Submitted:
19 September 2023
Posted:
19 September 2023
You are already at the latest version
Abstract
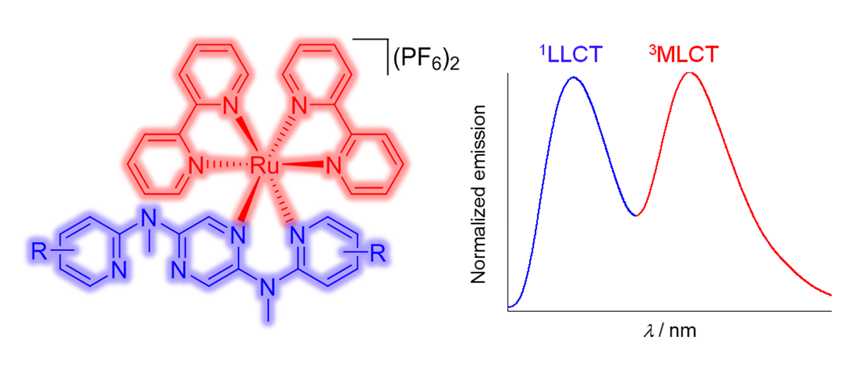
Keywords:
1. Introduction
2. Experimental Section
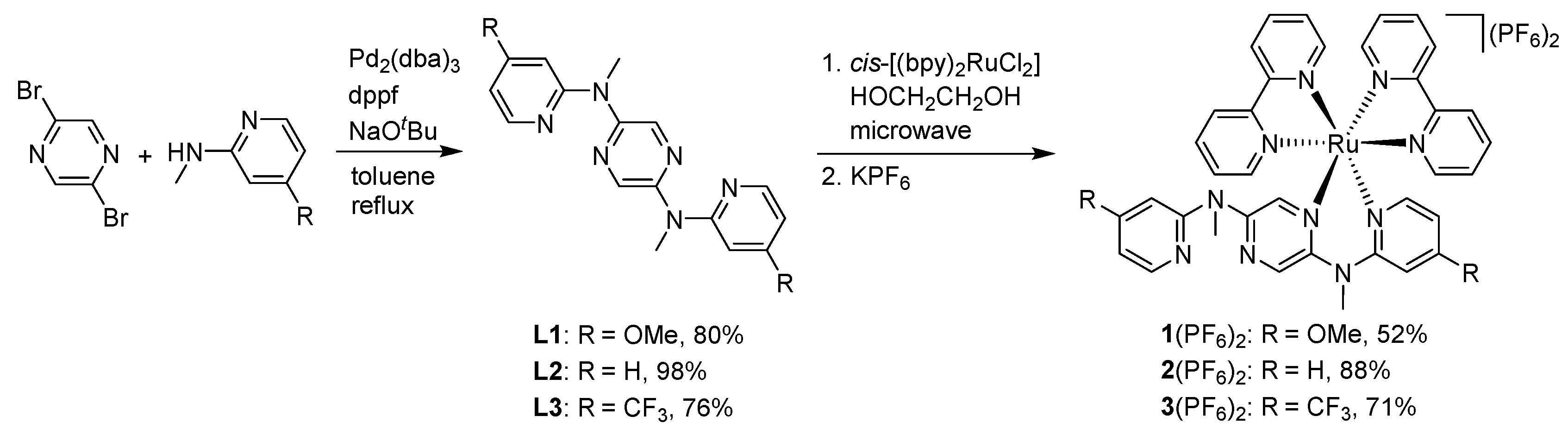
2.1. Synthetic Details
2.1.1. Synthesis of 2,5-di(N-methyl-N′-(4-methoxy-2-pyridyl)amino)pyrazine (L1)
2.1.2. Synthesis of 2,5-di(N-methyl-N′-(4-(trifluoromethyl)-2-pyridyl)amino)pyrazine (L3)
2.1.3. Synthesis of 1(PF6)2
2.1.4. Synthesis of 3(PF6)2
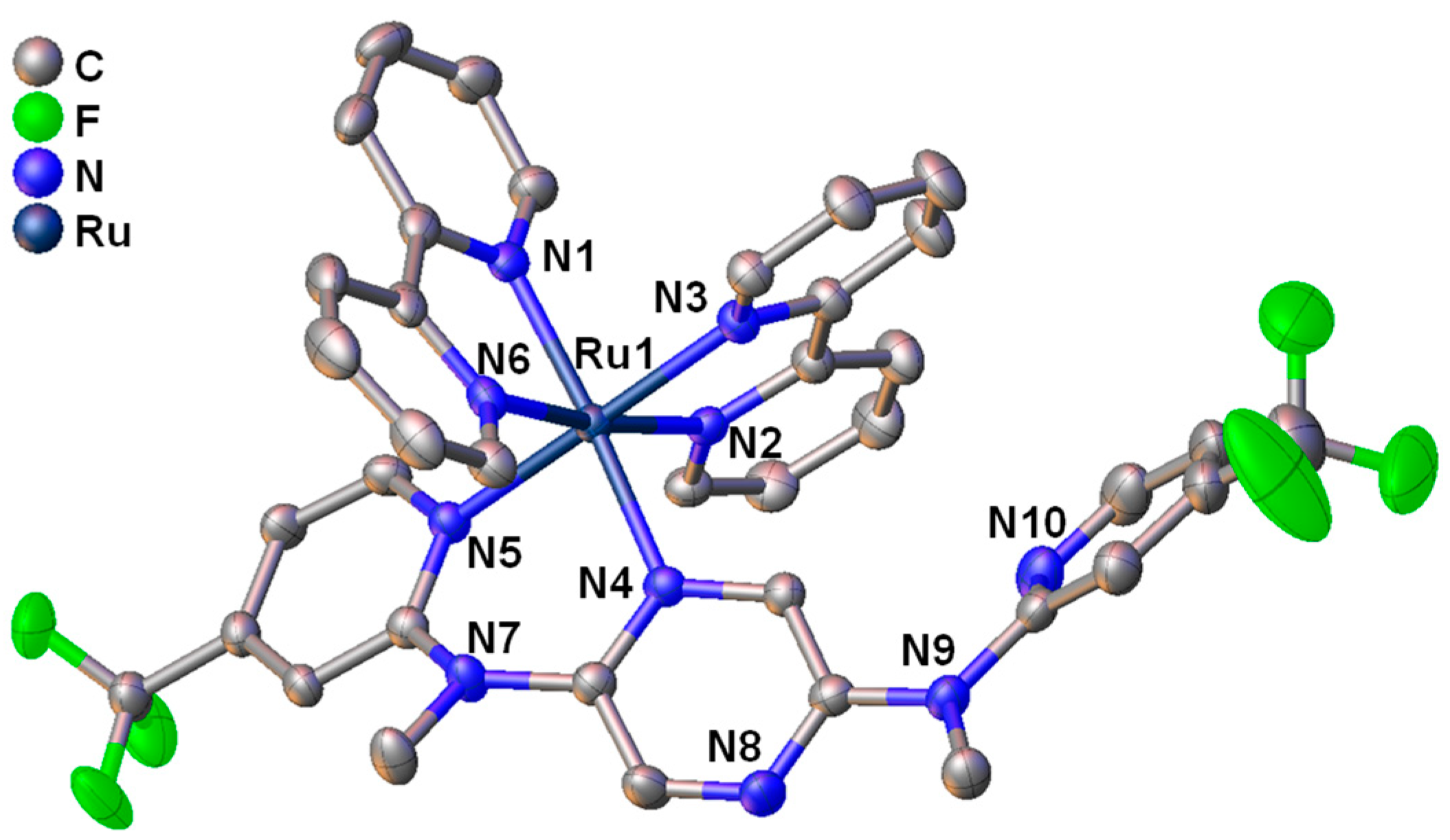
2.2. X-ray Crystallography
2.3. Spectroscopic Measurements
2.4. Electrochemical Measurements
2.5. DFT and TDDFT Calculations
2.6. HPLC Analysis
3. Results and Discussions
3.1. Studies on Preparation and Single Crystal X-ray Analysis
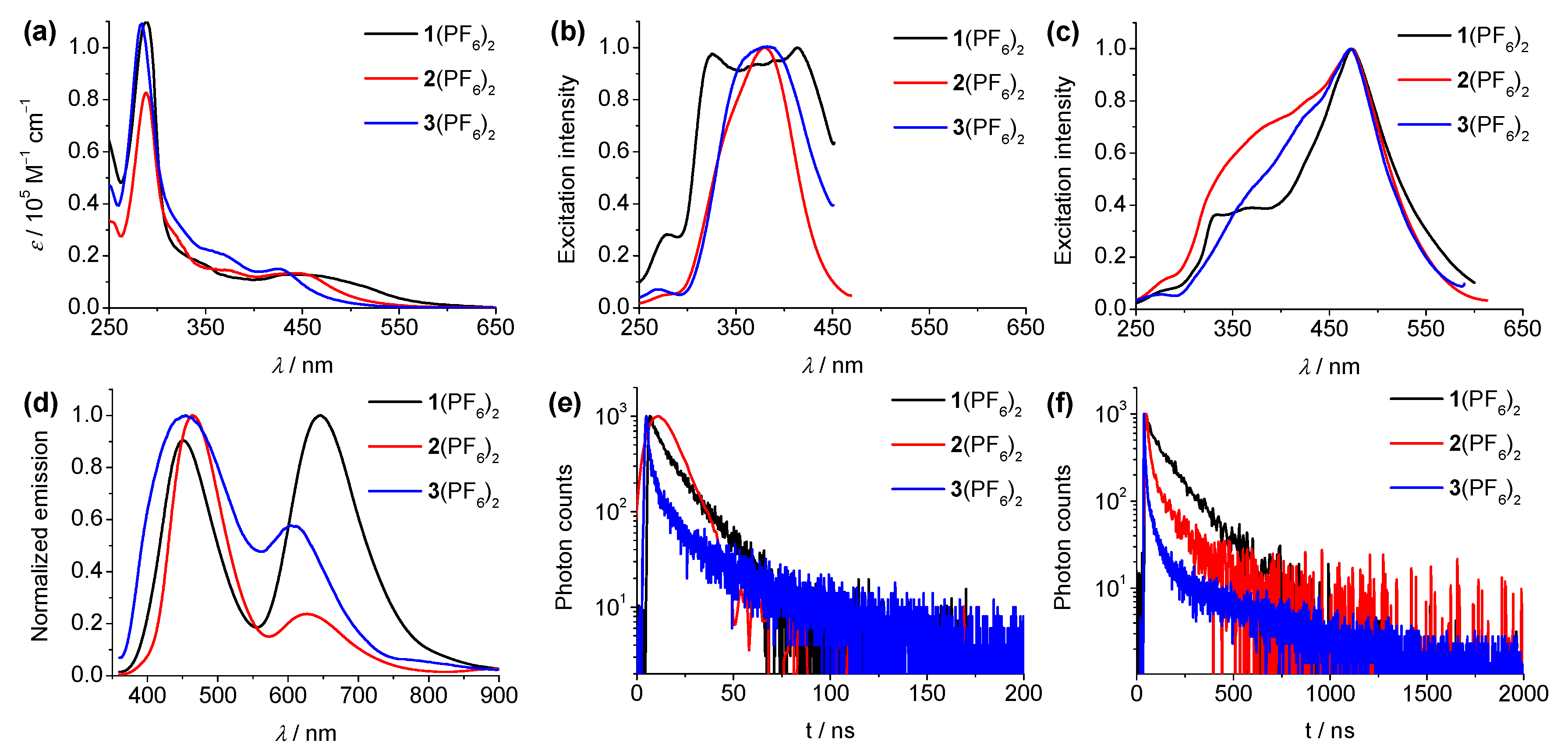
3.2. Spectroscopic Studies
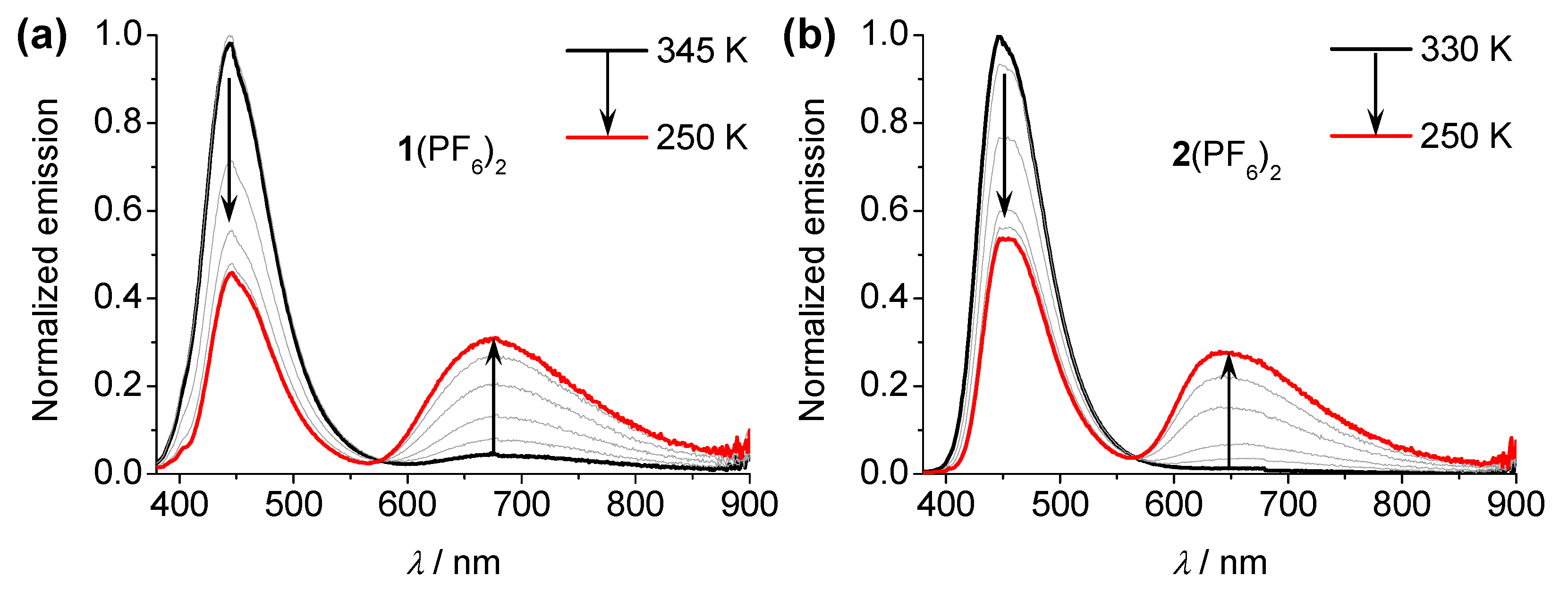
3.3. Temperature-Dependent Emission Spectral Studies
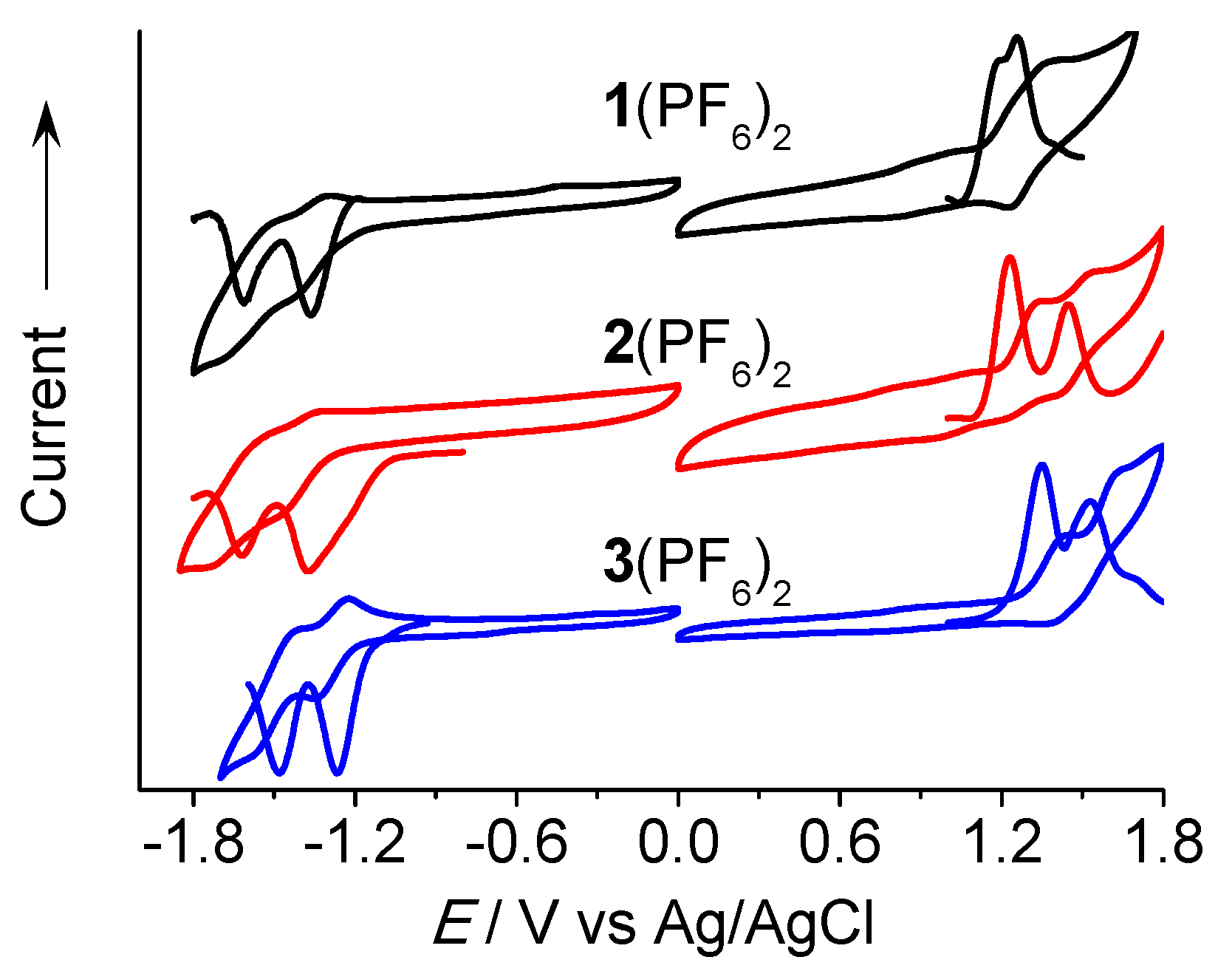
3.4. Electrochemical Studies
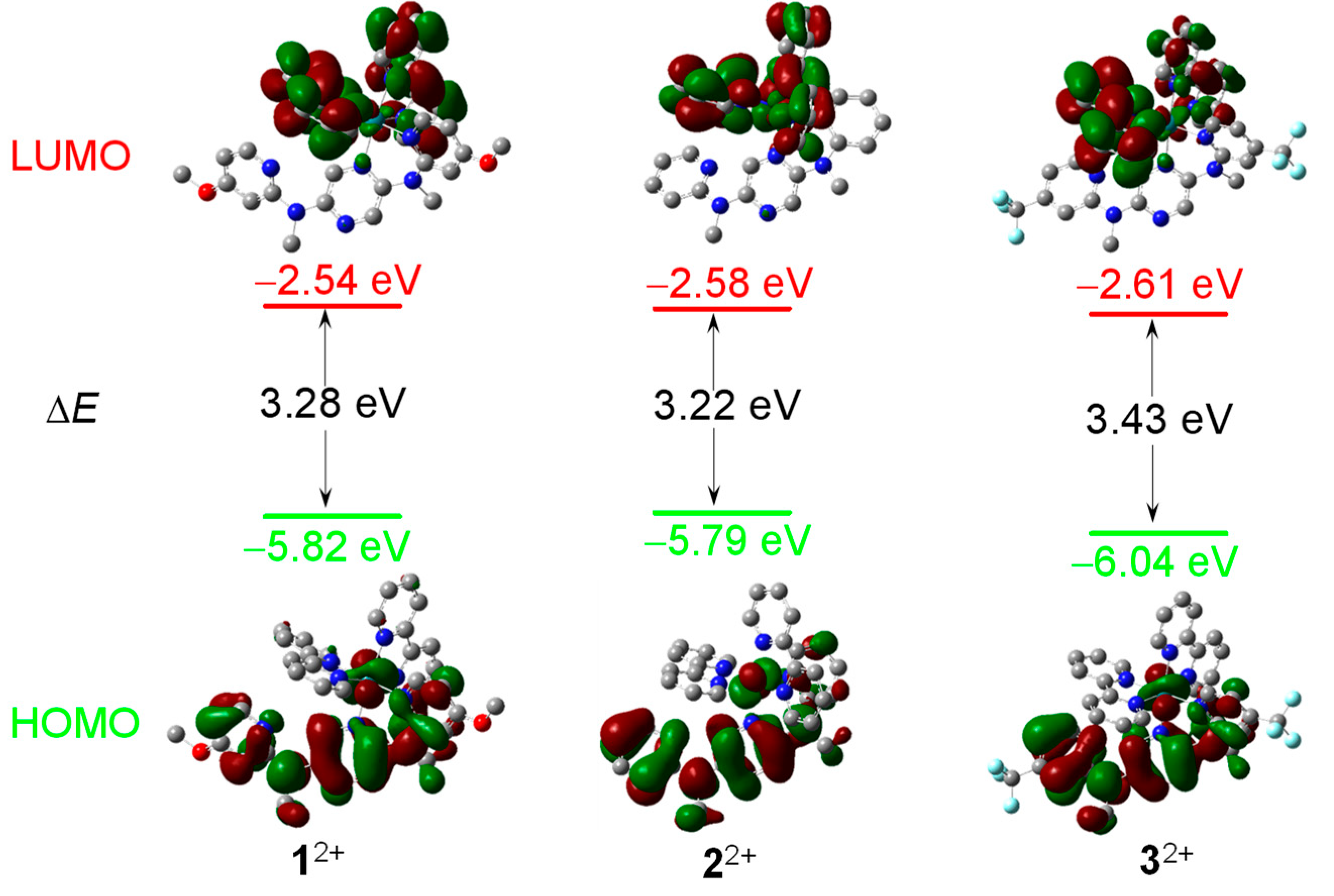
3.5. DFT and TD-DFT calculations
4. Conclusions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]
- Behera, S.K.; Park, S.Y.; Gierschner, J. Dual Emission: Classes, Mechanisms, and Conditions. Angew. Chem. Int. Ed. 2020, 60, 22624–22638. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-S.; Fu, H.; Zhang, Y.; Jakubek, Z.J.; Tao, Y.; Wang, S. A Dual Emissive BODIPY Dye and Its Use in Functionalizing Highly Monodispersed PbS Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 11658–11662. [Google Scholar] [CrossRef] [PubMed]
- Swamy P, C.A.; Mukherjee, S.; Thilagar, P. Dual emissive borane–BODIPY dyads: molecular conformation control over electronic properties and fluorescence response towards fluoride ions. Chem. Commun. 2013, 49, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Fine-Tuning Dual Emission and Aggregation-Induced Emission Switching in NPI-BODIPY Dyads. Chem. – A Eur. J. 2014, 20, 9052–9062. [Google Scholar] [CrossRef]
- Li, R.; Gong, Z.-L.; Tang, J.-H.; Sun, M.-J.; Shao, J.-Y.; Zhong, Y.-W.; Yao, J. Triarylamines with branched multi-pyridine groups: modulation of emission properties by structural variation, solvents, and tris(pentafluorophenyl)borane. Sci. China Chem. 2018, 61, 545–556. [Google Scholar] [CrossRef]
- Matsuo, K.; Saito, S.; Yamaguchi, S. Photodissociation of B–N Lewis Adducts: A Partially Fused Trinaphthylborane with Dual Fluorescence. J. Am. Chem. Soc. 2014, 136, 12580–12583. [Google Scholar] [CrossRef]
- Wu, P.; Hou, X.; Xu, J.-J.; Chen, H.-Y. Ratiometric fluorescence, electrochemiluminescence, and photoelectrochemical chemo/biosensing based on semiconductor quantum dots. Nanoscale 2016, 8, 8427–8442. [Google Scholar] [CrossRef]
- Ventura, B.; Durola, F.; Frey, J.; Heitz, V.; Sauvage, J.-P.; Flamigni, L. Near-infrared dual luminescence from an extended zinc porphyrin. Chem. Commun. 2012, 48, 1021–1023. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Yue, M.; Jiang, Y.-X.; Zhao, C.-H.; Liao, Y.-Y.; Lei, X.-W.; Yue, C.-Y. Combining Dual-Light Emissions to Achieve Efficient Broadband Yellowish-Green Luminescence in One-Dimensional Hybrid Lead Halides. Inorg. Chem. 2021, 60, 1491–1498. [Google Scholar] [CrossRef]
- Blakley, R.L.; Myrick, M.L.; DeArmond, M.K. Interligand and charge-transfer emission from [Ru(bpy)(HDPA)2]2+: a dual emitting ruthenium(II) complex. J. Am. Chem. Soc. 1986, 108, 7843–7844. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Li, M.; Zheng, J.; Huang, X.-C.; Li, D. A dual-emitting Cu6–Cu2–Cu6 cluster as a self-calibrated, wide-range luminescent molecular thermometer. Chem. Commun. 2014, 50, 9115–9118. [Google Scholar] [CrossRef] [PubMed]
- Glazer, E.C.; Magde, D.; Tor, Y. Ruthenium Complexes That Break the Rules: Structural Features Controlling Dual Emission. J. Am. Chem. Soc. 2007, 129, 8544–8551. [Google Scholar] [CrossRef] [PubMed]
- Keyes, T.E.; O’Connor, C.; Vos, J.G. Evidence for the presence of dual emission in a ruthenium(II) polypyridyl mixed ligand complex. Chem. Commun. 1998, 889–890. [Google Scholar] [CrossRef]
- Zambrana, J.L.; Ferloni, E.X.; Colis, J.C.; Gafney, H.D. Multiple Charge-Transfer Emissions from Different Metal−Ligand Pairs in Ruthenium Diimines. Inorg. Chem. 2007, 47, 2–4. [Google Scholar] [CrossRef]
- Song, L.-q.; Feng, J.; Wang, X.-s.; Yu, J.-h.; Hou, Y.-j.; Xie, P.-h.; Zhang, B.-w.; Xiang, J.-f.; Ai, X.-c.; Zhang, J.-p. Dual Emission from 3MLCT and 3ILCT Excited States in a New Ru(II) Diimine Complex. Inorg. Chem. 2003, 42, 3393–3395. [Google Scholar] [CrossRef]
- Kumar, S.; Hisamatsu, Y.; Tamaki, Y.; Ishitani, O.; Aoki, S. Design and Synthesis of Heteroleptic Cyclometalated Iridium(III) Complexes Containing Quinoline-Type Ligands that Exhibit Dual Phosphorescence. Inorg. Chem. 2016, 55, 3829–3843. [Google Scholar] [CrossRef]
- Kozhevnikov, D.N.; Kozhevnikov, V.N.; Shafikov, M.Z.; Prokhorov, A.M.; Bruce, D.W.; Williams, J.A.G. Phosphorescence vs Fluorescence in Cyclometalated Platinum(II) and Iridium(III) Complexes of (Oligo)thienylpyridines. Inorg. Chem. 2011, 50, 3804–3815. [Google Scholar] [CrossRef]
- Cao, Y.; Wolf, M.O.; Patrick, B.O. Dual-Emissive Platinum(II) Metallacycles with Thiophene-Containing Bisacetylide Ligands. Inorg. Chem. 2016, 55, 8985–8993. [Google Scholar] [CrossRef]
- Hudson, Z.M.; Zhao, S.-B.; Wang, R.-Y.; Wang, S. Switchable Ambient-Temperature Singlet–Triplet Dual Emission in Nonconjugated Donor–Acceptor Triarylboron–PtII Complexes. Chem. Eur. J. 2009, 15, 6131–6137. [Google Scholar] [CrossRef]
- Cheng, Y.-M.; Yeh, Y.-S.; Ho, M.-L.; Chou, P.-T.; Chen, P.-S.; Chi, Y. Dual Room-Temperature Fluorescent and Phosphorescent Emission in 8-Quinolinolate Osmium(II) Carbonyl Complexes: Rationalization and Generalization of Intersystem Crossing Dynamics. Inorg. Chem. 2005, 44, 4594–4603. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Zhao, Z.; Li, X.; Yu, X.; Huo, P.; Jin, Q.; Liu, Z.; Bian, Z.; Huang, C. Two-Coordinate Copper(I)-NHC Complexes: Novel Dual-Emissive Property and Ultralong Room Temperature Phosphorescence. Angew. Chem., Int. Ed. 2020, 59, 8210–8217. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Nagano, S.; Kinoshita, T. Dual Emission from Precious Metal-Free Luminophores Consisting of C, H, O, Si, and S/P at Room Temperature. Chem. Eur. J. 2020, 26, 5162–5167. [Google Scholar] [CrossRef] [PubMed]
- Gitlina, A.Y.; Ivonina, M.V.; Sizov, V.V.; Starova, G.L.; Pushkarev, A.P.; Volyniuk, D.; Tunik, S.P.; Koshevoy, I.O.; Grachova, E.V. A rare example of a compact heteroleptic cyclometalated iridium(iii) complex demonstrating well-separated dual emission. Dalton Trans. 2018, 47, 7578–7586. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-J.; Meng, G.; Li, Y.; Wang, N.; Chen, P.; Wang, S.; Yin, X. Millisecond Time-scale Photoluminescence of B–N-doped Tetrathienonaphthalene with Borane/Amine Substituents. Inorg. Chem. 2020, 60, 1099–1106. [Google Scholar] [CrossRef]
- You, Y.; Han, Y.; Lee, Y.-M.; Park, S.Y.; Nam, W.; Lippard, S.J. Phosphorescent Sensor for Robust Quantification of Copper(II) Ion. J. Am. Chem. Soc. 2011, 133, 11488–11491. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhao, J. Ratiometric luminescent molecular oxygen sensors based on uni-luminophores of C⁁N Pt(ii)(acac) complexes that show intense visible-light absorption and balanced fluorescence/phosphorescence dual emission. Chem. Commun. 2011, 47, 11471–11473. [Google Scholar] [CrossRef]
- Martin, A.; Byrne, A.; Dolan, C.; Forster, R.J.; Keyes, T.E. Solvent switchable dual emission from a bichromophoric ruthenium–BODIPY complex. Chem. Commun. 2015, 51, 15839–15841. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, X.; Cao, T.; Zhang, K.Y.; Yang, L.; Liu, S.; Liang, H.; Yang, H.; Li, F.; Huang, W. Fluorescent/phosphorescent dual-emissive conjugated polymer dots for hypoxia bioimaging. Chem. Sci. 2015, 6, 1825–1831. [Google Scholar] [CrossRef]
- Gupta, S.K.; Haridas, A.; Choudhury, J. Remote Terpyridine Integrated NHC–IrIII Luminophores as Potential Dual-Emissive Ratiometric O2 Probes. Chem. Eur. J. 2017, 23, 4770–4773. [Google Scholar] [CrossRef]
- Lo, K.K.-W.; Zhang, K.Y.; Leung, S.-K.; Tang, M.-C. Exploitation of the Dual-emissive Properties of Cyclometalated Iridium(III)–Polypyridine Complexes in the Development of Luminescent Biological Probes. Angew. Chem. Int. Ed. 2008, 47, 2213–2216. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-Y.; Wu, S.-H.; Ma, J.; Gong, Z.-L.; Sun, T.-G.; Jin, Y.; Yang, R.; Sun, B.; Zhong, Y.-W. Ratiometric detection of amyloid-β aggregation by a dual-emissive tris-heteroleptic ruthenium complex. Chem. Commun. 2020, 56, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.G.; Ramu, V.; Meijer, A.J.H.M.; Das, A.; Thomas, J.A. A ratiometric sensor for DNA based on a dual emission Ru(dppz) light-switch complex. Dalton Trans. 2017, 46, 6079–6086. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Yang, R.; Sun, B.; Tang, J.-H.; Gong, Z.-L.; Ma, J.; Wang, L.; Liu, J.; Ma, D.-X.; Shao, J.-Y.; et al. Dual-Emissive Tris-Heteroleptic Ruthenium Complexes: Tuning the DNA-Triggered Ratiometric Emission Response by Ancillary Ligands. Inorg. Chem. 2021, 60, 14810–14819. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Bu, X.; Fu, Y.; Wang, Z.; Li, Q. Recent advances in dual-emission ratiometric fluorescence probes forchemo/biosensing and bioimaging of biomarkers. Coord. Chem. Rev. 2019, 383, 82–103. [Google Scholar] [CrossRef]
- Chen, Z.; Ho, C.; Wang, L.; Wong, W. Single-Molecular White-Light Emitters and Their Potential WOLED Applications. Adv. Mater. 2020, 32, e1903269. [Google Scholar] [CrossRef]
- Kawashiro, M.; Mori, T.; Ito, M.; Ando, N.; Yamaguchi, S. Photodissociative Modules that Control Dual-Emission Properties in Donor–π–Acceptor Organoborane Fluorophores. Angew. Chem., Int. Ed. 2023, 62, e202303725. [Google Scholar] [CrossRef]
- Cheng, X.; Yue, S.; Chen, R.; Yin, J.; Cui, B.-B. White Light-Emitting Diodes Based on One-Dimensional Organic–Inorganic Hybrid Metal Chloride with Dual Emission. Inorg. Chem. 2022, 61, 15475–15483. [Google Scholar] [CrossRef]
- Vázquez-Domínguez, P.; Journaud, O.; Vanthuyne, N.; Jacquemin, D.; Favereau, L.; Crassous, J.; Ros, A. Helical donor–acceptor platinum complexes displaying dual luminescence and near-infrared circularly polarized luminescence. Dalton Trans. 2021, 50, 13220–13226. [Google Scholar] [CrossRef]
- Huang, X.; Song, J.; Yung, B.C.; Huang, X.; Xiong, Y.; Chen, X. Ratiometric optical nanoprobes enable accurate molecular detection and imaging. Chem. Soc. Rev. 2018, 47, 2873–2920. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Son, J.B.; Filatov, M.; Choi, C.H.; Lee, N.K.; Lee, D. Single-Benzene Dual-Emitters Harness Excited-State Antiaromaticity for White Light Generation and Fluorescence Imaging. Angew. Chem. Int. Ed. 2023, 62, e202302107. [Google Scholar] [CrossRef] [PubMed]
- Magde, D.; Magde Jr., M.D.; Glazer, E.C. So-called “dual emission” for 3MLCT luminescence in ruthenium complex ions: What is really happening? Coord. Chem. Rev. 2016, 306, 447–467. [Google Scholar] [CrossRef]
- Shao, J.-Y.; Wu, S.-H.; Yang, R.; Zhong, Y.-W.; Gong, Z.-L. Dual-emissive transition-metal complexes and their applications as ratiometric photoluminescent probes. Sci. Sin. Chim. 2020, 50, 315–323. [Google Scholar] [CrossRef]
- Steube, J.; Kruse, A.; Bokareva, O.S.; Reuter, T.; Demeshko, S.; Schoch, R.; Cordero, M.A.A.; Krishna, A.; Hohloch, S.; Meyer, F.; et al. Janus-type emission from a cyclometalated iron(iii) complex. Nat. Chem. 2023, 15, 468–474. [Google Scholar] [CrossRef]
- Kwak, S.W.; Choi, B.H.; Lee, J.H.; Hwang, H.; Lee, J.; Kwon, H.; Chung, Y.; Lee, K.M.; Park, M.H. Synthesis and Dual-Emission Feature of Salen-Al/Triarylborane Dyads. Inorg. Chem. 2017, 56, 6039–6043. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Liu, H.-W.; Tang, M.-C.; Choi, A.W.-T.; Zhu, N.; Wei, X.-G.; Lau, K.-C.; Lo, K.K.-W. Dual-Emissive Cyclometalated Iridium(III) Polypyridine Complexes as Ratiometric Biological Probes and Organelle-Selective Bioimaging Reagents. Inorg. Chem. 2015, 54, 6582–6593. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Gao, P.; Sun, G.; Zhang, T.; Li, X.; Liu, S.; Zhao, Q.; Lo, K.K.-W.; Huang, W. Dual-Phosphorescent Iridium(III) Complexes Extending Oxygen Sensing from Hypoxia to Hyperoxia. J. Am. Chem. Soc. 2018, 140, 7827–7834. [Google Scholar] [CrossRef]
- Glazer, E.C.; Magde, D.; Tor, Y. Dual Emission from a Family of Conjugated Dinuclear RuII Complexes. J. Am. Chem. Soc. 2005, 127, 4190–4192. [Google Scholar] [CrossRef]
- Han, M.; Tian, Y.; Yuan, Z.; Zhu, L.; Ma, B. A Phosphorescent Molecular “Butterfly” that undergoes a Photoinduced Structural Change allowing Temperature Sensing and White Emission. Angew. Chem. Int. Ed. 2014, 53, 10908–10912. [Google Scholar] [CrossRef]
- Zhou, C.; Tian, Y.; Yuan, Z.; Han, M.; Wang, J.; Zhu, L.; Tameh, M.S.; Huang, C.; Ma, B. Precise Design of Phosphorescent Molecular Butterflies with Tunable Photoinduced Structural Change and Dual Emission. Angew. Chem. Int. Ed. 2015, 54, 9591–9595. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.C.; Bautista, D.; González-Herrero, P. Stereoselective Formation of Facial Tris-Cyclometalated PtIV Complexes: Dual Phosphorescence from Heteroleptic Derivatives. Chem. – A Eur. J. 2020, 26, 11307–11315. [Google Scholar] [CrossRef] [PubMed]
- Scattergood, P.A.; Ranieri, A.M.; Charalambou, L.; Comia, A.; Ross, D.A.W.; Rice, C.R.; Hardman, S.J.O.; Heully, J.-L.; Dixon, I.M.; Massi, M.; Alary, F.; Elliott, P.I.P. Unravelling the Mechanism of Excited-State Interligand Energy Transfer and the Engineering of Dual Emission in [Ir(C∧N)2(N∧N)]+ Complexes. Inorg. Chem. 2020, 59, 1785–1803. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, M.; Müller, C.; Cullen, A.A.; Brandon, M.P.; Dietzek, B.; Pryce, M.T. Photophysics of Ruthenium(II) Complexes with Thiazole π-Extended Dipyridophenazine Ligands. Inorg. Chem. 2020, 60, 760–773. [Google Scholar] [CrossRef]
- Kisel, K.S.; Melnikov, A.S.; Grachova, E.V.; Hirva, P.; Tunik, S.P.; Koshevoy, I.O. Linking ReI and PtII Chromophores with Aminopyridines: A Simple Route to Achieve a Complicated Photophysical Behavior. Chem. – A Eur. J. 2017, 23, 11301–11311. [Google Scholar] [CrossRef]
- Wu, S.-H.; Ma, D.-X.; Gong, Z.-L.; Ma, J.; Shao, J.-Y.; Yang, R.; Zhong, Y.-W. Synthesis, Photophysical, and Computational Studies of a Bridged IrIII-PtII Heterodimetallic Complex. Crystals 2021, 11, 236. [Google Scholar] [CrossRef]
- Liu, L.; Fang, H.; Chen, Q.; Chan, M.H.; Ng, M.; Wang, K.; Liu, W.; Tian, Z.; Diao, J.; Mao, Z.; et al. Multiple-Color Platinum Complex with Super-Large Stokes Shift for Super-Resolution Imaging of Autolysosome Escape. Angew. Chem. Int. Ed. 2020, 59, 19229–19236. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, L.-Y.; Dai, F.-R.; Shi, L.-X.; Chen, Z.-N. Preparation, Characterization, and Photophysical Properties of Pt-M (M = Ru, Re) Heteronuclear Complexes with 1,10-Phenanthrolineethynyl Ligands. Inorg. Chem. 2008, 47, 2811–2819. [Google Scholar] [CrossRef]
- Yao, L.-Y.; Yam, V.W.-W. Dual Emissive Gold(I)–Sulfido Cluster Framework Capable of Benzene–Cyclohexane Separation in the Solid State Accompanied by Luminescence Color Changes. J. Am. Chem. Soc. 2021, 143, 2558–2566. [Google Scholar] [CrossRef]
- Zhang, S.-S.; Su, H.-F.; Zhuang, G.-L.; Wang, X.-P.; Tung, C.-H.; Sun, D.; Zheng, L.-S. A hexadecanuclear silver alkynyl cluster based NbO framework with triple emissions from the visible to near-infrared II region. Chem. Commun. 2018, 54, 11905–11908. [Google Scholar] [CrossRef]
- Lei, Z.; Guan, Z.-J.; Pei, X.-L.; Yuan, S.-F.; Wan, X.-K.; Zhang, J.-Y.; Wang, Q.-M. An Atomically Precise Au10Ag2 Nanocluster with Red–Near-IR Dual Emission. Chem. Eur. J. 2016, 22, 11156–11160. [Google Scholar] [CrossRef]
- Shan, X.-C.; Jiang, F.-L.; Yuan, D.-Q.; Zhang, H.-B.; Wu, M.-Y.; Chen, L.; Wei, J.; Zhang, S.-Q.; Pan, J.; Hong, M.-C. A multi-metal-cluster MOF with Cu4I4 and Cu6S6 as functional groups exhibiting dual emission with both thermochromic and near-IR character. Chem. Sci. 2013, 4, 1484–1489. [Google Scholar] [CrossRef]
- Li, G.; Zhu, D.; Wang, X.; Su, Z.; Bryce, M.R. Dinuclear metal complexes: multifunctional properties and applications. Chem. Soc. Rev. 2020, 49, 765–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Shao, J.-Y.; Gong, Z.-L.; Chen, N.; Zhong, Y.-W. Tuning the dual emissions of a monoruthenium complex with a dangling coordination site by solvents, O2, and metal ions. Dalton Trans. 2017, 47, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Shao, J.-Y.; Dai, X.; Cui, X.; Su, H.; Zhong, Y.-W. Synthesis and Characterization of Trisbidentate Ruthenium Complexes of Di(pyrid-2-yl)-methylamine. Eur. J. Inorg. Chem. 2017, 2017, 3064−3071. [Google Scholar] [CrossRef]
- Ishida, H.; Tobita, S.; Hasegawa, Y.; Katoh, R.; Nozaki, K. Recent advances in instrumentation for absolute emission quantum yield measurements. Co-ord. Chem. Rev. 2010, 254, 2449–2458. [Google Scholar] [CrossRef]
- Climent, C.; Alam, P.; Pasha, S.S.; Kaur, G.; Choudhury, A.R.; Laskar, I.R.; Alemany, P.; Casanova, D. Dual emission and multi-stimuli-response in iridium(iii) complexes with aggregation-induced enhanced emission: applications for quantitative CO2 detection. J. Mater. Chem. C 2017, 5, 7784–7798. [Google Scholar] [CrossRef]
| Compound | λmax,abs [nm] (ε [105 M−1cm−1])b |
λmax,emi [nm]c |
τ [ns]d (air) |
τ [ns] (N2) |
τ [ns] (77K) |
Φ (N2)e | E1/2,anodic [V]f | E1/2,cathodic [V] |
|---|---|---|---|---|---|---|---|---|
| L1 | 310 (0.16), 360 (0.07) | 457 | ND | 5.6 | ND | 24% | +0.84 | ND |
| L2 | 310 (0.13), 361 (0.07) | 445 | ND | 11.5 | ND | 43% | +0.89 | ND |
| L3 | 295 (0.08), 329 (0.07), 353 (0.07) | 450 | ND | 0.4 | ND | 0.06% | +1.01 | ND |
| 1(PF6)2 | 289 (1.10), 350 (0.16), 467 (0.12) | 451/646 | 14/89 | 16/193 | 5.0/528 | 2.1% | +1.12, +1.26 | −1.36, −1.61 |
| 2(PF6)2 | 288 (0.83), 375 (0.14), 448 (0.13) | 465/627 | 10/58 | 13/219 | 5.0/2500 | 6.7% | +1.23, +1.44 | −1.37, −1.62 |
| 3(PF6)2 | 284 (1.09), 368 (0.21), 427 (0.15) | 455/608 | 10/24 | 15/189 | ND | 0.53% | +1.35, +1.53 | −1.27, −1.48 |
| Comp. | Sn | E [ev] | λ [nm] | f | Dominant transition(s) (% contributionb) | Assignmentc |
|---|---|---|---|---|---|---|
| 12+ | 1 | 2.65 | 468 | 0.0111 | HOMO → LUMO (61) | Ldapz-OMeLbpyCT |
| 2 | 2.72 | 455 | 0.0190 | HOMO−1 → LUMO+1 (41) | MLbpyCT | |
| 3 | 2.73 | 454 | 0.0144 | HOMO−2 → LUMO (36) | MLbpyCT | |
| 4 | 2.81 | 442 | 0.0378 | HOMO−2 → LUMO+1 (36) | MLbpyCT | |
| 7 | 3.04 | 409 | 0.0678 | HOMO−3 → LUMO (34) | MLbpyCT | |
| 8 | 3.11 | 399 | 0.0702 | HOMO−3 → LUMO+1 (28), HOMO−3 → LUMO (17) | MLbpyCT | |
| 9 | 3.19 | 389 | 0.0319 | HOMO → LUMO+2 (75) | ICT | |
| 10 | 3.33 | 373 | 0.0150 | HOMO−2 → LUMO+2 (59), HOMO−1 → LUMO+2 (22) | MLdapz-OMeCT/ICT | |
| 11 | 3.45 | 359 | 0.0596 | HOMO−1 → LUMO+2 (39) | MLdapz-OMeCT/ICT | |
| 12 | 3.53 | 351 | 0.0696 | HOMO−3 → LUMO+2 (69), HOMO−1 → LUMO+2 (16) | MLdapz-OMeCT/ICT | |
| 13 | 3.56 | 349 | 0.0102 | HOMO → LUMO+3 (18) | Ldapz-OMeLbpyCT | |
| 19 | 3.81 | 325 | 0.0183 | HOMO → LUMO+5 (52) | Ldapz-OMeLbpyCT | |
| 32+ | 2 | 2.81 | 441 | 0.0107 | HOMO−1 → LUMO+1 (35) | MLbpyCT |
| 3 | 2.83 | 438 | 0.0181 | HOMO−1 → LUMO (23), HOMO−1 → LUMO+1 (27) | MLbpyCT | |
| 4 | 2.93 | 423 | 0.0467 | HOMO → LUMO+1 (40) | Ldapz-CF3LbpyCT | |
| 7 | 3.13 | 396 | 0.0930 | HOMO−3 → LUMO (50) | MLbpyCT | |
| 9 | 3.17 | 391 | 0.0555 | HOMO−2 → LUMO+1 (37) | MLbpyCT | |
| 10 | 3.27 | 379 | 0.0406 | HOMO−1 → LUMO+3 (26), HOMO → LUMO+3 (33) | MLdapz-CF3CT/ICT | |
| 13 | 3.42 | 363 | 0.0945 | HOMO−2 → LUMO+2 (67), HOMO → LUMO+2 (14) | MLdapz-CF3CT/ICT | |
| 14 | 3.52 | 352 | 0.1135 | HOMO−3 → LUMO+2 (38), HOMO−1 → LUMO+2 (13) | MLdapz-CF3CT/ICT | |
| 16 | 3.58 | 347 | 0.0388 | HOMO−1 → LUMO+12 (21) | Ldapz-CF3LbpyCT | |
| 20 | 3.77 | 329 | 0.0310 | HOMO−1 → LUMO+4 (22), HOMO → LUMO+4 (12) | MLdapz-CF3CT/Ldapz-CF3LbpyCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





