Submitted:
27 September 2023
Posted:
28 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction to Neutrophil Extracellular Traps (NETs)
1.1. Neutrophil and Innate Immunity
1.2. Antimicrobial actions of Neutrophils:
1.3. Neutrophil Extracellular Traps (NETs)
1.4. NETosis Mechanism
1.5. NETosis Pathways
1.6. Physiological role of NETs
1.7. Controversial role of NETs
1.8. Detection of NETs
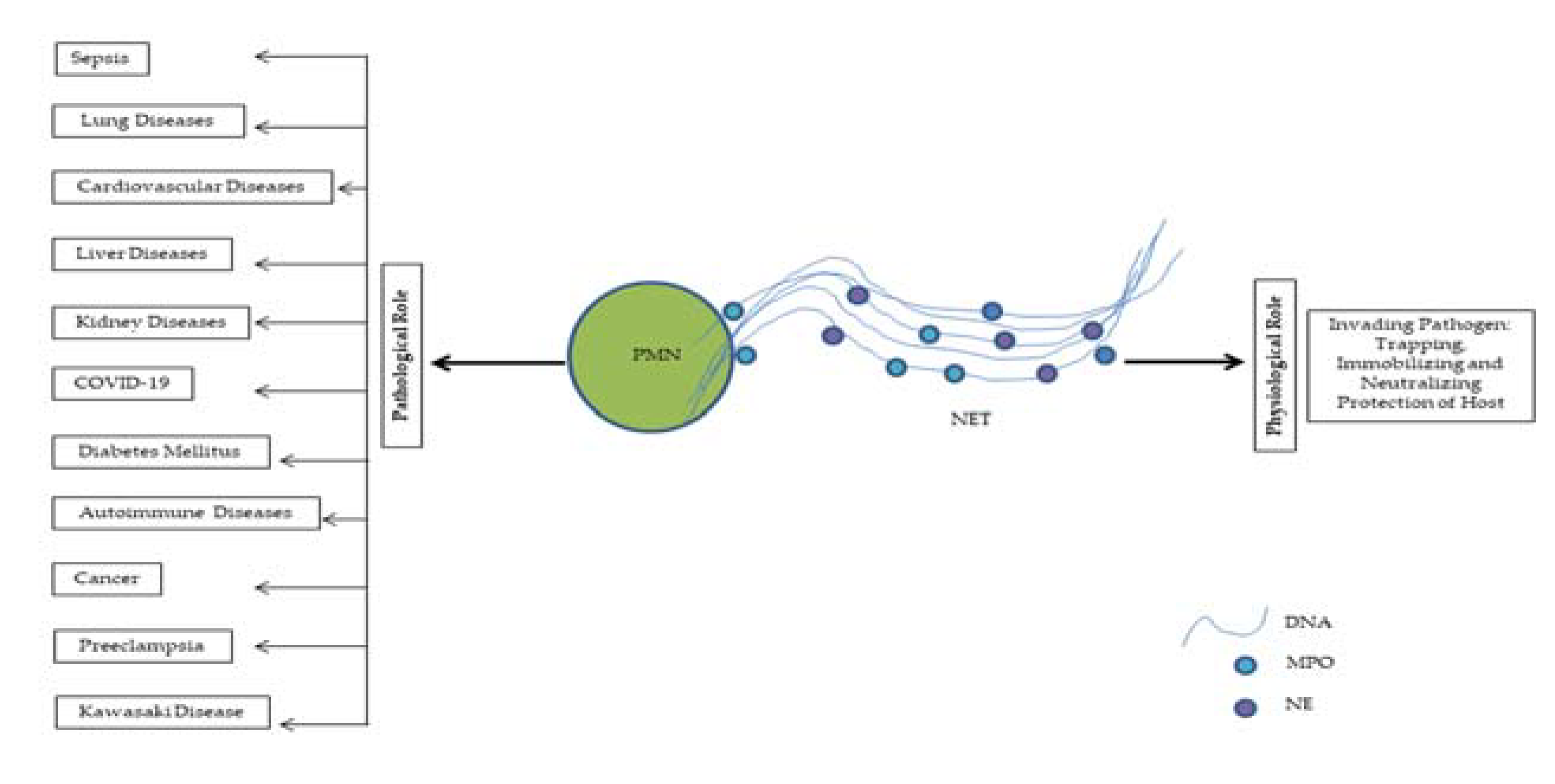
2. NETs in Clinical Settings
2.1. NETs in Sepsis
2.2. NETs in Lung Diseases
2.3. NETs in Cardiovascular Diseases
2.4. NETs in Liver Diseases
2.5. NETs in Kidney Diseases
2.6. NETs in COVID-19
2.7. NETs in Coagulopathy and Thrombotic microangiopathy
2.8. NETs in Diabetes
2.9. NETs in Autoimmune Diseases
2.10. NETs in Cancer
2.11. NETs in Preeclampsia
2.12. NETs in Kawasaki Disease
3. Evaluation of NETs Inhibition as Therapeutic Targets
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J Leukoc Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Pruchniak, MP.; Arazna, M.; Demkow, U. Life of neutrophil: from stem cell to neutrophil extracellular trap. Respir Physiol Neurobiol 2013, 187, 68–73. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types. Front Physiol 2018, 9, 113. [Google Scholar] [CrossRef]
- Phillipson, M; Kubes, P. The neutrophil in vascular inflammation. Nat Med 2011, 17, 1381–1390. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol 2012, 198, 773–783. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Greenlee-Wacker, MC. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev 2016, 273, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M. ; Mumby, S; Koenderman, L. Update on Neutrophil Function in Severe Inflammation. Front Immunol 2018, 9, 2171. [Google Scholar] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; et al. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Wang, X.; Yousefi, S.; Simon, HU. Necroptois and neutrophil-associated disorders. Cell Death Dis 2018, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.I.; Wang, Y.; Liu, Q.; Li, H-R.; Yu, C-M.; Li, P.; Deng, X-M.; Wang, J-F. Dysregulation of neutrophil death in sepsis. Front Immunol 2022, 13, 963955. [Google Scholar] [CrossRef]
- Ganesh, K.; Joshi, M.B. Neutrophil sub-types in maintaining immune homeostasis during steady state, infections and sterile inflammation. Inflamm Res 2023, 72, 1175–1192. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; Fresno, C.D.; Crainiciuc, G.; Cuartero, M.I.; Casanova-Acebes, M.; Weiss, L.A.; Huerga-Encabo, H.; Silvestre-Roig, C.; Rossaint, J.; Cossío, I.; Lechuga-Vieco, A.V.; García-Prieto, J.; Gómez-Parrizas, M.; Quintana, J.A.; Ballesteros, I.; Martin-Salamanca, S.; Aroca-Crevillen, A. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity 2019, 50, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Drifte, G,; Dunn-Siegrist, I. ; Tissières, P.; Pugin, J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med 2013, 41, 820–832. [Google Scholar] [CrossRef]
- Lipp, P.; Ruhnau, J.; Lange, A.; Vogelgesang, A.; Dressel, A.; Heckmann, M. Less Neutrophil Extracellular Trap Formation in Term Newborns than in Adults. Neonatology 2017, 111, 182–188. [Google Scholar] [CrossRef]
- Saitoh, T. , Komano, J.; Saitoh, Y. et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 2012, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Gazendam, R.P.; van de Geer, A.; Roos, D.; van den Berg, T.K.; Kuijpers, T.W. How Neutrophils Kill Fungi. Immunol Rev 2016, 273, 299–311. [Google Scholar] [CrossRef]
- Yufei, H.; Liu, J.; Chen, Y.; Yan, L.; Wu, J. Neutrophil Extracellular Traps in Candida albicans Infection. Front Immunol 2022, 13, 913028. [Google Scholar]
- Babatunde, K.A.; Adenuga, O.F. Neutrophils in malaria: A double-edged sword role. Front Immunol, 2022, 13, 922377. [Google Scholar] [CrossRef]
- Thiam, HR.; Wong, SL.; Wagner, D.D. Waterman CM. Cellular Mechanisms of NETosis. Annu Rev Cell Dev Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Keshari, R.S.; Jyoti, A.; Dubey, M.; et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS One 2012, 7, e48111. [Google Scholar] [CrossRef]
- Omeljaniuk, W.J.; Jabłońska, E.; Garley, M.; Pryczynicz, A.; Ratajczak-Wrona, W.; Socha, K.; Borawska, M.H.; Charkiewicz, A.E. Biomarkers of neutrophil extracellular traps (NETs) and nitric oxide-(NO)-dependent oxidative stress in women who miscarried. Sci Rep 2020, 10, 13088. [Google Scholar] [CrossRef]
- Su, M.; Chen, C.; Li, S.; Li, M.; Zeng, Z.; Zhang, Y.; Xia, L.; Li, X.; Zheng, D.; Lin, Q.; Fan, X.; Wen, Y.; Liu, Y.; Chen, F.; Luo, W.; Bu, Y.; Qin, J.; Guo, M.; Qiu, M.; Sun, L.; Liu, R.; Wang, P.; Hwa, J.; Tang, W.H. Gasdermin D-dependent platelet pyroptosis exacerbates NET formation and inflammation in severe sepsis. Nat Cardiovasc Res 2022, 1, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Schorn, C.; Janko, C.; Latzko, M.; Chaurio, R.; Schett, G. Herrmann M. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front Immunol 2012, 3, 277. [Google Scholar] [CrossRef] [PubMed]
- Behnen, M.; Leschczyk, C.; Möller, S.; et al. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcγRIIIB and Mac-1. J Immunol 2014, 193, 1954–1965. [Google Scholar] [CrossRef]
- Yalavarthi, S.; Gould, T.J.; Rao, A.N.; et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol 2015, 67, 2990–3003. [Google Scholar] [CrossRef]
- Ortiz-Espinosa, S.; Morales, X.; Senent, Y.; et al. Complement C5a induces the formation of neutrophil extracellular traps by myeloid-derived suppressor cells to promote metastasis. Cancer Lett 2022, 529, 70–84. [Google Scholar] [CrossRef]
- Park J, Wysocki RW, Amoozgar Z, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016, 8, 138. [Google Scholar]
- Rayes, R.F.; Mouhanna, J.G.; Nicolau, I.; et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight 2019, 5, e128008. [Google Scholar] [CrossRef]
- Boone, B.A.; Orlichenko, L.; Schapiro, N.E.; et al. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther 2015, 22, 326–334. [Google Scholar] [CrossRef]
- McDonald, B.; Urrutia, R.; Yipp, B.G.; Jenne, C.N.; Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012, 12, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Aube, F.A.; Bidias, A.; Pépin, G. Who and how, DNA sensors in NETs-driven inflammation. Front Immunol 2023, 14, 1190177. [Google Scholar] [CrossRef]
- Ravindran, M.; Khan, M.A.; Palaniyar, N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules 2019, 9, 365. [Google Scholar] [CrossRef]
- Azzouz, D.; Khan, M.A.; Sweezey, N.; Palaniyar, N. Two-in-one: UV radiation simultaneously induces apoptosis and NETosis. Cell Death Discov 2018, 4, 51. [Google Scholar] [CrossRef]
- Douda, D.N.; Yip, L.; Khan, M.A.; Grasemann, H.; Palaniyar, N. Akt is essential to induce NADPH-dependent NETosis and to switch the neutrophil death to apoptosis. Blood 2014, 123, 597–600. [Google Scholar] [CrossRef]
- Douda, D. N.; Khan, M. A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci U S A 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, A. S.; Slade, D. J.; Thompson, P. R.; Mowen, K. A. Activation of PAD4 in NET formation. Front Immunol 2012, 3, 360. [Google Scholar] [CrossRef] [PubMed]
- Metzler, K. D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep 2014, 2014. 8, 883–896. [Google Scholar] [CrossRef]
- Azzouz, D.; Palaniyar, N. ApoNETosis: discovery of a novel form of neutrophil death with concomitant apoptosis and NETosis. Cell Death Dis 2018, 9, 839. [Google Scholar] [CrossRef]
- Khan, MA.; Palaniyar, N. Transcriptional firing helps to drive NETosis. Sci Rep 2017, 7, 41749. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. ; Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.S.; O'Brien, X.M.; Johnson, C.M.; Lavigne, L.M.; Reichner, J.S. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J Immunol 2013, 190, 4136–4148. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: how vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front Immunol 2017, 8, 81. [Google Scholar] [CrossRef]
- Liang, C.; Lian, N.; Li. M. The emerging role of neutrophil extracellular traps in fungal infection. Front Cell Infect Microbiol, 2022, 12, 900895. [Google Scholar] [CrossRef]
- Omar, M.; Abdelal, H. NETosis in Parasitic Infections: A Puzzle That Remains Unsolved. Int J Mol Sci 2023, 24, 8975. [Google Scholar] [CrossRef]
- Diaz-Godinez, C.; Carrero, J.C. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. Biosci Rep 2019, 39, BSR20180916. [Google Scholar] [CrossRef]
- Bartneck, M.; Keul, H.A.; Zwadlo-Klarwasser, G.; Groll, J. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett 2010, 10, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Meurer, M.; Öhlmann, S.; Bonilla, M.C.; Valentin-Weigand, P.; Beineke, A.; Hennig-Pauka, I.; Schwerk, C.; Schroten, H.; Baums, C.G.; Köckritz-Blickwede, M.V.; de Buhr, N. Role of Bacterial and Host DNases on Host-Pathogen Interaction during Streptococcus suis Meningitis. Int J Mol Sci 2020, 21, 5289. [Google Scholar] [CrossRef] [PubMed]
- Nel, Jan G. ; Theron, Annette J.; Pool, Roger; Durandt, Chrisna; Tintinger, Gregory R.; Anderson, Ronald. Neutrophil extracellular traps and their role in health and disease. S Afr J Sci 2016, 112, 1–9. [Google Scholar]
- Liao, C.; Mao, F.; Qian, M.; Wang, X. Pathogen-Derived Nucleases: An Effective Weapon for Escaping Extracellular Traps. Front Immunol 2022, 13, 899890. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Neutrophils and Bacterial Immune Evasion. J Innate Immun 2018, 10, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Storisteanu, D.M.; M Pocock, J.M.; Cowburn, A.S.; Juss, J.K.; Nadesalingam, A.; Nizet, V.; Chilvers, E.R. Evasion of neutrophil extracellular traps by respiratory pathogens. Am J Respir Cell Mol Biol 2017, 56, 423–431. [Google Scholar] [CrossRef]
- Wartha, F.; Beiter, K.; Albiger, B.; Fernebro, J.; Zychlinsky, A.; Normark, S.; Henriques-Normark, B. Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol 2007, 9, 1162–1171. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S. E.; Wang, Q.; Gutierrez, M. G.; Brown, G. D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Low, C. Y.; Rotstein, C. Emerging fungal infections in immunocompromised patients. F1000 Med Rep 2011, 3, 14. [Google Scholar] [CrossRef]
- Zhong, H.; Lu, R.Y.; Wang, Y. Neutrophil extracellular traps in fungal infections: A seesaw battle in hosts. Front Immunol 2022, 13, 977493. [Google Scholar] [CrossRef]
- Marcos-Jubilar, M.; Lecumberri, R.; Páramo, J. Immunothrombosis: Molecular Aspects and New Therapeutic Perspectives. J Clin Med 2023, 12, 1399. [Google Scholar] [CrossRef]
- Iba, T.; Miki, T.; Hashiguchi, N.; Tabe, Y.; Nagaoka, I. Is the neutrophil a 'prima donna' in the procoagulant process during sepsis? Crit Care 2014, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Stiel, L.; Meziani, F.; Helms, J. Neutrophil Activation During Septic Shock. Shock 2018, 49, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Alcázar, M.; Kim, N.; Fuchs, T. A. Circulating Extracellular DNA: Cause or Consequence of Thrombosis? Semin Thromb Hemost 2017, 43, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.T.; Morton, B.; Alhamdi, Y.; Alsabani, M.; Lane, S.; Welters, I. D.; Wang, G.; Toh, C.H. A Novel Assay for Neutrophil Extracellular Trap Formation Independently Predicts Disseminated Intravascular Coagulation and Mortality in Critically Ill Patients. Am J Respir Crit Care Med 2019, 200, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fogg, D. K.; Kaplan, M. J. A novel image-based quantitative method for the characterization of NETosis. J Immunol Methods 2015, 423, 104–110. [Google Scholar] [CrossRef]
- Kano, H.; Aminul Huq, M.; Tsuda, M.; Noguchi, H.; Takeyama, N. Sandwich ELISA for Circulating Myeloperoxidase- and Neutrophil Elastase-DNA Complexes Released from Neutrophil Extracellular Traps. Advanced Techniques in Biology & Medicine 2016, 5, 196. [Google Scholar]
- Islam, M.M.; Salma, U.; Irahara, T.; Watanabe, E.; Takeyama, N. Quantifying Myeloperoxidase-DNA and Neutrophil Elastase-DNA Complexes from Neutrophil Extracellular Traps by Using a Modified Sandwich ELISA. J Vis Exp 2023, 195, 64644. [Google Scholar] [CrossRef]
- Sil, P.; Yoo, D. G.; Floyd, M.; Gingerich, A.; Rada, B. High Throughput Measurement of Extracellular DNA Release and Quantitative NET Formation in Human Neutrophils In Vitro. J Vis Exp 2016, 112, 52779. [Google Scholar]
- Lelubre, C.; Vincent, J.L. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol 2018, 14, 417–427. [Google Scholar] [CrossRef]
- Liaw, P. C.; Ito, T.; Iba, T.; Thachil, J.; Zeerleder, S. DAMP and DIC: The role of extracellular DNA and DNA-binding proteins in the pathogenesis of DIC. Blood Rev 2016, 30, 257–261. [Google Scholar] [CrossRef]
- Gardiner, E. E.; Andrews, R. K. Neutrophil extracellular traps (NETs) and infection-related vascular dysfunction. Blood Rev 2012, 26, 255–259. [Google Scholar] [CrossRef]
- Maruchi, Y.; Tsuda, M.; Mori, H.; Takenaka, N.; Gocho, T.; Huq, M.A.; Takeyama, N. Plasma myeloperoxidase-conjugated DNA level predicts outcomes and organ dysfunction in patients with septic shock. Crit Care 2018, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- Stiel, L.; Mayeur-Rousse, C.; Helms, J.; Meziani, F.; Mauvieux, L. First visualization of circulating neutrophil extracellular traps using cell fluorescence during human septic shock-induced disseminated intravascular coagulation. Thromb Res 2019, 183, 153–158. [Google Scholar] [CrossRef]
- Lenz, M.; Maiberger, T.; Armbrust, L.; Kiwit, A.; Von der Wense, A.; Reinshagen, K.; Elrod, J.; Boettcher, M. cfDNA and DNases: New Biomarkers of Sepsis in Preterm Neonates—A Pilot Study. Cells 2022, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Lerman, Y.V.; Kim, M. Neutrophil migration under normal and sepsis conditions. Cardiovasc Hematol Disord Drug Targets 2015, 15, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Joshi, M.B.; Philippova, M.; Erne, P.; Hasler, P.; Hahn, S.; Resink, T.J. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett 2010, 584, 3193–3197. [Google Scholar] [CrossRef]
- Iba, T.; Levi, M.; Levy, J.H. Intracellular communication and immunothrombosis in sepsis. J Thromb Haemost, 2022, 20, 2475–2484. [Google Scholar] [CrossRef]
- Ogura, H.; Gando, S.; Saitoh, D.; Takeyama, N.; Kushimoto, S.; Fujishima, S.; Mayumi, T.; Araki, T.; Ikeda, H.; Kotani, J.; Miki, Y.; Shiraishi, S.; Suzuki, K.; Suzuki, Y.; Takuma, K.; Tsuruta, R.; Yamaguchi, Y.; Yamashita, N.; Aikawa, N. ; Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother 2014, 20, 157–162. [Google Scholar] [CrossRef]
- Pfeiler, S.; Stark, K.; Massberg, S.; Engelmann, B. Propagation of thrombosis by neutrophils and extracellular nucleosome networks. Haematologica 2017, 102, 206–213. [Google Scholar] [CrossRef]
- Delabranche, X.; Stiel, L.; Severac, F.; Galoisy, A.C.; Mauvieux, L.; Zobairi, F.; Lavigne, T.; Toti, F.; Anglès-Cano, E.; Meziani, F.; Boisramé-Helms, J. Evidence of Netosis in Septic Shock-Induced Disseminated Intravascular Coagulation. Shock 2017, 47, 313–317. [Google Scholar] [CrossRef]
- Li, Z.; Delaney, M.K.; O'Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, H.; Qu, M.; Nan, K.; Cao, H.; Cata, J.P.; Chen, W.; Miao, C. Review: The Emerging Role of Neutrophil Extracellular Traps in Sepsis and Sepsis-Associated Thrombosis. Front Cell Infect Microbiol 2021, 11, 653228. [Google Scholar] [CrossRef] [PubMed]
- Sreeramkumar, V.; Adrover, J.M.; Ballesteros, I.; Cuartero, M.I.; Rossaint, J.; Bilbao, I.; Nácher, M.; Pitaval, C.; Radovanovic, I.; Fukui, Y.; McEver, R. P.; Filippi, M.D.; Lizasoain, I.; Ruiz-Cabello, J.; Zarbock, A.; Moro, M.A.; Hidalgo, A. Neutrophils scan for activated platelets to initiate inflammation. Science 2014, 346, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef]
- Carestia, A.; Kaufman, T.; Rivadeneyra, L.; Landoni, V.I.; Pozner, R.G.; Negrotto, S.; D'Atri, L.P.; Gómez, R.M.; Schattner, M. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J Leukoc Biol 2016, 99, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Szatmary, P.; Huang, W.; Criddle, D.; Tepikin, A.; Sutton, R. Biology, role and therapeutic potential of circulating histones in acute inflammatory disorders. J Cell Mol Med 2018, 22, 4617–4629. [Google Scholar] [CrossRef]
- Potey, P.M.; Rossi, A.G.; Lucas, C.D.; Dorward, D.A. Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential. J Pathol 2019, 247, 672–685. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A. S. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Ramji, H.F.; Hafiz, M.; Altaq, H.H.; Hussain, S.T.; Chaudry, F. Acute Respiratory Distress Syndrome; A Review of Recent Updates and a Glance into the Future. Diagnostics 2023, 13, 1528. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; Ranieri, M.; Rubenfeld, G.; Thompson, B.T.; Wrigge, H.; Slutsky, A.S.; Pesenti, A. ; Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA, 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol Med 2011, 17, 293–307. [Google Scholar] [CrossRef]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 2011, 179, 199–210. [Google Scholar] [CrossRef]
- Yang, S.C.; Yung-Fong Tsai, Y.F.; Yen-Lin Pan, Y.L.; Tsong-Long Hwang, T.L. Understanding the role of neutrophils in acute respiratory distress syndrome. BioMed J 2021, 44, 439–446. [Google Scholar] [CrossRef]
- Mikacenic, C.; Moore, R.; Dmyterko, V.; West, T.E.; Altemeier, W.A.; Liles, W.C.; Lood, C. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator-associated pneumonia. Crit Care 2018, 22, 358. [Google Scholar] [CrossRef]
- Scozzi, D.; Liao, F.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The role of neutrophil extracellular traps in acute lung injury. Front Immunol 2022, 13, 953195. [Google Scholar] [CrossRef]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 2012, 7, e32366. [Google Scholar] [CrossRef]
- Abrams, S. T.; Zhang, N.; Manson, J.; Liu, T.; Dart, C.; Baluwa, F.; Wang, S. S.; Brohi, K.; Kipar, A.; Yu, W.; Wang, G.; Toh, C. H. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 2013, 187, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Bosmann, M.; Grailer, J.J.; Ruemmler, R.; Russkamp, N.F.; Zetoune, F.S.; Sarma, J.V.; Standiford, T. J.; Ward, P.A. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J 2013, 27, 5010–5021. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Huq, M.A.; Islam, M.M.; Takeyama, N. Neutrophil extracellular traps are associated with altered human pulmonary artery endothelial barrier function. European Journal of Inflammation 2021, 19, 1–10. [Google Scholar] [CrossRef]
- Garcia, C.C.; Weston-Davies, W.; Russo, R.C.; Tavares, L.P.; Rachid, M.A.; Alves-Filho, J.C.; Machado, A.V.; Ryffel, B.; Nunn, M.A.; Teixeira, M.M. Complement C5 activation during influenza A infection in mice contributes to neutrophil recruitment and lung injury. PLoS One 2013, 8, e64443. [Google Scholar] [CrossRef]
- Lefrançais, E.; Mallavia, B.; Zhuo, H.; Calfee, C.S.; Looney, M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight 2018, 3, e98178. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alemán, S.R.; Campos-García, L.; Palma-Nicolas, J.P.; Hernández-Bello, R.; González, G.M.; Sánchez-González, A. Understanding the Entanglement: Neutrophil Extracellular Traps (NETs) in Cystic Fibrosis. Front Cell Infect Microbiol 2017, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Manzenreiter, R.; Kienberger, F.; Marcos, V.; Schilcher, K.; Krautgartner, W.D.; Obermayer, A.; Huml, M.; Stoiber, W.; Hector, A.; Griese, M.; Hannig, M.; Studnicka, M.; Vitkov, L.; Hartl, D. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros 2012, 11, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S. V.; Bruce, K. D. Bruce, The cystic fibrosis airway microbiome. Cold Spring Harb Perspect Med 2013, 3, a009738. [Google Scholar] [CrossRef]
- Yoo, D. G.; Floyd, M.; Winn, M.; Moskowitz, S.M.; Rada, B. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase-DNA and neutrophil elastase-DNA complexes. Immunol Lett 2014, 160, 186–194. [Google Scholar] [CrossRef]
- Piva, T.C.; Luft, C.; Antunes, K.H.; Marostica, P.J.C.; Pinto, L.A.; Donadio, M.V.F. Extracellular DNA in sputum is associated with pulmonary function and hospitalization in patients with cystic fibrosis. Respir Med 2020, 172, 106144. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Staab, D.; Zychlinsky, A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One 2011, 6, e28526. [Google Scholar] [CrossRef] [PubMed]
- Keir, H.R.; Chalmers, J.D. Neutrophil extracellular traps in chronic lung disease: implications for pathogenesis and therapy. Eur Respir Rev 2022, 31, 210241. [Google Scholar] [CrossRef]
- Rahman, S.; Gadjeva, M. Does NETosis Contribute to the Bacterial Pathoadaptation in Cystic Fibrosis? Front Immunol 2014, 5, 378. [Google Scholar] [CrossRef]
- Kim, H.Y.; DeKruyff, R.H.; Umetsu, D. T. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol 2010, 11, 577–584. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 18, 1469–1485. [Google Scholar] [CrossRef]
- Dworski, R.; Simon, H. U.; Hoskins, A.; Yousefi, S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol 2011, 127, 1260–1266. [Google Scholar] [CrossRef]
- Hosoki, K.; Ying, S.; Corrigan, C.; Qi, H.; Kurosky, A.; Jennings, K.; Sun, Q.; Boldogh, I.; Sur, S. Analysis of a Panel of 48 Cytokines in BAL Fluids Specifically Identifies IL-8 Levels as the Only Cytokine that Distinguishes Controlled Asthma from Uncontrolled Asthma, and Correlates Inversely with FEV1. PLoS One 2015, 10, e0126035. [Google Scholar] [CrossRef]
- Pablo-Torres, C.; Izquierdo, E.I.; Tan, T.J.; Obeso, D.; Layhadi, J.A.; Sánchez-Solares, J.; Mera-Berriatua, L.; Bueno-Cabrera, J.L.; Reaño-Martos, M.D.M.; Iglesias-Cadarso, A.; Barbas, C.; Gomez-Casado, C.; Villaseñor, A.; Barber, D.; Shamji, M.H.; Escribese, M.M. Deciphering the role of platelets in severe allergy by an integrative omics approach. Allergy 2023, 78, 1319–1332. [Google Scholar] [CrossRef]
- Bonaventura, A.; Montecucco, F.; Dallegri, F. Cellular recruitment in myocardial ischaemia/reperfusion injury. Eur J Clin Invest 2016, 46, 590–601. [Google Scholar] [CrossRef]
- Mozzini, C.; Pagani, M. Cardiovascular Diseases: Consider Netosis. Curr Probl Cardiol 2021, 47, 100929. [Google Scholar] [CrossRef]
- Distelmaier, K.; Winter, M. P.; Dragschitz, F.; Redwan, B.; Mangold, A.; Gleiss, A.; Perkmann, T.; Maurer, G.; Adlbrecht, C.; Lang, I. M. Prognostic value of culprit site neutrophils in acute coronary syndrome. Eur J Clin Invest 2014, 44, 257–265. [Google Scholar] [CrossRef]
- Borissoff, J.I.; Joosen, I.A.; Versteylen, M.O.; Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Gallant, M.; Martinod, K.; Ten Cate, H.; Hofstra, L.; Crijns, H.J.; Wagner, D.D.; Kietselaer, B.L.J.H. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol 2013, 33, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Megens, R.T.; Vijayan, S.; Lievens, D.; Döring, Y.; van Zandvoort, M.A.; Grommes, J.; Weber, C.; Soehnlein, O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb Haemost 2012, 107, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Oklu, R.; Stone, J.R.; Albadawi, H.; Watkins, M.T. Extracellular traps in lipid-rich lesions of carotid atherosclerotic plaques: implications for lipoprotein retention and lesion progression. J Vasc Interv Radiol 2014, 25, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhou, X.; Ji, W.J.; Lu, R.Y.; Zhang, Y.; Zhang, Y.D.; Ma, Y.Q.; Zhao, J.H.; Li, Y. M. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. Am J Physiol Heart Circ Physiol 2015, 308, H500–H509. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Wichapong, K.; Lee, E.Y.; Teulon, J.M.; Berrebeh, N.; Winter, J.; Adrover, J.M.; Santos, G.S.; Froese, A.; Lemnitzer, P.; Ortega-Gómez, A.; Chevre, R.; Marschner, J.; Schumski, A.; Winter, C.; Perez-Olivares, L.; Pan, C.; Paulin, N.; Schoufour, T.; … Soehnlein, O. . Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 2019, 569, 236–240. [Google Scholar] [CrossRef]
- Pérez-Sánchez, C.; Ruiz-Limón, P.; Aguirre, M.A.; Jiménez-Gómez, Y.; Arias-de la Rosa, I.; Ábalos-Aguilera, M.C.; Rodriguez-Ariza, A.; Castro-Villegas, M.C.; Ortega-Castro, R.; Segui, P.; Martinez, C.; Gonzalez-Conejero, R.; Rodríguez-López, S.; Gonzalez-Reyes, J.A.; Villalba, J.M.; Collantes-Estévez, E.; Escudero, A.; Barbarroja, N.; López-Pedrera, C. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in Rheumatoid Arthritis patients. J Autoimmun 2017, 82, 31–40. [Google Scholar] [CrossRef]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; Ritis, K. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J 2015, 36, 1405–1414. [Google Scholar] [CrossRef]
- Huang, H.; Tohme, S.; Al-Khafaji, A.B.; Tai, S.; Loughran, P.; Chen, L.; Wang, S.; Kim, J.; Billiar, T.; Wang, Y.; Tsung, A. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 2015, 62, 600–614. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.W.; Evankovich, J.; Yan, W.; Rosborough, B. R.; Nace, G.W.; Ding, Q.; Loughran, P.; Beer-Stolz, D.; Billiar, T.R.; Esmon, C.T.; Tsung, A. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J Immunol 2013, 191, 2665–2679. [Google Scholar] [CrossRef]
- Linkermann, A.; Chen, G.; Dong, G.; Kunzendorf, U.; Krautwald, S.; Dong, Z. Regulated cell death in AKI. J Am Soc Nephrol 2014, 25, 2689–2701. [Google Scholar] [CrossRef]
- Linkermann, A.; Stockwell, B.R.; Krautwald, S.; Anders, H.J. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol 2014, 14, 759–767. [Google Scholar] [CrossRef]
- Allam, R.; Scherbaum, C.R.; Darisipudi, M.N.; Mulay, S.R.; Hägele, H.; Lichtnekert, J.; Hagemann, J.H.; Rupanagudi, K.V.; Ryu, M.; Schwarzenberger, C.; Hohenstein, B.; Hugo, C.; Uhl, B.; Reichel, C.A.; Krombach, F.; Monestier, M.; Liapis, H.; Moreth, K.; Schaefer, L.; Anders, H.J. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol 2012, 23, 1375–1388. [Google Scholar] [CrossRef]
- Kumar, S.V.; Kulkarni, O.P.; Mulay, S.R.; Darisipudi, M.N.; Romoli, S.; Thomasova, D.; Scherbaum, C.R.; Hohenstein, B.; Hugo, C.; Müller, S.; Liapis, H.; Anders, H.J. Neutrophil Extracellular Trap-Related Extracellular Histones Cause Vascular Necrosis in Severe GN. J Am Soc Nephrol 2015, 26, 2399–2413. [Google Scholar] [CrossRef]
- Ekaney, M.L.; Otto, G.P.; Sossdorf, M.; Sponholz, C.; Boehringer, M.; Loesche, W.; Rittirsch, D.; Wilharm, A.; Kurzai, O.; Bauer, M.; Claus, R.A. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit Care 2014, 18, 543. [Google Scholar] [CrossRef]
- Nakazawa, D.; Kumar, S.V.; Marschner, J.; Desai, J.; Holderied, A.; Rath, L.; Kraft, F.; Lei, Y.; Fukasawa, Y.; Moeckel, G.W.; Angelotti, M.L.; Liapis, H.; Anders, H.J. Histones and Neutrophil Extracellular Traps Enhance Tubular Necrosis and Remote Organ Injury in Ischemic AKI. J Am Soc Nephrol 2017, 28, 1753–1768. [Google Scholar] [CrossRef]
- Allam, R.; Kumar, S.V.; Darisipudi, M.N.; Anders, H.J. Extracellular histones in tissue injury and inflammation. J Mol Med (Berl) 2014, 92, 465–472. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; Woods, R.J.; Kanthi, Y.; Knight, J.S. Neutrophil extracellular traps in COVID-19. JCI Insight, 2020, 5, e138999. [Google Scholar] [CrossRef]
- Janiuk, K.; Jabłońska, E.; & Garley, M.; & Garley, M. Significance of NETs Formation in COVID-19. Cells, 2021, 10, 151. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; Tian, D.S. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; Wang, J. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front Mol Biosci 2020, 7, 157. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.; Absalón-Aguilar, A.; Nuñez-Aguirre, M.; Pérez-Fragoso, A.; Carrillo-Vázquez, D.A.; Maravillas-Montero, J.L.; Mejía-Domínguez, N.R.; Llorente, L.; Alcalá-Carmona, B.; Lira-Luna, J.; Núñez-Álvarez, C.; Juárez-Vega, G.; Meza-Sánchez, D.; Hernández-Gilsoul, T.; Tapia-Rodríguez, M.; Gómez-Martín, D. Neutrophil Extracellular Traps Contribute to COVID-19 Hyperinflammation and Humoral Autoimmunity. Cells 2021, 10, 2545. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef]
- Borges, L.; Pithon-Curi, T.C.; Curi, R.; Hatanaka, E. COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps. Mediators Inflamm 2020, 2020, 8829674. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. ; COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; Cheng, Z.; Yu, T.; Xia, J.; Wei, Y.; Wu, W.; Xie, X.; Yin, W.; Li, H.; Liu, M.; Xiao, Y.; … Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; Loda, M.; Looney, M.R.; McAllister, F.; Rayes, R.; Renaud, S.; Rousseau, S.; Salvatore, S.; Schwartz, R.E.; Spicer, J.D.; Yost, C. C.; Weber, A.; Zuo, Y.; Egeblad, M. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Samstag, Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv Biol Regul 2020, 77, 100741. [Google Scholar] [CrossRef]
- Yaqinuddin, A.; Kashir, J. Novel therapeutic targets for SARS-CoV-2-induced acute lung injury: Targeting a potential IL-1β/neutrophil extracellular traps feedback loop. Med Hypotheses, 2020, 143, 109906. [Google Scholar] [CrossRef] [PubMed]
- Yaqinuddin, A.; Kvietys, P.; Kashir, J. COVID-19: Role of neutrophil extracellular traps in acute lung injury. Respir Investig 2020, 58, 419–420. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Allegra, A.G.; Musolino, C. Coagulopathy and thromboembolic events in patients with SARS-CoV-2 infection: pathogenesis and management strategies. Ann Hematol 2020, 99, 1953–1965. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; Cody, M.J.; Manne, B.K.; Portier, I.; Harris, E.S.; Petrey, A.C.; Beswick, E.J.; Caulin, A.F.; Iovino, A.; Abegglen, L.M.; Weyrich, A.S.; Rondina, M.T.; Egeblad, M.; Schiffman, J.D.; Yost, C.C. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood, 2020, 136, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Ledderose, S.; Schulz, H.; Scherer, C.; Rudelius, M.; Zoller, M.; Höchter, D.; Keppler, O.; Teupser, D.; Zwißler, B.; von Bergwelt-Baildon, M.; Kääb, S.; Massberg, S.; Pekayvaz, K.; Stark, K. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef]
- Ng, H.; Havervall, S.; Rosell, A.; Aguilera, K.; Parv, K.; von Meijenfeldt, F.A.; Lisman, T.; Mackman, N.; Thålin, C.; Phillipson, M. Circulating Markers of Neutrophil Extracellular Traps Are of Prognostic Value in Patients With COVID-19. Arterioscler Thromb Vasc Biol 2021, 41, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pérez, M.P.; Águila, S.; Reguilón-Gallego, L.; de Los Reyes-García, A.M.; Miñano, A.; Bravo-Pérez, C.; de la Morena, M.E.; Corral, J.; García-Barberá, N.; Gómez-Verdú, J.M.; Bernal, E.; Herranz, M.T.; Vicente, V.; Martínez, C.; González-Conejero, R.; Lozano, M. L. Neutrophil extracellular traps and von Willebrand factor are allies that negatively influence COVID-19 outcomes. Clin Transl Med 2021, 11, e268. [Google Scholar] [CrossRef]
- Gando, S.; Levi, M.; Toh, C.H. Disseminated intravascular coagulation. Nat Rev Dis Primers 2016, 2, 16037. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Jr, Wrobleski, S. K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A, 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- McDonald, B.; Davis, R.P.; Kim, S.J.; Tse, M.; Esmon, C.T.; Kolaczkowska, E.; Jenne, C. N. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood, 2017, 129, 1357–1367. [Google Scholar] [CrossRef]
- Tanaka, K.; Koike, Y.; Shimura, T.; Okigami, M.; Ide, S.; Toiyama, Y.; Okugawa, Y.; Inoue, Y.; Araki, T.; Uchida, K.; Mohri, Y.; Mizoguchi, A.; Kusunoki, M. In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model. PLoS One, 2014, 9, e111888. [Google Scholar] [CrossRef]
- Komissarov, A.A.; Florova, G.; Idell, S. Effects of extracellular DNA on plasminogen activation and fibrinolysis. J Biol Chem 2011, 286, 41949–41962. [Google Scholar] [CrossRef]
- Longstaff, C.; Varjú, I.; Sótonyi, P.; Szabó, L.; Krumrey, M.; Hoell, A.; Bóta, A.; Varga, Z.; Komorowicz, E.; Kolev, K. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem 2013, 288, 6946–6956. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Bhandari, A.A.; Wagner, D.D. Histones induce rapid and profound thrombocytopenia in mice. Blood 2011, 118, 3708–3714. [Google Scholar] [CrossRef]
- Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Thomas, G.M.; Martinod, K.; De Meyer, S.F.; Bhandari, A.A.; Wagner, D.D. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost 2012, 10, 136–144. [Google Scholar] [CrossRef]
- Iba, T.; Watanabe, E.; Umemura, Y.; Wada, T.; Hayashida, K.; Kushimoto, S. Japanese Surviving Sepsis Campaign Guideline Working Group for disseminated intravascular coagulation, & Wada, H. Sepsis-associated disseminated intravascular coagulation and its differential diagnoses. J Intensive Care 2019, 7, 32. [Google Scholar]
- Fuchs, T.A.; Kremer Hovinga, J.A.; Schatzberg, D.; Wagner, D.D.; Lämmle, B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood 2012, 120, 1157–1164. [Google Scholar] [CrossRef]
- Martinod, K.; Demers, M.; Fuchs, T.A.; Wong, S.L.; Brill, A.; Gallant, M.; Hu, J.; Wang, Y.; Wagner, D.D. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A, 2013, 110, 8674–8679. [Google Scholar] [CrossRef]
- Massberg, S.; Grahl, L.; von Bruehl, M.L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; Konrad, I.; Kennerknecht, E.; Reges, K.; Holdenrieder, S.; Braun, S.; Reinhardt, C.; Spannagl, M.; Preissner, K.T.; Engelmann, B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010, 16, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Alcázar, M.; Napirei, M.; Panda, R.; Köhler, E.C.; Kremer Hovinga, J.A.; Mannherz, H.G.; Peine, S.; Renné, T.; Lämmle, B.; Fuchs, T.A. Impaired DNase1-mediated degradation of neutrophil extracellular traps is associated with acute thrombotic microangiopathies. J Thromb Haemost 2015, 13, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Njeim, R.; Azar, W.S.; Fares, A.H.; Azar, S. T.; Kfoury Kassouf, H.; Eid, A.A. NETosis contributes to the pathogenesis of diabetes and its complications. J Mol Endocrinol 2020, 65, R65–R76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Zhong, L.; Ye, D.; Zhang, J.; Tu, Y.; Bornstein, S.R.; Zhou, Z.; Lam, K.S.; Xu, A. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes. Diabetes 2014, 63, 4239–4248. [Google Scholar] [CrossRef] [PubMed]
- Diana, J.; Simoni, Y.; Furio, L.; Beaudoin, L.; Agerberth, B.; Barrat, F.; Lehuen, A. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med 2013, 19, 65–73. [Google Scholar] [CrossRef]
- Menegazzo, L.; Ciciliot, S.; Poncina, N.; Mazzucato, M.; Persano, M.; Bonora, B.; Albiero, M.; Vigili de Kreutzenberg, S.; Avogaro, A.; Fadini, G.P. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol 2015, 52, 497–503. [Google Scholar] [CrossRef]
- Carestia, A.; Frechtel, G.; Cerrone, G.; Linari, M.A.; Gonzalez, C.D.; Casais, P.; Schattner, M. NETosis before and after Hyperglycemic Control in Type 2 Diabetes Mellitus Patients. PLoS One 2016, 11, e0168647. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 2015, 21, 815–819. [Google Scholar] [CrossRef]
- Fadini, G.P.; Menegazzo, L.; Rigato, M.; Scattolini, V.; Poncina, N.; Bruttocao, A.; Ciciliot, S.; Mammano, F.; Ciubotaru, C.D.; Brocco, E.; Marescotti, M.C.; Cappellari, R.; Arrigoni, G.; Millioni, R.; Vigili de Kreutzenberg, S.; Albiero, M.; Avogaro, A. NETosis Delays Diabetic Wound Healing in Mice and Humans. Diabetes 2016, 65, 1061–1071. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.E.; Gu, J.Y.; Yoo, H.J.; Park, S.H.; Kim, Y.I.; Nam-Goong, I.S.; Kim, E.S.; Kim, H.K. Evaluation of Circulating Markers of Neutrophil Extracellular Trap (NET) Formation as Risk Factors for Diabetic Retinopathy in a Case-Control Association Study. Exp Clin Endocrinol Diabetes 2016, 124, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, X.; Yin, Y.; Mai, Y.; Wang, D.; Zhang, X. Hyperglycemia Induces Neutrophil Extracellular Traps Formation Through an NADPH Oxidase-Dependent Pathway in Diabetic Retinopathy. Front Immunol 2018, 9, 3076. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, A.; Yamada, M.; Shida, H.; Nakazawa, D.; Kusunoki, Y.; Nakamura, A.; Miyoshi, H.; Tomaru, U.; Atsumi, T.; Ishizu, A. Circulating Neutrophil Extracellular Trap Levels in Well-Controlled Type 2 Diabetes and Pathway Involved in Their Formation Induced by High-Dose Glucose. Pathobiology 2016, 83, 243–251. [Google Scholar] [CrossRef]
- Alarcon, M.F.; McLaren, Z.; Wright, H.L. Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O. Front Immunol 2021, 12, 649693. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009, 15, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, Y.; Yin, S.W.; Gao, X. J.; Shi, W.W.; Wang, Y.; Huang, X.; Wang, L.; Zou, L.Y.; Zhao, J.H.; Huang, Y.J.; Shan, L.Y.; Gounni, A.S.; Wu, Y.Z.; Zhang, J.B. Neutrophil extracellular trap formation is associated with autophagy-related signalling in ANCA-associated vasculitis. Clin Exp Immunol 2015, 180, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; Thompson, P.; Chen, P.; Fox, D.A.; Pennathur, S.; Kaplan, M.J. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013, 5, 178ra40. [Google Scholar] [CrossRef]
- Garcia-Romo, G. S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; Barrat, F.J.; Banchereau, J.; Pascual, V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011, 3, 73ra20. [Google Scholar] [CrossRef]
- Papadaki, G.; Choulaki, C.; Mitroulis, I.; Verginis, P.; Repa, A.; Raptopoulou, A.; Boumpas, D.; Sidiropoulos, P. Enhanced release of neutrophil extracellular traps from peripheral blood neutrophils in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 2012, 71, A79. [Google Scholar] [CrossRef]
- Sur Chowdhury, C.; Giaglis, S.; Walker, U. A.; Buser, A. , Hahn, S.; Hasler, P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther 2014, 16, R122. [Google Scholar] [CrossRef]
- Wang, W.; Peng, W.; Ning, X. Increased levels of neutrophil extracellular trap remnants in the serum of patients with rheumatoid arthritis. Int J Rheum Dis 2018, 21, 415–421. [Google Scholar] [CrossRef]
- Willis, V. C.; Gizinski, A. M.; Banda, N. K.; Causey, C. P.; Knuckley, B.; Cordova, K. N.; Luo, Y.; Levitt, B.; Glogowska, M.; Chandra, P.; Kulik, L.; Robinson, W. H.; Arend, W. P.; Thompson, P. R.; Holers, V. M. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol 2011, 186, 4396–4404. [Google Scholar] [CrossRef]
- Chirivi, R. G. S.; Jenniskens, G. J.; Raats, J. M. H, Anti-Citrullinated Protein Antibodies as Novel Therapeutic Drugs in Rheumatoid Arthritis. J Clin Cell Immunol 2013, S6, 006. [Google Scholar]
- Leffler, J.; Martin, M.; Gullstrand, B.; Tydén, H.; Lood, C.; Truedsson, L.; Bengtsson, A. A.; Blom, A. M. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 2012, 188, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Manderson, A. P.; Carlucci, F.; Lachmann, P. J.; Lazarus, R. A.; Festenstein, R. J.; Cook, H. T.; Walport, M. J.; Botto, M. The in vivo expression of actin/salt-resistant hyperactive DNase I inhibits the development of anti-ssDNA and anti-histone autoantibodies in a murine model of systemic lupus erythematosus. Arthritis Res Ther 2006, 8, R68. [Google Scholar] [CrossRef] [PubMed]
- Knight, J. S.; Subramanian, V.; O'Dell, A. A.; Yalavarthi, S.; Zhao, W.; Smith, C. K.; Hodgin, J. B.; Thompson, P. R.; Kaplan, M. J. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis 2015, 74, 2199–2206. [Google Scholar] [CrossRef]
- Smith, H. A.; Kang, Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl) 2013, 91, 411–429. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, J. Neutrophil extracellular traps: New players in cancer research. Front Immunol 2022, 13, 937565. [Google Scholar] [CrossRef]
- Fridlender, Z. G.; Albelda, S. M. Tumor-associated neutrophils: friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef]
- Piccard, H.; Muschel, R. J.; Opdenakker, G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol 2012, 82, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L. The pro-tumor effect and the anti-tumor effect of neutrophils extracellular traps. Biosci Trends 2020, 13, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Berger-Achituv, S.; Brinkmann, V.; Abed, U. A.; Kühn, L. I.; Ben-Ezra, J.; Elhasid, R.; Zychlinsky, A. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol 2013, 4, 48. [Google Scholar] [CrossRef]
- Chen, CJ.; Wu, C.C.; Chang, C.Y.; Li, J.R.; Ou, Y.C.; Chen, W.Y.; Liao, S.L.; Wang, J.D. Metformin Mitigated Obesity-Driven Cancer Aggressiveness in Tumor-Bearing Mice. Int J Mol Sci 2022, 23, 9134. [Google Scholar] [CrossRef]
- Demers, M.; Krause, D. S.; Schatzberg, D.; Martinod, K.; Voorhees, J. R.; Fuchs, T. A.; Scadden, D. T.; Wagner, D. D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A 2012, 109, 13076–13081. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Franco, M. M.; Leon-Rodriguez, E.; Torres-Ruiz, J. J.; Gómez-Martín, D.; Angles-Cano, E.; de la Luz Sevilla-González, M. Neutrophil Extracellular Traps Associate with Clinical Stages in Breast Cancer. Pathol Oncol Res 2020, 26, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Najmeh, S.; Cools-Lartigue, J.; Rayes, R. F.; Gowing, S.; Vourtzoumis, P.; Bourdeau, F.; Giannias, B.; Berube, J.; Rousseau, S.; Ferri, L. E.; Spicer, J. D. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int J Cancer 2017, 140, 2321–2330. [Google Scholar] [CrossRef]
- Mishalian, I. , et al., Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother, 2013. 62(11): p. 1745-56.
- Sangaletti, S.; Tripodo, C.; Vitali, C.; Portararo, P.; Guarnotta, C.; Casalini, P.; Cappetti, B.; Miotti, S.; Pinciroli, P.; Fuligni, F.; Fais, F.; Piccaluga, P. P.; Colombo, M. P. Defective stromal remodeling and neutrophil extracellular traps in lymphoid tissues favor the transition from autoimmunity to lymphoma. Cancer Discov 2014, 4, 110–129. [Google Scholar] [CrossRef]
- McDonald, B.; Spicer, J.; Giannais, B.; Fallavollita, L.; Brodt, P.; Ferri, L. E. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer 2009, 125, 1298–1305. [Google Scholar] [CrossRef]
- Spicer, J. D.; McDonald, B.; Cools-Lartigue, J. J.; Chow, S. C.; Giannias, B.; Kubes, P.; Ferri, L. E. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res 2012, 72, 3919–3927. [Google Scholar] [CrossRef]
- Cools-Lartigue, J.; Spicer, J.; Najmeh, S.; Ferri, L. Neutrophil extracellular traps in cancer progression. Cell Mol Life Sci 2014, 71, 4179–4194. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A. M.; Rzymkiewicz, D. M.; Ji, H.; Gregory, A. D.; Egea, E. E.; Metz, H. E.; Stolz, D. B.; Land, S. R.; Marconcini, L. A.; Kliment, C. R.; Jenkins, K. M.; Beaulieu, K. A.; Mouded, M.; Frank, S. J.; Wong, K. K.; Shapiro, S. D. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med 2010, 16, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A. D.; Hale, P.; Perlmutter, D. H.; Houghton, A. M. Clathrin pit-mediated endocytosis of neutrophil elastase and cathepsin G by cancer cells. J Biol Chem 2012, 287, 35341–35350. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.; Carré, A.; Ardi, V.; Muramatsu, T.; Schmidt, J.; Pham, C.; Quigley, J.P. Neutrophil Elastase Facilitates Tumor Cell Intravasation and Early Metastatic Events. iScience 2020, 23, 101799. [Google Scholar] [CrossRef]
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin Cancer Biol 2019, 56, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L. M.; Tinkle, C. L.; Hanahan, D.; Werb, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000, 103, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights Into the Role of Matrix Metalloproteinases in Cancer and its Various Therapeutic Aspects: A Review. Front Mol Biosci 2022, 9, 896099. [Google Scholar] [CrossRef]
- Wilson, T. J.; Nannuru, K. C.; Futakuchi, M.; Singh, R. K. Cathepsin G-mediated enhanced TGF-beta signaling promotes angiogenesis via upregulation of VEGF and MCP-1. Cancer Lett 2010, 288, 162–169. [Google Scholar] [CrossRef]
- Morimoto-Kamata, R.; Mizoguchi, S.; Ichisugi, T.; Yui, S. Cathepsin G induces cell aggregation of human breast cancer MCF-7 cells via a 2-step mechanism: catalytic site-independent binding to the cell surface and enzymatic activity-dependent induction of the cell aggregation. Mediators Inflamm 2012, 2012, 456462. [Google Scholar] [CrossRef]
- Guan, X.; Lu, Y.; Zhu, H.; Yu, S.; Zhao, W. ; Chi,X. ; Xie, C.; Yin, Z. The Crosstalk Between Cancer Cells and Neutrophils Enhances Hepatocellular Carcinoma Metastasis via Neutrophil Extracellular Traps-Associated Cathepsin G Component: A Potential Therapeutic Target. J Hepatocell Carcinoma 2021, 8, 451–465. [Google Scholar]
- Redman, C.W.; Sargent, I.L. Sargent, Latest advances in understanding preeclampsia. Science 2005. 308, 1592–1594.
- Miranda, M.L.; Macher, H.C.; Muñoz-Hernández, R.; Vallejo-Vaz, A.; Moreno-Luna, R.; Villar, J.; Guerrero, J.M.; Stiefel, P. Role of circulating cell-free DNA levels in patients with severe preeclampsia and HELLP syndrome. Am J Hypertens 2013, 26, 1377–1380. [Google Scholar] [CrossRef]
- Gupta, A.; Hasler, P.; Gebhardt, S.; Holzgreve, W.; Hahn, S. Occurrence of neutrophil extracellular DNA traps (NETs) in pre-eclampsia: a link with elevated levels of cell-free DNA? Ann N Y Acad Sci 2006, 1075, 118–122. [Google Scholar] [CrossRef]
- Hasler, P.; Giaglis, S.; Hahn, S. Neutrophil extracellular traps in health and disease. Swiss Med Wkly 2016, 146, w14352. [Google Scholar] [CrossRef]
- Marder, W.; Knight, J.S.; Kaplan, M.J.; Somers, E.C.; Zhang, X.; O'Dell, A.A.; Padmanabhan, V.; Lieberman, R.W. Placental histology and neutrophil extracellular traps in lupus and pre-eclampsia pregnancies. Lupus Sci Med 2016, 3, e000134. [Google Scholar] [CrossRef]
- Saguil, A.; Fargo, M.; Grogan, S. Diagnosis and management of kawasaki disease. Am Fam Physician 2015, 91, 365–371. [Google Scholar]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; Kobayashi, T.; Wu, M.H.; Saji, T.T.; Pahl, E. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Tian, J.; Lv, H.T.; An, X.J.; Ling, N.; Xu, F. Endothelial microparticles induce vascular endothelial cell injury in children with Kawasaki disease. Eur Rev Med Pharmacol Sci 2016, 20, 1814–1818. [Google Scholar]
- Newburger, J.W.; Takahashi, M.; Burns, J.C. ; Kawasaki Disease. J Am Coll Cardiol 2016, 67, 1738–1749. [Google Scholar] [CrossRef]
- Richter, J.R.; Sanderson, R.D. The glycocalyx: pathobiology and repair. Matrix Biol Plus 2023, 17, 100128. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Yasudo, H.; Suzuki, Y.; Furuta, T.; Matsuguma, C.; Azuma, Y.; Miyake, A.; Okada, S.; Ichihara, K.; Ohga, S.; Hasegawa, S. Circulating endothelial glycocalyx components as a predictive marker of coronary artery lesions in Kawasaki disease. Int J Cardiol 2019, 292, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, Y.; Yang, X.; Yan, C.; Feng, Y. Recent Insights into Neutrophil Extracellular Traps in Cardiovascular Diseases. J Clin Med 2022, 11, 6662. [Google Scholar] [CrossRef]
- Hu, J.; Qian, W.; Yu, Z.; Xu, T.; Ju, L.; Hua, Q.; Wang, Y.; Ling, J.J.; Lv, H. Increased Neutrophil Respiratory Burst Predicts the Risk of Coronary Artery Lesion in Kawasaki Disease. Front Pediatr 2020, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Takeshita, S.; Kawamura, Y.; Kanai, T.; Tsujita, Y.; Nonoyama, S. Enhanced formation of neutrophil extracellular traps in Kawasaki disease. Pediatr Res 2020, 87, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Ding, M.; Fu, J.; Xiao, Y.; Chen, X.; Zhang, Q. Neutrophil extracellular trap from Kawasaki disease alter the biologic responses of PBMC. Biosci Rep 2020, 40, BSR20200928. [Google Scholar] [CrossRef] [PubMed]
- Zandstra, J.; van de Geer, A.; Tanck, M.W.T.; van Stijn-Bringas Dimitriades, D.; Aarts, C.E.M.; Dietz, S.M.; van Bruggen, R.; Schweintzger, N.A.; Zenz, W.; Emonts, M.; Zavadska, D.; Pokorn, M.; Usuf, E.; Moll, H.A.; Schlapbach, L.J.; Carrol, E.D.; Paulus, S.; Tsolia, M.; Fink, C.; Yeung, S.; Shimizu, C.; Tremoulet, A.; Galassini, R.; Wright, V.J.; Martinón-Torres, F.; Herberg, J.; Burns, J.; Levin, M.; Kuijpers, T.W.; EUCLIDS Consortium, PERFORM Consortium and UK Kawasaki Disease Genetics Study Network. Biomarkers for the Discrimination of Acute Kawasaki Disease From Infections in Childhood. Front Pediatr 2020, 22, 355. [Google Scholar] [CrossRef]
- Martinod, K.; Fuchs, T.A.; Zitomersky, N.L.; Wong, S.L.; Demers, M.; Gallant, M.; Wang, Y.; Wagner, D.D. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 2015, 125, 1948–1956. [Google Scholar] [CrossRef]
- Sorvillo, N.; Cherpokova, D.; Martinod, K.; Wagner, D.D. Extracellular DNA NET-Works With Dire Consequences for Health. Circ Res 2019, 125, 470–488. [Google Scholar] [CrossRef]
- Martinod, K.; Wagner, D.D. Thrombosis: tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Savchenko, A.S.; Borissoff, J.I.; Martinod, K.; De Meyer, S.F.; Gallant, M.; Erpenbeck, L.; Brill, A.; Wang, Y.; Wagner, D.D. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 2014, 123, 141–148. [Google Scholar] [CrossRef]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 2010, 107, 9813–9818. [Google Scholar] [CrossRef]
- Wong, S.L.; Wagner, D.D. Wagner, Peptidylarginine deiminase 4: a nuclear button triggering neutrophil extracellular traps in inflammatory diseases and aging. FASEB J 2018, 32, fj201800691R. [Google Scholar] [CrossRef]
- Patutina, O.; Mironova, N.; Ryabchikova, E.; Popova, N.; Nikolin, V.; Kaledin, V.; Vlassov, V.; Zenkova, M. Inhibition of metastasis development by daily administration of ultralow doses of RNase A and DNase I. Biochimie 2011, 93, 689–696. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.F.; Suidan, G.L.; Fuchs, T.A.; Monestier, M.; Wagner, D.D. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol 2012, 32, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 2013, 123, 3446–3458. [Google Scholar] [CrossRef] [PubMed]
| References | Year | Results |
|---|---|---|
| Martinod et al [230] | 2014 | NET inhibition by DNase I administration showed protective role against thrombosis in vivo murine model of ischemic stroke, myocardial infarction and DVT. |
| Brill et al [163] | 2012 | Infusion of DNase I protected mice from DVT through inhibition of NETs. |
| De Meyer et al [235] |
2012 | NET inhibition by DNase I improves ischemic stroke outcome in mice model. |
| Savchenko et al [231] |
2014 | DNase I therapy against NETs in mice model exerts cardioprotective effects and improve cardiac contractile function. |
| Papayannopoulos et al [107] | 2011 | DNase I therapy enhances sputum solubilization in CF patients |
| Wong et al[233] | 2018 | Elevated NETs contribute to diabetes complications and NET disruption by using DNase I therapy improves wound healing. |
| Patutina et al [234] | 2011 | DNase I treatment inhibits tumor progression and dissemination. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





