orange Dye from Aqueous Phase and CO2 from Gaseous Phase. J. Compos. Sci. 2023, 7. [Google Scholar] [CrossRef]
- Karthik, D.; Militky, J.; Wang, Y.; Venkataraman, M. Joule Heating of Carbon-Based Materials Obtained by Carbonization of Para-Aramid Fabrics. C 2023. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U. Bin; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biomass Convers. Biorefinery 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Kwa, A. Physical Properties of Starch / Powdered Activated Carbon. Polymers (Basel). 2021. [Google Scholar]
- Blachnio, M.; Derylo-Marczewska, A.; Charmas, B.; Zienkiewicz-Strzalka, M.; Bogatyrov, V.; Galaburda, M. Activated Carbon from Agricultural Wastes for Adsorption of Organic Pollutants; 2020; Vol. 25; ISBN 4881537563.
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, C. Research progress on modification of activated carbon. E3S Web Conf. 2018, 38, 2–5. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Alsohaimi, I.H.; Alhumaimess, M.S.; Hassan, H.M.A.; Reda, M.; Aldawsari, A.M.; Chen, Q.; Kariri, M.A. Chitosan Polymer Functionalized-Activated Carbon/Montmorillonite Composite for the Potential Removal of Lead Ions from Wastewater. Polymers (Basel). 2023, 15. [Google Scholar] [CrossRef]
- Mobarak, M.; Ali, R.A.M.; Seliem, M.K. Chitosan/activated coal composite as an effective adsorbent for Mn(VII): Modeling and interpretation of physicochemical parameters. Int. J. Biol. Macromol. 2021, 186, 750–758. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Surip, S.N.; Sabar, S. Adsorptive performance of carbon modified chitosan biopolymer for cationic dye removal: kinetic, isotherm, thermodynamic, and mechanism study. Int. J. Environ. Anal. Chem. 2022, 102, 6189–6203. [Google Scholar] [CrossRef]
- Elmaghraby, N.A.; Omer, A.M.; Kenawy, E.R.; Gaber, M.; Hassaan, M.A.; Ragab, S.; Hossain, I.; El Nemr, A. Electrospun cellulose acetate/activated carbon composite modified by EDTA (rC/AC-EDTA) for efficient methylene blue dye removal. Sci. Rep. 2023, 13, 1–16. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, S.K. Effect of Hydrothermal Carbonization Reaction Parameters on. Environ. Prog. Sustain. Energy 2014, 33, 676–680. [Google Scholar]
- de Freitas, F.P.; Carvalho, A.M.M.L.; Carneiro, A. de C.O.; de Magalhães, M.A.; Xisto, M.F.; Canal, W.D. Adsorption of neutral red dye by chitosan and activated carbon composite films. Heliyon 2021, 7. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




