1. Introduction
Central precocious puberty is a rare condition in childhood, occurring in approximately 1 in every 5000 to 1 in every 10,000 individuals, and results from the early maturation of the hypothalamic-pituitary-gonadal (HPG) axis 1, 2. Diagnosis of central precocious puberty (CPP) is typically made when secondary sexual characteristics appear before the age of 8 in females and before the age of 9 in males. There has been a global trend towards a decrease in the age of puberty onset, particularly among girls 3.
Gonadotropin-releasing hormone (GnRH) analogs are widely utilized in pediatric patients as depot formulations to manage CPP 4. These analogs work by suppressing gonadotropin and sex hormone secretion, thereby preventing the progression of advanced puberty and mitigating the impact on final adult height due to accelerated growth plate fusion 5. One commonly used GnRH analog is triptorelin acetate (TA), a synthetic nanopeptide that downregulates GnRH receptors in the pituitary 2. Although the majority of patients tolerate TA well, occasional adverse reactions do occur. Local effects like pain, flares, and subcutaneous nodules are commonly known and typically require no specific treatment 6, 7. However, more significant systemic reactions, including sterile abscess formation and hypersensitivity reactions, are exceedingly rare. Yet, if these reactions manifest, they can pose life-threatening risks 8, 9.
The primary objective of this study was to analyze ADRs associated with the administration of TA within the time frame of 2015 to 2022, focusing on a single pediatric tertiary medical center. Additionally, we discuss SADRs and share our insights on managing these adverse reactions.
2. Materials and Methods
2.1. Study design and population
Shenzhen Children's Hospital is a comprehensive facility for children's healthcare, encompassing medical services, wellness care, scientific investigation, educational activities, and recovery programs. Moreover, it stands as the exclusive 3A pediatric hospital in Guangdong Province. This study delineates ADRs related to triptorelin, as reported by healthcare practitioners at Shenzhen Children's Hospital during the period spanning September 2015 to December 2022. The reporting was executed through our internal electronic system designated for such purposes. Upon the occurrence of a suspected ADR within our hospital, a specialized physician evaluates the impact of each adverse incident on the patient. The assessment takes into account factors like impairment, probable cause, and the nature of the adverse occurrence. Subsequently, a comprehensive clinical dossier detailing the incident is documented. Records of instances that are identified as potentially ADR-positive by our clinical review team are subjected to further examination by pharmacologists. These experts identify any adverse events and compile a thorough report before transmitting it to the Adverse Drug Reaction Monitoring agency of Guangdong Province, China (GDADR). GDADR functions as the central repository for suspected ADR reports stemming from medications and vaccines submitted by all provinces within Guangdong, China. Specialists within GDADR then proceed to appraise the severity and validate the likelihood of the reported ADRs.
2.2. Evaluation criteria
An ADR is defined as unintended harmful responses to medications, occurring at customary treatment doses or due to interactions with other drugs, such as accidental overdose, substance misuse, and treatment ineffectiveness10. ADR severity is categorized as mild, moderate, or severe. Serious ADRs encompass instances that could be life-threatening (like liver failure, irregular heart rhythms, and specific allergic reactions), those resulting in enduring or significant incapacity, hospitalization, and those leading to birth anomalies.
2.3. Causality of ADRs
To further evaluate whether ADRs were induced by GnRH analogs, a causality assessment was conducted employing the validated Kramer's algorithm
10-13. Kramer's algorithm establishes six distinct criteria axes, each associated with its corresponding scoring system (
Table 1). The cumulative score correlates with the likelihood that the clinical indication signifies an ADR. Based on the total score, ADRs were categorized into four classes: (1) Definite: A reasonable temporal or spatial relationship between the drug and ADR, verified by de-challenge and re-challenge, and/or laboratory tests. (2) Probable: A rational time connection to drug administration, with no concurrent illnesses or suspicions of other drugs linked to the clinical occurrence. Additionally, a clinically logical response upon discontinuation is necessary. (3) Possible: A reasonable temporal connection to drug administration, but the event could also be explicable by concurrent illnesses or other drugs. (4) Unclear Causality: The clinical occurrence could be attributed to either the underlying ailment or other drugs
12.
History: Prior occurrences of ADR
No other factors related to the underlying disease, and no other drug-unrelated causes can explain the presence of the clinical indication in the patient.
Timing: Time elapsed between drug administration and manifestation of ADR; ADR either diminishes or disappears after reducing or discontinuing the drug.
De-challenge: ADR either diminishes or disappears after reducing or discontinuing the drug.
Re-challenge: ADR either diminishes or disappears after re-administering the drug.
Score Interpretation: Less than 0 indicates an unlikely adverse drug reaction; 0-3 suggests a possible reaction; 4 and 5 signify a probable reaction; 6 and 7 indicate a definite reaction.
2.4. Strategy of analysis
This study employed an analytical strategy that encompassed medical history and demographic details, including age, gender, weight, patient background, allergic history, injection dose and interval, treatment duration, information related to reactions such as ADR onset, clinical progression, administered ADR treatments, and patient outcomes post-management. The collected data were subjected to statistical analysis using a computerized system. The analysis procedure involved descriptive statistical techniques.
3. Results
3.1. Analysis of the complete data
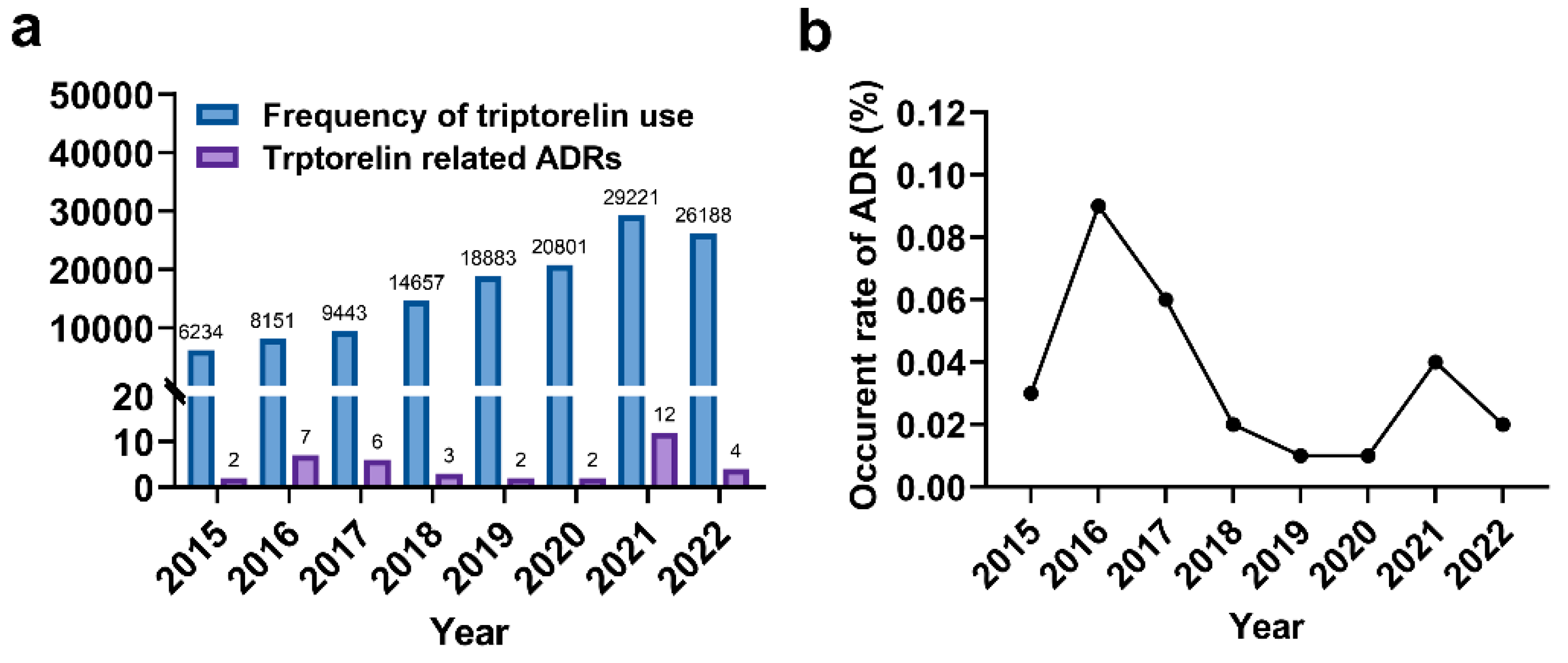
A total of 38 suspected ADRs cases were recorded among 33,045 patients who were prescribed TA between 2015 and 2022. Among these patients, 34 received intramuscular injections for central precocious puberty (CPP) treatment. Specifically, 31 patients were administered a monthly dosage of 3.75 mg, while 2 patients received 3 mg per month, and 1 patient was prescribed 2.6 mg per month. Additionally, 4 patients were subjected to subcutaneous injection for a stimulation test with a dosage below 0.1 mg. No patients had a history of previous TA-related adverse drug reactions.
The number of ADRs exhibited variation over the seven-year period, peaking at 12 in 2021 (
Figure 1a). The incidence rate of ADRs fluctuated between 0.02% and 0.09%, reaching its peak of 0.09% in 2015 (
Figure 1b). On average, patients' age was approximately 9.7 years. Female patients accounted for a larger share of ADR reports compared to males (32 versus 6) (
Table 2). A comparison of the ages of male and female patients revealed that girls experiencing ADRs were notably younger than boys (9.47 versus 11.67).
3.2. Analyses of ADR reports referring to causality and severity
Based on Kramer's algorithm, the majority of reported ADRs were categorized as "possible," scoring between 0 and 3. Only 7 cases were classified as "probable," scoring between 4 and 5. In terms of ADR severity, 12 patients encountered severe ADRs, accounting for 31.6% of the reported instances (
Table 2). Moderate adverse effects were experienced by more than two-thirds of patients (68.4%). Out of the 38 patients, 31 fully recovered, while one patient remained uncured due to permanent scarring at the injection site. The outcomes for six patients were unascertainable due to loss of communication.
3.3. Analyses of interval time between drug administration and ADRs
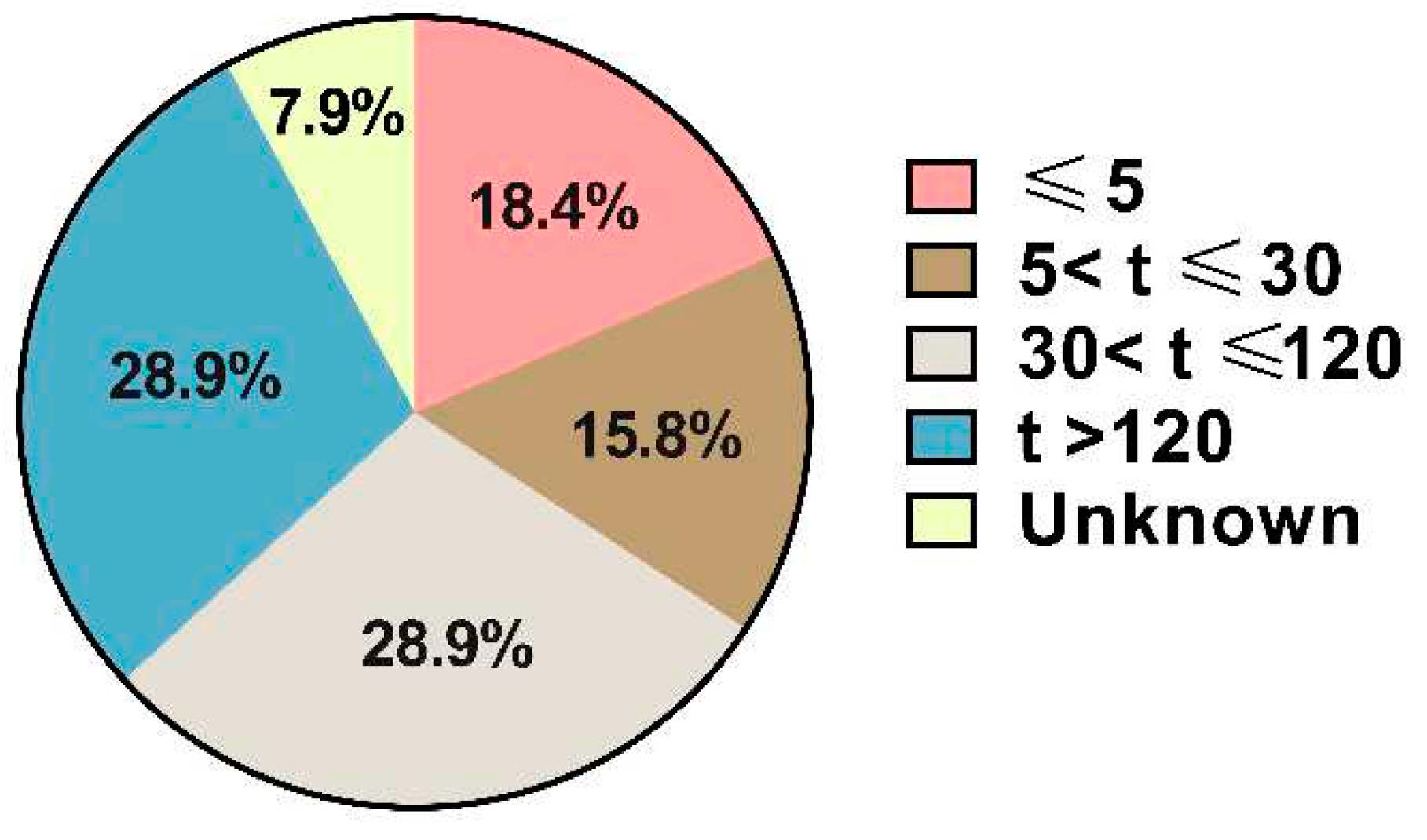
Out of the 38 cases collected, 18.4% occurred immediately after injection (within 5 minutes). An additional 6 patients experienced discomfort within 30 minutes (
Figure 2). The number of patients experiencing ADRs between 30 minutes and two hours after drug administration was equal to those encountering adverse reactions beyond 2 hours.
We also reviewed the medical history of these patients to verify prior triptorelin usage. As listed in
Table 3, eighteen patients had no history of TA administration. Two patients had received triptorelin injections over 10 times before experiencing adverse drug reactions. Additionally, six patients encountered ADRs due to a change in manufacturing from Diphereline (Ipsen Pharma Biotech) to Decapeptyl (Ferring GmbH). No further ADRs occurred when they reverted to Diphereline.
3.4. Analysis of ADRs involving organs and systems
Taking into account that a patient might experience multiple symptoms, we calculated the frequency of identical symptoms to offer a comprehensive overview of potential ADRs. The frequencies and proportions of ADRs related to organs and systems are outlined in
Table 4. As depicted, skin and subcutaneous tissue disorders were the predominant ADRs associated with TA. Common reactions in the skin included erythema and pruritus. The systemic reaction observed was pyrexia. There were two reported injection site reactions, characterized as injection site nodules and injection site pain. Digestive system-related ADRs constituted around 20.7% of the cases, while nervous system disorders represented 15.9%. Notably, four patients were diagnosed with hip synovitis, a phenomenon not previously documented.
3.5. Analysis of sever adverse reactions in TA Treatment
A total of 12 sever adverse drug reactions (SADRs) were documented over the 6-year period. Detailed patient information concerning SADRs linked to TA treatment is provided in
Table 4. Notable SADRs included conjunctivitis, hip synovitis, arthralgia, systemic anaphylactic reactions, anaphylactic shock, and injection site nodules, among others. Among these, two patients required immediate intervention in the rescue room due to unconsciousness and anaphylactic shock, and they eventually recovered following management. Seven patients achieved recovery through drug administration, while two patients recuperated without requiring specific treatment.
The three most frequent SADRs were anaphylactic reactions, hip synovitis, and arthralgia. Four patients encountered anaphylactic reactions, and within this group, two experienced anaphylactic shock. Hip synovitis was noted in four cases, with two patients unable to ambulate. Unfortunately, four patients were lost to follow-up due to personal reasons.
4. Discussion
ADRs includes unintended harmful events arising from the use of drugs at therapeutic doses for prophylaxis, diagnosis, treatment, or physiological modulation 14. Triptorelin, a GnRH analog, has been linked to adverse effects like elevated blood lipids, central obesity, and psychological reactions such as anxiety, panic, and depression8. The drug label for triptorelin acetate (Decapeptyl) notes potential ADRs like bleeding, vomiting, nausea, and allergic reactions in children. Given the growing prevalence of precocious puberty, the expanding clinical utilization of triptorelin underscores the significance of collecting and analyzing its adverse reactions when employed to treat CPP in children.
In this study, we conducted a retrospective analysis of 38 ADR cases and determined that the majority of triptorelin-related ADRs manifested rapidly. A noteworthy 18.4% of patients experienced ADRs immediately after injection (within 5 minutes), with the reactions quickly resolving either spontaneously or through medical intervention. However, the identification of twelve severe ADRs during triptorelin acetate administration demands a careful examination to unveil patterns and establish appropriate response protocols.
The World Health Organization (WHO) has categorized the clinical expressions and immune system-related adverse effects of GnRH analogs across 97 countries from 1988 to 2010 15. Notably, all five cases of severe anaphylactic reactions in this study occurred within an hour of administration, aligning with WHO-reported clinical manifestations. These occurrences are indicative of rapid immunoglobulin E (IgE)-mediated allergic reactions. This mechanism involves sensitization through allergen stimulation, triggering the attachment of IgE molecules to mast cells or basophilic granulocytes. Upon re-exposure to the allergen, the bridging of IgE with specific allergens prompts the release of allergenic mediators, thus initiating the entire allergic reaction 16-18.
While reports of ADRs related to triptorelin are infrequent compared to other GnRH drugs, the potential for severe allergic reactions in children necessitates proactive preventive measures. At the 2019 European Annual Meeting of Pediatric Endocrinology, Tarik Kirkgo proposed a comprehensive approach to managing type I allergic reactions triggered by GnRH drugs. In the event of a type I hypersensitivity reaction, evaluating the risk and benefit for the child is crucial, followed by skin prick tests (SPT) and intradermal tests (IDT). If these tests yield positive results, patients should be desensitized or administered medications under close medical supervision9. Allergic reactions can extend beyond active substances to include adjuvants and preservatives present in the drug product 19. Notably, six patients in our study experienced ADRs after a manufacturing change. To address this, we recommend conducting SPT and IDT before altering pharmaceutical manufacturers to identify potential causative allergens.
Hip synovitis denotes an acute inflammatory response within the synovium, typically characterized by hip pain, stiffness, and even impaired mobility. Although prior literature and instructions have reported multiple arthralgias and abnormal musculoskeletal joint responses to triptorelin, there has been no documentation regarding hip synovitis associated with the drug. The precise etiology remains unclear, making it a common cause of pediatric hip pain 20. Yassar Alamri's study identified significant eosinophil elevation in 15.6% of 103 children with hip synovitis, suggesting a potential link with eosinophilia 21. Eosinophils are blood cells capable of combating bacteria and parasites. Eosinophilia can arise from bacterial infections or allergic reactions 22,23. Among the four children with hip synovitis in our study, three displayed varying degrees of allergic reactions, and one exhibited leukocytosis. Given that anaphylactic reactions often generate numerous inflammatory factors; we hypothesize that the observed hip synovitis might stem from anaphylactic reactions. Treatment options such as dexamethasone and vitamin C were employed to mitigate inflammation and delay tissue oxidation, yielding acceptable and effective outcomes for the four patients with hip synovitis 24.
To enhance the prevention and management of adverse reactions related to hip inflammation caused by triptorelin, we have shared comprehensive information about children who experienced such reactions with the National Adverse Drug Reaction Surveillance System. We actively engage in communication with manufacturers and improve drug instructions. Prior to administering the medication, it is important to carefully inquire about the child's history of allergies. Post-medication administration, it is advisable to ensure the child remains under observation in the hospital for thirty minutes, during which relevant emergency measures should be readily available. Furthermore, considering the specific storage requirements of triptorelin (Decapeptyl injection) necessitating temperatures between 2-8℃, we believe it is imperative to uphold stringent drug quality control. We urge manufacturers to furnish a comprehensive report detailing pre-distribution inspections, as well as cold chain quality control data encompassing production, storage, and transportation. Additionally, it is vital to prohibit patients from self-administering the drug outside the hospital premises and minimize the time span between prescription and drug administration.
Nevertheless, our retrospective investigation presents certain limitations. One key limitation is the potential underreporting of suspected ADRs. Physicians may lack awareness of the necessity to report well-established adverse drug reactions, necessitating clearer reporting guidelines. Underreporting could also result from a misconception that a strong causal relationship between a drug and a presumed adverse reaction is required before reporting. Moreover, our study is based on a single-center retrospective analysis, warranting future research encompassing data from diverse healthcare facilities to enhance comprehensiveness.
5. Conclusions
In conclusion, we have documented 38 cases of adverse drug reactions associated with triptorelin acetate injection over a span of 7 years. Pediatricians must exercise heightened vigilance, particularly when potential risk factors are present, especially in cases where the patient is receiving triptorelin treatment for the first time. Administration of the drug should occur under strict medical supervision, with emergency medical supplies readily available to manage potential anaphylactic reactions. Although rare, most ADRs linked to triptorelin are type I anaphylaxis, which can be life-threatening. We recommend conducting skin prick tests and intradermal tests, especially when changing pharmaceutical manufacturers to mitigate potential ADRs stemming from adjuvants or preservatives in the drug product. Post-administration, diligent monitoring of the child's condition is essential, coupled with preparedness for prompt first-aid interventions.
Author Contributions
X. Lei and L. Yang were responsible for data collection and result organization. Z.Q. Cao, J.J. Chen, Z.B. Chen, and Z. Min drafted the manuscript. F.Z. Chen and T. Liu undertook the statistical analysis. X.Y. Liu and X.J. Li supervised the manuscript writing and critically revised it for intellectual content. All authors significantly contributed to the work, reviewed and approved the final manuscript.
Funding
This work was supported by Guangdong High-level Hospital Construction Fund.
Institutional Review Board Statement
This study followed the guidelines outlined in the Declaration of Helsinki and was approved by the Ethics Committee of the Shenzhen Children’s Hospital. An exemption for informed consent was applied and approved by the Ethics Committee because only clinical data and completed test results were collected.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zakharova L, Sharova V, Izvolskaia M. Mechanisms of Reciprocal Regulation of Gonadotropin-Releasing Hormone (GnRH)-Producing and Immune Systems: The Role of GnRH, Cytokines and Their Receptors in Early Ontogenesis in Normal and Pathological Conditions. Int J Mol Sci 2020; Volume 22. [CrossRef]
- Metbulut AP, Adiguzel KT, Islamoglu C, Boyraz M, Misirlioglu ED. Evaluation of Hypersensitivity Reactions with Leuprolide Acetate and Triptorelin Acetate in Children. Indian J Endocrinol Metab 2021; Volume 25: pp. 527-531. [CrossRef]
- Sorensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr 2012; Volume 77: pp. 137-145. [CrossRef]
- Martinerie L, de Mouzon J, Blumberg J, et al. Fertility of Women Treated during Childhood with Triptorelin (Depot Formulation) for Central Precocious Puberty: The PREFER Study. Horm Res Paediatr 2020; Volume 93: pp. 529-538. [CrossRef]
- Chan Ng P, Huang CH, Rajakulendran M, et al. Successful desensitization to gonadotropin-releasing hormone analogue triptorelin acetate using a sustained-release depot preparation. Pediatr Allergy Immunol 2018; Volume 29: pp. 660-663. [CrossRef]
- Cheuiche AV, da Silveira LG, de Paula LCP, Lucena IRS, Silveiro SP. Diagnosis and management of precocious sexual maturation: an updated review. Eur J Pediatr 2021; Volume 180: pp. 3073-3087. [CrossRef]
- Karamizadeh Z, Tabebordbar M, Saki F, Karamifar H, Amirhakimi G. The side effects of gonadotropin releasing hormone analog (diphereline) in treatment of idiopathic central precocious puberty. Acta Med Iran 2013; Volume 51: pp. 41-46.
- Lee JW, Kim HJ, Choe YM, et al. Significant adverse reactions to long-acting gonadotropin-releasing hormone agonists for the treatment of central precocious puberty and early onset puberty. Ann Pediatr Endocrinol Metab 2014; Volume 19: pp. 135-140.
- Kirkgoz T, Karakoc-Aydiner E, Bugrul F, et al. Management of Systemic Hypersensitivity Reactions to Gonadotropin-Releasing Hormone Analogues during Treatment of Central Precocious Puberty. Horm Res Paediatr 2020; Volume 93: pp. 66-72. [CrossRef]
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; Volume 356: pp. 1255-1259. [CrossRef]
- Kramer M, Leventhal J, Hutchinson T, Feinstein A. An Algorithm for the Operational Assessment of ADR-JAMA. JAMA 1979; Volume 242.
- Taofikat B. Agbabiaka JScEE. Methods for causality assessment of adverse drug reactions. Drug Safety 2008; Volume 31: pp. 21-37.
- Gallelli L, Ferreri G, Colosimo M, et al. Retrospective analysis of adverse drug reactions to bronchodilators observed in two pulmonary divisions of Catanzaro, Italy. Pharmacol Res 2003; Volume 47: pp. 493-499. [CrossRef]
- Edwards IR, Biriell C. Harmonisation in pharmacovigilance. Drug Saf 1994; Volume 10: pp. 93-102. [CrossRef]
- WHO. The database of the World Health Organization (WHO). Collaborating Centre for International Drug Monitoring. Uppsala, Sweden: Uppsala Monitoring Centre; 2010.
- Grant JP, Jr., Levinson AW. Anaphylaxis to leuprolide acetate depot injection during treatment for prostate cancer. Clin Genitourin Cancer 2007; Volume 5: pp. 284-286. [CrossRef]
- Luchinger AB, Mijatovic V, Rustemeyer T, Hompes PG. Anaphylactic reaction to different gonadotropin-releasing hormone agonists for the treatment of endometriosis. Am J Med Sci 2011; Volume 341: pp. 240-242. [CrossRef]
- Akin O, Yavuz ST, Hacihamdioglu B, Sari E, Gursel O, Yesilkaya E. Anaphylaxis to gonadorelin acetate in a girl with central precocious puberty. J Pediatr Endocrinol Metab 2015; Volume 28(11-12): pp. 1387-1389. [CrossRef]
- Okdemir D, Hatipoglu N, Akar HH, et al. A patient developing anaphylaxis and sensitivity to two different GnRH analogues and a review of literature. J Pediatr Endocrinol Metab 2015; Volume 28: pp. 923-925. [CrossRef]
- Whitelaw CC, Varacallo M. Transient Synovitis. [Updated 2022 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459181/.
- Alamri Y, Cockfield A. Peripheral eosinophilia in children with transient synovitis of the hip: 7-year experience from a single centre in New Zealand. J Child Orthop 2016; Volume 10(3): pp. 215-218. [CrossRef]
- Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev 2011; Volume 242: pp. 161-177. [CrossRef]
- Ramirez GA, Yacoub MR, Ripa M, et al. Eosinophils from Physiology to Disease: A Comprehensive Review. Biomed Res Int 2018. [CrossRef]
- Huebner KD, Shrive NG, Frank CB. Dexamethasone inhibits inflammation and cartilage damage in a new model of post-traumatic osteoarthritis. J Orthop Res 2014; Volume 32: pp. 566-572. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).