Submitted:
22 September 2023
Posted:
25 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and reagents
2.3. Brain microdialysis
2.4. Extracellular concentration of DA, 5-HT, glutamate, GABA and acetylcholine
2.5. Western blotting
2.6. Open Field Test
2.7. Statistical analysis
3. Results
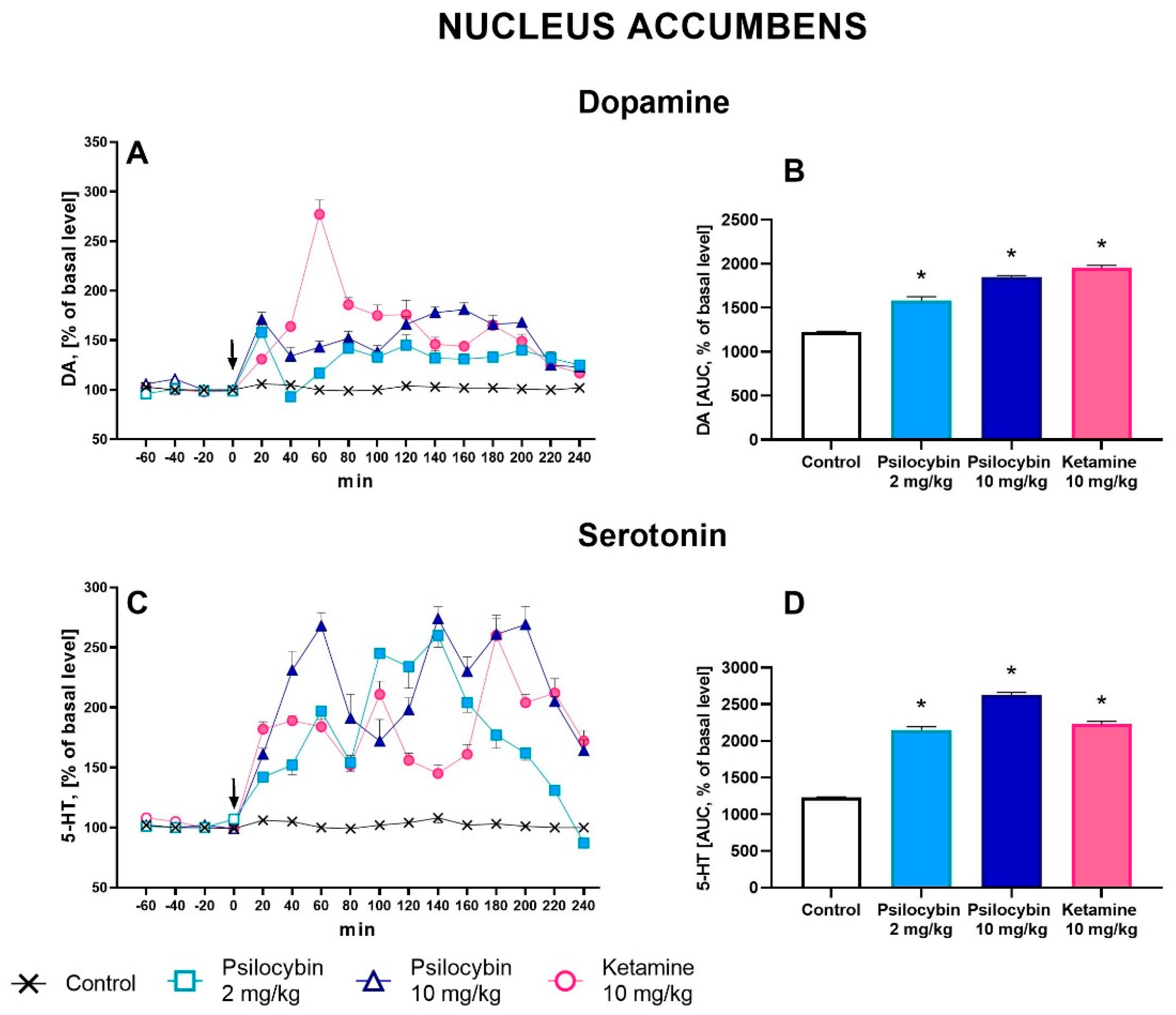
3.1. The effect of psilocybin and ketamine on extracellular levels of DA and 5-HT in the rat nucleus accumbens
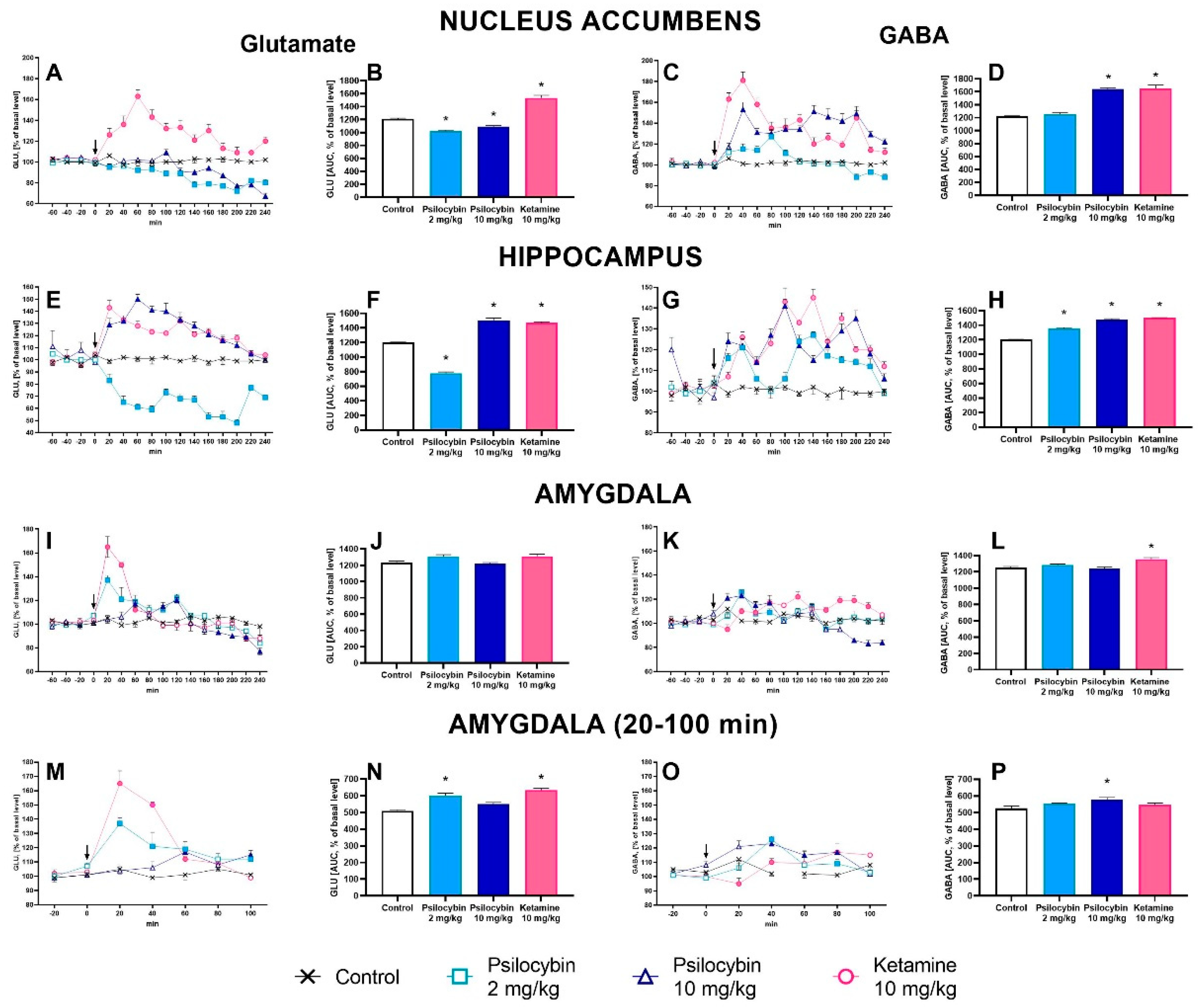
3.2. The effect of psilocybin and ketamine on extracellular levels of glutamate and GABA in the rat nucleus accumbens, hippocampus and amygdala
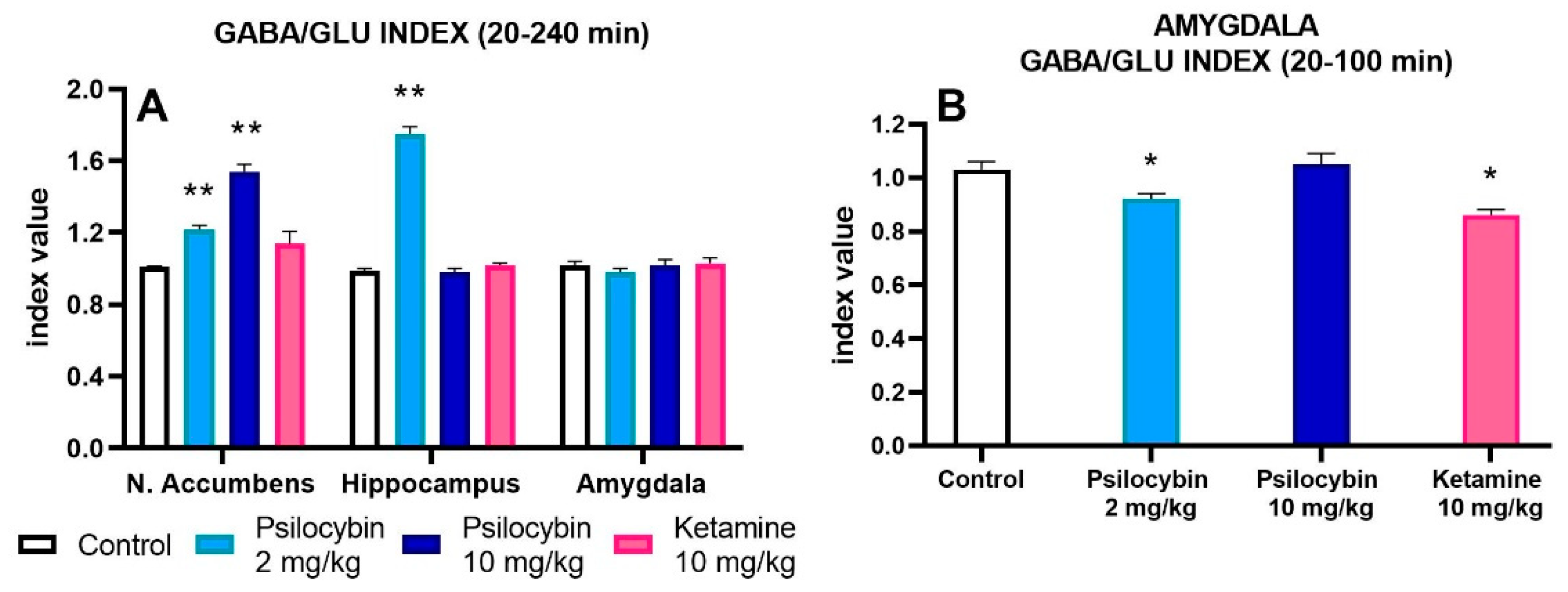
3.3. The effect of psilocybin and ketamine on GABA/glutamate ratio in the rat nucleus accumbens, hippocampus and amygdala
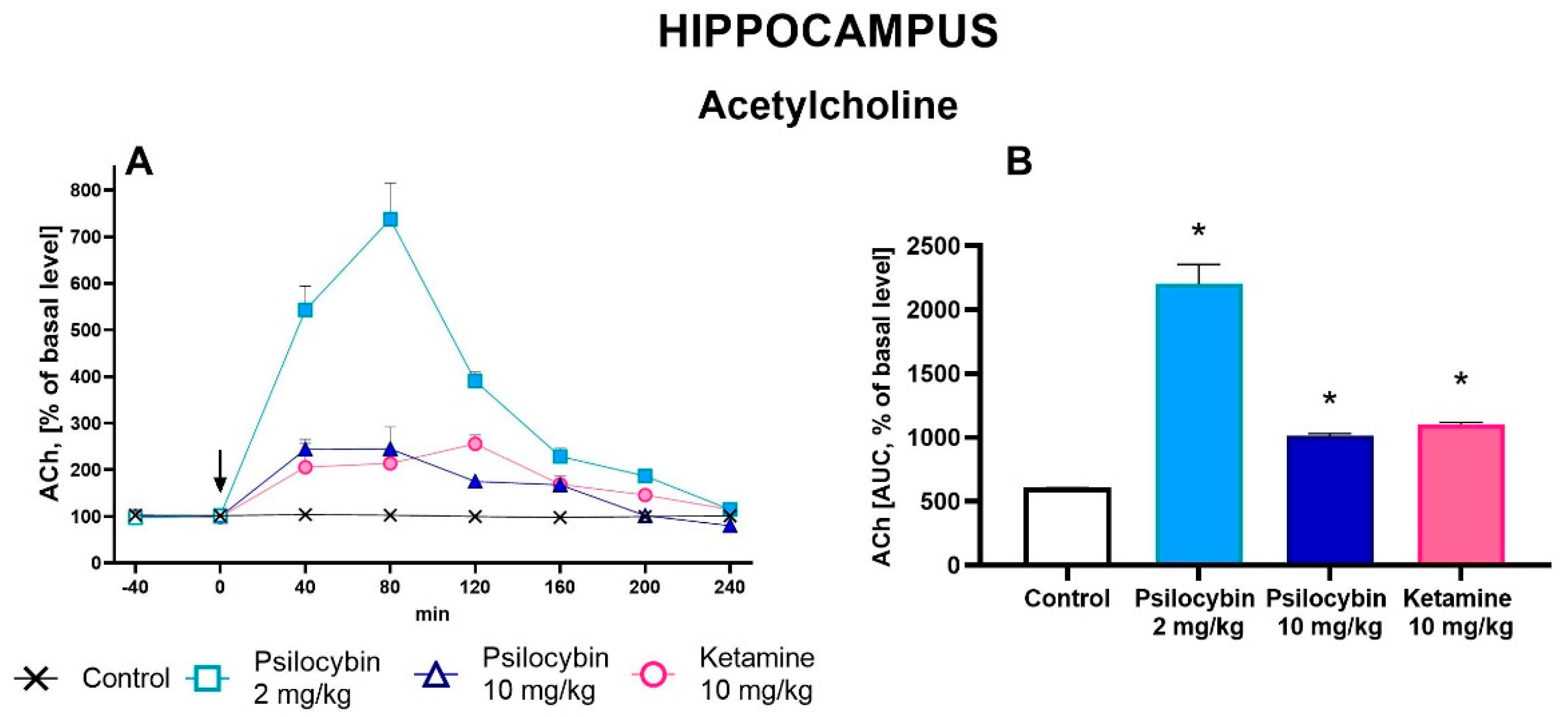
3.4. The effect of psilocybin and ketamine on extracellular levels of ACh in the rat hippocampus
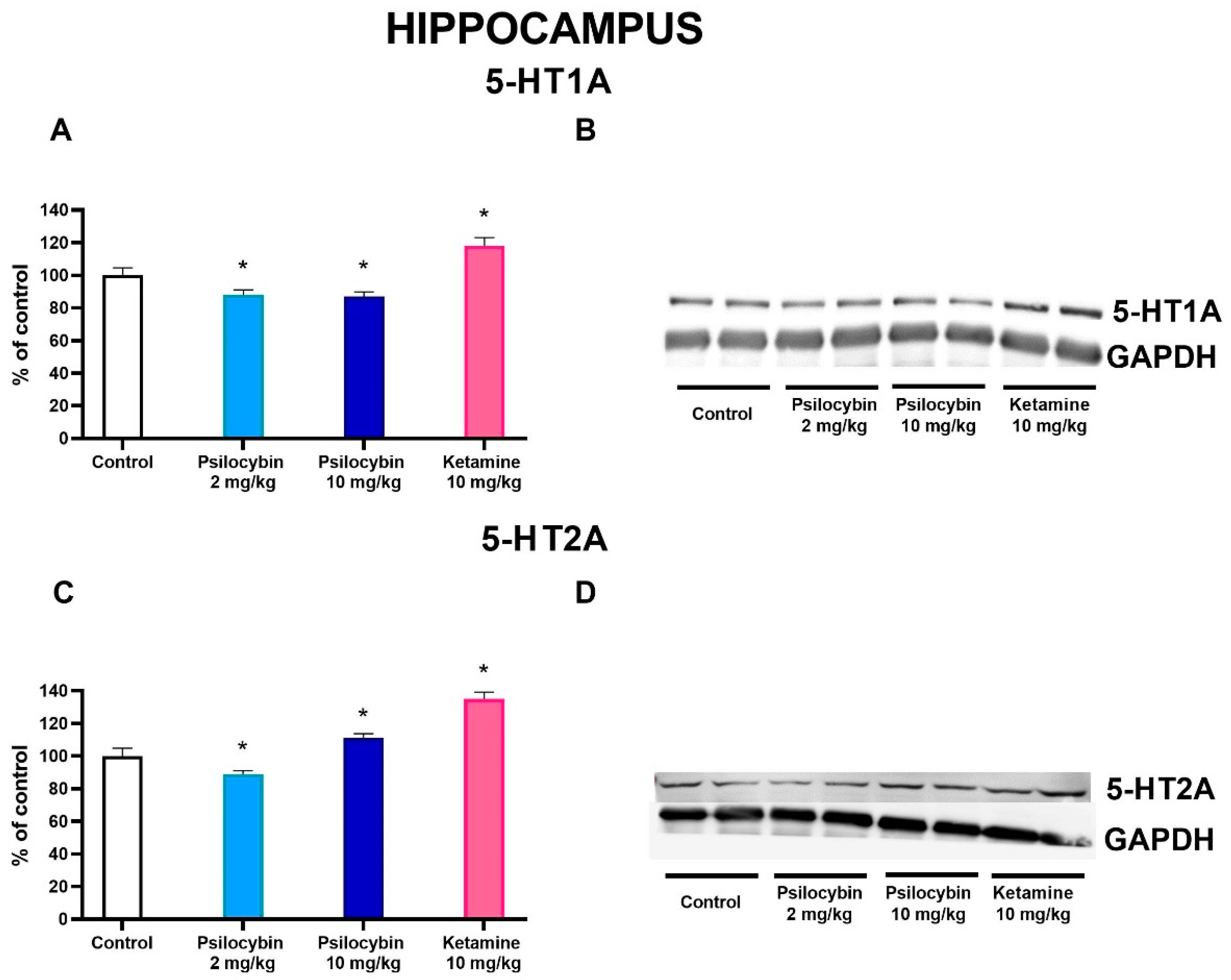
3.5. The effect of psilocybin and ketamine on 5-HT1A and 5-HT2A receptors level in the rat hippocampus
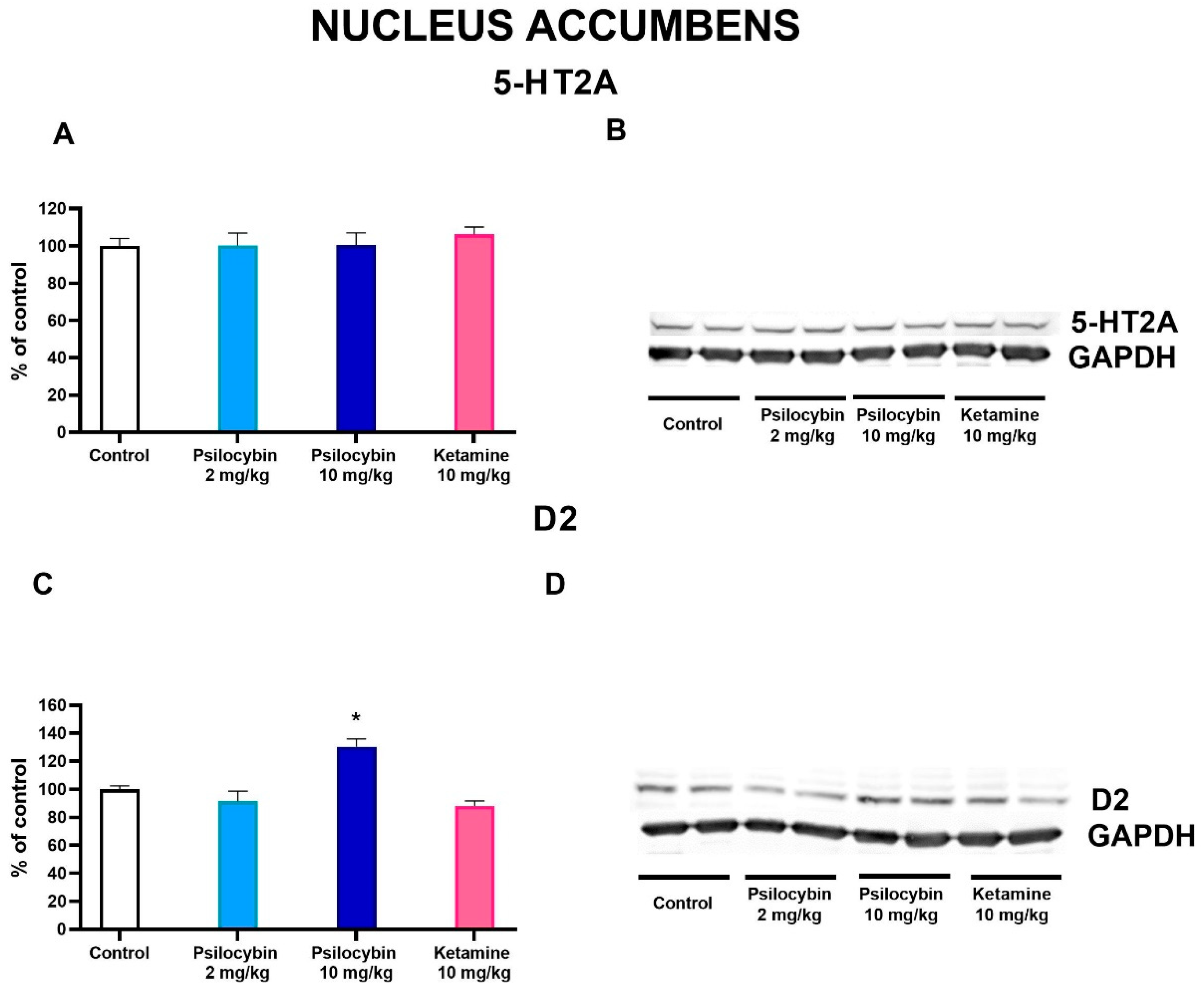
3.6. The effect of psilocybin and ketamine on D2 and 5-HT2A receptors level in the rat nucleus accumbens
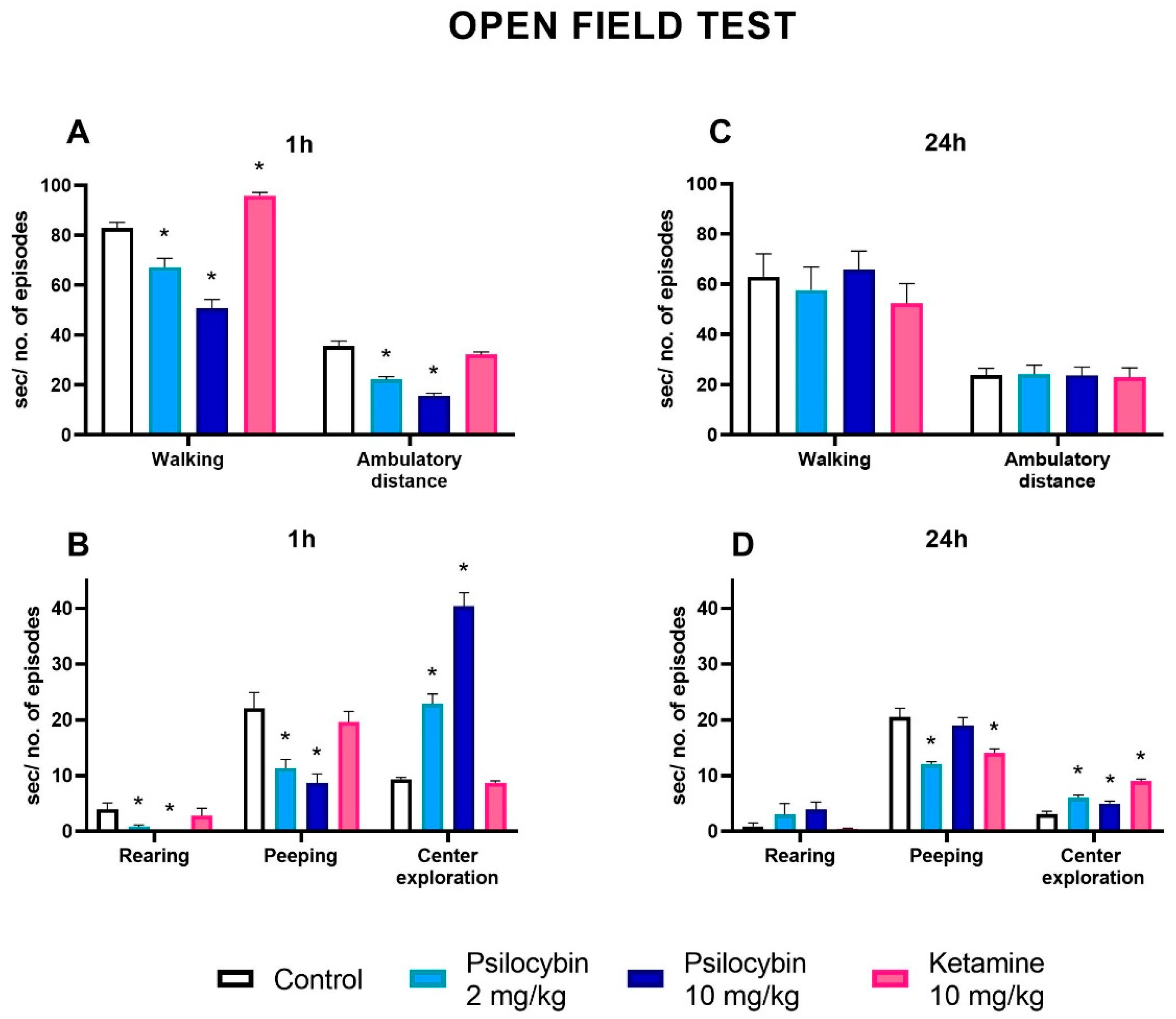
3.7. The effect of psilocybin and ketamine on activity of rats in the Open Field Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffen, A.; Nübel, J.; Jacobi, F.; Bätzing, J.; Holstiege, J. Mental and Somatic Comorbidity of Depression: A Comprehensive Cross-Sectional Analysis of 202 Diagnosis Groups Using German Nationwide Ambulatory Claims Data. BMC psychiatry. 2020, 20.1, 1-15. [CrossRef]
- Chand, S.P.; Arif, H. Depression. Treasure Island, FL: StatPearls Publishing. 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK430847 (accessed on January 2023).
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016, Mar;22(3):238-49. [CrossRef] [PubMed] [PubMed Central]
- Pandya, M.; Altinay, M.; Malone, D.A., Jr.; Anand, A. Where in the brain is depression? Curr Psychiatry Rep. 2012, Dec;14(6):634-42. [CrossRef] [PubMed] [PubMed Central]
- Trifu, S.C.; Trifu, A.C.; Aluaş, E.; Tătaru, M.A.; Costea, R.V. Brain changes in depression. Rom J Morphol Embryol. 2020, Apr-Jun;61(2):361-370. [CrossRef] [PubMed] [PubMed Central]
- Sheline, Y.I.; Gado, M.H.; Price, J.L. Amygdala core nuclei volumes are decreased in recurrent major depression. NeuroReport. 1998, 9(9):2023–2028, PubMed: 9674587. [CrossRef]
- Abdallah, C.G.; Jackowski, A.; Salas, R.; Gupta, S.; Sato, J.R.; Mao, X.; Coplan, J.D.; Shungu, D.C.; Mathew, SJ. The Nucleus Accumbens and Ketamine Treatment in Major Depressive Disorder. Neuropsychopharmacology. 2017, Jul;42(8):1739-1746. Epub 2017 Mar 8, PMID: 28272497; PMCID: PMC5518908. [CrossRef]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders. Arlington. [CrossRef]
- Xu, L.; Nan, J.; Lan, Y. The Nucleus Accumbens: A Common Target in the Comorbidity of Depression and Addiction. Front Neural Circuits. 2020, Jun 30;14:37. [CrossRef] [PubMed] [PubMed Central]
- Maeng, S.; Zarate, C.A. The Role of Glutamate in Mood Disorders: Results from the Ketamine in Major Depression Study and the Presumed Cellular Mechanism Underlying Its Antidepressant Effects. Curr. Psychiatry Rep. 2007, 9, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.C.; Henter, I.D.; Zarate, C.A. Targeting the Glutamatergic System to Treat Major Depressive Disorder: Rationale and Progress to Date. Drugs. 2012, 72, 1313–1333. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hu, Y.M.; Zhou, Z.Q.; Zhang, G.F.; Yang, J.J. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J Med Sci. 2013, Mar;118(1):3-8. Epub 2012 Sep 13, PMID: 22970723; 13, PMCID: PMC3572668. [CrossRef]
- Zhou, Y.; Wu, F.; Liu, W.; Zheng, W.; Wang, C.; Zhan, Y.; Lan, X.; Ning, Y. Volumetric changes in subcortical structures following repeated ketamine treatment in patients with major depressive disorder: A longitudinal analysis. Transl. Psychiatry. 2020, 10:264. [CrossRef]
- Abdallah, C.G.; Salas, R.; Jackowski, A.; Baldwin, P.; Sato, J.R.; Mathew, S.J. Hippocampal volume and the rapid antidepressant effect of ketamine. J. Psychopharmacol. 2015, May;29(5):591-5. Epub 2014 Aug 13. [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.L.; Wu, F.C.; Wang, C.Y.; Zheng, W.; Lan, X.F.; Deng, X.R.; Ning, Y.P. Relationship between hippocampal volume and inflammatory markers following six infusions of ketamine in major depressive disorder. J Affect Disord. 2020, Nov 1;276:608-615. Epub 2020 Jul 16, PMID: 32871692. [CrossRef]
- Hibicke, M.; Landry, A.N.; Kramer, H.M.; Talman, Z.K.; Nichols, C.D. Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem Neurosci. 2020, Mar 18;11(6):864-871. Epub 2020 Mar 5. [CrossRef] [PubMed]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, Jun 12;23(11):3170-3182. [CrossRef] [PubMed] [PubMed Central]
- Cameron, L.P.; Benson, C.J.; Dunlap, L.E.; Olson, D.E. Effects of N, N-Dimethyltryptamine on Rat Behaviors Relevant to Anxiety and Depression. ACS Chem. Neurosci. 2018, Jul 18;9(7):1582-1590. Epub 2018 Apr 24. [CrossRef] [PubMed] [PubMed Central]
- Hesselgrave, N.; Troppoli, T.A.; Wulff, A.B.; Cole, A.B.; Thompson, S.M. ; Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci U S A. 2021, Apr 27;118(17):e2022489118. [CrossRef] [PubMed] [PubMed Central]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, Apr 15;384(15):1402-1411. [CrossRef] [PubMed]
- Shirota, O.; Hakamata, W.; Goda, Y. Concise Large-Scale Synthesis of Psilocin and Psilocybin, Principal Hallucinogenic Constituents of “Magic Mushroom. ” J. Nat. Prod. 2003, 66, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Popik, P.; Hogendorf, A.; Bugno, R.; Khoo, S.Y.S.; Zajdel, P.; Malikowska-Racia, N.; Nikiforuk, A.; Golebiowska, J. Effects of Ketamine Optical Isomers, Psilocybin, Psilocin and Norpsilocin on Time Estimation and Cognition in Rats. Psychopharmacology (Berl). 2022. [CrossRef] [PubMed]
- Jefsen, O.; Højgaard, K.; Christiansen, S.L.; Elfving, B.; Nutt, D.J.; Wegener, G.; Müller, H.K. Psilocybin Lacks Antidepressant-like Effect in the Flinders Sensitive Line Rat. Acta Neuropsychiatr. 2019. [CrossRef] [PubMed]
- Paxinos, G.; Watson, G. The Rat Brain in Stereotaxic Coordinates. 1998, Academic Press: San Diego.
- Herian, M.; Skawski, M.; Wojtas, A.; Sobocińska, M.K.; Noworyta, K.; Gołembiowska, K. Tolerance to Neurochemical and Behavioral Effects of the Hallucinogen 25I-NBOMe. Psychopharmacology (Berl). 2021, 238, 2349–2364. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, A.; Herian, M.; Skawski, M.; Sobocińska, M.; González-Marín, A.; Noworyta-Sokołowska, K.; Gołembiowska, K. Neurochemical and Behavioral Effects of a New Hallucinogenic Compound 25B-NBOMe in Rats. Neurotox. Res. 2021, 39, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, A.; Bysiek, A.; Wawrzczak-Bargiela, A.; Szych, Z.; Majcher-Maślanka, I.; Herian, M.; Maćkowiak, M. ; Gołembiowska,K. Effect of Psilocybin and Ketamine on Brain Neurotransmitters, Glutamate Receptors, DNA and Rat Behavior. Int. J. Mol. Sci. 2022, 23, 6713. [Google Scholar] [CrossRef] [PubMed]
- Rogóz, Z.; Skuza, G. Anxiolytic-like Effects of Olanzapine, Risperidone and Fluoxetine in the Elevated plus-Maze Test in Rats. Pharmacol. Reports 2011, 63, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X.; Kometer, M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nature Neurosciences. 2010, 11, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Beique, J.C.; Imad, M.; Mladenovic, L.; Gingrich, J.A.; Andrade, R. Mechanism of 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc. Natl. Acad. Sci. USA. 2007, 104, 9870-5. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.L.; Kuypers, K.P.; Muller, F.; Reckweg, J.; Tse, D.H.Y.; Toennes, S.W.; Hutten, N.R.P.W.; Jansen, J.F.A.; Stiers, P.; Feilding, A.; Ramaekers, J.G. Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology. 2020, 45:2003–2011. [CrossRef]
- Abdallah, C.G.; Sanacora, G.; Duman, R.S.; Krystal, J.H. The Neurobiology of Depression, Ketamine and Rapid-Acting Antidepressants: Is it Glutamate Inhibition or Activation? Pharmacol. Ther. 2018, October ; 190: 148–158. [CrossRef]
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, M.H. The pharmacology of psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Rickli, A.; Moning, O.D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. European Neuropsychopharmacology. 2016, 26, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Celada, P.; Puig, M.V.; Artigas, F. Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci. 2013, 7:25. [CrossRef]
- Meccia, J.; Lopez, J.; Bagot, R.C. Probing the antidepressant potential of psilocybin: integrating insight from human research and animal models towards an understanding of neural circuit mechanisms. Psychopharmacology. 2023, 240:27–40. [CrossRef]
- Malenka, R.C.; Nestler, E.J.; Hyman, S.E. (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 147–148, 154–157, ISBN 9780071481274.
- Dailly, E.; Chenu, F.; Renard, C.E.; Bourin, M. Dopamine, depression and antidepressants. Fundam. Clin. Pharmacol. 2004, 18, 601–607. [Google Scholar] [CrossRef] [PubMed]
- De Deurwaerdère, P.; Di Giovanni, G. Serotonergic modulation of the activity of mesencephalic dopaminergic systems: Therapeutic implications. Progress in Neurobiology. 2017, 151: 175–236. [CrossRef]
- Sakashita, Y.; Abe, K.; Katagiri, N.; Kambe, T.; Saitoh, T.; Utsunomiya, I.; Horiguchi, Y.; Taguchi, K. Effect of psilocin on extracellular dopamine and serotonin levels in the mesoaccumbens and mesocortical pathway in awake rats. Biological and Pharmaceutical Bulletin, 2015, 38(1), 134-138. [CrossRef]
- Del Arco, A.; Mora, F. Prefrontal cortex–nucleus accumbens interaction: In vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacology, Biochemistry and Behavior. 2008, 90.2: 226–235. [CrossRef]
- Moulédous, L.; Roullet, P.; Guiard, B.P. Brain Circuits Regulated by the 5-HT2A Receptor: Behavioural Consequences on Anxiety and Fear Memory. 5-HT2A Receptors in the Central Nervous System. 2018, 231-258. [CrossRef]
- Miller, O.H.; Moran, J.T.; Hall, B.J. Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology. 2016, 100: 17-26. [CrossRef]
- Ago, Y.; Tanabe, W.; Higuchi, M.; Tsukada, S.; Tanaka, T.; Yamaguchi, T.; Igarashi, H.; Yokoyama, R.; Seiriki, K.; Kasai, A.; et al. (R)-Ketamine Induces a Greater Increase in Prefrontal 5-HT Release Than (S)-Ketamine and Ketamine Metabolites via an AMPA Receptor-Independent Mechanism. Int. J. Neuropsychopharm. 2019, 22(10): 665–674. [CrossRef]
- Chaudhury, D.; Walsh, J.J.; Friedman, A.K.; Juarez, B.; Ku, S.M.; Koo, J.W.; Ferguson, D.; Tsai, H.-C.; Pomeranz, L.; Christoffel, D.J.; et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013, 493: 532–536. [CrossRef]
- Walsh, J.J.; Friedman, A.K.; Sun, H.; Heller, E.A.; Ku, S.M.; Juarez, B.; Burnham, V.L.; Mazei-Robison, M.S.; Ferguson, D.; Golden, S.A.; et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014, 17: 27–29. [CrossRef]
- Wook Koo, J.; Labonte, B.; Engmann, O.; Calipari, E.S.; Juarez, B.; Lorsch, Z.; Walsh, J.J.; Friedman, A.K.; Yorgason, J.T.; Han, M.-H.; et al. Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress-induced depressive behaviors. Biol Psychiatry. 2016, 80.6: 469–478. [CrossRef]
- Vollenweider, F.X.; Smallridge, J.W. Classic Psychedelic Drugs: Update on Biological Mechanisms. Pharmacopsychiatry. 2022, 55: 121–138. [CrossRef]
- Beliveau, V.; Ganz, M.; Feng, L.; Ozenne, B.; Højgaard, L.; Fisher, PM.; Svarer, C.; Greve, D.N.; Knudsen, G.M. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. The Journal of Neuroscience. 2017, January 4, 37(1):120 –128. [CrossRef]
- Zhu, Z.; Wang, G.; Ma, K.; Cui, S.; Wang, J.-H. GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression. Oncotarget. 2017, 8: 35933-35945. [CrossRef]
- Dale, E.; Pehrson, A.L.; Jeyarajah, T.; Li, Y.; Leiser, S.C.; Smagin, G.; Olsen, C.K.; Sanchez, C. Effects of serotonin in the hippocampus: how SSRIs and multimodal antidepressants might regulate pyramidal cell function. CNS Spectrums. 2016, 21, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.; Nicoll, R.A. Pharmacologically distinct actions of serotonin on single pyramidal neurons of the rat hippocampus recorded in vitro. J Physiol. 1987, 394: 99–124. [CrossRef]
- Bombardi, C. Neuronal localization of 5-HT2A receptor immunoreactivity in the rat hippocampal region. Brain Res. Bull. 2012, 87(2–3): 259–273. [CrossRef]
- Abdallah, C.G.; Jackowski, A.; Sato, J.R.; Mao, X.; Kang, G.; Cheema, R.; Coplan, J.D.; Mathew, S.J.; Shungu, D.C. Prefrontal Cortical GABA Abnormalities Are Associated With Reduced Hippocampal Volume In Major Depressive Disorder. Eur. Neuropsychopharmacol. 2015, August ; 25(8): 1082–1090. [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012, 76.1: 116-129. [CrossRef]
- Gielow, M.R.; Zaborszky, L. The Input-Output Relationship of the Cholinergic Basal Forebrain. Cell Rep. 2017, 18: 1817-1830. [CrossRef]
- Fujii, T.; Yoshizawa, M.; Nakai, K.; Fujimoto, K.; Suzuki, T.; Kawashima, K. Demonstration of the facilitatory role of 8-OH-DPAT on cholinergic transmission in the rat hippocampus using in vivo microdialysis. Brain Res. 1997, Jul 4;761(2):244-9. [CrossRef]
- Nair, S.G.; Gudelsky, G.A. Activation of 5-HT2 receptors enhances the release of acetylcholine in the prefrontal cortex and hippocampus of the rat. Synapse. 2004, 53.4:202–207. [CrossRef]
- Pacheco, A.T.; Olson, R.J.; Garza, G.; Moghaddam, B. Acute psilocybin enhances cognitive flexibility in rats. Neuropsychopharmacology. 2023, 48:1011–1020. [CrossRef]
- Almeida, J.R.C.; Versace, A.; Hassel, S.; Kupfer, D.J.; Phillips, M.L. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol. Psychiatry. 2010, 67, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Barrett, F.S.; Doss, M.K.; Sepeda, N.D.; James, J.; Pekar, J.J.; Griffiths, R.R. Emotions and brain function are altered up to one month after a single high dose of psylocybin. Sci. Rep. 2020, 10:2214. [CrossRef]
- Liu, W.Z.; Huang, S.H.; Wang, Y.; Wang, C.Y.; Pan, H.Q.; Zhao, K.; Hu, P.; Pan, B.X.; Zhang, W.H. Medial prefrontal cortex input to basolateral amygdala controls acute stress-induced short-term anxiety-like behavior in mice. Neuropsychopharmacology. 2023, 48:734–744. [CrossRef]
- Pompeiano, M.; Palacios, J.M.; Mengod, G. Distribution of serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res. 1994, 23:163–178. [CrossRef]
- Cornea-Hebert, V.; Riad, M.; Wu, C.; Singh, S.K.; Descarries, L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. Journal of Comparative Neurology. 1999, 409.2: 187-209. [CrossRef]
- Nikolaus, S.; Müller, H.-W.; Hautzel, H. Different patterns of 5-HT receptor and transporter dysfunction in neuropsychiatric disorders – a comparative analysis of in vivo imaging findings. Rev. Neurosci. 2016, 27(1): 27–59. [CrossRef]
- Raval, N.R.; Johansen, A.; Donovan, L.L.; Ros, N.F.; Ozenne, B.; Hansen, H.D.; Knudsen, G.M. A Single Dose of Psilocybin Increases Synaptic Density and Decreases 5-HT2A Receptor Density in the Pig Brain. Int. J. Mol. Sci. 2021, 22, 835. [Google Scholar] [CrossRef] [PubMed]
- Wedzony, K.; Maćkowiak, M.; Czyrak, A.; Fijał, K.; Michalska, B. Single doses of MK-801, a non-competitive antagonist of NMDA receptors, increase the number of 5-HT1A serotonin receptors in the rat brain. Brain Res. 1997, 756: 84-91. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




