Submitted:
21 September 2023
Posted:
25 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Cell Culture
DsiRNA Transfection
Quantitative Real-Time PCR Analysis
Western Blot Analysis
Cell Viability Assays
Myotube Diameter Measurements
Genomic DNA Isolation and Mitochondrial DNA Copy Number Analysis
Statistical Analysis
3. Results
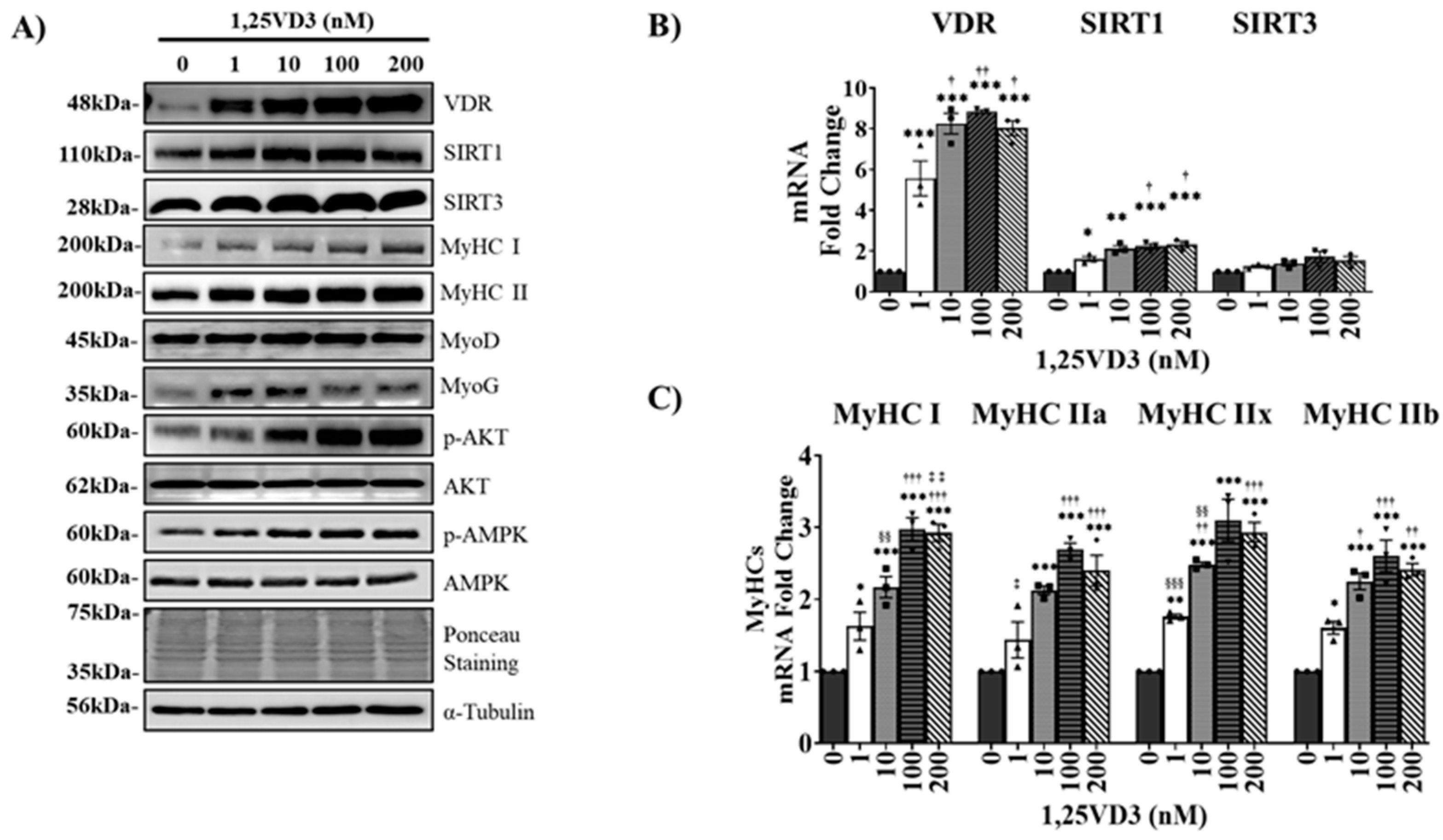
1,25. VD3 induces C2C12 Myotube Hypertrophy and Increases C2C12 Myogenic Differentiation via Upregulation of VDR, SIRT1 and SIRT3 Expression
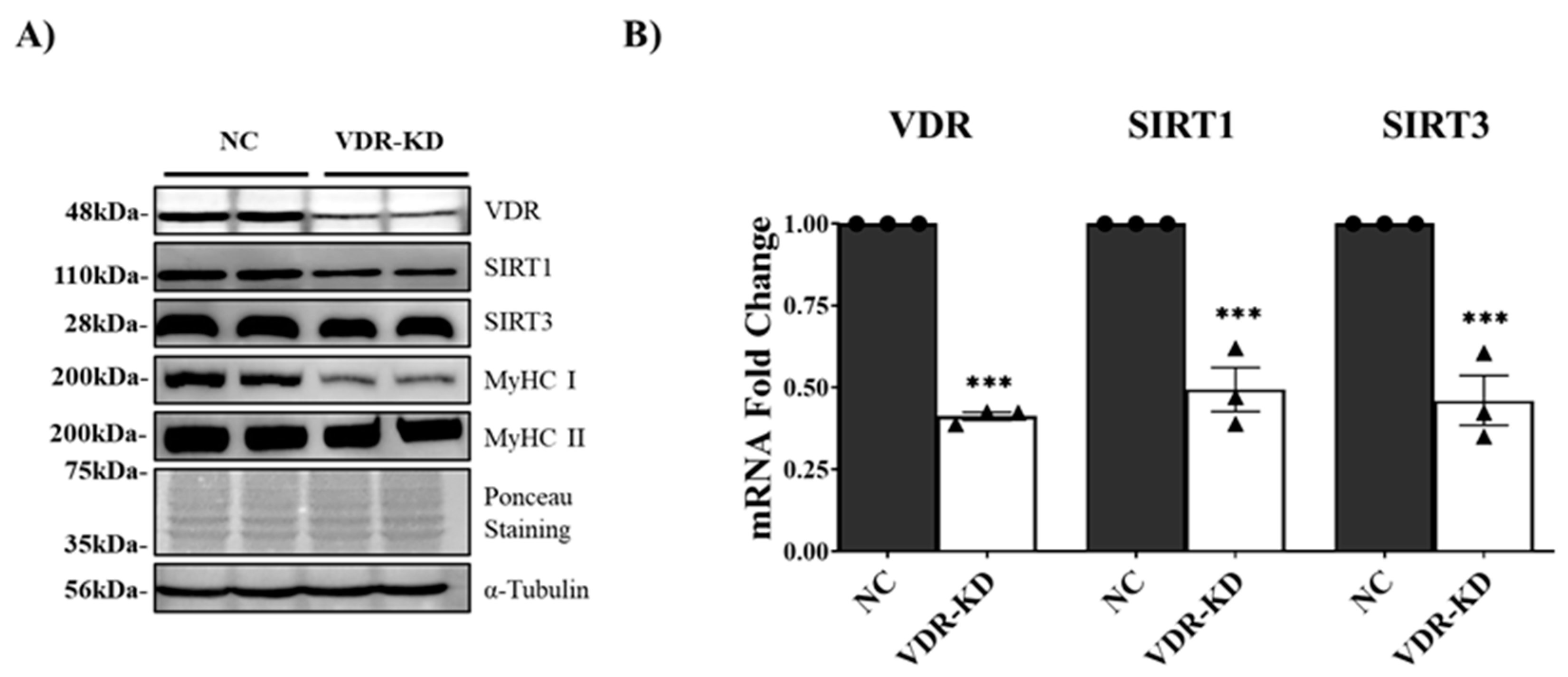
Transient VDR Knockdown Downregulates SIRT1 and SIRT3 Expression and Induces Myotube Atrophy.
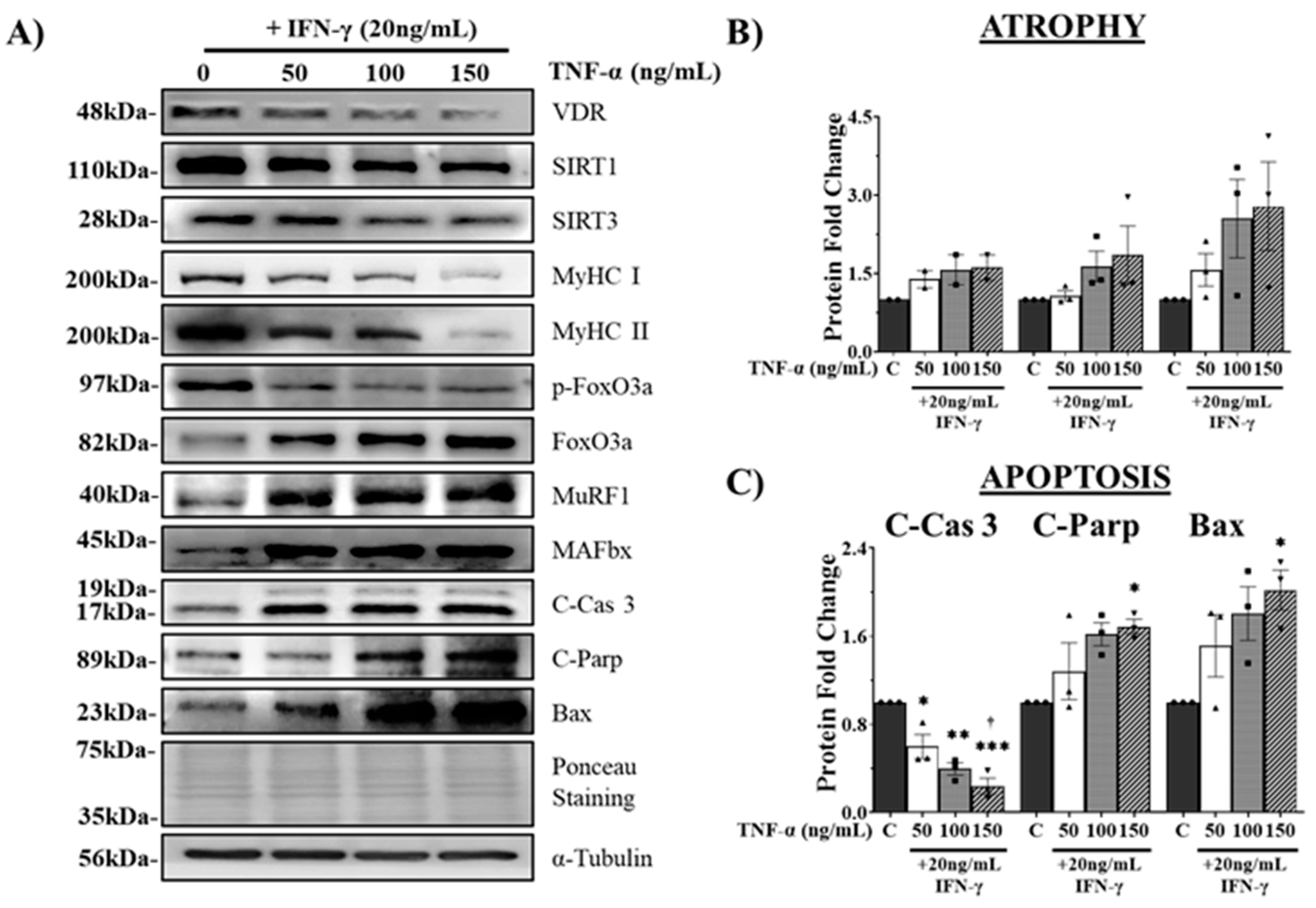
IFN-γ/TNF-α Treatment Induces Atrophy and Apoptosis in C2C12 Myotubes via Downregulation of VDR/SIRT1/SIRT3 Axis.
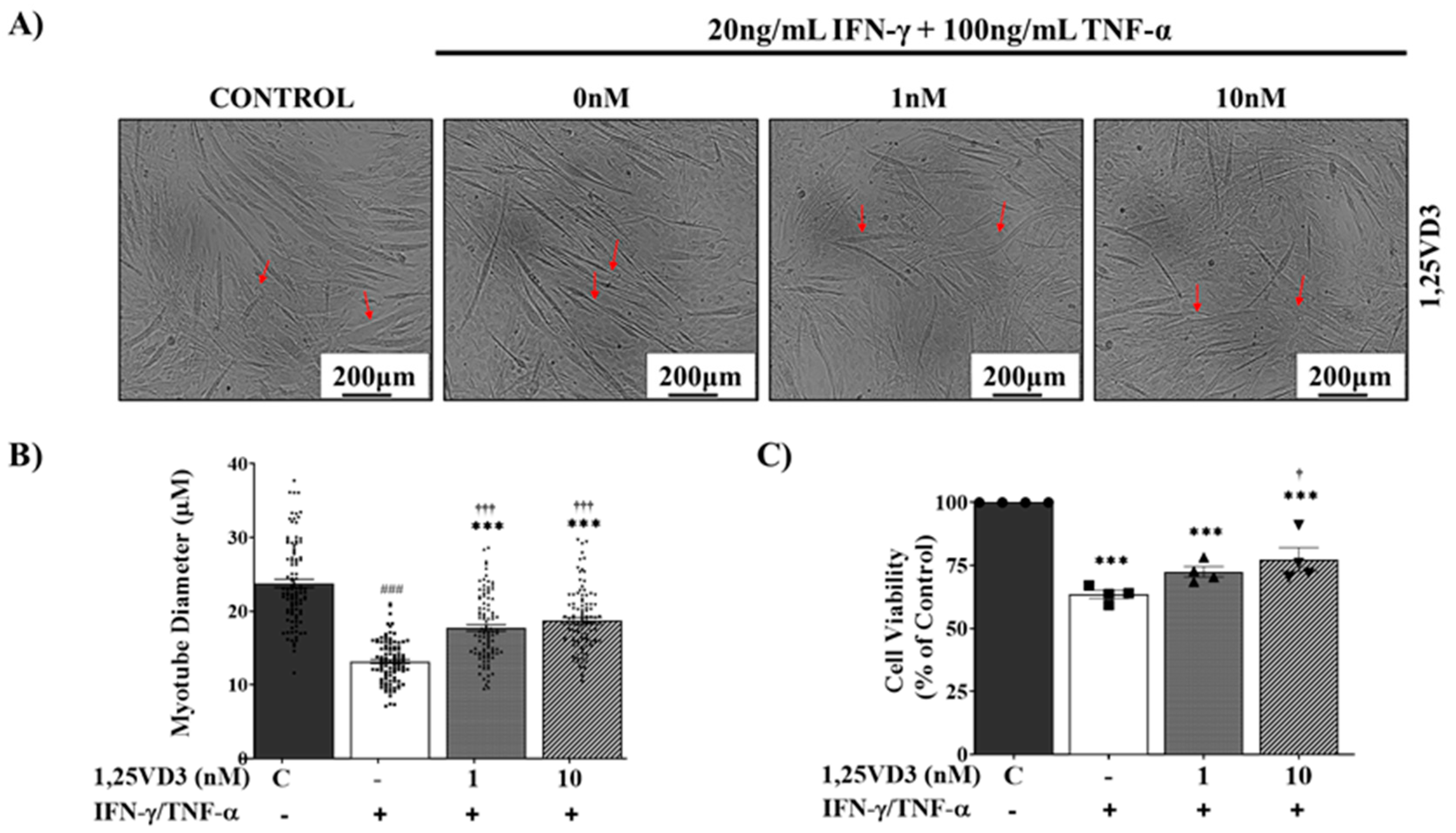
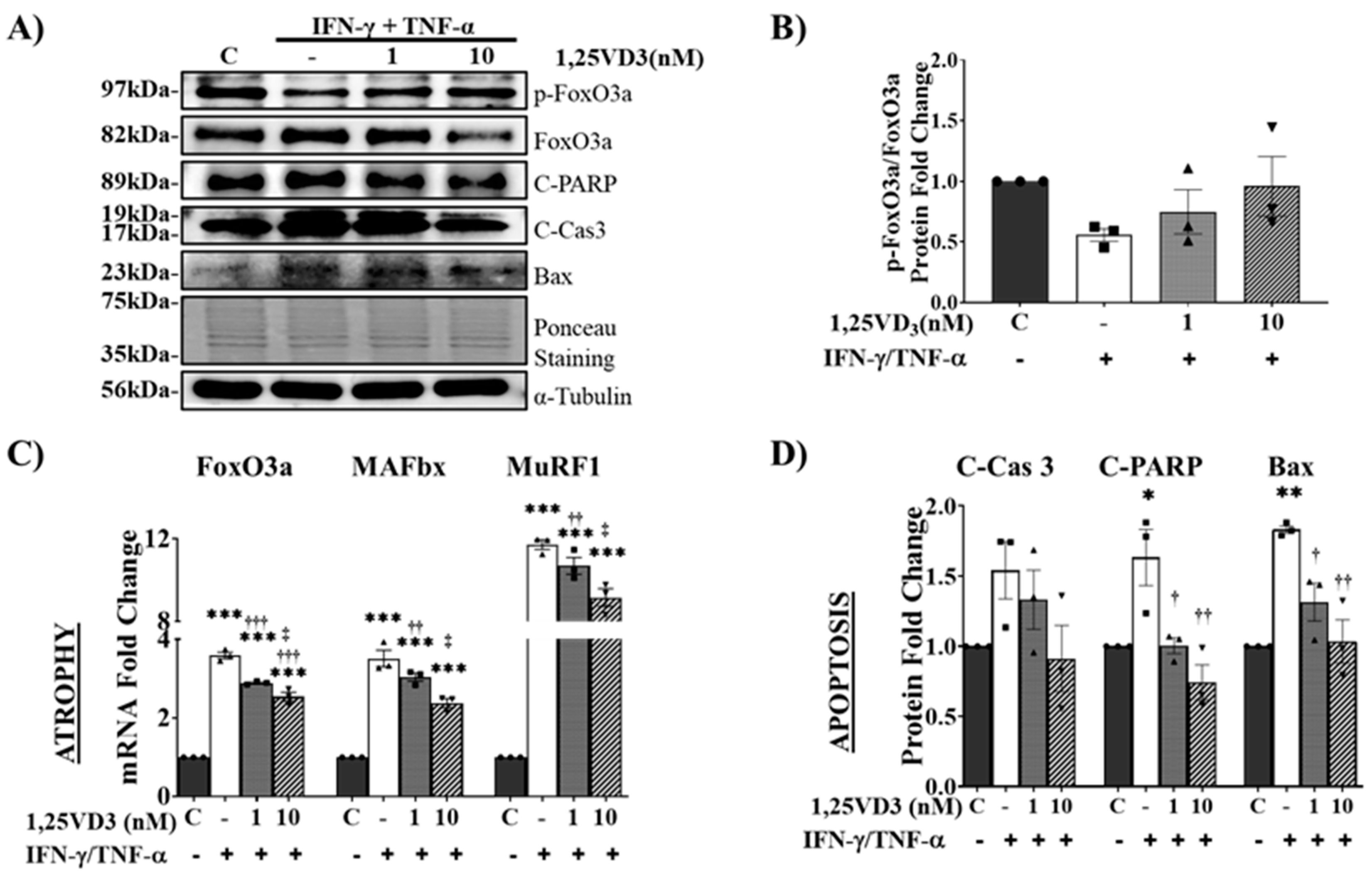
1,25VD3 Ameliorates Myotube Atrophy and Apoptosis Induced by IFN-γ/TNF-α Co-Treatment.
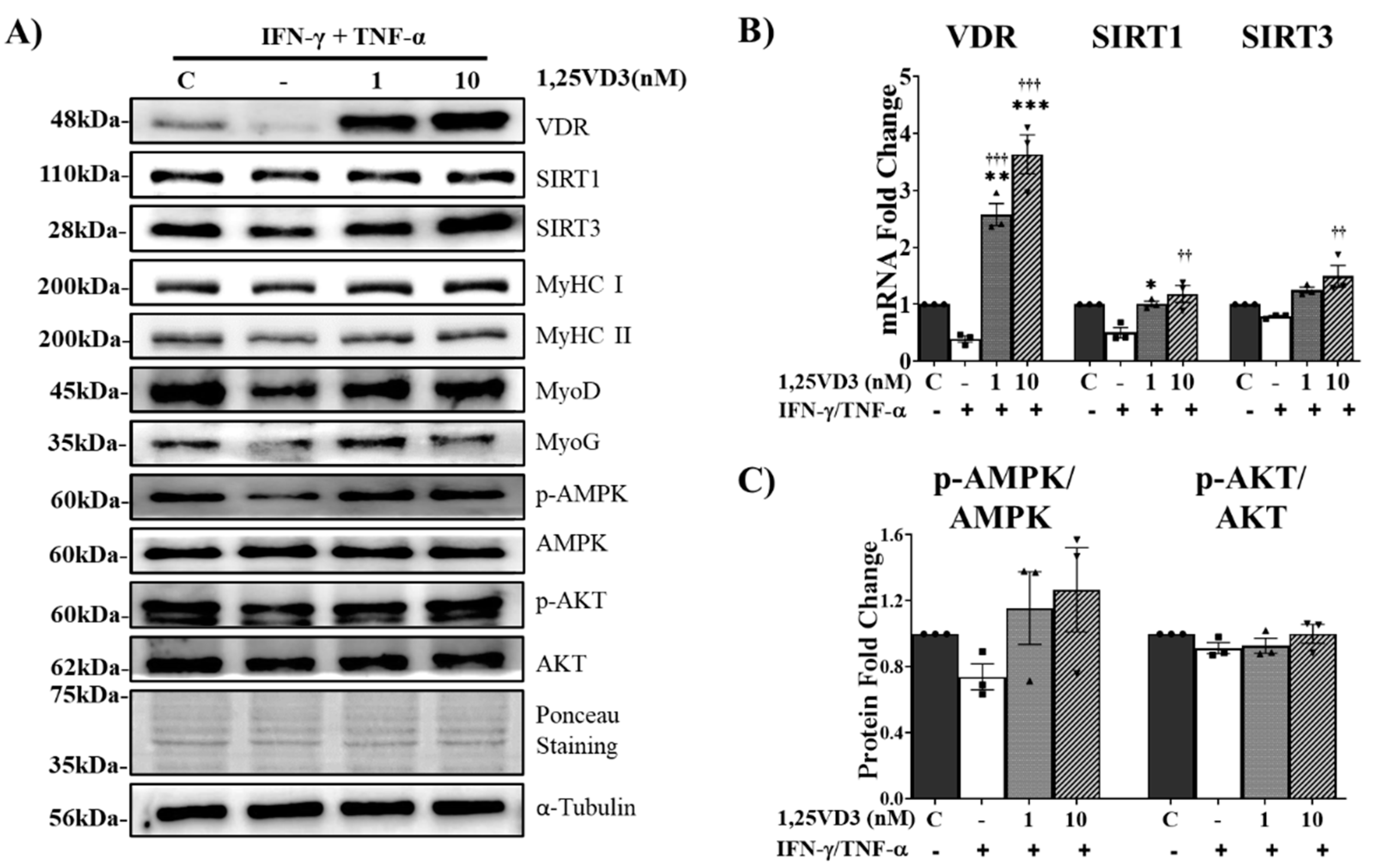
1,25. VD3 Protects against IFN-γ/TNF-α-Induced Muscle Cell Apoptosis and Atrophy by Upregulating the VDR/SIRT1/SIRT3 Axis and Its Downstream Targets.
Upregulation of VDR/SIRT1/SIRT3 Axis by 1,25VD3 Inhibits Atrophy and Apoptosis in C2C12 Myotubes.
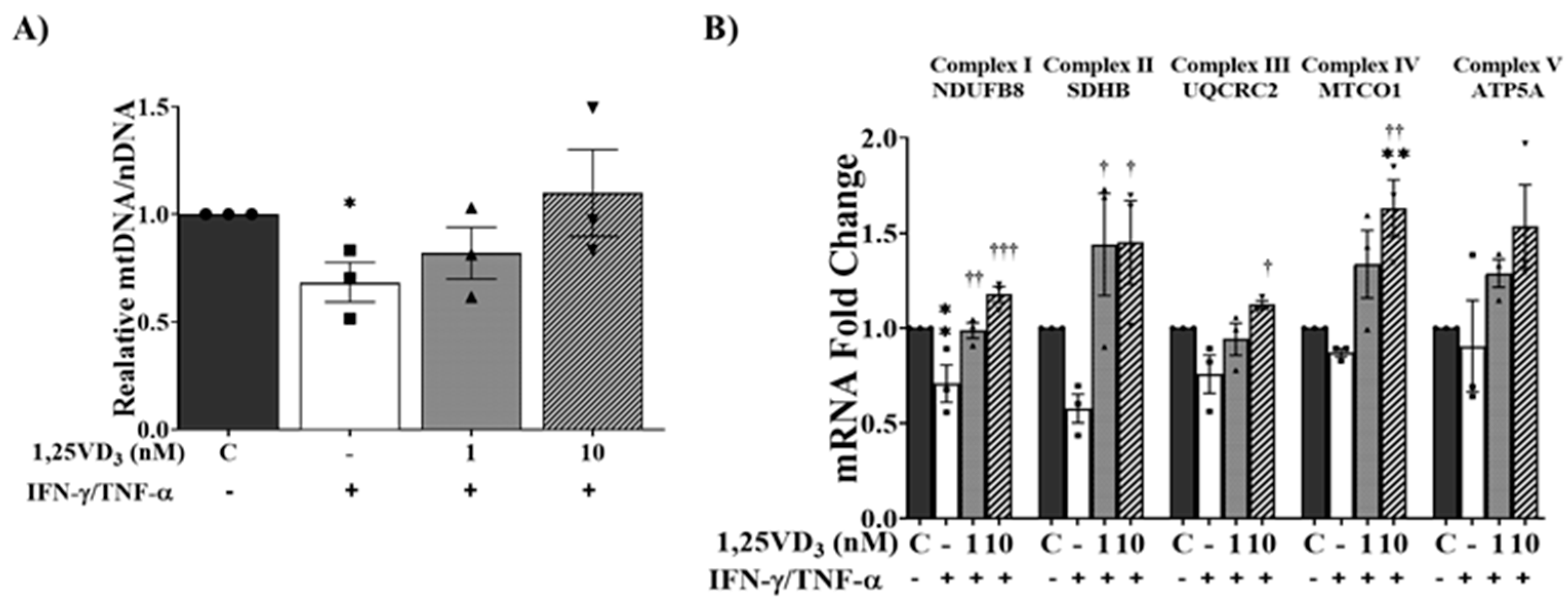
1,25. VD3 Increased Oxidative Phosphorylation Capacity in IFN-γ/TNF-α-Treated Myotubes.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [CrossRef]
- von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 2010, 1, 129-133. [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int 2015, 96, 183-195. [CrossRef]
- Anton, S.D.; Hida, A.; Mankowski, R.; Layne, A.; Solberg, L.M.; Mainous, A.G.; Buford, T. Nutrition and Exercise in Sarcopenia. Curr Protein Pept Sci 2018, 19, 649-667. [CrossRef]
- Roh, Y.H.; Hong, S.W.; Chung, S.W.; Lee, Y.S. Altered gene and protein expressions of vitamin D receptor in skeletal muscle in sarcopenic patients who sustained distal radius fractures. J Bone Miner Metab 2019, 37, 920-927. [CrossRef]
- Bass, J.J.; Nakhuda, A.; Deane, C.S.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; Kadi, F.; Andersen, D.; et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol Metab 2020, 42, 101059. [CrossRef]
- Bass, J.J.; Kazi, A.A.; Deane, C.S.; Nakhuda, A.; Ashcroft, S.P.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; et al. The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo. J Physiol 2021, 599, 963-979. [CrossRef]
- Wacker, M.; Holick, M.F. Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111-148. [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11. [CrossRef]
- Bischoff-Ferrari, H.A.; Borchers, M.; Gudat, F.; Durmuller, U.; Stahelin, H.B.; Dick, W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res 2004, 19, 265-269. [CrossRef]
- MacLaughlin, J.; Holick, M.F. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 1985, 76, 1536-1538. [CrossRef]
- Snijder, M.B.; van Schoor, N.M.; Pluijm, S.M.; van Dam, R.M.; Visser, M.; Lips, P. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab 2006, 91, 2980-2985. [CrossRef]
- Ceglia, L.; Niramitmahapanya, S.; da Silva Morais, M.; Rivas, D.A.; Harris, S.S.; Bischoff-Ferrari, H.; Fielding, R.A.; Dawson-Hughes, B. A randomized study on the effect of vitamin D(3) supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab 2013, 98, E1927-1935. [CrossRef]
- Halfon, M.; Phan, O.; Teta, D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int 2015, 2015, 953241. [CrossRef]
- Pfeifer, M.; Begerow, B.; Minne, H.W. Vitamin D and muscle function. Osteoporos Int 2002, 13, 187-194. [CrossRef]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447-476. [CrossRef]
- Vargas-Ortiz, K.; Perez-Vazquez, V.; Macias-Cervantes, M.H. Exercise and Sirtuins: A Way to Mitochondrial Health in Skeletal Muscle. Int J Mol Sci 2019, 20. [CrossRef]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschop, M.H. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 2012, 92, 1479-1514. [CrossRef]
- Kwon, Y.; Kim, J.; Lee, C.Y.; Kim, H. Expression of SIRT1 and SIRT3 varies according to age in mice. Anat Cell Biol 2015, 48, 54-61. [CrossRef]
- Gong, H.; Pang, J.; Han, Y.; Dai, Y.; Dai, D.; Cai, J.; Zhang, T.M. Age-dependent tissue expression patterns of Sirt1 in senescence-accelerated mice. Mol Med Rep 2014, 10, 3296-3302. [CrossRef]
- Foreman, N.A.; Hesse, A.S.; Ji, L.L. Redox Signaling and Sarcopenia: Searching for the Primary Suspect. Int J Mol Sci 2021, 22. [CrossRef]
- Frederick, D.W.; Loro, E.; Liu, L.; Davila, A., Jr.; Chellappa, K.; Silverman, I.M.; Quinn, W.J., 3rd; Gosai, S.J.; Tichy, E.D.; Davis, J.G.; et al. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab 2016, 24, 269-282. [CrossRef]
- Makanae, Y.; Ogasawara, R.; Sato, K.; Takamura, Y.; Matsutani, K.; Kido, K.; Shiozawa, N.; Nakazato, K.; Fujita, S. Acute bout of resistance exercise increases vitamin D receptor protein expression in rat skeletal muscle. Exp Physiol 2015, 100, 1168-1176. [CrossRef]
- Chang, E.; Kim, Y. Vitamin D Ameliorates Fat Accumulation with AMPK/SIRT1 Activity in C2C12 Skeletal Muscle Cells. Nutrients 2019, 11. [CrossRef]
- Yu, W.; Dong, X.; Dan, G.; Ye, F.; Cheng, J.; Zhao, Y.; Chen, M.; Sai, Y.; Zou, Z. Vitamin D3 protects against nitrogen mustard-induced apoptosis of the bronchial epithelial cells via activating the VDR/Nrf2/Sirt3 pathway. Toxicol Lett 2022, 354, 14-23. [CrossRef]
- Daly, R.M. Independent and combined effects of exercise and vitamin D on muscle morphology, function and falls in the elderly. Nutrients 2010, 2, 1005-1017. [CrossRef]
- Mankhong, S.; Kim, S.; Moon, S.; Kwak, H.B.; Park, D.H.; Kang, J.H. Experimental Models of Sarcopenia: Bridging Molecular Mechanism and Therapeutic Strategy. Cells 2020, 9. [CrossRef]
- Palacios, O.M.; Carmona, J.J.; Michan, S.; Chen, K.Y.; Manabe, Y.; Ward, J.L., 3rd; Goodyear, L.J.; Tong, Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009, 1, 771-783. [CrossRef]
- Safarpour, P.; Daneshi-Maskooni, M.; Vafa, M.; Nourbakhsh, M.; Janani, L.; Maddah, M.; Amiri, F.S.; Mohammadi, F.; Sadeghi, H. Vitamin D supplementation improves SIRT1, Irisin, and glucose indices in overweight or obese type 2 diabetic patients: a double-blind randomized placebo-controlled clinical trial. BMC Fam Pract 2020, 21, 26. [CrossRef]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch Biochem Biophys 2017, 615, 22-34. [CrossRef]
- Puangthong, C.; Sukhong, P.; Saengnual, P.; Srikuea, R.; Chanda, M. A single bout of high-intensity exercise modulates the expression of vitamin D receptor and vitamin D-metabolising enzymes in horse skeletal muscle. Equine Vet J 2021, 53, 796-805. [CrossRef]
- Nemeth, Z.; Patonai, A.; Simon-Szabo, L.; Takacs, I. Interplay of Vitamin D and SIRT1 in Tissue-Specific Metabolism-Potential Roles in Prevention and Treatment of Non-Communicable Diseases Including Cancer. Int J Mol Sci 2023, 24. [CrossRef]
- Karlic, H.; Varga, F. Impact of vitamin D metabolism on clinical epigenetics. Clin Epigenetics 2011, 2, 55-61. [CrossRef]
- Sabir, M.S.; Khan, Z.; Hu, C.; Galligan, M.A.; Dussik, C.M.; Mallick, S.; Stone, A.D.; Batie, S.F.; Jacobs, E.T.; Whitfield, G.K.; et al. SIRT1 enzymatically potentiates 1,25-dihydroxyvitamin D(3) signaling via vitamin D receptor deacetylation. J Steroid Biochem Mol Biol 2017, 172, 117-129. [CrossRef]
- Yang, J.; Zhang, Y.; Pan, Y.; Sun, C.; Liu, Z.; Liu, N.; Fu, Y.; Li, X.; Li, Y.; Kong, J. The Protective Effect of 1,25(OH)(2)D(3) on Myocardial Function is Mediated via Sirtuin 3-Regulated Fatty Acid Metabolism. Front Cell Dev Biol 2021, 9, 627135. [CrossRef]
- Kjobsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmoller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J 2018, 32, 1741-1777. [CrossRef]
- Frost, R.A.; Lang, C.H. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol (1985) 2007, 103, 378-387. [CrossRef]
- Girgis, C.M.; Cha, K.M.; So, B.; Tsang, M.; Chen, J.; Houweling, P.J.; Schindeler, A.; Stokes, R.; Swarbrick, M.M.; Evesson, F.J.; et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J Cachexia Sarcopenia Muscle 2019, 10, 1228-1240. [CrossRef]
- Tanaka, M.; Kishimoto, K.N.; Okuno, H.; Saito, H.; Itoi, E. Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve 2014, 49, 700-708. [CrossRef]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131-142. [CrossRef]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-alpha. Eur J Med Res 2017, 22, 25. [CrossRef]
- Marzetti, E.; Picca, A.; Marini, F.; Biancolillo, A.; Coelho-Junior, H.J.; Gervasoni, J.; Bossola, M.; Cesari, M.; Onder, G.; Landi, F.; et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: The frailty "cytokinome" at its core. Exp Gerontol 2019, 122, 129-138. [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 2013, 6, 25-39. [CrossRef]
- Garcia, L.A.; King, K.K.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 2011, 152, 2976-2986. [CrossRef]
- Braga, M.; Simmons, Z.; Norris, K.C.; Ferrini, M.G.; Artaza, J.N. Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocr Connect 2017, 6, 139-150. [CrossRef]
- Abdel Khalek, W.; Cortade, F.; Ollendorff, V.; Lapasset, L.; Tintignac, L.; Chabi, B.; Wrutniak-Cabello, C. SIRT3, a mitochondrial NAD(+)-dependent deacetylase, is involved in the regulation of myoblast differentiation. PLoS One 2014, 9, e114388. [CrossRef]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 2008, 283, 27628-27635. [CrossRef]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056-1060. [CrossRef]
- Lin, L.; Chen, K.; Abdel Khalek, W.; Ward, J.L., 3rd; Yang, H.; Chabi, B.; Wrutniak-Cabello, C.; Tong, Q. Regulation of skeletal muscle oxidative capacity and muscle mass by SIRT3. PLoS One 2014, 9, e85636. [CrossRef]
- Pillai, V.B.; Sundaresan, N.R.; Kim, G.; Gupta, M.; Rajamohan, S.B.; Pillai, J.B.; Samant, S.; Ravindra, P.V.; Isbatan, A.; Gupta, M.P. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem 2010, 285, 3133-3144. [CrossRef]
- Pillai, V.B.; Sundaresan, N.R.; Gupta, M.P. Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circ Res 2014, 114, 368-378. [CrossRef]
- Wang, X.; Hu, S.; Liu, L. Phosphorylation and acetylation modifications of FOXO3a: Independently or synergistically? Oncol Lett 2017, 13, 2867-2872. [CrossRef]
- Greer, E.L.; Oskoui, P.R.; Banko, M.R.; Maniar, J.M.; Gygi, M.P.; Gygi, S.P.; Brunet, A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 2007, 282, 30107-30119. [CrossRef]
- Marcus, J.M.; Andrabi, S.A. SIRT3 Regulation Under Cellular Stress: Making Sense of the Ups and Downs. Front Neurosci 2018, 12, 799. [CrossRef]
- Canto, C.; Sauve, A.A.; Bai, P. Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol Aspects Med 2013, 34, 1168-1201. [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399-412. [CrossRef]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857-868. [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: oxidative stress, energy metabolism and anabolic state. Eur J Appl Physiol 2019, 119, 825-839. [CrossRef]
- Motta, M.C.; Divecha, N.; Lemieux, M.; Kamel, C.; Chen, D.; Gu, W.; Bultsma, Y.; McBurney, M.; Guarente, L. Mammalian SIRT1 represses forkhead transcription factors. Cell 2004, 116, 551-563. [CrossRef]
- Miyamoto, T.; Kakizawa, T.; Hashizume, K. Inhibition of nuclear receptor signalling by poly(ADP-ribose) polymerase. Mol Cell Biol 1999, 19, 2644-2649. [CrossRef]
- Jurgensmeier, J.M.; Xie, Z.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A 1998, 95, 4997-5002. [CrossRef]
- Hu, J.; Wu, J.; Wan, F.; Kou, L.; Yin, S.; Sun, Y.; Li, Y.; Zhou, Q.; Wang, T. Calcitriol Alleviates MPP(+)- and MPTP-Induced Parthanatos Through the VDR/PARP1 Pathway in the Model of Parkinson's Disease. Front Aging Neurosci 2021, 13, 657095. [CrossRef]
- Rajamohan, S.B.; Pillai, V.B.; Gupta, M.; Sundaresan, N.R.; Birukov, K.G.; Samant, S.; Hottiger, M.O.; Gupta, M.P. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol 2009, 29, 4116-4129. [CrossRef]
- Marzetti, E.; Privitera, G.; Simili, V.; Wohlgemuth, S.E.; Aulisa, L.; Pahor, M.; Leeuwenburgh, C. Multiple pathways to the same end: mechanisms of myonuclear apoptosis in sarcopenia of aging. ScientificWorldJournal 2010, 10, 340-349. [CrossRef]
- Marzetti, E.; Hwang, J.C.; Lees, H.A.; Wohlgemuth, S.E.; Dupont-Versteegden, E.E.; Carter, C.S.; Bernabei, R.; Leeuwenburgh, C. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta 2010, 1800, 235-244. [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 2010, 12, 662-667. [CrossRef]
- Cohen, H.Y.; Lavu, S.; Bitterman, K.J.; Hekking, B.; Imahiyerobo, T.A.; Miller, C.; Frye, R.; Ploegh, H.; Kessler, B.M.; Sinclair, D.A. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell 2004, 13, 627-638. [CrossRef]
- Zhang, X.; Zanello, L.P. Vitamin D receptor-dependent 1 alpha,25(OH)2 vitamin D3-induced anti-apoptotic PI3K/AKT signaling in osteoblasts. J Bone Miner Res 2008, 23, 1238-1248. [CrossRef]
- Salles, J.; Chanet, A.; Guillet, C.; Vaes, A.M.; Brouwer-Brolsma, E.M.; Rocher, C.; Giraudet, C.; Patrac, V.; Meugnier, E.; Montaurier, C.; et al. Vitamin D status modulates mitochondrial oxidative capacities in skeletal muscle: role in sarcopenia. Commun Biol 2022, 5, 1288. [CrossRef]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J Clin Endocrinol Metab 2013, 98, E509-513. [CrossRef]
- Lombard, D.B.; Tishkoff, D.X.; Bao, J. Mitochondrial sirtuins in the regulation of mitochondrial activity and metabolic adaptation. Handb Exp Pharmacol 2011, 206, 163-188. [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109-1122. [CrossRef]
- Brenmoehl, J.; Hoeflich, A. Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion 2013, 13, 755-761. [CrossRef]
- Suwa, M.; Nakano, H.; Radak, Z.; Kumagai, S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle. Metabolism 2008, 57, 986-998. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




