1. Introduction
Cotton (Gossypium spp.) provides raw materials to many industries with its various economic features such as natural fiber to the textile sector, cotton seed to the oil sector, and pulp to the livestock sector. This industrial plant, which directly and indirectly affects the livelihood of many people around the world, is drought-tolerant and is cultivated by about 30 countries, primarily China, India, the USA, Pakistan, and Brazil due to their warm climate (Riaz et al. 2013; Statista, 2015). However, intensive cultivation also leads to the emergence of various biotic and abiotic diseases and pests. Abiotic stress factors such as salinity, and drought, prevent the water intake of the cotton, and biotic stress factors such as Verticillium, and Fusarium, xylem vessels are clogged and the plant may not receive sufficient water which causes a decrease in the turgor pressure that provides the verticality of the plants and causes the leaves to wilt and the fruits to be small (Mahmood et al. 2020). In the USA, which is one of the leaders in world cotton production, the production loss due to drought stress in the last 50 years is approximately 67% (Comas et al. 2013), while it is 34% in Pakistan (Dawn News 2016). Drought means that plants suffer from water shortages for a long period on a global scale because of the greenhouse gas effect as a result of human and non-human activities. Drought is one of the most important abiotic stress factors that cause 73% of product loss in cotton plants (Saranga et al. 2009).
Although cotton has a higher tolerance to drought compared to other cultivated industrial field crops when it is exposed to long-term drought stress, undesirable effects such as a decrease in yield, lower biomass, and stem weight, a slowdown in plant development, a decrease in fiber quality, and small bolls may occur [parida et al., 2007]. Drought stress affects cotton as other plants by limiting and adversely affecting parameters such as height, leaf weight, number of nodes, transpiration rate, photosynthesis rate, and stomatal conductivity [Parida et al., 2007, Asati et al., 2022, Zhang et al., 2013, Ranjan et al., 2012, Bowman et al., 2013]. In addition, when it is exposed to long-term drought stress, it closes its stomata, rolls its leaves, tends to have osmotic regulation, and tries to reach water/moisture in the depths of the soil [Hejnák et al.2015)] and the rate of photosynthesis is reduced [Chastain et al., 2014]. Pace et al., [1999] reported that short-term drought during the seedling period increased the cotton roots to reach the deep water but decreased the diameter of the roots. It is revealed that the cotton roots were affected by drought more than the root distribution [Malik et al., 1979]. Insufficient soil moisture reduces the elongation of the roots [Ball et al., 1994, Prior et al., 1995] and the shortened root system is developed approximately 42-70 days after the germination of the cotton [Malik et al., 1979]. This situation seems to coincide with the period of strong droughts.
The responses of the cotton plant to stress factors vary during different stages of development. The seedling stage is a period of high sensitivity to environmental conditions for many plant species, including cotton [Gutterman, 2002]. McCarter (1976) reported low temperature as the main factor affecting the degree of damage by pathogens such as Rhizoctonia and Pythium on cotton seedlings. Brand et al. (2016) discovered that low temperature at the seedling stage in cotton under chilling conditions is strongly associated with a low temperature of the seedling stage diseases such as dumping off, which causes root deterioration. Low temperature at the seedling stage of cotton has a big effect on the damage thresholds of pathogens such as Rhizoctonia and Pythium (Brown and McCarter, 1976). Also, Brown and Seedling emergence rates and development under controlled conditions can determine the sensitivity of varieties to cold weather (Singh et al., 2018; Reddy et al., 1192). When the cotton seeds emerged and were exposed to sub-optimal temperatures in late spring, seedling growth was adversely affected and significant decreases were observed in yield (Bradow and Bauer, 2010). When soil moisture tension generally exceeds 30-50 centibars, drought stress occurs, depending on soil type (Perry et al., 2012).
Various studies have been conducted to understand the genetic mechanism of drought tolerance. Studies such as developing tolerant cultivars to drought through crossbreeding [Kıvılcım et al., 2005)], hormonal regulation against drought and salinity stress [Riemann et al., 2005], understanding the drought tolerance mechanism of cotton and integrating it into variety development breeding programs [Fang & Xiong, 2015], increasing the tolerance to drought using the CRISPR/Cas9 technique [Long et al., 2018], performing Genome-wide associaition (GWAS) analyzes to determine the genetic variations and the relationship between drought and genetic variations of gene candidates that are responsible for some traits in Upland cotton [Hou et al.2018], performing linkage mapping and genome-wide association mapping (GWAS) for Verticillium wilt, thrips, salinity and drought tolerance [Abdelraheem, 2017], determining Quantitative trait loci (QTLs) in cotton germplasm related to tolerance to salinity and drought stress [Abdelraheem et al., 2016], performing Linkage mapping in Recombinant Inbred Lines (RIL) against drought [Abdelraheem et al., 2015], determining genetic stock materials and molecular markers that are tolerant to drought [Adams, 2011], comprehensive QTL analyzes of cotton's economic parameters under the influence of abiotic and biotic stress factors [Said et al., 2013], genome-wide (GWAS) drought-associated marker identification studies [Chen et al., 2013, Padmalatha et al., 2012, Park et al., 2012, Zhao et al., 2016, Li et al., 2020], drought stress generation and determination of tolerant genotypes in cotton plant using Polyethylene Glycol (PEG) [Zhang et al., 2007], determining drought stress related traits in seedling period [Li et al., 2019], strategies for coping with drought stress in cotton and their application to crop improvement programs [Li et al., 2019], Increasing drought tolerance of cotton genotypes by exposing them to salinity during the seedling period [Ullah et al., 2017], allelic genetic diversity of drought-resistant cotton genotypes [Javaid et al., 2017] and identifying cultivars carrying drought genes using Marker-assisted selection (MAS) in cotton [Ulloa et al., 2017] have been carried out.
Research conducted shows that the seedling stage is the period during which the plant reactions can be observed the best [Uniyal and Nautiyal, 1998)]. This study aims to investigate the drought-tolerant level of some elite breeding lines and some genetic stock cultivars of the Upland cotton (Gossypium hirsutum L.) species under artificial drought stress with limited irrigation methods during the seedling stage. In this study, unlike many studies not only are cultivars evaluated under drought stress conditions but also elit breeding lines’ genetic potential against drought is determined.
2. Materials and Methods
2.1. Plant Materials
All of the 93 cotton genetic materials used in the experiment are a panel of both accessions from advanced lines of F
3 and commercial varieties of
G. hirsutum L. cotton with allotetraploid (2n=4x=52) chromosome set with AD genome group. The genotypes were provided by East Mediterranean Transitional Zone Agricultural Research Institute, East Mediterranean Agricultural Research Institute, GAP International Agricultural Research and Training Institute, SET seed company (
www.settohum.com/), STARseed, MAY (
www.may.com.tr/), BASF (
www.agro.basf.com.tr) and Progen Inc. seed companies (
www.progenseed.com/). The commercial cultivars’ properties such as cotton seed yield, resistance to biotic and abiotic stresses, fiber yield and quality, adaption, and climate requirements are known. Due to the continuous segregation, the economic properties of the elite breeding lines are still not clear, this experiment provides information about individuals of advanced lines including reactions against drought.
2.2. Method
The pattern of the trial is a completed randomized plots trial design with 3 replications. The experiment was conducted at Bingol University, Genc Vocational School (Coordinates: 38 ° 442 58” N and 40 ° 32’ 11” E), climate chamber. The soil used is prepared by using peat, perlite, and soil at a ratio of 3:1:1. All three components were sterilized with an autoclave at 121°C for 15 minutes and transferred to 200 ml plastic pots. Triangular holes were made at the bottom of the plastic pots for the excess water to be drained. 4 seeds were sowed in each pot at a depth of about 2 cm and potting soil was brought to the field capacity. For control purposes, STV373 (G86), BA119 (G56, White gold), and TEX (G93) varieties adapted to the Southeastern Anatolia Region, where the experiment was established, were used. In the experiment, three pots were used for each genotype as 3 replications. The soil temperature was raised to 15°C for seeds to germinate and since the experiment was established in winter, the cotton seeds, which are likely to be dormant, were kept in a hot water bath device at 65°C for 15-30 minutes before sowing to break the dormancy. Then, the ambient temperature was adjusted to be between 28-38°C and the humidity was between 57-67% [Basal et al., 2005].
The LED lights were adjusted to provide daylight at 2.500 lux for 14-15 hours a day, and each pot was given 0.2 g of pure nitrogen-containing urea fertilizer for effective growth 14 days after planting. From the starting point of the experiment, cotton genotypes were irrigated with 100% irrigation every 10 days until true leaves bloomed. All genotypes were fully watered with 80 mL-1 of water (100% irrigation) until three true leaves appeared. For cotton plants to develop optimally, the soil should contain 60% humidity of the field capacity [Wrona, 2005]. As soon as all genotypes opened true leaves, the irrigation was limited. Then, water stress was applied at limited irrigation of 75% (60 mL-1), 50% (40 mL-1), and 25% (20 mL-1) of field capacity. The experiment was terminated on the 52th day.
The cotton plants were taken out of their pots intact and the soil was washed using tap water without damaging the roots, and parameters such as the Root Length (RL), Root Fresh Weight (RFW), Root Dry Weight (RDW), Shoot length (SL), Shoot Fresh Weight (SFW), Shoot dry weight (SDW) and Number of lateral roots (NLRs) of one plant from each replication genotype were measured. RL was calculated by direct measurement of fresh taproots, RFW by direct weighting of taproots, RDW was measured after dried in the oven after 24 hours at 60° C, NLRs by direct counting of roots before drying, SL by measuring with a ruler, SFW by calculation with a scale with 0.5 mg precision, SDW was measured after dried in oven after 24 hours at 60° C [Basal et al., 2005]
2.3. Data Analysis
Analysis of variance (ANOVA) using data from these drought-related parameters was carried out using the statistical software JUMP 17.0 (JMP®, Version <17>. SAS Institute Inc., Cary, NC, 1989–2021) [JMP, 17]. and the significance of the difference between the means of the parameters was investigated (p<0.05). The least significant difference (LSD) test was used to compare the means.
3. Results
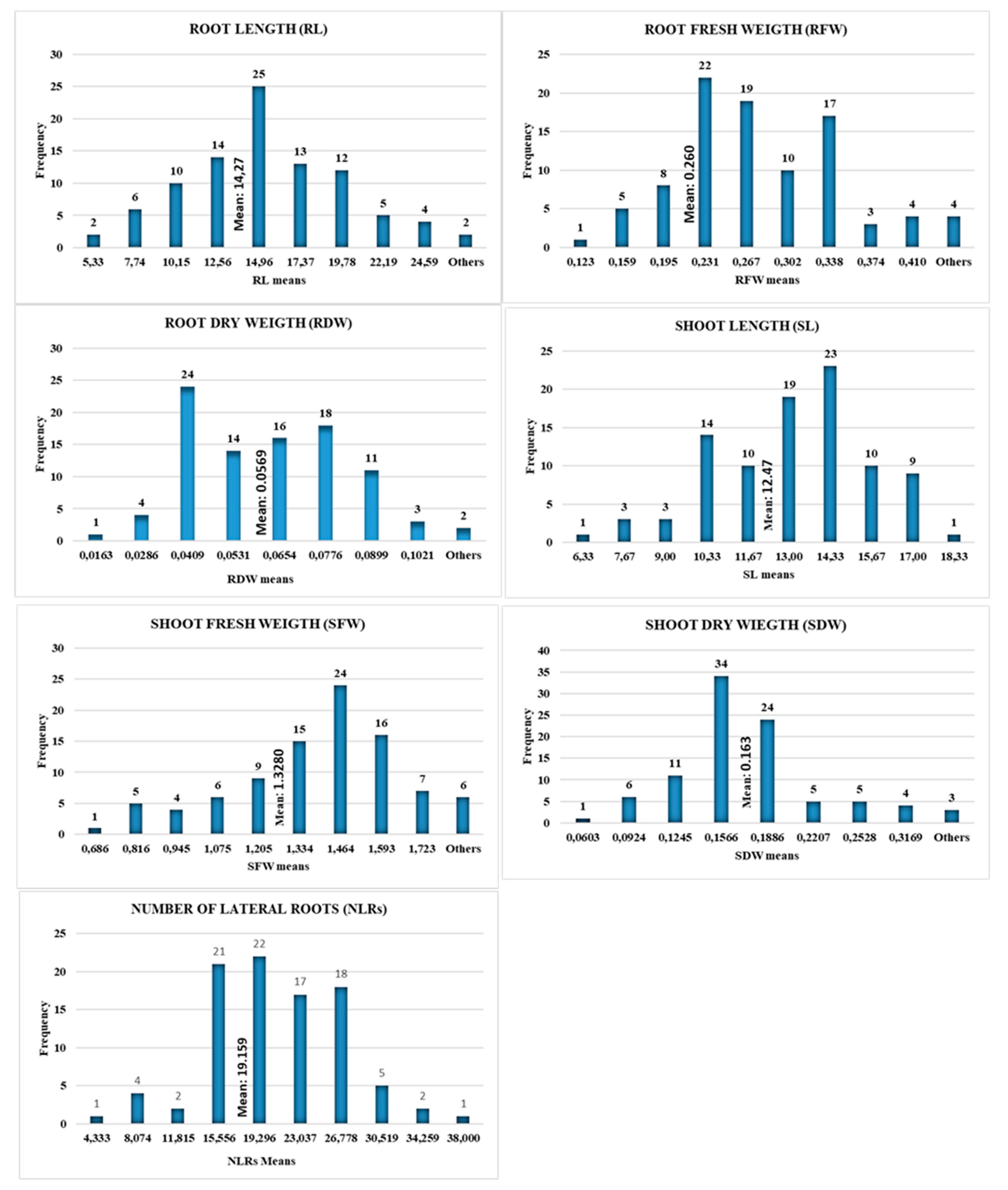
In the experiment, the RL, RFW, RDW, NLRs, SL, SFW, and SDW of the cotton germplasm panel were measured. As a result of the statistical analysis, it was observed that there was a highly significant difference between the means for each parameter (P<0.01).
The first plant organ that is exposed to water stress is the roots, and by transmitting this stress to the rest of the plant, it goes through Morphological, physiological, and metabolic changes and the plant starts to activate its mechanisms to cope with water stress (Kang et al, 2022). In this study, the genotypes with the longest roots (the ones with the highest tolerance to drought) were G56 (27 cm), and G44 (25,6), followed by GG5 and G86 cultivars with 24.3 and 23.6 cm root lengths. G76 and G41 had the shortest RL (5,3 cm), and it was followed by G35 line, G14 (5,7 cm), and G79, G73, and G7 (7,3 cm) cm) genotypes (
Supplementary Table S1). The mean RL of the population was 14.27 cm (
Figure 1,
Supplementary Table S1).
Figure 1A shows the mean distribution frequency of genotypes regarding the RL. The developed root system turns towards the direction where soil moisture is intense and solves the water problem of the plant, and the roots disperse to the deep and to the sides to enable the plant to hold onto the soil more strongly under harsh weather conditions. Similar studies have been conducted on the length and width of the roots.
The responses of plant roots under drought stress have become the focus of researchers in recent years (Gupta et al., 2020, Yamaguchi and Sharp, 2010, Hu and Xiong, 2014). A great deal of research is being conducted to understand the physiological, morphological, and metabolic reactions of plants against drought in roots (Kang et al. 2022; Xiong et al., 2018, Ahmad et al., 2016). Under drought stress, the roots of the plants tend to go deeper and aim to reach the underground water, and these behaviors of the roots also play an important role in activating other mechanisms related to drought [Španić et al., 2017]. Anjum et al [2011] indicated that a strong root system plays a very critical role in physiological functions, carbohydrate storage, uptaking, and absorption of water and nutrients from the soil. In the first stage of drought plants, slow down the elongation of the stem, and develop the root system to reach the deeper water [Kul et al., 2020].
Root weight is an indicator that the plant tries to develop the root system by transferring all its strength to the roots to absorb water from the soil under water stress. The mean RFW of the entire population in the study was 0.26 g. The lowest root Fresh weight was (0.123 g) recorded in G76 and G41 (0.136 g) Recombinant inbred lines (RIL) genotypes followed by G35 (0.140 g), and the highest root fresh weight was recorded in G1 (0.446 g) elite line, and these were followed by the cv. G56 (0.427 g), G44 (0.424 g), commercial cultivar G86 (0.411 g), G51 (0.397 g), and G88 (0.384 g) (
Supplementary Table S1,
Figure 1). The research showed that there is a positive correlation between root weight and drought and that the genotypes with quantitatively higher root weight had higher tolerance to drought stress than others. According to
Figure 1B RW-frequency histogram graph, the number of genotypes with a mean root weight above the mean of the whole population (0.260 g) is 79 (
Supplementary Table S1). This may be an indication that most of the individuals in the population can activate their tolerance mechanisms against drought stress. The cotton plant, which tries to develop and strengthen its root system when drought stress begins, completely stops the development of both roots and other organs under long-term drought stress [Anjum et al., 2011].
The number and development of lateral roots is an important indicator of drought tolerance. The development of the root system, which allows plants to stand, hold on to the soil, give yield, and grow and develop, plays an active role in the fight against agricultural drought. Wang et al. (2017) stated that many previous studies related to crop root system improvement suggest that increasing the number of lateral roots (NLRs) could characterize plant development and increase crop yield. In the current study, the G44 (38) RIL genotype had the most number of lateral roots (NLRs), and it was followed by G86 (33), Commercial cv. G56 (32), and elite lines G13 (30), G5 (29,33), and G49 (29), from the F
3 period of the segregation. The mean of lateral roots of all genotypes was 19.16 (
Supplementary Table S1). The number, development, and growth of lateral roots, are critical for the development and yield of field crops [Barber and Silberbush, 1984, Russel, 1977]. In another study, Forde and Lorenzo, (2001) stated that the lateral roots, which are important in plants with a taproot system, initially act as a taproot and take water and nutrients dissolved in water. To increase tolerance to drought stress, it was seen that researchers should focus on the development of genotypes of lateral root systems. It was seen that the drought-resistance RIL genotype G44 (38), G86 (33), and G56 (32) varieties, which have good adaptation in the Southeast Anatolia region of Turkey, where cotton is mainly cultivated, develop better lateral root systems under drought stress.
Root dry weight (RDW) is also one of the phenotypic markers used to see the reactions of the cotton against drought. Accordingly, the mean RDW of the population consisting of 93 genotypes was 0.06 g. The elite genotype G5 recorded the highest RDW value (0.127 g) followed by cv. G56 (0.112 g) and the elite breeding line G44 (0.099 g), G75 (0.092 g), and cv. G90 (0.091), and this value was followed by G85 (0.088 g), and G7 (0.085 g) breeding genotypes. It is possible to say that the drought resistance genes show themself in the phenotype of G5, G56, and G44 cultivars. Control cultivar G56 is well adapted to the Southeastern Anatolia and Mediterranean regions of Turkey, where cotton cultivation is most common, and shows tolerance above the general mean, are used as control varieties in the research (
Supplementary Table S1). The mean RDW of the 50 genotypes used was above the general mean. Liu and Stützel [2004) pointed out that under long-term drought stress root dry weight increased and leaf area decreased in China spinach.
The difference between the Shoot Length (SL) mean of the population was found to be highly significant (p<0.01). Accordingly, the mean total Shoot Length of the genotypes was 12.47 cm. G35 (18.33 cm) had the longest SL, and it was followed by G15-G29 (16.67 cm), and G76 (16.33 cm) breeding genotypes, respectively. Commercial cultivar G65 (6.33 cm) showed the lowest SL means followed by Elit lines G72-G32 (7.33 cm) and Local variety G22 (7.33) (
Supplementary Table S1).
The difference between the Shoot Fresh Weight (SFW) mean of the population was found to be highly significant (p<0.01). The mean SFW of 93 upland cotton genotypes was 1.33 grams. The highest SFW values were recorded in the genotypes Elite line G15 (1.853 g), G52 (1.815 g), and commercial variety G60 (1.805 g), respectively. These were followed by G31 (1,752 g), G41 (1.736 g), G47 (1,724 g), G14 (1,700 g) and the Commercial local variety G68 (1,691 g). The lowest SFW value was measured in RIL genotype G83 (0.686 g), followed by G2 (0.716 g), G75 (0.739 g), and G91 (0.740 g), respectively. As a result of statistical analysis, the difference between the mean SDW of the population was found to be statistically very significant (p<0.01). SDW mean of all genotypes was recorded as 0.16 g. The highest SDW value was recorded in G35 (0.349 g), the lowest in the G65 (0.060 g) genotype. While commercial cultivars such as G57 (0.337 g), G60 (0.294 g), and G66 (0.289 g) showed high mean SDW values, the elite lines of G52 (0.345 g) and G41 (0.314) showed very high SDW values. The second lowest SDW value was found at commercial variety G91 (0.078 g) and G4 (0.078 g), G20 (0.078 g) followed by G22 (0.081 g), and Cv. G90 (0.087 g) (
Table 2,
Figure 2)
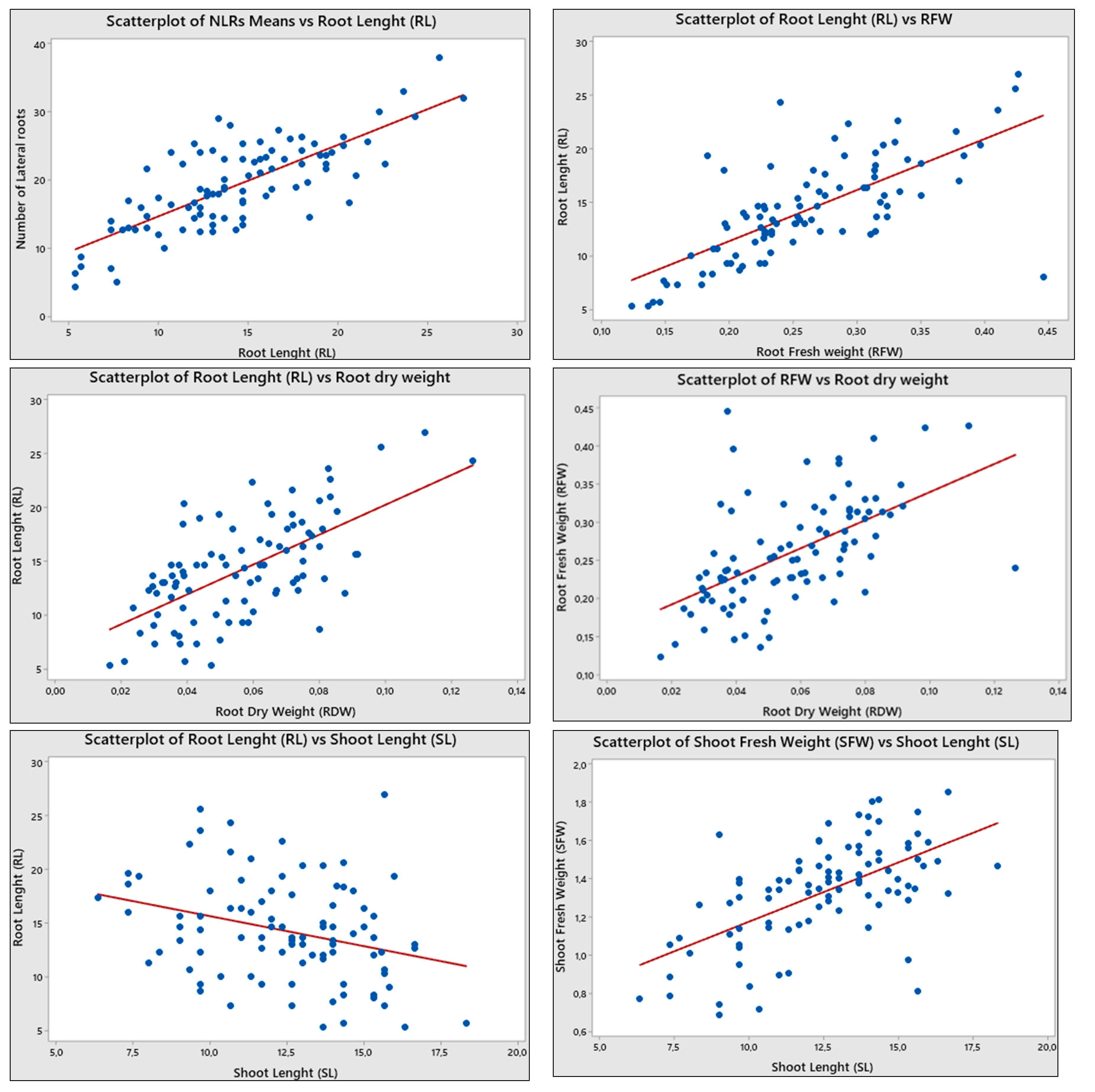
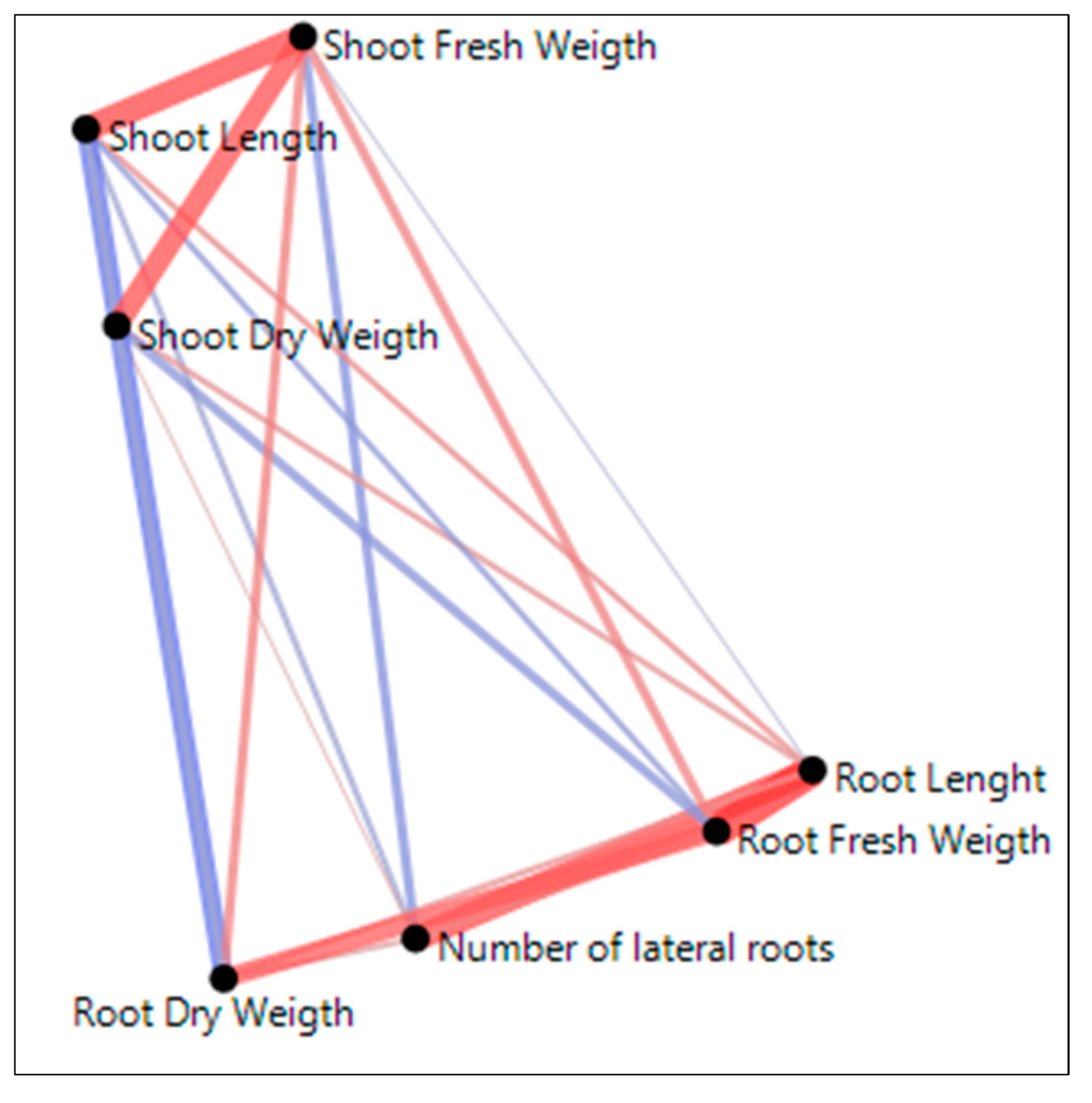
Scatter plot analysis is done to determine the relationship and relationship type between every two variables of drought indicators. Yi [2022] emphasized that the closer they get to the linear line, the more the relationship between parameters increases. According to
Figure 2 scatter plots and correlation diagram, while the strongest positive correlation is viewed between RL and RFW (0.823), followed by NLRs and RL (0.759), RFW and NLRs (0.679), RFW-RDW (0.642), RL-RDW (0.568) and NLRs-RDW (0.494); the strongest negative colerations took place between SL-RDW (-0.446) and followed by RFW-SL (-0.327), NLRs-SL )-0.318), NLRs-SFW (-0.219) and SDW-RFW (-0.143) (
Table 2,
Figure 2 and
Figure 3). Similar results were found in literature as Lesch and Corwin [2008] expressed the cotton yield and electrical conductivity of the saturation extract (Ece, dS/m), which is one of the causes of drought relation using Scatter plot analysis and indicated that until the level of 7.17 dS/m the cotton yield is increased and after that point yield started to decrease.
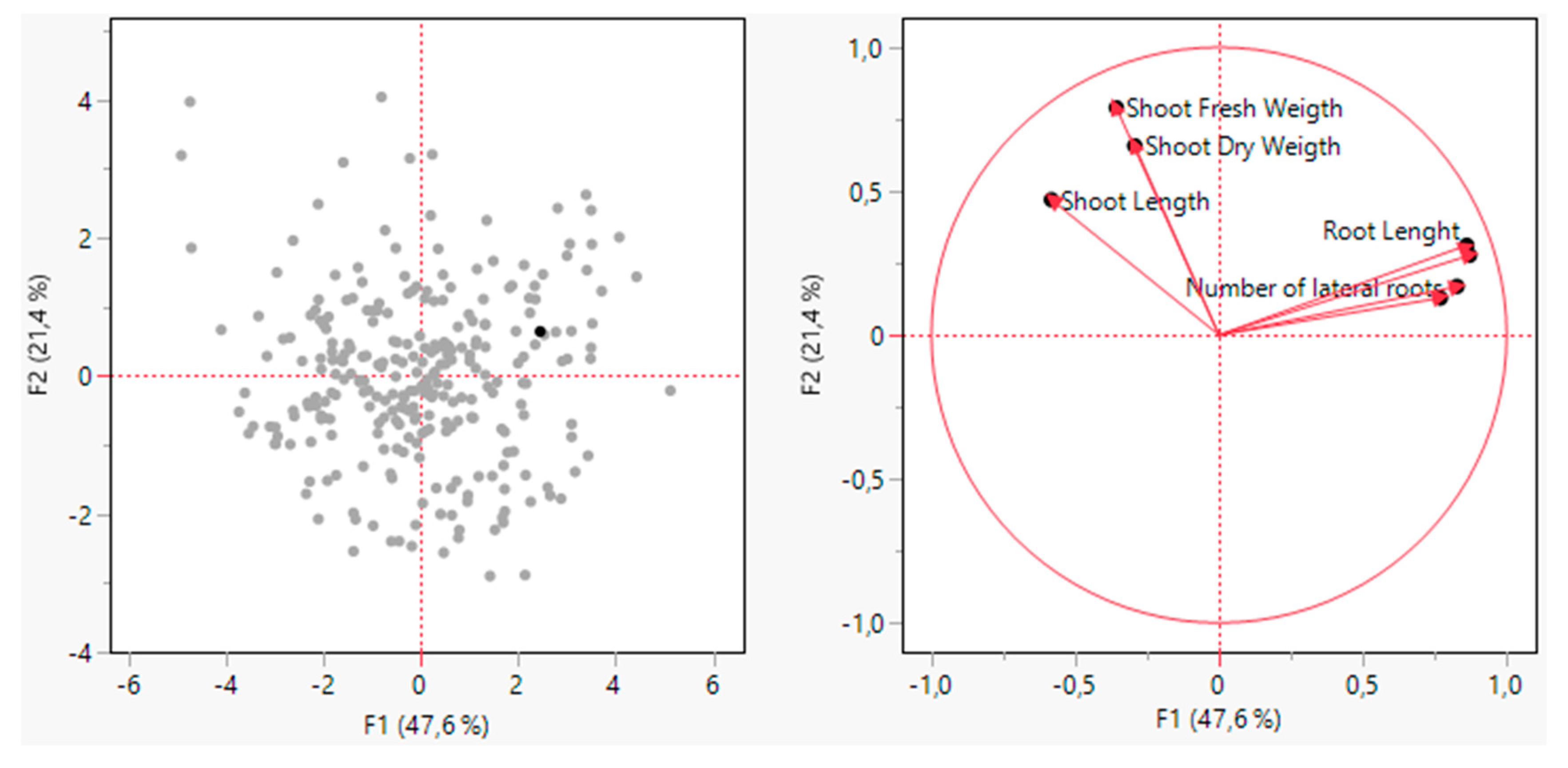
Principal component analysis (PCA) is a very common statistical analysis to reduce the dimensionality of large data sets. It’s a technique for identifying patterns of data, expressing the data, and highlighting their similarities and dissimilarities (Mishra et al., 2017). The graph (
Figure 4) is the loading plot from the PCA analysis. The results of PCA are given in
Figure 4. The PCA analysis was conducted based on the morphological drought parameters and in the correlation circle the first axes (F1) and the second axes (F2) represent the discriminant function analysis (DFA) respectively 47.6% and 21.4% of variable. Among the drought measurement parameters of the cotton plant's aboveground portion, the strongest positive correlation was found between Shoot dry weight (SDW) and Shoot fresh weight (SFW), while shoot length (SL) showed a positive correlation with both SFW and SDW (
Figure 4). Accordingly, as SL increases, it appears that SFW and SDW values increase positively. In terms of underground drought measurement morphological parameters, a very close positive correlation between them is observed. The strongest positive correlation was found between Root length (RL) and Root Fresh weight (RFW), while a significant positive correlation was also found between the number of lateral roots (NLRs) and Root dry weight (RDW). Generally, there is a negative correlation between aboveground drought markers and underground markers in cotton plants. This situation can be explained by the development of aboveground parts being hindered due to strengthening the roots of cotton plants under water stress, transferring photosynthesis products from aboveground parts to the roots to draw water from the soil more effectively (
Figure 4).
4. Discussion and Conclusions
When the cotton is exposed to drought stress, the Glucose (C
6H
12O
6) produced during the photosynthesis event is transmitted to the roots through the phloem transmission bundle and triggers the development of the root system. Photosynthesis plays a significant role in plant development and is most affected by water deficiency in the soil. Studies show that the biggest side effects of drought occur on photosynthesis and light utilization efficiency in cotton leaves [Tang et al., 2005, Ko and Piccinni, 2009]. Photosynthetic components react at certain rates and as in Liebig's Law of Minimum [Ebelhar et al., 2008], the rate of photosynthesis is determined by the lowest amount of water (H
2O). As photosynthesis takes place with water (H
2O), when a cotton plant under drought stress cannot get enough water, the turgor pressure drops, the defoliation, and the plant rolls its leaves to reduce the sun’s effects to minimize the plant’s water loss through transpiration [
14]. Since the photosynthesis rate of the plant whose photosynthesis products cannot be transferred to the plant roots, the nutrients previously stored in the leaves are transferred to the roots; hence, the fruits of the plant remain small, and the yield decreases [Wanjura et al., 1984, Peynircioğlu, 2014, Kaçar, 2007].
It’s a current trend in plant breeding to select the genotypes with the longest root as drought tolerance (Paez-Garcia et al., 2015, Comas et al., 2013). It was seen in this study that several elite breeding lines had a high tolerance to drought in some drought measurement parameters. Cv. Commercial variety G56 had the longest RL (27 cm) and was followed by G44 (25.67 cm), G5 (24.33 cm), and G86 (23.67 cm). Similar results were found by [Basal et al.2005] that are reported that taproots had a root length between 47.9 and 86.6 cm under full irrigation, and between 48.3 and 82.5 cm under restricted irrigation in their experiment. The higher root length means of Basal et al. (2005) compared to our results are believed to be dependent on the experimental conditions, trial materials, origins of the plant varieties used, amount of water used, and the duration of the experiment. Root Length growth has a great impact on uptaking nutrients and root system architecture (Tian et al., 2014, Sun et al., 2017). Only two cultivars were noted to have significant root shortening. In the study, although the humidity and temperature in the greenhouse can increase excessively during the day, the amount of soil mixture used was parallel to our study, and a longer taproot system was observed. Research showed that immersing the roots in depths where the moisture content is high can compensate for the moisture losses that occur through evapotranspiration [Nguyen et al., 2004]. In the current study, G76 and G41 advanced lines produced 5.33 cm RL as the lowest and followed by G35, G14 (5.7), G79, G73, and G7 (7.3 cm). Similar root length (RL) values were obtained by Pawar and Veena (2020) with the highest root length as 12.72, 12.17, and 12.88 cm, the lowest as 9.8, 9.85, and 10.5 cm root lengths with the PEG 6000 drought application. At the same time, Pawar and Veena revealed that the root length increased when the PEG 6000 application was increased by 10 % and decreased after that level. This situation can be explained with the plant root tolerance mechanism of the plant: The plant sents more photosynthesis products to the root in order to strengthen the roots and uptaking more water from the soil (Babu et al., 2014). In a study, Zahid et al. (2021) found a highly significant correlation between genotypes and watering, and in their study, they found the root length as 5,161 cm. According to the current study with a 27 cm mean root length, 5.161 cm is highly low and the irrigation period, the amount of water given in each irrigation, soil mixture, infiltration and germination ability, and speed can be shown as the reasons why it is found to be lower than our study. Wang et al [2009] reported that the distribution of photosynthetic products in the roots of beans increased under drought stress which did not last long, but the photosynthetic products in the production organs decreased significantly. It was observed that the plant tries to strengthen its root system under long-lasting drought stress; hence, severely reducing its yield and economic added value as a result of giving all its weight to the roots. However, it was reported that moderate water stress during the grain-filling period of the wheat plant increases the transport of photosynthetic products to the roots, and this provides a significant benefit to the wheat, as the wheat will have developed roots and thus take more moisture and therefore more nutrients from the soil [Proffitt et al., 1985]. Studies reported that a situation similar to the growth period of wheat can be observed in cotton, that moderate water stress causes photosynthetic products to be transported to the roots by vascular bundles and has a similar positive effect on buds and bolls [Shan and Zhang, 2006].
While breeding line cultivar G1 (0.446 g) has the weighest Root Fresh Weight value and is followed by Cv. G56 (0.427 g), G44 (0.424 g), commercial variety G86 (0.411 g), G51 (0.397 g), and G88 (0.384 g); and the lowest Root weight (RW) values belong to advanced lines G76 (0.123 g), G41 (0.136 g), G35 (0.140 g), G14 (0.145 g) and G47 (0.148 g). In our study on another drought indicator, root dry weight (RDW), the highest value was recorded in the advanced line G5 (0.127 g), followed by the control variety G56 (0.112 g), G44 (0.099 g), G75 (0.092 g), and control variety G90 (0.091 g). The lowest RDW values were recorded in the advanced line G76 (0.016 g), followed by genotypes G35 (0.021 g), G20 (0.024 g), G61 (0.026 g), G70 (0.028 g), G59-G15 (0.029 g), and G67 (0.030 g). Istiqomah et al. (2021) found a very significant difference between the applications in terms of dry root weight or total dry weight in the corn plant. Similar to our current study conducted by Shah et al. [2011] they obtained root weight ranging from 0.049 to 0.155 g under water stress in a trial conducted under normal and water stress for 52 days. A higher root weight uptake more plant nutrients from the soil and plays a key role in vegetative and generative development (Istiqomah et al., 2021). The fact that the ambient temperature fell below the optimum level due to unpredictable power cuts at night or on weekends in the experiment, which was established in laboratory conditions in the winter season, may have caused some drought measurement parameters to be below normal. Root growth is a critical process to be grown under sustainable temperatures [Mai et al., 2018, Gavelienė et al., 2022]. Zahid et al [2016] stated that 22-30°C is an optimal temperature for cotton root, shoot, and root/shoot (R/S) ratio, but a temperature between 32-40°C will restrict the distribution and growth of the root/shoot system. A similar approach was declared by Koevoets et al. [2016] as the optimal temperature promotes the increase of root/shoot ratio (R/S ratio), nevertheless, a temperature above optima temperature will decrease water absorption and plant nutrients by cotton plant root system as a results root system get weaker against drought [Luo et al., 2020]. The decrease in soil water content by evaporation significantly reduces root activities, but hydraulic lifting and transpiration by roots can carry groundwater to levels close to the soil surface (to 0-40 cm soil depth) [Chiatante et al., 1999].
Lateral roots and numbers play a key role in plants reaching and absorbing the water (Varney and Canny, 1993) and uptaking the plant nutrients such as phosphorus (Lambers et al., 2006). Hund et al. (2007) and Hund et al. (2008) Put forward that lateral root numbers can possibly have positive effects of vigorous growth under low temperatures in maize plants. Considering the studies in the literature, it is seen that the number of lateral roots is a drought marker that increases the tolerance of plants against drought stress. The number of lateral roots (NLRs) population means changed from 5 to 38. Advanced line G44 (38) showed the highest NLRs and this value was followed by G86 (33), Cv. Variety G56 (32), G13 (30), and G5 (29.33). The lowest NLRs values ranged from 5 to 10. In the current study, the lowest NLRs were recorded in G47 (5), followed by G76 (6.33), and G79 (7) (
Supplementary Table S1). McMichael et al. (1987) obtained similar NLRs values ranging from 4 to 50 at 30° C within 7 days after germination. The reason of the higher LRNs values may be associated with not applied drought stress in the first 7 days and plenty of water and plant nutrients. Gallardo et al. [
83] reported that enough water in soil accelerates the root development, and Luo et al. [2014] reported that moderate water stress in the middle and upper layers of the soil strengthens the root system in the early stage of cotton plant development and contributes significantly to reaching deep waters. four strands of cotton and generally 120 exotic cotton genotypes provided NLRs means changed from 3.5, 17.5, 25.97, and 49.75, respectively (McMichael et al. 1987). Lateral root initiation (LRI) and development is coordinated by many genes. In
Arabidopsis thaliana, there are more than 7 genes associated with lateral root growth (Benková et al., 2003; De Smet et al., 2006). Lateral root number and development is regulated by diverse many hormones and their interaction (Shkolnik et al., 2010; Nishiyama et al., 2011; Ha et al., 2012). Even though the current study breeding lines have developed good taproot systems, they may not show resistance when faced with high temperatures compared to commercial cultivars adapted in warm regions.
As a result of this study, it has been observed that Shoot Length (SL) during the seedling stage is one of the organs most affected by drought in cotton, the population means were obtained as 12.47, and genotype means ranged from 18.33 cm (G35) to 6.33 cm (G91). Iqbal et al. (2020) reported that drought during the seedling stage negatively affects both the shoot and root morphology of cotton, reducing shoot length by 29%. A study indicated that drought stress causes a significant reduction in shoot elongation and the percentage of decreasing SL ranging from 97.14 to 35.1 (Zahid et al., 2021). Simonneau et al. (1993) indicated that a reduction the in shoot or in the root may be caused by the imbalance of water.
The genotype G35 has emerged as the most tolerant genotype in both parameters, with a mean shoot length (SL) of 18.33 cm and shoot dry weight (SDW) of 0.349 g. Meanwhile, genotype G15 registers the second-highest mean SL, and the same genotype yields the highest mean shoot fresh weight (SFW) (
Supplementary Table S1). These values indicate that an increase in shoot length does not always correspond to the highest shoot fresh weight; however, there is a positive correlation between an increase in shoot length and shoot fresh weight. A similar situation is observed in the tolerant genotype G31 for the SL and SFW parameters. On the contrary, the genotype G91, which has the lowest SL value, becomes the fourth genotype with the lowest SFW means. Generally, genotypes with higher SL means have higher SFW means, and genotypes with lower SL means have lower SFW values. In the experiment, among plants that shade each other, those receiving less light exhibit higher shoot length but lower shoot fresh and shoot dry weights compared to plants receiving full light.
This situation can be explained by the hypothesis that as the amount of light increases, the plant enhances its photosynthetic rate and consequently increases its biomass. Based on the conducted phenotypic observations, it has been observed that under greenhouse conditions, cotton genotypes exhibit shorter shoot lengths compared to the same duration of laboratory trials. However, it is evident that the experiment established in greenhouse conditions has significantly longer root lengths and a higher lateral root count compared to the laboratory drought trial. This suggests that when the plant experiences actual water stress, it strengthens its roots and engages in coping mechanisms to deal with water scarcity. This response aims to minimize the impact of drought and indicates the activation of defense mechanisms to mitigate the effects of water stress (Çelik, 2023, not published).The genotype G35, which shows the highest value in shoot dry weight (SDW), also exhibits the highest value in the shoot length (SL) parameter as a drought marker. Genotypes G22 and G91 have low averages in both SL, SFW, and SDW parameters, indicating their low tolerance to drought stress (
Supplementary Table S1).
Genotypes G60, G52, and G41 have produced high averages in both shoot fresh weight (SFW) and SDW parameters. In genotype G52, as the SFW value increases, a proportional increase in SDW value is observed, indicating a positive correlation between them. On the other hand, the genotype G60, which shows the highest value in SFW, ranks sixth in SDW. This suggests that while the SFW value increases, it doesn't necessarily follow a linear correlation with SDW. In the SL marker, genotypes G35, G37, and G29, in the SFW marker, genotypes G15, G52, G60, G31, G41, and in the SDW marker, genotypes G35, G52, G57, G41, and G60, all appear to be more tolerant compared to the control genotypes G90, G56, and G5 (
Supplementary Table S1).
In this study, it has been observed that there is a negative relationship between root-related drought markers and shoot markers in terms of the obtained values. None of the genotypes that ranked highest in any of the root-related markers appeared as high-ranking in the shoot markers. This suggests that when the plant faces water stress, it initially focuses on strengthening its root system for a certain period, and later continues on strengthening the shoots. This behavior indicates that under abiotic stress conditions, the plant doesn't allocate resources evenly between shoots and roots, but rather prioritizes different aspects at different stages of stress.
5. Conclusions
Global warming, which occurs as a result of the greenhouse gas effect and various human activities, causes global climate change. As a result, precipitation regimes and precipitation areas varied. When the correlation between the decrease in Agricultural yield and human nutrition is considered, drought is a crucial issue that the whole world should focus on. Thus, it is necessary to use the available amount of water effectively to provide maximum benefit, to implement drip irrigation methods that will avoid water waste and provide high efficiency, and to increase the drought tolerance characteristics of hot climate plants to be grown in the drought season.
The current study aimed to determine the drought tolerance of the Gossypium hirsutum L. cotton species, which is the direct and indirect livelihood of millions of people. Root system parameters such as RL, RFW, RDW, LNRs, SL, SFW and SDW indicate that when the plant faced drought stress, a sugary compound, glucose (C6H12O6), which is produced of photosynthesis, for a while at the beginning of the stress, is transmitted from the leaves to the roots through the phloem conduction bundle and stored in the roots. In the current study, the first six genotypes with the highest values in each parameter and the genotypes that were common in two or more drought measurement parameters are considered genotypes with high drought tolerance.
In this study, genotypes with the highest values for multiple parameters and genotypes with the highest values for each drought measurement parameter are considered genotypes with high drought tolerance. In previous studies, parameters such as RL, RFW, RDW, NLRs, SL, SFW, and SDW have been used as markers for drought measurement, and certain genotypes, including control varieties (G56, G90, and G86), have shown high values for some of these parameters. Therefore, it is possible to state that some genotypes are tolerant to drought due to their high values in certain parameters.
Genotypes G44, G56, and NLRs exhibit common high values in all four drought indicators: NLRs, RL, RFW, and RDW. Genotypes G13 and G5 share high values in NLRs and RL, while genotype G5 coincides in NLRs, RL, and RDW. Genotypes G1, G56, G51, and G88 show the highest values in RFW, and genotypes G5, G56, G44, G75, and G90 consistently yield the highest means in the RDW parameter. These genotypes (G5, G56, G44, G75, G90) are considered to be highly drought-tolerant genotypes. Genotype G35, shows the highest mean values in both shoot length (SL) and shoot dry weight (SDW), genotype G15, shares high values in both SL and shoot fresh weight (SFW), and genotypes G41, G52, and G60 with high values in both SFW and SDW parameters, demonstrate high drought tolerance.
Conversely, genotypes G22 and G91, sharing the lowest means in all three shoot-related parameters, as well as genotypes G83, G2, and G75 in SFW, and genotypes G65 and G4 in SDW, exhibit low drought tolerance. The study's outcome suggests that commercial varieties showing high drought tolerance could potentially be used as parent plants in breeding programs aimed at developing drought-tolerant cotton varieties. It is also possible for the genes/QTLs responsible for this tolerance to transfer from the donor plants to the recipient plants through hybridization.
The drought-tolerant elite breeding lines are used to obtain Recombinant inbred lines (RIL), and after adaptation in different regions by determining some technological parameters such as the seed cotton yield, and fiber quality parameters will be able to release new cotton varieties. In order to develop varieties with high drought tolerance for the Marker-assisted selection (MAS) breeding program to be carried out, it is strongly recommended that they be used as parents after genetic diversity analyses have been carried out and the kinship distances between them have been determined. Additionally, as a result of the observations, measurements, and evaluations made in the current study, as well as the phenotypic observations made on perennial herbaceous plants in field conditions, it can be argued that one of the most important factors that convert a plant from an annual to a perennial is having a strong root system.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. All the data and materials are available
Author Contributions
Only one author contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Sadettin ÇELİK. The first draft of the manuscript was written by Sadettin ÇELİK who commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript
Acknowledgments
I am thankful for the support of the by East Mediterranean Transitional Zone Agricultural Research Institute, East Mediterranean Agricultural Research Institute, GAP International Agricultural Research, and Training Institutes SET seed, MAY, BASF, and Progen Inc. seed companies which provided me cotton seed and to the Bingol University, Genç Vaccaional school personnel and students for assistance during all processes of the experiment
Conflicts of Interest
The authors declare no conflict or interest.
References
- Riaz, M.; Farooq, J.; Sakhawat, G.; Mahmood, A.; Sadiq, M.A. and Yaseen, M.; 2013. Genotypic variability for root/shoot parameters under water stress in some advanced lines of cotton (Gossypium hirsutum L.). Genet. Mol. Res, 2013, 12(1), pp.552-61. [CrossRef]
- Statista. http://www.statista.com/statistics/263055/cotton-production-worldwide-by-top-countries/. 2015.
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; & Dierig, D.A. Root traits contributing to plant productivity under drought. Frontiers in plant science, 2013.4, 442. [CrossRef]
- Dawn, news. Cotton production plummets. 2016.34pc. http://www.dawn.com/news/1240448.
- Guo, X.; Ullah, A.; Siuta, D.; Kukfisz, B.; Iqbal, S. Role of WRKY Transcription Factors in Regulation of Abiotic Stress Responses in Cotton. Life 2022, 12, 1410. [CrossRef]
- Mushtaq, N.; Iqbal, S.; Hayat, F.; Raziq, A.; Ayaz, A.; Zaman, W. Melatonin in Micro-Tom Tomato: Improved Drought Tolerance via the Regulation of the Photosynthetic Apparatus, Membrane Stability, Osmoprotectants, and Root System. Life 2022, 12, 1922. [CrossRef]
- Saranga, Y.; Paterson, A.H. and Levi, A. Bridging classical and molecular genetics of abiotic stress resistance in cotton. In Genetics and genomics of cotton (pp. 337-352). Springer, 2009, New York, NY. [CrossRef]
- Parida, A.K.; Dagaonkar, V.S.; Phalak, M.S.; Umalkar, G.V. and Aurangabadkar, L.P. Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotechnology Reports, 2007, 1(1), pp.37-48. [CrossRef]
- Asati, R.; Tripathi, M.K.; Tiwari, S.; Yadav, R.K.; Tripathi, N. Molecular Breeding and Drought Tolerance in Chickpea. Life 2022, 12, 1846. [CrossRef]
- Zhang, X.; Yao, D.; Wang, Q.; Xu, W.; Wei, Q.; Wang, C., ... & Li, F. mRNA-seq analysis of the Gossypium arboreum transcriptome reveals tissue selective signaling in response to water stress during the seedling stage. PloS one, 2013, 8(1), e54762. [CrossRef]
- Ranjan, A.; Nigam, D.; Asif, M.H.; Singh, R.; Ranjan, S.; Mantri, S.; Pandey, N.; Trivedi, I.; Rai, K.M.; Jena, S.N. and Koul, B. Genome-wide expression profiling of two accessions of G. herbaceum L. in response to drought. BMC genomics, 2012, 13(1), pp.1-18. [CrossRef]
- Bowman, M.J.; Park, W.; Bauer, P.J.; Udall, JA.; Page, JT.; Raney, J.; ... & Campbell, B. T. RNA-Seq transcriptome profiling of upland cotton (Gossypium hirsutum L.) root tissue under water-deficit stress. PLoS One, 2013. 8(12), e82634. [CrossRef]
- Hejnák, V.; Tatar, Atasoy, G.D.; Martinková, J.; Çelen, A.E.; Hniliˇcka, F.; Skalický, M. Growth, and photosynthesis of upland and Pima cotton: Response to drought and heat stress. Plant Soil Environ, 2015, 62, 507–514. [CrossRef]
- Chastain, D.R.; Snider, J.L.; Collins, G.D.; Perry, C.D.; Whitaker, J.; & Byrd, S. A. Water deficit in field-grown Gossypium hirsutum primarily limits net photosynthesis by decreasing stomatal conductance, increasing photorespiration, and increasing the ratio of dark respiration to gross photosynthesis. Journal of plant physiology, 2014. 171(17), 1576-1585. [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A. and Du, X. Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells, 2020,9(1), p.105. [CrossRef]
- Divya, K.; Jami, S.K.; & Kirti, P.B. Constitutive expression of mustard annexin, AnnBj1 enhances abiotic stress tolerance and fiber quality in cotton under stress. Plant molecular biology, 2010. 73(3), 293-308. [CrossRef]
- Zhang, F.; Li, S.; Yang, S.; Wang, L. and Guo, W. Overexpression of a cotton annexin gene, GhAnn1, enhances drought and salt stress tolerance in transgenic cotton. Plant Mol. Biol. 2015, 87, 47–67. [CrossRef]
- Ma, L.F.; Li, Y.; Chen, Y. and Li, X.B. Improved drought and salt tolerance of Arabidopsis thaliana by ectopic expression of a cotton (Gossypium hirsutum) CBF gene. Plant Cell, Tissue and Organ Culture (PCTOC),2016, 124(3), pp.583-598. [CrossRef]
- Danquah, A.; de Zélicourt, A.; Boudsocq, M.; Neubauer, J.; Frei dit Frey, N.; Leonhardt, N.; ... & Colcombet, J. Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana. The Plant Journal, 2015. 82(2), 232-244. [CrossRef]
- Li, F.; Li, M.; Wang, P.; Cox Jr, K.L.; Duan, L.; Dever, J.K.; Shan, L.; Li, Z. and He, P. 2017. Regulation of cotton (Gossypium hirsutum) drought responses by mitogen-activated protein (MAP) kinase cascade-mediated phosphorylation of Gh WRKY 59. New Phytologist, 2017 215(4), pp.1462-1475. [CrossRef]
- Wang, C.; Lu, W.; He, X.; Wang, F.; Zhou, Y.; Guo, X., & Guo, X. The cotton mitogen-activated protein kinase kinase 3 functions in drought tolerance by regulating stomatal responses and root growth. Plant and Cell Physiology, 2016, 57(8), 1629-1642. [CrossRef]
- Chen, X.; Wang, J.; Zhu, M.; Jia, H.; Liu, D.; Hao, L.; & Guo, X. A cotton Raf-like MAP3K gene, GhMAP3K40, mediates reduced tolerance to biotic and abiotic stress in Nicotiana benthamiana by negatively regulating growth and development. Plant Science, 2015. 240, 10-24. [CrossRef]
- Wang, N. N., Zhao, L. L., Lu, R., Li, Y., & Li, X. B. Cotton mitogen-activated protein kinase4 (GhMPK4) confers the transgenic Arabidopsis hypersensitivity to salt and osmotic stresses. Plant Cell, Tissue and Organ Culture (PCTOC), 2015, 123(3), 619-632. [CrossRef]
- Zhang, J.; Zou, D.; Li, Y.; Sun, X.; Wang, N.N.; Gong, S.Y. ... & Li, X.B. GhMPK17, a cotton mitogen-activated protein kinase, is involved in plant response to high salinity and osmotic stresses and ABA signaling. PloS one, 2014, 9(4), e95642. [CrossRef]
- Long, L.; Gao, W.; Xu, L.; Liu, M.; Luo, X.; He, X.; Yang, X.; Zhang, X. and Zhu, L. 2014. GbMPK3, a mitogen-activated protein kinase from cotton, enhances drought and oxidative stress tolerance in tobacco. Plant Cell, Tissue and Organ Culture (PCTOC), 2014, 116(2), pp.153-162. [CrossRef]
- Li, Y.; Zhang, L.; Wang, X.; Zhang, W.; Hao, L.; Chu, X. and Guo, X. Cotton Gh MPK 6a negatively regulates osmotic tolerance and bacterial infection in transgenic Nicotiana benthamiana, and plays a pivotal role in development. The FEBS journal, 2013, 280(20), pp.5128-5144. [CrossRef]
- Fang, Y.; & Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cellular and molecular life sciences, 2015. 72(4), 673-689.. Cell. Mol. Life Sci. 72, 673–689. [CrossRef]
- Zhao, Y.; Wang, H.; Chen, W.; Zhao, P.; Gong, H.; Sang, X.; & Cui, Y. Regional association analysis-based fine mapping of three clustered QTL for verticillium wilt resistance in cotton (G. hirsutum. L). BMC genomics, 2017, 18(1), 1-12. [CrossRef]
- Vinod, K.K. Association mapping in crop plants. Bioinform Tools Genom Res., 2011.
- Kıvılcım, N.; Şahin, A.; Ekşi, İ.; Özbek, N.; Yolcu, S.; Naza, İ.; Bilgen, F.; İmamoğlu, A.; Sezener, V.; Gençyılmaz, E.; Coşkun, R. Development of drought-tolerant cotton varieties by crossbreeding. Ministry of Agriculture and Rural Affairs, General Directorate of Agricultural Research, Nazilli Cotton Research Institute, Project report, 2005, TAGEM project no: 97/03/01/004.
- Riemann, M.; Dhakarey, R.; Hazman, M.; Miro, B.; Kohli, A. and Nick, P. Exploring jasmonates in the hormonal network of drought and salinity responses. Frontiers in plant science, 2015 6, p.1077. [CrossRef]
- Long, L.; Guo, D.D.; Gao, W.; Yang, W.W.; Hou, L.P.; Ma, X.N.; Miao, Y.C.; Botella, J.R. and Song, C.P. Optimization of CRISPR/Cas9 genome editing in cotton by improved sgRNA expression. Plant Methods, 2018, 14(1), pp.1-9. [CrossRef]
- Hou, S.; Zhu, G.; Li, Y.; Li, W.; Fu, J.; Niu, E.; Li, L.; Zhang, D. and Guo, W. Genome-wide association studies reveal genetic variation and candidate genes of drought stress related traits in cotton (Gossypium hirsutum L.). Frontiers in Plant Science, 2018. 9, p.1276. [CrossRef]
- Abdelraheem, A.R. A joint genetic linkage mapping and genome-wide association study of drought and salinity tolerance and Verticillium wilt and thrips resistance in cotton. New Mexico State University (Ph.D. dissertation). New Mexico State University. 2017. USA. http://www.secheresse.info/spip.php?article67309.
- Abdelraheem, A.; Percy, R.; Gore, M.; Dever, J.; Fang, D.; & Zhang, J. F. Genetic analysis for yield, fiber quality and abiotic stress tolerance in Pima cotton. In Proc Beltwide Cotton Conf. 2016. (Vol. 4, No. 6). https://www.cotton.org/beltwide/.
- Abdelraheem, A.; Mahdy, E.; & Zhang, J. The first linkage map for a recombinant inbred line population in cotton (Gossypium barbadense) and its use in studies of PEG-induced dehydration tolerance. Euphytica, 2015, 205(3), 941-958. [CrossRef]
- Adams, N. Identification of cotton germplasm and molecular markers for drought tolerance (MS. Thesis), New Mexico State Univ., Las Cruces, 2011. NM, USA. [CrossRef]
- Said, J.I.; Lin, Z.; Zhang, X.; Song, M. and Zhang, J. A comprehensive meta QTL analysis for fiber quality, yield, yield related and morphological traits, drought tolerance, and disease resistance in tetraploid cotton. BMC genomics, 2013, 14(1), pp.1-22. [CrossRef]
- Chen, Y.; Liu, Z.H.; Feng, L.; Zheng, Y.; Li, D.D.; & Li, X.B. Genome-wide functional analysis of cotton (Gossypium hirsutum) in response to drought. PLoS One, 2013. 8(11), e80879. [CrossRef]
- Padmalatha, K.V.; Dhandapani, G.; Kanakachari, M.; Kumar, S.; Dass, A.; Patil, D.P.; Rajamani, V.; Kumar, K.; Pathak, R.; Rawat, B. and Leelavathi, S. Genome-wide transcriptomic analysis of cotton under drought stress reveal significant down-regulation of genes and pathways involved in fibre elongation and up-regulation of defense responsive genes. Plant molecular biology, 2012, 78(3), pp.223-246. [CrossRef]
- Park, W.; Scheffler, B.E.; Bauer, P.J. and Campbell, B.T. Genome-wide identification of differentially expressed genes under water deficit stress in upland cotton (Gossypium hirsutum L.). BMC plant biology, 2012, 12(1), pp.1-12. [CrossRef]
- Zhao, G.; Song, Y.; Wang, C.; Butt, H. I.; Wang, Q.; Zhang, C.; ... & Li, F. Genome-wide identification and functional analysis of the TIFY gene family in response to drought in cotton. Molecular Genetics and Genomics, 2016, 291(6), 2173-2187. [CrossRef]
- Li, B.; Chen, L.; Sun, W.; Wu, D.; Wang, M.; Yu, Y.; Chen, G.; Yang, W.; Lin, Z.; Zhang, X. and Duan, L.; 2020. Phenomics-based GWAS analysis reveals the genetic architecture for drought resistance in cotton. Plant biotechnology journal, 2020,18(12), pp.2533-2544. [CrossRef]
- Zhang, X.Y.; Liu, C.L.; Wang, J.J.; Li, F.G. & Ye, W.E. Evaluation to the drought tolerance of cotton by PEG water-stress. J Cotton Sci, 2007, 19, 205-209. [CrossRef]
- Li, H.M.; Liu, S.D.; Ge, C.W.; Zhang, X.M.; Zhang, S.P.; Chen, J.; Shen, Q.; Ju, F.Y.; Yang, Y.F.; Li, Y. and Liu, R.H. Association Analysis of Drought Tolerance and Associated Traits in Upland Cotton at the Seedling. International journal of molecular sciences, 2019, 20(16), p.3888. [CrossRef]
- Ullah, A.; Sun, H.; Yang, X. and Zhang, X. Drought coping strategies in cotton: increased crop per drop. Plant biotechnology journal, 2017, 15(3), pp.271-284. [CrossRef]
- Javaid, A.; Awan, F.S.; Azhar, F.M. and Khan, I.A. Assessment of allelic diversity among drought-resistant cotton genotypes using microsatellite markers. Genet Mol Res, 2017, 16(2). [CrossRef]
- Ulloa, M.; De Santiago, L.M.; Hulse-Kemp, A.M.; Stelly, D.M. and Burke, J.J. Enhancing Upland cotton for drought resilience, productivity, and fiber quality: comparative evaluation and genetic dissection. Molecular Genetics and Genomics, 2020,295(1), pp.155-176. [CrossRef]
- Gutterman, Y. Survival strategies of annual desert plants. Springer Science & Business Media.2002. https://link.springer.com/book/ 10.1007/978-3-642-55974-7.
- Uniyal, R.C. and Nautiyal, A.R. Seed germination and seedling extension growth in Ougeinia dalbergioides Benth. under water and salinity stress. New Forests, 1998, 16(3), pp.265-272. [CrossRef]
- Basal, H.; Smith, C.W.; Thaxton, P.S.; & Hemphill, J.K. Seedling drought tolerance in upland cotton. Crop Science, 2005. 45(2), 766-771. [CrossRef]
- Wrona, 2000.
- JMP®, Version <17x>. SAS Institute Inc, Cary, NC, 1989–2021.Where <17> is the version of JMP being cited.
- Španić, V.; Ižaković, M. and Marček, T. Wheat germination and seedlings under PEG-induced conditons. Agronomski glasnik: Glasilo Hrvatskog agronomskog društva, 2017, 79(3), pp.99-109. [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. African Journal of Agricultural Research, 2011. 6 (9): 2026-2032. [CrossRef]
- Pace, P.F.; Cralle, H.T.; El-Halawany, S.H.; Cothren, J.T. and Senseman, S.A. Drought-induced changes in shoot and root growth of young cotton plants. J. Cotton Sci, 1999, 3(4), pp.183-187. http://journal.cotton.org.
- Malik, R.S.; Dhankar, J.S. and Turner, N.C. Influence of soil water deficits on root growth of cotton seedlings. Plant and soil, 1979, 53(1), pp.109-115. [CrossRef]
- Ball, R.A.; Oosterhuis, D.M.;& Mauromoustakos, A. Growth dynamics of the cotton plant during water-deficit stress. Agronomy journal,1994. 86(5), 788-795. [CrossRef]
- Prior, S.A.; Rogers, H.H.; Runion, G.B.; Kimball, B.A.; Mauney, J.R.; Lewin, K.F.; Nagy, J. and Hendrey, G.R. Free-air carbon dioxide enrichment of cotton: Root morphological characteristics (Vol. 24, No. 4, pp. 678-683). American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America. 1995. [CrossRef]
- Wang, Y.; Meng, Z.; Liang, C.; Meng, Z.; Wang, Y.; Sun, G.; Zhu, T.; Cai, Y.; Guo, S.; Zhang, R. and Lin, Y. Increased lateral root formation by CRISPR/Cas9-mediated editing of arginase genes in cotton. Sci China Life Sci, 2017, 60, 524–527. [CrossRef]
- Barber, S.A.; & Silberbush, M. Plant root morphology and nutrient uptake. Roots, nutrient and water influx, and plant growth, 1984. 49, 65-87. https://cris.bgu.ac.il/en/publications/plant-root-morphology-and-nutrient-uptake.
- Russel, R.S. Plant root ¢~stems, their fimction, and interaction with the soil. McGraw-Hill Books, 1977, London.
- Forde, B.; and Lorenzo, H. The nutritional control of root development. Plant Soil. 2001 232: 51–68. [CrossRef]
- Kul, R.; Ekinci, M.; Turan, M.; Ors, S.; & Yildirim, E. How abiotic stress conditions affects plant roots. Plant Roots, 2020, 6-10. [CrossRef]
- Liu, F.; Stützel, H. Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Scientia Horticulturae, 2004, 102(1):15-27. [CrossRef]
- Minitab, LLC. Minitab. 2021, Retrieved from https://www.minitab.com.
- Yi, M. https://chartio.com/learn/charts/what-is-a-scatter-plot/ (Access: 11.07.2022, 09:50 a.m). 2022.
- Corwin, D.L.; & Lesch, S.M. Application of Geo-referenced Geophysical Measurements to Precision Agriculture. Fast Times: News for the Near Surface Geophysical Sciences,2008. 13(2), 29-37. Apparent Soil Electrical Conductivity (ECa) Directed Soil Sampling.
- Wanjura, D.F.; Kelly, C.A.; Wendt, C.W.; Hatfield, J.L. Canopy temperature and water stress of cotton crops with complete and partial ground cover. Irrigation Science, 1984, 5(1), 37-46. [CrossRef]
- Peynircioğlu, C. The determination of cotton (Gossypium hirsutum L.) for improvement of drought tolerant cotton varieties (Master's thesis), Adnan Menderes University, Turkey, 2014, https: //tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp.
- Kaçar, M.M. Investıgatıon of cotton water stress ındex varıatıons Under dıfferent water and fertılızer systems. Çukurova University, Institute of Science and Technology, Department of Agricultural Structures and Irrigation (Master Thesis) Turkey. 2007, https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp.
- Nguyen, T.T.T.; Klueva, N.; Chamareck, V.; Aarti, A.; Magpantay, G.; Millena, A.C.M.; Pathan, M.S. and . Saturation mapping of QTL regions and identification of putative candidate genes for drought tolerance in rice. Molecular Genetics and Genomics, 2004, 272(1), pp.35-46. [CrossRef]
- Zahid, Z.; Khan, M.K.R.; Hameed, A.; Akhtar, M.; Ditta, A.; Hassan, H.M., & Farid, G. Dissection of drought tolerance in upland cotton through morpho-physiological and biochemical traits at seedling stage. Frontiers in Plant Science, 2021,12, 627107. [CrossRef]
- Wang, L.; Wang, P.C., Zhang, T.; Zhang, H.Y.; Ding, S.Y. Effect of short-term drought and rewatering during the pod-setting stage on leaf photosynthesis and yield of the soybean. Acta Ecol. Sin, 2009, 29, 3328-3334.
- Proffitt, A.P.B.; Berliner, P.R. and Oosterhuis, D.M. 1985. A Comparative Study of Root Distribution and Water Extraction Efficiency by Wheat Grown Under High-and Low-Frequency Irrigation 1. Agronomy Journal, 1985, 77(5), pp.655-662. [CrossRef]
- Shan, L.; Zhang, S.Q. Is possible to save large irrigation water? The situation and prospect of water-saving agriculture in China. Chinese J. Nature, 2006, 28: 71-74. [CrossRef]
- Shah, A.R.; Khan, T.M.; Sadaqat, H.A. and Chatha, A.A. Alterations in Leaf Pigments in Cotton (Gossypium hirsutum) Genotypes Subjected to Drought Stress Conditions. International Journal of Agriculture & Biology, 2011, 13(6). http://www.fspublishers.org/.
- Mai, W.; Xue, X.; Feng, G.; Yang, R. and Tian, C. 2018. Can optimization of phosphorus input lead to high productivity and high phosphorus use efficiency of cotton through maximization of root/mycorrhizal efficiency in phosphorus acquisition?. Field Crops Research, 2018 216, pp.100-108. [CrossRef]
- Gavelienė, V.; Jurkonienė, S.; Jankovska-Bortkevič, E.; & Švegždienė, D. Effects of Elevated Temperature on Root System Development of Two Lupine Species. Plants, 2022. 11(2), 192. [CrossRef]
- Zahid, K.R.; Ali, F.; Shah, F.; Younas, M.; Shah, T.; Shahwar, D.; ... & Wu, W. Response and tolerance mechanism of cotton Gossypium hirsutum L. to elevated temperature stress: a review. Frontiers in plant science, 2016, 7, 937. [CrossRef]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; & Testerink, C. Roots withstanding their environment: exploiting root system architecture responses to abiotic stress to improve crop tolerance. Frontiers in plant science, 2016, 7, 1335. [CrossRef]
- Luo, H.; Xu, H.; Chu, C.; He, F. and Fang, S. High temperature can change root system architecture and intensify root interactions of plant seedlings. Frontiers in plant science, 2020, 11, p.160. [CrossRef]
- Gallardo, M.; Turner, N.C.; Ludwig, C. Water relations, gas exchange and abscisic acid content of Lupinus cosentinii leaves in response to drying different proportions of the root system. – J. Exp. Bot. 1994. 45: 909-918. [CrossRef]
- Luo, H.; Zhang, H.; Han, H.; Hu, Y.; Zhang, Y. and Zhang, W. Effects of water storage in deeper soil layers on growth, yield, and water productivity of cotton (Gossypium hirsutum L.) in arid areas of northwestern. China. Irrigation and Drainage, 2014, 63(1), pp.59-70. [CrossRef]
- Chiatante, D.; Di Iorio, A.; Maiuro, L.; & Scippa, S.G. Effect of water stress on root meristems in woody and herbaceous plants during the first stage of development. Plant and Soil, 1999. 217(1), 159-172. [CrossRef]
- Tang, L.S.; Li, Y. and Zhang, J. Physiological and yield responses of cotton under partial rootzone irrigation. Field Crops Research, 2005, 94(2-3), pp.214-223. [CrossRef]
- Ko, J.; Piccinni, G. Characterizing leaf gas exchange responses of cotton to full and limited irrigation conditions. – Field Crop. 2009, Res. 112: 77-89, 2009. [CrossRef]
- Ebelhar, S.; Chesworth, W.; Paris, Q. Law of the Minimum. In: Chesworth, W. (eds) Encyclopedia of Soil Science. Encyclopedia of Earth Sciences Series. Springer, 2008, Dordrecht. [CrossRef]
- Brand, D., C. Wijewardana, W. Gao, and K.R. Reddy. 2016. Interactive effects of carbon dioxide, low temperature, and ultraviolet-B radiation on cotton seedling root and shoot morphology and growth. Front. Earth Sci. 10:607-620.
- Brown, E., and S. McCarter. 1976. Effect of a seedling disease caused by Rhizoctonia solani on subsequent growth and yield of cotton. Phytopathology 66:111-115.
- Singh, B., E. Norvell, C. Wijewardana, T. Wallace, D. Chastain, and K.R. Reddy. 2018. Assessing morphological characteristics of elite cotton lines from different breeding programs for low temperature and drought tolerance. J. Agron. Crop. Sci. 4:467-476.
- Reddy, K., H. Hodges, J. McKinion, and G. Wall. 1992. Temperature effects on Pima cotton growth and development. Agron. J. 84:237-243.
- Bradow, J.M., and P.J. Bauer. 2010. Germination and seedling development. In: J.M. Stewart, D.M. Oosterhuis, J.J. Heitholt, and J.R. Mauney, editors, Physiology of Cotton. Springer, Netherlands. p. 48-56.
- Perry, C., E. Barnes, D. Munk, K. Fisher, and P. Bauer. 2012. Cotton irrigation management for humid regions. Cotton Incorporated, Cary, NC.
- Iqbal A, Dong Q, Wang X, Gui H, Zhang H, Zhang X, Song M (2020) High Nitrogen Enhance Drought Tolerance in Cotton through Antioxidant Enzymatic Activities, Nitrogen Metabolism and Osmotic Adjustment. Plants. 9(2):178. [CrossRef]
- Simonneau T, Habib R, Goutouly JP and Huguet JG (1993). Diurnal changes in stem diameter depend upon variations in water content: direct evidence in peach trees. J. Exp. Bot. 44, 615–621.
- Zahid Z, Khan MK, Hameed A, Akhtar M, Ditta A, Hassan HM, Farid G (2021) Dissection of drought tolerance in upland cotton through morpho-physiological and biochemical traits at seedling stage. Frontiers in Plant Science. 2021 Mar 12;12:627107.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).