1. Introduction
Tarwi (
Lupinus mutabilis Sweet), also known by other common names such as tauri or chocho [
1], is a species native to the Andean region of Peru, Bolivia, and Ecuador that has been cultivated since pre-Incan times [
2,
3]. The indigenous populations of this region used tarwi as one of their main sources of food and income. The ancestral knowledge about its cultivation, conservation, and various forms of utilization are still maintained by indigenous people in the Andean culture.
L. mutabilis is the only species of the genus
Lupinus that has been domesticated and cultivated in South America [
4]. Currently, it is planted mainly in the Andean region of Ecuador, Peru, and Bolivia and is it used for human consumption in several ways of food preparation [
5].
The cultivation of tarwi is essential to conserve the agroecological systems in the highlands. Through the nodules formed in their roots by the bacteria
Rhizobium, the plant has the capacity to fix from 120 to 160 kg ha
-1 of Nitrogen (N) in the soil per year [
6]; even fixing up to 500 kg ha
-1 of N per year [
7]. In addition, the plant is adapted to produce under poor soil conditions (low nutrient content) and low-input agricultural systems [
8]. In the “chakra” (Andean name for a typical farmer plot), tarwi is typically cultivated as a monoculture or in association with other crops like maize (
Zea mayz) quinoa (
Chenopodium quinua), popatoes (
Solanum tuberosum) and cereals [
9].
Tarwi is considered of paramount importance for food security in the Andean region [
10]. The seed has a high protein content of 41-51%, which exceeds that of any other
Lupinus species [
11]; additionally, it has an oil content of 14-24% [
12], high content of calcium, fiber, iron, zinc and do not contain gluten [
13]. Therefore, its cultivation and consumption are considered strategic, especially for nursing mothers and growing children as one of the alternatives to combat child malnutrition [
14]. The consumption of
L. mutabilis, as part of a healthy diet for human, prevents and treats metabolic diseases such as obesity, diabetes, and cardiovascular diseases [
14].
Tarwi grains are raw materials with great industrial potential in the production of processed or semi-processed foods [
14]. The commercialization of proteins of vegetable origin and their derivatives has increased significantly in recent years: in 2012 they moved about 1.7 million metric tons, and it is expected that there will be an increase of 5.5% in the coming years; being North America and Europe the markets with the highest consumption [
16]. Tarwi paste is useful in making beverages, yogurts and jams; meanwhile, the flour is mainly used in the preparation of protein-rich and gluten-free bread, cakes, cookies, cakes, noodles, chips, instant soups and sausages [
17], it is excellent option for people with intolerance gluten (celiac disease)
Although tarwi has been considered an ideal food for its nutritional value, industrial potential, and environmental benefits, the study and genetic improvement of the crop is still limited, lacking high-yielding, early-maturing varieties, and a consistent breeding history [
18]. This review describes the breeding methods used in
L. mutabilis and the released cultivars during 40 years of research in Ecuador, Bolivia, and Peru; countries recognized as centers of origin of the crop. Additionally, the future of tarwi breeding is analyzed.
Much of the information contained in this review, referring to the genetic improvement carried out in the Andean region, has not been widely disseminated in the international scientific community. The publication of the progress obtained in the improvement of tarwi will be used by breeders in the Andean Region and breeders from other parts of the world who have already shown interest in generating new varieties of L mutabilis.
2. Tarwi plant characteristics
L. mutabilis (2n=48) [
18], taxonomically belongs to the Fabaceae Family [
2] and is primarily an autogamous species with hermaphroditic flowers, but a certain level of cross-pollination can occur (5 to 10%), this level can increase depending on the genotype and the ecological conditions where it is planted [
19]. The most important characteristic of tarwi is the high nutritional value, its seeds have high protein, fat and fiber content (50%, 20%, 7%) that can surpass other grains that are commonly used in the food industry such as soybeans (
Glycine max) (40%, 18%, 4%), beans (
Phaseolus vulgaris) (22%, 1.6%, 4%) and peanuts
(Arachis hypogaea) (27%, 42%, 2%) [
2].
L. mutabilis is generally an annual plant that varies in height from 0.50 m to more than 2 m depending on the genotype and the environment in which it is grown [
20]. The root is taper, vigorous and deep, which can extend up to 45-50 cm deep [
4].
The secondary roots have symbiotic nodules with bacteria of the genus
Rhizobium [
21].
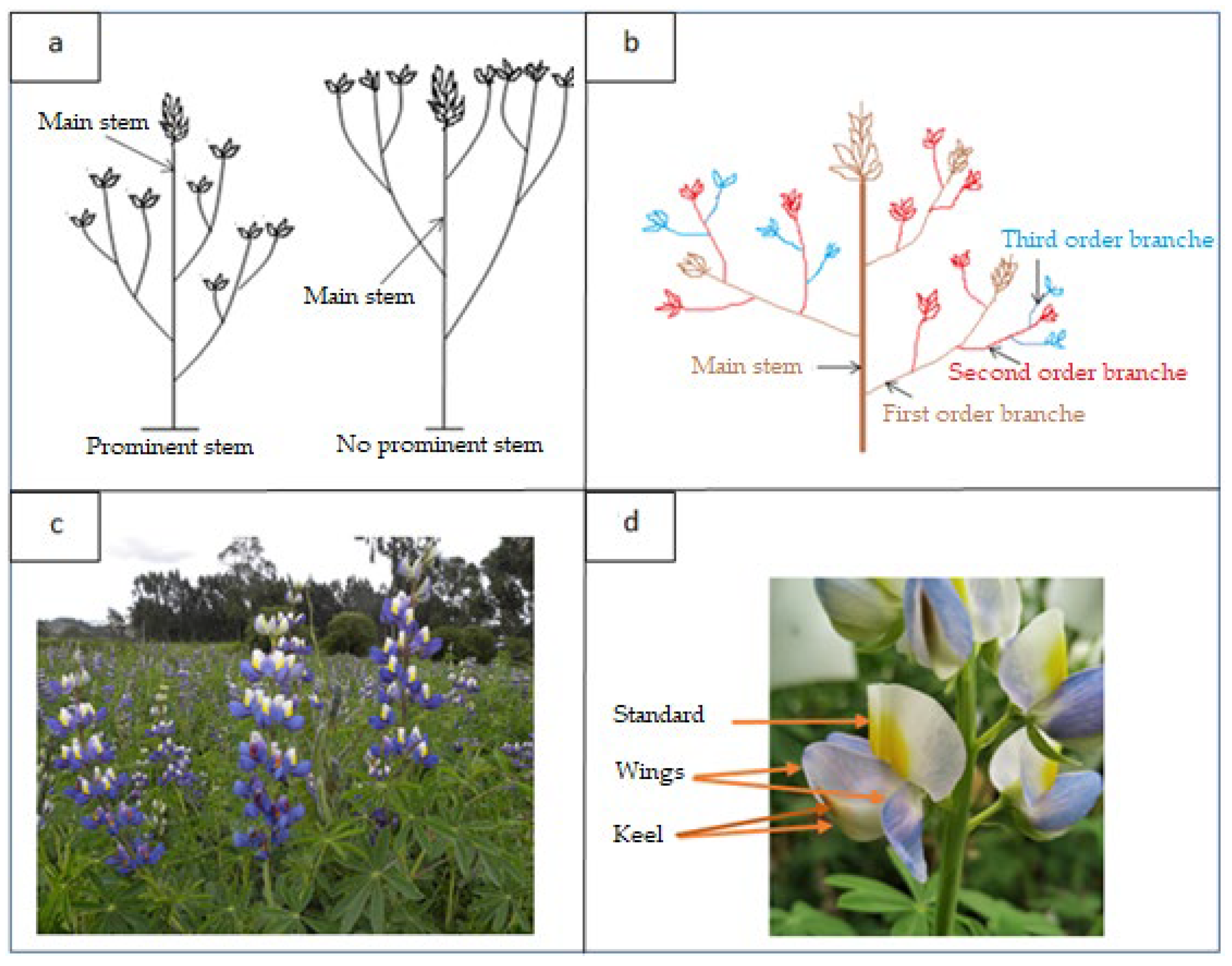
The stems are erect, cylindrical and have a main axis that can be prominent or not prominent with ramifications [
2] (
Figure 1a). The stem of the tarwi is generally very woody, with a high content in fiber and cellulose. The number of branches varies from a few to 52 branches. The number of pods and fruitful branches has a positive correlation with high production [
4]. The stem and its ramifications determine the architecture of the plant [
19].
The branching of the tarwi is sympodial, typically alternate; axillary buds are born on the central stem sides, forming the first order branches; in turn, they form second-order sub-branches, these branches give rise to third-order branches [
4] (
Figure 1b).
Tarwi has terminal inflorescences of flowers arranged vertically (
Figure 1c) [
2]. The variation in the number of clusters depends on the number of branches and each cluster can develop up to 20 flowers which bloom at different times.
The flowers are bilaterally symmetrical, each flower has a corolla with five petals, one for the standard, two for the keel and two for the wings (
Figure 1d). The keel surrounds the pistil and the ten stamens [
22]. The color of the corolla can be blue, white, pink or violet [
23] (
Figure 2a). The flower color intensity varies from the beginning of its formation to maturation, starting from a light color to very intense (
Figure 2b).
The fruit is an elliptical pod. The seeds are accommodated in the pod in a row in a size that varies from 4 to 15 mm (
Figure 2c). The young pods are very pubescent, and as it matures there is considerable loss of pubescence. The shape of the seeds can be ellipsoidal, lenticular, rounded and others in a semi-square shape. The seed color is varied, especially due to their distribution that can be like an eyebrow, mustache, crescent, marbled [
22]. The grain color is regulated by several genes, in some cases the dark colors are dominant over the light ones, however, the white color has a double recessive behavior [
4].
3. The cultivation of tarwi in Ecuador, Peru, and Bolivia
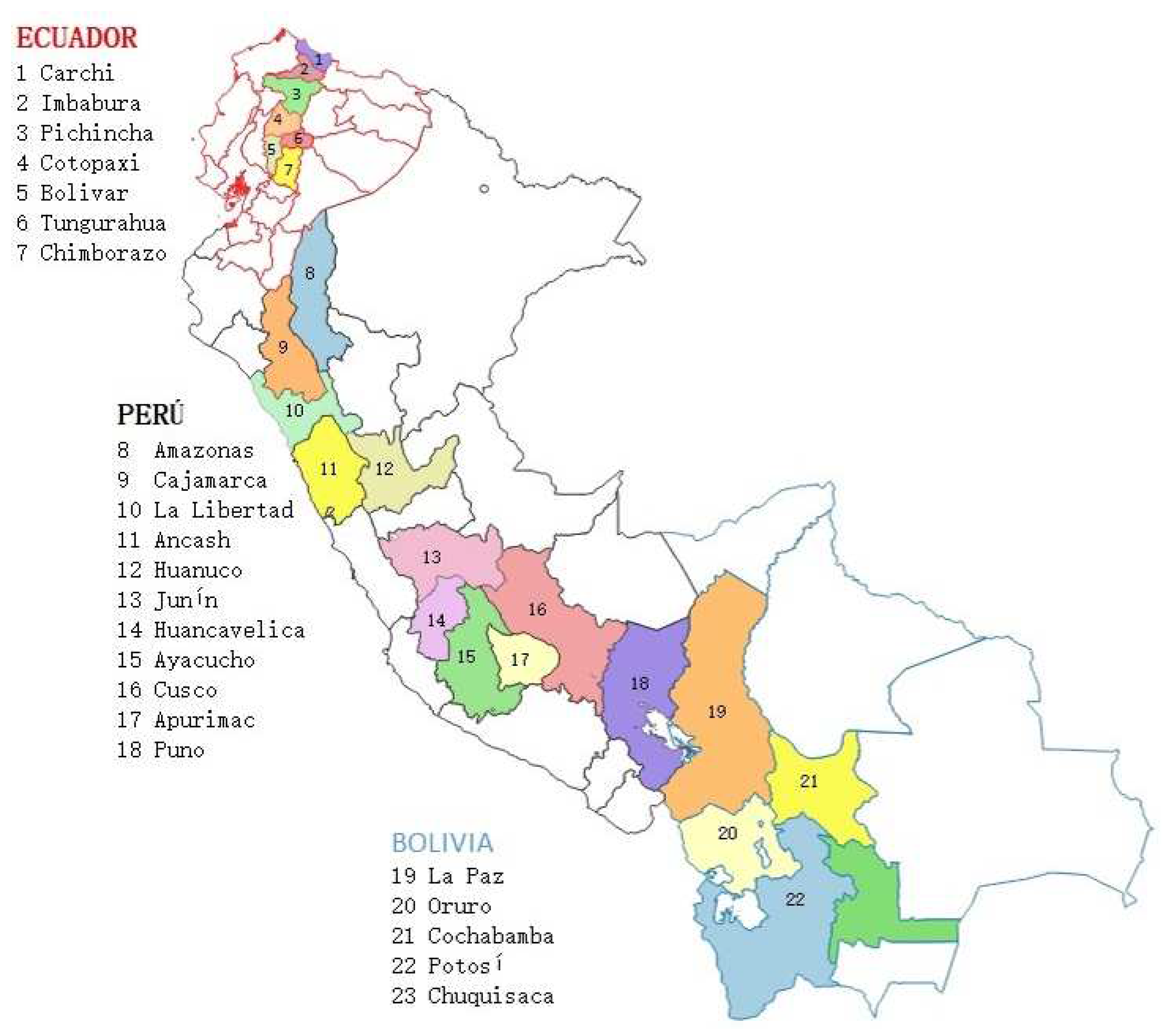
Bolivia has an approximate area of 1,895 ha of tarwi [
24,
25], with an average yield of 637 kg ha
-1 [
24]. It is grown in the cold areas of the Altiplano and in the inter-Andean valleys, at altitudes between 2,500 and 4,000 masl, specifically in the Departments of Cochabamba, La Paz, Potosí, and Chuquisaca [
26] (
Figure 3).
In Ecuador, the approximate area of tarwi cultivation is 6,000 ha, and the average yield is 400 kg ha
-1 [
27]; however, improved varieties with a potential yield of 1,500 kg ha
-1 are currently being introduced [
3]. Tarwi cultivation is concentrated in the inter-Andean region, especially in the provinces of Cotopaxi (Juan Montalvo and Alaquez) and Chimborazo (Palmira and Riobamba). Although to a lower extension, it is also cultivated in other agroecological zones of the highlands, such as Pichincha (Cayambe), Bolivar (Guaranda), Imbabura (Cotacachi), Tungurahua (Quero), and Carchi (Bolívar) [
3]. The tarwi-producing areas in these provinces are located between 2,700 and 3,800 masl, mainly in areas with low rainfall [
23]. A potential area of 140,712 ha for the cultivation of tarwi has been estimated for the highlands of Ecuador [
9].
In Peru, tarwi is cultivated in the Sierra (highland) of Cajamarca, La Libertad, Amazonas, Huanuco, Huancavelica, Ancash, Ayacucho, Junin, Pasco, Apurimac, Cusco, and Puno [
28]. The approximate area is 10,628 ha, and the average yield of 1,335 kg ha
-1, with a maximum yield of 2,232 kg ha
-1 in Apurímac, and a minimum yield of 657 kg ha
-1 in Amazonas [
3].
Annual per capita consumption in Ecuador is 4 kg year
-1, being the highest in the region. In Bolivia and Peru consumption does not exceed 0.2 kg year
-1 and 0.5 kg year
-1, respectively [
3].
4. Genetic Diversity of L. mutabilis in the Andean Region
L. mutabilis was domesticated in the highlands of northern Peru, most likely in the Department of Cajamarca, with
L. piurensis being the most likely wild progenitor of tarwi.
L. piurensis is a wild
Lupinus species endemic to the western edges of the Andes, from Cajamarca through Piura in Peru to the southern Loja in Ecuador, between 1,650 and 3,300 masl [
29]. The Andes has the greatest genetic diversity of
L. mutabilis [
20], with a wide variety in plant morphology and ecological adaptation, differentiating three subspecies [
19]:
L. mutabilis, chocho common name (northern Peru and Ecuador), with a high degree of branching, very late maturing, greater hairiness in leaves and stems; some ecotypes behave as biennials, tolerant to anthracnose.
L. mutabilis, tarwi common name (central and southern Peru), with few branches, medium late maturing, somewhat tolerant to anthracnose.
L. mutabilis, tauri common name (altiplano of Peru and Bolivia), with few branches, smaller plant (1-1.40 m), with a developed main stem, susceptible to anthracnose.
Additionally, in Bolivia, according to the vegetative cycle, the geographical region of adaptation and diversity, the cultivated tarwi is divided into three races [
21]:
Early Titicaca, late Titicaca, Cochabamba
Early South (Chuquisaca-Potosí), and
Late South (Potosí).
Germplasm collections of
L. mutabilis were initiated in 1974 by Dr. Oscar Blanco at the University of Cusco (Peru) and shortly thereafter, collections were conducted in Bolivia and Ecuador. In addition to the Germplasm Banks of Peru, Ecuador, and Bolivia, there are smaller collections in Chile, Argentina, Colombia, Australia, Russia, Poland, Germany, Spain, Hungary, the United Kingdom, and Portugal [
18]. However, the number of accessions conserved in these centers mainly represents the varieties and/or species collected by researchers in the area, and not the total existing agro-biodiversity [
2].
In South America, the main centers for the conservation of germplasm of
L. mutabilis are the PROINPA Foundation (Toralapa and Pairumani) in Bolivia; the Instituto Nacional de Innovación Agraria (INIA) in Peru with locations in Kayra (Cusco), Santa Ana (Huancayo), Camacani (Puno), Canaan (Ayacucho), and Baños del Inca (Cajamarca); the Gorbea Experimental Station (Temuco) in Chile; and Instituto Nacional de Investigaciones Agropecuarias (INIAP) in Mejía (Santa Catalina Experimental Station), in Ecuador [
2,
30].
In Peru there is the largest collection of germplasm of
L. mutabilis, with more than 2000 accessions collected [
6]. The Santa Catalina Experimental Station of INIAP-Ecuador has a collection of 529 accessions of 17 species of
Lupinus. Out of them, 257 accessions were collected in Ecuador and 272 in other countries. In a preliminary characterization of 120 accessions of
L. mutabilis, a wide variability in earliness, reaction to diseases, and flower color was found [
31]. In Bolivia, a collection of 595 accessions is maintained in germplasm banks located in La Paz and Cochabamba [
6,
32] (
Table 1).
Tarwi diversity identified in gene banks is wide, as is shown by several phenotypic traits, such as growth periods, branching patterns (branches from 0 to 52), color and shape of grains and flowers, and flowering times [
31,
33,
34]. This wide diversity has also been revealed by molecular markers. The genetic variability of 30 accessions of Peruvian origin was evaluated by using 65 ISRR (Inter Simple Sequence Repeat) primers, of which ISSR primers (AG)
8YT and (AG)
8YC were the most polymorphic, both within each accession and between accessions; whereas, the rest of ISSR primers showed a high content of intra-accession polymorphic information (
Table 2). The average percentage of polymorphism was 58.82 [
34]
Similar results were obtained when using six ISSR primers (
Table 2) to characterize the genetic variability of 23 accessions of
L. mutabilis. These primers revealed an average polymorphism of 30.55%. In this study, primers (AG)
8YG, (AG)
8YC and HVH(TG)
7 were the most suitable to distinguish accessions. Additionally, they verified that genetic variability did not correlate with phenotypic variability, indicating the need to incorporate more molecular markers [
35]. In the same study, the genome size of
L. mutabilis ranged between 1.94 pg/2C and 2.13 pg/2C and, according to the authors, it was the first intraspecific analysis of the genome size of
L. mutabilis, which represented an overall average size of 2.05 pg (2001.2 Mbp) [
35].
Two recent studies evaluated accessions from the Ecuadorian germplasm bank on four environments (Portugal, Ecuador and two locations in The Netherlands), and high phenotypic variability has been reported for some characteristics such as flowering time, plant height, number of branch orders, vegetative yield, pods and seeds on the central axis, total number of pods and seeds per plant and seed weight. High heritability values were reported for flowering (0.88), plant height (0.82) and production of pods and seeds on the main stem (0.6), as well as strong correlation between plant height, flowering time and seed yield was reported [
36,
37]. In addition, using GWAS (genome wide association study) 10 Markers linked to flowering time, plant height, vegetative yield, and number of pods and seeds on the main stem were identified (
Table 3). The most of the SNPs reported were identified as single location markers. The reference genome used in this study was
L angistifolius [
37].
L. mutabilis shows a wide diversity in plant architecture, plant height, grain and flower color [
13,
34]; likewise, it varies in crop cycle, protein, oil and alkaloid content, yield, and tolerance to insect pests and diseases [
1]. This variability has been detected within and between populations. This species exhibits 5 to 10% of intra-specific cross-pollination and inter-specific crosses with wild
Lupinus species [
22]. This event is interesting from the point of view of plant breeding since genes of interest from wild species could be introduced into the tarwi; but, on the other hand, this complicates the development of uniform and stable genotypes.
5. Breeding methods used in the region
The genetic variability that has been found in the germplasm of
L. mutabilis collected in Ecuador, Peru and Bolivia have allowed a wide margin of selection to identify materials with good agronomic characteristics [
22,
38]. Therefore, the genetic improvement of
L. mutabilis in the Andes was based mainly on selection within native ecotypes to adapt lines to specific conditions. In this way, several cultivars have been obtained by selection within heterogeneous populations and not properly as a result of hybridization programs [
39].
Mass selection has been employed as the main breeding method for the development of new tarwi cultivars in Ecuador, Peru and Bolivia. The method consisted on evaluating landraces or accessions from the germplasm bank in farmers' fields and experimental stations, selecting the best individual plants. In the following cycle, the seeds of the selected plants were sown in individual rows (plant-row), and the best plants were selected again. This evaluation and selection process was carried out for several cycles until promising lines were obtained. Subsequently, these lines were evaluated in yield trials in several locations or environments to identify the best genotype [
13,
40,
41,
42,
43].
In 2008, breeders of INIAP (Ecuador) began the improvement of tarwi by hybridization, making the first crosses among accessions from the germplasm bank to generate new cultivars that could improve over the performance of the widely used commercial cultivar “INIAP 450 Andino” [
23,
43]. Currently, two promising lines have been generated by the Genealogical or Pedigree Method [
44] and they have been tested in several locations. However, from the best of our knowledge, no improved varieties of
L. mutabilis have been released by hybridization in the Andean Region. Additionally, molecular tools and mapping populations to study the genetics basis of
L. mutabilis are not available. Molecular studies with tarwi have focused on understanding the phylogeny [
29,
45] and characterizing the genetic variability of this species [
34,
35].
6. Breeding targets in the region
The most important traits evaluated in tarwi have been earliness, plant architecture, yield, protein and oil content, tolerance to anthracnose, plant height and alkaloid content [
3,
40,
43,
46,
47,
48].
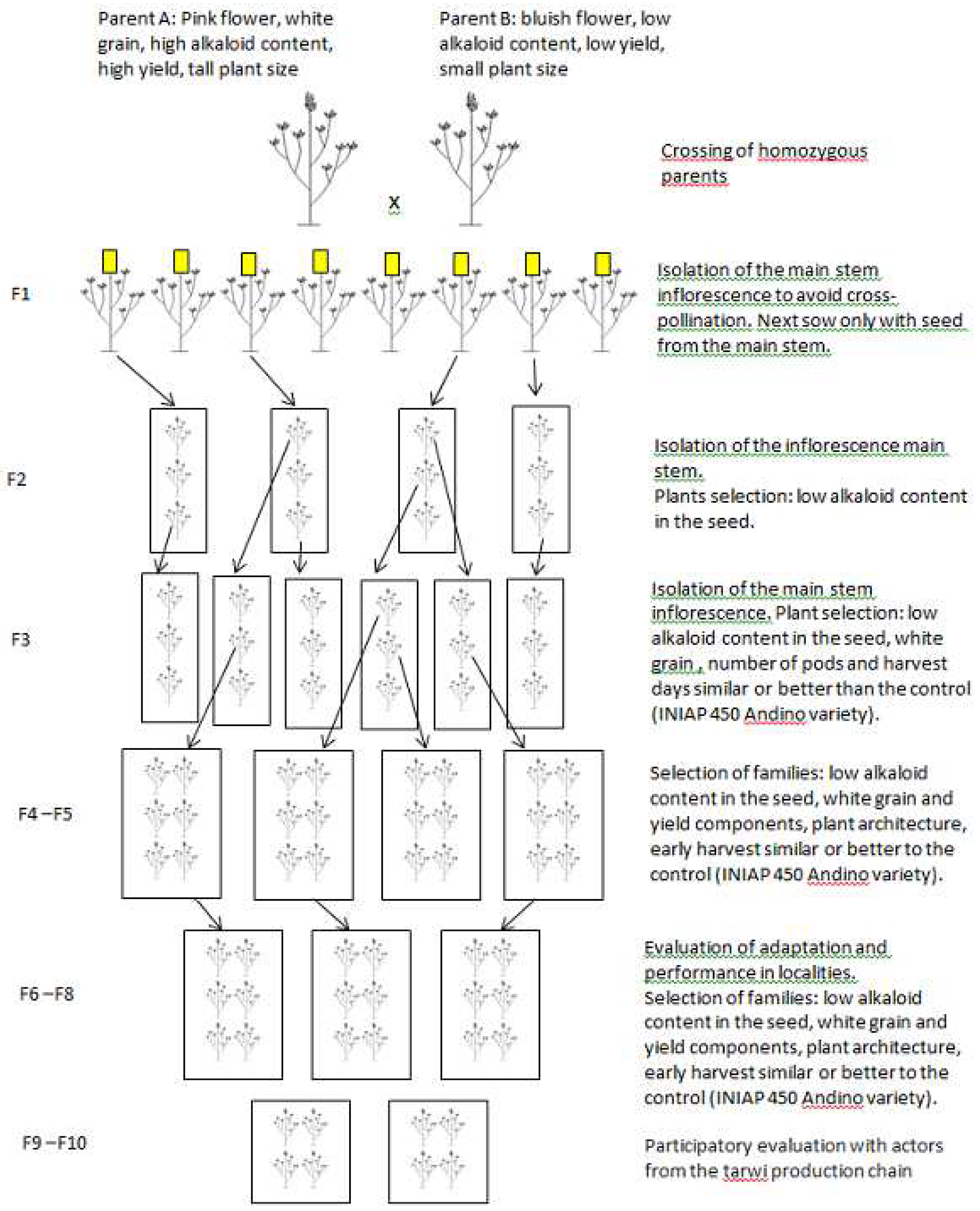
6.1. Breeding for low content alkaloid
The selection of tarwi genotypes with low alkaloid content has been an important objective since the beginning of the breeding programs in Bolivia and Peru [
4,
13,
38,
40]; while in Ecuador, the breeding (by crossing) for low alkaloid content began in 2018 [
10] (
Figure 4) [
49]. The presence of alkaloids in the grains of Tarwi has been a key limiting factor to the wide-scale expansion of this crop [
22,
50,
51]. The content and composition of alkaloids in grains depend on many factors, including the genotype, biotic/abiotic stress, and edaphoclimatic conditions [
49,
52]. The content of alkaloids in the Peruvian germplasm showed great variation and presented quantitative inheritance [
4]. Later, it was discovered that the inheritance of the trait was recessive, since only 12% of the F2 plants had low alkaloid content [
4]. The main alkaloids reported in
L. mutabilis are lupanine, sparteine, 3-hydroxylupanine, 13-hydroxylupanine and 4-hydroxylupanine [
50]. Although the chemistry of quinolizidine alkaloids has been extensively studied, the information of the genetic basis and proteins involved in the production of the alkaloids in
L. mutabilis remains unknown [
49].
6.2. Breeding for resistance to anthracnose
Anthracnose is the most important disease in
L. mutabilis, causing significant yield losses [
35,
38,
53,
54]. Anthracnose was previously reported to be caused by the fungus
Colletotrichum gloeosporioides [
55], but more recent research claimed that the disease is caused by
C. acutatum [
53,
55,
57]. The environmental conditions that favor the development of the disease are high precipitation and high temperature [
58].
Typical symptoms of the disease include twisting of stems, petioles, and pods with necrosis. When mature, the acervuli produce aggregates of conidia in orange mucilaginous masses. Tissues above the infection area could collapse [
35,
54] (
Figure 5). The pathogen can enter the interior of the infected pod and affects the seed, being the main way of spreading the disease [
58]. Contaminated seeds appear thin (sucked). Plants that are born from infected seeds show symptoms in the cotyledons and stems, sometimes killing the plant [
22].
One of the objectives of the Breeding Program of tarwi in the Andean region has been the development of cultivars with resistance to anthracnose [
3,
23,
43,
48]; however, so far, only one variety has been reported with tolerance to anthracnose, the Peruvian variety ‘Huamachuco’ [
2] (
Table 4).
In Ecuador, studies have been carried out using artificial inoculations in the field and greenhouse since 2007, to identify plants with resistance or tolerance to anthracnose in several accessions from the INIAP Germplasm Bank.
As a result of several studies using accessions from the germplasm bank of Ecuador and isolates of
C. acutatum, an inoculation methodology has been defined to allow the selection of genotypes with resistance or tolerance to anthracnose. In addition, a series of steps has been described for the development of a breeding program to obtain varieties resistant to anthracnose in Ecuador [
53] (
Table 5).
7. Improved varieties of L. mutabilis obtained in the Andean region
All commercial varieties have been generated from the selection of the best local ecotypes or landraces, however many of these have not been used extensively [
13]. In Potosí, local varieties known as ‘Chumpi tarwi’ are planted, which have a dark brown grain color and are widespread in the region. Other varieties, such as ‘Tarwi Ñawi’ (dark-colored in the embryo part) and ‘Blanca’ (large white seed), are not widely used [
61]. In 2013, the Foundation PROINPA began the genetic improvement of tarwi, evaluating local genotypes collected in different parts of the country [
3]. Among the limitations of tarwi cultivation, it was identified that in conditions of fertile soil and high humidity, the plants grew too tall (up to 2.5 m), their cycle was quite long and produced non-uniform maturation, which made mechanized cultivation impossible. Therefore, among the main improvement objectives, the PROINPA Foundation considered obtaining plants for an early cycle, with uniform maturation and a high yield [
13], managing to generate two cultivars that are in the process of being officially registered with the names de ‘Jayata’ [
3,
40] and ‘Candela’ [
13] (
Table 6).
Between the years 1980 and 1991, the National Institute of Agrarian Research (INIA) and the National Universities released white and other colored grain varieties [
62]; however, these varieties have not been adequately disseminated and it is difficult to find pure materials [
22]. Furthermore, they have rarely resulted in the official registration of cultivars [
21]. In later years, a series of varieties with outstanding characteristics in terms of yield, crop cycle, and lower alkaloid content were released, but with enormous susceptibility to insect pests, diseases, and adverse weather conditions [
63]. Currently, there are few varieties from Cusco, Huancayo, and La Libertad that have reached yields of over 3000 kg ha
-1 [
22] (
Table 6). Despite the numbers of new cultivars released in Peru, it can be affirmed that tarwi cultivation is still carried out with local ecotypes, typical of each region, that are characterized by being heterogeneous populations, very late and very susceptible to anthracnose, rust, ascochyta and insect pests [
62].
The history of plant breeding for L. mutabilis began in 1983, with the collection and formation of the germplasm bank at the INIAP-Santa Catalina Experimental Station. Between 1987 and 1996, promising materials were characterized and selected. In 1999, the first improved variety (by mass selection) ‘INIAP 450 Andino’ was released, coming from an accession of Peruvian origin. This is an early variety (six to seven months of cultivation), of wide adaptability, with a large grain size, white grain color, and high yield. Today, it is estimated that more than 70% of the planted area in the country is grown with this variety (5000 ha approx.).
In 2010, in a joint effort with the University of Bolívar, using mass selection, the second improved variety “INIAP 451 Guranguito” was released in the province of Bolívar [
43] (
Table 6). This variety has tolerance to foliar diseases, large grain size, and white grain color, intermediate cycle, and market acceptance [
64].
It is important to highlight the importance of the ‘Inti’ variety, which is the first variety of
L. mutabilis with low alkaloid content (0.0075%), generated at the Gorbea Experimental Station in Chile. The ‘Inti’ cultivar was evaluated in the Peruvian Andes, where the yields obtained were in the range from 121 to 1216 kg ha
-1 [
12]. In addition, ‘Inti’ showed an enormous susceptibility to insect pest, diseases, and adverse weather conditions [
47].
8. Brief overview of tarwi breeding progress in Europe
There is a growing interest for
L. mutabilis in Europe to generate cultivars that adapt to Mediterranean environmental conditions. In the 1990s, two European research projects took place: “
Lupinus mutabilis: Its adaptation and production under European pedoclimatic conditions” and “Adaptation of
L. mutabilis to European soil and climate conditions”. The interest of Europe continued in 2020 with the LIBBIO European project (No 720726, Horizon 2020), entitled "
Lupinus mutabilis for Increased Biomass from marginal lands and value for BIOrefineries" (
http://www.libbio.net). In these projects, several ecotypes of
L. mutabilis have been phenotypically and genetically characterized in Germany, the United Kingdom, Poland, France, Portugal, Greece, Spain, Austria, Iceland, the Netherlands, and Romania. After some years of experimentation and research, some
L. mutabilis accessions have finally been identified; as a result, they are partially adapted to the European agroecological conditions [
65].
The investment that European countries have made in research on the cultivation of tarwi shows that it has great commercial potential, however, in the Andean region, being the center of origin, its cultivation is still undervalued, without greater investment for research.
Lupinus species from Mediterranean origin, such as
L. albus and
L. angustifolius, are better studied and they have a wide selection of genomic tools and molecular markers available for traits such as alkaloid content, flowering time, and resistance to anthracnose disease [
66]. These resources could be applied to
L. mutabilis to significantly accelerate the improvement of tarwi in the Andean Region.
9. Breeding perspectives.
The high content of quinolizidine alkaloids in the seed is one of the most important factors that have slowed down the potential use of this crop. Before consumption or processing, an alkaloid elimination process is necessary, which involves time (about 3 to 7 days), use of natural resources such as water, and additional financial resources for adequate equipment and infrastructure. This process makes the final product more expensive. Additionally, the susceptibility to biotic and abiotic factors, the indeterminate growth and non-uniform maturation of the central axis and lateral branches (which complicates the mechanization of the crop), are other aspects that prevent the further commercial development of the crop [
38]. These limitations could be overcome through diverse breeding strategies. The wide genetic variability preserved in the germplasm of
L. mutabilis and in the wild species of
Lupinus that grow in the Andes, offers the opportunity to carry out intra- and inter-specific crosses to generate promising materials with great potential for the region.
Within the collections of L. mutabilis, conserved in the germplasm banks of the Andean region, there are accessions and/or varieties with low content of alkaloids, earliness, high yield and plant architecture. These genotypes are important in breeding programs to generate "sweet" varieties. However, before starting crosses, homozygous materials must be obtained (for the characteristics of interest) ensuring self-fertilization of selected plants during several reproductive cycles. Thanks to the degree of cross-pollination, it is very likely that some plants of tarwi present characteristics of interest, even if they show recessive inheritance as presumably is the content of alkaloids in tarwi; therefore, they will segregate, and after a few production cycles the number of plants with these characteristics could decrease drastically.
On the other hand, the importance of wild species lies in the wide geographic variability of adaptation, so they can be sources of genes for resistance to biotic and abiotic factors. Currently, preliminary characterization studies of these species are already available in Bolivia [
67], Peru [
68] and Ecuador [
69]. Wild species from the Andean region are presumed to share the same chromosome number as
L. mutabilis [
22,
70]. With wild species, evaluations must be carried out for resistance to diseases and pests of economic importance. In the case of anthracnose (the most important disease), specific inoculation methodologies are already available that would allow the evaluation and selection of resistant genotypes. Otherwise, the information related to the study of insect pests, evaluation methodologies and selection of resistant cultivars is almost nil, despite the fact that insect pests are important limiting factors in some tarwi production areas.
Another important aspect will be to evaluate the behavior of sweet varieties or accessions in terms of susceptibility to pests and diseases, for which standardized evaluation methodologies will be important. This will better guide the selection of parents in the breeding program and agronomic management in the field for future varieties with low alkaloid content.
To optimize this genetic improvement process, it is necessary to include molecular tools and generate genomic information that is still very scarce for
L. mutabilis. Currently, the New Generation Sequencing technologies allow the generation of a large amount of genomic information at a lower cost compared to previous technologies. For example, genome-wide association studies (GWAS) to identify and establish genetic markers [
18]. In addition, it is necessary to initiate crosses between homozygous plants for the characteristics of interest, to generate mapping populations and, together with the use of molecular tools, obtain genomic information that allows accelerating genetic improvement. It is also important to mention that genomic information is available for
L. angustifloius and
L. albus that can be applied to the improvement of
L. mutabilis.
One of the breeding methods that has been widely used in
L. mutabilis is mutation induction [
10,
71,
72,
73], through which materials with a low content of alkaloids and plants without branching have been obtained. It is feasible the use of this method to further expand the variability of
L. mutabilis for these and other characteristics of interest. Branchless plants provide the opportunity to obtain new varieties for mechanized harvesting.
At the moment, the availability of molecular resources for breeding of L. mutabilis remains limited, without a reference genome, without genetics map and without reported genes. However, the use of new technologies, such as gene editing, offers the opportunity to generate commercial varieties in the short and medium term. In the Andean region, adapted and highly productive but bitter varieties are cultivated. With gene editing it is possible to optimize the generation of sweet varieties that commercially promote the cultivation of tarwi, not only regionally but globally. For this, it is essential to advance with genomic information of this crop.
10. Conclusions
At least 25 varieties of L. mutabilis have been generated in the highlands of Ecuador, Peru, and Bolivia in the last 40 years. These varieties were developed primarily using mass selection breeding methods. Some of the ecotypes or varieties generated by these breeding methods are not officially registered or released. To date, no varieties that come from crosses or hybridization have been released, although in Ecuador there are already two promising lines developed by crosses using the Pedigree breeding method.
Despite the achievements shown in terms of the number of varieties released, tarwi still has some limitations for its cultivation: no varieties resistant to anthracnose have been obtained; the vast majority of varieties grown in the Andean region have high alkaloid content. There is a variety with low alkaloid content, but it is susceptible to diseases and pests. No breeding studies for pest resistance have been conducted; the use of molecular tools for breeding of L. mutabilis in the Andean region is almost null.
Due to the multiple advantages in agriculture, and benefits in nutrition and medicine, L. mutabilis is gaining global importance; however, it will be necessary to implement new breeding strategies and start studies at the molecular level; for example, GWAS and gene editing, that will optimize genetic gains and reduce the time to obtain new varieties.
Author Contributions
Conceptualization, D.R., J.L.Z., A.T. and S.P.; methodology and search of literature, D.R.; writing—original draft preparation, D.R.; writing—review and editing, D.R., J.L.Z., A.T. and S.P.; supervision, J.L.Z., S.P. and A.M. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jacobsen, S.-E., and Mujica, A. El Tarwi (Lupinus mutabilis Sweet.) y sus parientes silvestres. Botánica Económica de los Andes Centrales. 2006; 458–482.
- Mujica, A. 2018. Capítulo 1. La planta de Tarwi. En: Lupinus mutabilis (Tarwi), Leguminosa andina con gran potencial industrial. Amparo Zavaleta (Compiladora). 1° Ed. Fondo Editorial de la Universidad Nacional Mayor de San Marcos. Lima. ISBN 978-9972-46-620-5.
- Mercado, G. Memoria foro virtual: Los caminos del tarwi y la integración andina: Bolivia, Peru y Ecuador. Bolivia: IPDRS. 60 p. 2018.
- Blanco, O. Genetic variability of tarwi (Lupinus mutabilis Sweet). In: Agricultural and Nutritional Aspects of Lupines, R Gross and ES Bunting (editors). GTZ, Eschborn, Germany. pp. 33–49. 1982.
- Camarena, F. El tarwi o chocho. In: Paradigmas: Desafíos y oportunidades de los cultivos andinos frente a los tratados de libre comercio (pp. 32-36). Lima: Concytec. 2011.
- Jacobsen, S.-E., and Mujica, A. Geographical distribution of the Andean lupin (Lupinus mutabilis Sweet). Plant Genet. Res. 2008, 1–8.
- Canahua-Murillo, A.; Roman-Canahua, P. Tarwi. Leguminosa andina de gran potencial. Revista de AGROECOLOGIA, 2016, 32 (2).
- Lucas, M.M.; Stoddard, F.; Annicchiarico, P.; Frias, J.; Martinez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Frias, J.; Martinez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Peralta, E.; Mazón, N.; Murillo, A.; Rodríguez, D. Manual Agrícola de Granos Andinos: Chocho, Quinua, Amaranto y Ataco. Cultivos, variedades y costos de producción. Cuarta Edición. Publicación Miscelánea No. 69. INIAP. Programa Nacional de Leguminosas y granos Andinos. Estación experimental Santa Catalina. Quito, Ecuador. 72 p. 2014.
- Sinche-Serra, M; Velazquez, J; Aguilar-Aguilar, J.; Ramirez, T. Promising Lines of Lupinus mutabilis Sweet Derived from Mutation Induction with Ionizing Radiations In: Abstracts of International Symposium on Plant Mutation Breeding and Biotechnology, Viena, Austria, 27-31 August 2018, IAEA-CN-263-115.
- Molina-Poveda, C.; Lucas, M.; Jover, M. Evaluation of the potential of Andean lupin meal (Lupinus mutabilis Sweet) as an alternative to fish meal in juvenile Litopenaeus vannamei diets. Aquaculture 2013, 410-411, 148–156. [Google Scholar] [CrossRef]
- Gross, R.; Von Baer, E.; Koch, R.; Marquard, L.; Trugo, L.; Wink, M. Chemical composition of a new variety of the Andean lupin (Lupinus mutabilis cv. Inti) with low alkaloid content. Journal of Food Composition and Analysis 1988, 1(4), 353–361. [Google Scholar] [CrossRef]
- Gabriel, J.; Vallejos, J.; Mamani, P.; Angulo, A. Mejora genética del tarwi (Lupinus mutabilis Sweet) en Bolivia. Revista de Agricultura (Bolivia). 2018, Nro. 57, 1–9. [Google Scholar]
- Baldeón, M.; Fornasini, M.; Muñoz, E.; Villacrés, E. Medical advances in the consumption of Lupinus mutabilis sweet, chocho/tarwi, in Ecuador. In: Abstract Book XV International Lupin Conference: Developing lupin crop into a modern and sustainable food and feed source. Cochabamba, Bolivia, 18-21 March 2019; pp 38.
- Intiquilla, A.; Flores-Fernandez, C. Jiménez, K; Iris, A. 2018. Capítulo 4. Potencial biotecnológico de las semillas de tarwi. En: Lupinus mutabilis (Tarwi), Leguminosa andina con gran potencial industrial. Amparo Zavaleta (Compiladora). 1° Ed. Fondo Editorial de la Universidad Nacional Mayor de San Marcos. Lima. ISBN 978-9972-46-620-5.
- Vegas, N.; Vegas, C.; Peña, P. 2018. Capítulo 3. Potencial tecnológico de las semillas de tarwi. En: Lupinus mutabilis (Tarwi), Leguminosa andina con gran potencial industrial. Amparo Zavaleta (Compiladora). 1° Ed. Fondo Editorial de la Universidad Nacional Mayor de San Marcos. Lima. ISBN 978-9972-46-620-5.
- Zamora, H.; Zamora-Burbano, A.; Ribeiro, L.; Lopes, T.; Coral, J.; Pasquini, D. Potencial do tremoço andino (Lupinus mutabilis) para produção de biodiesel via rota metílica: Uma revisão. Rev. Virtual Quim. 2020, 12(4), 852–866. [Google Scholar]
- Gulisano, A.; Alves, S.; Martins, J.N.; Trindade, L.M. Genetics and Breeding of Lupinus mutabilis: An Emerging Protein Crop. Front. Plant Sci. 2019, 10, 1385. [Google Scholar] [CrossRef]
- Gross, R. El cultivo y la utilización del Tarwi: Lupinus mutabilis Sweet. Estudio FAO. Producción y Protección Vegetal, N° 36. Roma, Italia. 1982.
- Rojas, J. El cultivo de Tarwi (Lupinus mutabilis Sweet) en el Estado Plurinacional de Bolivia. Info INIAF [online]. 2016, vol.1, n.7, pp. 88-100. Available online: http://www.revistasbolivianas.org.bo/pdf/rciii/v1n7/v1n7_a14.pdf. (accessed on 09 May 2021).
- Tapia, M. and Fries A. Guía de campo de los cultivos andinos. FAO. ANPE. Lima, Peru. 2007. [Google Scholar]
- Tapia, M. E. El tarwi, lupino Andino. Tarwi, tauri o chocho, 2015. Available online: http://fadvamerica.org/wp-content/uploads/2017/04/TARWI-espanol.pdf. (accessed on 14 May 2021).
- Peralta, E. El chocho en Ecuador “estado del arte”. Quito, Ecuador: INIAP, PRONALAEG. 2016.
- Chipana, G.; Trigo, R.; Bosque, H.; Jacobsen, S. E.; Mercado, G.; Rodríguez, J.; Callisaya, I.; Contreras, E.; Condori, J. EL tarwi (Lupinus mutabilis) y su importancia social y económica en las familias del altiplano norte de Bolivia. In: Abstract Book XV International Lupin Conference: Developing lupin crop into a modern and sustainable food and feed source. Cochabamba, Bolivia, 18-21 March 2019; pp 54.
- Vicente, J. El cultivo de Tarwi (Lupinus mutabilis Sweet) en el Estado Plurinacional de Bolivia. Revista Científica de Investigación INFO-INIAF, 2016, 88-100.
- Gandarillas, A.; Vallejos, J.; Mamani, O. El tarwi: Un cultivo con nuevas oportunidades en Bolivia. Revista de Agricultura (Bolivia) 2018, Nro. 57, 31–39. [Google Scholar]
- INEC (Instituto Nacional de Estadísticas y Censos). (2000). III Censo Nacional Agropecuario. Resultados Nacionales. Ecuador. 56 p.
- Jacobsen, S. E. and Mujica, A. Geographical distribution of the Andean lupin (Lupinus mutabilis Sweet). In: Book of proceedings of the 7th ESA Congress: European agriculture in a global context (pp. 931-932). KVL, Copenhagen. Denmark, 11-15 July 2004.
- Atchison, G.W.; Nevado, B.; Eastwood, R.J.; Contreras-Ortiz, N.; Reynel, C.; Madriñán, S.; Filatov, D.A.; Hughes, C.E. Lost crops of the Incas: Origins of domestication of the Andean pulse crop tarwi, Lupinus mutabilis. Am J Bot. 2016, 103(9), 1592–606. [Google Scholar] [CrossRef]
- Huaringa, A. Caracterización fenotípica preliminar de 14 ecotipos de tarwi provenientes de Puno, Cusco y La Libertad y Evaluación preliminar de la capacidad simbiótica de cepas nativas de los ecotipos de tarwi. In: Simposio Regional del Chocho o Tarwi. Quito, Ecuador, 29 november - 1 december 2016.
- Rivera, M.; Pinzón, J.; Caicedo, C.; Murillo, A.; Mazón, N.; Peralta, E. Catálogo del banco de germoplasma de chocho (Lupinus mutabilis Sweet) y otras especies de lupinos. Quito. Ecuador: INIAP, Estación Experimental Santa Catalina, Programa Nacional de Leguminosas. 1998.
- GRIN-GLOBAL (2018). Banco Nacional de Germoplasma de Bolivia. Available online: http://germoplasma.iniaf.gob.bo/gringlobal/search.aspx (accessed on 14 May 2021).
- Neves-Martins, J.; Silva, P.; Sousa, R. Evaluation of Lupinus mutabilis accessions for protein and oil in Portugal. In: Agrimed Research Programme - Lupinus mutabilis: its adaptation and production under European pedoclimatic conditions (Commission of the European Communities). EUR 14102, Luxembourg. pp. 1–10. 1992.
- Chirinos-Arias, M.; Jiménez, E. J.; Vilca-Machaca, S. L. Analysis of Genetic Variability among thirty accessions of Andean Lupin (Lupinus mutabilis Sweet) using ISSR molecular markers. Scientia Agropecuaria 2015, 6, 17–30. [Google Scholar] [CrossRef]
- Guilengue, N.; Alves, S.; Talhinhas, P.; Neves-Martins, J. Genetic and Genomic Diversity in a Tarwi (Lupinus mutabilis Sweet) Germplasm Collection and Adaptability to Mediterranean Climate Conditions. Agronomy 2020, 10(1), 21. [Google Scholar] [CrossRef]
- Gulisano A, Alves S, Rodriguez D, Murillo A, van Dinter BJ, Torres AF, Gordillo-Romero M, Torres ML, Neves-Martins J, Paulo MJ, Trindade LM. Diversity and Agronomic Performance of Lupinus mutabilis Germplasm in European and Andean Environments. Front Plant Sci. 2022 Jun 10;13:903661. [CrossRef]
- Gulisano A, Lippolis A, van Loo EN, Paulo M-J and Trindade LM (2023) A genome wide association study to dissect the genetic architecture of agronomic traits in Andean lupin (Lupinus mutabilis). Front. Plant Sci. 13:1099293. [CrossRef]
- Tapia, M. (2000). Cultivos Andinos Sub explotados y su aporte a la alimentación. Segunda Edición. FAO, Santiago, Chile.
- Cowling, W.; Buirchell, B.; Tapia, M. Lupin. Lupinus L.Promoting the conservation and use of underutilized and neglected crops. 23. IPK, Gatersleben/IPGRI, Rome, Italy. 100 p. 1998.
- Vallejos, J., Gabriel, J., Angulo, A., Cadima, X., Mamani, P. New tarwi (Lupinus mutabilis Sweet) crop obtained by stratified masal selection. In: Abstract Book XV International Lupin Conference: Developing Lupin crop into a modern and sustainable food and feed source. Cochabamba, Bolivia, 18-21 March 2019; pp 114.
- Caicedo, C; Murillo, A.; Peralta, E.; Pinzón, J.; Rivera, M. INIAP-450 Andino, variedad de chocho para la zona centro/norte de la Sierra ecuatoriana. Revista informativa del Instituto Nacional Autónomo de Investigaciones Agropecuarias. Nro. 14. Quito-Ecuador, pp. 28-29. 2000.
- Lalama, M. and Nieto, C. Avance de la investigación en cultivos andinos en INIAP. III Congreso Internacional de Cultivos Andinos. IBTA – CIID. La Paz, Bolivia, 8–12 Febrary 1982; pp 425-432.
- Peralta, E.; Murillo, A.; Mazón, N. LINEA DEL TIEMPO. Mejoramiento genético de los granos andinos en Ecuador: quinua, chocho, amaranto y ataco. Programa de Leguminosas y granos Andinos, INIAP, Estación Experimental Santa Catalina. Publicación miscelánea No. 420. Quito, Ecuador. 2015.
- Poehlman, J.M. and Sleeper. D.A. Breeding Field Crops, 4 th Edition, Iowa State University Press, USA. 1995.
- Eastwood, R. J., and C. E. Hughes . Origins of domestication of Lupinus mutabilis in the Andes. In Lupins for health and wealth, Proceedings of the 12th International Lupin conference , 2008 , 373 – 379 , Freemantle, Western Australia , International Lupin Association.
- Mujica, A.; Chura, E.; Apaza, J.; Chuquimia, D.; Moscoso, G.; Calandri, E.; Montoya, P.; Grasso, F.; Guzmán; C. Selección de cultivares nacionales de tarwi (Lupinus mutabilis sweet) por rendimiento, precocidad, contenido de aceite y proteína en Puno, Peru. In: Abstract Book XV International Lupin Conference: Developing Lupin crop into a modern and sustainable food and feed source. Cochabamba, Bolivia, 18-21 March 2019; pp 166.
- Mujica, A., Moscoso, G., Chuquimia, D., Romero, T., Astete, A., Calandri, E., Montoya, P. Capítulo 1. Selección de cultivares de tarwi (Lupinus mutabilis Sweet.) por rendimiento, precocidad, contenido de aceite y proteína en Puno Peru. In: Agrárias: pesquisa e inovaco nas que alimentam o mundo. Curitiba-PR, Brasil; pp. 1-13. 2021.
- Murillo, A.; Rivera, M; Peralta, E.; Mazon, N.; Vargas, F. Avances preliminares en el mejoramiento genético de chocho (Lupinus mutabilis Sweet) para resistencia a antracnosis. XII Congreso Internacional de Cultivos Andinos. INIAP, PUCE. Quito Ecuador, 23 -27 July 2006.
- Frick, K.; Kamphuis, L. G.; Siddique, K. H.; Singh, K. B.; and Foley, R. C. Quinolizidine Alkaloid Biosynthesis in Lupins and Prospects for Grain Quality Improvement. Front Plant Sci. 2017, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Larenas, F.; Linnemann, A.; Nout, M.; Koziol, M.; van Boekel, A. Lupinus mutabilis: Composition, Uses, Toxicology, and Debittering. Critical Reviews in Food Science and Nutrition 2016, 56(9), 1454–1487. [Google Scholar] [CrossRef]
- Rychel, S.; Książkiewicz, M. Development of gene-based molecular markers tagging low alkaloid pauper locus in white lupin (Lupinus albus L.). Journal of Applied Genetics 2019, 60, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Avendaño, P.; Tarvainen, M.; Suomela, JP.; Glorio-Paulet, P.; Yang, B.; and Repo-Carrasco-Valencia, R. (2016). Profile and Content of Residual Alkaloids in Ten Ecotypes of Lupinus mutabilis Sweet after Aqueous Debittering Process. Plant Foods Hum Nutr 2020, 75, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Falconí, C. Lupinus mutabilis in Ecuador with special emphasis on anthracnose resistance. Ph.D. Thesis, Wageningen University, Wageningen NL. 2012.
- Guilengue, N.; Neves-Martins, J.; Talhinhas, P. Response to Anthracnose in a Tarwi (Lupinus mutabilis) Collection Is Influenced by Anthocyanin Pigmentation. Plants 2020 a, 9, 583. [Google Scholar] [CrossRef]
- Brown, A.; Sreenivasaprasad, S.; Timmer, L. Molecular characterization of slow-growing orange and key lime anthracnose strains of Colletotrichum from citrus as C. acutatum. Phytopathology 1996, 86, 523–527. [Google Scholar] [CrossRef]
- Huaringa, J.; Ubillus, M.; Rojas, V.; Sotelo, M. Rendimiento en grano seco, desamargado y proteína de cinco ecotipos promisorios de tarwi, Lupinus mutabilis Swwet cultivados en Vicos Marcará, Ancash, Peru. In Libro de Resúmenes del VII Congreso Mundial de la Quinua y otros Granos Andinos. Iquique, Chile, 25-28 March 2019.
- Gondran J, Bateman GL, Milford GFJ and 24 others. An thracnose of white lupin: European prospects for a future sustainable crop. In Proceedings of the 8th International Lupin Conference. Pacific Grove, California, USA, 11-16 May 1996.
- Plata, G. and Gandarillas, A. Enfermedades que afectan al cultivo del tarwi (Lupinu mutabilis) en Bolivia. Revista de Agricultura (Bolivia) 2018, Nro. 57, 62-72.
- Rodríguez, D.; Vega, L.; Murillo, A.; Peralta, E. Identificación de fuentes de resistencia a la antracnosis (Colletotrihum acutatum) del chocho Lupinus mutabilis Sweet) en el banco de germoplasma del INIAP. In: Memorias IV Congreso Mundial de la Quinua y I Simposio de Granos Andinos. Ibarra, Ecuador, 8-12 July 2013.
- Guaytarilla, P. and Falconí, C. (2014). Selección por arquitectura de la planta y resistencia a la Antracnosis de 7 Genotipos de Chocho (Lupinus mutabilis). In: Memorias IX Congreso de Ciencia y Tecnología ESPE. Sangolqui, Ecuador, 28-30 May 2014; pp.63-70.
- PADER – COSUDE. Cadena de Valor de Tarwi. Taller “Agenda de responsabilidad compartida de la cadena de valor de tarwi”. Mancomunidad del Altiplano Andino de Norte Potosí. Bolivia. 58p. 2006.
- Huaringa, J.; Ubillus, M.; Rojas, V.; Sotelo, M. Rendimiento en grano seco, desamargado y proteína de cinco ecotipos promisorios de tarwi, Lupinus mutabilis Swwet cultivados en Vicos Marcará, Ancash, Peru. In Libro de Resúmenes del VII Congreso Mundial de la Quinua y otros Granos Andinos. Iquique, Chile, 25-28 March 2019.
- Mujica, A.; Jacobsen, S.; Izquierdo, J. Andean lupin (Lupinus mutabilis Sweet) forty years of research in Peru. In: Proceed ings X International Lupin Conference. Wild and Cultivated Lupins from the Tropics to the Poles. Laugarvatn, Iceland, 19–24 June 2002. p. 106.
- Peralta, E.; Rivera, M.; Murillo, A.; Mazón, N.; Monar, C. INIAP 451 Guaranguito, Nueva variedad de chocho para la provincia de Bolívar. Boletín Divulgativo No. 382. INIAP, Estación Experimental Santa Catalina: Quito, Ecuador. 2010.
- Bebeli, P.J.; Lazaridi, E.; Chatzigeorgiou, T.; Suso, M.-J.; Hein, W.; Alexopoulos, A.A.; Canha, G.; van Haren, R.J.F.; Jóhannsson, M.H.; Mateos, C.; Neves-Martins, J.; Prins, U.; Setas, F.; Simioniuc, D.P.; Talhinhas, P.; van den Berg, M. State and Progress of Andean Lupin Cultivation in Europe: A Review. Agronomy. 2020, 10, 1038. [Google Scholar] [CrossRef]
- Abraham, E. M.; Ganopoulos, I.; Madesis, P.; Mavromatis, A.; Mylona, P.; Nianiou- Obeidat, I.; et al. The Use of Lupin as a Source of Protein in Animal Feeding: Genomic Tools and Breeding Approaches. Intern. J. Mol. Sci. 2019, 20(4), 851. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A. and Alcon, M. Obtención de fenotipos similares al tarwi domesticado a partir de sus parientes silvestres. In: Abstract Book XV International Lupin Conference: Developing Lupin crop into a modern and sustainable food and feed source. Cochabamba, Bolivia, 18-21 March 2019; pp 107.
- Tapia, M. Biodiversidad del Género Lupinus en los Andes. In: Abstract Book XV International Lupin Conference: Developing Lupin crop into a modern and sustainable food and feed source. Cochabamba, Bolivia, 18-21 March 2019; pp 138.
- Morales, T. and Quintana, F. Lupinus silvestres del Ecuador. In: Abstract Book XV International Lupin Conference: Developing Lupin crop into a modern and sustainable food and feed source. Cochabamba, Bolivia, 18-21 March 2019; pp 131.
- Bonifacio, A.; Aroni, G.; Villca, M. Adaptación y perspectivas de aprovechamiento del lupino silvestre en sistemas de producción del altiplano. Revista de Agricultura (Bolivia) 2018. Nro 57. Pp. 10-18.
- Pakendorf, K.W., and Rensburg, P.J. van. (1981). Selection for low alkaloid mutants in Lupinus mutabilis In: Abstracts of International Symposium on Induced Mutation as a Tool for Crop Plant Improvement, Viena, Austria, 9-13 March 1981, STI/PUB/591.
- Stawwinski, S.; Rybinski, W; Starzycki, M. Mutants of Andean lupin (Lupinus mutabilis Sweet) with improved architecture habit In: the XVIth EUCARPIA Genetic Resources Section workshop, Poznan, Poland, 16-20 May 2001. Pp. 93-95.
- Galek R., Sawicka-Sienkiewicz E., Zalewski D., Stawiński S., Spychała K. Searching for low alkaloid forms in the Andean lupin (Lupinus mutabilis) collection. Czech J. Genet. Plant Breed. 2017, 53: 55-62. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).