Submitted:
26 September 2023
Posted:
28 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Study design
3. Material and methods
3.1. Routine sample processing startegy
3.2. Genexus analysis
4. Results
4.1. Hot spot mutations
4.2. Fusions rearrangements
5. Discussion
Author Contributions
Funding
Patient consent for publication
Ethics approval
Competing interests
References
- Yates, L.; Seoane, J.; Le Tourneau, C.; Siu, L.; Marais, R.; Michiels, S.; Soria, J.; Campbell, P.; Normanno, N.; Scarpa, A.; et al. The European Society for Medical Oncology (ESMO) Precision Medicine Glossary. Ann. Oncol. 2017, 29, 30–35. [Google Scholar] [CrossRef]
- Ciardiello, F.; Arnold, D.; Casali, P.G.; Cervantes, A.; Douillard, J.-Y.; Eggermont, A.; Eniu, A.; McGregor, K.; Peters, S.; Piccart, M.; et al. Delivering precision medicine in oncology today and in future—the promise and challenges of personalised cancer medicine: a position paper by the European Society for Medical Oncology (ESMO). Ann. Oncol. 2014, 25, 1673–1678. [Google Scholar] [CrossRef]
- Di Nicolantonio, F.; Martini, M.; Molinari, F.; Sartore-Bianchi, A.; Arena, S.; Saletti, P.; De Dosso, S.; Mazzucchelli, L.; Frattini, M.; Siena, S.; et al. Wild-Type BRAF Is Required for Response to Panitumumab or Cetuximab in Metastatic Colorectal Cancer. J. Clin. Oncol. 2008, 26, 5705–5712. [Google Scholar] [CrossRef] [PubMed]
- Lièvre, A.; Bachet, J.B.; Boige, V.; Cayre, A.; Le Corre, D.; Buc, E.; Ychou, M.; Bouché, O.; Landi, B.; Louvet, C.; André, T.; Bibeau, F.; Diebold, M.D.; Rougier, P.; Ducreux, M.; Tomasic, G.; Emile, J.F.; Penault-Llorca, F.; Laurent-Puig, P. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008, 26, 374–9. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011, 364, 2507–16. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R. Targeted therapies in gastrointestinal stromal tumors. Semin. Diagn. Pathol. 2008, 25, 295–303. [Google Scholar] [CrossRef]
- Fusco, N.; Malapelle, U.; Fassan, M.; Marchiò, C.; Buglioni, S.; Zupo, S.; Criscitiello, C.; Vigneri, P.; Tos, A.P.D.; Maiorano, E.; et al. PIK3CA Mutations as a Molecular Target for Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-R.; Schultheis, A.M.; Yu, H.; Mandelker, D.; Ladanyi, M.; Büttner, R. Precision medicine in non-small cell lung cancer: Current applications and future directions. Semin. Cancer Biol. 2020, 84, 184–198. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular Testing Guideline for the Selection of Patients With Lung Cancer for Treatment With Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.J.; Arcila, M.E.; Corless, C.; Kamel-Reid, S.; Lubin, I.M.; Pfeifer, J.; Temple-Smolkin, R.L.; Voelkerding, K.V.; Nikiforova, M.N. Guidelines for Validation of Next-Generation Sequencing-Based Oncology Panels: A Joint Consensus Recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn. 2017, 19, 341–365. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, A.R.; Hamilton, S.R.; Allegra, C.J.; Grody, W.; Cushman-Vokoun, A.M.; Funkhouser, W.K.; Kopetz, S.E.; Lieu, C.; Lindor, N.M.; Minsky, B.D.; et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 1453–1486. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; Nishiwaki, Y.; Ohe, Y.; Yang, J.J.; Chewaskulyong, B.; Jiang, H.; Duffield, E.L.; Watkins, C.L.; Armour, A.A.; Fukuoka, M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009, 361, 947–57. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G.; Johnson, B.E.; Berry, L.D.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Varella-Garcia, M.; Franklin, W.A.; Aronson, S.L.; Su, P.-F.; et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA 2014, 311, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Brusco, L.; Shaw, K.; Horombe, C.; Kopetz, S.; Davies, M.A.; Routbort, M.; Piha-Paul, S.A.; Janku, F.; Ueno, N.; et al. Feasibility of Large-Scale Genomic Testing to Facilitate Enrollment Onto Genomically Matched Clinical Trials. J. Clin. Oncol. 2015, 33, 2753–2762. [Google Scholar] [CrossRef]
- Sundaresan, T.K.; Sequist, L.V.; Heymach, J.V.; Riely, G.J.; Jänne, P.A.; Koch, W.H.; Sullivan, J.P.; Fox, D.B.; Maher, R.; Muzikansky, A.; Webb, A.; Tran, H.T.; Giri, U.; Fleisher, M.; Yu, H.A.; Wei, W.; Johnson, B.E.; Barber, T.A.; Walsh, J.R.; Engelman, J.A.; Stott, S.L.; Kapur, R.; Maheswaran, S.; Toner, M.; Haber, D.A. Detection of T790M, the Acquired Resistance EGFR Mutation, by Tumor Biopsy versus Noninvasive Blood-Based Analyses. Clin Cancer Res. 2016, 22, 1103–10. [Google Scholar] [CrossRef]
- Roy-Chowdhuri, S.; Chow, C.; Kane, M.K.; Yao, H.; Wistuba, I.I.; Krishnamurthy, S.; Stewart, J.; Staerkel, G. Optimizing the DNA yield for molecular analysis from cytologic preparations. Cancer Cytopathol. 2015, 124, 254–260. [Google Scholar] [CrossRef]
- Pepe, F.; De Luca, C.; Smeraglio, R.; Pisapia, P.; Sgariglia, R.; Nacchio, M.; Russo, M.; Serra, N.; Rocco, D.; Battiloro, C.; et al. Performance analysis of SiRe next-generation sequencing panel in diagnostic setting: focus on NSCLC routine samples. J. Clin. Pathol. 2018, 72, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Velizheva, N.P.; Rechsteiner, M.P.; Wong, C.E.; Zhong, Q.; Rössle, M.; Bode, B.; Moch, H.; Soltermann, A.; Wild, P.J.; Tischler, V. Cytology smears as excellent starting material for next-generation sequencing-based molecular testing of patients with adenocarcinoma of the lung. Cancer Cytopathol. 2016, 125, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef]
- Pisapia, P.; Pepe, F.; Iaccarino, A.; Sgariglia, R.; Nacchio, M.; Conticelli, F.; Salatiello, M.; Tufano, R.; Russo, G.; Gragnano, G.; et al. Next Generation Sequencing in Cytopathology: Focus on Non-Small Cell Lung Cancer. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Hayashi, H.; Tanishima, S.; Fujii, K.; Mori, R.; Okada, C.; Yanagita, E.; Shibata, Y.; Matsuoka, R.; Amano, T.; Yamada, T.; et al. Clinical impact of a cancer genomic profiling test using an in-house comprehensive targeted sequencing system. Cancer Sci. 2020, 111, 3926–3937. [Google Scholar] [CrossRef]
- Kou, T.; Kanai, M.; Yamamoto, Y.; Kamada, M.; Nakatsui, M.; Sakuma, T.; Mochizuki, H.; Hiroshima, A.; Sugiyama, A.; Nakamura, E.; et al. Clinical sequencing using a next-generation sequencing-based multiplex gene assay in patients with advanced solid tumors. Cancer Sci. 2017, 108, 1440–1446. [Google Scholar] [CrossRef]
- Pisapia, P.; Pepe, F.; Baggi, A.; Barberis, M.; Galvano, A.; Gristina, V.; Mastrilli, F.; Novello, S.; Pagni, F.; Pasini, S.; et al. Next generation diagnostic algorithm in non-small cell lung cancer predictive molecular pathology: The KWAY Italian multicenter cost evaluation study. Crit. Rev. Oncol. 2022, 169, 103525. [Google Scholar] [CrossRef]
- Low, S.-K.; Ariyasu, R.; Uchibori, K.; Hayashi, R.; Chan, H.T.; Chin, Y.M.; Akita, T.; Harutani, Y.; Kiritani, A.; Tsugitomi, R.; et al. Rapid genomic profiling of circulating tumor DNA in non-small cell lung cancer using Oncomine Precision Assay with GenexusTM integrated sequencer. Transl. Lung Cancer Res. 2022, 11, 711–721. [Google Scholar] [CrossRef]
- Ilié, M.; Hofman, V.; Bontoux, C.; Heeke, S.; Lespinet-Fabre, V.; Bordone, O.; Lassalle, S.; Lalvée, S.; Tanga, V.; Allegra, M.; et al. Setting Up an Ultra-Fast Next-Generation Sequencing Approach as Reflex Testing at Diagnosis of Non-Squamous Non-Small Cell Lung Cancer; Experience of a Single Center (LPCE, Nice, France). Cancers 2022, 14, 2258. [Google Scholar] [CrossRef]
- Sheffield, B.S.; Beharry, A.; Diep, J.; Perdrizet, K.; Iafolla, M.A.J.; Raskin, W.; Dudani, S.; Brett, M.A.; Starova, B.; Olsen, B.; et al. Point of Care Molecular Testing: Community-Based Rapid Next-Generation Sequencing to Support Cancer Care. Curr. Oncol. 2022, 29, 1326–1334. [Google Scholar] [CrossRef]
- Malapelle, U.; Mayo de-Las-Casas, C.; Rocco, D.; Garzon, M.; Pisapia, P.; Jordana-Ariza, N.; Russo, M.; Sgariglia, R.; De Luca, C.; Pepe, F.; Martinez-Bueno, A.; Morales-Espinosa, D.; González-Cao, M.; Karachaliou, N.; Viteri Ramirez, S.; Bellevicine, C.; Molina-Vila, M.A.; Rosell, R.; Troncone, G. Development of a gene panel for next-generation sequencing of clinically relevant mutations in cell-free DNA from cancer patients. Br J Cancer. 2017, 116, 802–810. [Google Scholar] [CrossRef]
- Pepe, F.; De Luca, C.; Smeraglio, R.; Pisapia, P.; Sgariglia, R.; Nacchio, M.; Russo, M.; Serra, N.; Rocco, D.; Battiloro, C.; et al. Performance analysis of SiRe next-generation sequencing panel in diagnostic setting: focus on NSCLC routine samples. J. Clin. Pathol. 2018, 72, 38–45. [Google Scholar] [CrossRef]
- Malapelle, U.; Pepe, F.; Pisapia, P.; Sgariglia, R.; Nacchio, M.; De Luca, C.; Lacalamita, R.; Tommasi, S.; Pinto, R.; Palomba, G.; et al. Harmonization of Next-Generation Sequencing Procedure in Italian Laboratories: A Multi-Institutional Evaluation of the SiRe® Panel. Front. Oncol. 2020, 10, 236. [Google Scholar] [CrossRef]
- Yang, S.R.; Schultheis, A.M.; Yu, H.; Mandelker, D.; Ladanyi, M.; Büttner, R. Precision medicine in non-small cell lung cancer: current applications and future directions. Semin. Cancer Biol. Epub ahead of print. 2020. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.; Barlesi, F.; Lolkema, M.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Werner, R.; Connolly, A.; Bennett, M.; Hand, C.K.; Burke, L. Implementation of an ISO15189 accredited next-generation sequencing service with the fully automated Ion Torrent Genexus: the experience of a clinical diagnostic laboratory. J. Clin. Pathol. 2022. [Google Scholar] [CrossRef]
| ID | Sex | Age | Sample Type | Tumour | N.C. | Clinical Request |
|---|---|---|---|---|---|---|
| DNA 1* | M | 78 | Resection | CRC | 70.0% | RAS, BRAF |
| DNA 2* | M | 78 | Resection | CRC | 70.0% | RAS, BRAF |
| DNA 3 | M | 89 | Biopsy | CRC | 50.0% | RAS, BRAF |
| DNA 4 | F | 68 | Resection | NSCLC | 70.0% | EGFR, KRAS, BRAF |
| DNA 5 | M | 73 | Resection | CRC | 50.0% | RAS, BRAF |
| DNA 6 | M | 53 | Biopsy | NSCLC | 30.0% | EGFR, KRAS, BRAF |
| DNA 7 | M | 66 | Resection | CRC | 40.0% | RAS, BRAF |
| DNA 8 | F | 78 | Resection | CRC | 40.0% | RAS, BRAF |
| DNA 9 | F | 67 | Resection | NSCLC | 60.0% | EGFR, KRAS, BRAF |
| DNA 10 | F | 51 | Resection | CRC | 30.0% | RAS, BRAF |
| DNA 11 | M | 50 | Resection | CRC | 80.0% | c-KIT, PDGFRA |
| DNA 12 | F | 50 | Biopsy | NSCLC | 50.0% | EGFR, KRAS, BRAF |
| DNA 13 | M | 70 | Biopsy | NSCLC | 20.0% | EGFR, KRAS, BRAF |
| DNA 14 | F | 59 | Resection | NSCLC | 40.0% | EGFR, KRAS, BRAF |
| DNA 15 | M | 66 | Biopsy | NSCLC | 30.0% | EGFR, KRAS, BRAF |
| DNA 16 | M | 56 | Resection | CRC | 50.0% | RAS, BRAF |
| DNA 17 | M | 66 | Resection | NSCLC | 60.0% | EGFR, KRAS, BRAF |
| DNA 18 | F | 51 | Biopsy | CRC | 50.0% | RAS, BRAF |
| DNA 19 | F | 41 | Biopsy | BC | 30.0% | PIK3CA |
| DNA 20 | F | 82 | Biopsy | CRC | 30.0% | RAS, BRAF |
| DNA 21 | M | 67 | Biopsy | CRC | 50.0% | RAS, BRAF |
| DNA 22 | M | 82 | Resection | NSCLC | 80.0% | EGFR, KRAS, BRAF |
| DNA 23 | M | 74 | Resection | NSCLC | 70.0% | EGFR, KRAS, BRAF |
| DNA 24 | M | 74 | Resection | CRC | 40.0% | RAS, BRAF |
| DNA 25 | F | 44 | Biopsy | CRC | 40.0% | RAS, BRAF |
| DNA 26 | F | 69 | Biopsy | NSCLC | 60.0% | EGFR, KRAS, BRAF |
| DNA 27 | M | 54 | Resection | CRC | 30.0% | RAS, BRAF |
| DNA 28 | F | 74 | Resection | MM | 90.0% | BRAF, NRAS |
| DNA 29 | F | 63 | Biopsy | NSCLC | 40.0% | EGFR, KRAS, BRAF |
| DNA 30 | M | 56 | Resection | NSCLC | 50.0% | EGFR, KRAS, BRAF |
| DNA 31 | F | 52 | Resection | CRC | 60.0% | RAS, BRAF |
| DNA 32 | F | 45 | Resection | BC | 60.0% | PIK3CA |
| ID | Sex | Age | Sample Type | Tumour | N.C. | Clinical Request |
|---|---|---|---|---|---|---|
| RNA 1 | M | 56 | Resection | NSCLC | 60.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 2 | F | 58 | Biopsy | NSCLC | 70.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 3 | M | 77 | Biopsy | NSCLC | 25.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 4 | M | 79 | Resection | NSCLC | 70.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 5 | M | 79 | Biopsy | NSCLC | 30.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 6 | M | 59 | Biopsy | NSCLC | 30.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 7 | F | 70 | Biopsy | NSCLC | 50.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 8 | M | 62 | Biopsy | NSCLC | 25.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 9 | M | 61 | Biopsy | NSCLC | 40.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 10 | M | 66 | Resection | NSCLC | 60.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 11 | M | 68 | Biopsy | NSCLC | 40.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 12 | M | 64 | Biopsy | NSCLC | 50.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 13 | F | 65 | Biopsy | NSCLC | 60.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 14 | M | 58 | Biopsy | NSCLC | 20.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 15 | F | 79 | Biopsy | NSCLC | 50.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 16 | M | 52 | Biopsy | NSCLC | 50.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 17 | M | 67 | Resection | NSCLC | 60.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 18 | M | 87 | Biopsy | NSCLC | 40.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 19 | M | 25 | Biopsy | NSCLC | 60.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 20 | F | 60 | Biopsy | NSCLC | 30.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 21 | M | 60 | Resection | NSCLC | 60.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 22 | F | 36 | Biopsy | NSCLC | 30.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 23 | M | 66 | Biopsy | NSCLC | 60.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 24 | F | 47 | Biopsy | NSCLC | 50.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 25 | M | 67 | Biopsy | NSCLC | 30.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 26 | F | 64 | Biopsy | NSCLC | 10.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 27 | M | 54 | Biopsy | NSCLC | 40.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 28 | F | 37 | Biopsy | NSCLC | 50.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 29 | M | 79 | Biopsy | NSCLC | 50.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 30 | F | 71 | Biopsy | NSCLC | 30.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 31 | M | 68 | Biopsy | NSCLC | 50.0% | ALK, ROS1, RET, MET, NTRK |
| RNA 32 | F | 72 | Biopsy | NSCLC | 70.0% | ALK, ROS1, RET, MET, NTRK |
| DNA Analysis Technical Parameters - S5 Plus (SiRe™ Panel) vs Genexus (OPA Panel) | |||||||
|---|---|---|---|---|---|---|---|
| ID | Platform | Total Reads | Mean Read Length | Mapped Reads | On Target Reads | Mean Depth | Uniformity |
| DNA 1* | S5 Plus | 254212 | 126 | 253622 | 94.6% | 5712 | 100% |
| Genexus | 872831 | 76 | 736530 | 77.7% | 2044 | 99.1% | |
| DNA 2* | S5 Plus | 215464 | 128 | 215047 | 92.6% | 4740 | 100% |
| Genexus | 732691 | 84 | 663064 | 83.9% | 2034 | 98.8% | |
| DNA 3 | S5 Plus | 298541 | 135 | 297999 | 93.9% | 6662 | 100% |
| Genexus | 1143038 | 91 | 1076855 | 88.8% | 3528 | 98.1% | |
| DNA 4 | S5 Plus | 524926 | 155 | 523086 | 92.3% | 11489 | 100% |
| Genexus | 1419289 | 101 | 1393603 | 92.9% | 5210 | 98.1% | |
| DNA 5 | S5 Plus | 361148 | 137 | 360373 | 91.3% | 7830 | 100% |
| Genexus | 1094620 | 98 | 1064051 | 91.5% | 3810 | 98.6% | |
| DNA 6 | S5 Plus | 314176 | 128 | 313706 | 99.2% | 7406 | 100% |
| Genexus | 1090358 | 98 | 1049935 | 90.8% | 3837 | 99,0% | |
| DNA 7 | S5 Plus | 635201 | 142 | 634226 | 92.1% | 13911 | 100% |
| Genexus | 1002231 | 92 | 946318 | 88.9% | 3150 | 98.9% | |
| DNA 8 | S5 Plus | 524182 | 131 | 523608 | 93.0% | 11591 | 100% |
| Genexus | 1262760 | 95 | 1208543 | 90.9% | 4176 | 98.9% | |
| DNA 9 | S5 Plus | 942781 | 161 | 940605 | 94.6% | 21192 | 100% |
| Genexus | 1791041 | 97 | 1756414 | 93,0% | 6097 | 97.9% | |
| DNA 10 | S5 Plus | 393979 | 126 | 393371 | 89.5% | 8381 | 100% |
| Genexus | 989635 | 60 | 717385 | 64.9% | 1459 | 98.9% | |
| DNA 11 | S5 Plus | 451494 | 139 | 450779 | 94.4% | 10127 | 100% |
| Genexus | 776893 | 78 | 679358 | 80.4% | 1863 | 96.7% | |
| DNA 12 | S5 Plus | 88915 | 129 | 88784 | 98.0% | 2072 | 92.9% |
| Genexus | 1297992 | 91 | 1263558 | 92.7% | 3996 | 93.9% | |
| DNA 13 | S5 Plus | 296845 | 143 | 296434 | 96.2% | 6790 | 100% |
| Genexus | 1196122 | 99 | 1174442 | 92.7% | 4258 | 98.5% | |
| DNA 14 | S5 Plus | 37206 | 133 | 37173 | 95.2% | 842,7 | 97.6% |
| Genexus | 1125616 | 97 | 1093531 | 91.8% | 3824 | 98.6% | |
| DNA 15 | S5 Plus | 782397 | 150 | 780894 | 95.2% | 17703 | 100% |
| Genexus | 1465786 | 92 | 1423741 | 91.9% | 4574 | 95.3% | |
| DNA 16 | S5 Plus | 378978 | 140 | 378373 | 93.3% | 8402 | 100% |
| Genexus | 1084647 | 87 | 1012693 | 87.6% | 3054 | 98.2% | |
| DNA 17 | S5 Plus | 520304 | 135 | 519653 | 91.5% | 11317 | 100% |
| Genexus | 1048030 | 98 | 1016324 | 91.4% | 3617 | 98.8% | |
| DNA 18 | S5 Plus | 49127 | 138 | 49055 | 95.3% | 1113 | 97.6% |
| Genexus | 1294194 | 97 | 1256161 | 91.9% | 4435 | 98.9% | |
| DNA 19 | S5 Plus | 486407 | 147 | 485652 | 96.6% | 11165 | 97.6% |
| Genexus | 1343529 | 97 | 1311776 | 92.3% | 4658 | 99.4% | |
| DNA 20 | S5 Plus | 346019 | 131 | 345464 | 97.4% | 8010 | 97.6% |
| Genexus | 974476 | 71 | 759420 | 75.7% | 2023 | 98.8% | |
| DNA 21 | S5 Plus | 67488 | 130 | 67417 | 95.9% | 1540 | 97.6% |
| Genexus | 1150249 | 90 | 1094010 | 90.3% | 3519 | 98.8% | |
| DNA 22 | S5 Plus | 52080 | 170 | 51956 | 90.4% | 1119 | 100% |
| Genexus | 1494337 | 100 | 1470085 | 92.3% | 5451 | 97.9% | |
| DNA 23 | S5 Plus | 614960 | 141 | 613813 | 96.2% | 14059 | 97.6% |
| Genexus | 1574234 | 91 | 1510266 | 91.2% | 4865 | 97.7% | |
| DNA 24 | S5 Plus | 188967 | 136 | 188623 | 98.1% | 4407 | 97.6% |
| Genexus | 1093646 | 103 | 1071141 | 92.2% | 4072 | 99.1% | |
| DNA 25 | S5 Plus | 140163 | 145 | 139930 | 95.5% | 3183 | 97.6% |
| Genexus | 949852 | 94 | 911448 | 90,0% | 3064 | 99.4% | |
| DNA 26 | S5 Plus | 40233 | 142 | 40180 | 96.7% | 925,4 | 97.6% |
| Genexus | 1497022 | 99 | 1476425 | 93.7% | 5365 | 98.3% | |
| DNA 27 | S5 Plus | 153378 | 133 | 153236 | 96.0% | 3501 | 97.6% |
| Genexus | 1059772 | 95 | 1021186 | 90.2% | 3498 | 98.7% | |
| DNA 28 | S5 Plus | 155154 | 118 | 154695 | 96.5% | 3553 | 92.8% |
| Genexus | 424900 | 75 | 365139 | 79.3% | 994 | 97.4% | |
| DNA 29 | S5 Plus | 358001 | 160 | 356995 | 95.2% | 8095 | 100% |
| Genexus | 1165795 | 98 | 1134969 | 92.2% | 4075 | 98.4% | |
| DNA 30 | S5 Plus | 275579 | 149 | 274340 | 98.4% | 6428 | 100% |
| Genexus | 1080846 | 92 | 1034348 | 90.3% | 3392 | 98.4% | |
| DNA 31 | S5 Plus | 259364 | 130 | 258623 | 92.6% | 5702 | 100% |
| Genexus | 1109488 | 92 | 1054465 | 89.9% | 3457 | 98.9% | |
| DNA 32 | S5 Plus | 263420 | 126 | 262682 | 93.4% | 5841 | 97.6% |
| Genexus | 710181 | 82 | 631880 | 82.5% | 1893 | 96.7% | |
| ID | S5Plus (SiRe™ Panel) | Genexus (OPA Panel) |
|---|---|---|
| DNA 1* |
KRAS p.G12C 27.6% PIK3CA p.H1047R 35.0% |
KRAS p.G12C 32.9% PIK3CA p.H1047R 33.2% |
| DNA 2* |
KRAS p.G12C 37.2% PIK3CA p.H1047R 42.2% |
KRAS p.G12C 32.7% PIK3CA p.H1047R 36.4% |
| DNA 3 | KRAS p.G12D 20.7% | KRAS p.G12D 18.9% |
| DNA 4 | EGFR p.L858R 27.7% | EGFR p.L858R 18.9% |
| DNA 5 | KRAS p.G12V 34.5% | KRAS p.G12V 33.0% |
| DNA 6 | WT | WT |
| DNA 7 | KRAS p.G12D 57.2% | KRAS p.G12D 60.8% |
| DNA 8 | KRAS p.Q61K 16.8% | KRAS p.Q61K 19.3% |
| DNA 9 | WT | WT |
| DNA 10 | KRAS p.G12D 50.6% | KRAS p.G12D 55.3% |
| DNA 11 | c-KIT p.L576P 68.0% | c-KIT p.L576P 63.8% |
| DNA 12 | EGFR p.A767_V769dup 67.2% | EGFR p.A767_V769dup 72.8% |
| DNA 13 | WT | WT |
| DNA 14 | WT | WT |
| DNA 15 | BRAF p.K601E 16.3% | BRAF p.K601E 16.1% |
| DNA 16 |
KRAS p.G12D 9.3% KRAS p.G13D 14.1% |
KRAS p.G12D 8.2 KRAS p.G13D 12.1% |
| DNA 17 | KRAS p.Q61L 32.7% | KRAS p.Q61L 36.3% |
| DNA 18 | NRAS p.Q61K 19.3% | NRAS p.Q61K 18.2% |
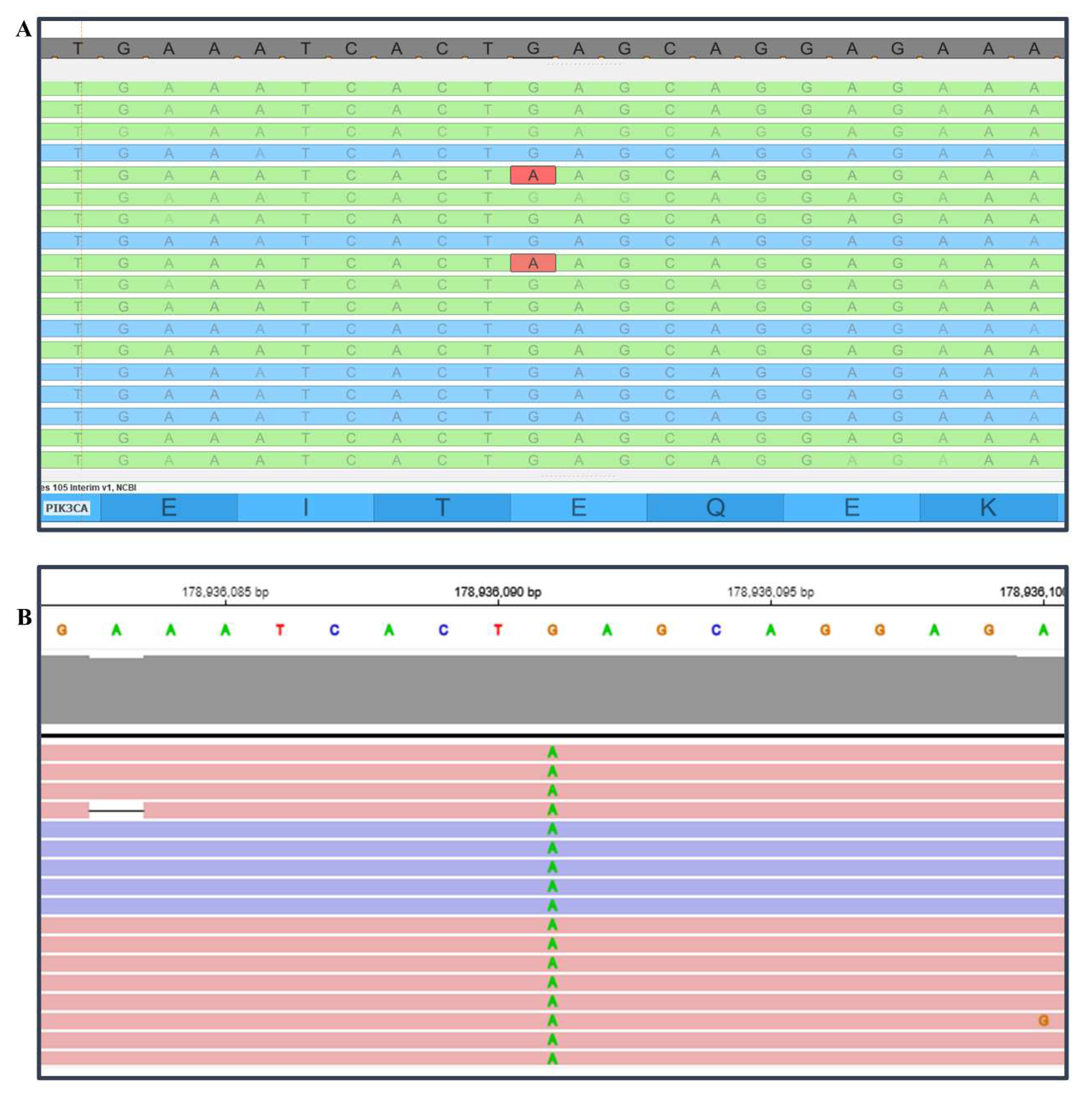
| DNA 19 | PIK3CA E545K 0.8%** | PIK3CA E545K 7.2% |
| DNA 20 | BRAF p.V600E 30.5% | BRAF p.V600E 30.0% |
| DNA 21 | NRAS p.Q61K 46.7% | NRAS p.Q61K 36.2% |
| DNA 22 |
KRAS p.G13D 47.4%*** KRAS p.G13E 47.9%*** |
KRAS p.G13D 41.9%*** KRAS p.G13E 42.0%*** |
| DNA 23 | WT | WT |
| DNA 24 | KRAS p.A146T 30.80% | KRAS p.A146T 26.4% |
| DNA 25 | WT | WT |
| DNA 26 | BRAF p.V600E 27.3% | BRAF p.V600E 30.3% |
| DNA 27 | KRAS p.G13D 14.9% | KRAS p.G13D 12.2% |
| DNA 28 | NRAS p.Q61R 34.3% | NRAS p.Q61R 28.2% |
| DNA 29 |
EGFR p.L858R 9.7% EGFR p.T790M 9.5% |
EGFR p.L858R 9.3% EGFR p.T790M 11.0% |
| DNA 30 | WT | WT |
| DNA 31 |
KRAS p.G12V 51.2% PIK3CA p.E545K 32.2% |
KRAS p.G12V 59.2% PIK3CA p.E545K 31.0% |
| DNA 32 | WT | WT |
| ID | Other Mutations (OPA Panel) |
|---|---|
| DNA 1* | MTOR p.R2217W 4.5% |
| DNA 2* |
TP53 p.G279E 4.8% TP53 p.V197M 4.0% |
| DNA 7 | TP53 p.H179Y 75.8% |
| DNA 9 | TP53 p.R273H 35.0% |
| DNA 12 | TP53 p.V197M 77.7% |
| DNA 14 | TP53 p.R273H 10.0% |
| DNA 16 |
CTNNB1 p.S45F 41.1% TP53 p.R175H 13.2% |
| DNA 18 | TP53 p.Y220C 19.7% |
| DNA 19 | TP53 p.L194F 9.9% |
| DNA 20 | TP53 p.P151S 54.7% |
| DNA 21 | TP53 p.K132R 51.4% |
| DNA 23 | TP53 p.C238S 25.3% |
| DNA 27 | CTNNB1 p.S45F 21.8% |
| DNA 30 | TP53 p.H179Y 24.6% |
| DNA 31 | TP53 p.Y220C 56.1% |
| DNA 32 | TP53 p.E285K 4.8% |
| RNA analysis Technical Parameters - S5 Plus (SiRe Fusion Panel) vs Genexus (OPA Panel) | ||||
|---|---|---|---|---|
| ID | Platform | Total Reads | Mean Read Length | Mapped Reads |
| RNA 1 | S5 Plus | 503832 | 92 | 489474 |
| Genexus | 2355408 | 99 | 170105 | |
| RNA 2 | S5 Plus | 829380 | 124 | 823978 |
| Genexus | 1748261 | 99 | 140327 | |
| RNA 3 | S5 Plus | 641591 | 89 | 348169 |
| Genexus | 2462555 | 104 | 54529 | |
| RNA 4 | S5 Plus | 254394 | 93 | 242076 |
| Genexus | 1667488 | 100 | 37387 | |
| RNA 5 | S5 Plus | 234803 | 67 | 176276 |
| Genexus | 1755508 | 91 | 111713 | |
| RNA 6 | S5 Plus | 357284 | 89 | 319350 |
| Genexus | 1542252 | 101 | 72995 | |
| RNA 7 | S5 Plus | 1070656 | 111 | 1067615 |
| Genexus | 1571469 | 100 | 150711 | |
| RNA 8 | S5 Plus | 535701 | 103 | 526127 |
| Genexus | 1737696 | 96 | 1029745 | |
| RNA 9 | S5 Plus | 494550 | 87 | 421901 |
| Genexus | 1634624 | 103 | 72104 | |
| RNA 10 | S5 Plus | 161964 | 100 | 153003 |
| Genexus | 1815512 | 96 | 51505 | |
| RNA 11 | S5 Plus | 190170 | 98 | 187044 |
| Genexus | 1597727 | 98 | 386493 | |
| RNA 12 | S5 Plus | 677654 | 91 | 513093 |
| Genexus | 1554237 | 101 | 171919 | |
| RNA 13 | S5 Plus | 765186 | 129 | 753177 |
| Genexus | 1777747 | 100 | 178846 | |
| RNA 14 | S5 Plus | 222717 | 103 | 217972 |
| Genexus | 1503566 | 102 | 48005 | |
| RNA 15 | S5 Plus | 490208 | 125 | 483482 |
| Genexus | 1523971 | 99 | 61024 | |
| RNA 16 | S5 Plus | 20405 | 91 | 17060 |
| Genexus | 1878041 | 97 | 42572 | |
| RNA 17 | S5 Plus | 367743 | 117 | 346142 |
| Genexus | 1769313 | 97 | 80920 | |
| RNA 18 | S5 Plus | 191027 | 99 | 189336 |
| Genexus | 1513615 | 97 | 365130 | |
| RNA 19 | S5 Plus | 240954 | 126 | 239481 |
| Genexus | 1744270 | 100 | 133226 | |
| RNA 20 | S5 Plus | 203214 | 86 | 195547 |
| Genexus | 1284559 | 94 | 173554 | |
| RNA 21 | S5 Plus | 195912 | 91 | 185689 |
| Genexus | 1940917 | 96 | 60947 | |
| RNA 22 | S5 Plus | 464854 | 119 | 462638 |
| Genexus | 1715374 | 98 | 294552 | |
| RNA 23 | S5 Plus | 258734 | 93 | 251939 |
| Genexus | 1644449 | 99 | 141394 | |
| RNA 24 | S5 Plus | 287598 | 104 | 284682 |
| Genexus | 1573653 | 103 | 68184 | |
| RNA 25 | S5 Plus | 297871 | 114 | 294124 |
| Genexus | 1587686 | 99 | 111160 | |
| RNA 26 | S5 Plus | 428858 | 118 | 426903 |
| Genexus | 1682103 | 100 | 185977 | |
| RNA 27 | S5 Plus | 173120 | 98 | 171187 |
| Genexus | 1471817 | 98 | 252247 | |
| RNA 28 | S5 Plus | 187176 | 145 | 185591 |
| Genexus | 1903859 | 98 | 126388 | |
| RNA 29 | S5 Plus | 311784 | 84 | 262726 |
| Genexus | 1839064 | 102 | 45998 | |
| RNA 30 | S5 Plus | 416422 | 93 | 393110 |
| Genexus | 1727113 | 101 | 57972 | |
| RNA 31 | S5 Plus | 240891 | 112 | 239186 |
| Genexus | 1598494 | 99 | 133522 | |
| RNA 32 | S5 Plus | 156106 | 63 | 97917 |
| Genexus | 1965363 | 93 | 52222 | |
| ID | S5Plus (SiRe Fusion Panel) | Genexus (OPA Panel) |
|---|---|---|
| RNA 1 | No Fusion | NTRK3 (ex14) - KANK1 (ex3) 1571 reads * |
| RNA 2 | No Fusion | No Fusion |
| RNA 3 | No Fusion | No Fusion |
| RNA 4 | No Fusion | No Fusion |
| RNA 5 | No Fusion | No Fusion |
| RNA 6 | No Fusion | No Fusion |
| RNA 7 | ALK (ex20) - EML4 (ex6) 601 reads | ALK (ex20) - EML4 (ex6) 353 reads |
| RNA 8 | No Fusion | No Fusion |
| RNA 9 | No Fusion | No Fusion |
| RNA 10 | No Fusion | No Fusion |
| RNA 11 | No Fusion | No Fusion |
| RNA 12 | No Fusion | No Fusion |
| RNA 13 | ALK (ex20) - unknown partner 149 reads | ALK (ex20) - DCTN1 (ex26) 2268 reads |
| RNA 14 | No Fusion | No Fusion |
| RNA 15 | No Fusion | No Fusion |
| RNA 16 | No Fusion | No Fusion |
| RNA 17 | No Fusion | No Fusion |
| RNA 18 | No Fusion | No Fusion |
| RNA 19 | ROS1 (ex34) - CD74 (ex6) 2208 reads | ROS1 (ex34) - CD74 (ex6) 1992 reads |
| RNA 20 | ALK (ex20) - EML4 (ex6) 43 reads | ALK (ex20) - EML4 (ex6) 1040 reads |
| RNA 21 | No Fusion | No Fusion |
| RNA 22 | ALK (ex20) - EML4 (ex13) 11335 reads | ALK (ex20) - EML4 (ex13) 7212 reads |
| RNA 23 | No Fusion | No Fusion |
| RNA 24 | RET (ex12) - KIF5B (ex15) 4063 reads | RET (ex12) - KIF5B (ex15) 2417 reads |
| RNA 25 | No Fusion | MET (ex13) - MET (ex15) 9638 reads |
| RNA 26 | No Fusion | No Fusion |
| RNA 27 | No Fusion | No Fusion |
| RNA 28 | ALK (ex20) - EML4 (ex20) 6293 reads | ALK (ex20) - EML4 (ex20) 1140 reads |
| RNA 29 | No Fusion | No Fusion |
| RNA 30 | No Fusion | No Fusion |
| RNA 31 | No Fusion | No Fusion |
| RNA 32 | RET (ex12) - CCDC6 (ex1) 494 reads | RET (ex12) - CCDC6 (ex1) 172 reads |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





