Submitted:
21 September 2023
Posted:
22 September 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.2. Materials
2.3. Methods
2.3.1. Deoxyribose Degradation Assay
2.3.2. Fenton reaction MTT assay
2.3.3. DPPH assay
2.3.4. ABTS assay
2.3.5. LDCL in presence of KO2
2.3.6. LDCL in presence of NaClO
2.4. Computational Details
2.4.1. Scaling the Wavenumbers
3. Results
3.1. Synthesis
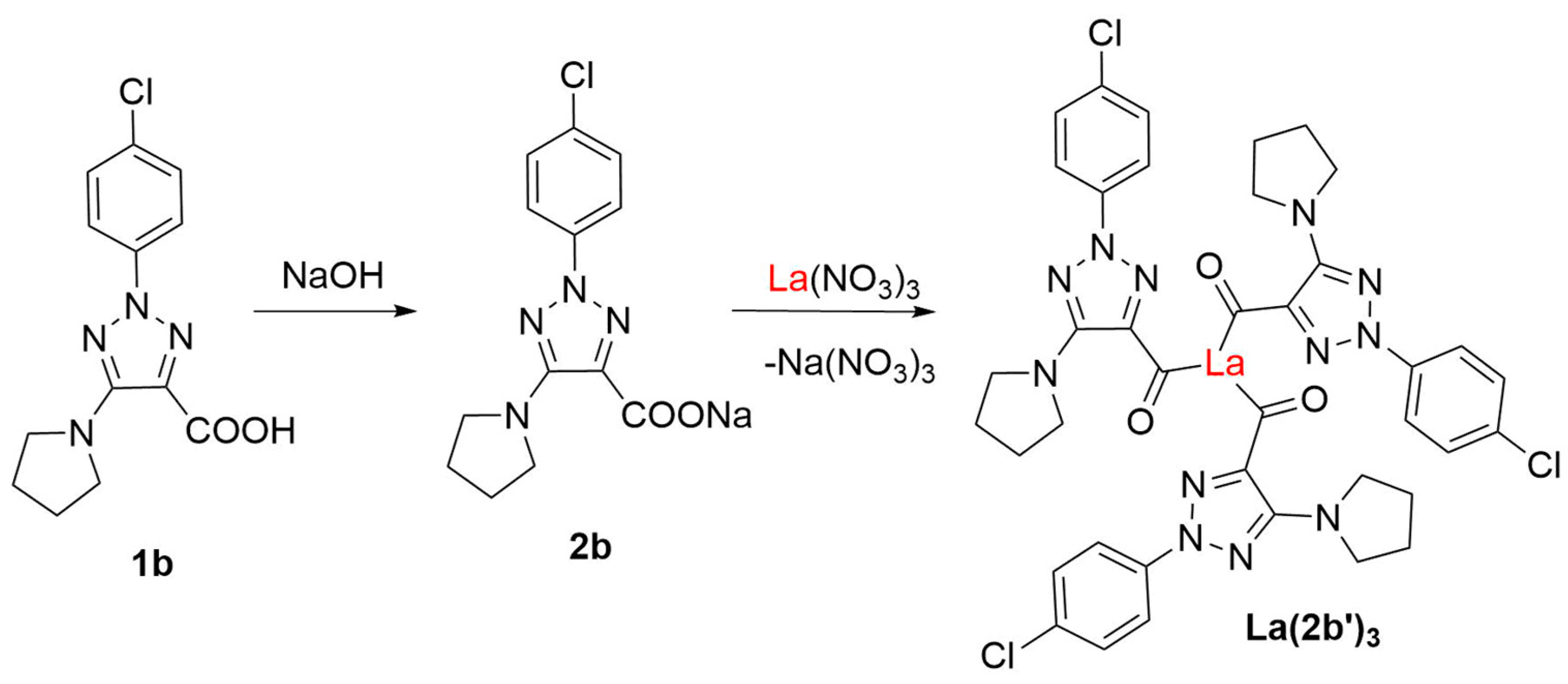
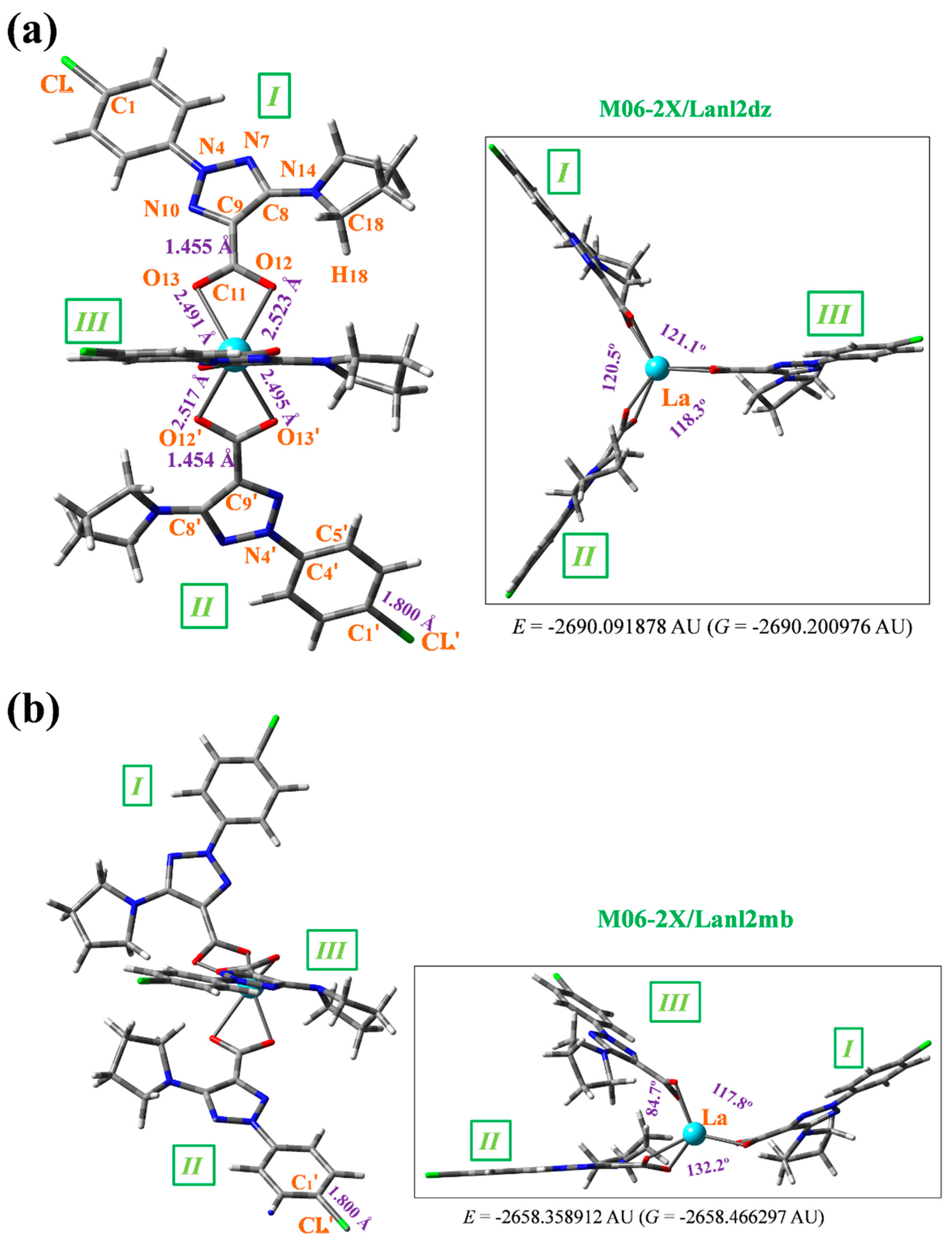
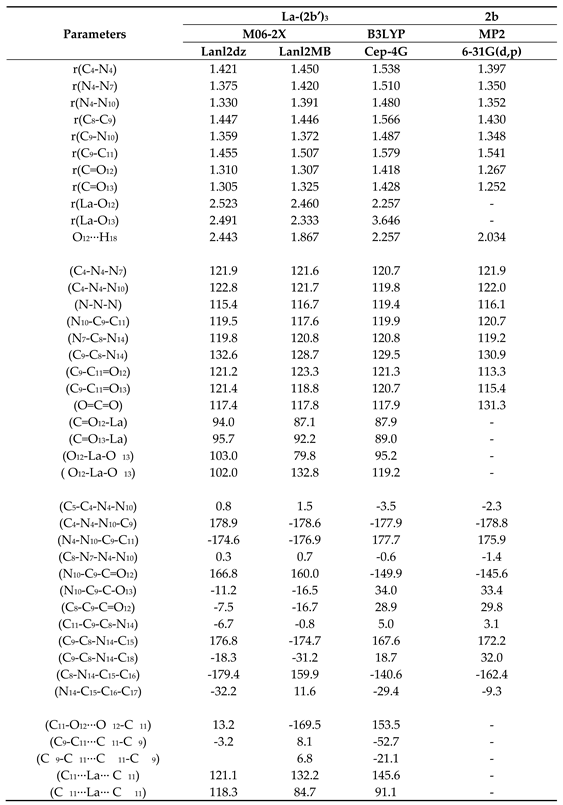
3.2. Molecular structure of the complex
3.3. Atomic charges and molecular properties calculation
| M06-2X | B3LYP | MP2 | ||
| atom | Lanl2dz | Lanl2MB | Cep-4G | 6-31G(d,p)a |
| La CL C1 C4 N4 N7 C8 C9 N10 C11 O12 O13 N14 |
3.164 −0.423 0.452 0.518 −0.566 −0.293 0.714 −0.835 0.340 1.821 −1.250 −1.266 −0.924 |
2.066 −0.496 0.476 0.723 −0.832 −0.069 0.465 −0.716 0.398 1.506 −0.925 −1.033 −0.771 |
2.884 −0.254 0.267 0.620 −0.742 −0.066 0.373 −0.844 0.401 1.381 −1.014 −1.089 −0.713 |
- −0.054 −0.080 0.210 −0.073 −0.402 0.419 0.031 −0.240 0.955 −0.887 −0.839 −0.557 |
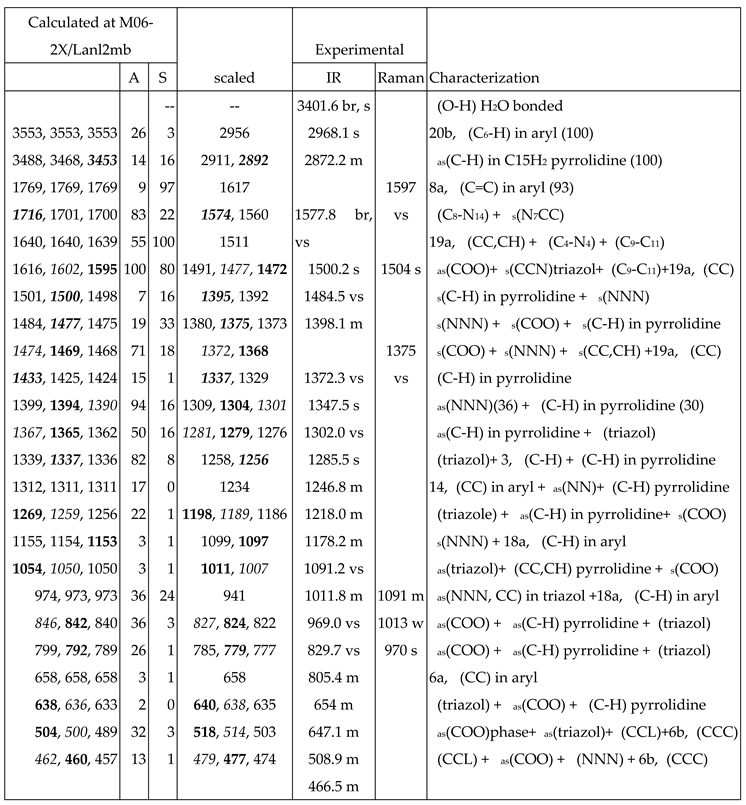
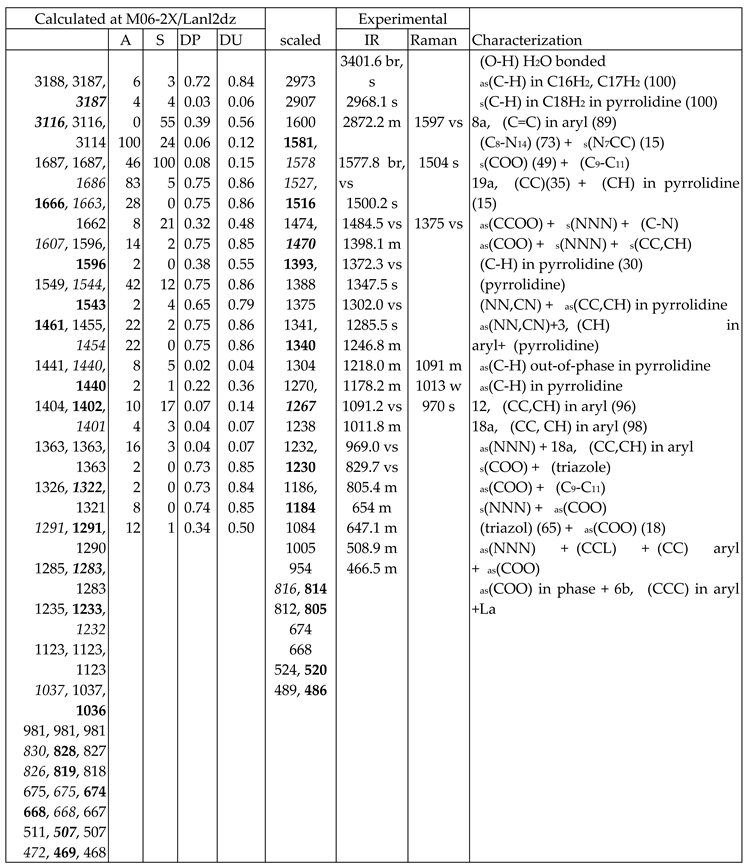
3.4. Vibrational Analysis
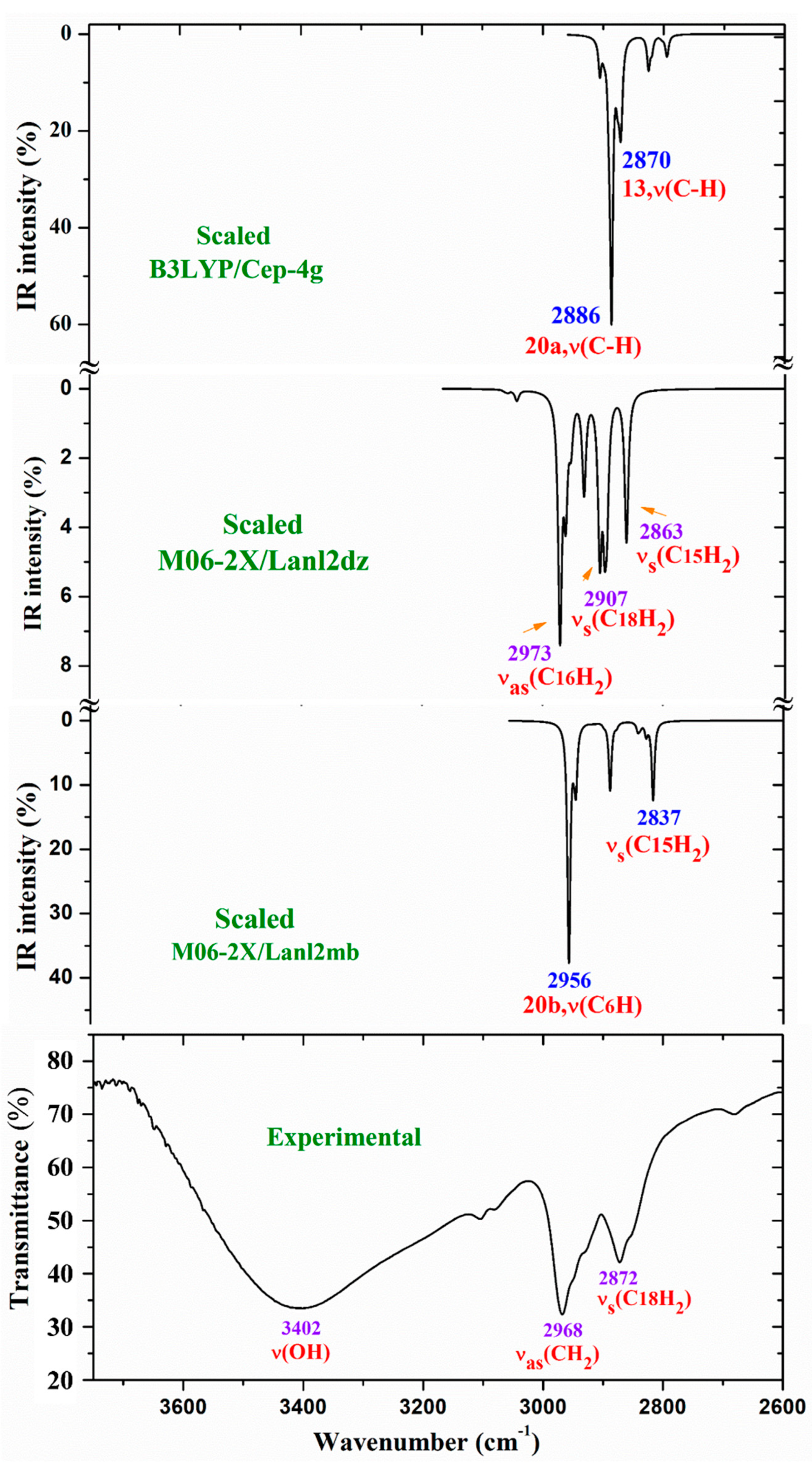
- A very broad band at ca. 3500 cm-1 is observed in the experimental spectrum, which can only correspond to the O-H stretching ν(O-H) mode of hydration water strongly H-bonded to the ligands in the La-(2b’)3 complex system. Because the spatial arrangement of the ligands in the complex, many holes appear in the structure that can be occupied by water molecules associated with the synthesis of the complex. Due to the large negative charge around the three carboxylate groups, water molecules can be H-bonded through their hydrogen atoms. Moreover, this band does not appear in the Raman spectrum, as it is expected.
- A broad and very strong band centered at 1577.8 cm-1 in the experimental spectrum, in which the in-plane bending δ(O-H) mode of these hydrated water molecules contributes to its broadness.
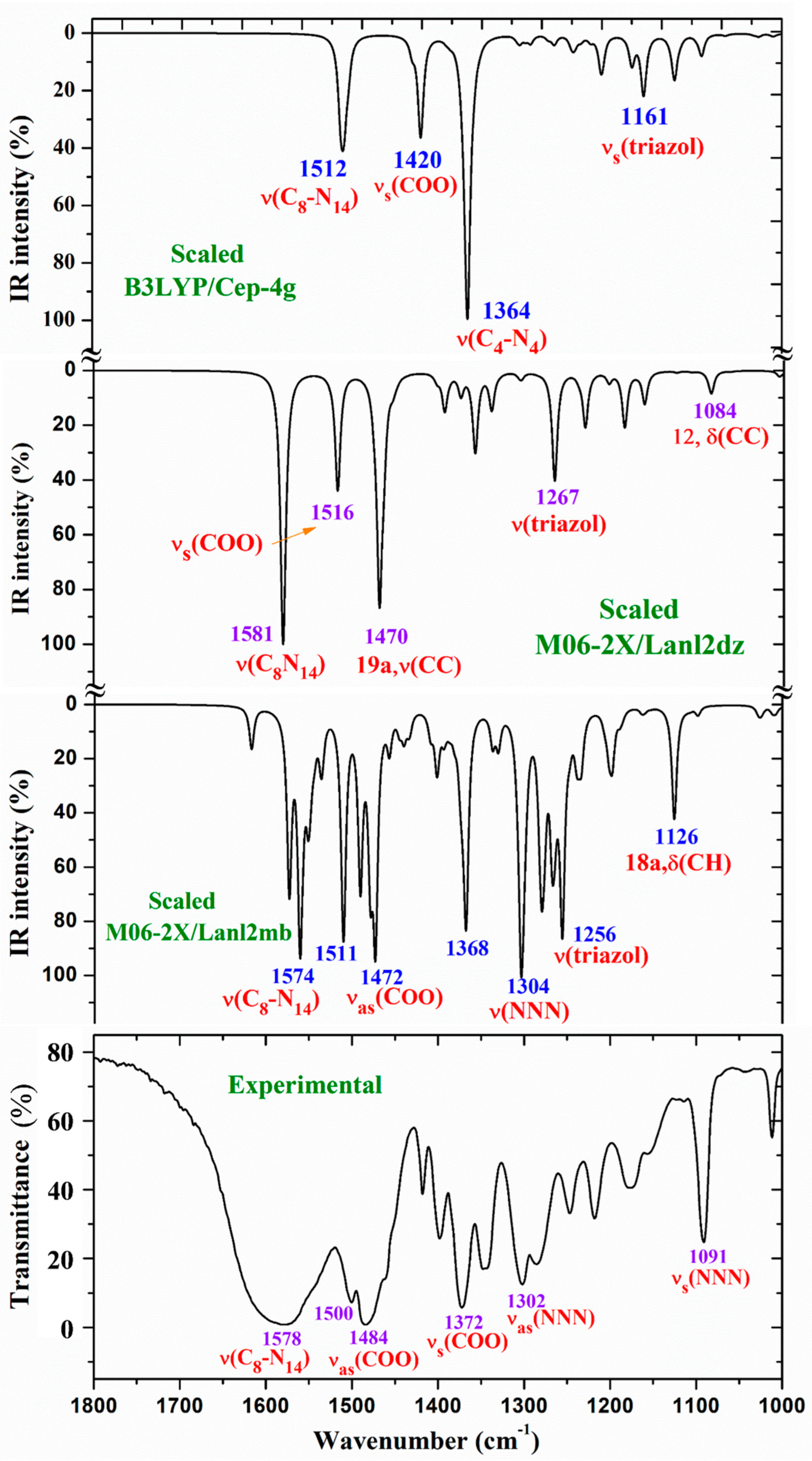
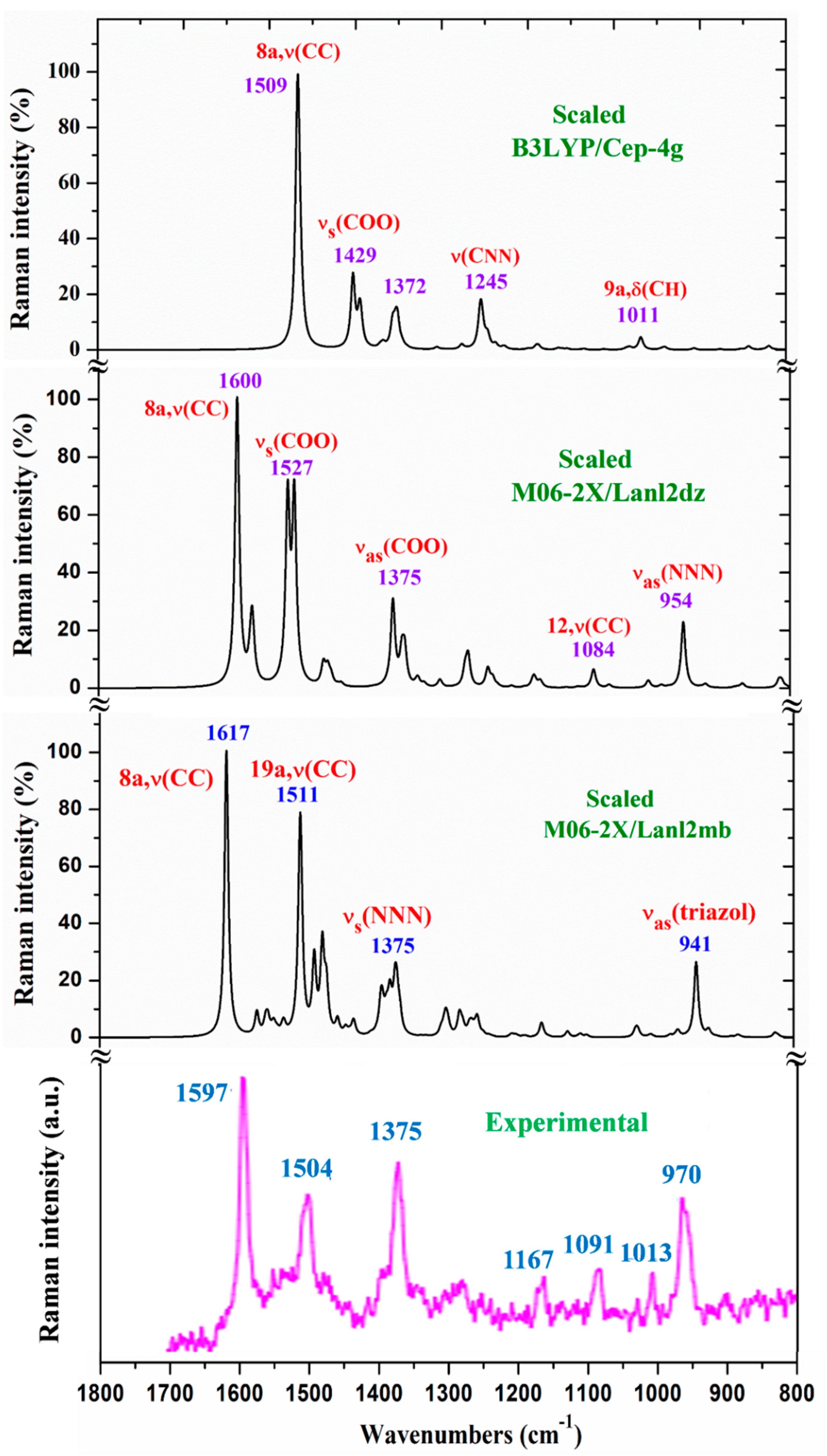
- A large similarity between the scaled spectra at the M06-2X/Lanl2dz and M06-2X/Lanl2mb levels with the experimental one appears, while the B3LYP/Cep-4g level differs remarkably. This feature is in accordance with a more symmetric and better optimized structure by M06-2X method, compared to B3LYP. The best accordance appears at the M06-2X/Lanl2mb level, although the characterization obtained at the M06-2X/Lanl2dz level was also used for the assignment of experimental spectrum.
- The coordination of the 2b ligands to the lanthanide ion noticeably changes the IR and Raman spectra. They seem different of those obtained with the 2b ligand molecule alone [22].
3.4.1. The carboxylate COO group modes
3.4.2. The triazole ring modes
3.4.3. The aryl ring modes
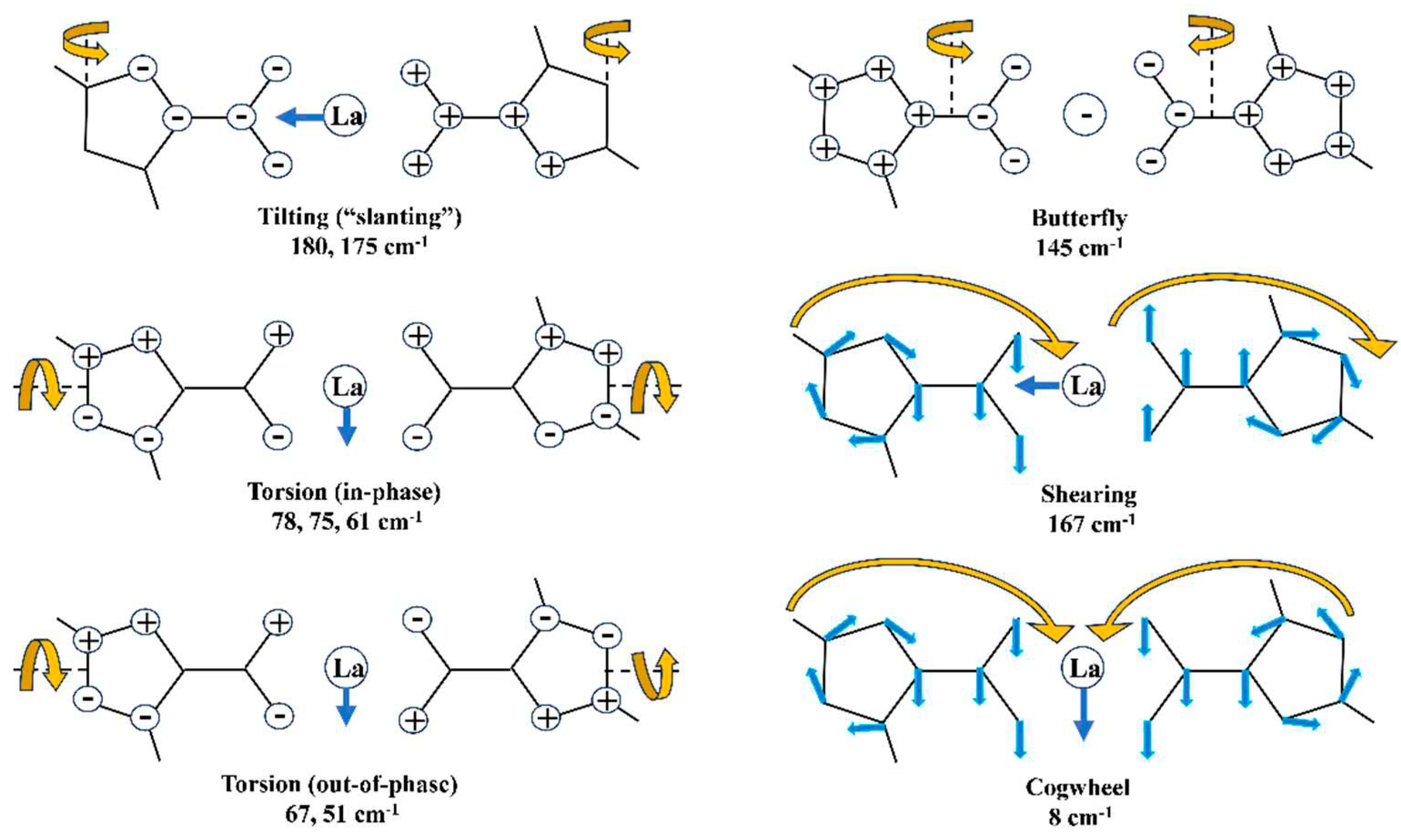
3.4.4. Low-frequency vibrations
3.5. Radical-Scavenging assays
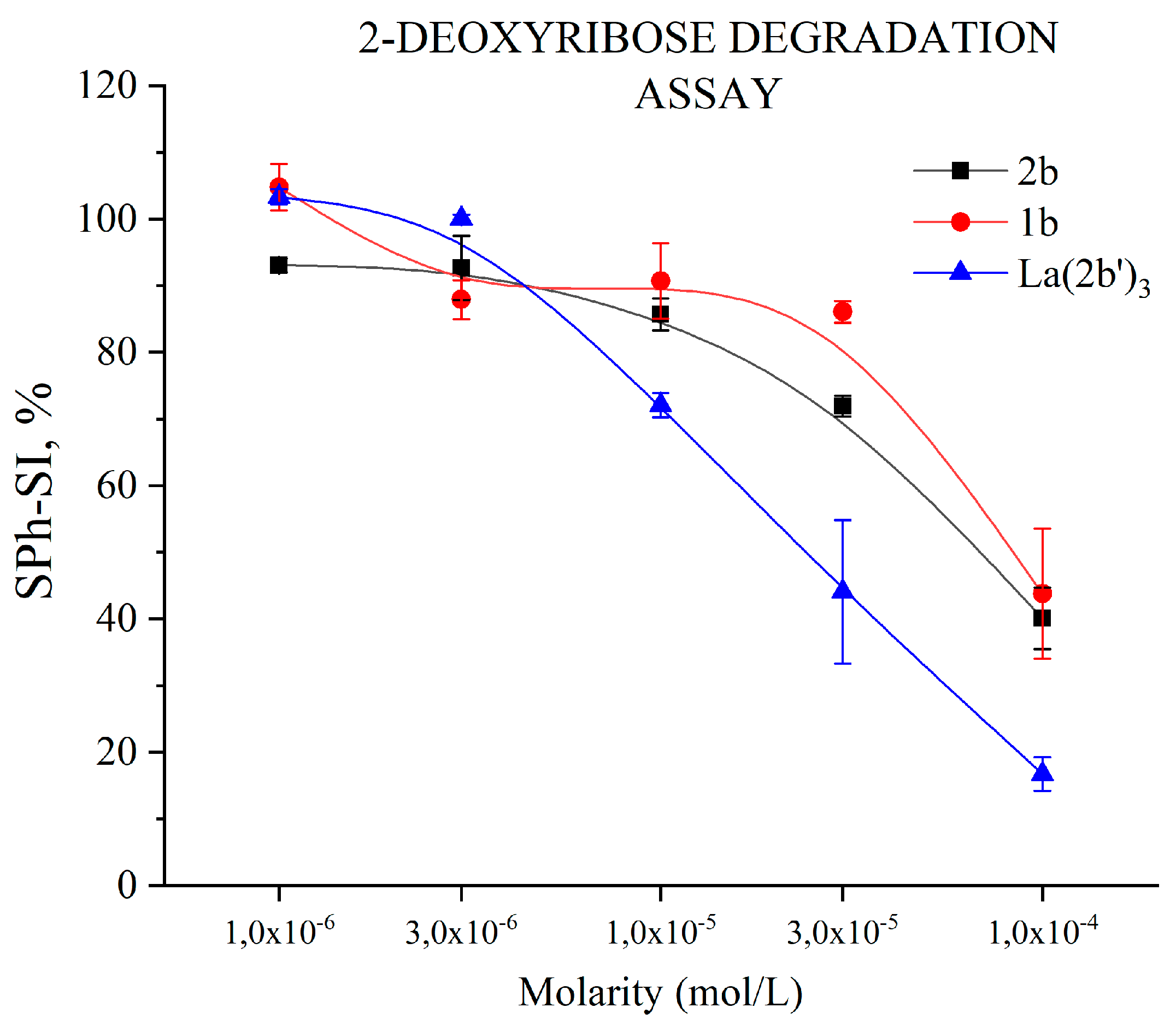
3.5.1. Impact of 1b, 2b and La(2b’)3 on 2-Deoxyribose degradation
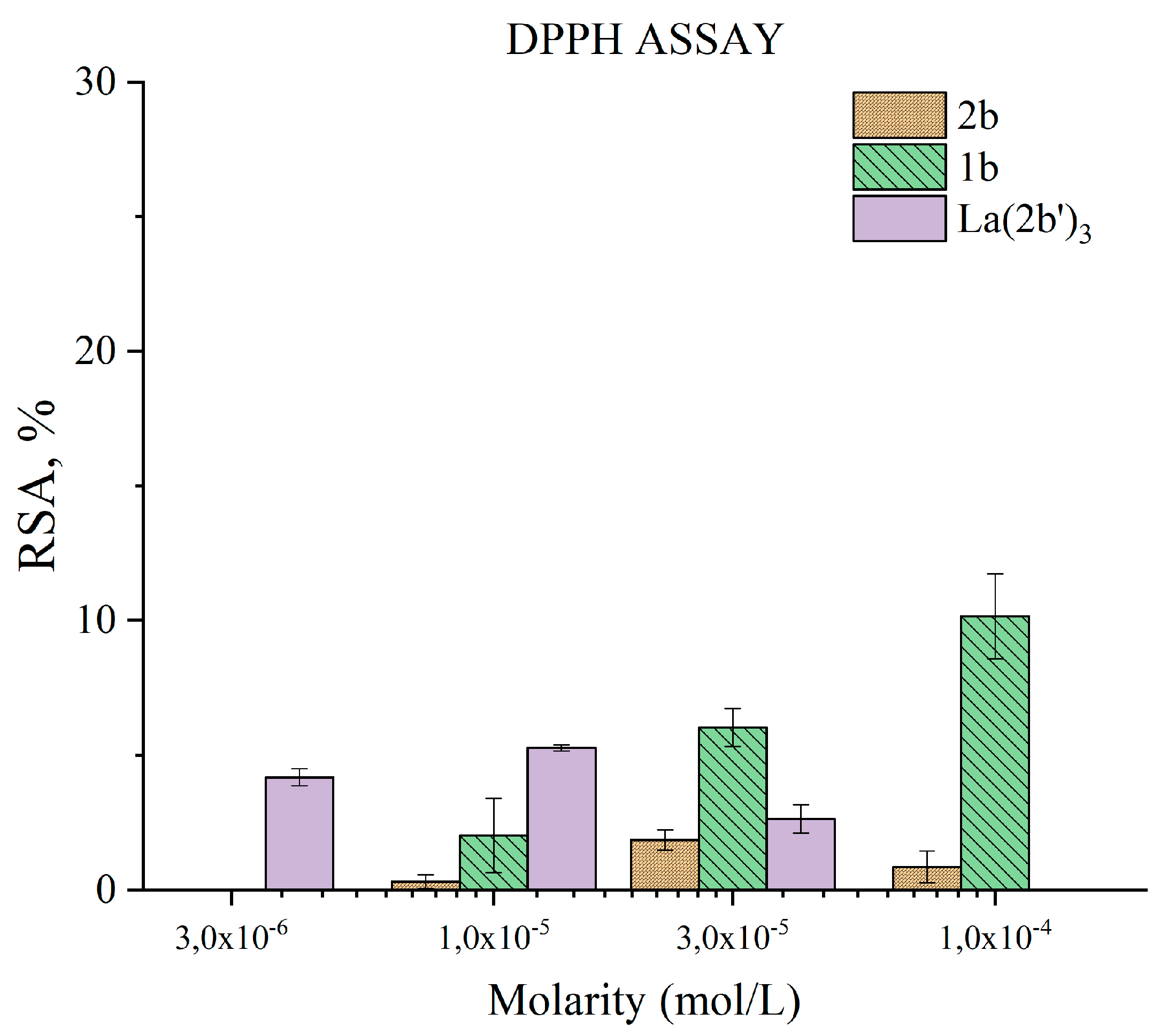
3.5.2. Impact of 1b, 2b, and La(2b’)3 on a model system containing the stable radical DPPH●
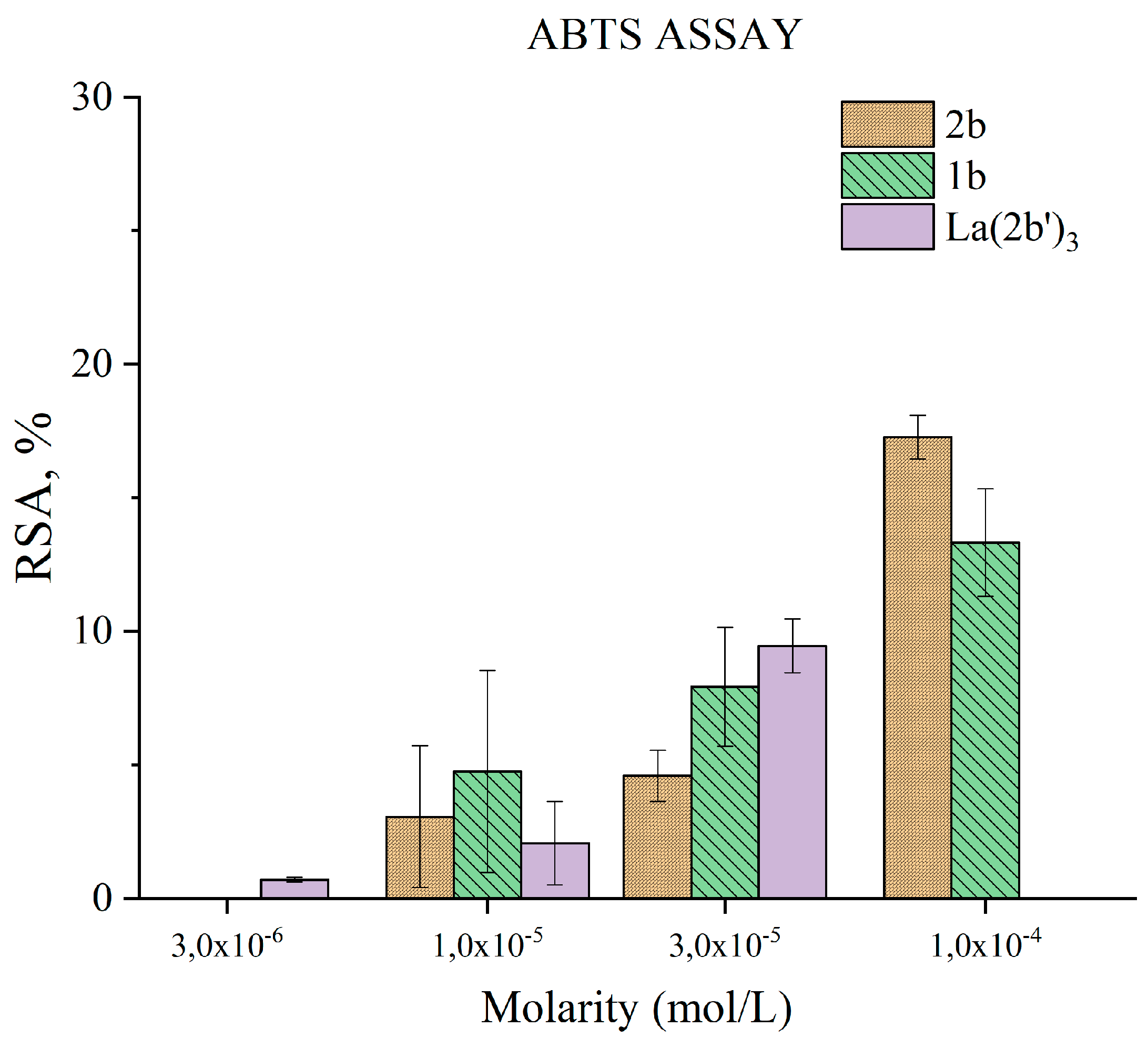
3.5.3. Impact of 2b, 1b and La(2b’)3 on a model system containing the stable radical ABTS●+
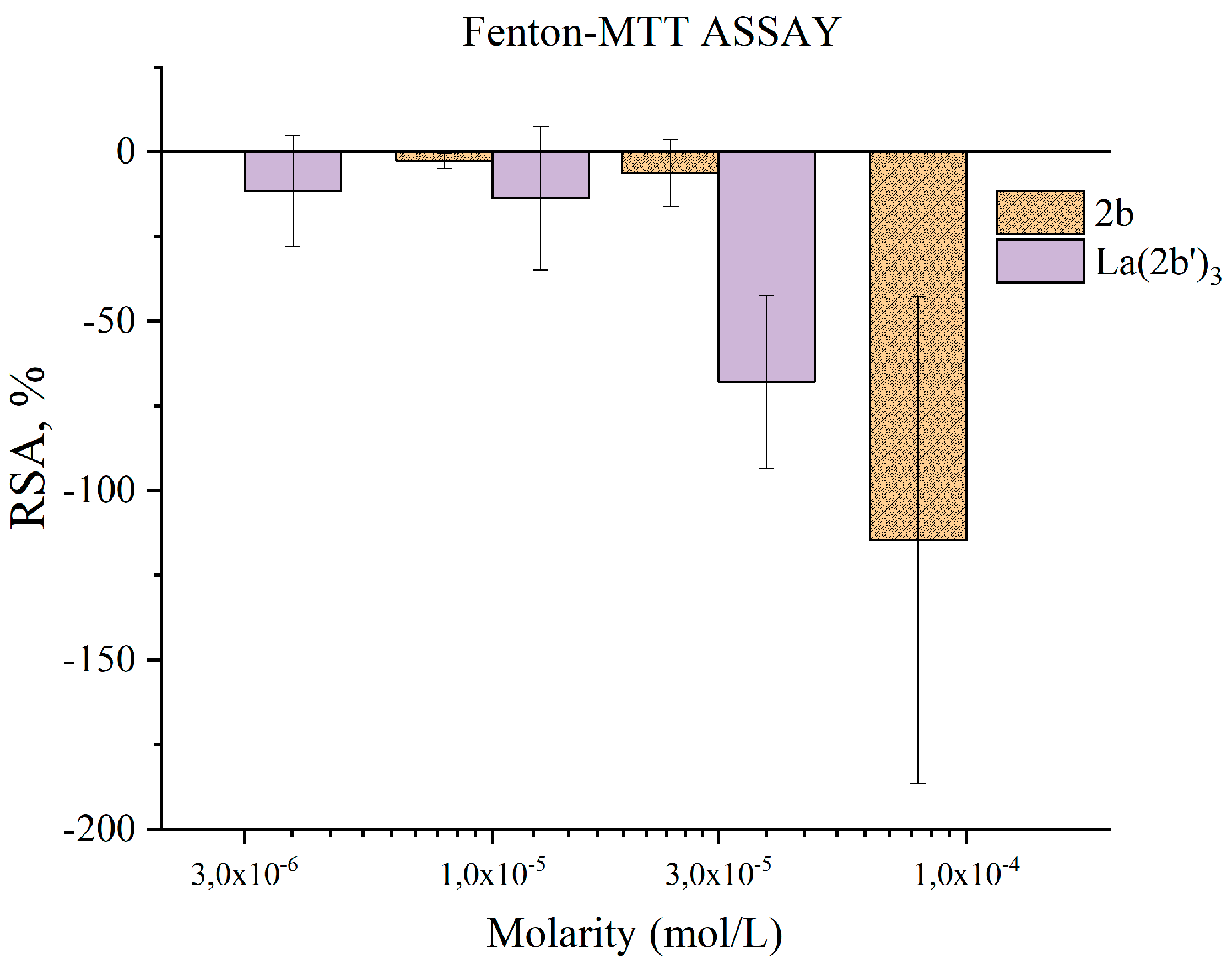
3.5.4. Impact of 2b and La(2b’)3 on MTT-formazan transformation via Fenton reaction derived hydroxyl radicals
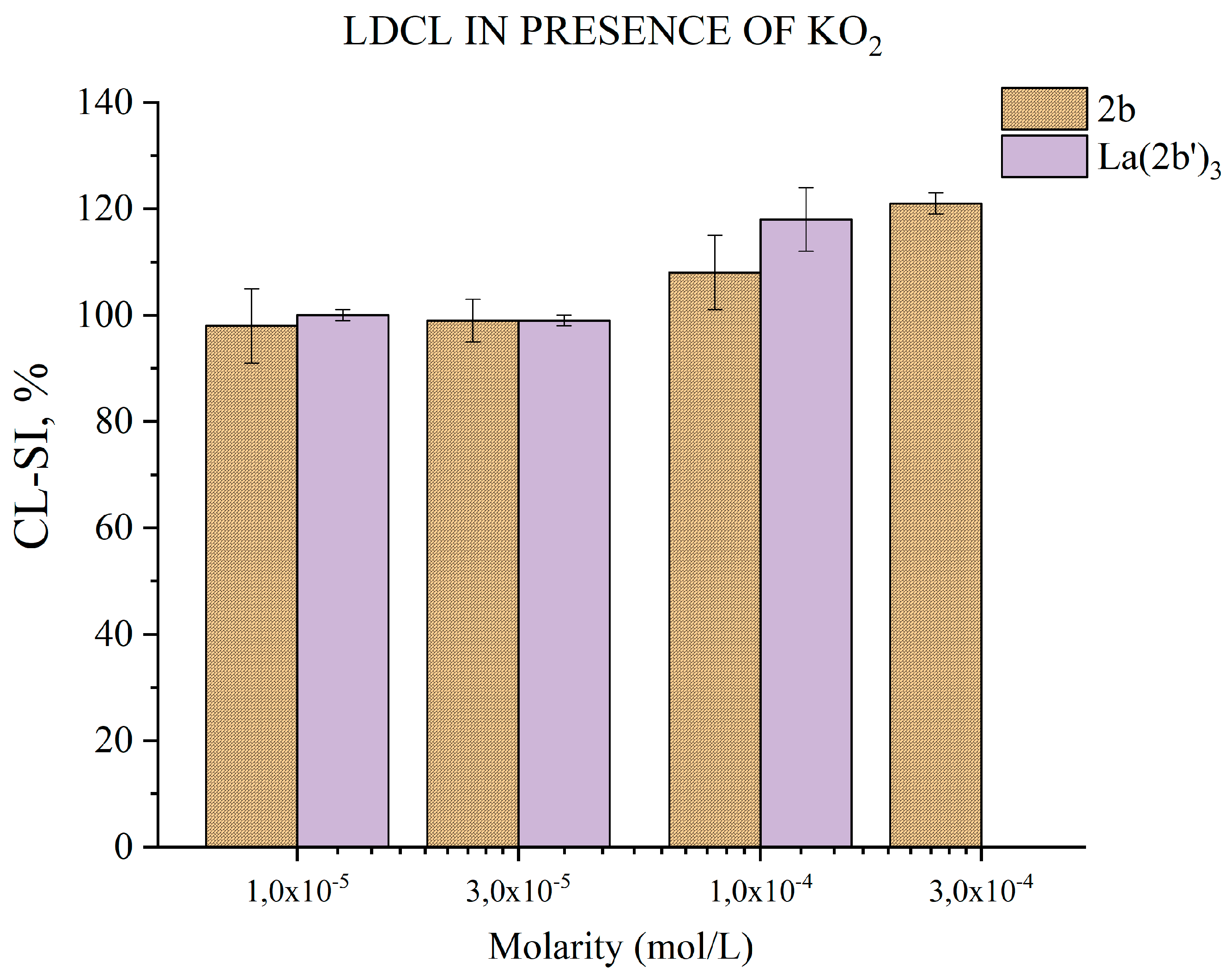
3.5.5. Impact of 2b and La(2b’)3 on LDCL in presence of KO2
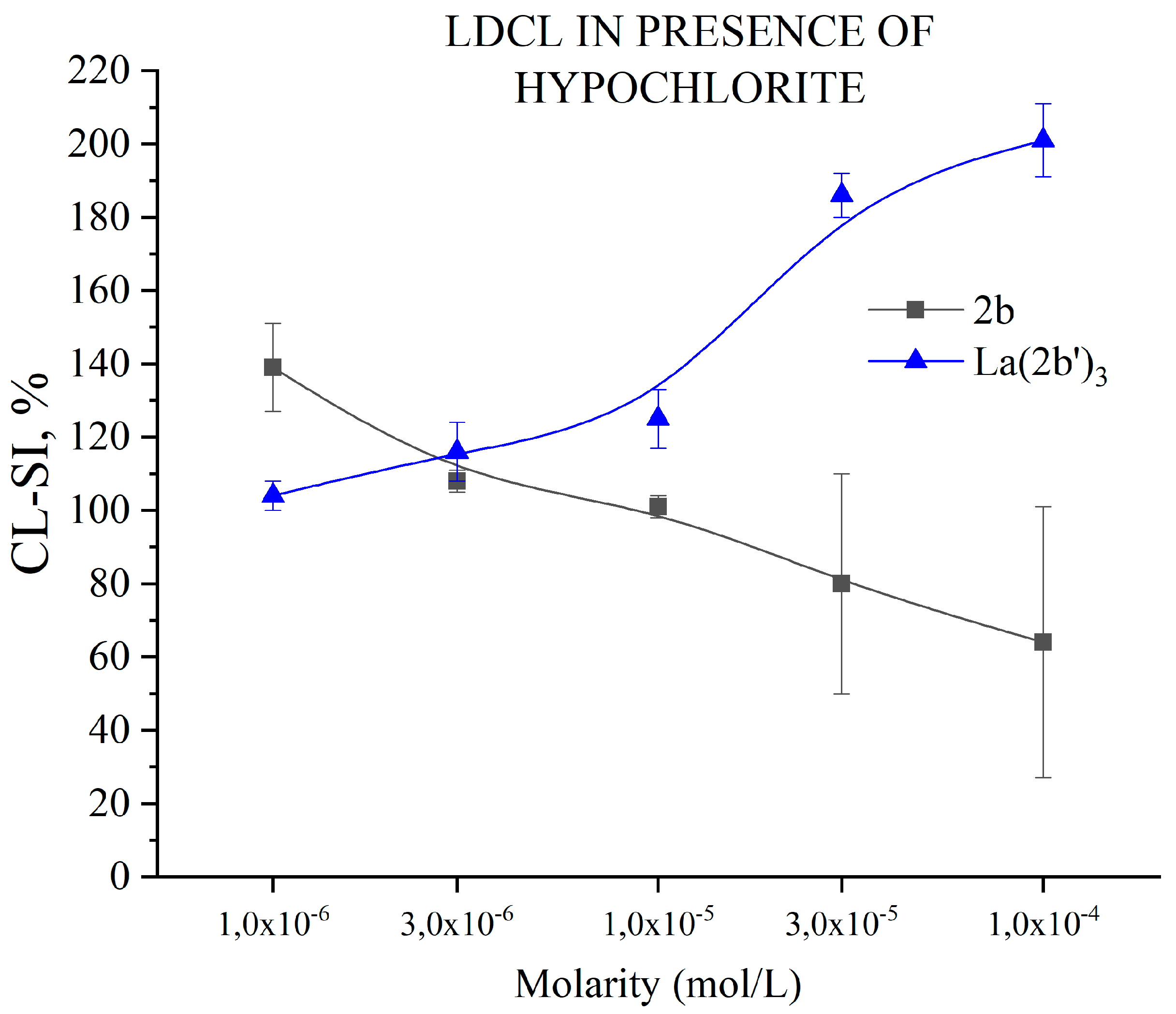
3.5.6. Impact of 2b and La(2b’)3 on LDCL in presence of NaClO
4. Discussion and Conclusions
- A structural study of the lanthanum(III) complex was carried out at three DFT levels. The starting structure optimized was that with the La(III) ion coordinated with three 2b ligands through the carboxylate group. The optimized structure at the M06-2X/lanl2dz level shows an almost symmetric arrangement. By rotation around the C9-C11 bond length another conformer can be obtained but it is less stable.
- Global chemical reactivity descriptors were calculated in the La(2b’)3 complex. The low energy gap calculated indicates a large chemical reactivity and small excitation energies to the manifold of excited states.
- The coordination of 2b ligand to La(III) ion noticeable changes its IR and Raman spectra, which seem different of those obtained with 2b alone.
- Several new scaling equations were used to improve the calculated spectra. The scaled spectra at M06-2X/Lanl2dz and M06-2X/Lanl2mb levels appear close to the experimental one, while those at the B3LYP/Cep-4g level differ remarkably. The best accordance corresponds to M06-2X/Lanl2mb level.
- The scaled wavenumbers of the most intense IR and Raman vibrations appear in good accordance by both, frequency and intensity, to the experimental most intense IR and Raman bands. Therefore, the spatial arrangement of the ligands in the synthetized La(III) complex was confirmed.
- The main low-lying molecular vibrations in the La(2b’)3 complex were characterized. Its number indicates a high flexibility of its molecular structure.
- The complex shows moderate HAT activity with the stable DPPH● radical, but the activity of ligand 2b in this model system is much lower, and almost zero. This lack of activity may be due to steric factors that hinder hydrogen transfer between the bulky DPPH● and the possible hydrogen-donating active sites in the 2b and La(2b’)3 molecules. When interacting with the small and mobile OH●, generated by UV-induced water radiolysis, both ligand and complex behave as scavengers (3·10−6 M or higher), the latter being the more potent at equimolar concentrations.
- The ligand and the complex participate in SET with the ABTS●+ radical-ion only moderately, in a concentration-dependent manner. Steric factors also seem to be at play here, as the complex appears to be less active than the ligand at three times the concentration.
- Both compounds appear to have little impact on KO2-derived superoxide. Only at the highest tested concentrations, 3·10-4 M for 2b and 1·10-4 M for La(2b’)3, do they seem to show a slight significant pro-oxidant effect.
- Though 2b and La(2b’)3 scavenge OH●, a different model system (Fenton reaction), generating the same RS yields completely opposite results. At low concentrations both compounds seem to be inactive. Increasing the molarity to 3·10-5 M and 1·10-4 M for La(2b’)3 and 2b, respectively. Yields results demonstrating that these compounds behave as pro-oxidants in this model system. As possible causes of this behavior, it is proposed that the ligand interacts with one or more components of the system, rather than the OH● generated by it.
- The La(III) ion dramatically changes the behavior of 2b toward hypochlorite once coordination has taken place. The higher the concentration, the better hypochlorite scavenger 2b seems to be. The behavior of its La(III) complex is the opposite, manifesting itself as a potent pro-oxidant at the highest concentration tested.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kostova, I.; Soni, R. Bioinorganic Chemistry. 2011.
- Goswami, A.K.; Kostova, I. Medicinal and Biological Inorganic Chemistry; Walter de Gruyter GmbH & Co KG, 2022. [Google Scholar]
- Patyal, M.; Kaur, K.; Bala, N.; Gupta, N.; Malik, A.K. Innovative Lanthanide Complexes: Shaping the future of cancer/tumor Chemotherapy. Journal of Trace Elements in Medicine and Biology 2023, 127277. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S. Applications of rare earth elements in cancer: Evidence mapping and scientometric analysis. Frontiers in Medicine 2022, 9, 946100. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Bettinelli, M.; Boffi, A.; Botta, M.; De Simone, G.; Luchinat, C.; Marengo, E.; Mei, H.; Aime, S. Rare earth elements (REE) in biology and medicine. Rendiconti Lincei. Scienze Fisiche e Naturali 2020, 31, 821–833. [Google Scholar] [CrossRef]
- Bao, G. Lanthanide complexes for drug delivery and therapeutics. Journal of Luminescence 2020, 228, 117622. [Google Scholar] [CrossRef]
- Fouad, R. Synthesis and characterization of lanthanide complexes as potential therapeutic agents. Journal of Coordination Chemistry 2020, 73, 2015–2028. [Google Scholar] [CrossRef]
- Paswan, S.; Anjum, A.; Yadav, N.; Jaiswal, N.; Singh, R.K.P. Synthesis, thermal, photo-physical, and biological properties of mononuclear Yb3+, Nd3+, and Dy3+ complexes derived from Schiff base ligands. Journal of Coordination Chemistry 2020, 73, 686–701. [Google Scholar] [CrossRef]
- Taha, Z.A.; Hijazi, A.K.; Al Momani, W.M. Lanthanide complexes of the tridentate Schiff base ligand salicylaldehyde-2-picolinoylhydrazone: Synthesis, characterization, photophysical properties, biological activities and catalytic oxidation of aniline. Journal of Molecular Structure 2020, 1220, 128712. [Google Scholar] [CrossRef]
- Wu, H.; Pan, G.; Bai, Y.; Zhang, Y.; Wang, H.; Shi, F.; Wang, X.; Kong, J. Study on synthesis, crystal structure, antioxidant and DNA-binding of mono-, di-and poly-nuclear lanthanides complexes with bis (N-salicylidene)-3-oxapentane-1, 5-diamine. Journal of Photochemistry and Photobiology B: Biology 2014, 135, 33–43. [Google Scholar] [CrossRef]
- Zou, H.-H.; Meng, T.; Chen, Q.; Zhang, Y.-Q.; Wang, H.-L.; Li, B.; Wang, K.; Chen, Z.-L.; Liang, F. Bifunctional mononuclear dysprosium complexes: single-ion magnet behaviors and antitumor activities. Inorganic Chemistry 2019, 58, 2286–2298. [Google Scholar] [CrossRef]
- Safronov, N.E.; Kostova, I.P.; Palafox, M.A.; Belskaya, N.P. Combined NMR Spectroscopy and Quantum-Chemical Calculations in Fluorescent 1, 2, 3-Triazole-4-carboxylic Acids Fine Structures Analysis. International Journal of Molecular Sciences 2023, 24, 8947. [Google Scholar] [CrossRef]
- Alam, M.M. 1, 2, 3-Triazole hybrids as anticancer agents: A review. Archiv der Pharmazie 2022, 355, 2100158. [Google Scholar] [CrossRef] [PubMed]
- Peica, N.; Kostova, I.; Kiefer, W. Theoretical and experimental studies on binding mode of 3, 5-pyrazoledicarboxylic acid in its new La (III) complex. Chemical physics 2006, 325, 411–421. [Google Scholar] [CrossRef]
- Hrimla, M.; Oubella, A.; Laamari, M.R.; Bahsis, L.; Ghaleb, A.; Auhmani, A.; Morjani, H.; Julve Olcina, M.; Stiriba, S.E.; Itto, M.Y.A. Click synthesis, anticancer activity, and molecular docking investigation of some functional 1, 2, 3-triazole derivatives. Biointerface Research in Applied Chemistry, 2022, vol. 12, num. 6, p. 7633-7667, 2022. [Google Scholar]
- Poonia, N.; Kumar, A.; Kumar, V.; Yadav, M.; Lal, K. Recent progress in 1H-1, 2, 3-triazoles as potential antifungal agents. Current Topics in Medicinal Chemistry 2021, 21, 2109–2133. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Sun, X.; Li, W.; Hou, G.; Gao, F. 1, 2, 3-Triazole-containing compounds as anti–lung cancer agents: Current developments, mechanisms of action, and structure–activity relationship. Frontiers in pharmacology 2021, 12, 661173. [Google Scholar] [CrossRef]
- Dong, G.; Jiang, Y.; Zhang, F.; Zhu, F.; Liu, J.; Xu, Z. Recent updates on 1, 2, 3-, 1, 2, 4-, and 1, 3, 5-triazine hybrids (2017–present): The anticancer activity, structure–activity relationships, and mechanisms of action. Archiv der Pharmazie 2023, 356, 2200479. [Google Scholar] [CrossRef]
- Wu, X.; Wang, J.; Xia, S.; Cheng, S.; Shi, Y. 1, 2, 3-Triazole Derivatives with Anti-breast Cancer Potential. Current Topics in Medicinal Chemistry 2022, 22, 1406–1425. [Google Scholar] [CrossRef]
- Slavova, K.I.; Todorov, L.T.; Belskaya, N.P.; Palafox, M.A.; Kostova, I.P. Developments in the application of 1, 2, 3-triazoles in cancer treatment. Recent Patents on Anti-Cancer Drug Discovery 2020, 15, 92–112. [Google Scholar] [CrossRef]
- Kostova, I.; Valcheva-Traykova, M. New samarium (III) complex of 5-aminoorotic acid with antioxidant activity. Applied Organometallic Chemistry 2015, 29, 815–824. [Google Scholar] [CrossRef]
- Palafox, M.A.; Belskaya, N.P.; Kostova, I.P. Study of the Molecular Architectures of 2-(4-Chlorophenyl)-5-(Pyrrolidin-1-Yl)-2H-1, 2, 3-Triazole-4-Carboxylic Acid as the Potential Anticancer Drug by Their Vibrational Spectra and Quantum Chemical Calculations. 2023.
- Rogachev, A.; Kuzmina, N.; Nemukhin, A. Theoretical modeling of the heterobimetallic complex [La (pta) 3Cu (salen)] and its precursors. Journal of alloys and compounds 2004, 374, 335–338. [Google Scholar] [CrossRef]
- Mehring, M.; Mansfeld, D.; Schürmann, M. The Stereochemical Activity of the Lone Pair in [Bi (NO3) 3 {(iPrO) 2 (O) PCH2P (O)(OiPr) 2} 2]—Comparison of Bismuth, Lanthanum and Praseodymium Nitrate Complexes. Zeitschrift für anorganische und allgemeine Chemie 2004, 630, 452–461. [Google Scholar] [CrossRef]
- Mishra, V.R.; Sekar, N. Photostability of coumarin laser dyes-a mechanistic study using global and local reactivity descriptors. Journal of fluorescence 2017, 27, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Chemical hardness and density functional theory. Journal of Chemical Sciences 2005, 117, 369–377. [Google Scholar] [CrossRef]
- Varsányi, G.; Láng, L.; Kovner, M.A.e.; Lempert, K. Assignment for vibrational spectra of seven hundred benzene derivatives. (No Title), 1974. [Google Scholar]
- Tammer, M. G. Sokrates: Infrared and Raman characteristic group frequencies: tables and charts: Wiley, Chichester, 2004. ISBN 0-470-09307-2, 347 pages, paperback; US $60. 2004.
- George, S. Infrared and Raman characteristic group frequencies: tables and charts. Wiley, Chichester 2001, 82, 85–87. [Google Scholar]
- Aziz, S.G.; Elroby, S.A.; Alyoubi, A.; Osman, O.I.; Hilal, R. Experimental and theoretical assignment of the vibrational spectra of triazoles and benzotriazoles. Identification of IR marker bands and electric response properties. Journal of molecular modeling 2014, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Törnkvist, C.; Bergman, J.; Liedberg, B. Geometry and vibrations of the 1, 2, 3-triazole anion. A theoretical and experimental study. The Journal of Physical Chemistry 1991, 95, 3119–3123. [Google Scholar] [CrossRef]
- El-Azhary, A.; Suter, H.; Kubelka, J. Experimental and theoretical investigation of the geometry and vibrational frequencies of 1, 2, 3-triazole, 1, 2, 4-triazole, and tetrazole anions. The Journal of Physical Chemistry A 1998, 102, 620–629. [Google Scholar] [CrossRef]
- Shishkin, O.V.; Pelmenschikov, A.; Hovorun, D.M.; Leszczynski, J. Theoretical analysis of low-lying vibrational modes of free canonical 2-deoxyribonucleosides. Chemical Physics 2000, 260, 317–325. [Google Scholar] [CrossRef]
- Hovorun, D.; Mishchuk, Y.R.; Yurenko, Y.P. Low-frequency Raman spectra of polycrystalline ribonucleosides. Biopolymers and Cell 2002, 18, 219–226. [Google Scholar] [CrossRef]
- Martel, P.; Hennion, B.; Durand, D.; Calmettes, P. Low-frequency vibrations of a nucleoside analog. Journal of Biomolecular Structure and Dynamics 1994, 12, 401–411. [Google Scholar] [CrossRef]
- Pelmenschikov, A.; Hovorun, D.M.; Shishkin, O.V.; Leszczynski, J. A density functional theory study of vibrational coupling between ribose and base rings of nucleic acids with ribosyl guanosine as a model system. The Journal of Chemical Physics 2000, 113, 5986–5990. [Google Scholar] [CrossRef]
- Palafox, M.A.; Nunez, J.; Gil, M. Theoretical quantum chemical study of benzoic acid: Geometrical parameters and vibrational wavenumbers. International journal of quantum chemistry 2002, 89, 1–24. [Google Scholar] [CrossRef]
- Zelsmann, H.; Mielke, Z. Far-infrared spectra of benzoic acid. Chemical physics letters 1991, 186, 501–508. [Google Scholar] [CrossRef]
- Todorov, L.; Hristova, N.; Belskaya, N.; Kostova, I. Antioxidant properties of a novel triazole ligand. Macedonian Pharmaceutical Bulletin 2022, 68, 2. [Google Scholar] [CrossRef]
- Galano, A. Free radicals induced oxidative stress at a molecular level: The current status, challenges and perspectives of computational chemistry based protocols. Journal of the Mexican Chemical Society 2015, 59, 231–262. [Google Scholar] [CrossRef]
- Kell, D.B. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC medical genomics 2009, 2, 1–79. [Google Scholar]
- Che, M.; Wang, R.; Li, X.; Wang, H.-Y.; Zheng, X.S. Expanding roles of superoxide dismutases in cell regulation and cancer. Drug discovery today 2016, 21, 143–149. [Google Scholar] [CrossRef]
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide dismutase 1 in health and disease: how a frontline antioxidant becomes neurotoxic. Angewandte Chemie International Edition 2021, 60, 9215–9246. [Google Scholar] [CrossRef]
- Pattison, D.; Davies, M. Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Current medicinal chemistry 2006, 13, 3271–3290. [Google Scholar] [CrossRef]
- Strzepa, A.; Pritchard, K.A.; Dittel, B.N. Myeloperoxidase: A new player in autoimmunity. Cellular immunology 2017, 317, 1–8. [Google Scholar] [CrossRef]
- Todorov, L.T.; Traykova, M.L.; Kostova, I.P. In Vitro Interaction of 5-aminoorotic Acid and Its Lanthanum (III) Complex With Superoxide and Hypochlorite Radicals. Der Pharma Chemica 2020, 12, 10. [Google Scholar]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. Journal of food science and technology 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. sci. technol 2004, 26, 211–219. [Google Scholar]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clinical biochemistry 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clinical biochemistry 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Analytical biochemistry 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Burns, W.G.; Sims, H.E. Effect of radiation type in water radiolysis. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases 1981, 77, 2803–2813. [Google Scholar] [CrossRef]
- Seminario, J.M. Modern density functional theory: a tool for chemistry; Elsevier, 1995. [Google Scholar]
- Palafox, M.A.; Iza, N. Tautomerism of the natural thymidine nucleoside and the antiviral analogue D4T. Structure and influence of an aqueous environment using MP2 and DFT methods. Physical Chemistry Chemical Physics 2010, 12, 881–893. [Google Scholar] [CrossRef]
- Brovarets’, O.h.O.; Hovorun, D.M. Prototropic tautomerism and basic molecular principles of hypoxanthine mutagenicity: An exhaustive quantum-chemical analysis. Journal of Biomolecular Structure and Dynamics 2013, 31, 913–936. [Google Scholar] [CrossRef]
- Riley, K.E.; Hobza, P. Noncovalent interactions in biochemistry. Wiley Interdisciplinary Reviews: Computational Molecular Science 2011, 1, 3–17. [Google Scholar] [CrossRef]
- Riley, K.E.; Pitonák, M.; Jurecka, P.; Hobza, P. Stabilization and structure calculations for noncovalent interactions in extended molecular systems based on wave function and density functional theories. Chemical Reviews 2010, 110, 5023–5063. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Applications and validations of the Minnesota density functionals. Chemical Physics Letters 2011, 502, 1–13. [Google Scholar] [CrossRef]
- Alcolea Palafox, M. Scaling factors for the prediction of vibrational spectra. I. Benzene molecule. International Journal of Quantum Chemistry 2000, 77, 661–684. [Google Scholar] [CrossRef]
- Palafox, M.A. DFT computations on vibrational spectra: Scaling procedures to improve the wavenumbers. Physical Sciences Reviews 2018, 3, 20170184. [Google Scholar] [CrossRef]
- Frisch, M.e.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16, revision C. 01. 2016. [Google Scholar]
- Srivastav, G.; Yadav, B.; Yadav, R.K.; Yadav, R. DFT studies of molecular structures conformers and vibrational characteristics of sulfanilamide. Computational and Theoretical Chemistry 2019, 1167, 112588. [Google Scholar] [CrossRef]

| Control | Sample | Blank | |
|---|---|---|---|
| Tested compound | no | 200 L | 200 L |
| MTT | 200 L | 200 L | 200 L |
| Fe2+/H2O2/Na2-EDTA | 100 L | 100 L | no |
| Ascorbic acid | 100 L | 100 L | no |
| Bi-destilled water | up to 2.0 mL | дo 2.0 mL | дo 2.0 mL |
| Blank | Control | Sample | |
|---|---|---|---|
| Tested compound | 200 L | no | 200 L |
| DPPH | no | 1800 L | 1800 L |
| Ethanol | 1800 L | no | no |
| Bi-distilled water | no | 200 L | no |
| Blank | Control | Sample | |
|---|---|---|---|
| Tested compound | 100 L | no | 100 L |
| R1 | 860 L | 860 L | 860 L |
| R2 | no | 40 L | 40 L |
| Bi-destilled water | 40 L | 100 L | no |
 |
| Molecular properties | M06-2X | B3LYP | |
|---|---|---|---|
| Lanl2dz | Lanl2MB | Cep-4G | |
| Rotational constants: A (GHz) B C |
0.019 0.017 0.012 |
0.040 0.015 0.014 |
0.035 0.013 0.012 |
| Cv (cal/mol·K) S (cal/mol·K) |
202.97 348.72 |
200.6 345.3 |
229.86 376.53 |
| Dipole moment (Debye) | 4.085 | 3.323 | 4.377 |
| HOMO LUMO Eg IP EA S |
-0.262 -0.052 0.210 0.262 0.052 0.157 0.105 0.052 |
-0.189 0.009 0.198 0.189 -0.009 0.090 0.099 0.049 |
-0.253 -0.146 0.107 0.253 0.146 0.199 0.054 0.027 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





