Submitted:
27 September 2023
Posted:
28 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
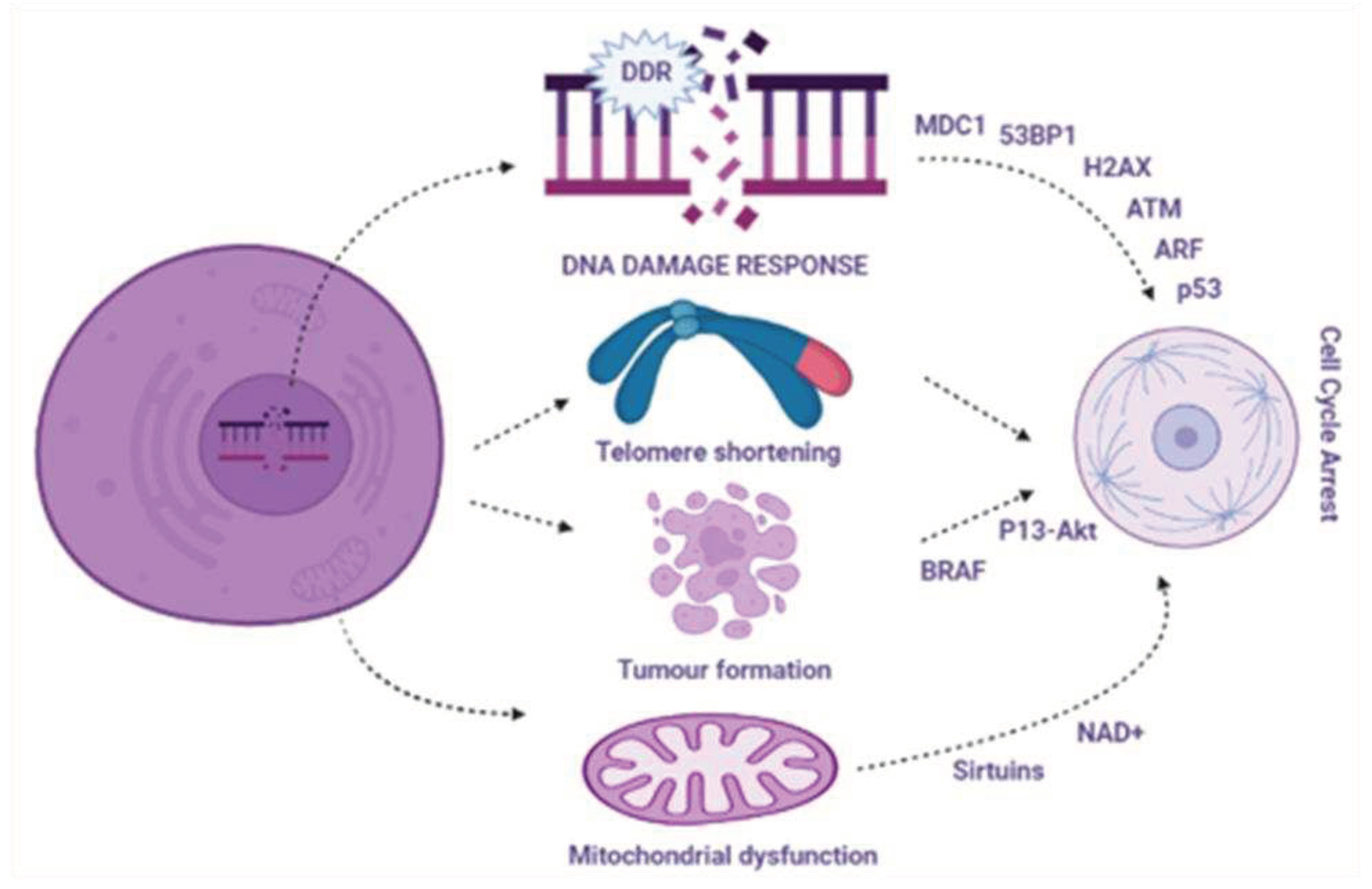
3.1. Signaling pathway involving aging and related aging inducers
3.2. DNA damage response in cellular aging
3.3. Telomere shortening and cellular ageing
3.4. Mitochondrial dysfunction and cellular aging
3.5. Oncogene-induced aging
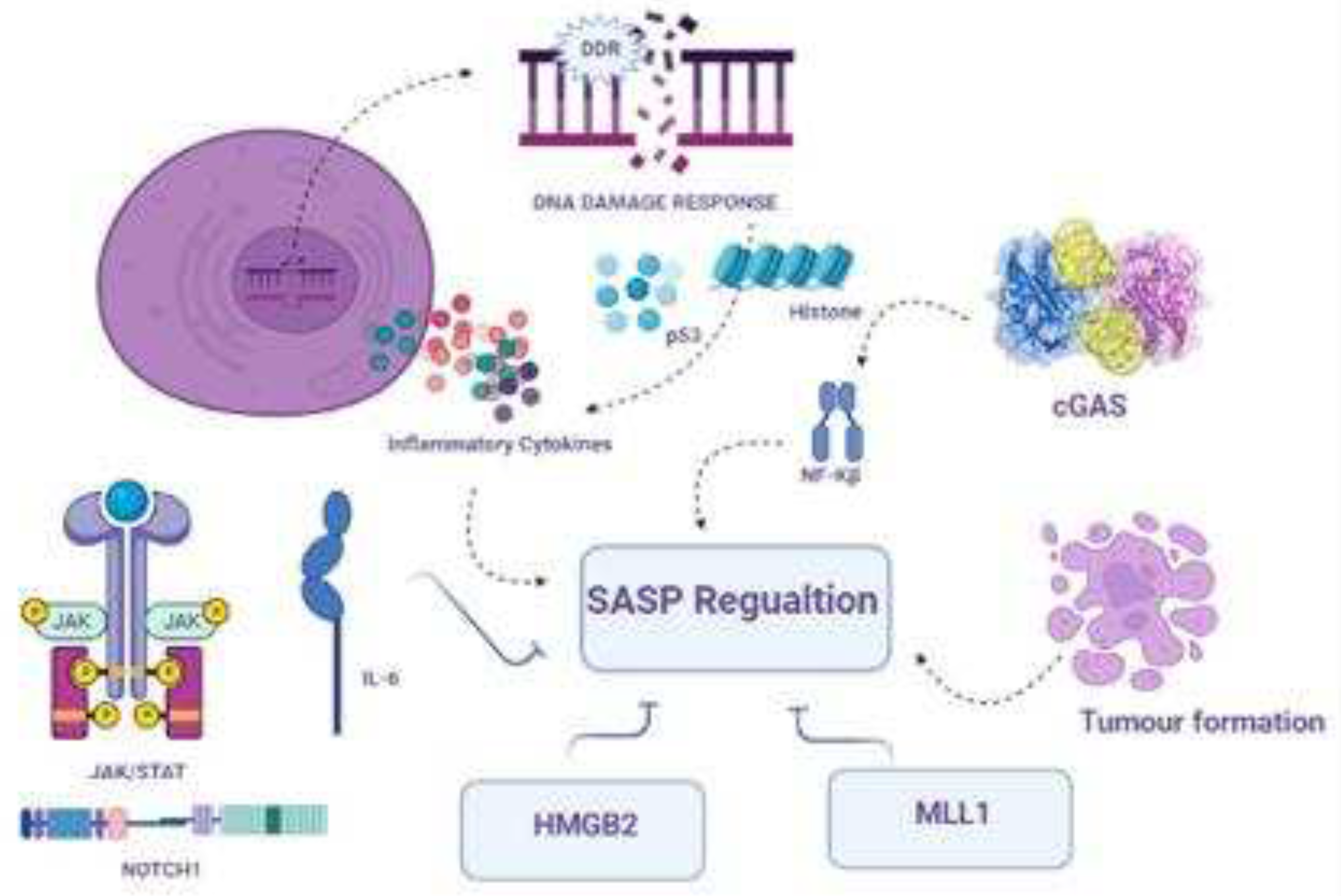
3.6. Chromatin changes in aging cells
3.7. SASP composition and regulation
3.8. Transcriptional and epigenetic control of SASP
3.9. SASP and non-specific immunity
3.10. Promising and detrimental outcomes of senescence in cells
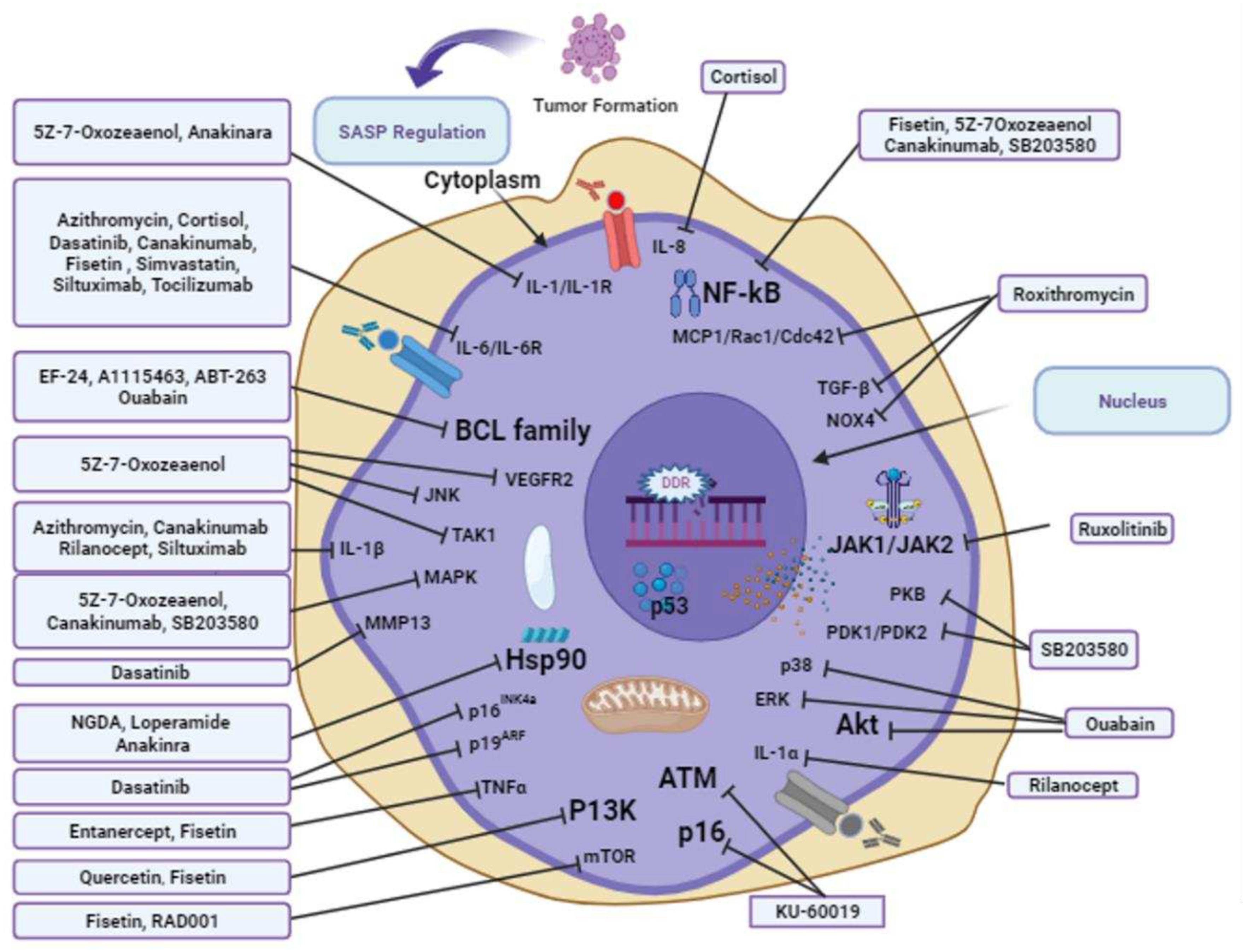
3.11. Senotherapeutic approaches to overcome aging
3.12. Potential geroprotective and anti-aging compounds
3.12.1. 7-Oxozeanol
3.12.2. A-1155463
3.12.3. ABT-263
3.12.4. Anakinra
3.12.5. Azithromycin
3.12.6. Canakinumab
3.12.7. Cortisol
3.12.8. Dasatinib and quercetin
3.12.9. EF24
3.12.10. Etanercept
3.12.11. Fisetin
3.12.12. KU-60019
3.12.13. Loperamide
3.12.14. NDGA
3.12.15. Ouabain
3.12.16. RAD001
3.12.17. Rilonacept
3.12.18. Roxithromycin
3.12.19. Ruxolitinib
3.12.20. SB203580
3.12.21. Simvastatin
3.12.22. Siltuximab
3.12.23. Tocilizumab
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liang, W.; Guan, H. Investigation and analysis on travel and health of the elderly in the old residential areas in Beijing. IRSPSD International; 2023, 11, 85–103. [CrossRef]
- López-Otín, C.; Pietrocola, F.; Roiz-Valle, D.; Galluzzi, L.; Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab 2023, 35, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Yeung, Y.T.; Muñoz, M.F.; Ayala, A.; Guerrero-Castilla, A.; Argüelles, S. Basic pathways and targets for antiaging intervention. Anti-Aging Pharmacology; Elsevier; 2023. pp. 13–40.
- Pezone, A.; Olivieri, F.; Napoli, M.V.; Procopio, A.; Avvedimento, E.V.; Gabrielli, A. Inflammation and DNA damage: cause, effect or both. Nat Rev Rheumatol 2023, 19, 200–211. [Google Scholar] [CrossRef]
- Liu, R.M. Aging, cellular senescence, and Alzheimer’s disease. Int J Mol Sci 2022, 23, 23(4):1989. [CrossRef]
- Vaddavalli, P.L.; Schumacher, B. The p53 network: cellular and systemic DNA damage responses in cancer and aging. Trends Genet 2022, 38, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Hu, J.; Li, N.; Gao, J.; Huo, R.; Peng, X.; Zhang, N.; Liu, Y.; Zhao, H.; Liu, R.; et al. The mechanism of stem cell aging. Stem Cell Rev Rep 2022, 18, 1281–1293. [Google Scholar] [CrossRef]
- Yoon, Y.S.; You, J.S.; Kim, T.K.; Ahn, W.J.; Kim, M.J.; Son, K.H.; Ricarte, D.; Ortiz, D.; Lee, S.J.; Lee, H.J. Senescence and impaired DNA damage responses in alpha-synucleinopathy models. Exp Mol Med 2022, 54, 115–128. [Google Scholar] [CrossRef]
- Wang, F.; Jin, S.; Mayca Pozo, F.M.; Tian, D.; Tang, X.; Dai, Y.; Yao, X.; Tang, J.; Zhang, Y. Chemical screen identifies shikonin as a broad DNA damage response inhibitor that enhances chemotherapy through inhibiting ATM and ATR. Acta Pharm Sin B 2022, 12, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- González-Bermúdez, L.; Genescà, A.; Terradas, M.; Martín, M. Role of H4K16 acetylation in 53BP1 recruitment to double-strand break sites in in vitro aged cells. Biogerontology 2022, 23, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Ho, T.L.F.; Hariharan, A.; Goh, H.C.; Wong, Y.L.; Verkaik, N.S.; Lee, M.Y.; Tam, W.L.; van Gent, D.C.; Venkitaraman, A.R.; et al. Rapid recruitment of p53 to DNA damage sites directs DNA repair choice and integrity. Proc Natl Acad Sci U S A 2022, 119, e2113233119. [Google Scholar] [CrossRef]
- Duan, J.L.; Ruan, B.; Song, P.; Fang, Z.Q.; Yue, Z.S.; Liu, J.J.; Dou, G.R.; Han, H.; Wang, L. Shear stress–induced cellular senescence blunts liver regeneration through Notch–sirtuin 1–P21/P16 axis. Hepatology 2022, 75, 584–599. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.Y.; O’Connell, J.F.; Egan, J.M.; Kim, Y. Black ginseng ameliorates cellular senescence via p53-p21/p16 pathway in aged mice. Biology 2022, 11, 1108. [Google Scholar] [CrossRef]
- Soto-Palma, C.; Niedernhofer, L.J.; Faulk, C.D.; Dong, X. Epigenetics, DNA damage, and aging. J Clin Invest 2022, 132, e158446. [Google Scholar] [CrossRef] [PubMed]
- Moser, R.; Gurley, K.E.; Nikolova, O.; Qin, G.; Joshi, R.; Mendez, E.; Shmulevich, I.; Ashley, A.; Grandori, C.; Kemp, C.J. Synthetic lethal kinases in Ras/p53 mutant squamous cell carcinoma. Oncogene 2022, 41, 3355–3369. [Google Scholar] [CrossRef] [PubMed]
- Bell, H.N.; Rebernick, R.J.; Goyert, J.; Singhal, R.; Kuljanin, M.; Kerk, S.A.; Huang, W.; Das, N.K.; Andren, A.; Solanki, S.; et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell 2022, 40, 185–200.e6. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol 2022, 24, 135–147. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Q.; Wang, Y.; Zhuang, Y.; Xu, L.; Ma, X.; Guan, D.; Zhou, J.; Liu, J.; Wu, X.; et al. Tert activates endogenous retroviruses to promote an immunosuppressive tumour microenvironment. EMBO Rep 2022, 23, e52984. [Google Scholar] [CrossRef]
- Makeeva, V.S. Ionizing radiation effects on telomeres. Biol Bull 2022, 49, 2257–2265. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Francia, S.; Capozzo, I.; Modafferi, S.; Iannelli, F.; Gioia, U., di Fagagna FdA. DROSHA and DICER RNA products control BMI1-dependent transcriptional repression at DNA damage sites. Europe PMC. 2022.
- Aguado, J.; d’Adda di Fagagna, F.; Wolvetang, E. Telomere transcription in ageing. Ageing Res Rev 2020, 62, 101115. [Google Scholar] [CrossRef]
- Vaena, S.; Chakraborty, P.; Lee, H.G.; Janneh, A.H.; Kassir, M.F.; Beeson, G.; Hedley, Z.; Yalcinkaya, A.; Sofi, M.H.; Li, H.; et al. Aging-dependent mitochondrial dysfunction mediated by ceramide signaling inhibits antitumor T cell response. Cell Rep 2021, 35, 109076. [Google Scholar] [CrossRef]
- Di Emidio, G.; Falone, S.; Artini, P.G.; Amicarelli, F.; D’Alessandro, A.M.; Tatone, C. Mitochondrial sirtuins in reproduction. Antioxidants (Basel) 2021, 10, 1047. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol 2021, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- l’Hôte v. Senolytic drug discovery and mechanisms of action in BRAF-V600E oncogene-induced senescence: Université Paris-Saclay; 2022.
- Helbling-Leclerc, A.; Garcin, C.; Rosselli, F. Beyond DNA repair and chromosome instability-Fanconi anaemia as a cellular senescence-associated syndrome. Cell Death Differ 2021, 28, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Krokidis, M.G.; Prasinou, P.; Efthimiadou, E.K.; Boari, A.; Ferreri, C.; Chatgilialoglu, C. Effects of aging and disease conditions in brain of tumor-bearing mice: evaluation of purine DNA damages and fatty acid pool changes. Biomolecules 2022, 12, 1075. [Google Scholar] [CrossRef]
- Pouikli, A.; Parekh, S.; Maleszewska, M.; Nikopoulou, C.; Baghdadi, M.; Tripodi, I.; Folz-Donahue, K.; Hinze, Y.; Mesaros, A.; Hoey, D.; et al. Chromatin remodeling due to degradation of citrate carrier impairs osteogenesis of aged mesenchymal stem cells. Nat Aging 2021, 1, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Castro-Obregón, S. Lamin B receptor: role on chromatin structure, cellular senescence and possibly aging. Biochem J 2020, 477, 2715–2720. [Google Scholar] [CrossRef]
- Lund, P.J.; Lopes, M.; Sidoli, S.; Coradin, M.; Vitorino, F.N.L.; Da Cunha, J.P.C.; Garcia, B.A. FGF-2 induces a failure of cell cycle progression in cells harboring amplified K-Ras, revealing new insights into oncogene-induced senescence. Mol Omics 2021, 17, 725–739. [Google Scholar] [CrossRef]
- Ashrafian, S.; Zarrineh, M.; Jensen, P.; Nawrocki, A.; Rezadoost, H.; Ansari, A.M.; Farahmand, L.; Ghassempour, A.; Larsen, M.R. Quantitative phosphoproteomics and Acetylomics of safranal anticancer effects in triple-negative breast cancer cells. J Proteome Res 2022, 21, 2566–2585. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y.; Yang, Y.; Yu, J.; Wu, J.; Liao, Z.; Guo, A.; Sun, Y.; Zhao, Y.; Chen, J.; et al. Lysyl oxidase-like 2 inhibitor rescues D-galactose-induced skeletal muscle fibrosis. Aging Cell 2022, 21, e13659. [Google Scholar] [CrossRef]
- Zhang, Y.; Amaral, M.L.; Zhu, C.; Grieco, S.F.; Hou, X.; Lin, L.; Buchanan, J.; Tong, L.; Preissl, S.; Xu, X.; et al. Single-cell epigenome analysis reveals age-associated decay of heterochromatin domains in excitatory neurons in the mouse brain. Cell Res 2022, 32, 1008–1021. [Google Scholar] [CrossRef]
- Zhang, N.; Shang, M.; Li, H.; Wu, L.; Dong, M.; Huang, B.; Lu, J.; Zhang, Y. Dual inhibition of H3K9me2 and H3K27me3 promotes tumor cell senescence without triggering the secretion of SASP. Int J Mol Sci 2022, 23, 3911. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Shang, M.; Han, Y.; Liu, J.; Liu, J.; Kong, S.H.; Hou, J.; Huang, B.; Lu, J.; Zhang, Y. EZH2–CCF–cGAS axis promotes breast cancer metastasis. Int J Mol Sci 2022, 23, 1788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Allis, C.D.; Wang, G.G. The language of chromatin modification in human cancers. Nat Rev Cancer 2021, 21, 413–430. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Z.; Shen, W.; Huang, G.; Sedivy, J.M.; Wang, H.; Ju, Z. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct Target Ther 2021, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Endicott, J.L.; Nolte, P.A.; Shen, H.; Laird, P.W. Cell division drives DNA methylation loss in late-replicating domains in primary human cells. Nat Commun 2022, 13, 6659. [Google Scholar] [CrossRef]
- Yu, Y.C.-Y.; Hui, T.Z.; Kao, T.H.; Liao, H.F.; Yang, C.Y.; Hou, C.C.; Hsieh, H.T.; Chang, J.Y.; Tsai, Y.T.; Pinskaya, M.; et al. Transient DNMT3L expression reinforces chromatin surveillance to halt senescence progression in mouse embryonic fibroblast. Front Cell Dev Biol 2020, 8, 103. [Google Scholar] [CrossRef]
- Chalmers, T.J.; Wu, L.E. Transposable elements cross kingdom boundaries and contribute to inflammation and ageing: somatic acquisition of foreign transposable elements as a catalyst of genome instability, epigenetic dysregulation, inflammation, senescence, and ageing. BioEssays 2020, 42, e1900197. [Google Scholar] [CrossRef]
- Metaxakis, A.; Gkikas, I.; Tavernarakis, N. The epigenetics of aging. Aging; Elsevier 2023, 333–358.
- Liu, H.; Zhao, H.; Sun, Y., Eds. Tumor microenvironment and cellular senescence: understanding therapeutic resistance and harnessing strategies. Semin Cancer Biol; Elsevier 2022, 86, 769–781. [CrossRef]
- Van Petten de Vasconcelos Azevedo, F.; Lopes, D.S.; Zóia, M.A.P.; Correia, L.I.V.; Saito, N.; Fonseca, B.B.; Polloni, L.; Teixeira, S.C.; Goulart, L.R.; de Melo Rodrigues Ávila, V. A New Approach to Inhibiting Triple-Negative Breast Cancer: in vitro, ex vivo and in vivo antiangiogenic Effect of BthTx-II, a PLA2-Asp-49 from Bothrops jararacussu Venom. Biomolecules 2022, 12, 258. [Google Scholar] [CrossRef]
- Tan, H.; Xu, J.; Liu, Y. Ageing, cellular senescence and chronic kidney disease: experimental evidence. Curr Opin Nephrol Hypertens 2022, 31, 235–243. [Google Scholar] [CrossRef]
- Liang, L.; Chai, Y.; Chai, F.; Liu, H.; Ma, N.; Zhang, H.; Zhang, S.; Nong, L.; Li, T.; Zhang, B. Expression of SASP, DNA damage response, and cell proliferation factors in early gastric neoplastic lesions: correlations and clinical significance. Pathol Oncol Res 2022, 28, 1610401. [Google Scholar] [CrossRef]
- Turley, J. Chitin-Derived STING Activators as Adjuvants and Therapeutics; Trinity College Dublin, 2021.
- Salech, F.; SanMartín, C.D.; Concha-Cerda, J.; Romero-Hernández, E.; Ponce, D.P.; Liabeuf, G.; Rogers, N.K.; Murgas, P.; Bruna, B.; More, J.; et al. Senescence markers in peripheral blood mononuclear cells in amnestic mild cognitive impairment and Alzheimer’s disease. Int J Mol Sci 2022, 23, 9387. [Google Scholar] [CrossRef]
- Morales-Valencia, J. From DNA Damage Repair to Cellular Plasticity: How Cancer Cells Respond to Genotoxic Stress; New York University, 2022.
- Morrugares Carmona, R. Determination of New DYRK2 Functions in Response to Genotoxic Stress, 2022.
- Josipovic, N.; Ebbesen, K.K.; Zirkel, A.; Danieli-Mackay, A.; Dieterich, C.; Kurian, L.; Hansen, T.B.; Papantonis, A. circRAB3IP modulates cell proliferation by reorganizing gene expression and mRNA processing in a paracrine manner. RNA 2022, 28, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): can it be controlled by senolysis? Inflamm Regen 2022, 42, 11. [Google Scholar] [CrossRef]
- Durinikova, E.; Reilly, N.M.; Buzo, K.; Mariella, E.; Chilà, R.; Lorenzato, A.; Dias, J.M.L.; Grasso, G.; Pisati, F.; Lamba, S.; et al. Targeting the DNA damage response pathways and replication stress in colorectal cancer. Clin Cancer Res 2022, 28, 3874–3889. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Guo, L.; Xu, W.; He, C.; Jia, X.; Zhao, Z.; Gu, Z. Aging-associated accumulation of mitochondrial DNA mutations in tumor origin. Life Med 2022, 1, 149–167. [Google Scholar] [CrossRef]
- Luzhin, A.; Rajan, P.; Safina, A.; Leonova, K.; Stablewski, A.; Wang, J.; Pal, M.; Kantidze, O.; Gurova, K. Comparison of cell response to chromatin and DNA damage. bioRxiv 2023, 01, 17.524424. [CrossRef]
- Chibaya, L.; Snyder, J.; Ruscetti, M., Eds. Senescence and the tumor-immune landscape: implications for cancer immunotherapy. Semin Cancer Biol; Elsevier 2022, 86, 827–845. [CrossRef]
- Ma, Y.; Li, S.; Ye, S.; Hu, D.; Wei, L.; Xiao, F. Hexavalent chromium triggers hepatocytes premature senescence via the GATA4/NF-κB signaling pathway mediated by the DNA damage response. Ecotoxicol Environ Saf 2022, 239, 113645. [Google Scholar] [CrossRef]
- Purohit, M.; Gupta, G.; Afzal, O.; Altamimi, A.S.A.; Alzarea, S.I.; Kazmi, I.; Almalki, W.H.; Gulati, M.; Kaur, I.P.; Singh, S.K.; et al. Janus kinase/signal transducers and activator of transcription (JAK/STAT) and its role in Lung inflammatory disease. Chem Biol Interact 2023, 371, 110334. [Google Scholar] [CrossRef]
- Molnár, A.Á.; Pásztor, D.; Merkely, B. Cellular senescence, aging and non-aging processes in calcified aortic valve stenosis: from Bench-Side to bedside. Cells 2022, 11, 3389. [Google Scholar] [CrossRef]
- Laubach, K.N.; Yan, W.; Kong, X.; Sun, W.; Chen, M.; Zhang, J.; Chen, X. p73α1, a p73 C-terminal isoform, regulates tumor suppression and the inflammatory response via Notch1. Proc Natl Acad Sci U S A 2022, 119, e2123202119. [Google Scholar] [CrossRef]
- Barrows, J.K.; Lin, B.; Quaas, C.E.; Fullbright, G.; Wallace, E.N.; Long, D.T. BRD4 promotes resection and homology-directed repair of DNA double-strand breaks. Nat Commun 2022, 13, 3016. [Google Scholar] [CrossRef]
- Markouli, M.; Strepkos, D.; Piperi, C. Impact of histone modifications and their therapeutic targeting in hematological malignancies. Int J Mol Sci 2022, 23, 13657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Jaganathan, A.; Sun, Y.; Ma, N.; Li, N.; Han, X.; Sun, X.; Yi, H.; Fu, S.; et al. BRD4-PRC2 represses transcription of T-helper 2-specific negative regulators during T-cell differentiation. EMBO J 2023, 42, e111473. [Google Scholar] [CrossRef]
- He, J.; Liu, M.W.; Wang, Z.Y.; Shi, R.J. Protective effects of the notoginsenoside R1 on acute lung injury by regulating the miR-128-2-5p/Tollip signaling pathway in rats with severe acute pancreatitis. Innate Immun 2022, 28, 19–36. [Google Scholar] [CrossRef]
- Yao, J.; Miao, Y.; Zhang, Y.; Zhu, L.; Chen, H.; Wu, X.; Yang, Y.; Dai, X.; Hu, Q.; Wan, M.; et al. Dao-Chi Powder ameliorates pancreatitis-induced intestinal and cardiac injuries via regulating the Nrf2-HO-1-HMGB1 signaling pathway in rats. Front Pharmacol 2022, 13, 922130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Simon, M.; Seluanov, A.; Gorbunova, V. DNA damage and repair in age-related inflammation. Nat Rev Immunol 2023, 23, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Frediani, E.; Scavone, F.; Laurenzana, A.; Chillà, A.; Tortora, K.; Cimmino, I.; Leri, M.; Bucciantini, M.; Mangoni, M.; Fibbi, G.; et al. Olive phenols preserve lamin B1 expression reducing cGAS/STING/NFκB-mediated SASP in ionizing radiation-induced senescence. J Cell Mol Med 2022, 26, 2337–2350. [Google Scholar] [CrossRef]
- Kirkland, J.L. Tumor dormancy and disease recurrence. Cancer Metastasis Rev 2023, 42, 9–12. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, K.; Silakari, O. The interplay between inflammatory pathways and COVID-19: A critical review on pathogenesis and therapeutic options. Microb Pathog 2021, 150, 104673. [Google Scholar] [CrossRef]
- Li, Y.; Adeniji, N.T.; Fan, W.; Kunimoto, K.; Török, N.J. Non-alcoholic fatty liver disease and liver fibrosis during aging. Aging Dis 2022, 13, 1239–1251. [Google Scholar] [CrossRef]
- Kim, K.H.; Cheng, N.; Lau, L.F. Cellular communication network factor 1-stimulated liver macrophage efferocytosis drives hepatic stellate cell activation and liver fibrosis. Hepatol Commun 2022, 6, 2798–2811. [Google Scholar] [CrossRef]
- Sun, G. The Role of BMP Signaling in DNA-Damage Responses During Zebrafish Heart Regeneration; Universität Ulm, 2022.
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: the path to the clinic. Nat Med 2022, 28, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer—role and therapeutic opportunities. Nat Rev Clin Oncol 2022, 19, 619–636. [Google Scholar] [CrossRef]
- Secomandi, L.; Borghesan, M.; Velarde, M.; Demaria, M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Hum Reprod Update 2022, 28, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Morsli, S.; Doherty, G.J.; Muñoz-Espín, D. Activatable senoprobes and senolytics: novel strategies to detect and target senescent cells. Mech Ageing Dev 2022, 202, 111618. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr Opin Immunol 2014, 27, 16–25. [Google Scholar] [CrossRef]
- Naylor, R.M.; Baker, D.J.; Van Deursen, J.M. Senescent cells: a novel therapeutic target for aging and age-related diseases. Clin Pharmacol Ther 2013, 93, 105–116. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: an expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Robbins, P.D.; Huffman, D.M. Heterochronic parabiosis: a valuable tool to investigate cellular senescence and other hallmarks of aging. Aging (Albany, NY) 2022, 14, 3325–3328. [Google Scholar] [CrossRef]
- Jeon, O.H.; Mehdipour, M.; Gil, T.H.; Kang, M.; Aguirre, N.W.; Robinson, Z.R.; Kato, C.; Etienne, J.; Lee, H.G.; Alimirah, F.; et al. Systemic induction of senescence in young mice after single heterochronic blood exchange. Nat Metab 2022, 4, 995–1006. [Google Scholar] [CrossRef]
- Yang, J.Y.; Deng, X.Y.; Li, Y.S.; Ma, X.C.; Feng, J.X.; Yu, B.; Chen, Y.; Luo, Y.L.; Wang, X.; Chen, M.L.; et al. Structure of Schlafen13 reveals a new class of tRNA/rRNA-targeting RNase engaged in translational control. Nat Commun 2018, 9, 1165. [Google Scholar] [CrossRef]
- Ninomiya-Tsuji, J.; Kajino, T.; Ono, K.; Ohtomo, T.; Matsumoto, M.; Shiina, M.; Mihara, M.; Tsuchiya, M.; Matsumoto, K. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 2003, 278, 18485–18490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Fu, D.; Xu, Q.; Cong, X.; Wu, C.; Zhong, X.; Ma, Y.; Lv, Z.; Chen, F.; Han, L.; et al. The senescence-associated secretory phenotype is potentiated by feedforward regulatory mechanisms involving Zscan4 and TAK1. Nat Commun 2018, 9, 1723. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.F.; Hasvold, L.; Wang, L.; Wang, X.; Petros, A.M.; Park, C.H.; Boghaert, E.R.; Catron, N.D.; Chen, J.; Colman, P.M.; et al. Discovery of a potent and selective BCL-XL inhibitor with in vivo activity. ACS Med Chem Lett 2014, 5, 1088–1093. [Google Scholar] [CrossRef]
- Sharma, A.K.; Roberts, R.L.; Benson Jr., R.D.; Pierce, J.L.; Yu, K.; Hamrick, M.W.; McGee-Lawrence, M.E. The senolytic drug navitoclax (ABT-263) causes trabecular bone loss and impaired osteoprogenitor function in aged mice. Front Cell Dev Biol 2020, 8, 354. [CrossRef]
- Kim, H.; Jang, J.; Song, M.J.; Park, C.H.; Lee, D.H.; Lee, S.H.; Chung, J.H. Inhibition of matrix metalloproteinase expression by selective clearing of senescent dermal fibroblasts attenuates ultraviolet-induced photoaging. Biomed Pharmacother 2022, 150, 113034. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Hurd, E.; Cush, J.; Schiff, M.; Weinblatt, M.E.; Moreland, L.W.; Kremer, J.; Bear, M.B.; Rich, W.J.; McCabe, D. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four–week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002, 46, 614–624. [Google Scholar] [CrossRef]
- Kuno, K.; Matsushima, K. The IL-1 receptor signaling pathway. J Leukoc Biol 1994, 56, 542–547. [Google Scholar] [CrossRef]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat Rev Cancer 2019, 19, 439–453. [Google Scholar] [CrossRef]
- Schett, G.; Dayer, J.M.; Manger, B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol 2016, 12, 14–24. [Google Scholar] [CrossRef]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLOS Biol 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef]
- Ozsvari, B.; Nuttall, J.R.; Sotgia, F.; Lisanti, M.P. Azithromycin and Roxithromycin define a new family of “senolytic” drugs that target senescent human fibroblasts. Aging (Albany, NY) 2018, 10, 3294–3307. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Li, W.C.; Tang, B.X.; Li, J.; Zang, Y. Roxithromycin attenuates bleomycin-induced pulmonary fibrosis by targeting senescent cells. Acta Pharmacol Sin 2021, 42, 2058–2068. [Google Scholar] [CrossRef]
- Dhimolea, E., Ed. Canakinumab. mAbs; Taylor & Francis 2010, 2, 3–13. [CrossRef]
- Kuemmerle-Deschner, J.B.; Ramos, E.; Blank, N.; Roesler, J.; Felix, S.D.; Jung, T.; Stricker, K.; Chakraborty, A.; Tannenbaum, S.; Wright, A.M.; et al. Canakinumab (ACZ885, a fully human IgG1 anti-IL-1β mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS). Arthritis Res Ther 2011, 13, R34. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S.; et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev 2011, 25, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Glynn, R.J.; Koenig, W.; Libby, P.; Everett, B.M.; Lefkowitz, M.; Thuren, T.; Cornel, J.H. Inhibition of interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol 2018, 71, 2405–2414. [Google Scholar] [CrossRef]
- Tasdemir, N.; Banito, A.; Roe, J.S.; Alonso-Curbelo, D.; Camiolo, M.; Tschaharganeh, D.F.; Huang, C.H.; Aksoy, O.; Bolden, J.E.; Chen, C.C.; et al. BRD4 connects enhancer remodeling to senescence immune SurveillanceBRD4. Cancer Discov 2016, 6, 612–629. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B. Human Anatomy and Physiology; Auflage: San Francisco, 2001. [Google Scholar]
- Laberge, R.M.; Zhou, L.; Sarantos, M.R.; Rodier, F.; Freund, A.; de Keizer, P.L.; Liu, S.; Demaria, M.; Cong, Y.S.; Kapahi, P.; et al. Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell 2012, 11, 569–578. [Google Scholar] [CrossRef]
- Ferguson-Smith, A.C.; Chen, Y.F.; Newman, M.S.; May, L.T.; Sehgal, P.B.; Ruddle, F.H. Regional localization of the interferon-β2B-cell stimulatory factor 2/hepatocyte stimulating factor gene to human chromosome 7p15-p21. Genomics 1988, 2, 203–208. [Google Scholar] [CrossRef]
- Chauntry, A.J.; Bishop, N.C.; Hamer, M.; Kingsnorth, A.P.; Chen, Y.L.; Paine, N.J. Sedentary behaviour is associated with heightened cardiovascular, inflammatory and cortisol reactivity to acute psychological stress. Psychoneuroendocrinology 2022, 141, 105756. [Google Scholar] [CrossRef]
- Han, X.; Lei, Q.; Xie, J.; Liu, H.; Li, J.; Zhang, X.; Zhang, T.; Gou, X. Potential regulators of the senescence-associated secretory phenotype during senescence and aging. J Gerontol A Biol Sci Med Sci 2022, 77, 2207–2218. [Google Scholar] [CrossRef]
- Rodier, F.; Muñoz, D.P.; Teachenor, R.; Chu, V.; Le, O.; Bhaumik, D.; Coppé, J.P.; Campeau, E.; Beauséjour, C.M.; Kim, S.H.; et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci 2011, 124, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Moghadam-Kia, S.; Werth, V.P. Prevention and treatment of systemic glucocorticoid side effects. Int J Dermatol 2010, 49, 239–248. [Google Scholar] [CrossRef]
- Novais, E.J.; Tran, V.A.; Johnston, S.N.; Darris, K.R.; Roupas, A.J.; Sessions, G.A.; Shapiro, I.M.; Diekman, B.O.; Risbud, M.V. Long-term treatment with senolytic drugs dasatinib and quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun 2021, 12, 5213. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front Immunol 2022, 13, 943321. [Google Scholar] [CrossRef]
- Chu, J.J.; Ji, W.B.; Zhuang, J.H.; Gong, B.F.; Chen, X.H.; Cheng, W.B.; Liang, W.D.; Li, G.R.; Gao, J.; Yin, Y. Nanoparticles-based anti-aging treatment of Alzheimer’s disease. Drug Deliv 2022, 29, 2100–2116. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, Y.; Zhang, R.; Zheng, G.; Zhou, D. The curcumin analog EF24 is a novel senolytic agent. Aging (Albany, NY) 2019, 11, 771–782. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS Journal 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Klareskog, L.; van der Heijde, D.; de Jager, J.P.; Gough, A.; Kalden, J.; Malaise, M.; Martín Mola, E.; Pavelka, K.; Sany, J.; Settas, L.; et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomized controlled trial. Lancet 2004, 363, 675–681. [Google Scholar] [CrossRef]
- Rider, P.; Carmi, Y.; Cohen, I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int J Cell Biol 2016, 2016, 9259646. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; De Bono, J.S.; Flechon, A.; Heidenreich, A.; Voog, E.; Davis, N.B.; Qi, M.; Bandekar, R.; Vermeulen, J.T.; Cornfeld, M.; et al. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur J Cancer 2012, 48, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.; Annett, S.; McClements, L.; Robson, T. Top notch targeting strategies in cancer: a detailed overview of recent insights and current perspectives. Cells 2020, 9, 1503. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, J.; Zitvogel, L.; Galluzzi, L.; Vacchelli, E.; Kroemer, G. Trial watch: STING agonists in cancer therapy. Oncoimmunology 2020, 9, 1777624. [Google Scholar] [CrossRef]
- Sahu, B.D.; Kalvala, A.K.; Koneru, M.; Mahesh Kumar, J.; Kuncha, M.; Rachamalla, S.S.; Sistla, R. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-κB activation and antioxidant defence. PLOS ONE 2014, 9, e105070. [Google Scholar] [CrossRef]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany, NY) 2017, 9, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Molagoda, I.M.N.; Jayasingha, J.A.C.C.; Choi, Y.H.; Jayasooriya, R.G.P.T.; Kang, C.H.; Kim, G.Y. Fisetin inhibits lipopolysaccharide-induced inflammatory response by activating β-catenin, leading to a decrease in endotoxic shock. Sci Rep 2021, 11, 8377. [Google Scholar] [CrossRef]
- Huang, C.; Filippone, N.R.; Reiner, T.; Roberts, S. Sensors and Inhibitors for the detection of ataxia telangiectasia mutated (ATM) protein kinase. Mol Pharm 2021, 18, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: the p53 family and its regulators provide endless cancer therapy opportunities. Exp Mol Med 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Aghali, A.; Koloko Ngassie, M.L.; Pabelick, C.M.; Prakash, Y.S. Cellular senescence in aging lungs and diseases. Cells 2022, 11, 1781. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, H.; Song, E.S.; Lee, Y.H.; Kuk, M.U.; Ko, G.; Kwon, H.W.; Byun, Y.; Park, J.T. Restoration of lysosomal and mitochondrial function through p38 mitogen-activated protein kinase inhibition ameliorates senescence. Rejuvenation Res 2022, 25, 291–299. [Google Scholar] [CrossRef]
- Kang, H.T.; Park, J.T.; Choi, K.; Kim, Y.; Choi, H.J.C.; Jung, C.W.; Lee, Y.S.; Park, S.C. Chemical screening identifies ATM as a target for alleviating senescence. Nat Chem Biol 2017, 13, 616–623. [Google Scholar] [CrossRef]
- Özeş, A.R.; Miller, D.F.; Özeş, O.N.; Fang, F.; Liu, Y.; Matei, D.; Huang, T.; Nephew, K.P. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene 2016, 35, 5350–5361. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Devesa, S.S.; Chang, B.L.; Bunker, C.H.; Cheng, I.; Cooney, K.; Eeles, R.; Fernandez, P.; Giri, V.N.; Gueye, S.M.; et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of african descent. Prostate Cancer 2013, 2013, 560857. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann-Stroissnigg, H.; Ling, Y.Y.; Zhao, J.; McGowan, S.J.; Zhu, Y.; Brooks, R.W.; Grassi, D.; Gregg, S.Q.; Stripay, J.L.; Dorronsoro, A.; et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun 2017, 8, 422. [Google Scholar] [CrossRef]
- Fleming, J.E.; Miquel, J.; Cottrell, S.F.; Yengoyan, L.S.; Economos, A.C. Is cell aging caused by respiration-dependent injury to the mitochondrial genome? Gerontology 1982, 28, 44–53. [Google Scholar] [CrossRef]
- Richie Jr., J.P.; Mills, B.J.; Lang, C.A. Dietary nordihydroguaiaretic acid increases the life span of the mosquito. Proc Soc Exp Biol Med 1986, 183, 81–85. [CrossRef]
- Harrison, D.E.; Strong, R.; Allison, D.B.; Ames, B.N.; Astle, C.M.; Atamna, H.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nadon, N.L.; et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 2014, 13, 273–282. [Google Scholar] [CrossRef]
- Lozano-Torres, B.; Estepa-Fernández, A.; Rovira, M.; Orzáez, M.; Serrano, M.; Martínez-Máñez, R.; Sancenón, F. The chemistry of senescence. Nat Rev Chem 2019, 3, 426–441. [Google Scholar] [CrossRef]
- Jenny, N.S. Inflammation in aging: cause, effect, or both? Discov Med 2012, 13, 451–460. [Google Scholar] [PubMed]
- Strong, R.; Miller, R.A.; Astle, C.M.; Floyd, R.A.; Flurkey, K.; Hensley, K.L.; Javors, M.A.; Leeuwenburgh, C.; Nelson, J.F.; Ongini, E.; et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 2008, 7, 641–650. [Google Scholar] [CrossRef]
- Guerrero, A.; Herranz, N.; Sun, B.; Wagner, V.; Gallage, S.; Guiho, R.; Wolter, K.; Pombo, J.; Irvine, E.E.; Innes, A.J.; et al. Cardiac glycosides are broad-spectrum senolytics. Nat Metab 2019, 1, 1074–1088. [Google Scholar] [CrossRef]
- Tedesco-Silva, H.; Saliba, F.; Barten, M.J.; De Simone, P.; Potena, L.; Gottlieb, J.; Gawai, A.; Bernhardt, P.; Pascual, J. An overview of the efficacy and safety of everolimus in adult solid organ transplant recipients. Transplant Rev (Orlando) 2022, 36, 100655. [Google Scholar] [CrossRef]
- Laberge, R.M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 2015, 17, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Shavlakadze, T.; Zhu, J.; Wang, S.; Zhou, W.; Morin, B.; Egerman, M.A.; Fan, L.; Wang, Y.; Iartchouk, O.; Meyer, A.; et al. Short-term low-dose mTORC1 inhibition in aged rats counter-regulates age-related gene changes and blocks age-related kidney pathology. J Gerontol A Biol Sci Med Sci 2018, 73, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.; Sundy, J.S.; Schumacher, H.R.; Murphy, F.; Bookbinder, S.; Biedermann, S.; Wu, R.; Mellis, S.; Radin, A. The interleukin 1 inhibitor Rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis 2009, 68, 1613–1617. [Google Scholar] [CrossRef]
- Hoffman, H.M.; Throne, M.L.; Amar, N.J.; Sebai, M.; Kivitz, A.J.; Kavanaugh, A.; Weinstein, S.P.; Belomestnov, P.; Yancopoulos, G.D.; Stahl, N.; et al. Efficacy and safety of Rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum 2008, 58, 2443–2452. [Google Scholar] [CrossRef]
- Liao, C.; Xiao, Y.; Liu, L. The dynamic process and its dual effects on tumors of therapy-induced senescence. Cancer Manag Res 2020, 12, 13553–13566. [Google Scholar] [CrossRef]
- Mesa, R.A.; Yasothan, U.; Kirkpatrick, P. Ruxolitinib. Nat Rev Drug Discov 2012, 11, 103–104. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Gasek, N.S.; Zhou, Y.; Cohn, R.L.; Martin, D.E.; Zuo, W.; Flynn, W.F.; Guo, C.; Jellison, E.R.; et al. Targeting p21Cip1 highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metab 2022, 34, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.N.; Talpaz, M.; Mead, A.J. Ruxolitinib is effective in patients with intermediate-1 risk myelofibrosis: a summary of recent evidence. Leuk Lymphoma 2016, 57, 2259–2267. [Google Scholar] [CrossRef]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A 2015, 112, E6301–E6310. [Google Scholar] [CrossRef]
- Xu, M.; Palmer, A.K.; Ding, H.; Weivoda, M.M.; Pirtskhalava, T.; White, T.A.; Sepe, A.; Johnson, K.O.; Stout, M.B.; Giorgadze, N.; et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 2015, 4, e12997. [Google Scholar] [CrossRef]
- Freund, A.; Patil, C.K.; Campisi, J. P38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J 2011, 30, 1536–1548. [Google Scholar] [CrossRef]
- Hou, J.; Cui, C.; Kim, S.; Sung, C.; Choi, C. Ginsenoside F1 suppresses astrocytic senescence-associated secretory phenotype. Chem Biol Interact 2018, 283, 75–83. [Google Scholar] [CrossRef]
- Lali, F.V.; Hunt, A.E.; Turner, S.J.; Foxwell, B.M. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J Biol Chem 2000, 275, 7395–7402. [Google Scholar] [CrossRef]
- Gordon, G.M.; LaGier, A.J.; Ponchel, C.; Bauskar, A.; Itakura, T.; Jeong, S.; Patel, N.; Fini, M.E. A cell-based screening assay to identify pharmaceutical compounds that enhance the regenerative quality of corneal repair. Wound Repair Regen 2016, 24, 89–99. [Google Scholar] [CrossRef] [PubMed]
- del Peso, Ld.; González-Garcıa, M.; Page, C.; Herrera, R.; Nuñez, G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 1997, 278, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Cesca, T.G.; Faqueti, L.G.; Rocha, L.W.; Meira, N.A.; Meyre-Silva, C.; De Souza, M.M.; Quintão, N.L.; Silva, R.M.; Filho, V.C.; Bresolin, T.M. Antinociceptive, anti-inflammatory and wound healing features in animal models treated with a semisolid herbal medicine based on Aleurites moluccana L. Willd. Euforbiaceae standardized leaf extract: semisolid herbal. J Ethnopharmacol 2012, 143, 355–362. [Google Scholar] [CrossRef]
- Liu, S.; Uppal, H.; Demaria, M.; Desprez, P.Y.; Campisi, J.; Kapahi, P. Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci Rep 2015, 5, 17895. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.B.; Hall, A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005, 21, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.; Yang, H.S.; Hinds, P.W. Cellular senescence requires CDK5 repression of Rac1 activity. Mol Cell Biol 2004, 24, 2808–2819. [Google Scholar] [CrossRef]
- Kim, K.H.; Chen, C.C.; Monzon, R.I.; Lau, L.F. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol 2013, 33, 2078–2090. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Qi, B.; Park, Y.C.; Irani, K. Constitutive activation of rac1 results in mitochondrial oxidative stress and induces premature endothelial cell senescence. Arterioscler Thromb Vasc Biol 2003, 23, e1–e6. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Debidda, M.; Witte, D.; Zheng, Y. Cdc42 GTPase-activating protein deficiency promotes genomic instability and premature aging-like phenotypes. Proc Natl Acad Sci U S A 2007, 104, 1248–1253. [Google Scholar] [CrossRef]
- Korneev, K.V.; Atretkhany, K.-S.N.; Drutskaya, M.S.; Grivennikov, S.I.; Kuprash, D.V.; Nedospasov, S.A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [Google Scholar] [CrossRef]
- Van Rhee, F.; Wong, R.S.; Munshi, N.; Rossi, J.F.; Ke, X.Y.; Fosså, A.; Simpson, D.; Capra, M.; Liu, T.; Hsieh, R.K.; et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol 2014, 15, 966–974. [Google Scholar] [CrossRef]
- Chen, R.; Chen, B. Siltuximab (CNTO 328): a promising option for human malignancies. Drug Des Devel Ther 2015, 9, 3455–3458. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Teachey, D.T.; Pequignot, E.; Frey, N.; Porter, D.; Maude, S.L.; Grupp, S.A.; June, C.H.; Melenhorst, J.J.; Lacey, S.F. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods 2016, 434, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Keystone, E.; Tony, H.P.; Cantagrel, A.; Van Vollenhoven, R.; Sanchez, A.; Alecock, E.; Lee, J.; Kremer, J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor BioLogicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008, 67, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kawamoto, S.; Ohtani, N.; Hara, E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci 2017, 108, 563–569. [Google Scholar] [CrossRef]
- Lau, L.; Porciuncula, A.; Yu, A.; Iwakura, Y.; David, G. Uncoupling the senescence-associated secretory phenotype from cell cycle exit via interleukin-1 inactivation unveils its protumorigenic role. Mol Cell Biol 2019, 39, e00586-18. [Google Scholar] [CrossRef]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat Rev Rheumatol 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Colich, N.L.; Rosen, M.L.; Williams, E.S.; McLaughlin, K.A. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychol Bull 2020, 146, 721–764. [Google Scholar] [CrossRef] [PubMed]
- Moulahoum, H.; Ghorbanizamani, F.; Khiari, Z.; Toumi, M.; Benazzoug, Y.; Tok, K.; Timur, S.; Zihnioglu, F. Artemisia alleviates AGE-induced liver complications via MAPK and RAGE signaling pathways modulation: a combinatorial study. Mol Cell Biochem 2022, 477, 2345–2357. [Google Scholar] [CrossRef]
- Furrer, R.; Handschin, C. Drugs, clocks and exercise in ageing: hype and hope, fact and fiction. J Physiol 2023, 601, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting senescence for the treatment of cancer. Nat Rev Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Li, X.; Xia, Y.; Li, H.; Li, X.; Yang, Z.Y.; Wang, Z.; Xu, C.; Fang, T.; Zhou, D.; et al. Bepotastine sensitizes ovarian cancer to PARP inhibitors through suppressing NF-κB-triggered SASP in cancer-associated fibroblasts. Mol Cancer Ther 2023, 22, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Sofiadis, K.; Josipovic, N.; Nikolic, M.; Kargapolova, Y.; Übelmesser, N.; Varamogianni-Mamatsi, V.; Zirkel, A.; Papadionysiou, I.; Loughran, G.; Keane, J.; et al. HMGB1 coordinates SASP-related chromatin folding and RNA homeostasis on the path to senescence. Mol Syst Biol 2021, 17, e9760. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Anil Kumar, N.V.; Thakur, G. Developments in the anticancer activity of structurally modified curcumin: an up-to-date review. Eur J Med Chem 2019, 177, 76–104. [Google Scholar] [CrossRef]
- Zhao, R.C.; Stambler, I. The urgent need for international action for anti-aging and disease prevention. Aging Dis 2020, 11, 212–215. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




