Submitted:
24 September 2023
Posted:
25 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
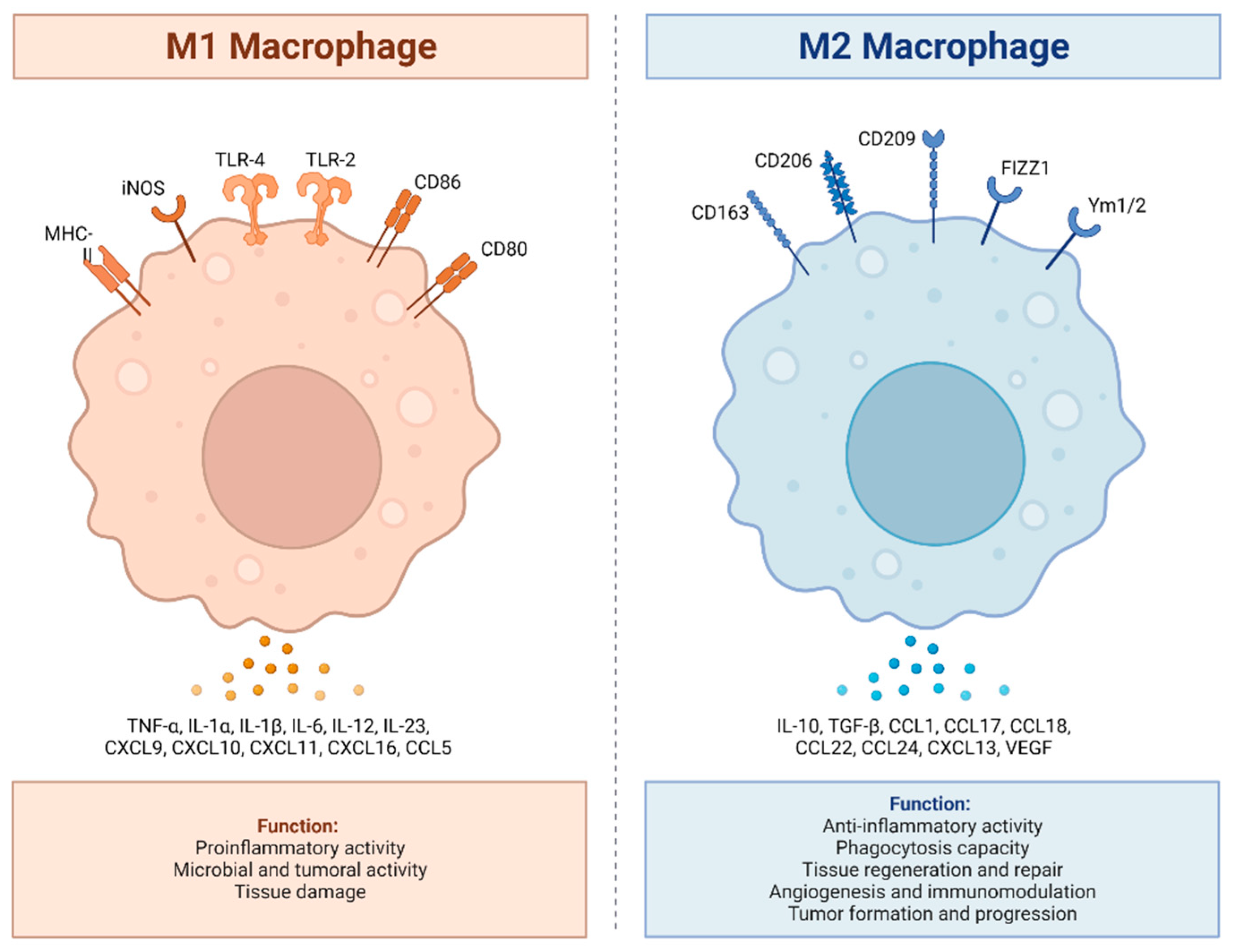
2. Activation and Polarization of Human Macrophages
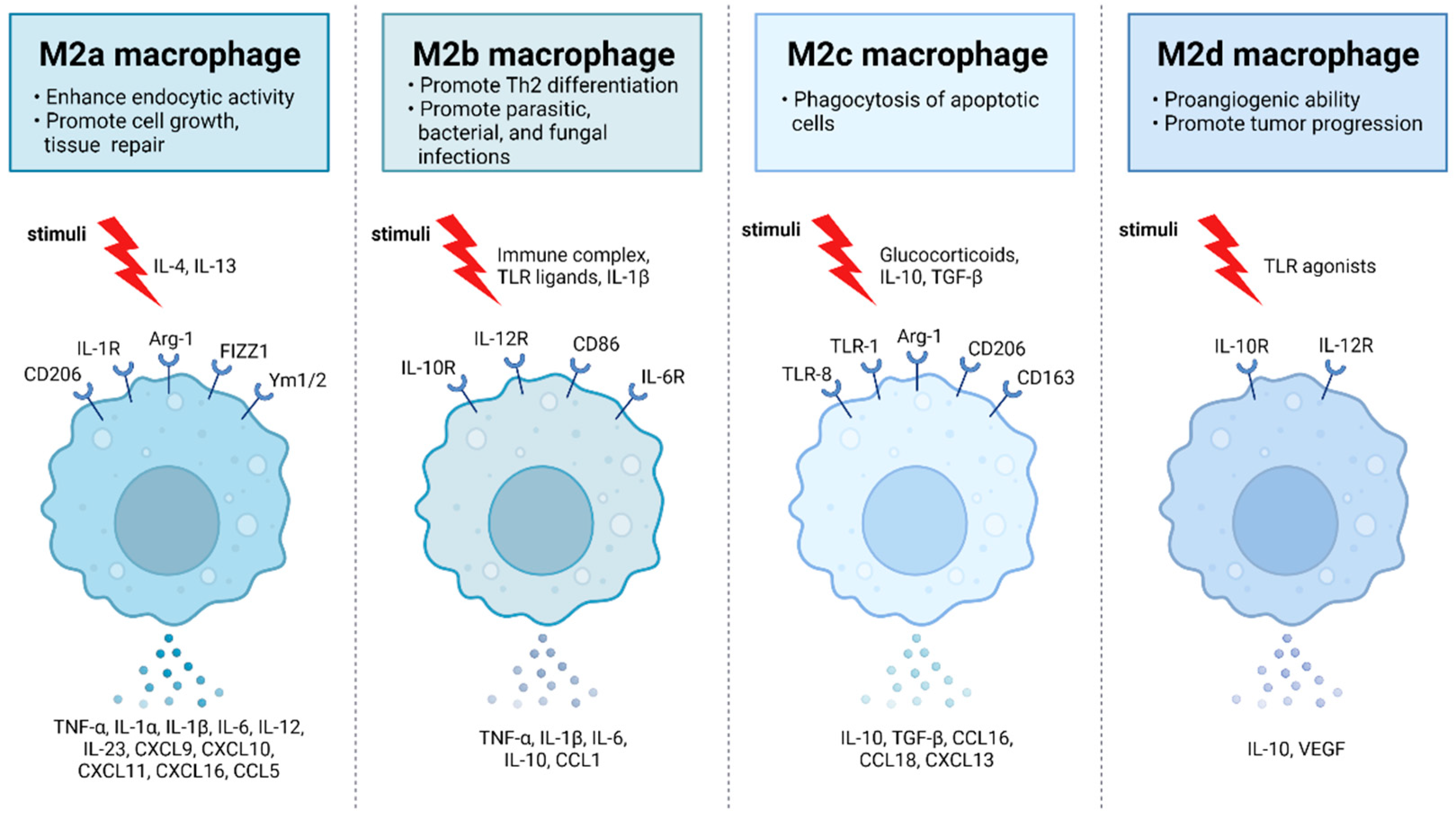
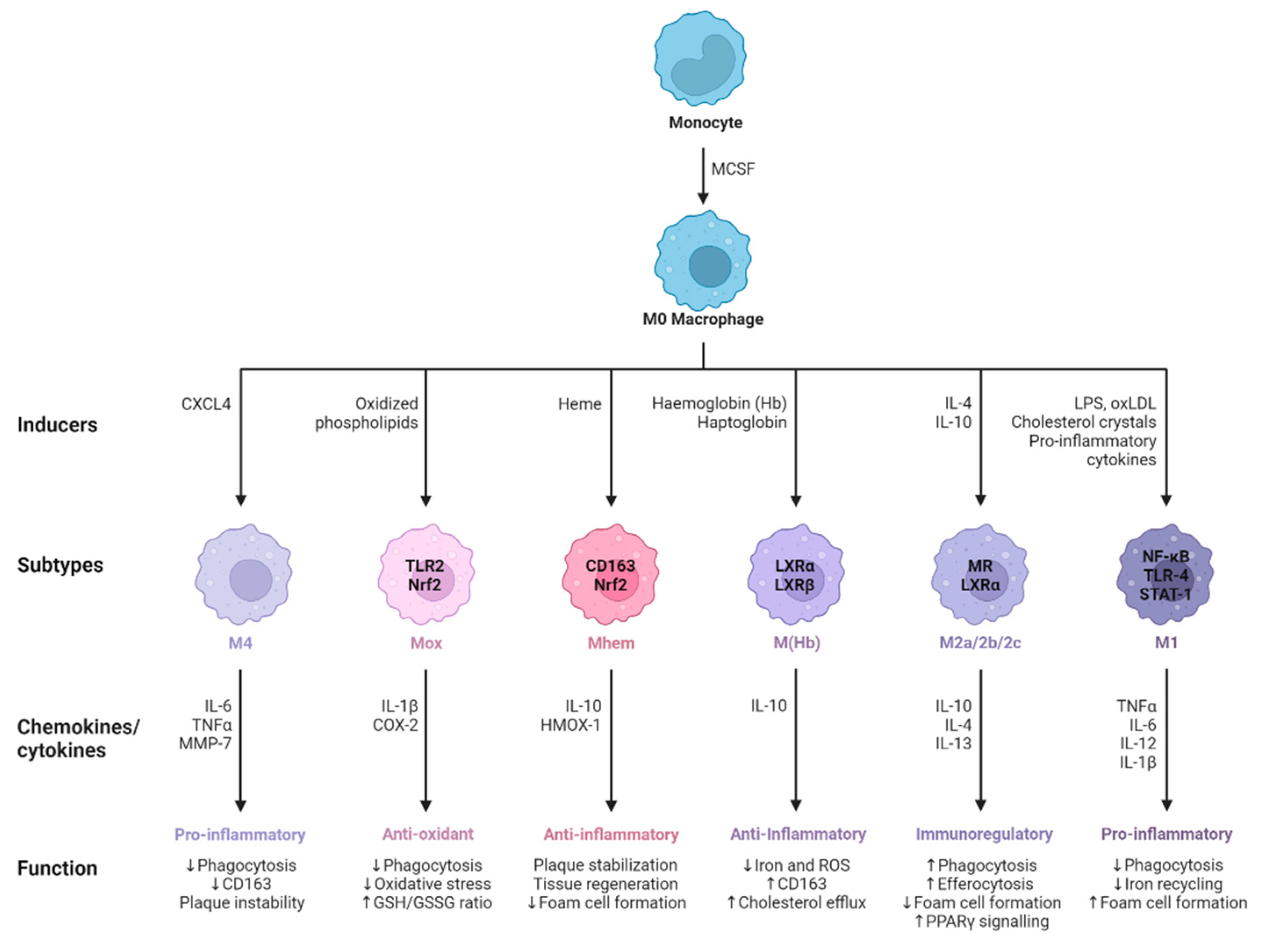
2.1. Polarization of Macrophages during Viral Infection
3. The Expression of nAChRs and the Potential Role of α7-nAChR in Shaping Macrophage Polarization through the Cholinergic Anti-Inflammatory Response
3.1. Expression of nAChRs in Macrophages
3.2. Cholinergic Machinery of Macrophages
3.3. The Cholinergic Anti-Inflammatory Pathway (CAP) Is Closely Associated with the Polarization of Macrophages
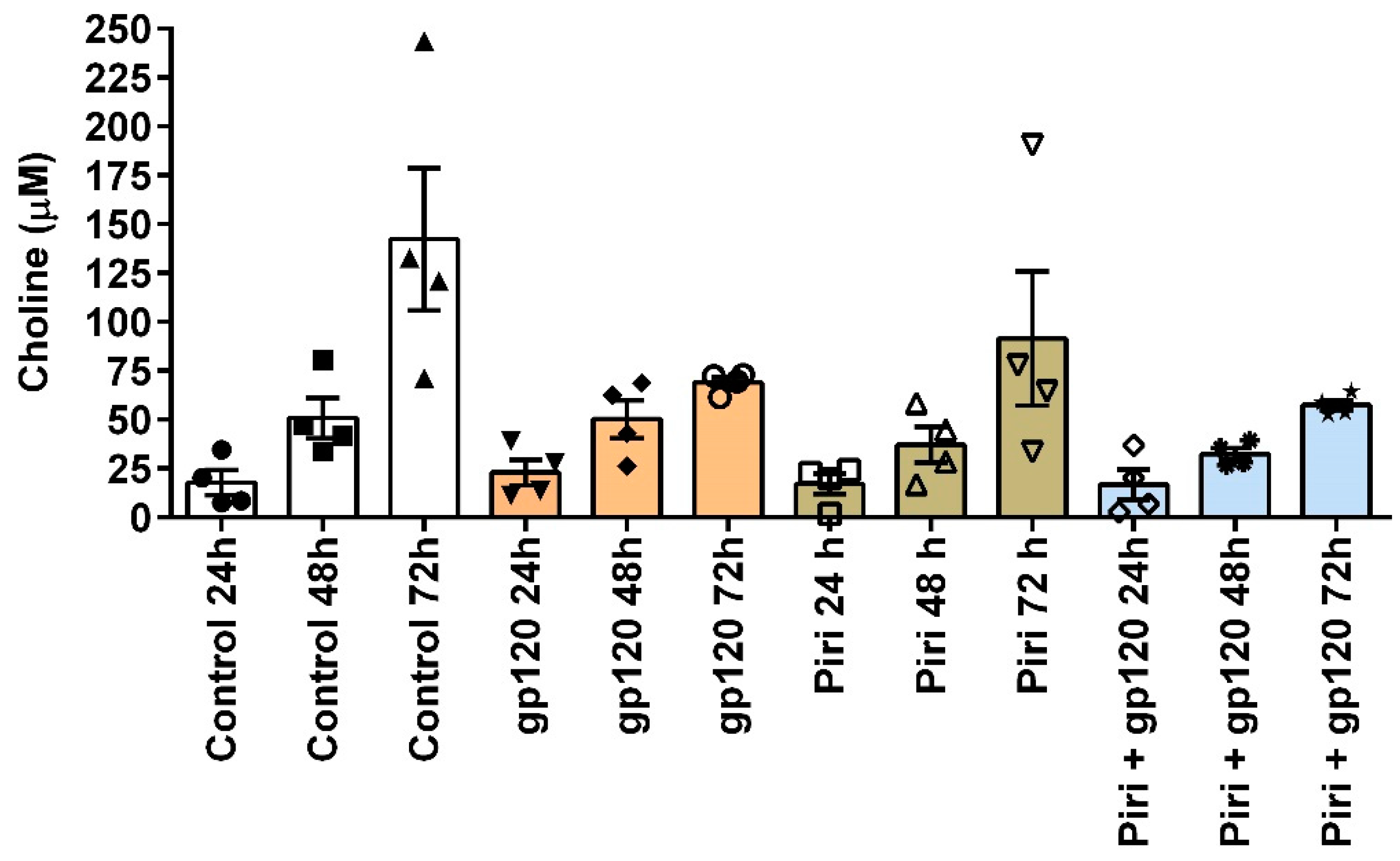
3.4. Experimental Challenges in Testing the Cholinergic Anti-Inflammatory Response in Macrophages: The Choline Issue
3.5. Function and Role of CHRFAM7A in Macrophage Polarization and Cholinergic Antiinflammatory Pathway Operation
4. Conclusions and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, E.Y.; Kim, S.J.; Ma, X.J. Regulation of Cytokine Production during Phagocytosis of Apoptotic Cells. Cell Res 2006, 16, 154–161. [CrossRef]
- Fagundes, D.L.G.; França, E.L.; Morceli, G.; Rudge, M.V.C.; Calderon, I. de M.P.; Honorio-França, A.C. The Role of Cytokines in the Functional Activity of Phagocytes in Blood and Colostrum of Diabetic Mothers. Journal of Immunology Research 2013, 2013, e590190. [CrossRef]
- Fadok, V.A.; McDonald, P.P.; Bratton, D.L.; Henson, P.M. Regulation of Macrophage Cytokine Production by Phagocytosis of Apoptotic and Post-Apoptotic Cells. Biochemical Society Transactions 1998, 26, 653–656. [CrossRef]
- Geiger, S.S.; Curtis, A.M.; O’Neill, L.A.J.; Siegel, R.M. Daily Variation in Macrophage Phagocytosis Is Clock-Independent and Dispensable for Cytokine Production. Immunology 2019, 157, 122–136. [CrossRef]
- Fu, Y.L.; Harrison, R.E. Microbial Phagocytic Receptors and Their Potential Involvement in Cytokine Induction in Macrophages. Frontiers in Immunology 2021, 12.
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic Acetylcholine Receptor Alpha7 Subunit Is an Essential Regulator of Inflammation. Nature 2003, 421, 384–388. [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus Nerve Stimulation Attenuates the Systemic Inflammatory Response to Endotoxin. Nature 2000, 405, 458–462. [CrossRef]
- Colin, S.; Chinetti-Gbaguidi, G.; Staels, B. Macrophage Phenotypes in Atherosclerosis. Immunol Rev 2014, 262, 153–166. [CrossRef]
- De Paoli, F.; Staels, B.; Chinetti-Gbaguidi, G. Macrophage Phenotypes and Their Modulation in Atherosclerosis. Circ J 2014, 78, 1775–1781. [CrossRef]
- Mushenkova, N.V.; Nikiforov, N.G.; Melnichenko, A.A.; Kalmykov, V.; Shakhpazyan, N.K.; Orekhova, V.A.; Orekhov, A.N. Functional Phenotypes of Intraplaque Macrophages and Their Distinct Roles in Atherosclerosis Development and Atheroinflammation. Biomedicines 2022, 10, 452. [CrossRef]
- Kadl, A.; Meher, A.K.; Sharma, P.R.; Lee, M.Y.; Doran, A.C.; Johnstone, S.R.; Elliott, M.R.; Gruber, F.; Han, J.; Chen, W.; et al. Identification of a Novel Macrophage Phenotype That Develops in Response to Atherogenic Phospholipids via Nrf2. Circ Res 2010, 107, 737–746. [CrossRef]
- Erbel, C.; Tyka, M.; Helmes, C.M.; Akhavanpoor, M.; Rupp, G.; Domschke, G.; Linden, F.; Wolf, A.; Doesch, A.; Lasitschka, F.; et al. CXCL4-Induced Plaque Macrophages Can Be Specifically Identified by Co-Expression of MMP7+S100A8+in Vitro and in Vivo. Innate Immun 2015, 21, 255–265. [CrossRef]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage Subsets in Atherosclerosis. Nat Rev Cardiol 2015, 12, 10–17. [CrossRef]
- Malyshev, I.; Malyshev, Y. Current Concept and Update of the Macrophage Plasticity Concept: Intracellular Mechanisms of Reprogramming and M3 Macrophage “Switch” Phenotype. Biomed Res Int 2015, 2015, 341308. [CrossRef]
- Kalish, S.; Lyamina, S.; Manukhina, E.; Malyshev, Y.; Raetskaya, A.; Malyshev, I. M3 Macrophages Stop Division of Tumor Cells In Vitro and Extend Survival of Mice with Ehrlich Ascites Carcinoma. Med Sci Monit Basic Res 2017, 23, 8–19. [CrossRef]
- Jackaman, C.; Yeoh, T.L.; Acuil, M.L.; Gardner, J.K.; Nelson, D.J. Murine Mesothelioma Induces Locally-Proliferating IL-10(+)TNF-α(+)CD206(-)CX3CR1(+) M3 Macrophages That Can Be Selectively Depleted by Chemotherapy or Immunotherapy. Oncoimmunology 2016, 5, e1173299. [CrossRef]
- Gleissner, C.A.; Shaked, I.; Erbel, C.; Böckler, D.; Katus, H.A.; Ley, K. CXCL4 Downregulates the Atheroprotective Hemoglobin Receptor CD163 in Human Macrophages. Circulation Research 2010, 106, 203–211. [CrossRef]
- Gleissner, C.A.; Shaked, I.; Little, K.M.; Ley, K. CXC Chemokine Ligand 4 Induces a Unique Transcriptome in Monocyte-Derived Macrophages. The Journal of Immunology 2010, 184, 4810–4818. [CrossRef]
- Erbel, C.; Wolf, A.; Lasitschka, F.; Linden, F.; Domschke, G.; Akhavanpoor, M.; Doesch, A.O.; Katus, H.A.; Gleissner, C.A. Prevalence of M4 Macrophages within Human Coronary Atherosclerotic Plaques Is Associated with Features of Plaque Instability. Int J Cardiol 2015, 186, 219–225. [CrossRef]
- Real, F.; Zhu, A.; Huang, B.; Belmellat, A.; Sennepin, A.; Vogl, T.; Ransy, C.; Revol, M.; Arrigucci, R.; Lombès, A.; et al. S100A8-Mediated Metabolic Adaptation Controls HIV-1 Persistence in Macrophages in Vivo. Nat Commun 2022, 13, 5956. [CrossRef]
- Maretti-Mira, A.C.; Golden-Mason, L.; Salomon, M.P.; Kaplan, M.J.; Rosen, H.R. Cholesterol-Induced M4-Like Macrophages Recruit Neutrophils and Induce NETosis. Front Immunol 2021, 12, 671073. [CrossRef]
- Yu, S.; Ge, H.; Li, S.; Qiu, H.-J. Modulation of Macrophage Polarization by Viruses: Turning Off/On Host Antiviral Responses. Front Microbiol 2022, 13, 839585. [CrossRef]
- Cassol, E.; Cassetta, L.; Rizzi, C.; Alfano, M.; Poli, G. M1 and M2a Polarization of Human Monocyte-Derived Macrophages Inhibits HIV-1 Replication by Distinct Mechanisms. J Immunol 2009, 182, 6237–6246. [CrossRef]
- Moyano, A.; Ferressini Gerpe, N.M.; De Matteo, E.; Preciado, M.V.; Chabay, P. M1 Macrophage Polarization Prevails in Epstein-Barr Virus-Infected Children in an Immunoregulatory Environment. Journal of Virology 2022, 96, e01434-21. [CrossRef]
- Jhan, M.-K.; Chen, C.-L.; Shen, T.-J.; Tseng, P.-C.; Wang, Y.-T.; Satria, R.D.; Yu, C.-Y.; Lin, C.-F. Polarization of Type 1 Macrophages Is Associated with the Severity of Viral Encephalitis Caused by Japanese Encephalitis Virus and Dengue Virus. Cells 2021, 10, 3181. [CrossRef]
- Lian, Q.; Zhang, K.; Zhang, Z.; Duan, F.; Guo, L.; Luo, W.; Mok, B.W.-Y.; Thakur, A.; Ke, X.; Motallebnejad, P.; et al. Differential Effects of Macrophage Subtypes on SARS-CoV-2 Infection in a Human Pluripotent Stem Cell-Derived Model. Nat Commun 2022, 13, 2028. [CrossRef]
- Tan, L.Y.; Komarasamy, T.V.; RMT Balasubramaniam, V. Hyperinflammatory Immune Response and COVID-19: A Double Edged Sword. Frontiers in Immunology 2021, 12.
- Embregts, C.W.E.; Begeman, L.; Voesenek, C.J.; Martina, B.E.E.; Koopmans, M.P.G.; Kuiken, T.; GeurtsvanKessel, C.H. Street RABV Induces the Cholinergic Anti-Inflammatory Pathway in Human Monocyte-Derived Macrophages by Binding to NAChr A7. Front Immunol 2021, 12, 622516. [CrossRef]
- Chrestia, J.F.; Oliveira, A.S.; Mulholland, A.J.; Gallagher, T.; Bermúdez, I.; Bouzat, C. A Functional Interaction Between Y674-R685 Region of the SARS-CoV-2 Spike Protein and the Human A7 Nicotinic Receptor. Mol Neurobiol 2022, 59, 6076–6090. [CrossRef]
- O’Brien, B.C.V.; Weber, L.; Hueffer, K.; Weltzin, M.M. SARS-CoV-2 Spike Ectodomain Targets A7 Nicotinic Acetylcholine Receptors. Journal of Biological Chemistry 2023, 104707. [CrossRef]
- Wen, J.; Zhao, C.; Chen, J.; Song, S.; Lin, Z.; Xie, S.; Qi, H.; Wang, J.; Su, X. Activation of A7 Nicotinic Acetylcholine Receptor Promotes HIV-1 Transcription. Cell Insight 2022, 1, 100028. [CrossRef]
- Atmeh, P.A.; Mezouar, S.; Mège, J.-L.; Atmeh, P.A.; Mezouar, S.; Mège, J.-L. Macrophage Polarization in Viral Infectious Diseases: Confrontation with the Reality. In Macrophages - Celebrating 140 Years of Discovery; IntechOpen, 2022 ISBN 978-1-80355-625-3.
- Cassetta, L.; Cassol, E.; Poli, G. Macrophage Polarization in Health and Disease. ScientificWorldJournal 2011, 11, 2391–2402. [CrossRef]
- Herbein, G.; Varin, A. The Macrophage in HIV-1 Infection: From Activation to Deactivation? Retrovirology 2010, 7, 33. [CrossRef]
- Chihara, T.; Hashimoto, M.; Osman, A.; Hiyoshi-Yoshidomi, Y.; Suzu, I.; Chutiwitoonchai, N.; Hiyoshi, M.; Okada, S.; Suzu, S. HIV-1 Proteins Preferentially Activate Anti-Inflammatory M2-Type Macrophages. J Immunol 2012, 188, 3620–3627. [CrossRef]
- Zhao, S.; Si, M.; Deng, X.; Wang, D.; Kong, L.; Zhang, Q. HCV Inhibits M2a, M2b and M2c Macrophage Polarization via HCV Core Protein Engagement with Toll-like Receptor 2. Exp Ther Med 2022, 24, 522. [CrossRef]
- Wang, Y.; Zheng, J.; Wang, X.; Yang, P.; Zhao, D. Alveolar Macrophages and Airway Hyperresponsiveness Associated with Respiratory Syncytial Virus Infection. Frontiers in Immunology 2022, 13.
- Tanmay, S.; Labrou, D.; Farsalinos, K.; Poulas, K. Is SARS-CoV-2 Spike Glycoprotein Impairing Macrophage Function via A7-Nicotinic Acetylcholine Receptors? Food Chem Toxicol 2021, 152, 112184. [CrossRef]
- Chernyavsky, A.I.; Arredondo, J.; Skok, M.; Grando, S.A. Auto/Paracrine Control of Inflammatory Cytokines by Acetylcholine in Macrophage-like U937 Cells through Nicotinic Receptors. Int. Immunopharmacol. 2010, 10, 308–315. [CrossRef]
- Mashimo, M.; Moriwaki, Y.; Misawa, H.; Kawashima, K.; Fujii, T. Regulation of Immune Functions by Non-Neuronal Acetylcholine (ACh) via Muscarinic and Nicotinic ACh Receptors. IJMS 2021, 22, 6818. [CrossRef]
- Richter, K.; Grau, V. Signaling of Nicotinic Acetylcholine Receptors in Mononuclear Phagocytes. Pharmacological Research 2023, 191, 106727. [CrossRef]
- Báez-Pagán, C.A.; Delgado-Vélez, M.; Lasalde-Dominicci, J.A. Activation of the Macrophage A7 Nicotinic Acetylcholine Receptor and Control of Inflammation. J Neuroimmune Pharmacol 2015. [CrossRef]
- Kabbani, N.; Nichols, R.A. Beyond the Channel: Metabotropic Signaling by Nicotinic Receptors. Trends Pharmacol Sci 2018, 39, 354–366. [CrossRef]
- Kabbani, N.; Nordman, J.C.; Corgiat, B.A.; Veltri, D.P.; Shehu, A.; Seymour, V.A.; Adams, D.J. Are Nicotinic Acetylcholine Receptors Coupled to G Proteins? Bioessays 2013, 35, 1025–1034. [CrossRef]
- King, J.R.; Kabbani, N. Alpha 7 Nicotinic Receptor Coupling to Heterotrimeric G Proteins Modulates RhoA Activation, Cytoskeletal Motility, and Structural Growth. J. Neurochem. 2016, 138, 532–545. [CrossRef]
- King, J.R.; Gillevet, T.C.; Kabbani, N. A G Protein-Coupled A7 Nicotinic Receptor Regulates Signaling and TNF-α Release in Microglia. FEBS Open Bio 2017, 7, 1350–1361. [CrossRef]
- AlQasrawi, D.; Qasem, A.; Naser, S.A. Divergent Effect of Cigarette Smoke on Innate Immunity in Inflammatory Bowel Disease: A Nicotine-Infection Interaction. Int J Mol Sci 2020, 21, 5801. [CrossRef]
- Siniavin, A.E.; Streltsova, M.A.; Kudryavtsev, D.S.; Shelukhina, I.V.; Utkin, Y.N.; Tsetlin, V.I. Activation of A7 Nicotinic Acetylcholine Receptor Upregulates HLA-DR and Macrophage Receptors: Potential Role in Adaptive Immunity and in Preventing Immunosuppression. Biomolecules 2020, 10. [CrossRef]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-Synthesizing T Cells Relay Neural Signals in a Vagus Nerve Circuit. Science 2011, 334, 98–101. [CrossRef]
- Reardon, C.; Duncan, G.S.; Brüstle, A.; Brenner, D.; Tusche, M.W.; Olofsson, P.; Rosas-Ballina, M.; Tracey, K.J.; Mak, T.W. Lymphocyte-Derived ACh Regulates Local Innate but Not Adaptive Immunity. Proc. Natl. Acad. Sci. U.S.A. 2013. [CrossRef]
- Knights, A.J.; Liu, S.; Ma, Y.; Nudell, V.S.; Perkey, E.; Sorensen, M.J.; Kennedy, R.T.; Maillard, I.; Ye, L.; Jun, H.; et al. Acetylcholine-Synthesizing Macrophages in Subcutaneous Fat Are Regulated by Β2 -Adrenergic Signaling. EMBO J 2021, 40, e106061. [CrossRef]
- Richter, K.; Koch, C.; Perniss, A.; Wolf, P.M.; Schweda, E.K.H.; Wichmann, S.; Wilker, S.; Magel, I.; Sander, M.; McIntosh, J.M.; et al. Phosphocholine-Modified Lipooligosaccharides of Haemophilus Influenzae Inhibit ATP-Induced IL-1β Release by Pulmonary Epithelial Cells. Molecules 2018, 23, 1979. [CrossRef]
- Delgado-Vélez, M.; Báez-Pagán, C.A.; Gerena, Y.; Quesada, O.; Santiago-Pérez, L.I.; Capó-Vélez, C.M.; Wojna, V.; Meléndez, L.; León-Rivera, R.; Silva, W.; et al. The A7-Nicotinic Receptor Is Upregulated in Immune Cells from HIV-Seropositive Women: Consequences to the Cholinergic Anti-Inflammatory Response. Clin Transl Immunology 2015, 4, e53. [CrossRef]
- Ríos, S.C.; Colón Sáez, J.O.; Quesada, O.; Figueroa, K.Q.; Lasalde Dominicci, J.A. Disruption of the Cholinergic Anti-Inflammatory Response by R5-Tropic HIV-1 Protein Gp120JRFL. J Biol Chem 2021, 100618. [CrossRef]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and Function of the Cholinergic System in Immune Cells. Front Immunol 2017, 8, 1085. [CrossRef]
- Wessler, I.K.; Kirkpatrick, C.J. The Non-Neuronal Cholinergic System: An Emerging Drug Target in the Airways. Pulm Pharmacol Ther 2001, 14, 423–434. [CrossRef]
- Koarai, A.; Traves, S.L.; Fenwick, P.S.; Brown, S.M.; Chana, K.K.; Russell, R.E.K.; Nicholson, A.G.; Barnes, P.J.; Donnelly, L.E. Expression of Muscarinic Receptors by Human Macrophages. Eur Respir J 2012, 39, 698–704. [CrossRef]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Physiological Functions of the Cholinergic System in Immune Cells. J Pharmacol Sci 2017, 134, 1–21. [CrossRef]
- de Oliveira, P.; Gomes, A.Q.; Pacheco, T.R.; Vitorino de Almeida, V.; Saldanha, C.; Calado, A. Cell-Specific Regulation of Acetylcholinesterase Expression under Inflammatory Conditions. Clin Hemorheol Microcirc 2012, 51, 129–137. [CrossRef]
- Liu, E.Y.L.; Xia, Y.; Kong, X.; Guo, M.S.S.; Yu, A.X.D.; Zheng, B.Z.Y.; Mak, S.; Xu, M.L.; Tsim, K.W.K. Interacting with A7 NAChR Is a New Mechanism for AChE to Enhance the Inflammatory Response in Macrophages. Acta Pharm Sin B 2020, 10, 1926–1942. [CrossRef]
- Li, S.; Qi, D.; Li, J.-N.; Deng, X.-Y.; Wang, D.-X. Vagus Nerve Stimulation Enhances the Cholinergic Anti-Inflammatory Pathway to Reduce Lung Injury in Acute Respiratory Distress Syndrome via STAT3. Cell Death Discov 2021, 7, 63. [CrossRef]
- Lee, R.H.; Vazquez, G. Evidence for a Prosurvival Role of Alpha-7 Nicotinic Acetylcholine Receptor in Alternatively (M2)-activated Macrophages. Physiol Rep 2013, 1. [CrossRef]
- Niu, X.-H.; Liu, R.-H.; Lv, X.; He, R.-L.; Lv, F.-Z.; Wu, S.-J.; Li, X.-Q.; Li, L.; Lin, J.-F. Activating A7nAChR Helps Post-Myocardial Infarction Healing by Regulating Macrophage Polarization via the STAT3 Signaling Pathway. Inflamm Res 2023, 72, 879–892. [CrossRef]
- Qian, Z.; Yang, H.; Li, H.; Liu, C.; Yang, L.; Qu, Z.; Li, X. The Cholinergic Anti-Inflammatory Pathway Attenuates the Development of Atherosclerosis in Apoe-/- Mice through Modulating Macrophage Functions. Biomedicines 2021, 9, 1150. [CrossRef]
- Suzuki, M.; Katayama, T.; Suzuki, C.; Nakajima, K.; Magata, Y.; Ogawa, M. Uptake of Nicotinic Acetylcholine Receptor Imaging Agent Is Reduced in the Pro-Inflammatory Macrophage. Nucl Med Biol 2021, 102–103, 45–55. [CrossRef]
- Han, X.; Li, W.; Li, P.; Zheng, Z.; Lin, B.; Zhou, B.; Guo, K.; He, P.; Yang, J. Stimulation of A7 Nicotinic Acetylcholine Receptor by Nicotine Suppresses Decidual M1 Macrophage Polarization Against Inflammation in Lipopolysaccharide-Induced Preeclampsia-Like Mouse Model. Frontiers in Immunology 2021, 12.
- Wang, J.; Lu, S.; Yang, F.; Guo, Y.; Chen, Z.; Yu, N.; Yao, L.; Huang, J.; Fan, W.; Xu, Z.; et al. The Role of Macrophage Polarization and Associated Mechanisms in Regulating the Anti-Inflammatory Action of Acupuncture: A Literature Review and Perspectives. Chinese Medicine 2021, 16, 56. [CrossRef]
- Torres-Rosas, R.; Yehia, G.; Peña, G.; Mishra, P.; del Rocio Thompson-Bonilla, M.; Moreno-Eutimio, M.A.; Arriaga-Pizano, L.A.; Isibasi, A.; Ulloa, L. Dopamine Mediates Vagal Modulation of the Immune System by Electroacupuncture. Nat Med 2014, 20, 291–295. [CrossRef]
- Alkondon, M.; Pereira, E.F.; Cortes, W.S.; Maelicke, A.; Albuquerque, E.X. Choline Is a Selective Agonist of Alpha7 Nicotinic Acetylcholine Receptors in the Rat Brain Neurons. Eur. J. Neurosci. 1997, 9, 2734–2742.
- Roci, I.; Watrous, J.D.; Lagerborg, K.A.; Jain, M.; Nilsson, R. Mapping Choline Metabolites in Normal and Transformed Cells. Metabolomics 2020, 16, 125. [CrossRef]
- Sanchez-Lopez, E.; Zhong, Z.; Stubelius, A.; Sweeney, S.R.; Booshehri, L.M.; Antonucci, L.; Liu-Bryan, R.; Lodi, A.; Terkeltaub, R.; Lacal, J.C.; et al. Choline Uptake and Metabolism Modulate Macrophage IL-1β and IL-18 Production. Cell Metab 2019, 29, 1350-1362.e7. [CrossRef]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington (DC), 1998; ISBN 978-0-309-06411-8.
- Song, M.; Xu, B.P.; Liang, Q.; Wei, Y.; Song, Y.; Chen, P.; Zhou, Z.; Zhang, N.; He, Q.; Liu, L.; et al. Association of Serum Choline Levels and All-Cause Mortality Risk in Adults with Hypertension: A Nested Case–Control Study. Nutrition & Metabolism 2021, 18, 108. [CrossRef]
- Elble, R.; Giacobini, E.; Higgins, C. Choline Levels Are Increased in Cerebrospinal Fluid of Alzheimer Patients. Neurobiology of Aging 1989, 10, 45–50. [CrossRef]
- Yang, Y.S.; Smucny, J.; Zhang, H.; Maddock, R.J. Meta-Analytic Evidence of Elevated Choline, Reduced N-Acetylaspartate, and Normal Creatine in Schizophrenia and Their Moderation by Measurement Quality, Echo Time, and Medication Status. Neuroimage Clin 2023, 39, 103461. [CrossRef]
- Judd, J.M.; Jasbi, P.; Winslow, W.; Serrano, G.E.; Beach, T.G.; Klein-Seetharaman, J.; Velazquez, R. Low Circulating Choline, a Modifiable Dietary Factor, Is Associated with the Pathological Progression and Metabolome Dysfunction in Alzheimer’s Disease. bioRxiv 2023, 2023.05.06.539713. [CrossRef]
- Mike, A.; Castro, N.G.; Albuquerque, E.X. Choline and Acetylcholine Have Similar Kinetic Properties of Activation and Desensitization on the Alpha7 Nicotinic Receptors in Rat Hippocampal Neurons. Brain Res 2000, 882, 155–168. [CrossRef]
- Gault, J.; Robinson, M.; Berger, R.; Drebing, C.; Logel, J.; Hopkins, J.; Moore, T.; Jacobs, S.; Meriwether, J.; Choi, M.J.; et al. Genomic Organization and Partial Duplication of the Human Alpha7 Neuronal Nicotinic Acetylcholine Receptor Gene (CHRNA7). Genomics 1998, 52, 173–185. [CrossRef]
- Riley, B.; Williamson, M.; Collier, D.; Wilkie, H.; Makoff, A. A 3-Mb Map of a Large Segmental Duplication Overlapping the Alpha7-Nicotinic Acetylcholine Receptor Gene (CHRNA7) at Human 15q13-Q14. Genomics 2002, 79, 197–209. [CrossRef]
- de Lucas-Cerrillo, A.M.; Maldifassi, M.C.; Arnalich, F.; Renart, J.; Atienza, G.; Serantes, R.; Cruces, J.; Sánchez-Pacheco, A.; Andrés-Mateos, E.; Montiel, C. Function of Partially Duplicated Human A77 Nicotinic Receptor Subunit CHRFAM7A Gene: Potential Implications for the Cholinergic Anti-Inflammatory Response. J. Biol. Chem. 2011, 286, 594–606. [CrossRef]
- Li, T.; Chen, W.; Zhang, Q.; Deng, C. Human-Specific Gene CHRFAM7A Mediates M2 Macrophage Polarization via the Notch Pathway to Ameliorate Hypertrophic Scar Formation. Biomedicine & Pharmacotherapy 2020, 131, 110611. [CrossRef]
- Courties, A.; Olmer, M.; Myers, K.; Ordoukhanian, P.; Head, S.R.; Natarajan, P.; Berenbaum, F.; Sellam, J.; Lotz, M.K. Human-Specific Duplicate CHRFAM7A Gene Is Associated with More Severe Osteoarthritis and Amplifies Pain Behaviours. Ann Rheum Dis 2023, 82, 710–718. [CrossRef]
- Maroli, A.; Di Lascio, S.; Drufuca, L.; Cardani, S.; Setten, E.; Locati, M.; Fornasari, D.; Benfante, R. Effect of Donepezil on the Expression and Responsiveness to LPS of CHRNA7 and CHRFAM7A in Macrophages: A Possible Link to the Cholinergic Anti-Inflammatory Pathway. J Neuroimmunol 2019, 332, 155–166. [CrossRef]
- Zhang, Y.; Qian, J.; Ren, H.; Meng, F.; Ma, R.; Xu, B. Human-Specific CHRFAM7A Protects against Radiotherapy-Induced Lacrimal Gland Injury by Inhibiting the P38/JNK Signalling Pathway and Oxidative Stress. Int J Clin Exp Pathol 2017, 10, 9001–9011.
- Zhou, B.; Zhang, Y.; Dang, X.; Li, B.; Wang, H.; Gong, S.; Li, S.; Meng, F.; Xing, J.; Li, T.; et al. Up-Regulation of the Human-Specific CHRFAM7A Gene Protects against Renal Fibrosis in Mice with Obstructive Nephropathy. Journal of Cellular and Molecular Medicine 2023, 27, 52–65. [CrossRef]
- Cao, X.; Wang, Y.; Gao, L. CHRFAM7A Overexpression Attenuates Cerebral Ischemia-Reperfusion Injury via Inhibiting Microglia Pyroptosis Mediated by the NLRP3/Caspase-1 Pathway. Inflammation 2021, 44, 1023–1034. [CrossRef]
- Horkowitz, A.P.; Schwartz, A.V.; Alvarez, C.A.; Herrera, E.B.; Thoman, M.L.; Chatfield, D.A.; Osborn, K.G.; Feuer, R.; George, U.Z.; Phillips, J.A. Acetylcholine Regulates Pulmonary Pathology During Viral Infection and Recovery. Immunotargets Ther 2020, 9, 333–350. [CrossRef]
- Islas-Weinstein, L.; Marquina-Castillo, B.; Mata-Espinosa, D.; Paredes-González, I.S.; Chávez, J.; Balboa, L.; Marín Franco, J.L.; Guerrero-Romero, D.; Barrios-Payan, J.A.; Hernandez-Pando, R. The Cholinergic System Contributes to the Immunopathological Progression of Experimental Pulmonary Tuberculosis. Front Immunol 2020, 11, 581911. [CrossRef]
- Ramirez, V.T.; Godinez, D.R.; Brust-Mascher, I.; Nonnecke, E.B.; Castillo, P.A.; Gardner, M.B.; Tu, D.; Sladek, J.A.; Miller, E.N.; Lebrilla, C.B.; et al. T-Cell Derived Acetylcholine Aids Host Defenses during Enteric Bacterial Infection with Citrobacter Rodentium. PLOS Pathogens 2019, 15, e1007719. [CrossRef]
- Matilla, M.A.; Velando, F.; Tajuelo, A.; Martín-Mora, D.; Xu, W.; Sourjik, V.; Gavira, J.A.; Krell, T. Chemotaxis of the Human Pathogen Pseudomonas Aeruginosa to the Neurotransmitter Acetylcholine. mBio 2022, 13, e0345821. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



