Submitted:
23 September 2023
Posted:
26 September 2023
You are already at the latest version
Abstract
Keywords:
1. Content
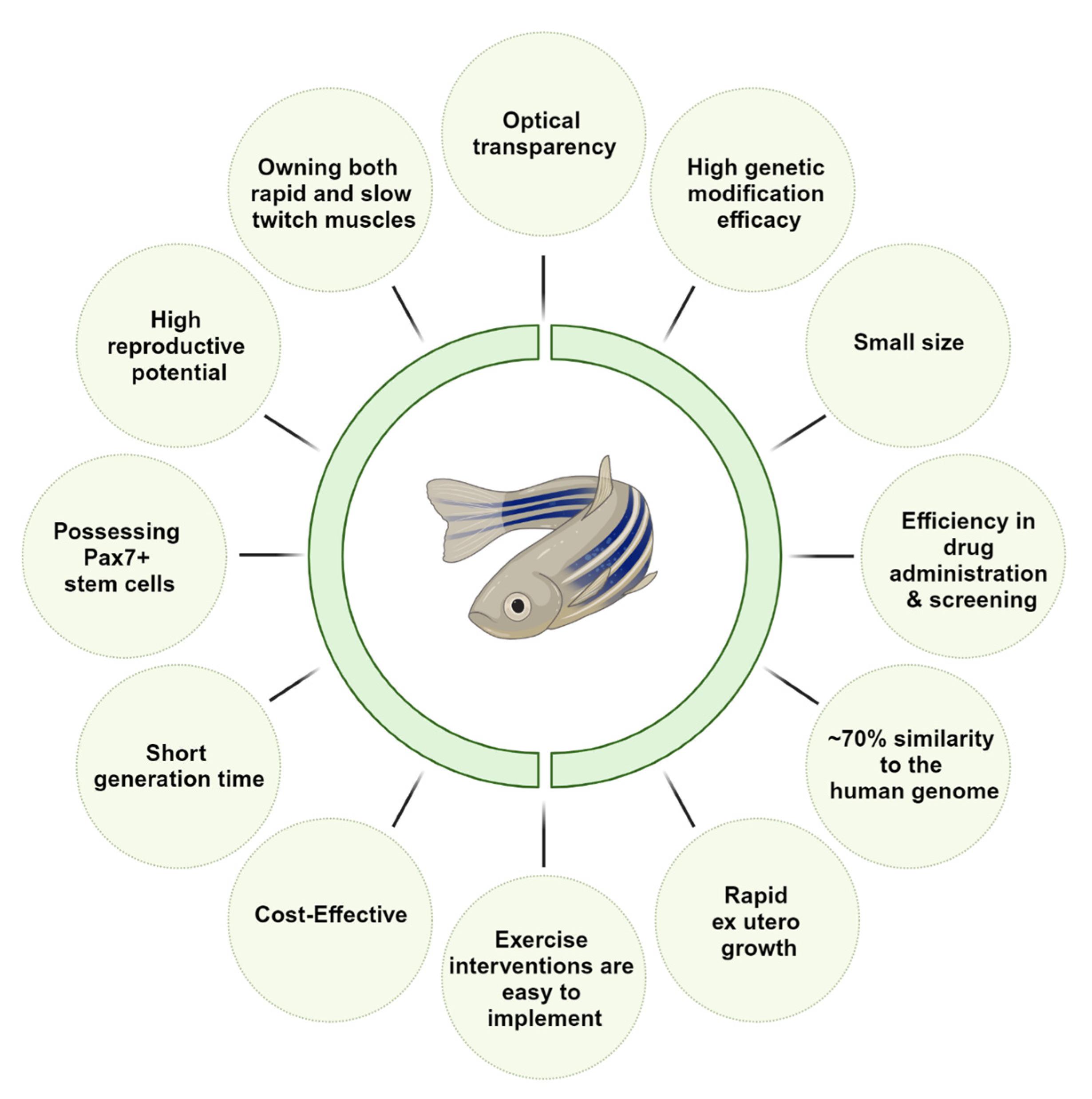
2. Zebrafish as an Animal Model of Sarcopenia
3. Current challenges and opportunities
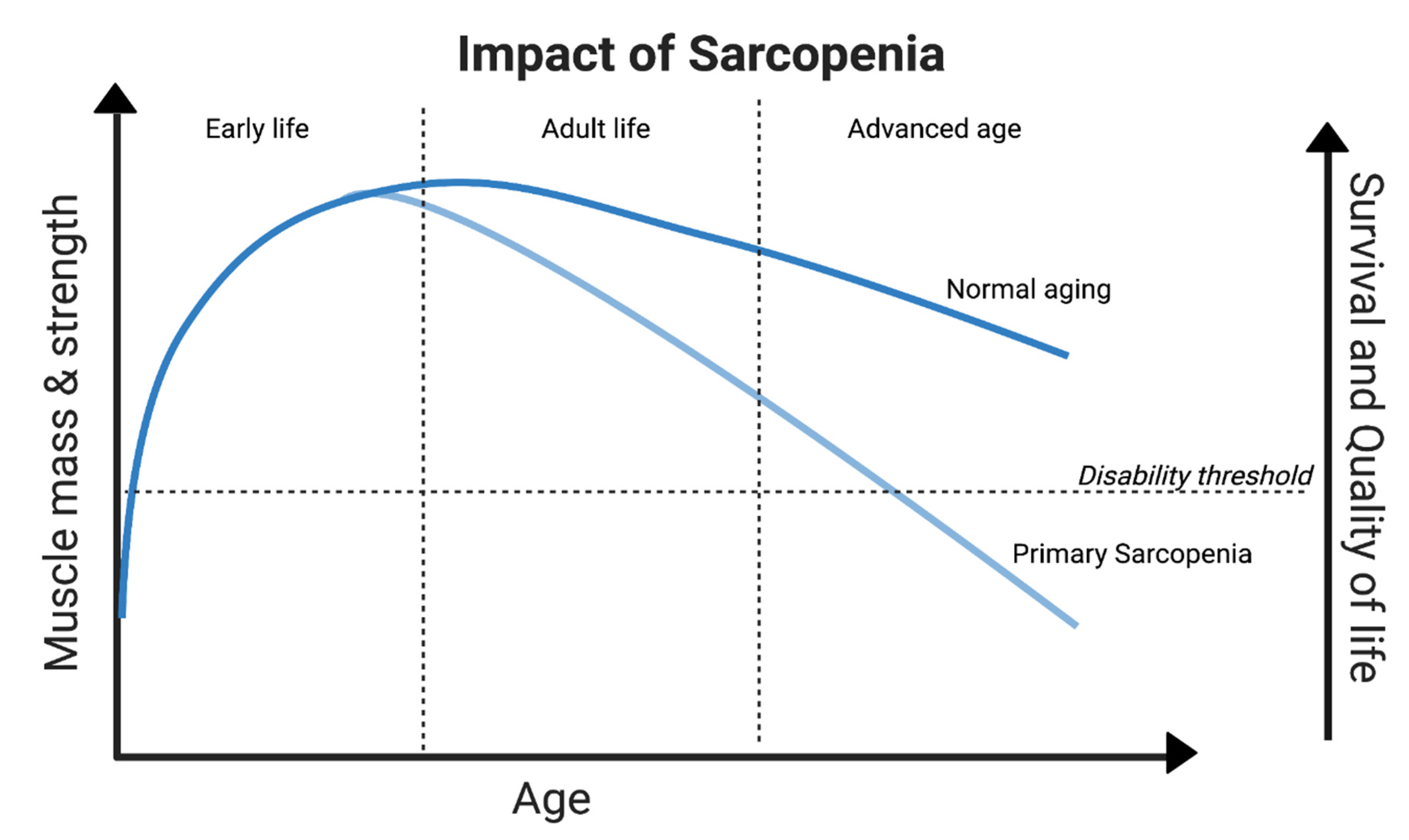
3.1. Aging and Sarcopenia
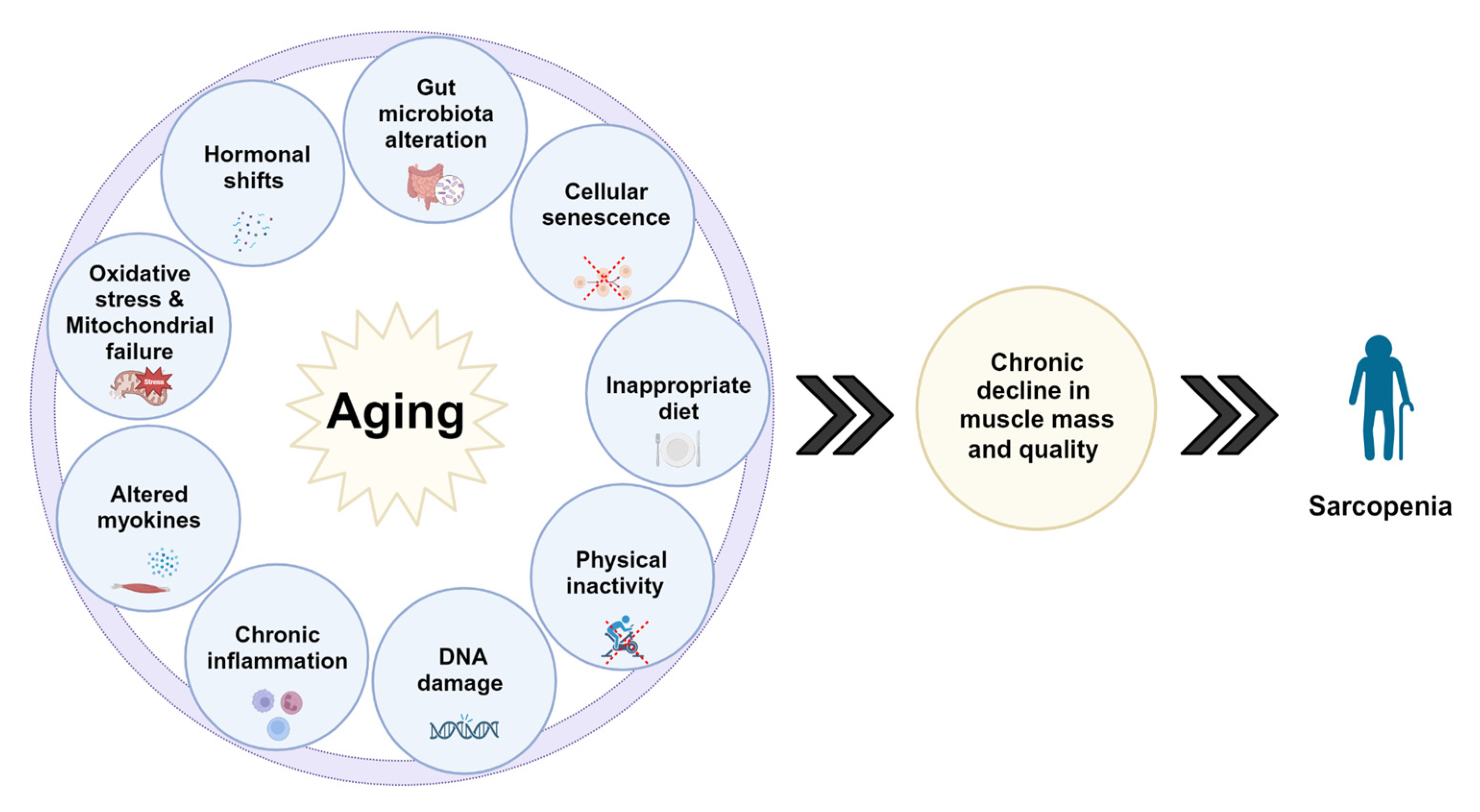
3.2. Pathogenesis
3.3. Diagnosis:
3.4. Treatment
4. Latest Zebrafish Advances in Sarcopenia
5. Future perspectives:
5.1. Novel pathways and molecules for improving mitochondrial function.
5.2. Identification of novel genes and candidates in population-wide studies
5.3. Complimentary with other animal models
6. Conclusion:
Author Contributions
Funding
Conflicts of Interest
References
- Bowley, G.; Kugler, E.; Wilkinson, R.; Lawrie, A.; van Eeden, F.; Chico, T.J.A.; Evans, P.C.; Noël, E.S.; Serbanovic-Canic, J. Zebrafish as a tractable model of human cardiovascular disease. Br. J. Pharmacol. 2021, 179, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Bashirzade, A.A.; Zabegalov, K.N.; Volgin, A.D.; Belova, A.S.; Demin, K.A.; de Abreu, M.S.; Babchenko, V.Y.; Bashirzade, K.A.; Yenkoyan, K.B.; Tikhonova, M.A.; et al. Modeling neurodegenerative disorders in zebrafish. Neurosci. Biobehav. Rev. 2022, 138, 104679. [Google Scholar] [CrossRef] [PubMed]
- Daya, A.; Donaka, R.; Karasik, D. Zebrafish models of sarcopenia. Dis Model Mech [Internet] 2020, 13, dmm042689. [Google Scholar] [CrossRef]
- Ichii, S.; Matsuoka, I.; Okazaki, F.; Shimada, Y. Zebrafish Models for Skeletal Muscle Senescence: Lessons from Cell Cultures and Rodent Models. Molecules 2022, 27, 8625. [Google Scholar] [CrossRef] [PubMed]
- Adhish, M.; Manjubala, I. Effectiveness of zebrafish models in understanding human diseases—A review of models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef]
- Kijima, Y.; Wantong, W.; Igarashi, Y.; Yoshitake, K.; Asakawa, S.; Suzuki, Y.; Watabe, S.; Kinoshita, S. Age-Associated Different Transcriptome Profiling in Zebrafish and Rats: an Insight into the Diversity of Vertebrate Aging. Mar. Biotechnol. 2022, 24, 1–16. [Google Scholar] [CrossRef]
- Johnston, I.A.; Bower, N.I.; Macqueen, D.J. Growth and the regulation of myotomal muscle mass in teleost fish. J. Exp. Biol. 2011, 214, 1617–1628. [Google Scholar] [CrossRef]
- Bhandari, S.; Kim, Y.-I.; Nam, I.-K.; Hong, K.; Jo, Y.; Yoo, K.-W.; Liao, W.; Lim, J.-Y.; Kim, S.-J.; Um, J.-Y.; et al. Loss of pex5 sensitizes zebrafish to fasting due to deregulated mitochondria, mTOR, and autophagy. Cell. Mol. Life Sci. 2023, 80, 1–20. [Google Scholar] [CrossRef]
- Guyon, J.R.; Steffen, L.S.; Howell, M.H.; Pusack, T.J.; Lawrence, C.; Kunkel, L.M. Modeling human muscle disease in zebrafish. Biochim Biophys Acta Mol Basis Dis [Internet] 2007, 1772, 205–15. [Google Scholar] [CrossRef]
- Tesoriero, C.; Greco, F.; Cannone, E.; Ghirotto, F.; Facchinello, N.; Schiavone, M.; Vettori, A. Modeling Human Muscular Dystrophies in Zebrafish: Mutant Lines, Transgenic Fluorescent Biosensors, and Phenotyping Assays. Int. J. Mol. Sci. 2023, 24, 8314. [Google Scholar] [CrossRef]
- Teame, T.; Zhang, Z.; Ran, C.; Zhang, H.; Yang, Y.; Ding, Q.; Xie, M.; Gao, C.; Ye, Y.; Duan, M.; et al. The use of zebrafish (Danio rerio) as biomedical models. Anim. Front. 2019, 9, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Christian, C.J.; Benian, G.M. Animal models of sarcopenia. Aging Cell [Internet] 2020, 19. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Je, J.-G.; Jeon, Y.-J.; Yang, H.-W. Zebrafish Model for Studying Dexamethasone-Induced Muscle Atrophy and Preventive Effect of Maca (Lepidium meyenii). Cells 2021, 10, 2879. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Schmeer, C.; Kretz, A.; Wengerodt, D.; Stojiljkovic, M.; Witte, O. W. Dissecting aging and senescence—current concepts and open lessons. Cells 2019, 8, 1446. [Google Scholar] [CrossRef]
- Hill, M.; Třískala, Z.; Honců, P.; Krejčí, M.; Kajzar, J.; Bičíková, M.; et al. Aging, hormones and receptors. Physiol Res 2020, 69 (Suppl 2), S255–72. [Google Scholar] [CrossRef]
- Ames, B.N. Prolonging healthy aging: Longevity vitamins and proteins. Proc. Natl. Acad. Sci. 2018, 115, 10836–10844. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in aging, health and diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Xing, Y.; Jia, J.; Tang, Y. B vitamins and prevention of cognitive decline and incident dementia: a systematic review and meta-analysis. Nutr Rev 2022, 80, 931–49. [Google Scholar] [CrossRef]
- Sun, R.; Wang, J.; Feng, J.; Cao, B. Zinc in Cognitive Impairment and Aging. Biomolecules 2022, 12, 1000. [Google Scholar] [CrossRef]
- Reuter, H.; Perner, B.; Wahl, F.; Rohde, L.; Koch, P.; Groth, M.; Buder, K.; Englert, C. Aging Activates the Immune System and Alters the Regenerative Capacity in the Zebrafish Heart. Cells 2022, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Monma, Y.; Shimada, Y.; Nakayama, H.; Zang, L.; Nishimura, N.; Tanaka, T. Aging-associated microstructural deterioration of vertebra in zebrafish. Bone Rep. 2019, 11, 100215. [Google Scholar] [CrossRef] [PubMed]
- Zambusi, A.; Burhan. P.; Di Giaimo, R.; Schmid, B.; Ninkovic, J. Granulins Regulate Aging Kinetics in the Adult Zebrafish Telencephalon. Cells 2020, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 1–8. [Google Scholar] [CrossRef]
- Cruz-Jentoft, AJ.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; et al. Sarcopenia: A time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle 2019, 10, 956–61. [Google Scholar] [CrossRef]
- Supriya, R.; Singh, K.P.; Gao, Y.; Gu, Y.; Baker, J.S. Effect of Exercise on Secondary Sarcopenia: A Comprehensive Literature Review. Biology 2021, 11, 51. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing 2022, 51. [Google Scholar] [CrossRef]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Joint Bone Spine 2019, 86, 309–14. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Colleluori, G.; Villareal, D.T. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp. Gerontol. 2021, 155, 111561–111561. [Google Scholar] [CrossRef] [PubMed]
- Goates, S.; Du, K.; Arensberg, M.B.; Gaillard, T.; Guralnik, J.; Pereira, S.L. ECONOMIC IMPACT OF HOSPITALIZATIONS IN US ADULTS WITH SARCOPENIA. J. Frailty Aging 2019, 8, 1–7. [Google Scholar] [CrossRef]
- Migliavacca, E.; Tay, S.K.H.; Patel, H.P.; Sonntag, T.; Civiletto, G.; McFarlane, C.; Forrester, T.; Barton, S.J.; Leow, M.K.; Antoun, E.; et al. Mitochondrial oxidative capacity and NAD+ biosynthesis are reduced in human sarcopenia across ethnicities. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- He, Y.; Xie, W.; Li, H.; Jin, H.; Zhang, Y.; Li, Y. Cellular Senescence in Sarcopenia: Possible Mechanisms and Therapeutic Potential. Front. Cell Dev. Biol. 2022, 9, 793088. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Dao, T.; Green, A.E.; A Kim, Y.; Bae, S.-J.; Ha, K.-T.; Gariani, K.; Lee, M.-R.; Menzies, K.J.; Ryu, D. Sarcopenia and Muscle Aging: A Brief Overview. Endocrinol. Metab. 2020, 35, 716–732. [Google Scholar] [CrossRef]
- Abdelrahman, Z.; Wang, X.; Wang, D.; Zhang, T.; Zhang, Y.; Wang, X.; Chen, Z. Identification of novel pathways and immune profiles related to sarcopenia. Front. Med. 2023, 10. [Google Scholar] [CrossRef]
- Ayobahan, S.U.; Eilebrecht, S.; Baumann, L.; Teigeler, M.; Hollert, H.; Kalkhof, S.; Eilebrecht, E.; Schäfers, C. Detection of biomarkers to differentiate endocrine disruption from hepatotoxicity in zebrafish (Danio rerio) using proteomics. Chemosphere 2019, 240, 124970. [Google Scholar] [CrossRef] [PubMed]
- Juvale, I.I.A.; Che Has, A.T. The potential role of miRNAs as predictive biomarkers in neurodevelopmental disorders. J Mol Neurosci 2021, 71, 1338–55, Available from: https://pubmed.ncbi.nlm.nih.gov/33774758/. [Google Scholar] [CrossRef]
- Wawruszak, A.; Okoń, E.; Dudziak, K. A Review of the Role of the Zebrafish (Danio reiro) in Preclinical and Clinical Models of Biomarker Identification and Drug Targeting, and Developments in Personalized Medicine in Breast Cancer. Experiment 2023, 29, e940550–1. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; et al. Oxidative stress, aging, and diseases. Clin Interv Aging 2018, 13, 757–72, Available from: https://pubmedncbinlmnihgov/29731617/. [Google Scholar] [CrossRef]
- Kadoguchi, T.; Shimada, K.; Miyazaki, T.; Kitamura, K.; Kunimoto, M.; Aikawa, T.; Sugita, Y.; Ouchi, S.; Shiozawa, T.; Yokoyama-Nishitani, M.; et al. Promotion of oxidative stress is associated with mitochondrial dysfunction and muscle atrophy in aging mice. Geriatr. Gerontol. Int. 2019, 20, 78–84. [Google Scholar] [CrossRef]
- Daussin, F.N.; Boulanger, E.; Lancel, S. From mitochondria to sarcopenia: Role of inflammaging and RAGE-ligand axis implication. Exp. Gerontol. 2021, 146, 111247. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Campanario, S.; Ramírez-Pardo, I.; Grima-Terrén, M.; Isern, J.; Muñoz-Cánoves, P. Stem cell aging in the skeletal muscle: The importance of communication. Ageing Res. Rev. 2021, 73, 101528. [Google Scholar] [CrossRef]
- Mankhong, S.; Kim, S.; Moon, S.; Kwak, H.-B.; Park, D.-H.; Kang, J.-H. Experimental Models of Sarcopenia: Bridging Molecular Mechanism and Therapeutic Strategy. Cells 2020, 9, 1385. [Google Scholar] [CrossRef]
- Pacifici, F.; Della-Morte, D.; Piermarini, F.; Arriga, R.; Scioli, M.G.; Capuani, B.; Pastore, D.; Coppola, A.; Rea, S.; Donadel, G.; et al. Prdx6 Plays a Main Role in the Crosstalk between Aging and Metabolic Sarcopenia. Antioxidants 2020, 9, 329. [Google Scholar] [CrossRef]
- Zhang, X.; Habiballa, L.; Aversa, Z.; Ng, Y.E.; Sakamoto, A.E.; Englund, D.A.; Pearsall, V.M.; White, T.A.; Robinson, M.M.; Rivas, D.A.; et al. Characterization of cellular senescence in aging skeletal muscle. Nat. Aging 2022, 2, 601–615. [Google Scholar] [CrossRef]
- Ebert, S.M.; Dierdorff, J.M.; Meyerholz, D.K.; Bullard, S.A.; Al-Zougbi, A.; DeLau, A.D.; Tomcheck, K.C.; Skopec, Z.P.; Marcotte, G.R.; Bodine, S.C.; et al. An investigation of p53 in skeletal muscle aging. J. Appl. Physiol. 2019, 127, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, D.; Yang, Y.; Xie, W.; He, M.; Yu, D.; Wu, Y.; Wang, X.; Xiao, W.; Li, Y. The role and therapeutic potential of stem cells in skeletal muscle in sarcopenia. Stem Cell Res. Ther. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Berberoglu, M.A.; Gallagher, T.L.; Morrow, Z.T.; Talbot, J.C.; Hromowyk, K.J.; Tenente, I.M.; Langenau, D.M.; Amacher, S.L. Satellite-like cells contribute to pax7-dependent skeletal muscle repair in adult zebrafish. Dev. Biol. 2017, 424, 162–180. [Google Scholar] [CrossRef]
- Ferreira, F.J.; Carvalho, L.; Logarinho, E.; Bessa, J. foxm1 Modulates Cell Non-Autonomous Response in Zebrafish Skeletal Muscle Homeostasis. Cells 2021, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.C.; de Castro, I.P.; Ferreira, M.G. Telomeres in aging and disease: lessons from zebrafish. Dis. Model. Mech. 2016, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Bayliss, P.E.; Uchiyama, J.; Koshimizu, E.; Qi, J.; Nanjappa, P.; Imamura, S.; Islam, A.; Neuberg, D.; Amsterdam, A.; et al. The Identification of Zebrafish Mutants Showing Alterations in Senescence-Associated Biomarkers. PLOS Genet. 2008, 4, e1000152–e1000152. [Google Scholar] [CrossRef]
- Henderson, T.D.; Choi, J.; Leonard, S.W.; Head, B.; Tanguay, R.L.; Barton, C.L.; Traber, M.G. Chronic Vitamin E Deficiency Dysregulates Purine, Phospholipid, and Amino Acid Metabolism in Aging Zebrafish Skeletal Muscle. Antioxidants 2023, 12, 1160. [Google Scholar] [CrossRef]
- Zou, Y.-Y.; Chen, Z.-L.; Sun, C.-C.; Yang, D.; Zhou, Z.-Q.; Xiao, Q.; Peng, X.-Y.; Tang, C.-F. A High-Fat Diet Induces Muscle Mitochondrial Dysfunction and Impairs Swimming Capacity in Zebrafish: A New Model of Sarcopenic Obesity. Nutrients 2022, 14, 1975. [Google Scholar] [CrossRef]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the 'anabolic resistance' of ageing. Nutr. Metab. 2011, 8, 68–68. [Google Scholar] [CrossRef]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef]
- E Yarasheski, K.; Bhasin, S.; Sinha-Hikim, I.; Pak-Loduca, J.; Gonzalez-Cadavid, N.F. Serum myostatin-immunoreactive protein is increased in 60-92 year old women and men with muscle wasting. Journal of nutrition health and aging 2002, 6, 343–348. [Google Scholar]
- Gao, Y.; Dai, Z.; Shi, C.; Zhai, G.; Jin, X.; He, J.; et al. Depletion of myostatin b promotes somatic growth and lipid metabolism in zebrafish. Front Endocrinol (Lausanne) 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Hellerstein, M.; Orwoll, E.; Cummings, S.; Cawthon, P.M. D3-Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J. Cachex- Sarcopenia Muscle 2019, 10, 14–21. [Google Scholar] [CrossRef]
- Lu, L.; Mao, L.; Feng, Y.; Ainsworth, B.E.; Liu, Y.; Chen, N. Effects of different exercise training modes on muscle strength and physical performance in older people with sarcopenia: a systematic review and meta-analysis. BMC Geriatr. 2021, 21, 1–30. [Google Scholar] [CrossRef]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006, 61, 1059–64. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I. Evolution of sarcopenia research. Appl Physiol Nutr Metab 2010, 35, 707–12. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Voulgaridou, G.; Kondyli, F.S.; Drakaki, M.; Sianidou, K.; Andrianopoulou, R.; Rodopaios, N.; Pritsa, A. Nutritional and Nutrition-Related Biomarkers as Prognostic Factors of Sarcopenia, and Their Role in Disease Progression. Diseases 2022, 10, 42. [Google Scholar] [CrossRef]
- Lin, S.; Ling, M.; Chen, C.; Cai, X.; Yang, F.; Fan, Y. Screening Potential Diagnostic Biomarkers for Age-Related Sarcopenia in the Elderly Population by WGCNA and LASSO. BioMed Res. Int. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Cesari, M.; Landi, F.; Bernabei, R.; Coelho-Júnior, H.J.; et al. Biomarkers of physical frailty and sarcopenia: Coming up to the place? Int J Mol Sci 2020, 21, 5635. [Google Scholar] [CrossRef]
- Feng, S.; Wang, S.; Wang, Y.; Yang, Q.; Wang, D.; Li, H. Identification and expression of carbonic anhydrase 2, myosin regulatory light chain 2 and selenium-binding protein 1 in zebrafish Danio rerio: Implication for age-related biomarkers. Gene Expr. Patterns 2018, 29, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.; King, M.; Dascombe, B.; Taylor, N.; Silva, D.d.O.; Holden, S.; Goff, A.; Takarangi, K.; Shields, N. Many physiotherapists lack preparedness to prescribe physical activity and exercise to people with musculoskeletal pain: A multi-national survey. Phys. Ther. Sport 2021, 49, 98–105. [Google Scholar] [CrossRef]
- Hurst, C.; Robinson, S.M.; Witham, M.D.; Dodds, R.M.; Granic, A.; Buckland, C.; De Biase, S.; Finnegan, S.; Rochester, L.; A Skelton, D.; et al. Resistance exercise as a treatment for sarcopenia: prescription and delivery. Age Ageing 2022, 51. [Google Scholar] [CrossRef]
- Tsekoura, M.; Billis, E.; Kastrinis, A.; Katsoulaki, M.; Fousekis, K.; Tsepis, E.; et al. The effects of exercise in patients with sarcopenia. In GeNeDis 2020; Springer International Publishing: Cham, 2021; pp. 281–90. [Google Scholar]
- Hasumura, T.; Meguro, S. Exercise quantity-dependent muscle hypertrophy in adult zebrafish (Danio rerio). J. Comp. Physiol. B 2016, 186, 603–614. [Google Scholar] [CrossRef]
- Rutkove, S.B.; Callegari, S.; Concepcion, H.; Mourey, T.; Widrick, J.; Nagy, J.A.; Nath, A.K. Electrical impedance myography detects age-related skeletal muscle atrophy in adult zebrafish. Sci. Rep. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; Zhu, N.; Xu, M.; Li, Y. Beneficial effects of whey protein peptides on muscle loss in aging mice models. Front. Nutr. 2022, 9, 897821. [Google Scholar] [CrossRef]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, S.; Tsatsakis, A.M.; Hayes, A.W.; Kouretas, D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Essential amino acids increase MicroRNA-499, −208b, and −23a and downregulate myostatin and myocyte enhancer factor 2C mRNA expression in human skeletal muscle. J Nutr 2009, 139, 2279–84. [Google Scholar] [CrossRef]
- Barbiera, A.; Pelosi, L.; Sica, G.; Scicchitano, B.M. Nutrition and microRNAs: Novel Insights to Fight Sarcopenia. Antioxidants 2020, 9, 951. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Papadimitriou, K.; Voulgaridou, G.; Georgaki, E.; Tsotidou, E.; Zantidou, O.; et al. Exercise and nutrition impact on osteoporosis and sarcopenia—the incidence of osteosarcopenia: A narrative review. Nutrients 2021, 13, 4499. [Google Scholar] [CrossRef]
- Bellanti, F.; Lo Buglio, A.; Vendemiale, G. Muscle delivery of mitochondria-targeted drugs for the treatment of sarcopenia: Rationale and perspectives. Pharmaceutics 2022, 14, 2588. [Google Scholar] [CrossRef]
- Hardee, J.P.; Lynch, G.S. Current pharmacotherapies for sarcopenia. Expert Opin Pharmacother 2019, 20, 1645–57. [Google Scholar] [CrossRef]
- Najjar, F.; Rizk, F.; Carnac, G.; Nassar, R.; Jabak, S.; Sobolev, AP.; et al. Protective effect ofRhus coriariafruit extracts against hydrogen peroxide-induced oxidative stress in muscle progenitors and zebrafish embryos. PeerJ 2017, 5, e4144. [Google Scholar] [CrossRef] [PubMed]
- Turkel, I.; Ozerklig, B.; Yılmaz, M.; Ulger, O.; Kubat, G.B.; Tuncer, M. Mitochondrial transplantation as a possible therapeutic option for sarcopenia. J Mol Med 2023, 101, 645–69. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Pan, M.; Zhou, M.; Tang, Q.; Chen, M.; Hong, W.; et al. Mitochondria transplantation from stem cell for mitigating sarcopenia. Aging Dis 2023. Available from: https://pubmed.ncbi.nlm.nih.gov/37196123. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Rutkove, S.; Lupton, E.C.; Padilla, C.J.; Arnold, W.D. Potential utility of electrical impedance myography in evaluating age-related skeletal muscle function deficits. Front Physiol 2021, 12. [Google Scholar] [CrossRef]
- Cebrián-Ponce, Á.; Irurtia, A.; Carrasco-Marginet, M.; Saco-Ledo, G.; Girabent-Farrés, M.; Castizo-Olier, J. Electrical impedance myography in health and physical exercise: A systematic review and future perspectives. Front Physiol 2021, 12. [Google Scholar] [CrossRef]
- Rutkove, S.B.; Chen, Z.-Z.; Pandeya, S.; Callegari, S.; Mourey, T.; Nagy, J.A.; et al. Surface electrical impedance myography detects skeletal muscle atrophy in aged wildtype zebrafish and aged gpr27 knockout zebrafish. Biomedicines 2023, 11, 1938, Available from: https://pubmed.ncbi.nlm.nih.gov/37509577/. [Google Scholar] [CrossRef]
- Sun, C.-C.; Yang, D.; Chen, Z.-L.; Xiao, J.-L.; Xiao, Q.; Li, C.-L.; et al. Exercise intervention mitigates zebrafish age-related sarcopenia via alleviating mitochondrial dysfunction. FEBS J 2023, 290, 1519–30. [Google Scholar] [CrossRef]
- Rogeri, P.S.; Zanella, R. , Jr, Martins, G.L.; Garcia, M.D.A.; Leite, G.; Lugaresi, R.; et al. Strategies to prevent sarcopenia in the aging process: Role of protein intake and exercise. Nutrients 2021, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Van houcke, J.; De Groef, L.; Dekeyster, E.; Moons, L. The zebrafish as a gerontology model in nervous system aging, disease, and repair. Ageing Res Rev 2015, 24 Pt B, 358–68, Available from: https://pubmed.ncbi.nlm.nih.gov/26538520/. [Google Scholar] [CrossRef]
- Shah, R.R.; Nerurkar, N.L.; Wang, C.C.; Galloway, J.L. Tensile properties of craniofacial tendons in the mature and aged zebrafish. J Orthop Res 2015, 33, 867–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-L.; Guo, C.; Zou, Y.-Y.; Feng, C.; Yang, D.-X.; Sun, C.-C.; et al. Aerobic exercise enhances mitochondrial homeostasis to counteract D-galactose-induced sarcopenia in zebrafish. Exp Gerontol 2023, 180, 112265. [Google Scholar] [CrossRef]
- Willems, S.M.; Wright, D.J.; Day, F.R.; Trajanoska, K.; Joshi, P.K.; Morris, J.A.; et al. Large-scale GWAS identifies multiple loci for hand grip strength providing biological insights into muscular fitness. Nat Commun 2017, 8. [Google Scholar] [CrossRef]
- Kwon, R.Y.; Watson, C.J.; Karasik, D. Using zebrafish to study skeletal genomics. Bone [Internet] 2019, 126, 37–50. [Google Scholar] [CrossRef]
- Xiao, S.-M.; Kung, A.W.C.; Gao, Y.; Lau, K.-S.; Ma, A.; Zhang, Z.-L.; et al. Post-genome wide association studies and functional analyses identify association of MPP7 gene variants with site-specific bone mineral density. Hum Mol Genet 2012, 21, 1648–57. [Google Scholar] [CrossRef]
- Polačik, M.; Blažek, R.; Reichard, M. Laboratory breeding of the short-lived annual killifish Nothobranchius furzeri. Nat Protoc 2016, 11, 1396–413. [Google Scholar] [CrossRef]
- Kim, Y.; Nam, HG.; Valenzano, DR. The short-lived African turquoise killifish: an emerging experimental model for ageing. Dis Model Mech 2016, 9, 115–29. [Google Scholar] [CrossRef]
- Hu, C.-K.; Brunet, A. The African turquoise killifish: A research organism to study vertebrate aging and diapause. Aging Cell 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Królak, M. The epizootiological situation with regard to animal brucellosis in Poland 1985. Przegl Epidemiol 1987, 41. [Google Scholar]
- Cencioni, C.; Heid, J.; Krepelova, A.; Rasa, S.M.M.; Kuenne, C.; Guenther, S.; et al. Aging triggers H3K27 trimethylation hoarding in the chromatin of Nothobranchius furzeri skeletal muscle. Cells 2019, 8, 1169. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, D.; De Felice, E.; Attanasio, C.; Palladino, A.; Schiano, V.; Mollo, E.; et al. Central and peripheral NPY age-related regulation: A comparative analysis in fish translational models. Int J Mol Sci 2022, 23, 3839. [Google Scholar] [CrossRef] [PubMed]
| Title | Authors | Zebrafish animals used | Intervention and/or exposure | Results |
|---|---|---|---|---|
| Electrical impedance myography detects age-related skeletal muscle atrophy in adult zebrafish | Rutkove SB, Callegari S, Concepcion H, Mourey T, Widrick J, Nagy JA, Nath AK. | Wildtype casper (6 & 33 months) and Tübingen (4 & 24 months) zebrafish | Relationship between swimming efficacy, age, and EIM measures | EIM effectively correlates with age-related muscle atrophy in adult zebrafish. |
| Exercise intervention mitigates zebrafish age-related sarcopenia via alleviating mitochondrial dysfunction | Sun CC, Yang D, Chen ZL, Xiao JL, Xiao Q, Li CL, Zhou ZQ, Peng XY, Tang CF, Zheng L | AB strain male zebrafish (21 &6 months) | swimming efficacy on mitochondrial homeostasis and protein regulation in sarcopenia | Exercise reduced age-related muscle atrophy by improving muscle structure, decreasing protein breakdown, and restoring mitochondrial activity. |
| A High-Fat Diet Induces Muscle Mitochondrial Dysfunction and Impairs Swimming Capacity in Zebrafish: A New Model of Sarcopenic Obesity | Zou YY, Chen ZL, Sun CC, Yang D, Zhou ZQ, Xiao Q, Peng XY, Tang CF. | Adult male AB strain zebrafish (4 months) | High-fat diet (16 weeks) induced sarcopenic obesity models and its effect on swimming capacity and muscle atrophy | High-fat diet for an extended period of time resulted in muscular atrophy, reduced swimming ability, increased body weight, higher muscle triglycerides, fatty liver characteristics, and downregulation of mitochondrial and fatty acid metabolism genes. |
| Surface Electrical Impedance Myography Detects Skeletal Muscle Atrophy in Aged Wildtype Zebrafish and Aged gpr27 Knockout Zebrafish | Rutkove SB, Chen ZZ, Pandeya S, Callegari S, Mourey T, Nagy JA, Nath AK. | Tübingen-strain wild type (8,12 & 36 months) and gpr27 knockout zebrafish | Muscle atrophy detection in young, aged and mutant zebrafish by surface EIM(sEIM) | sEIM effectively recognized muscle structural changes. |
| Aerobic exercise enhances mitochondrial homeostasis to counteract D-galactose-induced sarcopenia in zebrafish | Chen ZL, Guo C, Zou YY, Feng C, Yang DX, Sun CC, Wen W, Jian ZJ, Zhao Z, Xiao Q, Zheng L, Peng XY, Zhou ZQ, Tang CF. | Wild-type male AB strain zebrafish (7 months) | Correlation between D-galactose induced sarcopenia models and aerobic activity | Aerobic exercise enhances muscle function and quality by regulating miR-128/IGF-1 pathway and improves mitochondrial homeostasis in aging muscle. |
| Chronic Vitamin E Deficiency Dysregulates Purine, Phospholipid, and Amino Acid Metabolism in Aging Zebrafish Skeletal Muscle | Henderson TD, Choi J, Leonard SW, Head B, Tanguay RL, Barton CL, Traber MG. | Zebrafish (55 dpf) supplemented for 12 or 18 months | Vitamin E inadequate and adequate diet for 12 or 18 months | The metabolic pathway alterations in skeletal muscle observed in aging and vitamin E deprivation exhibit some similarities but also demonstrate distinct modifications. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





