Submitted:
27 September 2023
Posted:
28 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
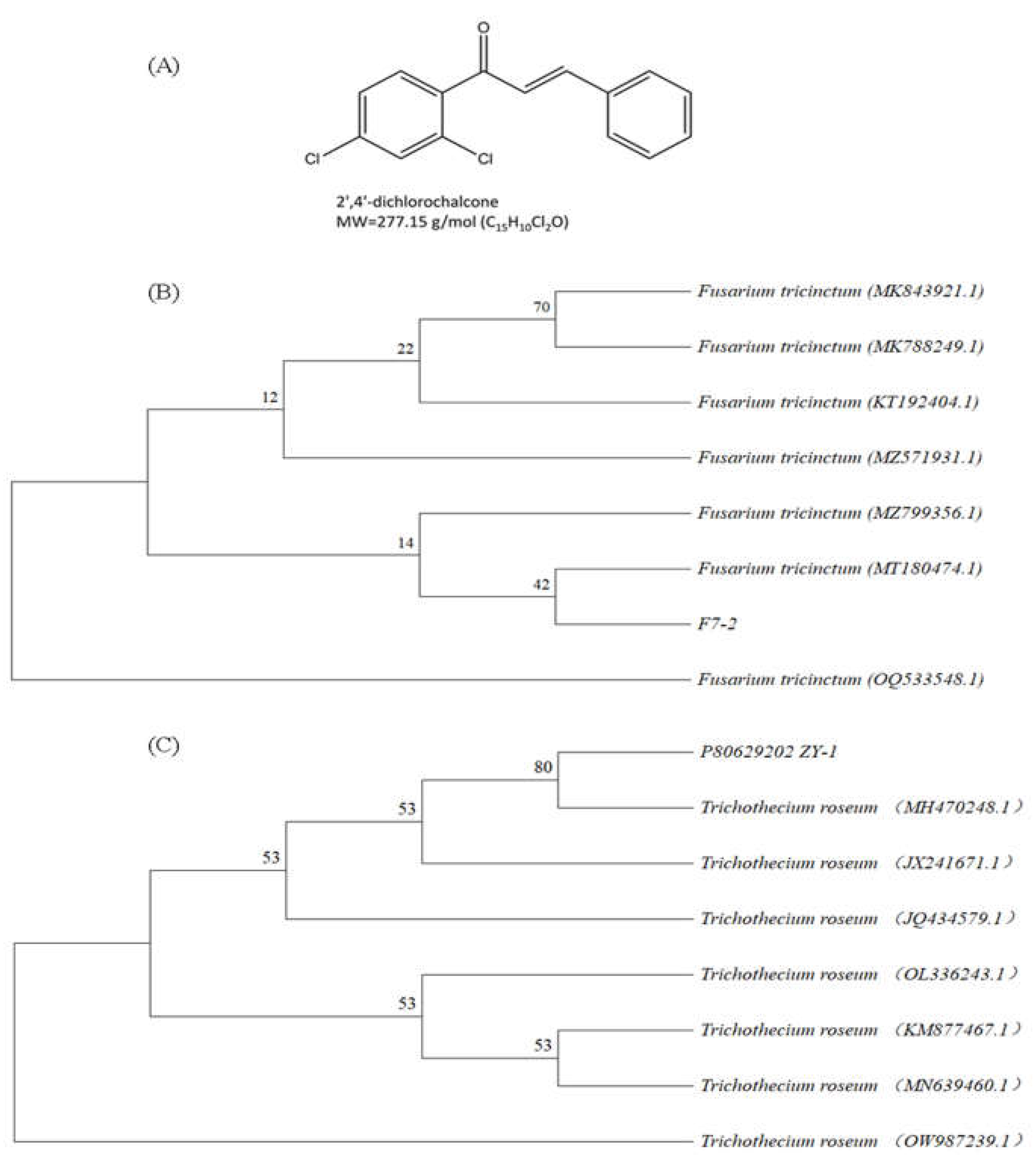
2.1. Biological materials and test reagents
2.2. Methods
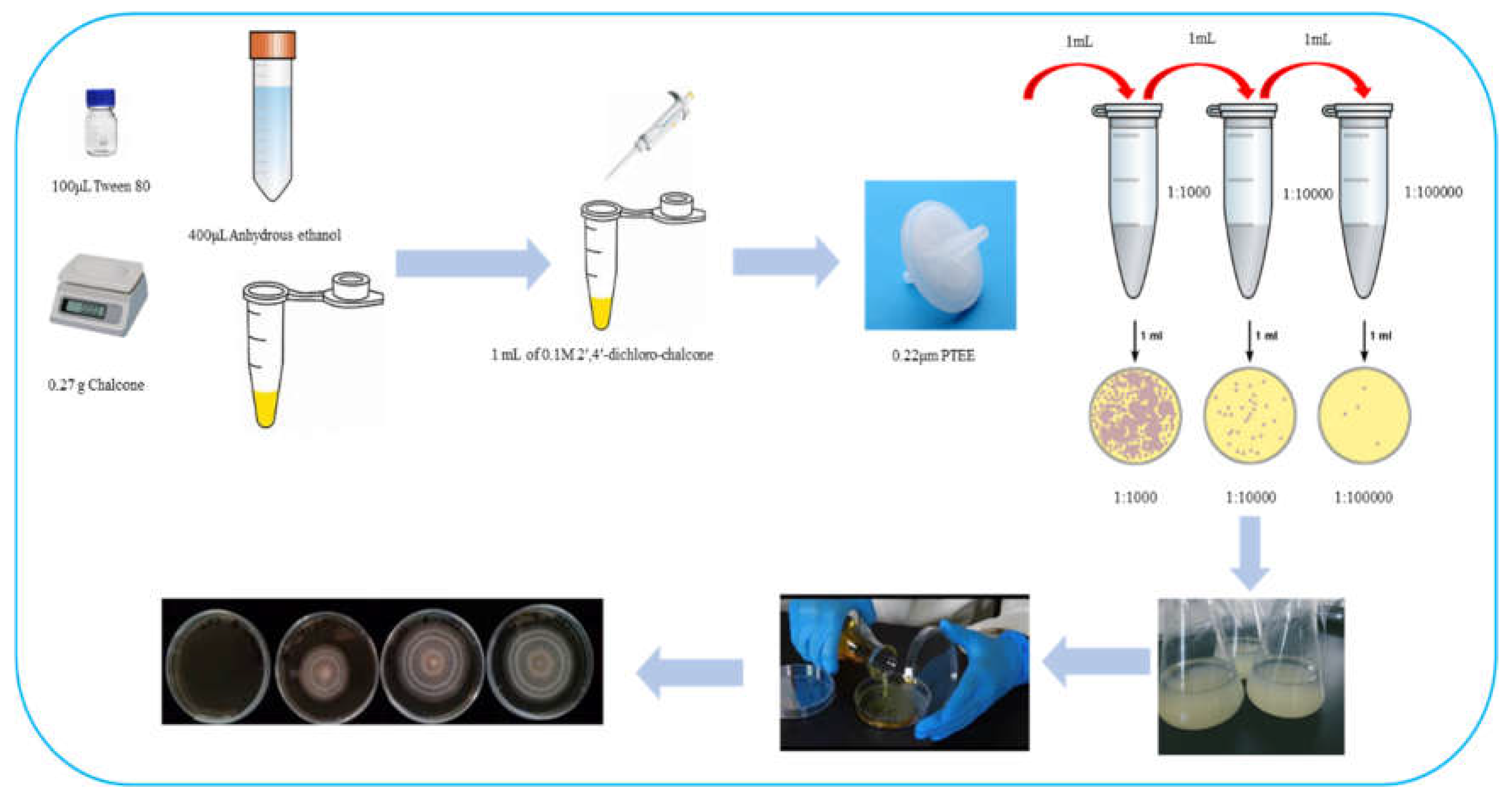
2.2.1. Configuration of 2ʹ,4ʹ-dichloro-chalcone stock solution and dosing medium preparation
2.2.2. Preparation of spore suspension of F. tricinctum and T. roseum
2.2.3. Determination of the effects of 2ʹ,4ʹ-dichloro-chalcone on F. tricinctum and T. roseum mycelial growth and sporulation
2.2.4. The effects of 2ʹ,4ʹ-dichloro-chalcone on hyphal cell membrane permeability
2.2.5. Kinetics of oxygen consumption rates in total and cyanide resistant respiration in F. tricinctum and T. roseum
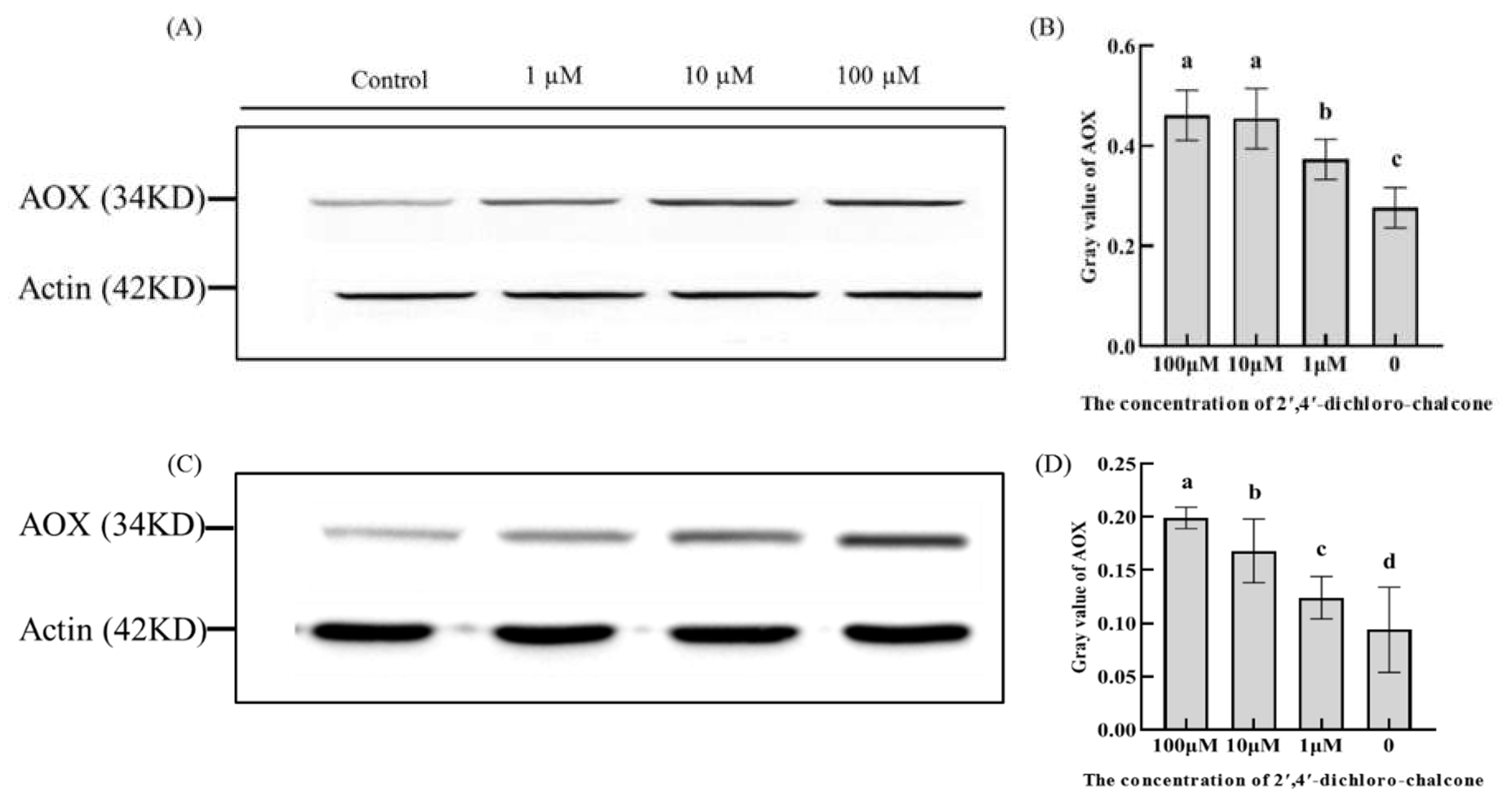
2.2.6. Protein immunoblotting analysis of AOX expression in F. tricinctum and T. roseum
2.2.7. Measurement of ROS production in F. tricinctum and T. roseum
2.2.8. Determination of F. tricinctum and T. roseum pathogenicities in vivo
2.3. Statistical analysis
3. Results
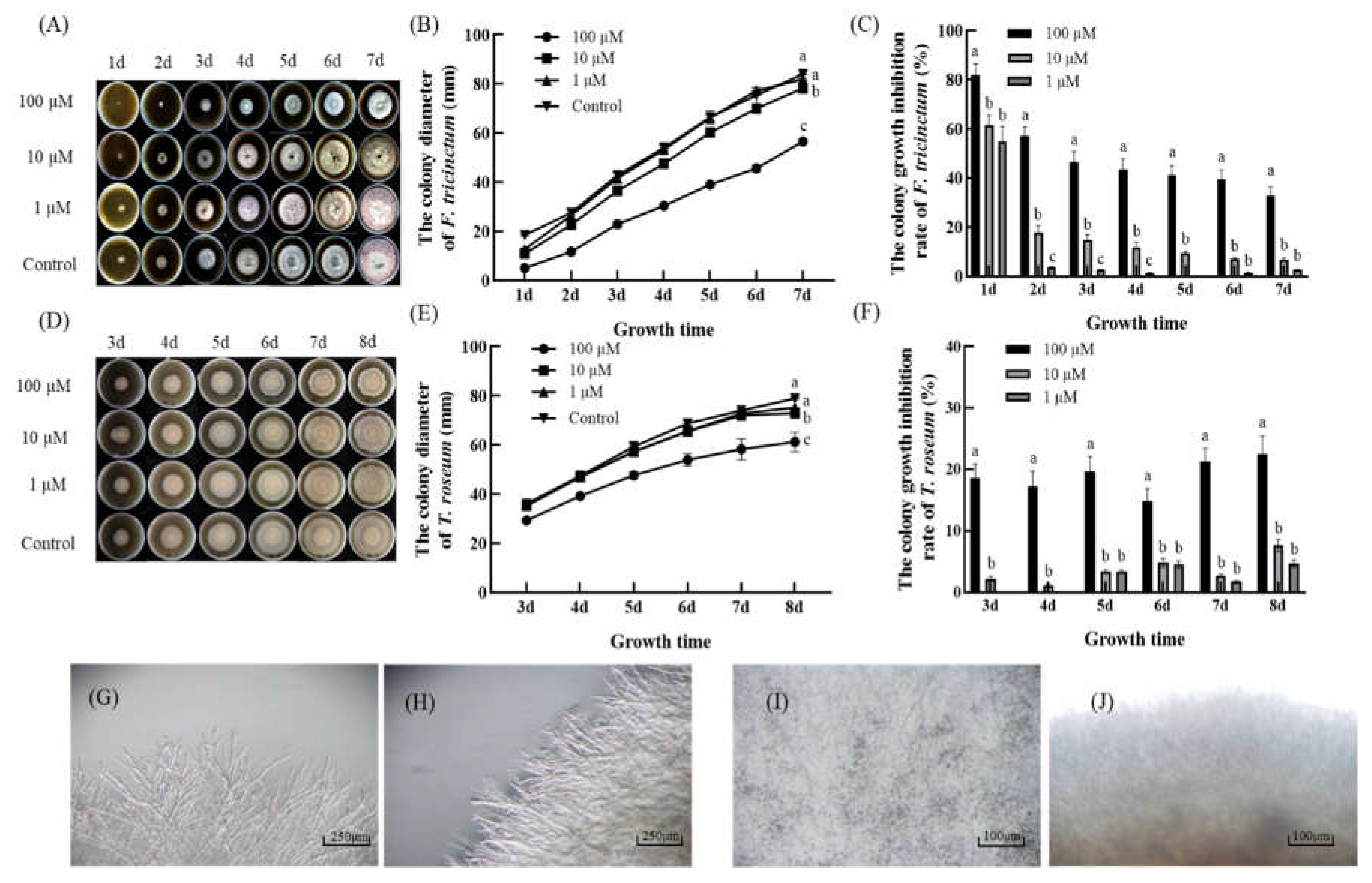
3.1. Effect of 2ʹ,4ʹ-dichloro-chalcone on mycelial growth of F. tricinctum and T. roseum
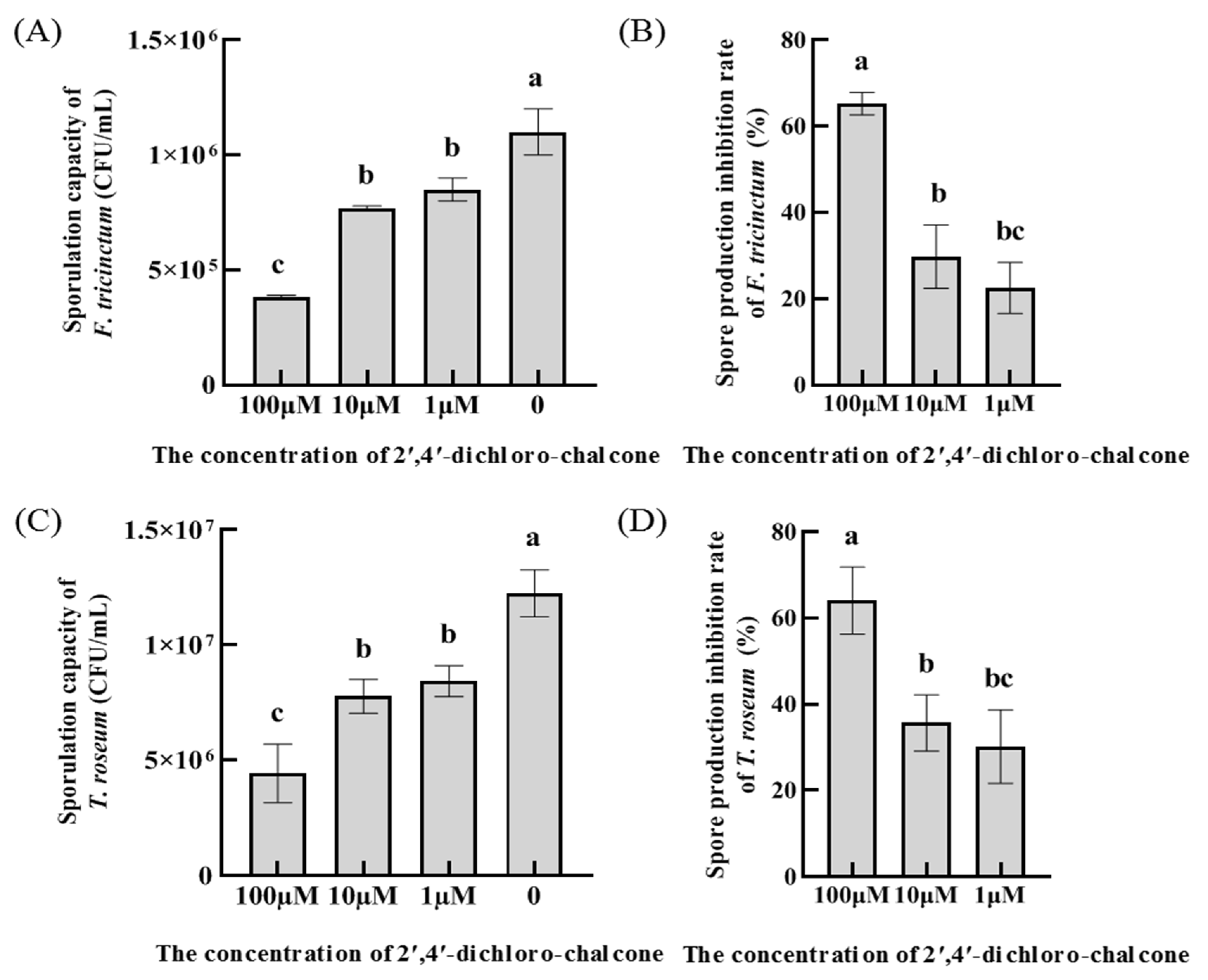
3.2. Effect of 2ʹ,4ʹ-dichloro-chalcone on spore production in F. tricinctum and T. roseum
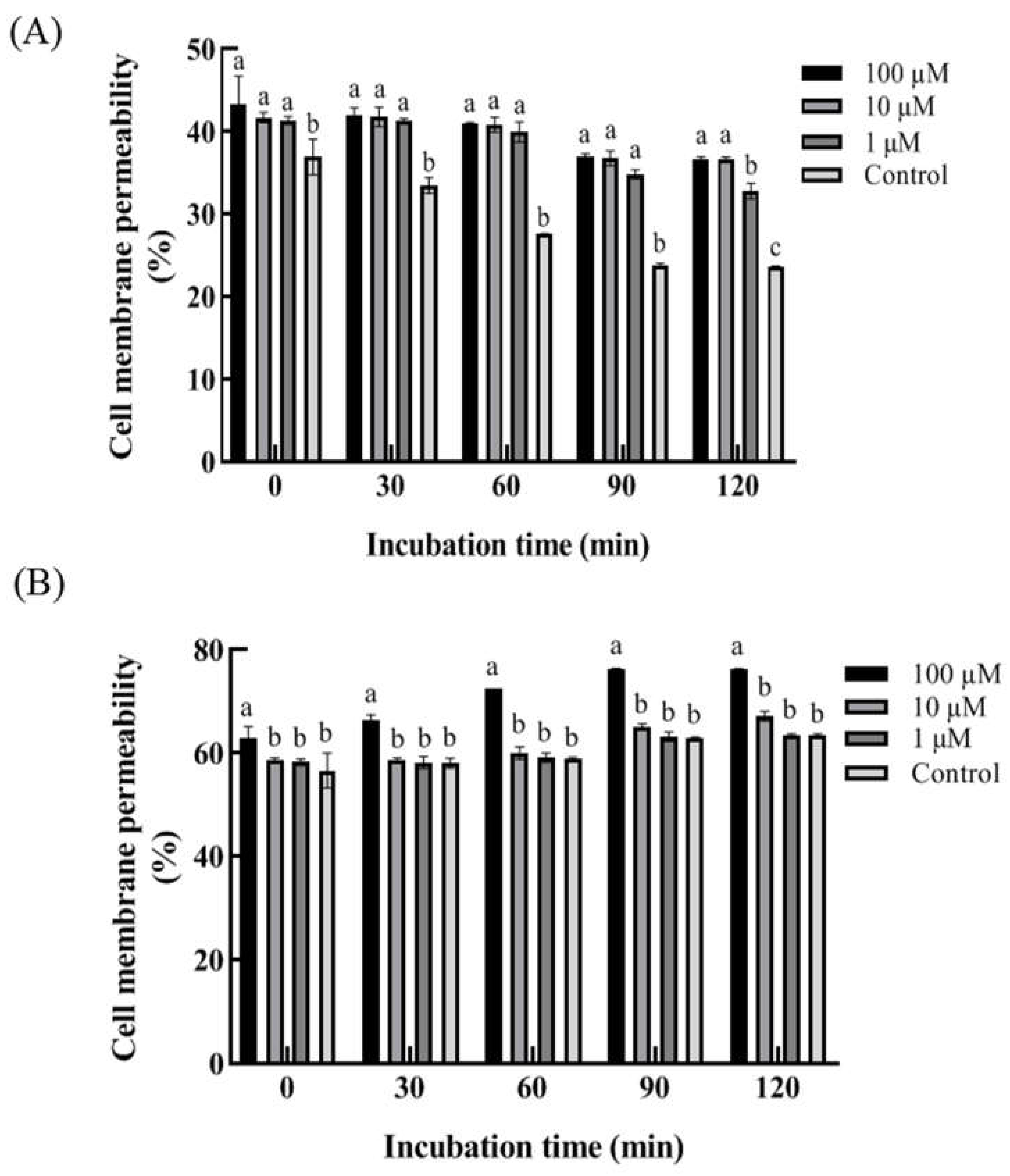
3.3. Effect of 2ʹ,4ʹ-dichloro-chalcone on membrane permeability of F. tricinctum and T. roseum
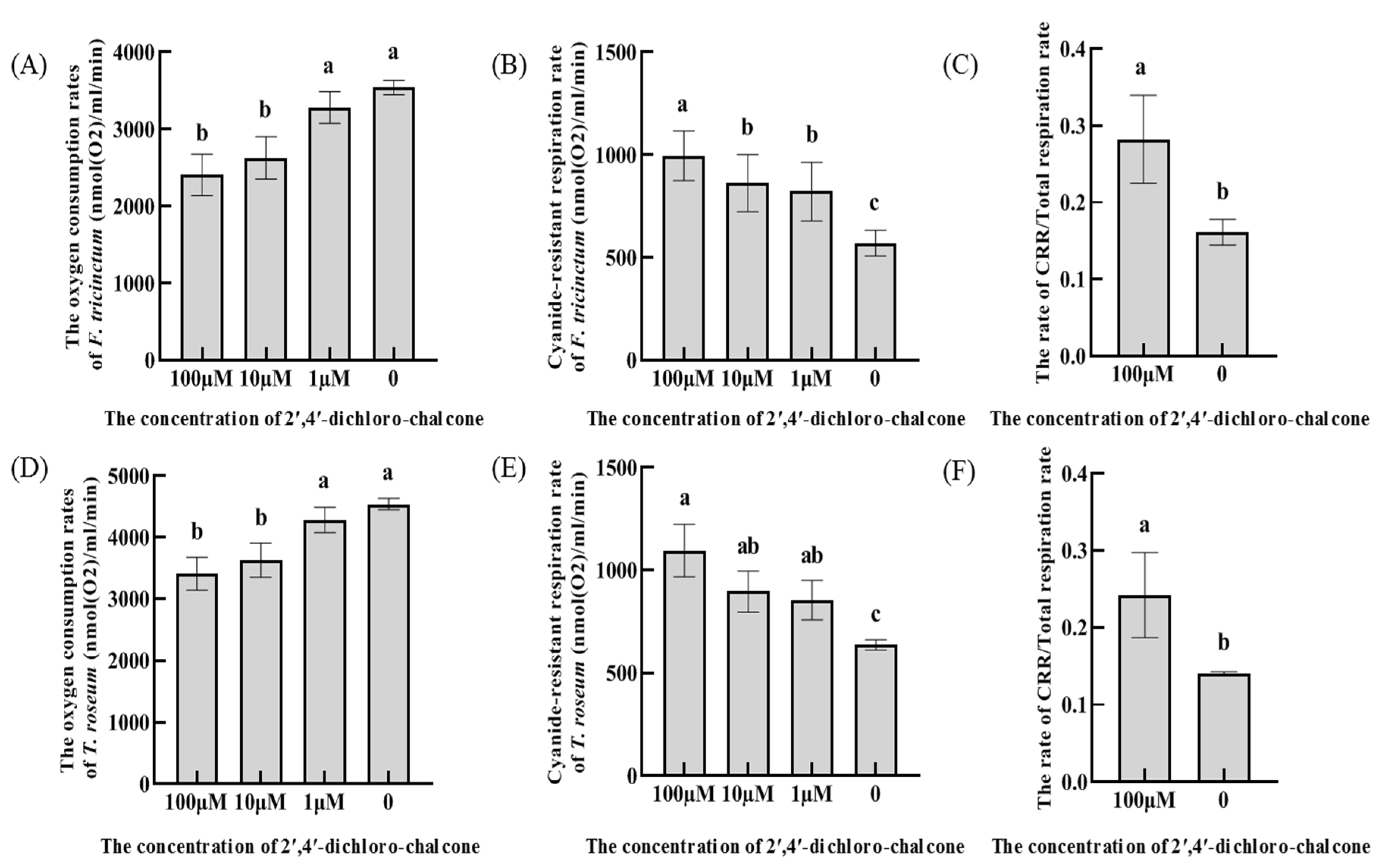
3.4. Effect of 2ʹ,4ʹ-dichloro-chalcone on the oxygen consumption rates in respiratory pathways of F. tricinctum and T. roseum
3.5. Effect of 2ʹ,4ʹ-dichloro-chalcone on the oxygen consumption rates in respiratory pathways of F. tricinctum and T. roseum
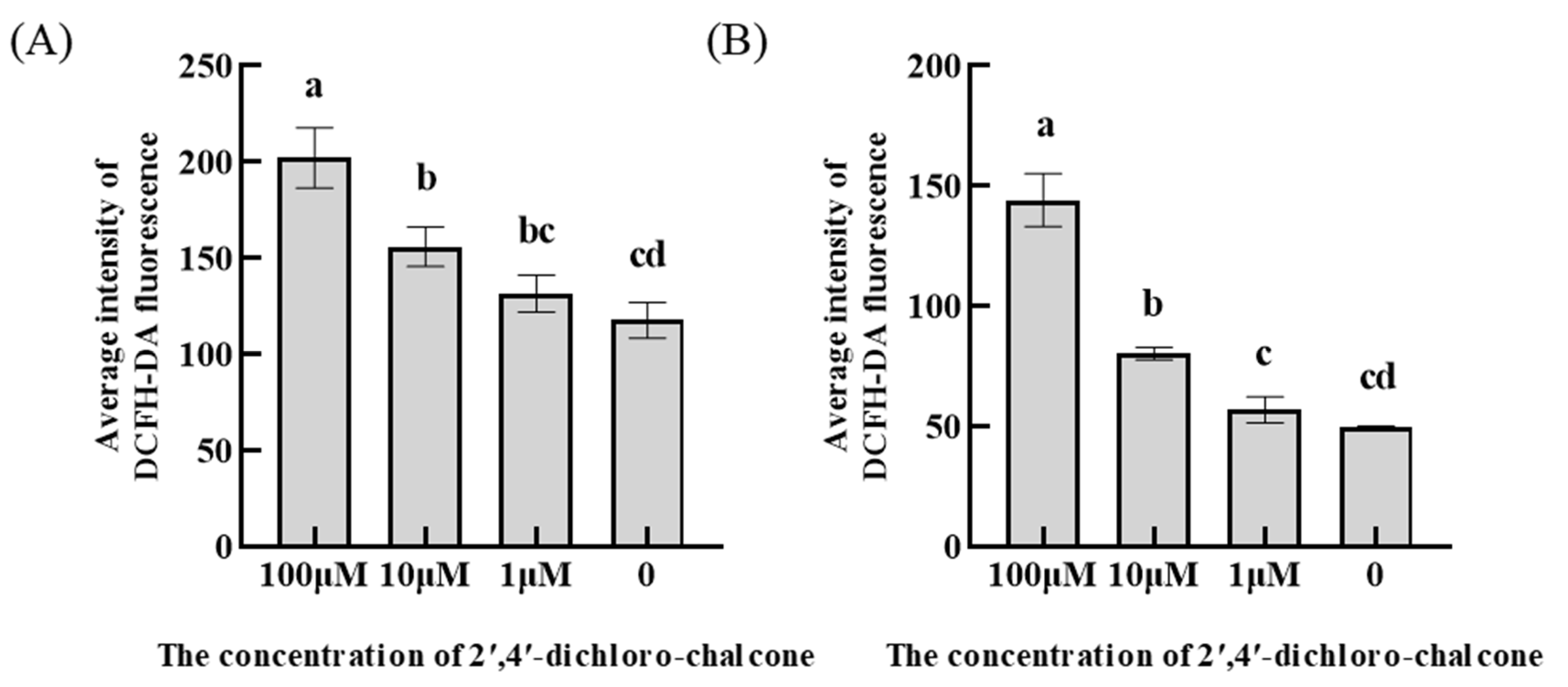
3.6. Effect of 2ʹ,4ʹ-dichloro-chalcone on ROS accumulation in F. tricinctum and T. roseum
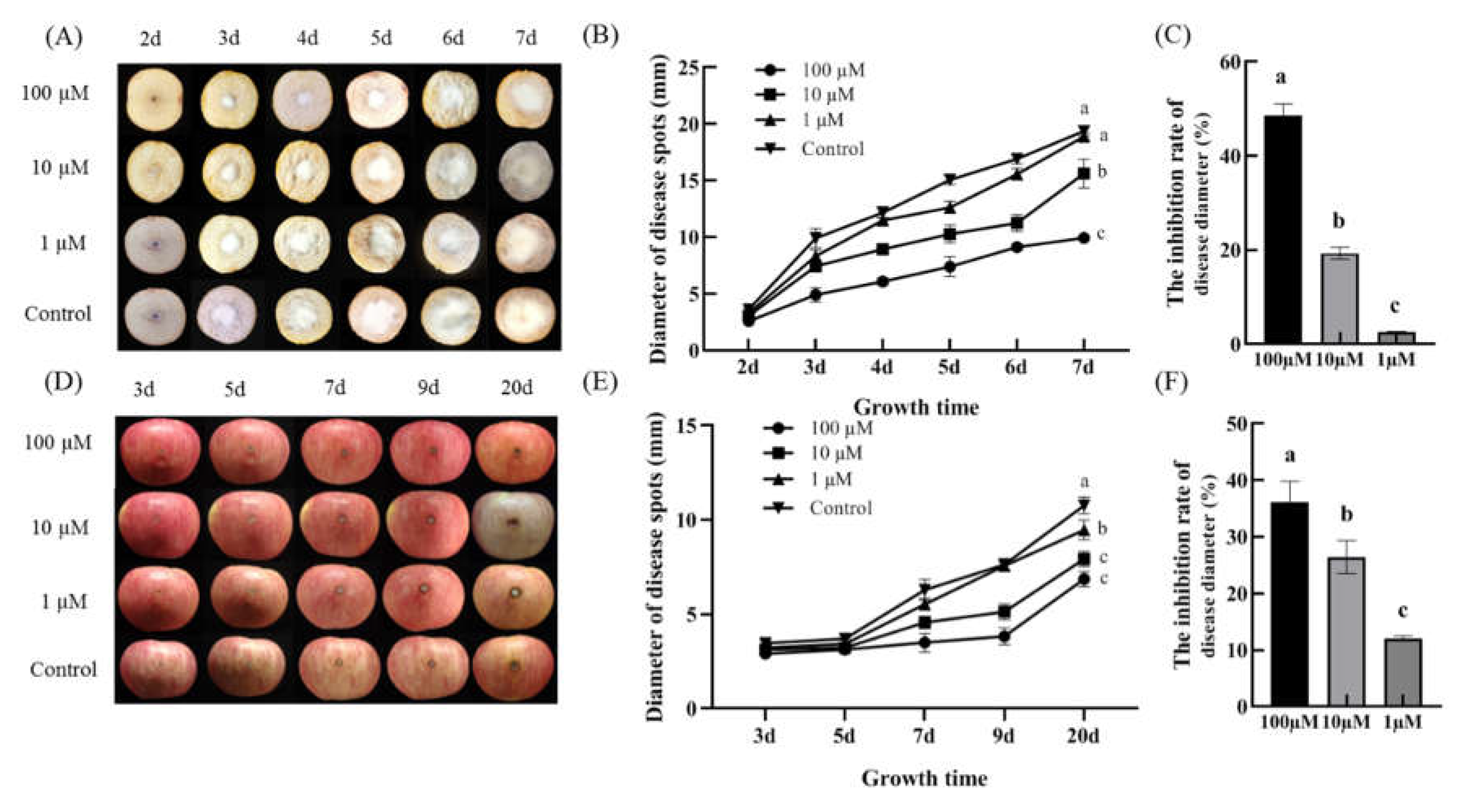
3.7. Effect of 2ʹ,4ʹ-dichloro-chalcone on the pathogenicities of F. tricinctum and T. roseum
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, F.; Xu, J.H.; Zhang, X.; Wang, S.F.; Xing, Y.J.; Mokoena, M.P.; Olaniran, A.O.; Shi, J.R. Gramineous weeds near paddy fields are alternative hosts for the Fusarium graminearum species complex that causes fusarium head blight in rice. Plant Pathol. 2020, 69, 433–441. [Google Scholar] [CrossRef]
- Amato, B.; Pfohl, K.; Tonti, S.; Nipoti, P.; Prodi, A. Fusarium proliferatum and fumonisin B1 co-occur with Fusarium species causing Fusarium Head Blight in durum wheat in Italy. J Appl Bot Food Qual. 2015, 88, 228–233. [Google Scholar]
- Arino, A.A.; Bullerman, L.B. Fungal colonization of corn grown in nebraska in relation to year, genotype and growing conditions1. J. Food Prot. 1994, 57, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Gachango, E.; Hanson, L.E.; Rojas, A.; Hao, J.J.; Kirk, W.W. Fusarium spp. causing dry rot of seed potato tubers in michigan and their sensitivity to fungicides. Plant Dis. 2012, 96, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, D.; Vieira, P.; Pandey, R.; Slovin, J.; Kamo, K. Symptom development in response to combined infection of in vitro-grown lilium longiflorum with pratylenchus penetrans and soilborne fungi collected from diseased roots of field-grown lilies. Plant Dis. 2017, 101, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Tsukiyama, R.I.; Katsura, H.; Tokuriki, N.; Kobayashi, M. Antibacterial activity of licochalcone A against spore-forming bacteria. Antimicrob. Agents Chemother. 2002, 46, 1226–1230. [Google Scholar] [CrossRef]
- Dai, P.; Jiang, Y.; Liang, X.; Gleason, M.L.; Sun, G. Trichothecium roseum enters 'fuji' apple cores through stylar fissures. Plant Dis. 2019, 104, 1060–1068. [Google Scholar] [CrossRef]
- May-De Mio, L.L.; Negri, G.; Michailides, T.J. Effect of Trichothecium roseum, lime sulphur and phosphites to control blossom blight and brown rot on peach. Can. J. Plant. Pathol. 2014, 36, 428–437. [Google Scholar]
- Gong, D.; Bi, Y.; Li, S.; Li, Y.; Wang, Y. Trichothecium roseum infection promotes ripening of harvested muskmelon fruits and induces the release of specific volatile compound. J Plant Pathol. 2019, 101, 529–538. [Google Scholar] [CrossRef]
- Ghazanfar, M.U.; Hussain, M.; Hamid, M.I.; Ansari, S.U. Utilization of biological control agents for the management of postharvest pathogens of tomato. Pak. J. Bot. 2016, 48, 2093–2100. [Google Scholar]
- Zhu, Y.H.; Shao, Y.Y.; Li, L.; Zhao, L.; Zhang, M.J.; Dong, C.M. The plant growth-promoting endophytic Fusarium oxysporum GG22 enhances Rehmannia glutinosa secondary metabolites accumulation. Ind. Crops Prod. 2022, 182, 114881. [Google Scholar] [CrossRef]
- Rizk, S.A.; Elsayed, G.A.; El-Hashash, M.A. One-pot synthesis, spectroscopic characterization and DFT study of novel 8-azacoumarin derivatives as eco-friendly insecticidal agents. J. Iran. Chem. Soc. 2018, 15, 2093–2105. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.J.; Chen, X.Y.; Fu, X.Y.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules. 2021, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, B.K.; Atli, O.; Gormus, G.; Ozkay, Y.; Kaplancikli, Z.A. Synthesis and Evaluation of Heterocycles Based Chalcone Derivatives as Anti-proliferative Agents. Anti-Cancer Agents Med. Chem. 2018, 18, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.; Joshi, D. Antibacterial and antifungal screening of newly synthesized benzimidazole-clubbed chalcone derivatives. Med. Chem. 2013, 22, 3688–3697. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Sood, A.K.; Goyal, K.; Singh, A.; Sharma, V.; Guliya, N.; Gulati, S.; Kumar, S. Chalcone Scaffolds as Anticancer Drugs: A Review on Molecular Insight in Action of Mechanisms and Anticancer Properties. Anti-Cancer Agents Med. Chem. 2021, 21, 1650–1670. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.N.; Cortes, J.C.; Ribas, J.C.; Castelli, M.V.; Zacchino, S.A.; Dominguez, J.N.; Lobo, G.; Charris-Charris, J.; Devia, C.; Rodriguez, A.M.; et al. In vitro antifungal evaluation and structure-activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorgan Med Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Mellado, M.; Werner, E.; Said, B.; Godoy, P.; Caro, N.; Besoain, X.; Montenegro, I.; Madrid, A. Sonochemical Synthesis of 2'-Hydroxy-Chalcone Derivatives with Potential Anti-Oomycete Activity. Antibiotics-Basel. 2020, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Kakati, D.; Sarma, R.K.; Saikia, R.; Barua, N.C.; Sarma, J.C. Rapid microwave assisted synthesis and antimicrobial bioevaluation of novel steroidal chalcones. Steroids. 2013, 78, 321–326. [Google Scholar] [CrossRef]
- Wang, Z.W.; Sun, Y.J.; Zhang, L.; Zhang, P. Synthesis and Antifungal Activity of Chalcones Containing Triazole Moiety. Chin J Synth Chem. 2023, 31, 405–414. [Google Scholar]
- Liu, J.B. Bioactivities of chalcones with halogen atom and their thiosemicarbazide derivatives. Chemistry. 2017, 8, 77–83. [Google Scholar]
- Sivakumar, P.M.; Iyer, G.; Natesan, L.; Doble, M. 3 '-Hydroxy-4-methoxychalcone as a potential antibacterial coating on polymeric biomaterials. Appl. Surf. Sci. 2010, 256, 6018–6024. [Google Scholar] [CrossRef]
- Hu, Z. Preparation and properties of pH-responsive chalcone-loaded PVA/PAA functional films. Hefei, China, 2021.
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Bolchacova, E.; Voigt, K.; Crous, P.W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci. 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, K.H.; Liu, X.; Zhu, Y.P.; Liu, C.H. Comparative Functional Genome Analysis Reveals the Habitat Adaptation and Biocontrol Characteristics of Plant Growth-Promoting Bacteria in NCBI Databases. Microbiol. Spectrum 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Copsey, A.C.; Young, L.; Barsottini, M.R.O.; Albury, M.S.; Moore, A.L. Comparison of the Kinetic Parameters of Alternative Oxidases from Trypanosoma brucei and Arabidopsis thaliana-A Tale of Two Cavities. Front. Plant Sci. 2021, 12, 744218. [Google Scholar] [CrossRef]
- Feng, H.Q.; Sun, K.; Wei, Y.; Wang, R.F.; Jia, L.Y.; Zhang, J.P.; Li, Y. Role of cyanide-resistant respiration during light-induced attraction of predators to herbivore-infested leaves. Photosynthetica. 2013, 51, 583–592. [Google Scholar] [CrossRef]
- Affourtit, C.; Moore, A.L. Purification of the plant alternative oxidase from Arum maculatum: measurement, stability and metal requirement. Acta Biochim. Biophys. Sin. 2004, 1608, 181–189. [Google Scholar] [CrossRef]
- Xin, Y.H.; Yang, D.; Yang, J.; Zhang, T.D.; Wang, Y.; Zhang, J.H.; Shi, Y.F. Effects of chalcone derivatives on mitochondrial structure and function of Aspergillus niger. Mycosystema. 2021, 40, 2144–2122. [Google Scholar]
- Zhu, Y.; Zhu, F.P.; Chen, F.; Shi, G.Y.; Zhao, Y.T.; Zhang, F.; Gong, W.J.; Zhang, W.; Wang, X.J.; Li, Y. C. Isolation, identification and pathogenicity of pathogens causing dry rot of stored potato tubers in Gansu Province. J Food Saf Qual. 2023, 14, 240–248. [Google Scholar]
- Ge, Y.H.; Deng, H.W.; Bi, Y.; Li, C.Y.; Liu, Y.Y.; Dong, B.Y. Postharvest ASM dipping and DPI pre-treatment regulated reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruit. Postharvest Biol. Technol. 2015, 99, 160–167. [Google Scholar] [CrossRef]
- Liu, J.J.; Hagberg, I.; Novitsky, L.; Hadj-Moussa, H.; Avis, T.J. Interaction of antimicrobial cyclic lipopeptides from Bacillus subtilis influences their effect on spore germination and membrane permeability in fungal plant pathogens. Fungal Biol. 2014, 118, 855–861. [Google Scholar] [CrossRef] [PubMed]
- El-Khoury, R.; Rak, M.; Benit, P.; Jacobs, H.T.; Rustin, P. Cyanide resistant respiration and the alternative oxidase pathway: A from to mammals. Bba-Bioenergetics. 2022, 1863, 148567. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Dixit, S.K.; Bhattacharya, A.; Mishra, L.C.; Sharma, M.; Awasthi, S.K.; Bhasin, V.K. Antimalarial activity of newly synthesized chalcone derivatives in vitro. Chem Biol Drug Des. 2012, 80, 340–347. [Google Scholar] [CrossRef]
- Shakil, N.A.; Singh, M.; Kumar, J.; Sathiyendiran, M.; Kumar, G.; Singh, M.; Pandey, R.P.; Pandey, A.; Parmar, V.S. Microwave synthesis and antifungal evaluations of some chalcones and their derived diaryl-cyclohexenones. J Environ Sci Heal B. 2010, 45, 524–530. [Google Scholar] [CrossRef]
- Shakhatreh, M.A.; Al-Smadi, M.L.; Khabour, O.F.; Shuaibu, F.A.; Hussein, E.I.; Alzoubi, K.H. ; Study of the antibacterial and antifungal activities of synthetic benzyl bromides, ketones, and corresponding chalcone derivatives. Drug Des Dev Ther. 2016, 10, 3653–3660. [Google Scholar] [CrossRef]
- Kucerova-Chlupacova, M.; Vyskovska-Tyllova, V.; Richterova-Finkova, L.; Kunes, J.; Buchta, V.; Vejsova, M.; Paterova, P.; Semelkova, L.; Jandourek, O.; Opletalova, V. Novel halogenated pyrazine-based chalcones as potential antimicrobial drugs. Molecules. 2016, 21, 1421. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.; Loh, W.S.; Ooi, C.; Quah, C.; Fun, H.K. Heteroaryl chalcones: design, synthesis, x-ray crystal structures and biological evaluation. Molecules 2013, 18, 12707–12724. [Google Scholar] [CrossRef] [PubMed]
- Erguden, B.; Unver, Y. Phenolic chalcones lead to ion leakage from gram-positive bacteria prior to cell death. Arch. Microbiol. 2021, 204, 3. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. Bmc Complem Altern M. 2018, 18, 252. [Google Scholar] [CrossRef]
- Jian, W.; Zhang, D.W.; Zhu, F.; Wang, S.X.; Zhu, T.; Pu, X.J.; Zheng, T.; Feng, H.; Lin, H.H. ; Nitrate reductase-dependent nitric oxide production is required for regulation alternative oxidase pathway involved in the resistance to Cucumber mosaic virus infection in Arabidopsis. Plant Growth Regul. 2015, 77, 99–107. [Google Scholar] [CrossRef]
- Xu, T.; Yao, F.; Liang, W.S.; Li, Y.H.; Li, D.R.; Wang, H.; Wang, Z.Y. Involvement of alternative oxidase in the regulation of growth, development, and resistance to oxidative stress of Sclerotinia sclerotiorum. J. Microbiol. 2012, 50, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest. Manage. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Banno, S.; Yamashita, K.; Fukumon, F.; Okada, K.; Uekusa, H.; Takagaki, M.; Kimura, M.; Fujimura, M. ; Characterization of QoI resistance in Botrytis cinerea and identification of two types of mitochondrial cytochrome b gene. Plant Pathol. 2009, 58, 120–129. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.T.; Liang, W.S.; Yao, F.; Li, Y.H.; Li, D.R.; Wang, H.; Wang, Z.Y. Involvement of alternative oxidase in the regulation of Sclerotinia sclerotiorum sensitivity to fungicides of azoxystrobin and procymidone. J Microbiol. 2013, 51, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS One 2012, 7, 30147. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Kondo, K.; Uehara, N.; Otokozawa, S.; Tsuji, N.; Yagihashi, A.; Watanabe, N. Endogenous reactive oxygen species is an important mediator of miconazole antifungal effect. Antimicrob. Agents Chemother. 2002, 46, 3113–3117. [Google Scholar] [CrossRef]
- Pozniakovsky, A.I.; Knorre, D.A.; Markova, O.V.; Hyman, A.A.; Skulachev, V.P.; Severin, F.F. Role of mitochondria in the pheromone- and amiodaroneinduced programmed death of yeast. J. Cell Biol. 2005, 168, 257–269. [Google Scholar] [CrossRef]
- Chai, N.N.; Sun, A.M.; Zhu, X.H.; Li, Y.P.; Wang, R.R.; Zhang, Y.; Mao, Z.W. Antifungal evaluation of quinoline-chalcone derivatives combined with FLC against drug-resistant Candida albicans. Bioorg Med Chem Lett. 2023, 86, 129242. [Google Scholar] [CrossRef]
- Bila, N.M.; Costa-Orlandi, C.B.; Vaso, C.O.; Bonatti, J.L.C.; Regasini, L.O.; Fontana, C.R.; Fusco-Almeida, A.M. 2-Hydroxychalcone as a Potent Compound and Photosensitizer Against Dermatophyte Biofilms. Front. Cell. Infect. Microbiol. 2021, 11, 679470. [Google Scholar]
- Wang, Y.; Zhang, W.; Dong, J.; Gao, J. Design, synthesis and bioactivity evaluation of coumarin-chalcone hybrids as potential anticancer agents. Bioorg. Chem. 2019, 95, 103530. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.S.; Jin, H.; Tao, T.; Hou, T.P. Synthesis and Anti-fungal Activity of Chalcones with Pyridyls. J. Shangdong Agric. Univ. 2016, 47, 166–171. [Google Scholar]
- Helmerhorst, E.J.; Stan, M.; Murphy, M.P.; Sherman, F.; Oppenheim, F.G. The concomitant expression and availability of conventional and alternative, cyanide-insensitive, respiratory pathways in Candida albicans. Mitochondrion. 2005, 5, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Serrano, M.; L'Haridon, F.; Tjamos, S.E.; Metraux, J.P. Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry. 2015, 112, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Marschall, R.; Tudzynski, P. A new and reliable method for live imaging and quantification of reactive oxygen species in Botrytis cinerea Technological advancement. Fungal Genet Biol. 2014, 71, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Nair, A.M.; Verma, P.K. Surviving the odds: From perception to survival of fungal phytopathogens under host-generated oxidative burst. Plant Commun. 2021, 2, 100142. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




