Submitted:
09 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General Consideration
2.2. Synthesis
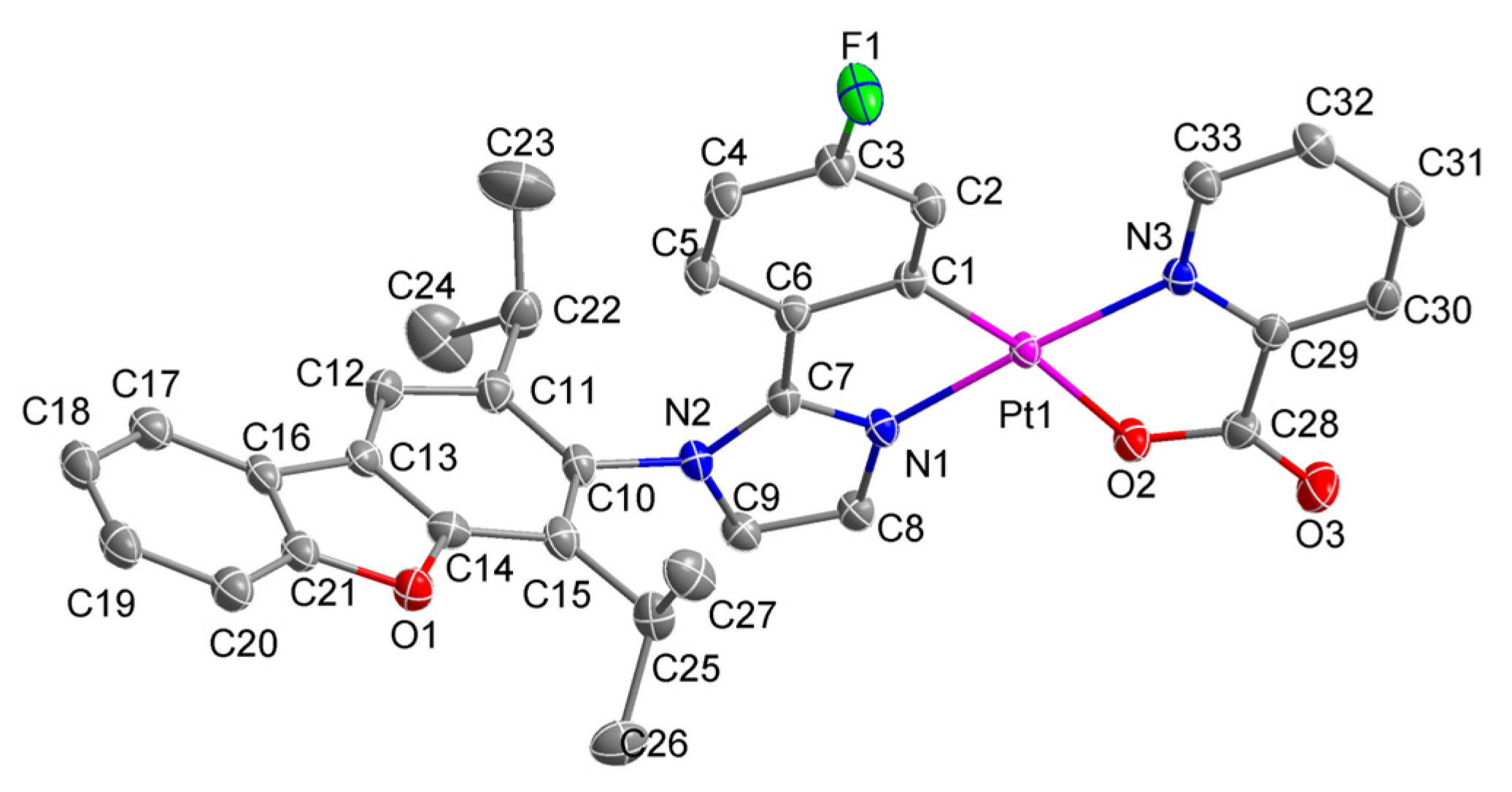
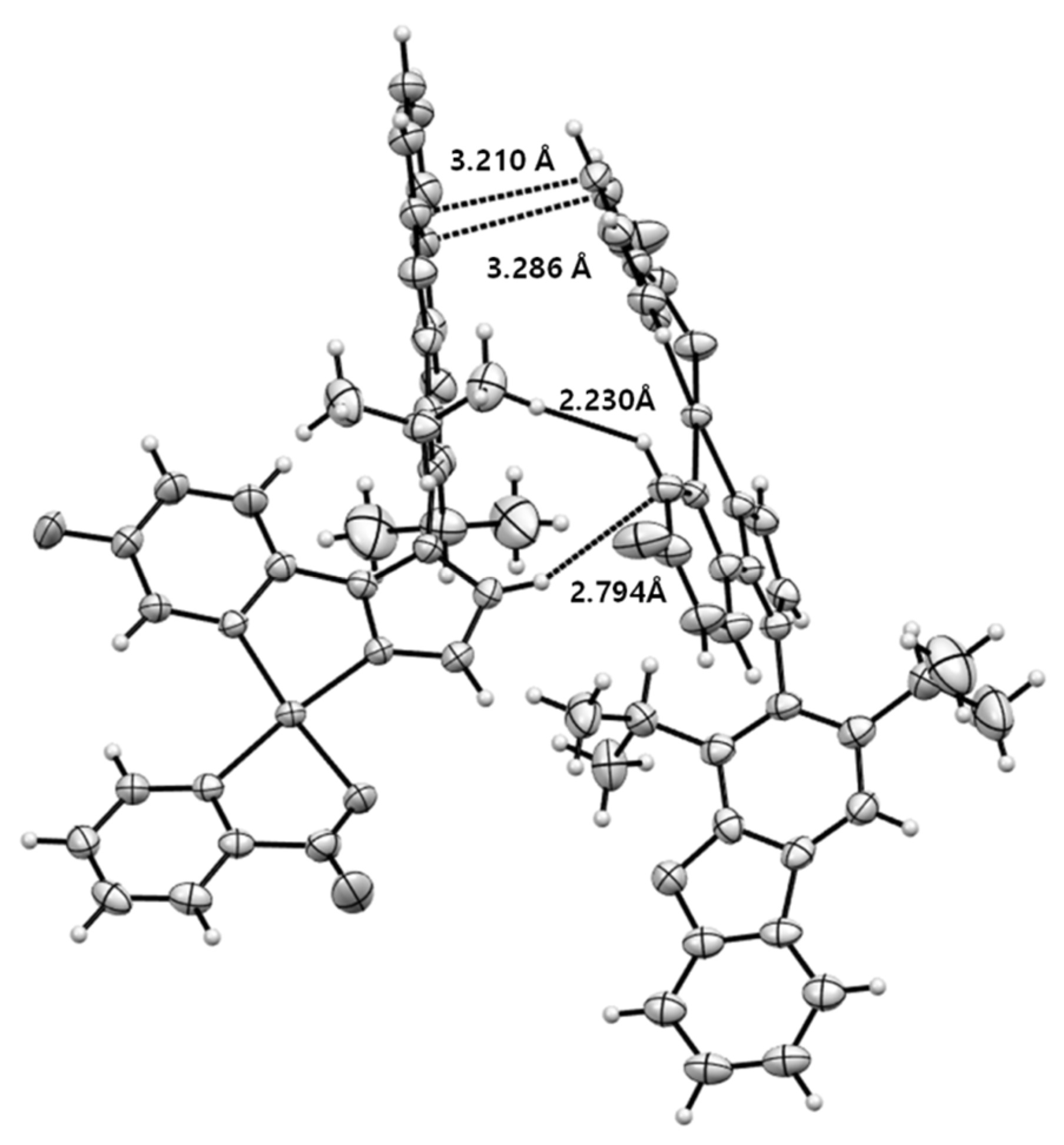
2.3. Single crystal X-ray analysis
2.4. Device Fabrication and Measurement
- Mixed host: mCBP/CNmCBPCN
- Dopant and doping ratio : compound 1 and 3 and 10 weight %
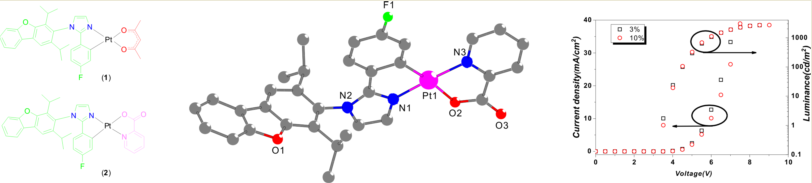
3. Results and Discussion
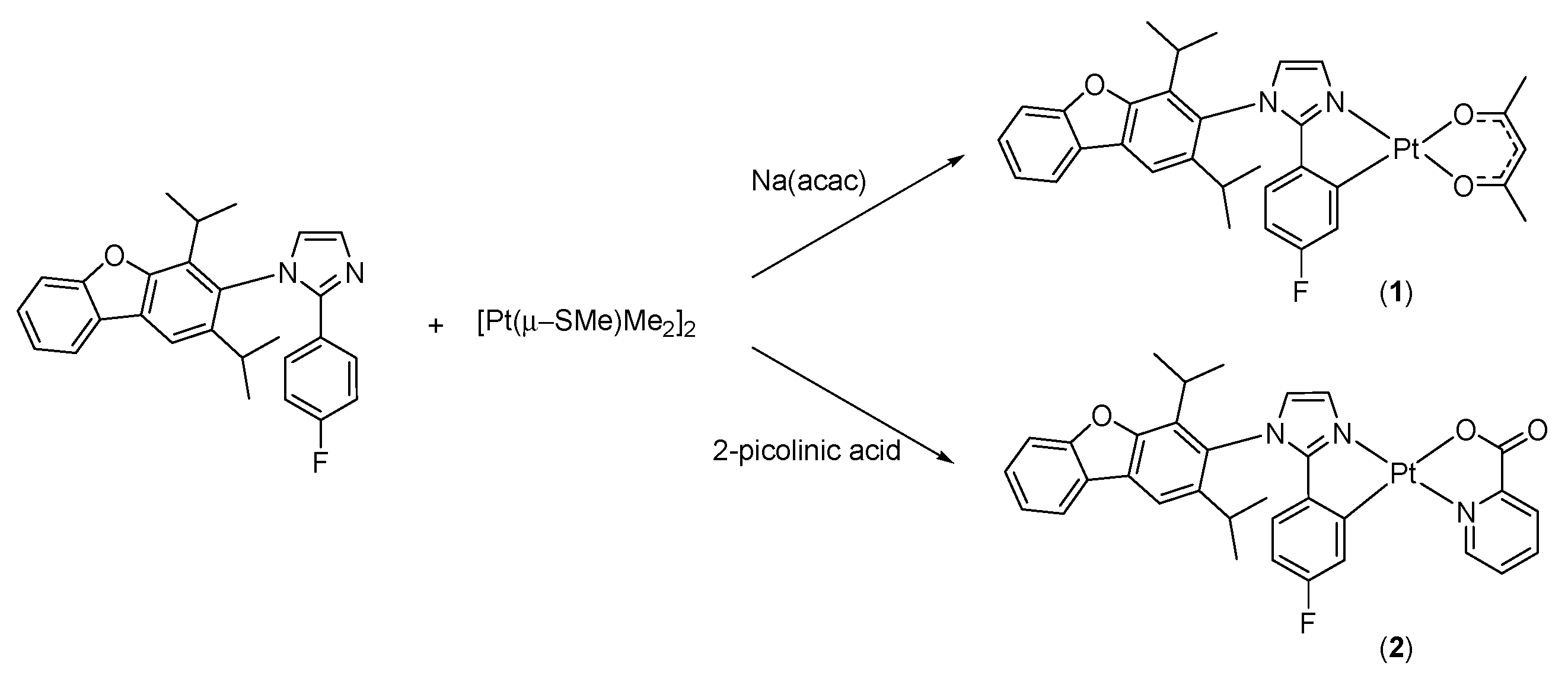
3.1. Synthesis and Structure
3.2. Photophysical and Electrochemical Properties
3.3. TD-DFT calculations
3.4. PhOLEDs performances
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- A Mao, P.; Liu, C.; Li, X.; Liu, M.; Chen, Q.; Han, M.; Maier, S. A.; Sargent, E. H.; Zhang, S. Single-step-fabricated disordered metasurfaces for enhanced light extraction from LEDs. Light Sci. Appl. 2021, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Filimonova, A. A.; Barbasova, T. A.; Shnayder, D. A. Outdoor lighting system upgrading based on smart grid concept. Energy Procedia, 2017, 111, 678–688. [Google Scholar] [CrossRef]
- Shahzad, K.; Čuček, L.; Sagir, M.; Ali, N.; Rashid, M. I.; Nazir, R.; Nizami, A. S.; Al- Turaif, H. A.; Ismail, I. M. I. An ecological feasibility study for developing sustainable street lighting system J. Clean. Prod., 2018, 175, 683–695. [Google Scholar] [CrossRef]
- Bladh, M.; Krantz, H. Towards a bright future? Household use of electric light: A microlevel study. Energy Pol. 2008, 36, 3521–3530. [Google Scholar] [CrossRef]
- Ramchandra, P. Organic light emitting diode devices: An energy efficient solid state lighting for applications. Renew. Sustain. Energy Rev., 2020, 133, 110043. [Google Scholar]
- Ho, S.-J.; Hsu, H.-C.; Yeh, C.-W.; Chen, H.-S. Inkjet-printed salt-encapsulated quantum dot film for UV-Based RGB color-converted Micro-Light Emitting Diode Displays. ACS Appl. Mater. Interfaces 2020, 12, 33346–33351. [Google Scholar] [CrossRef]
- Baranoff, E.; Curchod, B. F. E. FIrpic: archetypal blue phosphorescent emitter for electroluminescence. Dalton Trans., 2015, 44, 8318–8329. [Google Scholar] [CrossRef]
- Lee, S. J.; Park, K. M.; Yang, K.; Kang, Y. Blue Phosphorescent Ir(III) Complex with High Color Purity: fac-Tris(2′,6′-difluoro-2,3′- bipyridinato-N,C-4′)iridium(III). Inorg. Chem. 2009, 48, 1030−1037. [Google Scholar] [CrossRef]
- Yook, K. S.; Jeon, S. O.; Joo, C. W. J.; Lee, J. Y. High efficiency deep blue phosphorescent organic light-emitting diodes Org. Electron., 2009, 10, 170–173. [Google Scholar]
- Jayabharathi, J.; Thanikachalam, V.; Thilagavathy, S. Phosphorescent organic light-emitting devices: Iridium based emitter materials - An overview. Coord. Chem. Rev. 2023, 483, 215100. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, W.; Su, W.; Liu, Y.; Shen, Q.; Liao, L.; Zhou, M. Highly efficient phosphorescent organic light-emitting diodes using a homoleptic iridium(III) complex as a sky-blue dopant. Org. Electron., 2013, 14, 2596–2601. [Google Scholar] [CrossRef]
- Kang, J.; Park, K.-M.; Lee, K. H.; Lee, J. Y.; Kang, Y. Improvement in color purity and lifetime of blue PHOLEDs using a homoleptic iridium(III) complex with fluorinated dibenzofuranyl-imidazole ligand. Dyes Pigm. 2021, 190, 109334. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, X.; Fu, C.; Chen, Z.; Hu, J.; Xu, Z.; Tang, D. Design of stable platinum(II) complexes exhibited various colors via auxiliary ligand and electron-donating/withdrawing groups: A theoretical investigation. Org. Electron., 2019, 71, 251–257. [Google Scholar] [CrossRef]
- Kang, J.; Zaen, R; Lee, J. H.; Hwang, H.; Park, K.-M.; Kim, S. C.; Lee, J. Y.; Kang, Y. Effect of ancillary ligand on the photoluminescent and electroluminescent properties of blue Ir(III) complexes bearing main bipyridine ligand. Chem. Eng. J. 2022, 431, 134249. [Google Scholar] [CrossRef]
- Hill, G. S.; Irwin, M. J.; Levy, C. J.; Rendina, L. M.; Puddephatt, R. J.; Andersen, R. A.; Mclean, L. Inorg. Synth. 2007, 32, 149.
- APEX2: Data collection and Processing Software Ver. 2009.1-0; Bruker AXS Inc.: Madison, WI, 2008.
- SADABS: Empirical Absorption and Correction Software Ver 2.03; Bruker AXS Inc.: Madison, WI, 1999.
- Sheldrick, G. M. SHELXS-2014. Program for Structure Solution; Universität of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sheldrick, G. M. SHELXL-2014. Program for Structure Refinement; Universität of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Brandenburg, K. DIAMOND: Crystal Impact GbR: Bonn, Germany, 1998.
- Kang, J.; Kim, S.-C.; Lee J., Y.; Kang, Y. Blue phosphorescent platinum(II) complexes based on tetradentate ligand with rigid and highly distorted linkage for efficient organic light-emitting diodes. Dyes Pigm. 2022, 207, 110770. [Google Scholar] [CrossRef]
- Kang, J.; Zaen, R.; Park, K.-M.; Lee, K.H.; Lee, J.Y.; Kang, Y. Cyclometalated platinum(II) β-diketonate complexes with extremely high external quantum efficiency for white organic light-emitting diodes. Adv. Opt. Mater. 2021, 9, 2101233. [Google Scholar] [CrossRef]
- Ko, S.-B.; Lu, J.-S.; Kang, Y.; Wang, S. Impact of a Picolinate Ancillary Ligand on Phosphorescence and Fluoride Sensing Properties of BMes2-Functionalized Platinum(II) Compounds. Organometallics 2013, 32, 599–608. [Google Scholar] [CrossRef]
- Kim, H.; Hong, S.; Kim, S.-C.; Lee J., Y.; Kang, Y. Novel blue-phosphorescent platinum(II) ternary complexes with β-diketonate and fluorinated phenylimidazole for organic light-emitting diodes. Can. J. Chem. 2023, 101, 163–170. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Chan, S.-C.; Chan, M.C.W.; Hou, Y.-J.; Zhu, N.; Che, C.-M.; Liu, Y.; Wang, Y. Structural, Photophysical, and Electrophosphorescent Properties of Platinum(II) Complexes Supported by Tetradentate N2O2 Chelates. Chem. - Eur. J. 2003, 9, 1263–1272. [Google Scholar] [CrossRef]
- Zhu, Z.-Q.; Park, C.-D.; Klimes, K.; Li, J. Highly Efficient Blue OLEDs Based on Metal-Assisted Delayed Fluorescence Pd(II) Complexes. Adv. Optical Mater. 2019, 7, 1801518. [Google Scholar] [CrossRef]
- Zhu, Z.-Q.; Fleetham, T.; Turner, E.; Li, J. Harvesting all electrogenerated excitons through metal assisted delayed fluorescent materials. Adv. Mater. 2015, 27, 2533–2537. [Google Scholar] [CrossRef] [PubMed]
- Hofbeck, T.; Monkowius, U.; Yersin, H. Highly efficient luminescence of Cu(I) compounds: thermally activated delayed fluorescence combined with short-lived phosphorescence. J. Am. Chem. Soc. 2015, 137, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Zaen, R.; Park, K.-M.; Lee, K.H.; Lee, J. Y.; Kang, Y. Blue phosphorescent Ir(III) complexes achieved with over 30% external quantum efficiency. Adv. Opt. Mater. 2019, 7, 1901387. [Google Scholar] [CrossRef]
- Kang, Y.; Chang, Y.-L.; Lu, J.-S.; Ko, S.-B.; Rao, Y.; Varlan, M.; Lu, Z.-H.; Wang, S. Highly efficient blue phosphorescent and electroluminescent Ir(III) compounds. J. Mater. Chem. C, 2013, 1, 441–450. [Google Scholar] [CrossRef]
- Pommerehne, J.; Vestweber, H.; Guss, W.; Mahrt, R. F.; Bässler, H.; Porsch, M.; Daub, J. Efficient two layer leds on a polymer blend basis. Adv. Mater. 1995, 7, 551–554. [Google Scholar] [CrossRef]
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa et al., Gaussian 03, Revision C.02, Gaussian, Inc., Wallingford, CT 2004.uthor 1, A.B.; Author 2, C.D. Title of the article. Abbreviated Journal Name Year, Volume, page range.
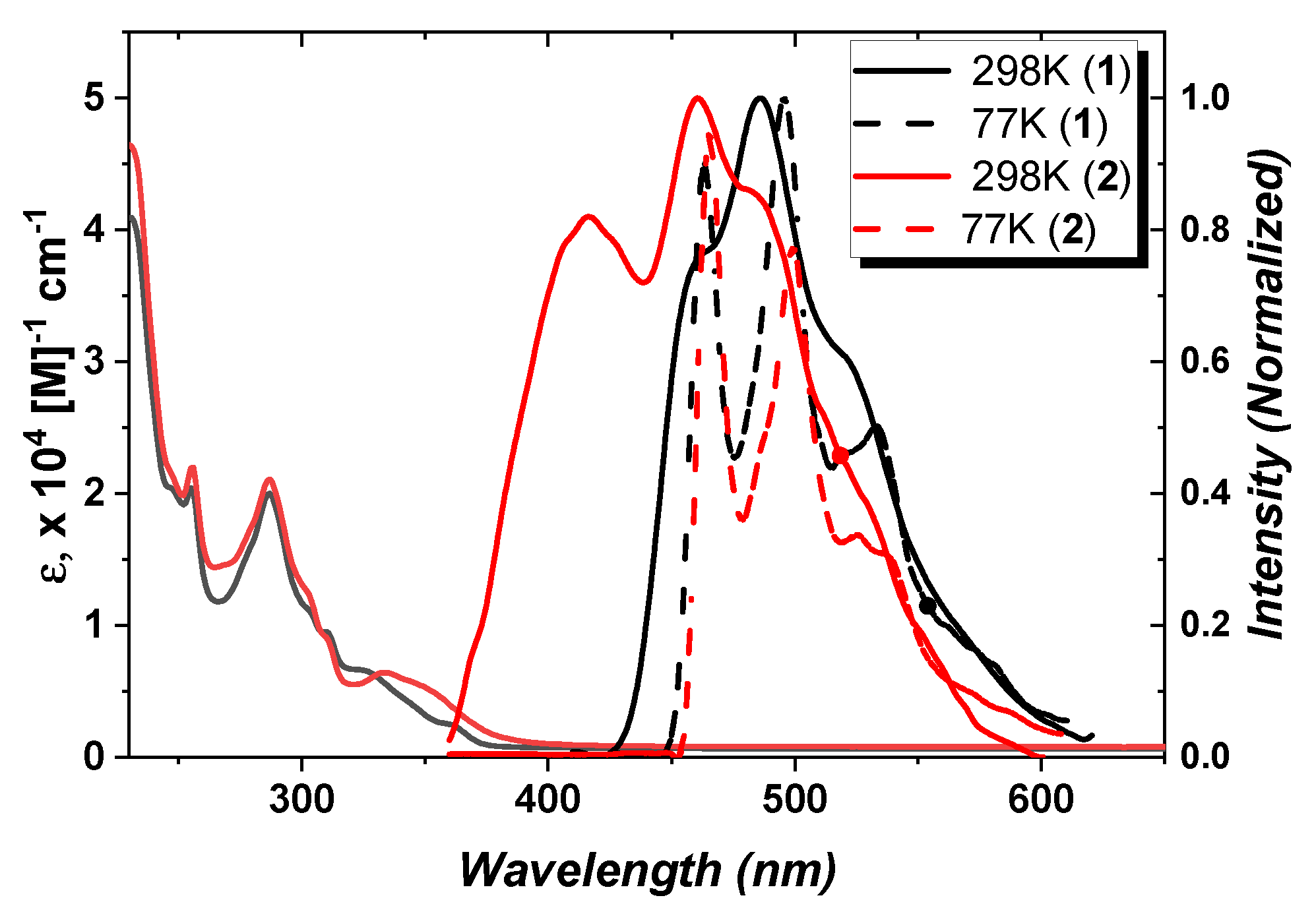
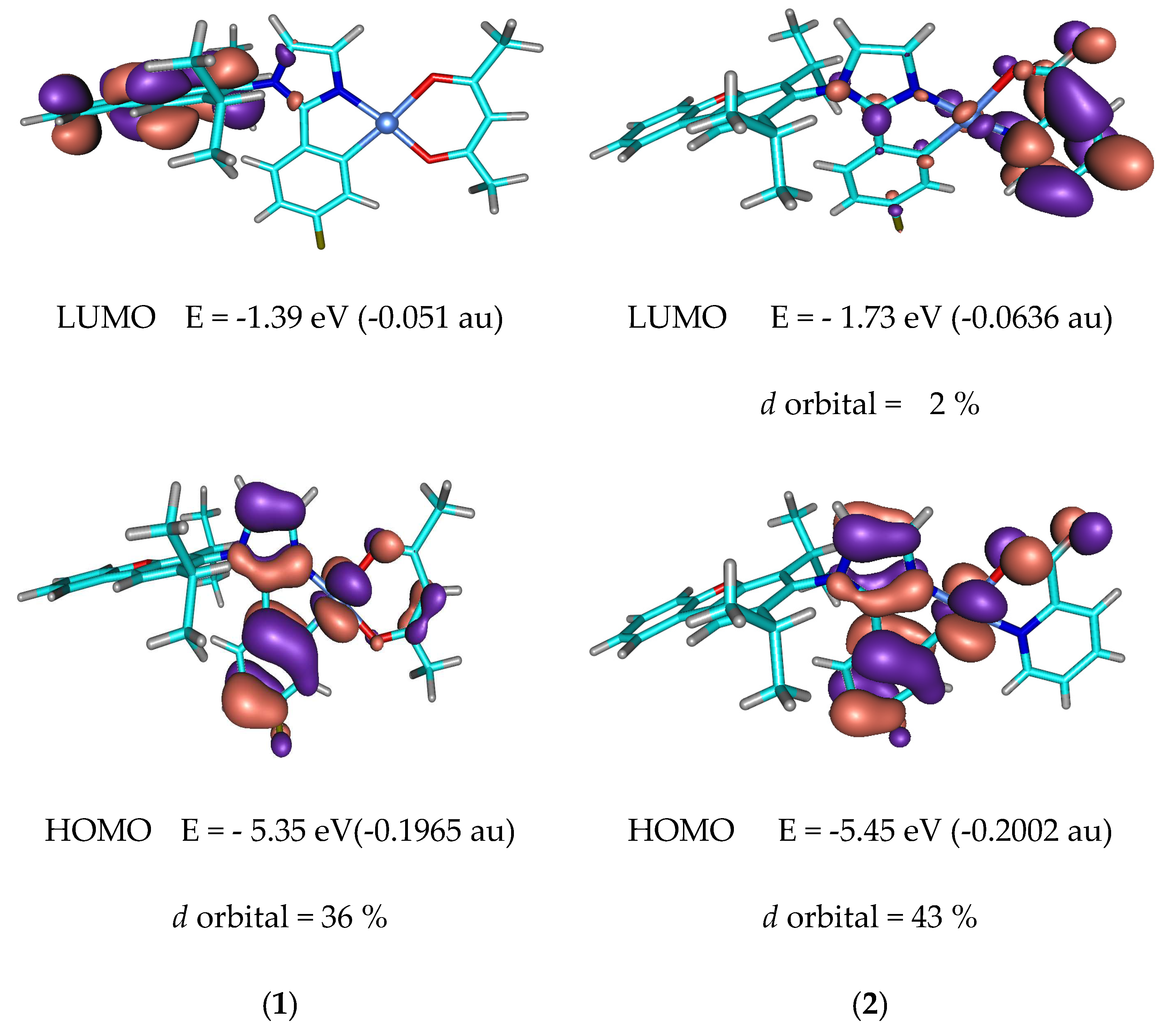
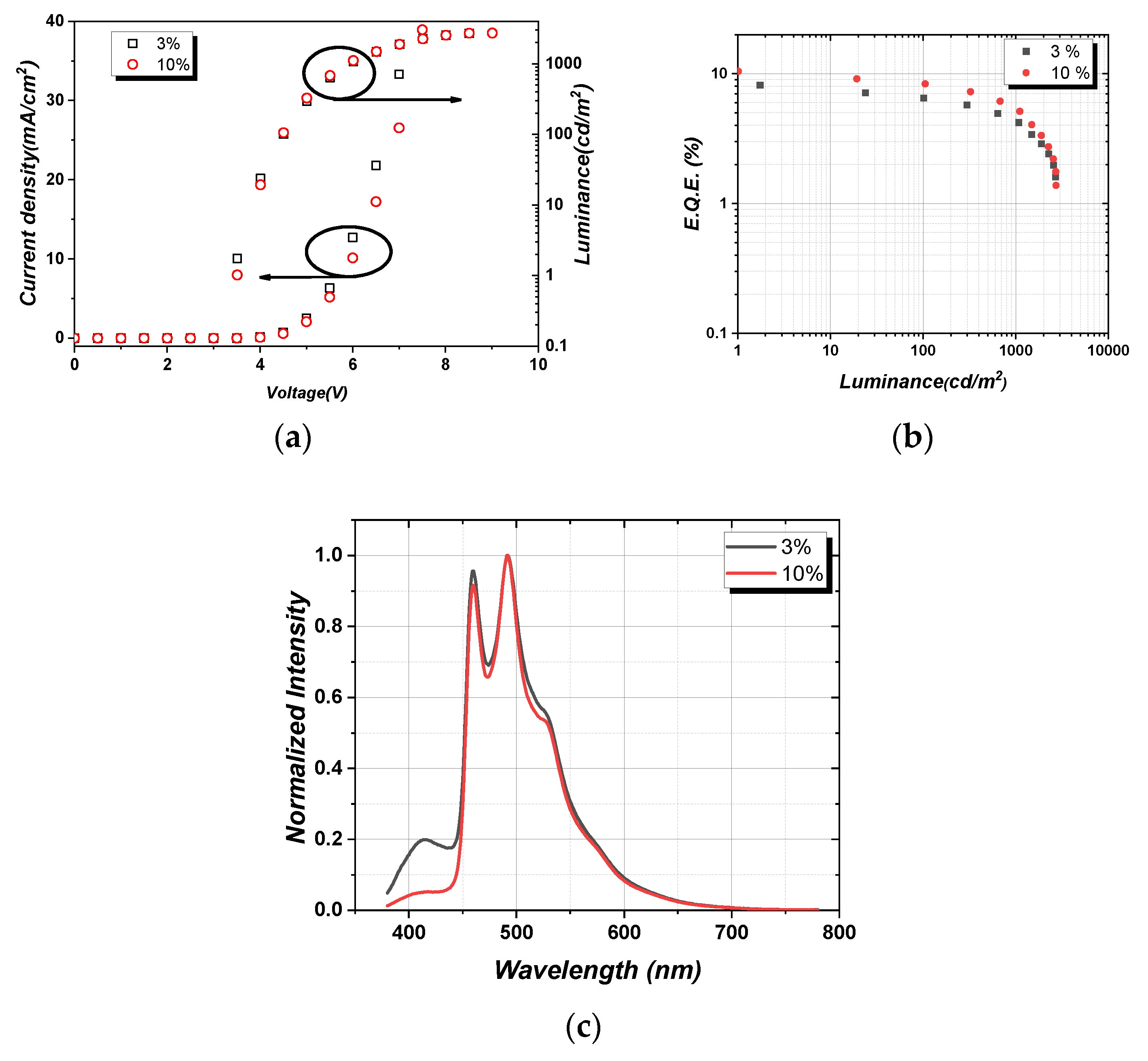
| Absorptiona)λmax [nm], ε[105M-1cm-1] | Emission,a) 298K, | Eg/ET1 [eV] c) | τ(obs)[μs] | |||
|---|---|---|---|---|---|---|
| λmax [nm] | ΦPLb) | |||||
| 1 | 289 (0.19), 326 (0.06), 364(0.02) | 485/(494,sh) | 0.37 | 3.09/2.74 | 6.1 | |
| 2 | 289 (0.46), 343 (0.30), 409(0.06) | 482/(507,sh) | 0.28 | 3.14/2.67 | 28.5 | |
| Experiment | TD-DFT result | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| EOx(V) a) | HOMO (eV) | LUMO (eV) | Egb) (eV) |
HOMO (eV) | LUMO (eV) | HOMO-LUMO gap (eV) | %H → L (S0→ S1) | Oscillator strength (S0 → S1) |
|
| 1 | 0.93 | -5.73 | -2.64 | 3.09 | -5.35 | -1.39 | 3.96 | 95.9 | 0.0001 |
| 2 | 1.03 | -5.83 | -2.96 | 2.87 | -5.45 | -1.73 | 3.72 | 94.8 | 0.0054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





