1. Introduction
Natural Sleep, a phenomenon observed in all animals, particularly mammals, continues to be a scientific enigma. Extensive research has consistently unveiled distinct manifestations of sleep in vertebrate and invertebrate animals (Eban-Rothschild et al., 2018). In mammals, vigilance states are typically characterized through the use of electroencephalogram (EEG) and electromyogram (EMG) recordings, which measure global cortical and muscular activities, respectively. This classification delineates vigilance states into three main phases: wakefulness, non-rapid eye movement sleep (NREMS), and rapid eye movement sleep (REMS) (Weber and Dan, 2016).
The wakeful state displays heterogeneity characterized by unsynchronized EEG oscillations of low amplitude and mixed frequencies, along with variable muscle activity. NREMS is defined by high-amplitude, low-frequency delta oscillations (0.5–4 Hz) and spindles (bursts of 7–15 Hz oscillations) in the EEG, accompanied by reduced postural muscle tone. During REMS, the EEG predominantly exhibits theta (5-10 Hz) and gamma oscillations (25-80 Hz), while the axial posture muscles experience a complete loss of muscle tone (Eban-Rothschild et al., 2018) (
Figure 1).
The mouse EEG data were recorded at the Department of Anesthesiology & Intensive Care, School of Medicine, Technical University of Munich, under license: ROB-55.2-2532.Vet_02-19-121. The human EEG recordings for (C) and (D) are sourced from the Sleep-EDF Database v1.0.0 (Kemp et al., 2018).In contrast to humans, who typically experience 5 to 6 sleep cycles alternating between NREMS and REMS during monophasic sleep, rodents follow a polyphasic sleep pattern distributed across their circadian behavior (Sulaman et al., 2023). Understanding the neural mechanisms underpinning this behavior is of profound scientific interest and clinical significance, given that sleep plays a vital role in cognitive and physiological functions as well as overall health (Gupta et al., 2013; Kahn, 2023).
Early anatomists and neurologists, including Purkinje, expressed skepticism regarding the existence of specific neural mechanisms responsible for controlling wakefulness and sleep (Scammell et al., 2017). Nevertheless, pioneering work by von Economo in the early last century (ECONOMO, 1930) laid the foundation for our understanding of the neuronal mechanisms underlying sleep-wake regulation in mammals. Following the discovery of the ascending reticular activating system in 1949 (Moruzzi and Magoun, 1949), we have made significant strides in unraveling the neural circuits that support wakefulness. In contrast, elucidating the neural mechanisms responsible for generating sleep has proven to be more challenging. Recent studies utilizing techniques like cell- and region-specific pharmacological manipulations have led to transformative advancements in our understanding of sleep regulation. They have unveiled numerous brain systems that selectively modulate our arousal states. However, the specific neuronal populations responsible for initiating and maintaining NREMS or REMS, as well as their interconnections, remain largely speculative. The brain nuclei responsible for controlling sleep-wake states are typically heterogeneous, housing mixed neuronal populations that can maintain various vigilance states (Eban-Rothschild et al., 2018; Weber and Dan, 2016).
Enhancing our understanding of the brain mechanisms that regulate sleep-wake behavior holds promise for gaining new insights into the functions of sleep and advancing the development of more effective treatments for sleep disorders.
In this paper, we provide a concise overview of sleep-wake regulatory areas and explore the anatomy of the subiculum, along with its primary functions. By reviewing the anatomical connections between the subiculum and the regions involved in regulating sleep-wake behavior, we also address observed changes in the subiculum among individuals with sleep disorders. Additionally, we examine the state-dependent alterations in subicular function observed in sleep-deprived mice. These findings underscore the importance of investigating the interaction between the subiculum and sleep regulation.
2. Sleep-wake promoting areas
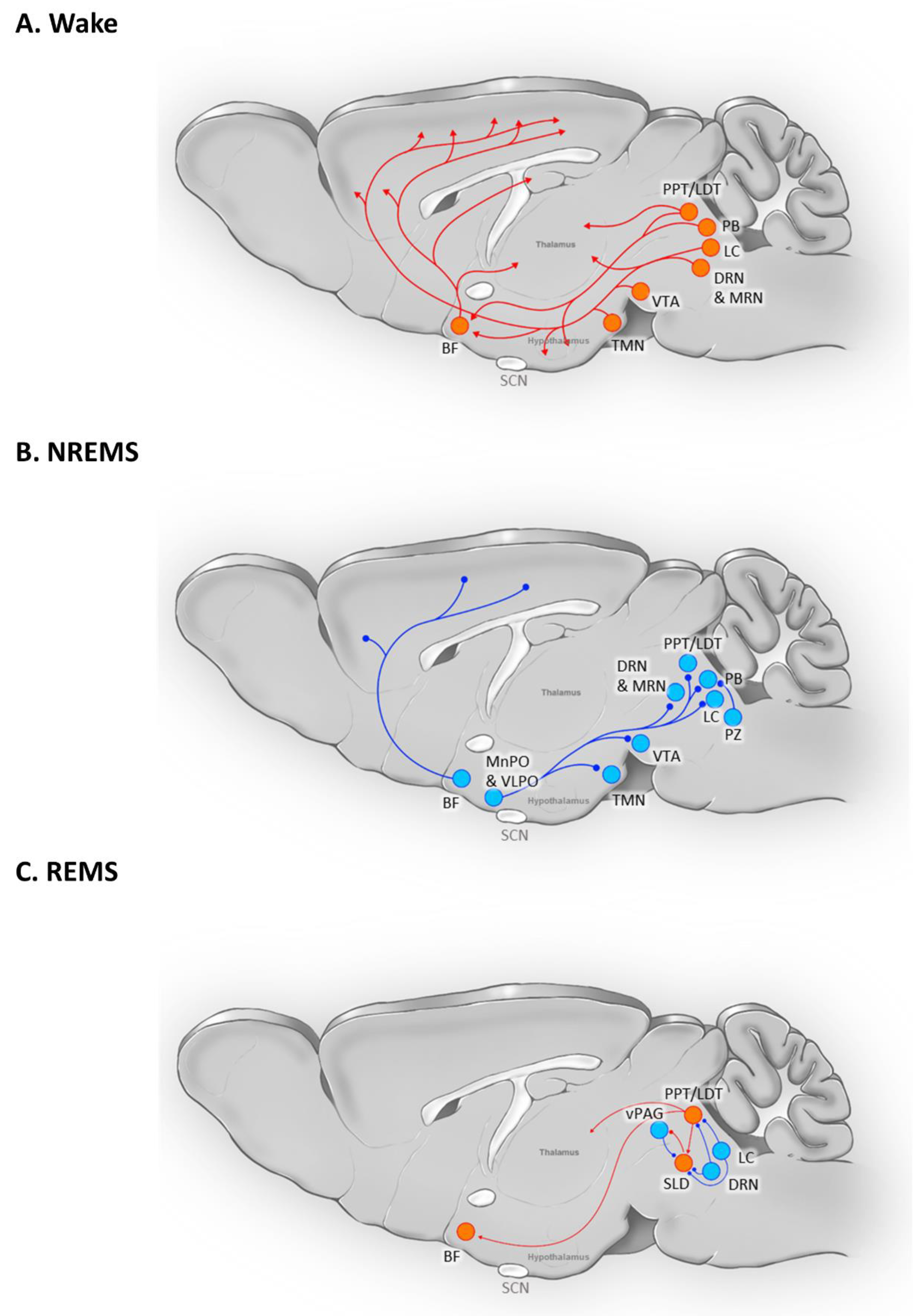
The regulation of the transition from wakefulness to sleep involves complex interactions between physiological conditions, sleep homeostasis, and intrinsic circadian fluctuations. Multiple brain regions play crucial roles in this process, with some areas serving dual functions in promoting sleep and maintaining wakefulness (
Figure 2).
BF: Basal Forebrain; DRN: Dorsal Raphe Nucleus; LC: Locus Coeruleus; LDT: Laterodorsal Tegmentum; MRN: Median Raphe Nucleus; PB: Parabrachial Nucleus; PPT: Pedunculopontine Nucleus; PZ: Parafacial Zone; SCN: Suprachiasmatic Nucleus; SLD: Sub-Laterodorsal (Tegmental) Nucleus; TMN: Tuberomammillary Nucleus; vPAG: Ventral Periaqueductal Gray; VTA: Ventral Tegmental Area
One such region is the lateral hypothalamus (LH), housing both sleep-active and wake-active neurons. Sleep-active neurons expressing melanin-concentrating hormone (MCH) in the LH are most active during REMS (Hassani et al., 2009; Kroeger et al., 2019; Varin et al., 2018). These neurons promote the initiation of REMS by inhibiting wake-promoting neurons in various brain areas, including the tuberomammillary nucleus (TMN), the locus coeruleus (LC), and the dorsal raphe nucleus (DRN) (Jego et al., 2013). The chronic activity of MCH neurons influences not only REMS but also NREMS (Konadhode et al., 2013). Additionally, hypocretin/orexin neurons in the LH play a crucial role in regulating the sleep-wake cycle (Latifi et al., 2018). Loss of these neurons leads to narcolepsy in various species, including humans (Thannickal et al., 2000) and rodents (Chemelli et al., 1999). Glutamatergic and GABAergic neurons in the LH region are crucial in inducing and maintaining wakefulness, and their inhibition enhances sleep (Herrera et al., 2016; Venner et al., 2016).
Von Economo's research in 1930 unveiled a correlation between damage to the preoptic hypothalamus area (POA) and insomnia in human patients (ECONOMO, 1930). The ventral lateral preoptic (VLPO) and median preoptic nucleus (MnPO) regions contain a dense population of neurons responsible for regulating sleep (Horner and Peever, 2017). Selective lesions in the VLPO significantly reduce NREMS (Lu et al., 2000). During sleep, GABAergic neurons in the VLPO and MnPO regions inhibit arousal regulatory systems, including the LH, posterior hypothalamus, DRN, LC, ventral periaqueductal gray matter (vPAG), and parabrachial nucleus (PB), thereby promoting sleep (Weber and Dan, 2016).
Adjacent to the POA, the basal forebrain (BF), is a critical region for both sleep and wakefulness (Takahashi et al., 2009). The majority of BF neurons are GABAergic, with a small fraction expressing acetylcholine or glutamate (Sulaman et al., 2023). Cholinergic neurons are active during wakefulness and REMS, inhibiting slow-wave delta oscillations (Han et al., 2014). BF GABAergic neurons exhibit mixed functions, with parvalbumin-expressing (PV) GABAergic neurons involved in rapid transitions from NREMS to wakefulness (McKenna et al., 2020), while somatostatin-expressing (SOM) GABAergic neurons promote NREMS (Xu et al., 2015). Glutamatergic neurons in the BF influence REMS by modulating theta rhythm (Xu et al., 2015).
The LC serves as a vital arousal-center, with its noradrenergic neurons promoting wakefulness (Liang et al., 2021). Their activity decreases during NREMS and ceases during REMS (Aston-Jones and Bloom, 1981; Hobson et al., 1975). Within the ventral tegmental area (VTA), a region containing numerous dopaminergic, glutamatergic, and GABAergic neurons, the dopaminergic neurons predominantly regulate sleep and wakefulness (Morales and Margolis, 2017). Optogenetic and chemogenetic stimulation of VTA dopaminergic and glutamatergic neurons induces wakefulness (Eban-Rothschild et al., 2016; Oishi et al., 2017), while inhibiting these neurons results in robust NREMS activation (Eban-Rothschild et al., 2016; Yu et al., 2019). GABAergic VTA neurons restrict wakefulness by inhibiting arousal-promoting VTA glutamatergic and/or dopaminergic neurons, as well as through projections to the LH (Yu et al., 2019).
The dorsal and median raphe nuclei (DRN and MRN) contribute to sleep regulation through serotonergic neurons (Sulaman et al., 2023). The firing pattern of DRN serotonergic neurons increases during wakefulness and decreases during REMS (Trulson and Jacobs, 1979). These serotonergic neurons promote relaxed wakefulness while inhibiting REMS (Jacobs and Fornal, 1993). Rasmussen et al. reported a group of presumed 5-HT neurons in the MRN with similar activity patterns to "classic" 5-HT neurons in the DRN (Rasmussen et al., 1984). Recent research in mice has shown that GABAergic neurons in the LH selectively suppress GABAergic neurons in the DRN, leading to increased activity in a significant portion of LH neurons, thereby promoting arousal (Gazea et al., 2021). MRN suppresses or blocks theta rhythm during NREMS, while silencing the MRN during REMS allows its expression (Vertes, 2010).
The brainstem pedunculopontine (PPT) and laterodorsal tegmental (LDT) nuclei house cholinergic neurons, maximally active during wakefulness and REMS, and GABAergic neurons, active solely during REMS (Boucetta et al., 2014). In the parafacial zone (PZ), GABAergic/glycinergic neurons regulate NREM sleep, with their stimulation leading to prolonged NREM sleep (Anaclet et al., 2014; Anaclet et al., 2018). The glutamatergic sublaterodorsal nucleus (SLD) is involved in muscle atony during REMS (Boissard et al., 2002).
3. Subiculum, and its interaction with sleep
The hippocampus, dentate gyrus (DG), and subiculum together constitute the hippocampal formation, which plays a pivotal role in functions such as learning, memory, and orientation (van Strien et al., 2009). Among these components, the subiculum stands out as the principal output region of the hippocampal formation. It serves not only as a relay for information but also as a unique processing region (Matsumoto et al., 2019).
3.1. Anatomy of subiculum
3.1.1. Structure and cell architecture
The subiculum is comprised of three distinct layers, arranged from outermost to innermost: the molecular layer, the pyramidal cell layer, and the polymorphic layer (O'Mara et al., 2001; O'Mara, 2005). Despite its adjacency to sector CA1, the subiculum exhibits distinct molecular profiles. Studies demonstrate a clear cytoarchitectural boundary between the subiculum and CA1, underscoring their separate molecular organizations. Some molecular biomarkers, such as fibronectin1 and SMI-32, are used to differentiate subiculum from CA1 (Lein et al., 2004) or subregions within the subicular complex, respectively (Ding, 2013). Traditionally, the subiculum was not extensively divided and was roughly segmented into proximal and distal subiculum, based on its proximity to CA1 (Fujise et al., 1995; Ishihara and Fukuda, 2016), or along the dorso-ventral axis into dorsal and ventral subiculum (Fei et al., 2021). Using single-cell RNA sequencing along the dorso-ventral axis, 27 distinct transcriptomic cell types were identified in subiculum (and prosubiculum) (Ding et al., 2020).
The predominant neuronal population in the subiculum consists of principal pyramidal neurons, which release glutamate as their neurotransmitter. These pyramidal neurons are regulated by various GABAergic interneurons. Most pyramidal cells in the subiculum have a primary apical dendrite that is innervated by the hippocampus through the molecular layer, and they also possess axonal collaterals that are entering the alveus (Harris et al., 2001). Varicosities and axonal extensions within the pyramidal cell layer and apical dendritic region suggest intrinsic connectivity, while the presence of these features on axons projecting to the presubiculum, entorhinal cortex (EC), or CA1 classifies them as projection cells (Harris et al., 2001). Pyramidal cells of the subiculum can be categorized into two main groups: bursting neurons and regular spiking neurons (Böhm et al., 2015).
Within the subiculum, GABAergic interneurons form intricate circuits, including feedforward, feedback, and disinhibitory connections. The three primary subclasses of subicular interneurons (INs) expressing PV (PV-INs), SOM (SOM-INs), or vasoactive intestinal peptide (VIP-INs) (Kepecs and Fishell, 2014), play potentially opposing roles in subicular circuits. PV-INs, referred to as fast-spiking INs, represent the most abundant population of GABAergic neurons in the subiculum and are primarily located within the pyramidal cell layer (Fei et al., 2021). The majority of SOM-INs in the subiculum are regular spiking neurons, including bistratified cells and oriens-lacunosum moleculare (O-LM) interneurons (Pelkey et al., 2017). O-LM-INs, primarily located in the polymorphic layer, co-express nicotinic acetylcholine receptor alpha2 subunits (Leão et al., 2012; Nichol et al., 2018) and play a pivotal role in the subicular feedback inhibitory circuit (Pelkey et al., 2017). VIP-INs, originating from the caudal ganglionic eminence (Miyoshi et al., 2015), exhibit firing patterns that can be irregular, bursting, or stuttering (Pelkey et al., 2017). These VIP-INs often co-express calretinin and are key participants in the subiculum's disinhibitory circuitry (Rahimi et al., 2023). A recent finding identified a new group of VIP-INs that co-express muscarinic receptor 2 (M2R) and project from CA1 to subiculum (Luo et al., 2019). Unlike GABAergic neurons of the hippocampus (Klausberger and Somogyi, 2008), VIP interneurons of the subiculum exhibit higher activity levels during quiet wakefulness (Luo et al., 2019). Although GABAergic interneurons constitute only 10% to 15% of the total neuronal population in the hippocampus (Bezaire and Soltesz, 2013), their extensive anatomical and physiological diversity enables them to exert significant regulatory control over nearly all aspects of cellular and circuit functions in the subiculum (Pelkey et al., 2017).
3.1.2. The primary afferent and efferent connections of the subiculum
The subiculum receives its primary input from two critical regions, namely the CA1 region and the EC (O'Mara, 2005; Witter, 2006). Notably, pyramidal neurons located in the proximal CA1, close to sector CA2, innervate the distal subiculum (adjacent to the presubiculum), while pyramidal neurons of the distal CA1 innervate the proximal portion of the subiculum close to sector CA1 (Matsumoto et al., 2019). Cells in layer III of the lateral and medial EC (LEC and MEC) mainly terminate in the stratum lacunosum-moleculare of area CA1 and the molecular layer of the subiculum (Witter et al., 2000).
While there is accumulating evidence indicating that the subiculum also sends backward projections to sector CA1, the major outputs from the subiculum are directed towards the EC, exhibiting a precise spatial organization. The proximal half of the dorsal subiculum innervates the LEC, whereas the distal part is connected to the MEC (Witter, 2006). Projections from the ventral hippocampus target both the dorsal and ventral MEC (Ohara et al., 2023). Additionally, the ventral subiculum is composed of multiple distinct neuronal populations that send parallel, long-range projections to various areas, including the prefrontal cortex, nucleus accumbens shell (Wee and MacAskill, 2020), amygdalohippocampal area, antero-dorsal thalamic nucleus, medial hypothalamus (Tang et al., 2016) and several brain regions involved in sleep regulation, which will be elaborated upon
Section 3.3.
3.2. The principal function of the subiculum
Lesioning the subiculum while minimizing damage to the adjacent hippocampal regions presents technical challenges. A pioneering study of Hagan and colleagues in 1992 was among the first to investigate the role of the subiculum in spatial navigation (Hagan et al., 1992). Through bilateral ibotenic acid injections in rats, they induced lesions encompassing the EC and subiculum. These rats exhibited impaired exploration, as evidenced by reduced motility and rearing in an open field arena. This observation has been subsequently confirmed by several other studies. The subiculum receives inputs from grid cells located in the MEC and place cells from the CA1 area of the hippocampus (Brotons-Mas et al., 2017). Dorsal subiculum neurons demonstrate a noise-resistant representation of place, speed, and trajectory, often as accurate as or more accurate than hippocampal CA1 neurons (Kitanishi et al., 2021). Additionally, it has been found that the activity of the ventral subiculum-ventral striatum pathway after learning is crucial for spatial memory consolidation and learning-induced plasticity (Torromino et al., 2019).
In addition to its role in spatial memory consolidation, which is dependent on specific tasks and training (Contreras et al., 2018), the ventral subiculum has been extensively studied for its involvement in contextual memory, reward and motivation processing, emotional regulation, and the stress response. For instance, the ventral subiculum has been implicated in the context-dependent renewal of extinguished Pavlovian conditioned responding to food cues (Anderson and Petrovich, 2017). Electrical stimulation of this region has been shown to reinvigorate behavior after the failure to achieve a goal in a food reward paradigm (Lindenbach et al., 2019). Ventral subicular lesion have been found to impair pro-social empathy-like behavior in adult Wistar rats (Subhadeep et al., 2022). Optogenetic activation of the circuit from the ventral subiculum to the ventral lateral septum triggers delayed but robust excessive grooming patterns, closely resembling those evoked by emotional stress (Mu et al., 2020). A direct circuit from the ventral subiculum to the anterior hypothalamic nucleus has been identified as essential for anxiety-like behavioral avoidance (Yan et al., 2022).
It is important to note that poor sleep quality can directly or indirectly affect all these primary functions of the subiculum. Recent research has indicated that sleep facilitates spatial memory (Simon et al., 2022) and poor sleep quality can impact spatial orientation (Valera et al., 2016), suggesting a potential alteration in subicular function. Insufficient sleep has been shown to affect how individuals learn from reward or punishment (Gerhardsson et al., 2021), and REMS deprivation can alter reward memory (Kaveh Shahveisi 2022). Interactions between insomnia, sleep duration and emotional processes have also been observed (Baglioni et al., 2023), and several studies in humans have linked fear and extinction recall/retention to both REMS and SWS (Bottary et al., 2023). However, it is crucial to recognize that various brain regions participate in the execution of these cognitive functions. Consequently, further research is necessary to explore whether the literature suggests anatomical and/or functional connections between the subiculum and sleep-wake behavior.
3.3. The anatomical connections between the subiculum and sleep-wake regulating areas
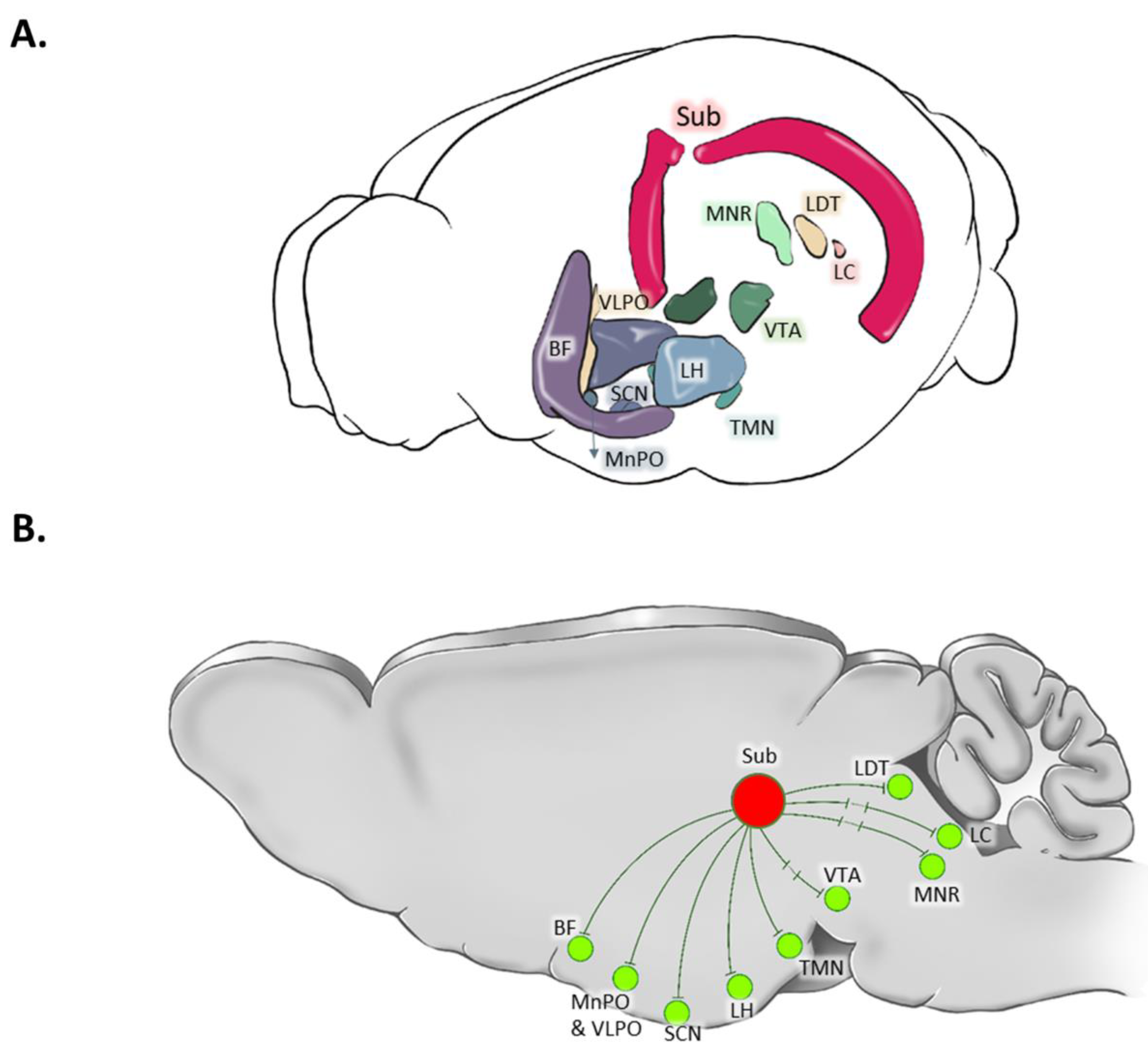
The anatomical connections of the subiculum with various brain areas involved in sleep-wake regulation have been comprehensively explored, as summarized in
Table 1 and illustrated in
Figure 3. These connections provide insights into the potential involvement of the subiculum in sleep-wake regulation.
1. MnPO: The temporal two-thirds of the subiculum are connected to the MnPO, as confirmed by anterograde Phaseolus vulgaris leucoagglutinin (PHA-L) and retrograde cholera toxin B subunit (CTB) injections (Kishi et al., 2000). Additionally, the ventral subiculum’s connection to the MnPO was identified using wheat germ agglutinin (Chiba and Murata, 1985) and pseudorabies virus injections (Westerhaus and Loewy, 1999). Furthermore, employing retrograde and anterograde axonal transport techniques (utilizing true blue, SITS, or wheat germ agglutinin), the MnPO displayed connections with the ventral subiculum (Simerly and Swanson, 1986).
2. VLPO: A direct connection between the VLPO and the ventral subiculum was described using retrograde tracer CTB subunit (Chou et al., 2002).
3. Hypothalamus: The ventral subiculum was found to have connections with various hypothalamic regions, including the anterior, tuberal, and mammillary regions, established through anterograde PHA-L and retrograde CTB subunit tracings (Kishi et al., 2000).
4. LH: Connections between the LH and the ventral subiculum were indicated by retrograde labelling through CTB-488 microinjections (Mu et al., 2020) , rabies virus injections (Shien Wee and MacAskill, 2021), and anterograde PHA-L tract-tracing (Köhler, 1990).
5. BF: Anterograde PHA-L tract-tracing provided evidence of connections between the subiculum and the BF (Yu et al., 2023).
6. VTA: The VTA exhibited disynaptic connections to the ventral subiculum, as demonstrated by anterograde PHA-L and retrograde CTB tracing techniques (Glangetas et al., 2015) and anterograde transsynaptic tracing using adeno-associated virus serotype 1 (Umaba et al., 2021).
7. LC: While some authors found a monosynaptic connection between the LC and the subiculum using uptake labeling and radioautography (Oleskevich et al., 1989), along with retrograde transport of horseradish peroxidase (Loy et al., 1980; Segal and Landis, 1974), others claimed second-order synapses (from the ventral subiculum, using herpes simplex virus 1 tracing technique) (Tang et al., 2016).
8. MRN: The medial part of the raphe nucleus was found to be connected to the ventral subiculum, as identified through Herpes simplex virus 1 tracing (Tang et al., 2016).
9. LDT: Using the Herpes simplex virus 1 tracing technique, connections between the LDT and the ventral subiculum were identified (Tang et al., 2016).
10. SCN: The SCN displayed connections with the ventral subiculum, as revealed through retrograde CTB tracing (Krout et al., 2002).
These findings provide insight into the intricate connection of the subiculum, particularly ventral subiculum, to sleep-wake promoting areas, hinting at its potential involvement in regulating sleep and wakefulness.
3.4. Clinical studies
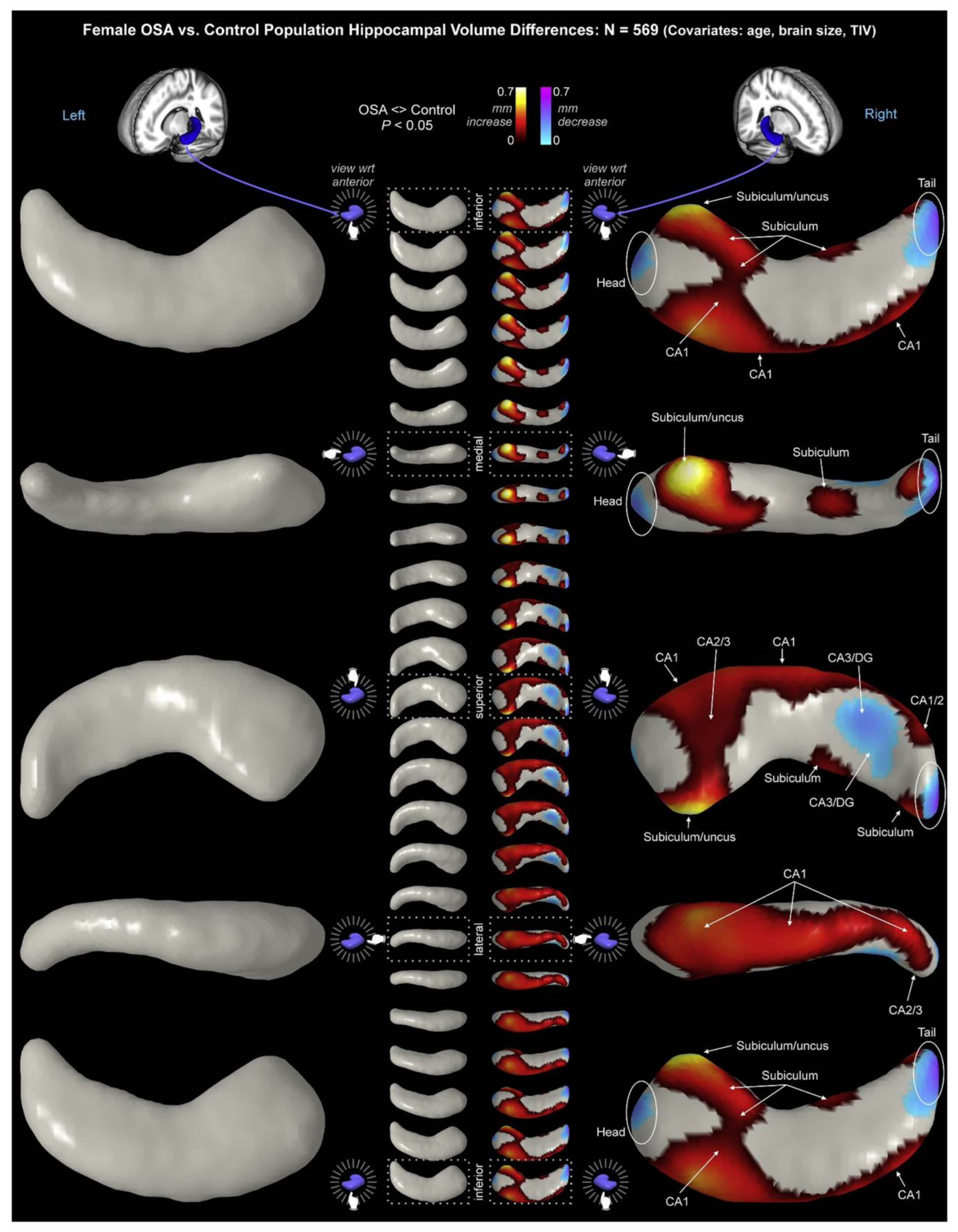
Structural changes in the subiculum have been observed in patients with sleep disorders, specifically Obstructive Sleep Apnea (OSA). Macey et al. collected high-resolution magnetic resonance imaging (MRI) images from 66 newly-diagnosed, untreated OSA patients (mean age ± SD: 46.3 ± 8.8 years; 50 males) and 59 healthy age-matched control participants (46.8 ± 9.0 years; 38 males) (Macey et al., 2018). Male patients exhibited higher bilateral volume throughout CA1 and subiculum, with greater right-hemispheric increases and lower bilateral volumes in the mid- and posterior-CA3/DG. Female patients showed right-hemispheric differences with increased volumes in CA1 and the subiculum/uncus, and decreased volumes in the posterior CA3/DG (
Figure 4).
Lee et al. calculated cortical thickness and hippocampal subfield volumes from images of 45 controls (age 15.43 ± 1.73 years, 21 males) and 53 adolescent children with OSA (age 15.26 ± 1.63 years, 32 males) to investigate the association of childhood OSA with alterations in cortical structure and hippocampal subfield structural changes (Lee et al., 2023). They observed that in adolescents with OSA, only the volume of the right-hemispheric subiculum-head area of the hippocampus was larger compared to the control group. This enlargement was positively correlated with both the apnea-hypopnea index (AHI) and the arousal index.
Recently, the association of sleep-disorder-related breathing impairments and medial temporal lobe atrophy in cognitively unimpaired amyloid-positive older adults was assessed (André et al., 2023). Data were collected between 2016 and 2020 as part of the Age-Well randomized controlled trial conducted under the Medit-Ageing European project. The AHI interacted with the volumes of the EC, subiculum, CA1, and DG. This interaction revealed that in individuals with amyloid-positive status, higher severity of sleep apnea was associated with reduced volumes of sub-regions in the medial temporal lobe. Notably, this relationship did not hold true for individuals who were amyloid-negative.
Subicular volume changes were also reported in connection with other sleep impairments. To assess the relationship between sleep duration, sleep impairments, perceived stress, and hippocampal subfield volumes in later life, adults (aged 68.8 ± 7.3; 46% males) from the Irish Longitudinal Study on Ageing completed a questionnaire along with multiparametric brain MRI (Looze et al., 2022). No cross-sectional and follow-up associations between sleep and total hippocampal volume, and between stress and total hippocampal volume, were found. In contrast, long sleep duration (≥9-10 hours per night) was linked to smaller volumes of the molecular layer, hippocampal tail, presubiculum, and subiculum. On the other hand, the combination of short sleep duration (≤6 hours) and higher perceived stress was associated with smaller volumes of CA1, molecular layer, subiculum, and hippocampal tail. Sleep impairments, independently and in conjunction with higher stress, together with the severity of sleep impairments were associated with smaller volumes of these same subfields. Similarly, Liu et al. reported that poor sleep was associated with smaller hippocampal subfields in healthy elderly individuals (Liu et al., 2021). Sleep quality was self-assessed using the Pittsburgh Sleep Quality Index (PSQI), and hippocampal volumes were measured from MRI data. A total of 67 cognitively normal elderly individuals aged 60-83 years were classified into 30 normal sleepers with a PSQI <5 and 37 poor sleepers with a PSQI ≥5. Compared to normal sleepers, poor sleepers exhibited significantly lower normalized volumes in the left CA1, DG, and subiculum, and the global PSQI was negatively associated with the normalized volumes of these regions (only in the left hemisphere).
Only one study assessed the changes in subiculum in connection to sleep in healthy individuals (Andrade et al., 2011). Young healthy subjects underwent simultaneous EEG and functional MRI measurements under resting conditions during the descent to sleep stage N3. The hippocampal function was integrated to variable strength in the default mode network in wakefulness and sleep stage N1 but not in slow wave sleep (SWS). While the cornu ammonis exhibited the strongest functional connectivity with the default mode network during wakefulness, the subiculum dominated hippocampal functional connectivity to frontal brain regions during sleep stage N2.
3.5. Animal studies
The pioneering study by Hagan et al. in 1992 explored the role of the subiculum in spatial navigation and also reported changes in sleep behavior in rats (Hagan et al., 1992). Bilateral ibotenic acid injections induced lesions in the EC/subiculum, resulting in increased diurnal SWS and spindle incidence, along with decreased REMS. Additionally, there was a reduction in theta power during REMS and quiet waking, but not during SWS. Subsequent studies did not investigate the potential role of the subiculum/EC in sleep regulation. However, a systematic approach involving immediate-early gene mapping, laser microdissection, cDNA microarrays, and in situ hybridization revealed significant molecular changes in the subiculum after 6 hours of sleep deprivation (Thompson et al., 2010), highlighting neuronal interactions between the subiculum and sleep. Additionally, observations indicated that a substantial set of genes and proteins in the ventral hippocampus (containing the ventral subiculum) exhibited circadian oscillations in healthy mice, which could influence various circadian rhythms in the brain (Debski et al., 2020). Despite the limited exploration of the direct relationship between the subiculum and sleep-wake behavior, the role of the subiculum in generating sleep oscillations has been well discussed.
Ibotenic acid lesioning of the ventral subiculum increased the absolute theta power in the CA1 area, with no noticeable change in its relative power (Laxmi et al., 2000). Conversely, this lesioning led to a decrease in both the absolute and relative power of EC theta power, indicating that the subicular output may play a modulatory role in the synchronous neuronal activity of EC and CA1-pyramidal cells during REMS. Bandarabadi et al. explored the dynamics of theta-gamma band interactions, utilizing multiple frequency and temporal scales during simultaneous recordings from hippocampal CA3, CA1, subiculum, and parietal cortex in freely moving mice (Bandarabadi et al., 2019). Interestingly, they found that coupling during REMS was significantly stronger than during active wake within the subiculum and parietal cortex, but no such differences were observed within CA3 and CA1. The theta power exhibited no significant changes across REMS, except within the subiculum with notable alterations. Additionally, the theta phase significantly modulated the ultrahigh gamma band (160-250 Hz) in pyramidal cell layers of CA3 and the subiculum exclusively. It was suggested that the role of the subiculum in theta- and gamma oscillations is modulated by cholinergic inputs to the ventral subiculum, as the elimination of these inputs significantly reduced subicular theta- and enhanced gamma activity during active wake and REMS states (Rastogi et al., 2014). Intriguingly, an autoradiographic study revealed that after 96 hours of REMS deprivation, a decrease in muscarinic receptor binding in the EC and subiculum could be recorded, but not in other parts of the hippocampal formation (Nunes et al., 1994).
Recent work by Raquet et al. demonstrated that neonatal exposure to the novel sedative/hypnotic drug, 3β-OH, resulted in reduced subicular delta- and sigma band oscillations during NREMS (Fine-Raquet et al., 2023). In contrast, a previous study by the same authors found that neonatal exposure to the common anesthetic agent ketamine increased subicular gamma band oscillations during NREMS and significantly suppressed subicular long-term potentiation in adolescent rats (Manzella et al., 2020). These findings suggest that exposure to different sedative/hypnotic agents during a critical period of brain development may induce distinct functional changes in subiculum circuitry that persist into adolescence. Recently, through anatomically restricted inactivation of VIP-INs in the ventral subiculum of epileptic mice, we observed a significant shift in the circadian rhythm of seizures (Rahimi et al., 2023), implying a prominent interaction between the ventral subiculum and circadian rhythm. However, the epileptic brain exhibits distinct functional connectivity compared to a non-epileptic one (Morgan et al., 2015); therefore, in-depth studies in normal behaving animals are essential to reveal the complexities and mechanisms underlying this relationship.
4. Conclusions and perspectives
The subiculum presents an enticing area for future research endeavors. Investigations on the precise neurophysiological mechanisms governing the subiculum's interactions with sleep-regulatory regions, as well as the molecular and cellular processes occurring within the subiculum during sleep-wake transitions, hold the promise of deeper insights into sleep neurobiology. In addition, pharmacological and neuromodulatory interventions targeting this region may offer innovative approaches to ameliorate sleep disorders and enhance overall sleep quality.
In conclusion, the subiculum might emerge as a pivotal, yet understudied player in the neural orchestration of sleep-wake behavior. Its complexity and clinical implications beckon for continued scientific inquiry. As we advance our understanding of this brain region, we may gain a more profound understanding of the fundamental processes governing sleep.
References
- Anaclet, C., Ferrari, L., Arrigoni, E. et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nature neuroscience, 2014, 17: 1217-1224. [CrossRef]
- Anaclet, C., Griffith, K. and Fuller, P. M. Activation of the GABAergic Parafacial Zone Maintains Sleep and Counteracts the Wake-Promoting Action of the Psychostimulants Armodafinil and Caffeine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 2018, 43: 415-425. [CrossRef]
- Anderson, L. C. and Petrovich, G. D. Sex specific recruitment of a medial prefrontal cortex-hippocampal-thalamic system during context-dependent renewal of responding to food cues in rats. Neurobiology of learning and memory, 2017, 139: 11-21. [CrossRef]
- Andrade, K. C., Spoormaker, V. I., Dresler, M. et al. Sleep spindles and hippocampal functional connectivity in human NREM sleep. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2011, 31: 10331-10339.
- André, C., Kuhn, E., Rehel, S. et al. Association of Sleep-Disordered Breathing and Medial Temporal Lobe Atrophy in Cognitively Unimpaired Amyloid-Positive Older Adults. Neurology, 2023. [CrossRef]
- Aston-Jones, G. and Bloom, F. E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. The Journal of neuroscience : the official journal of the Society for Neuroscience, 1981, 1: 876-886. [CrossRef]
- Baglioni, C., Johann, A. F., Benz, F. et al. Interactions between insomnia, sleep duration and emotional processes: An ecological momentary assessment of longitudinal influences combining self-report and physiological measures. Journal of sleep research, 2023: e14001.
- Bandarabadi, M., Boyce, R., Gutierrez Herrera, C. et al. Dynamic modulation of theta–gamma coupling during rapid eye movement sleep. Sleep, 2019, 42. [CrossRef]
- Bezaire, M. J. and Soltesz, I. Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus, 2013, 23: 751-785. [CrossRef]
- Böhm, C., Peng, Y., Maier, N. et al. Functional Diversity of Subicular Principal Cells during Hippocampal Ripples. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2015, 35: 13608-13618.
- Boissard, R., Gervasoni, D., Schmidt, M. H., Barbagli, B., Fort, P. and Luppi, P. H. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. The European journal of neuroscience, 2002, 16: 1959-1973. [CrossRef]
- Bottary, R. Bottary, R., Straus, L. D. and Pace-Schott, E. F. The Impact of Sleep on Fear Extinction. Current topics in behavioral neurosciences, 2023.
- Boucetta, S., Cissé, Y., Mainville, L., Morales, M. and Jones, B. E. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2014, 34: 4708-4727.
- Brotons-Mas, J. R., Schaffelhofer, S., Guger, C., O'Mara, S. M. and Sanchez-Vives, M. V. Heterogeneous spatial representation by different subpopulations of neurons in the subiculum. Neuroscience, 2017, 343: 174-189. [CrossRef]
- Chemelli, R. M., Willie, J. T., Sinton, C. M. et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell, 1999, 98: 437-451. [CrossRef]
- Chiba, T. and Murata, Y. Afferent and efferent connections of the medial preoptic area in the rat: A WGA-HRP study. Brain Research Bulletin, 1985, 14: 261-272. [CrossRef]
- Chou, T. C., Bjorkum, A. A., Gaus, S. E., Lu, J., Scammell, T. E. and Saper, C. B. Afferents to the Ventrolateral Preoptic Nucleus. The Journal of Neuroscience, 2002, 22: 977-990. [CrossRef]
- Contreras, M., Pelc, T., Llofriu, M., Weitzenfeld, A. and Fellous, J.-M. The ventral hippocampus is involved in multi-goal obstacle-rich spatial navigation. Hippocampus, 2018, 28: 853-866. [CrossRef]
- Debski, K. J., Ceglia, N., Ghestem, A. et al. The circadian dynamics of the hippocampal transcriptome and proteome is altered in experimental temporal lobe epilepsy. Science advances, 2020, 6. [CrossRef]
- Ding, S. L. Comparative anatomy of the prosubiculum, subiculum, presubiculum, postsubiculum, and parasubiculum in human, monkey, and rodent. The Journal of comparative neurology, 2013, 521: 4145-4162. [CrossRef]
- Ding, S. L., Yao, Z., Hirokawa, K. E. et al. Distinct Transcriptomic Cell Types and Neural Circuits of the Subiculum and Prosubiculum along the Dorsal-Ventral Axis. Cell reports, 2020, 31: 107648. [CrossRef]
- Eban-Rothschild, A., Appelbaum, L. and Lecea, L. de. Neuronal Mechanisms for Sleep/Wake Regulation and Modulatory Drive. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 2018, 43: 937-952.
- Eban-Rothschild, A., Rothschild, G., Giardino, W. J., Jones, J. R. and Lecea, L. de. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nature neuroscience, 2016, 19: 1356-1366. [CrossRef]
- ECONOMO, C. V. SLEEP AS A PROBLEM OF LOCALIZATION. The Journal of Nervous and Mental Disease, 1930, 71: 249-259.
- Fei, F., Wang, X., Wang, Y. and Chen, Z. Dissecting the role of subiculum in epilepsy: Research update and translational potential. Progress in neurobiology, 2021, 201: 102029. [CrossRef]
- Fine-Raquet, B., Manzella, F. M., Joksimovic, S. M. et al. Neonatal exposure to a neuroactive steroid alters low-frequency oscillations in the subiculum. Experimental biology and medicine (Maywood, N.J.), 2023, 248: 578-587. [CrossRef]
- Fujise, N., Hunziker, W., Heizmann, C. W. and Kosaka, T. Distribution of the calcium binding proteins, calbindin D-28K and parvalbumin, in the subicular complex of the adult mouse. Neuroscience research, 1995, 22: 89-107. [CrossRef]
- Gazea, M., Furdan, S., Sere, P. et al. Reciprocal Lateral Hypothalamic and Raphe GABAergic Projections Promote Wakefulness. The Journal of Neuroscience, 2021, 41: 4840-4849. [CrossRef]
- Gerhardsson, A., Porada, D. K., Lundström, J. N., Axelsson, J. and Schwarz, J. Does insufficient sleep affect how you learn from reward or punishment? Reinforcement learning after 2 nights of sleep restriction. Journal of sleep research, 2021, 30: e13236.
- Glangetas, C., Fois, G. R., Jalabert, M. et al. Ventral Subiculum Stimulation Promotes Persistent Hyperactivity of Dopamine Neurons and Facilitates Behavioral Effects of Cocaine. Cell Reports, 2015, 13: 2287-2296. [CrossRef]
- Gupta, R., Lahan, V. and Goel, D. Prevalence of restless leg syndrome in subjects with depressive disorder. Indian journal of psychiatry, 2013, 55: 70-73. [CrossRef]
- Hagan, J. J., Verheijck, E. E., Spigt, M. H. and Ruigt, G. S. F. Behavioural and electrophysiological studies of entorhinal cortex lesions in the rat. Physiology & Behavior, 1992, 51: 255-266. [CrossRef]
- Han, Y., Shi, Y. F., Xi, W. et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Current biology : CB, 2014, 24: 693-698. [CrossRef]
- Harris, E., Witter, M. P., Weinstein, G. and Stewart, M. Intrinsic connectivity of the rat subiculum: I. Dendritic morphology and patterns of axonal arborization by pyramidal neurons. The Journal of comparative neurology, 2001, 435: 490-505. [CrossRef]
- Hassani, O. K., Lee, M. G. and Jones, B. E. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106: 2418-2422. [CrossRef]
- Herrera, C. G., Cadavieco, M. C., Jego, S., Ponomarenko, A., Korotkova, T. and Adamantidis, A. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nature neuroscience, 2016, 19: 290-298. [CrossRef]
- Hobson, J. A., McCarley, R. W. and Wyzinski, P. W. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science (New York, N.Y.), 1975, 189: 55-58. [CrossRef]
- Horner, R. L. and Peever, J. H. Brain Circuitry Controlling Sleep and Wakefulness. Continuum (Minneapolis, Minn.), 2017, 23: 955-972. [CrossRef]
- Ishihara, Y. and Fukuda, T. Immunohistochemical investigation of the internal structure of the mouse subiculum. Neuroscience, 2016, 337: 242-266. [CrossRef]
- Jacobs, B. L. and Fornal, C. A. 5-HT and motor control: a hypothesis. Trends in Neurosciences, 1993, 16: 346-352. [CrossRef]
- Jego, S., Glasgow, S. D., Herrera, C. G. et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nature neuroscience, 2013, 16: 1637-1643. [CrossRef]
- Kahn, M. Insomnia in Infancy, Childhood, and Adolescence. Sleep medicine clinics, 2023, 18: 135-145.
- Kemp, B. Kemp, B., Aeilko Zwinderman, Tuk, B., Kamphuisen, H. and Oberyé, J. (2018). The Sleep-EDF Database [Expanded].
- Kepecs, A. and Fishell, G. Interneuron cell types are fit to function. Nature, 2014, 505: 318-326.
- Kishi, T., Tsumori, T., Ono, K., Yokota, S., Ishino, H. and Yasui, Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. The Journal of comparative neurology, 2000, 419: 205-222. [CrossRef]
- Kitanishi, T., Umaba, R. and Mizuseki, K. Robust information routing by dorsal subiculum neurons. Science advances, 2021, 7. [CrossRef]
- Klausberger, T. and Somogyi, P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science (New York, N.Y.), 2008, 321: 53-57. [CrossRef]
- Köhler, C. Subicular projections to the hypothalamus and brainstem: some novel aspects revealed in the rat by the anterograde Phaseolus vulgaris leukoagglutinin (PHA-L) tracing method. Progress in brain research, 1990, 83: 59-69.
- Konadhode, R. R., Pelluru, D., Blanco-Centurion, C. et al. Optogenetic stimulation of MCH neurons increases sleep. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2013, 33: 10257-10263.
- Kroeger, D., Bandaru, S. S., Madara, J. C. and Vetrivelan, R. Ventrolateral periaqueductal gray mediates rapid eye movement sleep regulation by melanin-concentrating hormone neurons. Neuroscience, 2019, 406: 314-324. [CrossRef]
- Krout, K. E., Kawano, J., Mettenleiter, T. C. and Loewy, A. D. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience, 2002, 110: 73-92. [CrossRef]
- Latifi, B., Adamantidis, A., Bassetti, C. and Schmidt, M. H. Sleep-Wake Cycling and Energy Conservation: Role of Hypocretin and the Lateral Hypothalamus in Dynamic State-Dependent Resource Optimization. Frontiers in neurology, 2018, 9: 790. [CrossRef]
- Laxmi, T. R., Meti, B. L. and Bindu, P. N. Ventral subicular lesion alters rhythmical slow wave activity (θ) of CA1 area of hippocampus and entorhinal cortex. Brain Research, 2000, 869: 236-240. [CrossRef]
- Leão, R. N., Mikulovic, S., Leão, K. E. et al. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nature neuroscience, 2012, 15: 1524-1530. [CrossRef]
- Lee, M.-H., Sin, S., Lee, S., Wagshul, M. E., Zimmerman, M. E. and Arens, R. Cortical thickness and hippocampal volume in adolescent children with obstructive sleep apnea. Sleep, 2023, 46. [CrossRef]
- Lein, E. S., Zhao, X. and Gage, F. H. Defining a molecular atlas of the hippocampus using DNA microarrays and high-throughput in situ hybridization. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2004, 24: 3879-3889. [CrossRef]
- Liang, Y., Shi, W., Xiang, A., Hu, D., Wang, L. and Zhang, L. The NAergic locus coeruleus-ventrolateral preoptic area neural circuit mediates rapid arousal from sleep. Current biology : CB, 2021, 31: 3729-3742.e5. [CrossRef]
- Lindenbach, D., Seamans, J. K. and Phillips, A. G. Activation of the ventral subiculum reinvigorates behavior after failure to achieve a goal: Implications for dopaminergic modulation of motivational processes. Behavioural brain research, 2019, 356: 266-270. [CrossRef]
- Liu, C., Lee, S. H., Hernandez-Cardenache, R., Loewenstein, D., Kather, J. and Alperin, N. Poor sleep is associated with small hippocampal subfields in cognitively normal elderly individuals. Journal of sleep research, 2021, 30: e13362. [CrossRef]
- Looze, C. de, Feeney, J. C., Scarlett, S. et al. Sleep duration, sleep problems, and perceived stress are associated with hippocampal subfield volumes in later life: findings from The Irish Longitudinal Study on Ageing. Sleep, 2022, 45. [CrossRef]
- Loy, R., Koziell, D. A., Lindsey, J. D. and Moore, R. Y. Noradrenergic innervation of the adult rat hippocampal formation. Journal of Comparative Neurology, 1980, 189: 699-710. [CrossRef]
- Lu, J., Greco, M. A., Shiromani, P. and Saper, C. B. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2000, 20: 3830-3842.
- Luo, X., Muñoz-Pino, E., Francavilla, R., Vallée, M., Droit, A. and Topolnik, L. Transcriptomic profile of the subiculum-projecting VIP GABAergic neurons in the mouse CA1 hippocampus. Brain structure & function, 2019, 224: 2269-2280. [CrossRef]
- Macey, P. M., Prasad, J. P., Ogren, J. A. et al. Sex-specific hippocampus volume changes in obstructive sleep apnea. NeuroImage. Clinical, 2018, 20: 305-317. [CrossRef]
- Manzella, F. M., Joksimovic, S. M., Orfila, J. E. et al. Neonatal Ketamine Alters High-Frequency Oscillations and Synaptic Plasticity in the Subiculum But Does not Affect Sleep Macrostructure in Adolescent Rats. Frontiers in systems neuroscience, 2020, 14: 26. [CrossRef]
- Matsumoto, N., Kitanishi, T. and Mizuseki, K. The subiculum: Unique hippocampal hub and more. Neuroscience research, 2019, 143: 1-12. [CrossRef]
- McKenna, J. T., Thankachan, S., Uygun, D. S. et al. Basal Forebrain Parvalbumin Neurons Mediate Arousals from Sleep Induced by Hypercarbia or Auditory Stimuli. Current biology : CB, 2020, 30: 2379-2385.e4.
- Miyoshi, G., Young, A., Petros, T. et al. Prox1 Regulates the Subtype-Specific Development of Caudal Ganglionic Eminence-Derived GABAergic Cortical Interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2015, 35: 12869-12889. [CrossRef]
- Morales, M. and Margolis, E. B. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nature reviews. Neuroscience, 2017, 18: 73-85. [CrossRef]
- Morgan, V. L., Abou-Khalil, B. and Rogers, B. P. Evolution of functional connectivity of brain networks and their dynamic interaction in temporal lobe epilepsy. Brain connectivity, 2015, 5: 35-44. [CrossRef]
- Moruzzi, G. and Magoun, H. W. Brain stem reticular formation and activation of the EEG. Electroencephalography and clinical neurophysiology, 1949, 1: 455-473. [CrossRef]
- Mu, M.-D., Geng, H.-Y., Rong, K.-L. et al. A limbic circuitry involved in emotional stress-induced grooming. Nature communications, 2020, 11: 2261. [CrossRef]
- Nichol, H., Amilhon, B., Manseau, F., Badrinarayanan, S. and Williams, S. Electrophysiological and Morphological Characterization of Chrna2 Cells in the Subiculum and CA1 of the Hippocampus: An Optogenetic Investigation. Frontiers in cellular neuroscience, 2018, 12: 32. [CrossRef]
- Nunes, G. P., Tufik, S. and Nobrega, J. N. Decreased muscarinic receptor binding in rat brain after paradoxical sleep deprivation: an autoradiographic study. Brain Research, 1994, 645: 247-252. [CrossRef]
- Ohara, S., Rannap, M., Tsutsui, K.-I., Draguhn, A., Egorov, A. V. and Witter, M. P. Hippocampal-medial entorhinal circuit is differently organized along the dorsoventral axis in rodents. Cell Reports, 2023, 42: 112001. [CrossRef]
- Oishi, Y., Suzuki, Y., Takahashi, K. et al. Activation of ventral tegmental area dopamine neurons produces wakefulness through dopamine D(2)-like receptors in mice. Brain structure & function, 2017, 222: 2907-2915. [CrossRef]
- Oleskevich, S., Descarries, L. and Lacaille, J. C. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. The Journal of Neuroscience, 1989, 9: 3803-3815. [CrossRef]
- O'Mara, S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. Journal of anatomy, 2005, 207: 271-282. [CrossRef]
- O'Mara, S. M., Commins, S., Anderson, M. and Gigg, J. The subiculum: a review of form, physiology and function. Progress in neurobiology, 2001, 64: 129-155. [CrossRef]
- Pelkey, K. A., Chittajallu, R., Craig, M. T., Tricoire, L., Wester, J. C. and McBain, C. J. Hippocampal GABAergic Inhibitory Interneurons. Physiological reviews, 2017, 97: 1619-1747.
- Rahimi, S., Salami, P., Matulewicz, P. et al. The Impact of subicular VIP-expressing interneurons on seizure dynamics in temporal lobe epilepsy: Insights from preclinical models. bioRxiv, 2023: 2023.05.30.542857.
- Rasmussen, K., Heym, J. and Jacobs, B. L. Activity of serotonin-containing neurons in nucleus centralis superior of freely moving cats. Experimental neurology, 1984, 83: 302-317. [CrossRef]
- Rastogi, S., Unni, S., Sharma, S., Laxmi, T. R. and Kutty, B. M. Cholinergic immunotoxin 192 IgG-SAPORIN alters subicular theta–gamma activity and impairs spatial learning in rats. Neurobiology of Learning and Memory, 2014, 114: 117-126.
- Scammell, T. E., Arrigoni, E. and Lipton, J. O. Neural Circuitry of Wakefulness and Sleep. Neuron, 2017, 93: 747-765. [CrossRef]
- Segal, M. and Landis, S. Afferents to the hippocampus of the rat studied with the method of retrograde transport of horseradish peroxidase. Brain Research, 1974, 78: 1-15. [CrossRef]
- Shien Wee, R. W. and MacAskill, A. Hippocampal Circuits for the Hunger-Dependent Control of Feeding Behaviour. Journal of the Endocrine Society, 2021, 5: A540-A540. [CrossRef]
- Simerly, R. B. and Swanson, L. W. The organization of neural inputs to the medial preoptic nucleus of the rat. Journal of Comparative Neurology, 1986, 246: 312-342. [CrossRef]
- Simon, K. C., Clemenson, G. D., Zhang, J. et al. Sleep facilitates spatial memory but not navigation using the Minecraft Memory and Navigation task. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119: e2202394119. [CrossRef]
- Subhadeep, D., Srikumar, B. N., Shankaranarayana Rao, B. S. and Kutty, B. M. Ventral subicular lesion impairs pro-social empathy-like behavior in adult Wistar rats. Neuroscience letters, 2022, 776: 136535. [CrossRef]
- Sulaman, B. A., Wang, S., Tyan, J. and Eban-Rothschild, A. Neuro-orchestration of sleep and wakefulness. Nature neuroscience, 2023, 26: 196-212. [CrossRef]
- Takahashi, K., Lin, J.-S. and Sakai, K. Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience, 2009, 161: 269-292. [CrossRef]
- Tang, H., Wu, G.-S., Xie, J. et al. Brain-wide map of projections from mice ventral subiculum. Neuroscience Letters, 2016, 629: 171-179. [CrossRef]
- Thannickal, T. C., Moore, R. Y., Nienhuis, R. et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron, 2000, 27: 469-474. [CrossRef]
- Thompson, C. L., Wisor, J. P., Lee, C.-K. et al. Molecular and anatomical signatures of sleep deprivation in the mouse brain. Frontiers in neuroscience, 2010, 4: 165. [CrossRef]
- Torromino, G., Autore, L., Khalil, V. et al. Offline ventral subiculum-ventral striatum serial communication is required for spatial memory consolidation. Nature communications, 2019, 10: 5721. [CrossRef]
- Trulson, M. E. and Jacobs, B. L. Raphe unit activity in freely moving cats: Correlation with level of behavioral arousal. Brain Research, 1979, 163: 135-150. [CrossRef]
- Umaba, R., Kitanishi, T. and Mizuseki, K. Monosynaptic connection from the subiculum to medial mammillary nucleus neurons projecting to the anterior thalamus and Gudden's ventral tegmental nucleus. Neuroscience research, 2021, 171: 1-8. [CrossRef]
- Valera, S., Guadagni, V., Slone, E. et al. Poor sleep quality affects spatial orientation in virtual environments. Sleep science (Sao Paulo, Brazil), 2016, 9: 225-231. [CrossRef]
- van Strien, N. M., Cappaert, N. L. M. and Witter, M. P. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nature Reviews Neuroscience, 2009, 10: 272-282. [CrossRef]
- Varin, C., Luppi, P. H. and Fort, P. Melanin-concentrating hormone-expressing neurons adjust slow-wave sleep dynamics to catalyze paradoxical (REM) sleep. Sleep, 2018, 41. [CrossRef]
- Venner, A., Anaclet, C., Broadhurst, R. Y., Saper, C. B. and Fuller, P. M. A Novel Population of Wake-Promoting GABAergic Neurons in the Ventral Lateral Hypothalamus. Current biology : CB, 2016, 26: 2137-2143. [CrossRef]
- Vertes, R. P. Serotonergic Regulation of Rhythmical Activity of the Brain, Concentrating on the Hippocampus. In: C. P. Müller and B. L. Jacobs (eds) Handbook of the behavioral neurobiology of serotonin. Academic Press, London, 2010: 277-292.
- Weber, F. and Dan, Y. Circuit-based interrogation of sleep control. Nature, 2016, 538: 51-59. [CrossRef]
- Wee, R. W. S. and MacAskill, A. F. Biased Connectivity of Brain-wide Inputs to Ventral Subiculum Output Neurons. Cell Reports, 2020, 30: 3644-3654.e6. [CrossRef]
- Westerhaus, M. J. and Loewy, A. D. Sympathetic-related neurons in the preoptic region of the rat identified by viral transneuronal labeling. Journal of Comparative Neurology, 1999, 414: 361-378.
- Witter, M. P. Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behavioural brain research, 2006, 174: 251-264. [CrossRef]
- Witter, M. P., Wouterlood, F. G., Naber, P. A. and van Haeften, T. Anatomical organization of the parahippocampal-hippocampal network. Annals of the New York Academy of Sciences, 2000, 911: 1-24. [CrossRef]
- Xu, M., Chung, S., Zhang, S. et al. Basal forebrain circuit for sleep-wake control. Nature neuroscience, 2015, 18: 1641-1647. [CrossRef]
- Yan, J.-J., Ding, X.-J., He, T. et al. A circuit from the ventral subiculum to anterior hypothalamic nucleus GABAergic neurons essential for anxiety-like behavioral avoidance. Nature communications, 2022, 13: 7464. [CrossRef]
- Yu, N., Song, H., Chu, G. et al. Basal Forebrain Cholinergic Innervation Induces Depression-Like Behaviors Through Ventral Subiculum Hyperactivation. Neuroscience Bulletin, 2023, 39: 617-630. [CrossRef]
- Yu, X., Li, W., Ma, Y. et al. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nature neuroscience, 2019, 22: 106-119. [CrossRef]
Figure 1.
Similarities and differences in sleep and wake patterns between mice and humans. (A) Representative EEG (Electroencephalogram), its density spectral array (DSA), and corresponding EMG (Electromyogram) recordings from a mouse during Wake, NREMS (Non-Rapid Eye Movement Sleep), and REMS (Rapid Eye Movement Sleep). The mouse EEG signals were amplified 1000 times before digitization. Notably, during REMS, theta frequency power is significantly higher compared to NREMS and Wake, as indicated in the DSA. (B) Color-coded brain states (hypnogram) during a continuous 23-hour recording from a mouse under a light-dark cycle. Mice, being nocturnal animals, tend to sleep more during the light cycle. Their sleep patterns are characterized by fragmented sleep, featuring short sleep bouts and frequent awakenings. (C) Examples of a human EEG, its DSA, and corresponding EMG recordings during Wake, NREMS (stage 3), and REMS. REMS exhibits desynchronized rhythms similar to Wake but can be distinguished by EMG activity. (D) Color-coded brain states (hypnogram) during a continuous 23-hour recording from a healthy human subject. In humans, sleep is typically consolidated with rare awakenings during the night. REMS sleep occurs regularly approximately every 90 minutes.
Figure 1.
Similarities and differences in sleep and wake patterns between mice and humans. (A) Representative EEG (Electroencephalogram), its density spectral array (DSA), and corresponding EMG (Electromyogram) recordings from a mouse during Wake, NREMS (Non-Rapid Eye Movement Sleep), and REMS (Rapid Eye Movement Sleep). The mouse EEG signals were amplified 1000 times before digitization. Notably, during REMS, theta frequency power is significantly higher compared to NREMS and Wake, as indicated in the DSA. (B) Color-coded brain states (hypnogram) during a continuous 23-hour recording from a mouse under a light-dark cycle. Mice, being nocturnal animals, tend to sleep more during the light cycle. Their sleep patterns are characterized by fragmented sleep, featuring short sleep bouts and frequent awakenings. (C) Examples of a human EEG, its DSA, and corresponding EMG recordings during Wake, NREMS (stage 3), and REMS. REMS exhibits desynchronized rhythms similar to Wake but can be distinguished by EMG activity. (D) Color-coded brain states (hypnogram) during a continuous 23-hour recording from a healthy human subject. In humans, sleep is typically consolidated with rare awakenings during the night. REMS sleep occurs regularly approximately every 90 minutes.
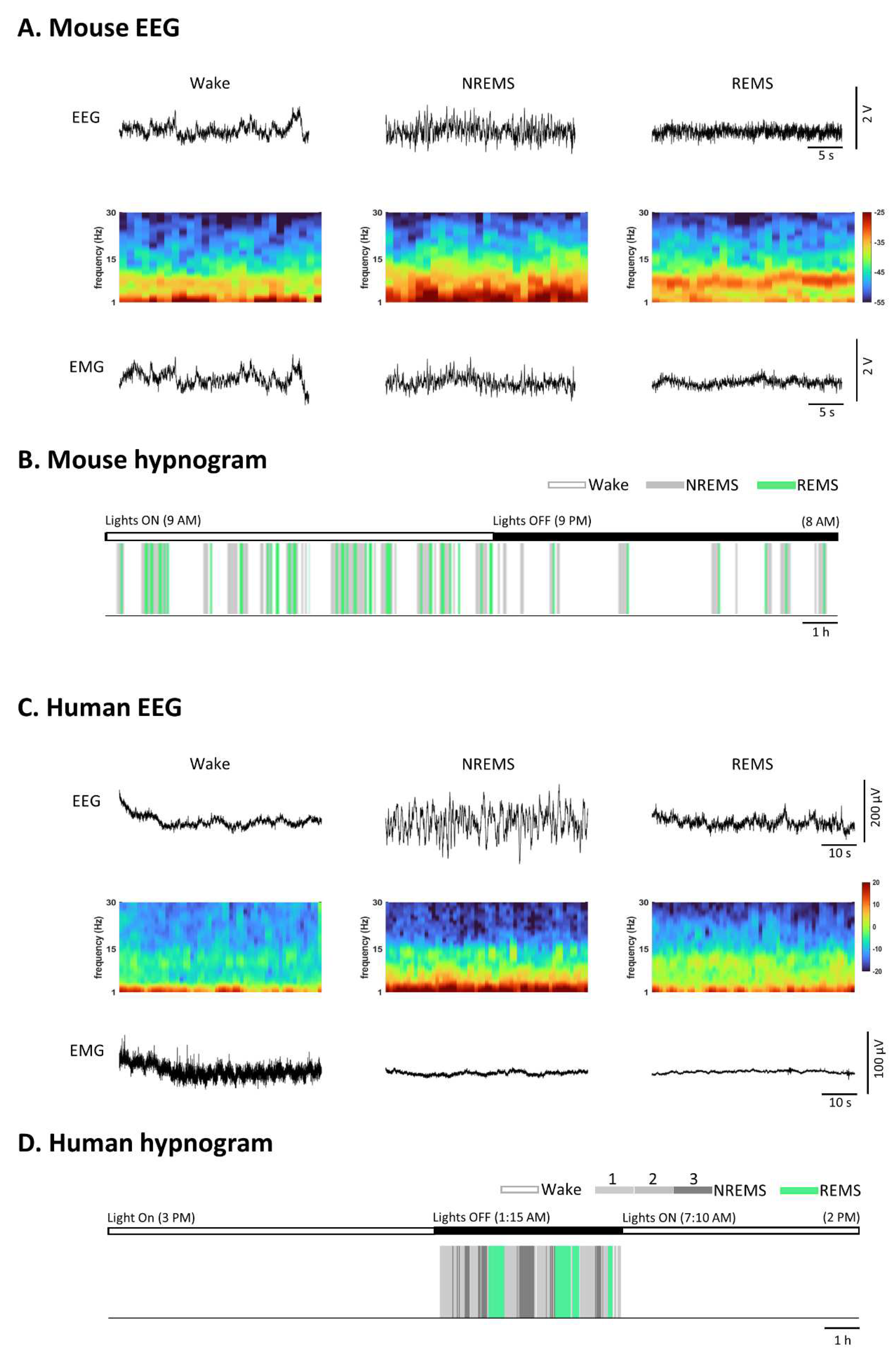
Figure 2.
Brain circuit involved in (A) Wake (B) NREMS, and (C) REMS. The depicted brain regions are intricately connected and can serve dual roles in both promoting sleep and maintaining wakefulness. Certain medulla oblongata nuclei, such as the Dorsal Paragigantocellular Nucleus, Lateral Paragigantocellular Nucleus, Gigantocellular Nucleus, and Gigantocellular Nucleus Alpha, along with components of the peripheral nervous system like premotor neurons and spinal motor neurons, contribute to motor inhibition during REMS. However, these elements have been excluded from (C) for the sake of this review’s focus. The figure is partially adopted from Scammell et al. (Scammell et al., 2017) and has undergone extensive modifications.
Figure 2.
Brain circuit involved in (A) Wake (B) NREMS, and (C) REMS. The depicted brain regions are intricately connected and can serve dual roles in both promoting sleep and maintaining wakefulness. Certain medulla oblongata nuclei, such as the Dorsal Paragigantocellular Nucleus, Lateral Paragigantocellular Nucleus, Gigantocellular Nucleus, and Gigantocellular Nucleus Alpha, along with components of the peripheral nervous system like premotor neurons and spinal motor neurons, contribute to motor inhibition during REMS. However, these elements have been excluded from (C) for the sake of this review’s focus. The figure is partially adopted from Scammell et al. (Scammell et al., 2017) and has undergone extensive modifications.
Figure 3.
Three-dimensional (3D) reconstruction and the schematic connections of the subiculum to the brain areas involved in the regulation of vigilance states. In the 3D reconstruction, the subiculum (Sub) is highlighted by red color. Disynaptic connection is shown by sign. BF: Basal Forebrain; LC: Locus Coeruleus; LH: Lateral Hypothalamus; LDT: Laterodorsal Tegmentum; MRN: Median Raphe Nucleus; SCN: Suprachiasmatic Nucleus; SLD: Sub-Laterodorsal (Tegmental) Nucleus; TMN: Tuberomammillary Nucleus; VTA: Ventral Tegmental Area.
Figure 3.
Three-dimensional (3D) reconstruction and the schematic connections of the subiculum to the brain areas involved in the regulation of vigilance states. In the 3D reconstruction, the subiculum (Sub) is highlighted by red color. Disynaptic connection is shown by sign. BF: Basal Forebrain; LC: Locus Coeruleus; LH: Lateral Hypothalamus; LDT: Laterodorsal Tegmentum; MRN: Median Raphe Nucleus; SCN: Suprachiasmatic Nucleus; SLD: Sub-Laterodorsal (Tegmental) Nucleus; TMN: Tuberomammillary Nucleus; VTA: Ventral Tegmental Area.
Figure 4.
Hippocampal volume changes in female with obstructive sleep apnea (OSA) relative to controls, with age and total intracranial volume (TIV) as covariates (adopted from Macey et al. (Macey et al., 2018)). Interestingly, in female patients, subiculum/uncus showed the most significant alternation in comparison to other regions of the hippocampal formation.
Figure 4.
Hippocampal volume changes in female with obstructive sleep apnea (OSA) relative to controls, with age and total intracranial volume (TIV) as covariates (adopted from Macey et al. (Macey et al., 2018)). Interestingly, in female patients, subiculum/uncus showed the most significant alternation in comparison to other regions of the hippocampal formation.
Table 1.
Neuronal connectivity between the subiculum and sleep-wake promoting areas.
Table 1.
Neuronal connectivity between the subiculum and sleep-wake promoting areas.
| Species |
Which region of subiculum |
Which area |
Which technique |
Monosynaptic or disynaptic |
Reference |
| rat |
temporal two-thirds |
medial preoptic region |
anterograde Phaseolus vulgaris leucoagglutinin (PHA-L) and retrograde cholera toxin B subunit |
monosynaptic |
(Kishi et al., 2000) |
| rat |
Not specified |
MnPO |
wheat germ agglutinin conjugated to horseradish peroxidase |
monosynaptic |
(Chiba and Murata, 1985) |
| rat |
ventral |
MnPO |
pseudorabies virus injections |
monosynaptic |
(Westerhaus and Loewy, 1999) |
| rat |
ventral |
MnPO |
retrograde and anterograde axonal transport techniques (true blue, SITS, or wheat germ agglutinin) |
monosynaptic |
(Simerly and Swanson, 1986) |
| rat |
ventral |
VLPO |
retrograde tracer CTB subunit |
monosynaptic |
(Chou et al., 2002) |
| rat |
full longitudinal extent |
anterior, tuberal, and mammillary regions of hypothalamus |
anterograde Phaseolus vulgaris leucoagglutinin (PHA-L) and retrograde cholera toxin B (CTB) subunit |
monosynaptic |
(Kishi et al., 2000) |
| rat |
ventral |
LH |
Retrograde labelling by CTB-488 microinjection |
monosynaptic |
(Mu et al., 2020) |
| mouse |
ventral |
LH |
Rabies virus injection |
monosynaptic |
(Shien Wee and MacAskill, 2021) |
| rat |
ventral |
LH |
anterograde PHA-L tract-tracing method |
monosynaptic |
(Köhler, 1990) |
| mouse |
ventral |
BF |
retrograde and anterograde virus tracing method |
monosynaptic |
(Yu et al., 2023) |
| mouse |
ventral |
VTA |
anterograde PHA-L and retrograde CTB |
disynaptic (through BNST) |
(Glangetas et al., 2015) |
| rat |
dorsal |
VTA |
anterograde transsynaptic tracing using adeno-associated virus serotype 1 |
disynaptic (through medial mammillary nucleus) |
(Umaba et al., 2021) |
| rat |
Not specified |
LC |
Uptake labeling and radioautography |
monosynaptic |
(Oleskevich et al., 1989) |
| rat |
Not specified |
LC |
retrograde transport of horseradish peroxidase |
monosynaptic |
(Loy et al., 1980) |
| rat |
Not specified |
LC |
retrograde transport of horseradish peroxidase |
monosynaptic |
(Segal and Landis, 1974) |
| mouse |
ventral |
LC |
Herpes simplex virus 1 strain H129 with an inserted fluorescent protein gene |
disynaptic |
(Tang et al., 2016) |
| mouse |
ventral |
MRN |
Herpes simplex virus 1 strain H129 with an inserted fluorescent protein gene |
disynaptic |
(Tang et al., 2016) |
| mouse |
ventral |
LDT |
Herpes simplex virus 1 strain H129 with an inserted fluorescent protein gene |
monosynaptic |
(Tang et al., 2016) |
| rat |
ventral |
SCN |
retrograde CTB |
monosynaptic |
(Krout et al., 2002) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).