Submitted:
25 September 2023
Posted:
26 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Diabetes
2.1. Chronic inflammation as a cause of diabetes
2.2. Understanding the relationship between hypertension and type 2 diabetes mellitus
3. The Landscape of ROS
3.1. Sources of free radicals in the cells
3.1.1. Mitochondria
3.1.2. Peroxisome
3.1.3. Endoplasmic reticulum
4. Visiting the most recent antioxidant of medicinal plants and their therapeutic potential and mechanisms in diabetes
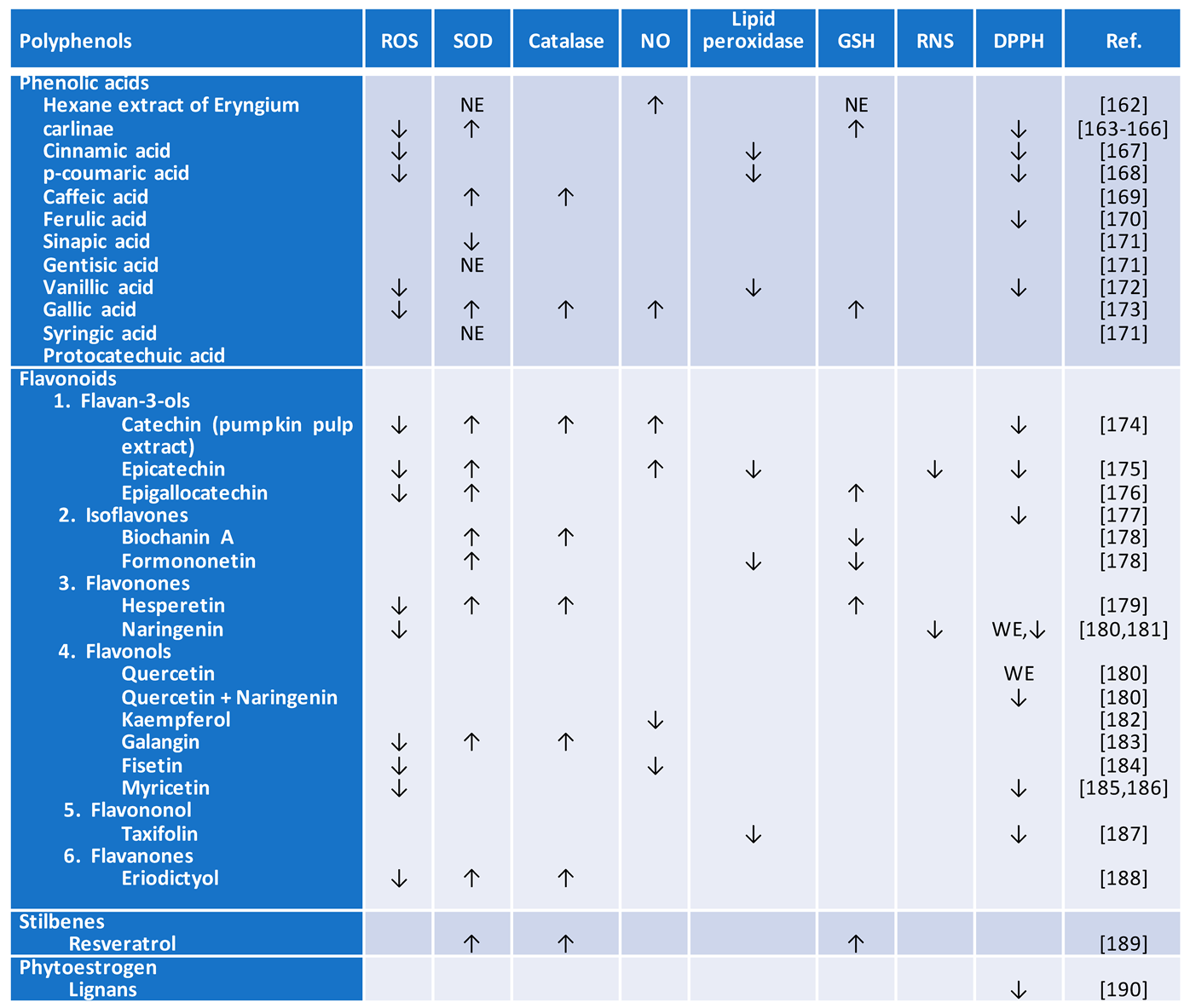
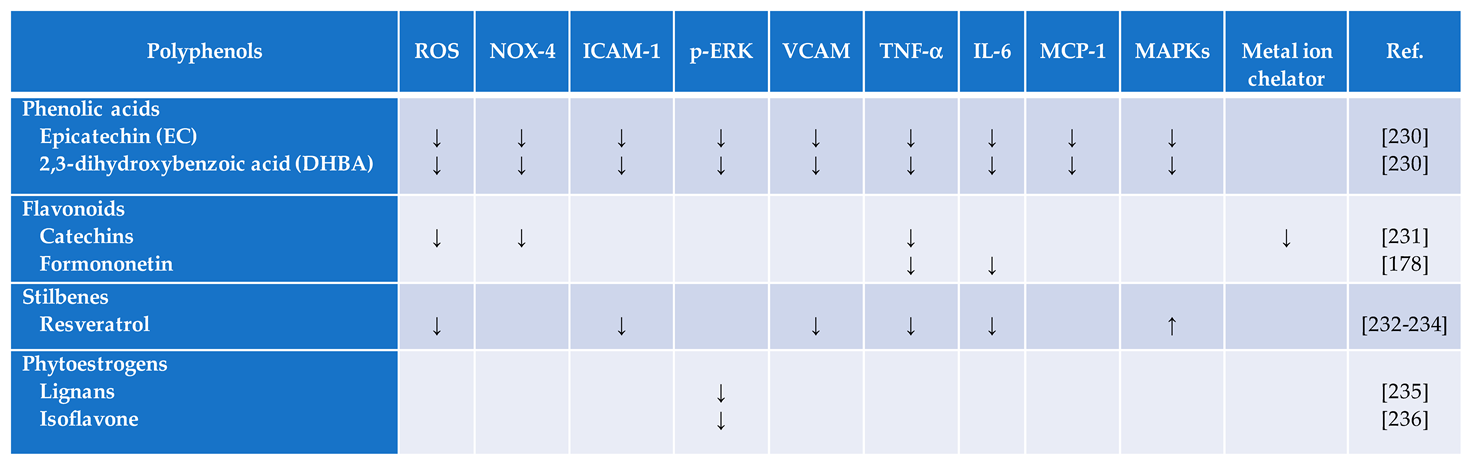
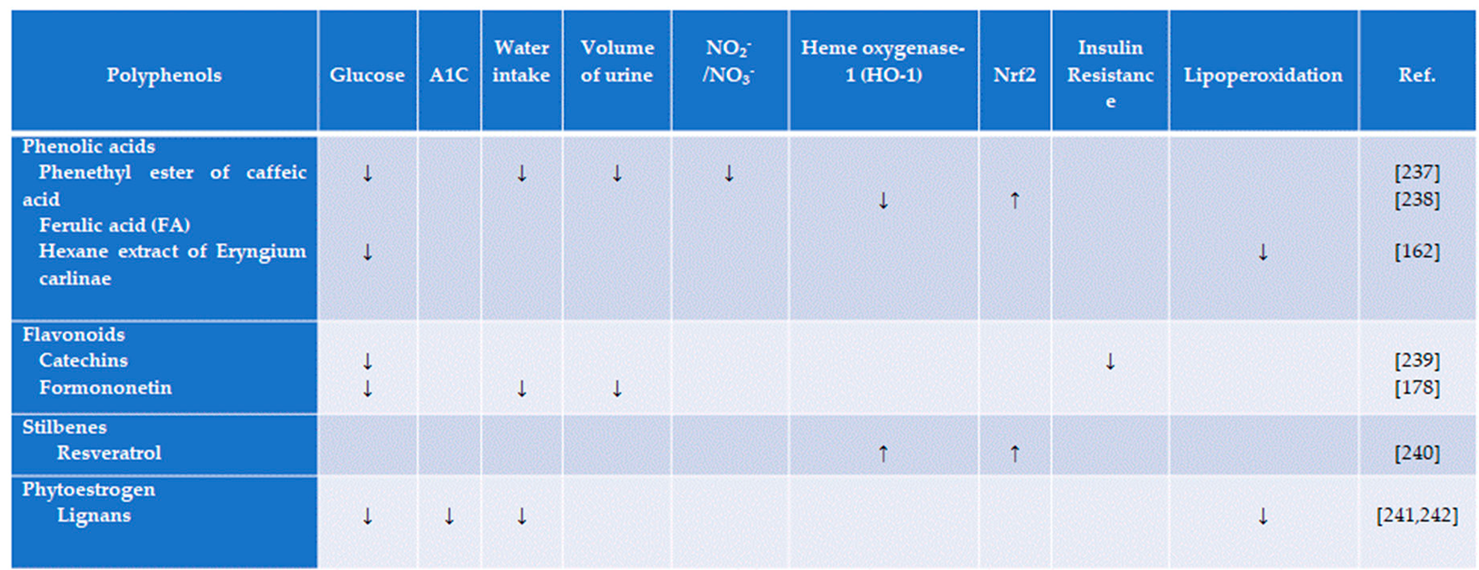
4.1. Flavonoids
4.2. Catechins
4.3. Phytoestrogens
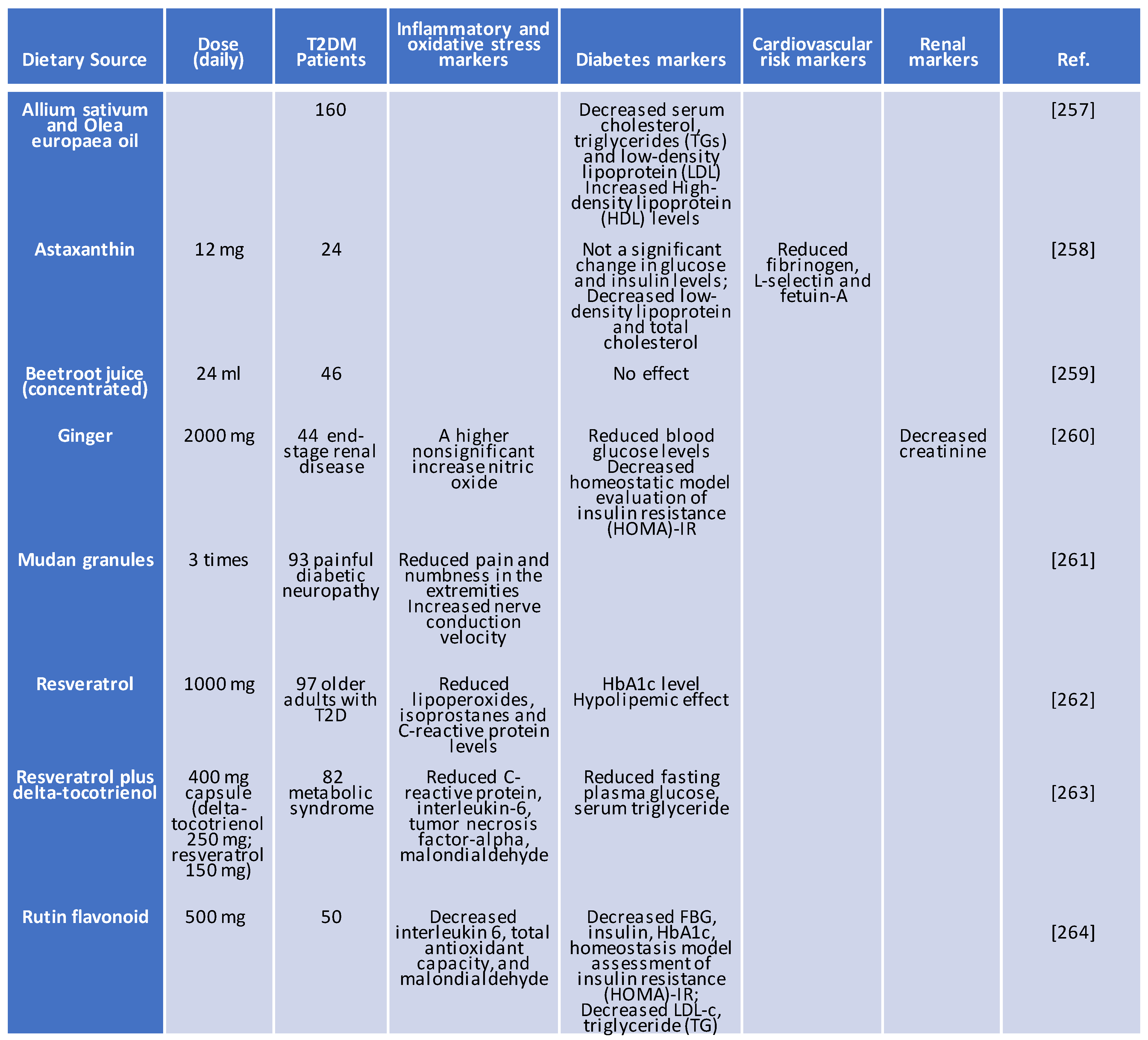
5. Clinical trial of antioxidant therapy in patients with diabetes
6. Conclusions and future implications
Author Contributions
Funding
Conflicts of Interest
References
- García-Cerrillo, D.; Noriega-Cisneros, R.; Peña-Montes, D.; Huerta-Cervantes, M.; Silva-Ríos, M.; Salgado-Garciglia, R.; Montoya-Pérez, R.; Saavedra-Molina, A. Antioxidant effects of Eryngium carlinae in diabetic rats. Asian Journal of Applied Sciences 2018, 6. [Google Scholar]
- Pagliari, S.; Forcella, M.; Lonati, E.; Sacco, G.; Romaniello, F.; Rovellini, P.; Fusi, P.; Palestini, P.; Campone, L.; Labra, M.; et al. Antioxidant and Anti-Inflammatory Effect of Cinnamon (Cinnamomum verum J. Presl) Bark Extract after In Vitro Digestion Simulation. Foods 2023, 12. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Babickaite, L.; Majiene, D.; Ramanauskiene, K. Comparison of Ethanolic and Aqueous Populus balsamifera L. Bud Extracts by Different Extraction Methods: Chemical Composition, Antioxidant and Antibacterial Activities. Pharmaceuticals (Basel) 2021, 14. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Rimkiene, L.; Ramanauskiene, K. Evaluation of Chemical Composition, Sun Protection Factor and Antioxidant Activity of Lithuanian Propolis and Its Plant Precursors. Plants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Oladimeji, O.H.; Owere, P.O.; Anthony, P.C. Acetylation of Cinnamic Acid and Evaluation of Antioxidant Activity of the Resultant Derivative. Biomedical Journal of Scientific & Technical Research 2021, 39, 31084–31088. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim Acta A Mol Biomol Spectrosc 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Bian, Y.Y.; Guo, J.; Majeed, H.; Zhu, K.X.; Guo, X.N.; Peng, W.; Zhou, H.M. Ferulic acid renders protection to HEK293 cells against oxidative damage and apoptosis induced by hydrogen peroxide. In Vitro Cell Dev Biol Anim 2015, 51, 722–729. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH•) tests. Journal of the American Oil Chemists' Society 2002, 79, 1191. [Google Scholar] [CrossRef]
- Chandrasekar, V.; Belur, P.D.; Regupathi, I. Effect of hydroxybenzoic acids antioxidants on the oxidative stability of sardine oil. Resource-Efficient Technologies 2016, 2, S114–S118. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Advances 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, X.; Wu, W.; Qin, R. Effect of Syringic acid on antioxidant biomarkers and associated inflammatory markers in mice model of asthma. Drug Dev Res 2019, 80, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.S.; Lee, J.Y.; Saitoh, S.I.; Miyake, K.; Kang, K.W.; Choi, Y.J.; Hwang, D.H. Suppression of MyD88- and TRIF-dependent signaling pathways of toll-like receptor by (−)-epigallocatechin-3-gallate, a polyphenol component of green tea. Biochemical Pharmacology 2006, 72, 850–859. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Zhang, W.; Zhao, P.; He, B.; Wu, N.; Han, P. Epigallocatechin-3-O-gallate (EGCG) attenuates FFAs-induced peripheral insulin resistance through AMPK pathway and insulin signaling pathway in vivo. Diabetes Res Clin Pract 2011, 93, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Hamza Sherif, S.; Gebreyohannes, B. Synthesis, Characterization, and Antioxidant Activities of Genistein, Biochanin A, and Their Analogues. Journal of Chemistry 2018, 2018, 4032105. [Google Scholar] [CrossRef]
- Jain, P.G.; Nayse, P.G.; Patil, D.J.; Shinde, S.D.; Surana, S.J. The possible antioxidant capabilities of formononetin in guarding against streptozotocin-induced diabetic nephropathy in rats. Future Journal of Pharmaceutical Sciences 2020, 6, 53. [Google Scholar] [CrossRef]
- Samie, A.; Sedaghat, R.; Baluchnejadmojarad, T.; Roghani, M. Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life Sci 2018, 210, 132–139. [Google Scholar] [CrossRef]
- Baranowska, M.; Koziara, Z.; Suliborska, K.; Chrzanowski, W.; Wormstone, M.; Namieśnik, J.; Bartoszek, A. Interactions between polyphenolic antioxidants quercetin and naringenin dictate the distinctive redox-related chemical and biological behaviour of their mixtures. Scientific Reports 2021, 11, 12282. [Google Scholar] [CrossRef]
- Rashmi, R.; Bojan Magesh, S.; Mohanram Ramkumar, K.; Suryanarayanan, S.; Venkata SubbaRao, M. Antioxidant Potential of Naringenin Helps to Protect Liver Tissue from Streptozotocin-Induced Damage. Rep Biochem Mol Biol 2018, 7, 76–84. [Google Scholar]
- Tran, M.H.; Nguyen, H.D.; Kim, J.C.; Choi, J.S.; Lee, H.K.; Min, B.S. Phenolic glycosides from Alangium salviifolium leaves with inhibitory activity on LPS-induced NO, PGE(2), and TNF-alpha production. Bioorg Med Chem Lett 2009, 19, 4389–4393. [Google Scholar] [CrossRef]
- Aloud, A.A.; Veeramani, C.; Govindasamy, C.; Alsaif, M.A.; El Newehy, A.S.; Al-Numair, K.S. Galangin, a dietary flavonoid, improves antioxidant status and reduces hyperglycemia-mediated oxidative stress in streptozotocin-induced diabetic rats. Redox Rep 2017, 22, 290–300. [Google Scholar] [CrossRef]
- Maher, P. Modulation of the Neuroprotective and Anti-inflammatory Activities of the Flavonol Fisetin by the Transition Metals Iron and Copper. Antioxidants 2020, 9, 1113. [Google Scholar] [PubMed]
- Awad, H.M.; Boersma, M.G.; Vervoort, J.; Rietjens, I.M. Peroxidase-catalyzed formation of quercetin quinone methide–glutathione adducts. Archives of Biochemistry and Biophysics 2000, 378, 224–233. [Google Scholar]
- Park, K.-S.; Chong, Y.; Kim, M.K. Myricetin: biological activity related to human health. Applied Biological Chemistry 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant activity of taxifolin: an activity–structure relationship. Journal of Enzyme Inhibition and Medicinal Chemistry 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Buranasudja, V.; Muangnoi, C.; Sanookpan, K.; Halim, H.; Sritularak, B.; Rojsitthisak, P. Eriodictyol Attenuates H(2)O(2)-Induced Oxidative Damage in Human Dermal Fibroblasts through Enhanced Capacity of Antioxidant Machinery. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Hu, H.-C.; Lei, Y.-H.; Zhang, W.-H.; Luo, X.-Q. Antioxidant and Anti-inflammatory Properties of Resveratrol in Diabetic Nephropathy: A Systematic Review and Meta-analysis of Animal Studies. Frontiers in Pharmacology 2022, 13. [Google Scholar] [CrossRef]
- Lu, Y.; Xue, Y.; Chen, S.; Zhu, H.; Zhang, J.; Li, X.-N.; Wang, J.; Liu, J.; Qi, C.; Du, G.; et al. Antioxidant Lignans and Neolignans from Acorus tatarinowii. Scientific Reports 2016, 6, 22909. [Google Scholar] [CrossRef]
- Alvarez Cilleros, D.; Lopez-Oliva, M.E.; Martin, M.A.; Ramos, S. (-)-Epicatechin and the colonic metabolite 2,3-dihydroxybenzoic acid protect against high glucose and lipopolysaccharide-induced inflammation in renal proximal tubular cells through NOX-4/p38 signalling. Food Funct 2020, 11, 8811–8824. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Jing, H.; Gan, L.; Li, H.; Luo, B. Resveratrol attenuated estrogen-deficient-induced cardiac dysfunction: role of AMPK, SIRT1, and mitochondrial function. Am J Transl Res 2016, 8, 2641–2649. [Google Scholar]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed Pharmacother 2021, 143, 112164. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and Ophthalmic Diseases. Nutrients 2016, 8, 200. [Google Scholar]
- Michalak, B.; Filipek, A.; Chomicki, P.; Pyza, M.; Woźniak, M.; Żyżyńska-Granica, B.; Piwowarski, J.P.; Kicel, A.; Olszewska, M.A.; Kiss, A.K. Lignans From Forsythia x Intermedia Leaves and Flowers Attenuate the Pro-inflammatory Function of Leukocytes and Their Interaction With Endothelial Cells. Frontiers in Pharmacology 2018, 9. [Google Scholar] [CrossRef]
- Kuryłowicz, A. The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment-A Narrative Review. Int J Mol Sci 2020, 22. [Google Scholar] [CrossRef]
- Sorrenti, V.; Raffaele, M.; Vanella, L.; Acquaviva, R.; Salerno, L.; Pittala, V.; Intagliata, S.; Di Giacomo, C. Protective Effects of Caffeic Acid Phenethyl Ester (CAPE) and Novel Cape Analogue as Inducers of Heme Oxygenase-1 in Streptozotocin-Induced Type 1 Diabetic Rats. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Kose, T.; Sharp, P.A.; Latunde-Dada, G.O. Phenolic Acids Rescue Iron-Induced Damage in Murine Pancreatic Cells and Tissues. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, C.; Ye, J.; Lv, Y.; Wang, L.; Chen, Z.; Jiang, Z. Protection of Porcine Intestinal-Epithelial Cells from Deoxynivalenol-Induced Damage by Resveratrol via the Nrf2 Signaling Pathway. J Agric Food Chem 2019, 67, 1726–1735. [Google Scholar] [CrossRef]
- Fukumitsu, S.; Aida, K.; Shimizu, H.; Toyoda, K. Flaxseed lignan lowers blood cholesterol and decreases liver disease risk factors in moderately hypercholesterolemic men. Nutrition Research 2010, 30, 441–446. [Google Scholar] [CrossRef]
- Memon, A.R.; Rajput, M.A.; Rizwan, F.; Akram, M.; Rizwan, M.; Iqbal, Z. Effect of Allium sativum and Olea europaea on serum lipids in patients with diabetes mellitus. J Taibah Univ Med Sci 2023, 18, 420–426. [Google Scholar] [CrossRef]
- Ciaraldi, T.P.; Boeder, S.C.; Mudaliar, S.R.; Giovannetti, E.R.; Henry, R.R.; Pettus, J.H. Astaxanthin, a natural antioxidant, lowers cholesterol and markers of cardiovascular risk in individuals with prediabetes and dyslipidaemia. Diabetes Obes Metab 2023, 25, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, L.; Sohrab, G.; Hedayati, M.; Ebrahimof, S.; Emami, G.; Razavion, T. Effects of concentrated beetroot juice consumption on glycemic control, blood pressure, and lipid profile in type 2 diabetes patients: randomized clinical trial study. Ir J Med Sci 2023, 192, 1143–1153. [Google Scholar] [CrossRef]
- Rostamkhani, H.; Veisi, P.; Niknafs, B.; Jafarabadi, M.A.; Ghoreishi, Z. The effect of zingiber officinale on prooxidant-antioxidant balance and glycemic control in diabetic patients with ESRD undergoing hemodialysis: a double-blind randomized control trial. BMC Complement Med Ther 2023, 23, 52. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, Q.; Liu, M.; Tan, M.; Zhang, X.; Wu, R. Efficacy and safety of Mudan granules for painful diabetic peripheral neuropathy: A protocol for a double-blind randomized controlled trial. Medicine (Baltimore) 2022, 101, e28896. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Nunez, V.M. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Fatima, S.; Khan, D.A.; Aamir, M.; Pervez, M.A.; Fatima, F. delta-Tocotrienol in Combination with Resveratrol Improves the Cardiometabolic Risk Factors and Biomarkers in Patients with Metabolic Syndrome: A Randomized Controlled Trial. Metab Syndr Relat Disord 2023, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H.; Moradi, L.; Zaman, F.; Zare Javid, A. The effects of rutin flavonoid supplement on glycemic status, lipid profile, atherogenic index of plasma, brain-derived neurotrophic factor (BDNF), some serum inflammatory, and oxidative stress factors in patients with type 2 diabetes mellitus: A double-blind, placebo-controlled trial. Phytother Res 2023, 37, 271–284. [Google Scholar] [CrossRef]
- National Diabetes Statistics Report 2020, Estimates of Diabetes and its burden in the United States. 2020.
- Perng, W.; Conway, R.; Mayer-Davis, E.; Dabelea, D. Youth-Onset Type 2 Diabetes: The Epidemiology of an Awakening Epidemic. Diabetes Care 2023, 46, 490–499. [Google Scholar] [CrossRef]
- Willemsen, G.; Ward, K.J.; Bell, C.G.; Christensen, K.; Bowden, J.; Dalgård, C.; Harris, J.R.; Kaprio, J.; Lyle, R.; Magnusson, P.K.; et al. The Concordance and Heritability of Type 2 Diabetes in 34,166 Twin Pairs From International Twin Registers: The Discordant Twin (DISCOTWIN) Consortium. Twin Res Hum Genet 2015, 18, 762–771. [Google Scholar] [CrossRef]
- Almgren, P.; Lehtovirta, M.; Isomaa, B.; Sarelin, L.; Taskinen, M.R.; Lyssenko, V.; Tuomi, T.; Groop, L. Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia 2011, 54, 2811–2819. [Google Scholar] [CrossRef]
- Mambiya, M.; Shang, M.; Wang, Y.; Li, Q.; Liu, S.; Yang, L.; Zhang, Q.; Zhang, K.; Liu, M.; Nie, F.; et al. The Play of Genes and Non-genetic Factors on Type 2 Diabetes. Frontiers in Public Health 2019, 7. [Google Scholar] [CrossRef]
- Udler, M.S. Type 2 Diabetes: Multiple Genes, Multiple Diseases. Curr Diab Rep 2019, 19, 55. [Google Scholar] [CrossRef]
- Caulfield, J.I.; Aizenbud, L.; Perdigoto, A.L.; Meffre, E.; Jilaveanu, L.; Michalek, D.A.; Rich, S.S.; Aizenbud, Y.; Adeniran, A.; Herold, K.C.; et al. Germline genetic variants are associated with development of insulin-dependent diabetes in cancer patients treated with immune checkpoint inhibitors. J Immunother Cancer 2023, 11. [Google Scholar] [CrossRef]
- Kamiya, J.; Aoki, Y. Associations between hyperglycaemia and somatic transversion mutations in mitochondrial DNA of people with diabetes mellitus. Diabetologia 2003, 46, 1559–1566. [Google Scholar] [CrossRef]
- Voight, B.F.; Scott, L.J.; Steinthorsdottir, V.; Morris, A.P.; Dina, C.; Welch, R.P.; Zeggini, E.; Huth, C.; Aulchenko, Y.S.; Thorleifsson, G.; et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 2010, 42, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Billings, L.K.; Florez, J.C. The genetics of type 2 diabetes: what have we learned from GWAS? Ann N Y Acad Sci 2010, 1212, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Williams, L.; Vuguin, P.M.; Charron, M.J. Minireview: Epigenetic programming of diabetes and obesity: animal models. Endocrinology 2012, 153, 1031–1038. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. International Journal of Molecular Sciences 2021, 22, 1786. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M.; Schmidt, A.M. Oxidative Stress and Diabetic Complications. Circulation Research 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Tesauro, M.; Alessandro, R.; Ghirlanda, G.; Cardillo, C. Oxidative Stress in Diabetes: Implications for Vascular and Other Complications. International Journal of Molecular Sciences 2013, 14, 21525–21550. [Google Scholar]
- Kelsey, M.M.; Zaepfel, A.; Bjornstad, P.; Nadeau, K.J. Age-related consequences of childhood obesity. Gerontology 2014, 60, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Afkarian, M. Diabetic kidney disease in children and adolescents. Pediatr Nephrol 2015, 30, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, L.G.; Jensen, B.W.; Angquist, L.; Osler, M.; Sorensen, T.I.A.; Baker, J.L. Change in Overweight from Childhood to Early Adulthood and Risk of Type 2 Diabetes. The New England journal of medicine 2018, 378, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: from basics to birth and beyond. Journal of developmental origins of health and disease 2017, 8, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.A.; Filippi, B.M.; Kang, G.M.; Kim, M.S.; Lam, T.K. Insulin action in the hypothalamus and dorsal vagal complex. Experimental physiology 2014, 99, 1104–1109. [Google Scholar] [CrossRef]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther 2008, 88, 1322–1335. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, T.; Ismail, A.A.; Rashid, A.R. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab 2007, 9, 767–780. [Google Scholar] [CrossRef]
- Chow, L.S.; Chen, H.; Miller, M.E.; Marcovina, S.M.; Seaquist, E.R. Biomarkers related to severe hypoglycaemia and lack of good glycaemic control in ACCORD. Diabetologia 2015, 58, 1160–1166. [Google Scholar] [CrossRef]
- Fanelli, C.; Calderone, S.; Epifano, L.; De Vincenzo, A.; Modarelli, F.; Pampanelli, S.; Perriello, G.; De Feo, P.; Brunetti, P.; Gerich, J.E.; et al. Demonstration of a critical role for free fatty acids in mediating counterregulatory stimulation of gluconeogenesis and suppression of glucose utilization in humans. J Clin Invest 1993, 92, 1617–1622. [Google Scholar] [CrossRef]
- Cao, C.; Koh, H.E.; Van Vliet, S.; Patterson, B.W.; Reeds, D.N.; Laforest, R.; Gropler, R.J.; Mittendorfer, B. Increased plasma fatty acid clearance, not fatty acid concentration, is associated with muscle insulin resistance in people with obesity. Metabolism 2022, 132, 155216. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine 2018, 54, 287–293. [Google Scholar] [CrossRef]
- King, G.L. The role of hyperglycaemia and hyperinsulinaemia in causing vascular dysfunction in diabetes. Ann Med 1996, 28, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation. International Journal of Molecular Sciences 2022, 23, 7273. [Google Scholar]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. American Journal of Physiology-Lung Cellular and Molecular Physiology 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Frontiers in Cell and Developmental Biology 2021, 9. [Google Scholar] [CrossRef]
- Kakimoto, M.; Inoguchi, T.; Sonta, T.; Yu, H.Y.; Imamura, M.; Etoh, T.; Hashimoto, T.; Nawata, H. Accumulation of 8-hydroxy-2'-deoxyguanosine and mitochondrial DNA deletion in kidney of diabetic rats. Diabetes 2002, 51, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Nekhaeva, E.; Bodyak, N.D.; Kraytsberg, Y.; McGrath, S.B.; Van Orsouw, N.J.; Pluzhnikov, A.; Wei, J.Y.; Vijg, J.; Khrapko, K. Clonally expanded mtDNA point mutations are abundant in individual cells of human tissues. Proc Natl Acad Sci U S A 2002, 99, 5521–5526. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, W.; Meng, P.; Wang, P.; Zhou, C.; Yao, Y.; Wu, S.; Wang, Y.; Zhao, J.; Zou, D.; et al. Immune-related somatic mutation genes are enriched in PDACs with diabetes. Transl Oncol 2019, 12, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.M.; Simpson, C.L.; Stewart, J.A., Jr. The Role of AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. J Diabetes Res 2016, 2016, 6809703. [Google Scholar] [CrossRef]
- Agrawal, A.; Cha-Molstad, H.; Samols, D.; Kushner, I. Overexpressed nuclear factor-κB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPβ and signal transducer and activator of transcription-3. Immunology 2003, 108, 539–547. [Google Scholar] [CrossRef]
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F.H. Inflammatory Cytokines and the Risk to Develop Type 2 Diabetes: Results of the Prospective Population-Based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-κβ: A Potential Target in the Management of Vascular Complications of Diabetes. Front Pharmacol 2017, 8, 798. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 2007, 9, 2277–2293. [Google Scholar] [CrossRef] [PubMed]
- Kairisalo, M.; Korhonen, L.; Blomgren, K.; Lindholm, D. X-linked inhibitor of apoptosis protein increases mitochondrial antioxidants through NF-kappaB activation. Biochem Biophys Res Commun 2007, 364, 138–144. [Google Scholar] [CrossRef]
- Pham, C.G.; Bubici, C.; Zazzeroni, F.; Papa, S.; Jones, J.; Alvarez, K.; Jayawardena, S.; De Smaele, E.; Cong, R.; Beaumont, C.; et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell 2004, 119, 529–542. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in Oxidative Stress. Curr Opin Toxicol 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur Cardiol 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Liu, C.; Dong, W.; Li, J.; Kong, Y.; Ren, X. O-GlcNAc Modification and Its Role in Diabetic Retinopathy. Metabolites 2022, 12, 725. [Google Scholar] [CrossRef]
- Wu, T.; Qiao, S.; Shi, C.; Wang, S.; Ji, G. Metabolomics window into diabetic complications. Journal of Diabetes Investigation 2018, 9, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, A.R.; Savina, A.; Vermeulen, M.; Pérez, L.; Geffner, J.; Hermine, O.; Rosenzweig, S.D.; Faure, F.; Amigorena, S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood 2008, 112, 4712–4722. [Google Scholar] [CrossRef] [PubMed]
- Chougnet, C.A.; Thacker, R.I.; Shehata, H.M.; Hennies, C.M.; Lehn, M.A.; Lages, C.S.; Janssen, E.M. Loss of Phagocytic and Antigen Cross-Presenting Capacity in Aging Dendritic Cells Is Associated with Mitochondrial Dysfunction. The Journal of Immunology 2015, 195, 2624–2632. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, X. Fatty Acid β-Oxidation in Kidney Diseases: Perspectives on Pathophysiological Mechanisms and Therapeutic Opportunities. Frontiers in Pharmacology 2022, 13. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. Journal of Hepatology 2015, 62, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biology International 2018, 42, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ Res 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Schossleitner, K.; Kral-Pointner, J.B.; Salzmann, M.; Schrammel, A.; Schmid, J.A. More than Just a Monolayer: the Multifaceted Role of Endothelial Cells in the Pathophysiology of Atherosclerosis. Current Atherosclerosis Reports 2022, 24, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Kornowski, R.; Hong, M.K.; Tio, F.O.; Bramwell, O.; Wu, H.; Leon, M.B. In-Stent Restenosis: Contributions of Inflammatory Responses and Arterial Injury to Neointimal Hyperplasia. Journal of the American College of Cardiology 1998, 31, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Che Man, R.; Sulaiman, N.; Ishak, M.F.; Bt Hj Idrus, R.; Abdul Rahman, M.R.; Yazid, M.D. The Effects of Pro-Inflammatory and Anti-Inflammatory Agents for the Suppression of Intimal Hyperplasia: An Evidence-Based Review. Int J Environ Res Public Health 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020; Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services: Atlanta, GA, USA, 2020. [Google Scholar]
- Favre, G.A.; Esnault, V.L.M.; Obberghen, E.V. Modulation of glucose metabolism by the renin-angiotensin-aldosterone system. American Journal of Physiology-Endocrinology and Metabolism 2015, 308, E435–E449. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Cheung, B.M.; Li, C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep 2012, 14, 160–166. [Google Scholar] [CrossRef]
- Dregan, A.; Charlton, J.; Chowienczyk, P.; Gulliford, M.C. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation 2014, 130, 837–844. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol 2013, 149, 84–91. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, L.; Shi, J.; Chen, X.; Li, Y.; Ma, B.; Zhang, Y. The risk of metabolic syndrome in patients with rheumatoid arthritis: a meta-analysis of observational studies. PLoS One 2013, 8, e78151. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nature Medicine 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory Cytokine Concentrations Are Acutely Increased by Hyperglycemia in Humans. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; González, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertension Research 2011, 34, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Lacy, F.; Kailasam, M.T.; O'Connor, D.T.; Schmid-Schönbein, G.W.; Parmer, R.J. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 2000, 36, 878–884. [Google Scholar] [CrossRef]
- Redón, J.; Oliva, M.R.; Tormos, C.; Giner, V.; Chaves, J.; Iradi, A.; Sáez, G.T. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 2003, 41, 1096–1101. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Kurz, S.; Münzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 1996, 97, 1916–1923. [Google Scholar] [CrossRef]
- Jung, O.; Schreiber, J.G.; Geiger, H.; Pedrazzini, T.; Busse, R.; Brandes, R.P. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation 2004, 109, 1795–1801. [Google Scholar] [CrossRef]
- Terada, Y.; Yayama, K. Angiotensin II-Induced Vasoconstriction via Rho Kinase Activation in Pressure-Overloaded Rat Thoracic Aortas. Biomolecules 2021, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Kang, B.P.; Cheung, S.; Opawumi, D.; Meggs, L.G. Angiotensin II promotes glucose-induced activation of cardiac protein kinase C isozymes and phosphorylation of troponin I. Diabetes 2001, 50, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Smith, R.D.; Balla, T.; Catt, K.J. Angiotensin II activates mitogen-activated protein kinase via protein kinase C and Ras/Raf-1 kinase in bovine adrenal glomerulosa cells. Endocrinology 1998, 139, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Togliatto, G.; Lombardo, G.; Brizzi, M.F. The Future Challenge of Reactive Oxygen Species (ROS) in Hypertension: From Bench to Bed Side. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef]
- Millatt, L.J.; Abdel-Rahman, E.M.; Siragy, H.M. Angiotensin II and nitric oxide: a question of balance. Regul Pept 1999, 81, 1–10. [Google Scholar] [CrossRef]
- Chen, K.; Pittman, R.N.; Popel, A.S. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal 2008, 10, 1185–1198. [Google Scholar] [CrossRef]
- Kawanami, D.; Takashi, Y.; Muta, Y.; Oda, N.; Nagata, D.; Takahashi, H.; Tanabe, M. Mineralocorticoid Receptor Antagonists in Diabetic Kidney Disease. Frontiers in Pharmacology 2021, 12. [Google Scholar] [CrossRef]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef]
- Gaede, P.; Vedel, P.; Larsen, N.; Jensen, G.V.; Parving, H.H.; Pedersen, O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003, 348, 383–393. [Google Scholar] [CrossRef]
- Sena, C.M.; Leandro, A.; Azul, L.; Seica, R.; Perry, G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front Physiol 2018, 9, 1668. [Google Scholar] [CrossRef]
- Tekin, K.; Tekin, M.I. Chapter 3 - Oxidative stress and diabetic retinopathy. In Pathology, Preedy, V.R., Ed.; Academic Press: 2020; pp. 29-37.
- Davi, G.; Falco, A.; Patrono, C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal 2005, 7, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int J Biomed Sci 2008, 4, 89–96. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Moron, E.; Abad-Jimenez, Z.; Maranon, A.M.; Iannantuoni, F.; Escribano-Lopez, I.; Lopez-Domenech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J Clin Med 2019, 8. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ Res 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Bolisetty, S.; Jaimes, E.A. Mitochondria and reactive oxygen species: physiology and pathophysiology. Int J Mol Sci 2013, 14, 6306–6344. [Google Scholar] [CrossRef]
- Venditti, P.; Di Stefano, L.; Di Meo, S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 2013, 13, 71–82. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mohammad, G. Mitochondrial Fragmentation in a High Homocysteine Environment in Diabetic Retinopathy. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Nadalutti, C.A.; Ayala-Pena, S.; Santos, J.H. Mitochondrial DNA damage as driver of cellular outcomes. Am J Physiol Cell Physiol 2022, 322, C136–C150. [Google Scholar] [CrossRef]
- Duraisamy, A.J.; Mohammad, G.; Kowluru, R.A. Mitochondrial fusion and maintenance of mitochondrial homeostasis in diabetic retinopathy. Biochim Biophys Acta Mol Basis Dis 2019, 1865, 1617–1626. [Google Scholar] [CrossRef]
- So, S.; Lee, S.; Lee, Y.; Han, J.; Kang, S.; Choi, J.; Kim, B.; Kim, D.; Yoo, H.J.; Shim, I.K.; et al. Dysfunctional pancreatic cells differentiated from induced pluripotent stem cells with mitochondrial DNA mutations. BMB Rep 2022, 55, 453–458. [Google Scholar] [CrossRef]
- Neginskaya, M.A.; Pavlov, E.V.; Sheu, S.S. Electrophysiological properties of the mitochondrial permeability transition pores: Channel diversity and disease implication. Biochim Biophys Acta Bioenerg 2021, 1862, 148357. [Google Scholar] [CrossRef]
- Scialo, F.; Fernandez-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front Physiol 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Manford, A.G.; Mena, E.L.; Shih, K.Y.; Gee, C.L.; McMinimy, R.; Martinez-Gonzalez, B.; Sherriff, R.; Lew, B.; Zoltek, M.; Rodriguez-Perez, F.; et al. Structural basis and regulation of the reductive stress response. Cell 2021, 184, 5375–5390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, Y.; Kim, I.M.; Weintraub, N.L.; Tang, Y. The Impaired Bioenergetics of Diabetic Cardiac Microvascular Endothelial Cells. Front Endocrinol (Lausanne) 2021, 12, 642857. [Google Scholar] [CrossRef]
- Barnes, P.J. Pulmonary Diseases and Ageing. Subcell Biochem 2019, 91, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Mehrabi Nasab, E.; Athari, S.M.; Athari, S.S. Mitochondria signaling pathways in allergic asthma. J Investig Med 2022, 70, 863–882. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Mathur, A.; Kakkar, P. Critical role of mitochondrial dysfunction and impaired mitophagy in diabetic nephropathy. J Cell Physiol 2019, 234, 19223–19236. [Google Scholar] [CrossRef]
- Ding, L.; Sun, W.; Balaz, M.; He, A.; Klug, M.; Wieland, S.; Caiazzo, R.; Raverdy, V.; Pattou, F.; Lefebvre, P.; et al. Peroxisomal β-oxidation acts as a sensor for intracellular fatty acids and regulates lipolysis. Nature Metabolism 2021, 3, 1648–1661. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-S.; Dighe, P.A.; Mezera, V.; Monternier, P.-A.; Brand, M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. Journal of Biological Chemistry 2017, 292, 16804–16809. [Google Scholar] [CrossRef] [PubMed]
- Schrader, M.; Fahimi, H.D. Peroxisomes and oxidative stress. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2006, 1763, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999, 20, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 1995, 270, 12953–12956. [Google Scholar] [CrossRef]
- Pascual-Ahuir, A.; Manzanares-Estreder, S.; Proft, M. Pro- and Antioxidant Functions of the Peroxisome-Mitochondria Connection and Its Impact on Aging and Disease. Oxidative Medicine and Cellular Longevity 2017, 2017, 9860841. [Google Scholar] [CrossRef]
- Kim, J.; Bai, H. Peroxisomal Stress Response and Inter-Organelle Communication in Cellular Homeostasis and Aging. Antioxidants 2022, 11, 192. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, R.; Torreggiani, M.; Ting, A.; Xiong, H.; Striker, G.E.; Vlassara, H.; Zheng, F. Induction of Diabetes in Aged C57B6 Mice Results in Severe Nephropathy: An Association with Oxidative Stress, Endoplasmic Reticulum Stress, and Inflammation. The American Journal of Pathology 2010, 176, 2163–2176. [Google Scholar] [CrossRef]
- Cunard, R.; Sharma, K. The endoplasmic reticulum stress response and diabetic kidney disease. American Journal of Physiology-Renal Physiology 2011, 300, F1054–F1061. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Huh, W.K.; Falvo, J.V.; Gerke, L.C.; Carroll, A.S.; Howson, R.W.; Weissman, J.S.; O'Shea, E.K. Global analysis of protein localization in budding yeast. Nature 2003, 425, 686–691. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Medicine and Cellular Longevity 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Zito, E. ERO1: A protein disulfide oxidase and H2O2 producer. Free Radical Biology and Medicine 2015, 83, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, H.M.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic Reticulum Stress and Associated ROS. Int J Mol Sci 2016, 17, 327. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful Plant Antioxidants: A New Biosustainable Approach to the Production of Rosmarinic Acid. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Murtaza, G.; Liu, G.; Rahu, N.; Saleem Kalhoro, M.; Hussain Kalhoro, D.; Adebowale, T.O.; Usman Mazhar, M.; Rehman, Z.U.; et al. Flavonoids and type 2 diabetes: Evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol Res 2020, 152, 104629. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: an overview. J Nutr Sci 2016, 5, e47. [Google Scholar] [CrossRef]
- Zou, J.; Yu, X.; Qu, S.; Li, X.; Jin, Y.; Sui, D. Protective effect of total flavonoids extracted from the leaves of Murraya paniculata (L.) Jack on diabetic nephropathy in rats. Food Chem Toxicol 2014, 64, 231–237. [Google Scholar] [CrossRef]
- Jin, Y.; Arroo, R. The protective effects of flavonoids and carotenoids against diabetic complications-A review of in vivo evidence. Front Nutr 2023, 10, 1020950. [Google Scholar] [CrossRef]
- Nyakundi, B.B.; Yang, J. Uses of Papaya Leaf and Seaweed Supplementations for Controlling Glucose Homeostasis in Diabetes. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Han, X.; Wu, Y.C.; Meng, M.; Sun, Q.S.; Gao, S.M.; Sun, H. Linarin prevents LPS-induced acute lung injury by suppressing oxidative stress and inflammation via inhibition of TXNIP/NLRP3 and NF-kappaB pathways. Int J Mol Med 2018, 42, 1460–1472. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shan, M.Y.; Tang, C.Y.; Cheng, M.Y.; Chen, B.; Yan, J.; Xu, Z.H. Linarin Ameliorates Diabetic Liver Injury by Alleviating Oxidative Stress and Inflammation through the Inhibition of AKR1B1. Comb Chem High Throughput Screen 2023. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mitchell, A.E. Quercetin and Isorhamnetin Glycosides in Onion (Allium cepa L.): Varietal Comparison, Physical Distribution, Coproduct Evaluation, and Long-Term Storage Stability. Journal of Agricultural and Food Chemistry 2011, 59, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Abdel Motaal, A.; Salem, H.H.; Almaghaslah, D.; Alsayari, A.; Bin Muhsinah, A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Shati, A.A.; El-Askary, H. Flavonol Glycosides: In Vitro Inhibition of DPPIV, Aldose Reductase and Combating Oxidative Stress are Potential Mechanisms for Mediating the Antidiabetic Activity of Cleome droserifolia. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Kalai, F.Z.; Boulaaba, M.; Ferdousi, F.; Isoda, H. Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Zhang, X.F.; Tang, Y.J.; Guan, X.X.; Lu, X.; Li, J.; Chen, X.L.; Deng, J.L.; Fan, J.M. Flavonoid constituents of Amomum tsao-ko Crevost et Lemarie and their antioxidant and antidiabetic effects in diabetic rats - in vitro and in vivo studies. Food Funct 2022, 13, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci 2017, 7, 50. [Google Scholar] [CrossRef]
- Alam, W.; Rocca, C.; Khan, H.; Hussain, Y.; Aschner, M.; De Bartolo, A.; Amodio, N.; Angelone, T.; Cheang, W.S. Current Status and Future Perspectives on Therapeutic Potential of Apigenin: Focus on Metabolic-Syndrome-Dependent Organ Dysfunction. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Teixeira, J.A.; Lyoussi, B. Effect of antioxidant-rich propolis and bee pollen extracts against D-glucose induced type 2 diabetes in rats. Food Res Int 2020, 138, 109802. [Google Scholar] [CrossRef]
- Bakour, M.; Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Ousaaid, D.; Teixeira, J.A.; Lyoussi, B. Exploring the Palynological, Chemical, and Bioactive Properties of Non-Studied Bee Pollen and Honey from Morocco. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Luo, Z.; Fu, C.; Li, T.; Gao, Q.; Miao, D.; Xu, J.; Zhao, Y. Hypoglycemic Effects of Licochalcone A on the Streptozotocin-Induced Diabetic Mice and Its Mechanism Study. J Agric Food Chem 2021, 69, 2444–2456. [Google Scholar] [CrossRef]
- Luo, Z.; Li, T.; Gao, Q.; Chen, Y.; Su, G.; Zhao, Y. Impact of licochalcone A on the progression of diabetic nephropathy in type 2 diabetes mellitus of C57BL/6 mice. Food Funct 2021, 12, 10676–10689. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Y.; Shen, Q.; Liu, G.; Ye, J.; Sun, G.; Sun, X. Myricitrin Attenuates High Glucose-Induced Apoptosis through Activating Akt-Nrf2 Signaling in H9c2 Cardiomyocytes. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, Q.; Chen, Y.; Pan, R.; Kuang, S.; Liu, G.; Sun, G.; Sun, X. Myricitrin Alleviates Oxidative Stress-induced Inflammation and Apoptosis and Protects Mice against Diabetic Cardiomyopathy. Sci Rep 2017, 7, 44239. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Solid Lipid Nanoparticles of Myricitrin Have Antioxidant and Antidiabetic Effects on Streptozotocin-Nicotinamide-Induced Diabetic Model and Myotube Cell of Male Mouse. Oxid Med Cell Longev 2018, 2018, 7496936. [Google Scholar] [CrossRef]
- Amri, J.; Alaee, M.; Babaei, R.; Salemi, Z.; Meshkani, R.; Ghazavi, A.; Akbari, A.; Salehi, M. Biochanin-A has antidiabetic, antihyperlipidemic, antioxidant, and protective effects on diabetic nephropathy via suppression of TGF-β1 and PAR-2 genes expression in kidney tissues of STZ-induced diabetic rats. Biotechnology and Applied Biochemistry 2022, 69, 2112–2121. [Google Scholar] [PubMed]
- Ram, C.; Gairola, S.; Verma, S.; Mugale, M.N.; Bonam, S.R.; Murty, U.S.; Sahu, B.D. Biochanin A Ameliorates Nephropathy in High-Fat Diet/Streptozotocin-Induced Diabetic Rats: Effects on NF-kB/NLRP3 Axis, Pyroptosis, and Fibrosis. Antioxidants 2023, 12, 1052. [Google Scholar]
- Oza, M.J.; Kulkarni, Y.A. Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomedicine & Pharmacotherapy 2018, 107, 1119–1127. [Google Scholar]
- Sadri, H.; Goodarzi, M.T.; Salemi, Z.; Seifi, M. Antioxidant effects of biochanin A in streptozotocin induced diabetic rats. Brazilian Archives of Biology and Technology 2017, 60. [Google Scholar] [CrossRef]
- Mou, X.; Zhou, D.-Y.; Zhou, D.-Y.; Ma, J.-R.; Liu, Y.-H.; Chen, H.-P.; Hu, Y.-B.; Shou, C.-M.; Chen, J.-W.; Liu, W.-H.; et al. Serum TGF-β1 as a Biomarker for Type 2 Diabetic Nephropathy: A Meta-Analysis of Randomized Controlled Trials. PLOS ONE 2016, 11, e0149513. [Google Scholar] [CrossRef] [PubMed]
- Bagang, N.; Gupta, K.; Singh, G.; Kanuri, S.H.; Mehan, S. Protease-activated receptors in kidney diseases: A comprehensive review of pathological roles, therapeutic outcomes and challenges. Chem Biol Interact 2023, 377, 110470. [Google Scholar] [CrossRef]
- Lossi, L. The concept of intrinsic versus extrinsic apoptosis. Biochem J 2022, 479, 357–384. [Google Scholar] [CrossRef]
- Tay, K.-C.; Tan, L.T.-H.; Chan, C.K.; Hong, S.L.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Formononetin: A Review of Its Anticancer Potentials and Mechanisms. Frontiers in Pharmacology 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.G.; Nayse, P.G.; Patil, D.J.; Shinde, S.D.; Surana, S.J. The possible antioxidant capabilities of formononetin in guarding against streptozotocin-induced diabetic nephropathy in rats. Future Journal of Pharmaceutical Sciences 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 2008, 283, 20015–20026. [Google Scholar] [CrossRef]
- Oza, M.J.; Kulkarni, Y.A. Formononetin treatment in type 2 diabetic rats reduces insulin resistance and hyperglycemia. Frontiers in pharmacology 2018, 9, 739. [Google Scholar] [CrossRef]
- Zhang, L.; Keung, W.; Samokhvalov, V.; Wang, W.; Lopaschuk, G.D. Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim Biophys Acta 2010, 1801, 1–22. [Google Scholar] [CrossRef]
- Lv, J.; Zhuang, K.; Jiang, X.; Huang, H.; Quan, S. Renoprotective effect of formononetin by suppressing Smad3 expression in Db/Db mice. Diabetes, Metabolic Syndrome and Obesity 2020, 3313-3324.
- Zhou, Z.; Zhou, X.; Dong, Y.; Li, M.; Xu, Y. Formononetin ameliorates high glucose-induced endothelial dysfunction by inhibiting the JAK/STAT signaling pathway. Molecular Medicine Reports 2019, 20, 2893–2901. [Google Scholar] [CrossRef]
- Lee, A.; Gu, H.; Gwon, M.-H.; Yun, J.-M. Hesperetin suppresses LPS/high glucose-induced inflammatory responses via TLR/MyD88/NF-κB signaling pathways in THP-1 cells. Nutr. Res. Pract 2021, 15, 591–603. [Google Scholar]
- Tian, M.; Han, Y.-B.; Zhao, C.-C.; Liu, L.; Zhang, F.-L. Hesperidin alleviates insulin resistance by improving HG-induced oxidative stress and mitochondrial dysfunction by restoring miR-149. Diabetology & Metabolic Syndrome 2021, 13, 1–11. [Google Scholar]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.H. Kaempferol Inhibits Pancreatic Cancer Cell Growth and Migration through the Blockade of EGFR-Related Pathway In Vitro. PLOS ONE 2016, 11, e0155264. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomedicine & Pharmacotherapy 2019, 117, 109086. [Google Scholar] [CrossRef]
- Timmer, J.C.; Salvesen, G.S. Caspase substrates. Cell Death & Differentiation 2007, 14, 66–72. [Google Scholar] [CrossRef]
- Alkhalidy, H.; Moore, W.; Zhang, Y.; McMillan, R.; Wang, A.; Ali, M.; Suh, K.-S.; Zhen, W.; Cheng, Z.; Jia, Z.; et al. Small Molecule Kaempferol Promotes Insulin Sensitivity and Preserved Pancreatic <b><i>β</i></b>-Cell Mass in Middle-Aged Obese Diabetic Mice. Journal of Diabetes Research 2015, 2015, 532984. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, G.; Al-Numair, K.S.; Alsaif, M.A.; Veeramani, C. Antidiabetic effect of kaempferol a flavonoid compound, on streptozotocin-induced diabetic rats with special reference to glycoprotein components. Progress in Nutrition 2015, 17, 50–57. [Google Scholar]
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R.P.; Zhen, W.; Zhou, K.; Liu, D. The flavonoid kaempferol ameliorates streptozotocin-induced diabetes by suppressing hepatic glucose production. Molecules 2018, 23, 2338. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Tekade, R.K.; Kalia, K. Kaempferol in ameliorating diabetes-induced fibrosis and renal damage: an in vitro and in vivo study in diabetic nephropathy mice model. Phytomedicine 2020, 76, 153235. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Z.; Zhao, X.; Xie, H.; Du, L.; Gao, H.; Xie, C. Mechanisms of Kaempferol in the treatment of diabetes: A comprehensive and latest review. Frontiers in Endocrinology 2022, 13, 990299. [Google Scholar] [PubMed]
- Abukhalil, M.H.; Althunibat, O.Y.; Aladaileh, S.H.; Al-Amarat, W.; Obeidat, H.M.; Alayn'Al-marddyah, A.; Hussein, O.E.; Alfwuaires, M.A.; Algefare, A.I.; Alanazi, K.M. Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress, inflammation and apoptosis in rats. Biomedicine & Pharmacotherapy 2021, 138, 111410. [Google Scholar]
- Al-Amarat, W.; Abukhalil, M.H.; Althunibat, O.Y.; Alfwuaires, M.A.; Alnamshan, M.M.; Alqosaibi, A.I.; Ahmeda, A.F.; Kamel, E.M.; Arab, H.H.; Mahmoud, A.M. Galangin attenuates liver injury, oxidative stress and inflammation, and upregulates Nrf2/HO-1 signaling in streptozotocin-induced diabetic rats. Processes 2021, 9, 1562. [Google Scholar]
- Kong, Y.; Feng, Z.; Chen, A.; Qi, Q.; Han, M.; Wang, S.; Zhang, Y.; Zhang, X.; Yang, N.; Wang, J.; et al. The Natural Flavonoid Galangin Elicits Apoptosis, Pyroptosis, and Autophagy in Glioblastoma. Frontiers in Oncology 2019, 9. [Google Scholar] [CrossRef]
- Kalhotra, P.; Chittepu, V.C.; Osorio-Revilla, G.; Gallardo-Velázquez, T. Discovery of galangin as a potential DPP-4 inhibitor that improves insulin-stimulated skeletal muscle glucose uptake: a combinational therapy for diabetes. International journal of molecular sciences 2019, 20, 1228. [Google Scholar]
- Deacon, C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Frontiers in Endocrinology 2019, 10. [Google Scholar] [CrossRef]
- Aloud, A.A.; Chinnadurai, V.; Govindasamy, C.; Alsaif, M.A.; Al-Numair, K.S. Galangin, a dietary flavonoid, ameliorates hyperglycaemia and lipid abnormalities in rats with streptozotocin-induced hyperglycaemia. Pharmaceutical biology 2018, 56, 302–308. [Google Scholar] [PubMed]
- Haddad, A.Q.; Venkateswaran, V.; Viswanathan, L.; Teahan, S.J.; Fleshner, N.E.; Klotz, L.H. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis 2006, 9, 68–76. [Google Scholar] [CrossRef]
- Sung, B.; Pandey, M.K.; Aggarwal, B.B. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol 2007, 71, 1703–1714. [Google Scholar] [CrossRef]
- Hou, D.X.; Fukuda, M.; Johnson, J.A.; Miyamori, K.; Ushikai, M.; Fujii, M. Fisetin induces transcription of NADPH:quinone oxidoreductase gene through an antioxidant responsive element-involved activation. Int J Oncol 2001, 18, 1175–1179. [Google Scholar] [CrossRef]
- Constantin, R.P.; Constantin, J.; Pagadigorria, C.L.S.; Ishii-Iwamoto, E.L.; Bracht, A.; Ono, M.d.K.C.; Yamamoto, N.S. The actions of fisetin on glucose metabolism in the rat liver. Cell Biochemistry and Function 2010, 28, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutrition & metabolism 2015, 12, 1–20. [Google Scholar]
- Prasath, G.S.; Pillai, S.I.; Subramanian, S.P. Fisetin improves glucose homeostasis through the inhibition of gluconeogenic enzymes in hepatic tissues of streptozotocin induced diabetic rats. European journal of pharmacology 2014, 740, 248–254. [Google Scholar] [PubMed]
- Li, Y.; Ding, Y. Minireview: Therapeutic potential of myricetin in diabetes mellitus. Food Science and Human Wellness 2012, 1, 19–25. [Google Scholar]
- Lalitha, N.; Sadashivaiah, B.; Ramaprasad, T.R.; Singh, S.A. Anti-hyperglycemic activity of myricetin, through inhibition of DPP-4 and enhanced GLP-1 levels, is attenuated by co-ingestion with lectin-rich protein. PloS one 2020, 15, e0231543. [Google Scholar]
- Li, Y.; Zheng, X.; Yi, X.; Liu, C.; Kong, D.; Zhang, J.; Gong, M. Myricetin: a potent approach for the treatment of type 2 diabetes as a natural class B GPCR agonist. Faseb j 2017, 31, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, Y.; Li, X.; Zhu, L.; Wang, X.; Li, L.; Sun, H.; Han, X.; Li, J. Myricetin relieves the symptoms of type 2 diabetes mice and regulates intestinal microflora. Biomedicine & Pharmacotherapy 2022, 153, 113530. [Google Scholar]
- Les, F.; Casedas, G.; Gomez, C.; Moliner, C.; Valero, M.S.; Lopez, V. The role of anthocyanins as antidiabetic agents: from molecular mechanisms to in vivo and human studies. J Physiol Biochem 2021, 77, 109–131. [Google Scholar] [CrossRef]
- Casedas, G.; Les, F.; Gomez-Serranillos, M.P.; Smith, C.; Lopez, V. Anthocyanin profile, antioxidant activity and enzyme inhibiting properties of blueberry and cranberry juices: a comparative study. Food Funct 2017, 8, 4187–4193. [Google Scholar] [CrossRef]
- Khan, D.; Sharif, A.; Zafar, M.; Akhtar, B.; Akhtar, M.F.; Awan, S. Delonix regia a Folklore Remedy for Diabetes; Attenuates Oxidative Stress and Modulates Type II Diabetes Mellitus. Curr Pharm Biotechnol 2020, 21, 1059–1069. [Google Scholar] [CrossRef]
- Meng, Q.; Qi, X.; Fu, Y.; Chen, Q.; Cheng, P.; Yu, X.; Sun, X.; Wu, J.; Li, W.; Zhang, Q.; et al. Flavonoids extracted from mulberry (Morus alba L.) leaf improve skeletal muscle mitochondrial function by activating AMPK in type 2 diabetes. J Ethnopharmacol 2020, 248, 112326. [Google Scholar] [CrossRef] [PubMed]
- Thaipitakwong, T.; Numhom, S.; Aramwit, P. Mulberry leaves and their potential effects against cardiometabolic risks: a review of chemical compositions, biological properties and clinical efficacy. Pharm Biol 2018, 56, 109–118. [Google Scholar] [CrossRef]
- Thaipitakwong, T.; Supasyndh, O.; Rasmi, Y.; Aramwit, P. A randomized controlled study of dose-finding, efficacy, and safety of mulberry leaves on glycemic profiles in obese persons with borderline diabetes. Complement Ther Med 2020, 49, 102292. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Ma, Z.; Li, Y.; Guo, J.; Meng, Q.; Bian, H. A novel formula from mulberry leaf ameliorates diabetic nephropathy in rats via inhibiting the TGF-beta1 pathway. Food Funct 2015, 6, 3307–3315. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Zhu, X.; Li, S.; Shi, X.; Li, Z.; Ai, M.; Sun, J.; Hou, B.; Cai, W.; et al. Protective Effects and Mechanisms of Vaccarin on Vascular Endothelial Dysfunction in Diabetic Angiopathy. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, X.; Zhu, X.; Wen, Y.; Zhu, M.; Cai, W.; Hou, B.; Xu, F.; Qiu, L. Vaccarin alleviates endothelial inflammatory injury in diabetes by mediating miR-570-3p/HDAC1 pathway. Front Pharmacol 2022, 13, 956247. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Lu, S.S.; Wang, H.L.; Li, G.; He, Y.F.; Liu, X.Y.; Rong, R.; Li, J.; Lu, X.C. Effects of the fenugreek extracts on high-fat diet-fed and streptozotocin-induced type 2 diabetic mice. Animal Model Exp Med 2018, 1, 68–73. [Google Scholar] [CrossRef]
- Rehman, K.; Chohan, T.A.; Waheed, I.; Gilani, Z.; Akash, M.S.H. Taxifolin prevents postprandial hyperglycemia by regulating the activity of alpha-amylase: Evidence from an in vivo and in silico studies. J Cell Biochem 2019, 120, 425–438. [Google Scholar] [CrossRef]
- Sun, X.; Chen, R.C.; Yang, Z.H.; Sun, G.B.; Wang, M.; Ma, X.J.; Yang, L.J.; Sun, X.B. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem Toxicol 2014, 63, 221–232. [Google Scholar] [CrossRef]
- Konate, K.; Yomalan, K.; Sytar, O.; Zerbo, P.; Brestic, M.; Patrick, V.D.; Gagniuc, P.; Barro, N. Free Radicals Scavenging Capacity, Antidiabetic and Antihypertensive Activities of Flavonoid-Rich Fractions from Leaves of Trichilia emetica and Opilia amentacea in an Animal Model of Type 2 Diabetes Mellitus. Evid Based Complement Alternat Med 2014, 2014, 867075. [Google Scholar] [CrossRef]
- Xu, J.; Jordan, R.B. Kinetics and mechanism of the oxidation of 2,3-dihydroxybenzoic acid by iron(III). Inorganic Chemistry 1988, 27, 4563–4566. [Google Scholar] [CrossRef]
- Álvarez-Cilleros, D.; Martín, M.; Ramos, S. (-)-Epicatechin and the Colonic 2,3-Dihydroxybenzoic Acid Metabolite Regulate Glucose Uptake, Glucose Production, and Improve Insulin Signaling in Renal NRK-52E Cells. Mol Nutr Food Res 2018, 62. [Google Scholar] [CrossRef]
- Sugihara, N.; Ohnishi, M.; Imamura, M.; Furuno, K. Differences in antioxidative efficiency of catechins in various metal-induced lipid peroxidations in cultured hepatocytes. Journal of health science 2001, 47, 99–106. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Sohal, R.S.; Arnold, L.A.; Sohal, B.H. Age-related changes in antioxidant enzymes and prooxidant generation in tissues of the rat with special reference to parameters in two insect species. Free Radic Biol Med 1990, 9, 495–500. [Google Scholar] [CrossRef]
- Lopez-Torres, M.; Perez-Campo, R.; Barja de Quiroga, G. Aging in brown fat: antioxidant defenses and oxidative stress. Mech Ageing Dev 1991, 59, 129–137. [Google Scholar] [CrossRef]
- Lopez-Torres, M.; Perez-Campo, R.; Barja de Quiroga, G. Effect of natural ageing and antioxidant inhibition on liver antioxidant enzymes, glutathione system, peroxidation, and oxygen consumption in Rana perezi. J Comp Physiol B 1991, 160, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-H.; Yeh, C.-T.; Yen, G.-C. Anthocyanins Induce the Activation of Phase II Enzymes through the Antioxidant Response Element Pathway against Oxidative Stress-Induced Apoptosis. Journal of Agricultural and Food Chemistry 2007, 55, 9427–9435. [Google Scholar] [CrossRef] [PubMed]
- Renjini, R.; Gayathri, N.; Nalini, A.; Srinivas Bharath, M.M. Oxidative damage in muscular dystrophy correlates with the severity of the pathology: role of glutathione metabolism. Neurochem Res 2012, 37, 885–898. [Google Scholar] [CrossRef]
- Samanta, L.; Roy, A.; Chainy, G.B. Changes in rat testicular antioxidant defence profile as a function of age and its impairment by hexachlorocyclohexane during critical stages of maturation. Andrologia 1999, 31, 83–90. [Google Scholar] [CrossRef]
- Giusti, L.; Gabriele, M.; Penno, G.; Garofolo, M.; Longo, V.; Del Prato, S.; Lucchesi, D.; Pucci, L. A Fermented Whole Grain Prevents Lipopolysaccharides-Induced Dysfunction in Human Endothelial Progenitor Cells. Oxid Med Cell Longev 2017, 2017, 1026268. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, A.; Zyryanov, G.V.; Bhagath, Y.B.; Manjula, K. Antioxidants: Structure-activity of plant polyphenolics. Vitam Horm 2023, 121, 395–411. [Google Scholar] [CrossRef]
- Stryjecka, M.; Krochmal-Marczak, B.; Cebulak, T.; Kiełtyka-Dadasiewicz, A. Assessment of Phenolic Acid Content and Antioxidant Properties of the Pulp of Five Pumpkin Species Cultivated in Southeastern Poland. International Journal of Molecular Sciences 2023, 24, 8621. [Google Scholar] [PubMed]
- Uchiyama, Y.; Suzuki, T.; Mochizuki, K.; Goda, T. Dietary supplementation with a low dose of (-)-epigallocatechin-3-gallate reduces pro-inflammatory responses in peripheral leukocytes of non-obese type 2 diabetic GK rats. J Nutr Sci Vitaminol (Tokyo) 2013, 59, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, Y.; Suzuki, T.; Mochizuki, K.; Goda, T. Dietary supplementation with (-)-epigallocatechin-3-gallate reduces inflammatory response in adipose tissue of non-obese type 2 diabetic Goto-Kakizaki (GK) rats. J Agric Food Chem 2013, 61, 11410–11417. [Google Scholar] [CrossRef]
- Elbling, L.; Weiss, R.M.; Teufelhofer, O.; Uhl, M.; Knasmueller, S.; Schulte-Hermann, R.; Berger, W.; Micksche, M. Green tea extract and (-)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. Faseb j 2005, 19, 807–809. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Afshari, G.; Mard, S.A.; Khodadadi, A.; Hashemitabar, M. Preventive effects of procyanidin A2 on glucose homeostasis, pancreatic and duodenal homebox 1, and glucose transporter 2 gene expression disturbance induced by bisphenol A in male mice. J Physiol Pharmacol 2016, 67, 243–252. [Google Scholar]
- Wen, L.; Wu, D.; Tan, X.; Zhong, M.; Xing, J.; Li, W.; Li, D.; Cao, F. The Role of Catechins in Regulating Diabetes: An Update Review. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Madkhali, H.; Tarawneh, A.; Ali, Z.; Le, H.V.; Cutler, S.J.; Khan, I.A.; Shariat-Madar, Z. Identification of Human Kinin-Forming Enzyme Inhibitors from Medicinal Herbs. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Draganescu, D.; Andritoiu, C.; Hritcu, D.; Dodi, G.; Popa, M.I. Flaxseed Lignans and Polyphenols Enhanced Activity in Streptozotocin-Induced Diabetic Rats. Biology (Basel) 2021, 10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





