1. Introduction
Cervical cancer is on the top 4 most common malignant tumor for female patients and one of the leading causes of death from cancer among women. The World Health Organization reported more than half a million new cases in 2020 and a high cancer-related mortality rate (342.000 deaths).1,2 The essential cause of cervical cancer has been identified as persistent infection with high-risk human papillomavirus (HPV).3,4 Thus, in order to detect and treat the precursor lesion, cervical intra-epithelial lesion (CIN), is essential in order for any screening program to succeed and can prevent the onset of cervical cancer.
The traditional diagnostic procedure known as a colposcopy is carried out after a negative cervical cancer screening test. It involves magnifying the view of the cervix by up to 30 times with a colposcope. The squamocolumnar junction (SCJ) and the transition zone are primarily the focus of the study. The dynamic area known as the transformation zone is where glandular cells eventually give way to squamous cells through metaplastic processes, increasing the risk of neoplasia5. The cervix is examined during colposcopy after a 35% acetic acid solution is applied (visualization of the cervix with acetic acid- VIA). The acidic solution dehydrates cells after about 30 to 90 seconds, causing squamous cells with relatively big or dense nuclei to reflect light and look white6. These are known as "acetowhite changes." Furthermore, against this white background, abnormal blood vessels and vascular patterns become clearer and more visible. Similarly, Lugol's iodine can be administered to the cervix, making dysplastic lesions easier to identify. Lugol's iodine is a substance that becomes brown or black when it comes into contact with glycogen, which is found in adult squamous epithelium. Because of weak cellular differentiation, precancerous lesions and cancer contain little or no glycogen and will turn into various hues of yellow following Lugol application7.
Colposcopy is widely used as part of screening programs to detect and treat such lesions, mainly high-grade cervical intra-epithelial grade 2+ lesions (HG-CIN, CIN 2+) and cervical cancer. Its sensitivity for detecting high-grade lesions ranges between 55.9% to 60% 8-11 and can reach 85.6% if the number of retrieved biopsies is increased or endocervical curettage is added12-14. Sauvaget et al15 conducted the most complete meta-analysis in which colposcopy was utilized as a primary screening modality. According to the authors, the overall sensitivity is 80%, the specificity is 92%, the the negative predictive value is 99% and the positive predictive value is 10%15. Furthermore, they concluded that region, screening provider training level, setting and size of study population had no effect on colposcopy accuracy. The substantial negative predictive value reported by Sauvaget et al15 was replicated by Sankaranarayanan et al16 in an Indian longitudinal study. Only 25 of the 23,000 VIA-negative women examined in this trial had cervical cancer within the subsequent eight years. This means that women who have had a colposcopy and had a negative screening result are unlikely to suffer from cervical cancer in the near future16.
However, effectiveness depends on the experience and training of the colposcopist, the prevalence of the disease in the study population, and the number of biopsies performed17. Ιnadequate evaluation of a colposcopic image can result either in failure to detect disease or unnecessary treatment in the absence of the disease. Electrical impedance spectroscopy (EIS), as a real-time adjunct to colposcopy, has been reported to improve the performance of colposcopy17-19. In fact, studies have demonstrated that EIS has been frequently employed in melanoma patients to differentiate between normal and abnormal skin lesions based on their modified cellular structure as a result of transformation into cancer.20,21 EIS, according to researchers, could be used in gynecology and constitutes a promising and quick approach for detecting aberrant cellular alterations in cervical epithelium.22-25 Apart from loss of stratification and differentiation and higher nuclear cytoplasmic ratio, it appears that CIN development can cause an up to six-fold increase in extracellular space, which decreases impedance at low frequencies in CIN. As a result, the original squamous epithelium has a high impedance, but high-grade CIN has a lower impedance.24,25
The primary endpoint of our study was to evaluate the effectiveness of the combination of colposcopy and EIS in detecting high-grade intraepithelial cervical lesions in patients referred to colposcopy due to an initial diagnosis of a low-grade lesion on cytology. Therefore, our ultimate goal was to identify patients underdiagnosed or patients not indicated to undergo further evaluation, thus, reducing morbidity, economic cost, and psychological stress.
2. Materials and Methods
This study was conducted in the Colposcopy Unit of «Alexandra» General University Hospital in real-world settings. All eligible women were initially referred to colposcopies to our institution due to abnormal cytology results and subsequently, electrical impedance measurements were received from each woman with a specific measuring device.
To evaluate the diagnostic method, an analysis was performed at 3 levels:
1. ZedScan data
2. Colposcopy results with the classic application of Lugol solution.
3. Histopathological analysis of tissues with suspected damage.
Furthermore, based on the international literature and to ensure the participants' safety, we thought it is reasonable, when colposcopy was negative and a high-grade disease was found only by a ZedScan-directed biopsy to be also regarded as an increase in detection. A patient was also considered negative for HSIL if the colposcopy was normal, there were no visible lesions, the ZedScan test was negative, and the patient had been referred with low-grade cytology (ASCUS, LSIL). To summarize, each patient served as their own control, having undergone both procedures during the same examination, enabling for a comparison of the rate of detection for high-grade lesions for colposcopy alone versus colposcopy in combination with ZedScan.
In total, 101 women were recruited to participate in the study from 2019 to 2022. Approval for this study was obtained by the University’s Scientific Board and Ethics Committee. Furthermore, we received written consent from all our patients.
Inclusion criteria: abnormal cervical cytology, LSIL, ASCUS, cervical inflammatory changes
Exclusion criteria: pregnancy, vaginal bleeding or active menstruation, cervical cancer, use of vaginal contraceptives or vaginal medication, refusal to participate in the study.
The same diagnostic approach was applied to all women and all diagnostic maneuvers were performed by two senior gynecologists. Colposcopies using Videocolposcope HD-1000 with IRIS software (Medicom, Wroclaw, Poland) was conducted according to established protocols of our hospital colposcopy unit.
Initially, all women underwent a standard colposcopy with acetic acid for an overview of the cervix and visualization of the possible epithelial lesions. Next, EIS was performed with ZedScan (Zilico Limited, Manchester, UK).
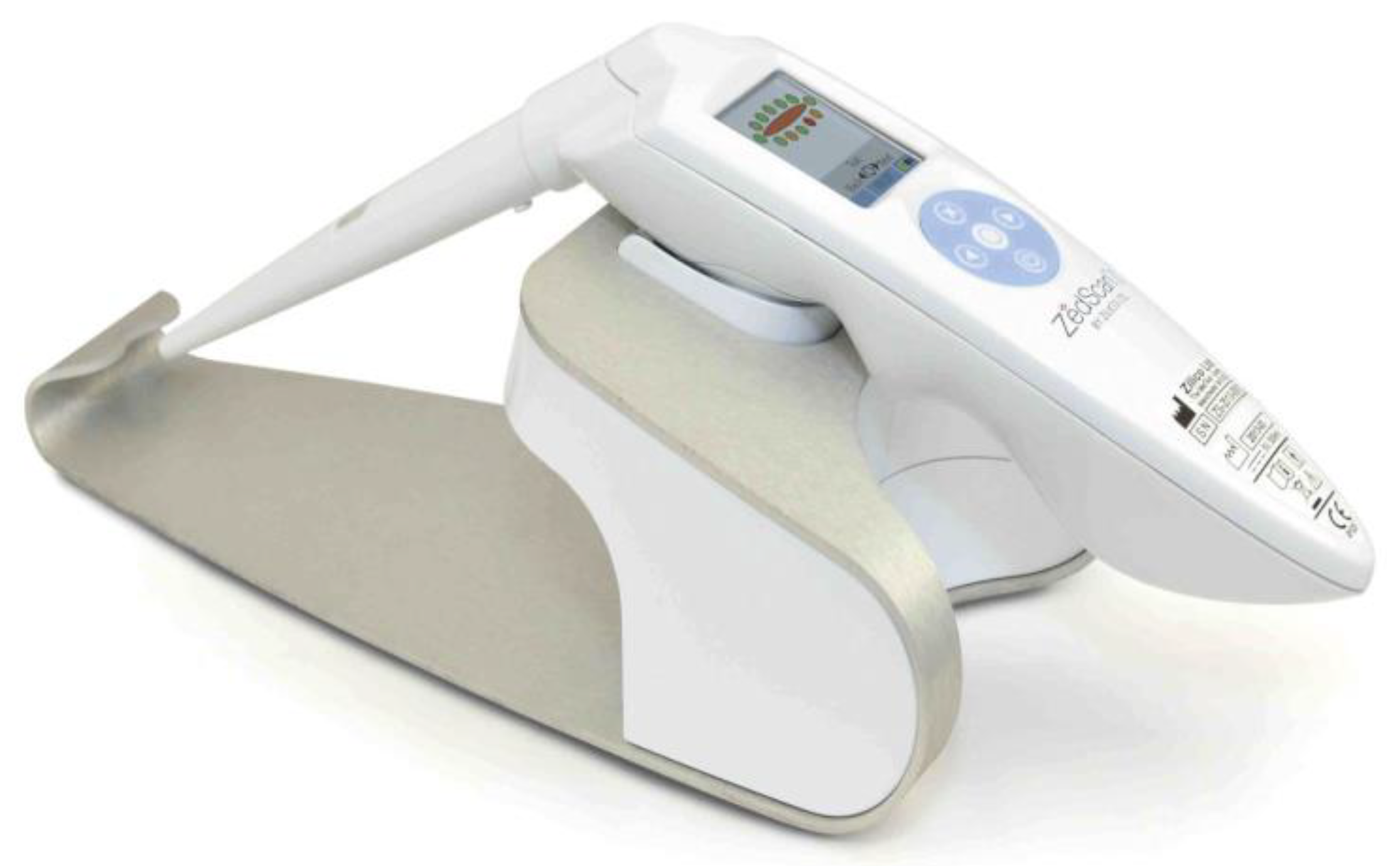
The device ZedScan consisted of a hand-held unit with a single-use sensor on the tip of the unit and an integrated screen in the area of the handle (
Figure 1). The whole cervical transformation zone (TZ) was observed and 12 measurements were taken from the TZ after 5% acetic acid was applied to the cervix. Measurements were presented and recorded on the hand-held unit's screen.
For the interpretation of the results 3 colors, red, amber, and green were used to identify areas with the highest probability of HSIL occurrence. The red color is interpreted as the area with the highest probability for HSIL and the green color as the area with the lowest probability. Intermediate states are depicted in amber and are associated with a low probability of HSIL.
The diagnostic procedure was completed by the application of potassium iodide to the surface of the cervix, identifying abnormalities on the cervical epithelium using the standard method. HSIL was confirmed by diagnostic cervical biopsies or cone biopsies. All biopsy specimens were analyzed by histopathologists with expertise in cervical pathology.
ZedScan's positivity for CIN was determined based on color changes, with amber or red indicating a positive result, and green representing a negative result. In cases where colposcopy yielded negative results but CIN2+ disease was identified through a biopsy guided by ZedScan, it was categorized as an enhanced CIN2+ detection attributable to ZedScan. Conversely, when both colposcopy and ZedScan were negative, patients were classified as negative for CIN2+. Notably, even in instances of negative results, due to the presence of suspicious cytology (ASCUS, LSIL), biopsies were invariably conducted. The ultimate endpoint for comparison of diagnostic efficacy between colposcopy and ZedScan was the histology reports resulting from these biopsies.
Colposcopy was completed by applying Lugol's solution to the cervical surface, which assisted in the identification of possible abnormalities without the need for staining. Biopsies were conducted based on colposcopic impressions and/or ZedScan results, and the excised tissues were histologically evaluated at the Hospital's Pathology Lab.
For cytological assessment, the collected samples were preserved in a PreservCyt/ThinPrep solution (Cytyc Corporation, Marlborough, Massachusetts, United States of America, 1987). The Pap test employed Liquid-Based Cytology (LBC) technology and was processed using the Thin Prep 2000 Processor (Cytyc Corporation, Marlborough, Massachusetts, United States of America, 1987), as per the manufacturer's guidelines. Thin-layer slides were Pap stained and thoroughly evaluated by a team of professional cytologists specialized in cervical pathology, in accordance with the standards established in the Bethesda System for Reporting Cervical Cytology, third edition, 2015. Cytological findings were classified as (a) low-grade squamous intraepithelial lesions (LSIL), (b) atypical squamous cells of undetermined significance (ASC-US), (c) negative for intraepithelial lesion or malignancy (NILM), (d) atypical squamous cells of undetermined significance without excluding high-grade squamous intraepithelial lesions (ASC-H), (e) high-grade squamous intraepithelial lesions (HSIL), and (f) squamous cervical carcinomas (SCC).
The Clinic's Laboratory of Gynecologic Oncology performed the molecular analyses. 1 mm of each Thin Prep sample was transferred to an Aptima Specimen Transfer Tube and processed using the Panther system (Hologic, Marlborough, Massachusetts, USA, 1985). The mRNA Aptima assay (Hologic, Marlborough, Massachusetts, United States of America, 1985) functioned as a qualitative method to detect mRNA from 14 different hr-HPVs (HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), utilizing specific probes targeting viral E6 and E7 mRNAs. An amplification process involving transcription-mediated amplification (TMA) was used to amplify DNA copies, which subsequently served as templates for RNA amplification. Probe hybridization was then employed for detection (measured as relative light units, RLU). Controls were integrated to establish a cut-off level, and outcomes were presented as signal-to-cut-off (S/CO) ratios. The study initially adhered to a recommended S/CO ratio cutoff value of 1.0, where an S/CO above 1.0 was automatically regarded as a positive result by the analyzer's algorithm. The Aptima HPV assay was meticulously conducted in accordance with the manufacturer's provided instructions.
Statistical Analysis
The study employed a range of statistical methods to analyze the data in an appropriate manner. Qualitative variables were presented as absolute and relative frequencies while quantitative variables were presented as mean values with standard deviations (SD). Proportions were compared using Chi-square tests. The predictive capabilities of ZED scan and colposcopy were assessed through Receiver Operating Characteristic (ROC) analysis, with the AUC (area under the curve) serving as a measure of overall performance. Logistic regression models assisted in generating linear predictors and comparing AUC values.
The effectiveness of the ZED scan and colposcopy was gauged using metrics like sensitivity, specificity, and positive and negative predictive values. To evaluate the agreement between the ZED scan, colposcopy, and histology, the Kappa coefficient (K) was utilized. The highest Kappa value attainable was 1, signifying perfect agreement; K values equal to or greater than 0.75 indicated excellent agreement, while values surpassing 0.4 suggested acceptable reliability.
All reported p-values were two-tailed, and a result was considered statistically significant if it was <= 0.05. The analyses were performed using the SPSS software for statistical analysis (version 22.0, SPSS Inc, Chicago, Illinois, United States of America, 1968). Overall, the study employed a comprehensive range of statistical techniques to evaluate the performance and reliability of the ZED scan and colposcopy in a rigorous and systematic manner.
3. Results
The study comprised of a selected cohort of 101 patients, with an average age of 39.8 years and a standard deviation of 11.2 years, spanning a range from 20 to 64 years. 96 of the 101 women examined were Caucasian. The average age of starting sexual contacts was 17 years and of the patients 48% had less than 3 sexual partners and 42% more than 3. 78% reported systematic contacts without condom use. 68 of the 101 women are married and 52 report having had only one sexual partner in the last 10 years. Of the patients, 63 have at least one child and 47 have at least one vaginal delivery.
From the other demographics, it is reported that 38% of the women were obese with a BMI >25, 9% underweight and the rest in the normal range. 61% of the sample are smokers and 34% suffer from chronic diseases. Detected comorbidities include Systemic Lupus Erythematosus (SLE), type I and II diabetes, and ulcerative colitis. Of the patients, 45% report a history of fungal vaginitis, 31% report a pathological culture result of vaginal fluid for which they received antibiotics. It is worth noting that 62% report skipping an annual gynecological examination and 28% of women did not undergo a pap test in the last 10 years. Of the examinees only 14 have been vaccinated with Gardasil, all before the age of starting sexual contacts.
All individuals were enrolled based on their referral for colposcopy due to an initial diagnosis of low-grade cervical intraepithelial lesion (LGSIL). The subsequent analysis targeted on the outcomes of three diagnostic methodologies: ZedScan, colposcopy, and biopsy, with the resulting data presented in
Table 1.
Table 1.
ZedScan, colposcopy and biopsy results (N=101).
Table 1.
ZedScan, colposcopy and biopsy results (N=101).
| |
N |
% |
| ZED SCAN |
|
|
| Normal |
29 |
28.7 |
| LGSIL |
17 |
16.8 |
| HGSIL |
55 |
54.5 |
| Colposcopy |
|
|
| Normal |
34 |
33.7 |
| LGSIL |
31 |
30.7 |
| HGSIL |
36 |
35.6 |
| Biopsy |
|
|
| Normal |
25 |
24.8 |
| CIN 1 |
31 |
30.7 |
| CIN 2-CIN 3 |
45 |
44.6 |
Table 2.
ZED SCAN and colposcopy outcome in association with biopsy results.
Table 2.
ZED SCAN and colposcopy outcome in association with biopsy results.
| |
|
|
| |
BIOPSY |
RESULTS |
|
|
| |
Normal |
CIN 1 |
CIN 2-CIN 3 |
| |
N |
% |
N |
% |
N |
% |
| ZED SCAN |
|
|
|
|
|
|
| Normal |
21 |
84.0 |
6 |
19.4 |
2 |
4.4 |
| LGSIL |
1 |
4.0 |
16 |
51.6 |
0 |
0.0 |
| HGSIL |
3 |
12.0 |
9 |
29.0 |
43 |
95.6 |
| Colposcopy |
|
|
|
|
|
|
| Normal |
23 |
92.0 |
11 |
35.5 |
0 |
0.0 |
| LGSIL |
2 |
8.0 |
18 |
58.1 |
11 |
24.4 |
| HGSIL |
0 |
0.0 |
2 |
6.5 |
34 |
75.6 |
Table 3.
ZED SCAN in association with colposcopy outcome.
Table 3.
ZED SCAN in association with colposcopy outcome.
| |
Colposcopy |
| |
Normal |
LGSIL |
HGSIL |
| |
N |
% |
N |
% |
N |
% |
| ZED SCAN |
|
|
|
|
|
|
| Normal |
23 |
67.6 |
4 |
12.9 |
2 |
5.6 |
| LGSIL |
8 |
23.5 |
9 |
29 |
0 |
0 |
| HGSIL |
3 |
8.8 |
18 |
58.1 |
34 |
94.4 |
Table 4.
Sensitivity, Specificity, PPV and NPV for ZED SCAN and Colposcopy.
Table 4.
Sensitivity, Specificity, PPV and NPV for ZED SCAN and Colposcopy.
| |
Sensitivity % |
Specificity % |
PPV % |
NPV % |
AUC (95% CI) |
P |
| ZED SCAN |
89.5 |
84.0 |
94.4 |
72.4 |
0.87 (0.78 – 0.96) |
<0.001 |
| Colposcopy |
85.5 |
92.0 |
97.0 |
67.6 |
0.89 (0.81 – 0.97) |
<0.001 |
Within this patient pool, 45 cases exhibited CIN2 during histopathological examination, with high-grade colposcopy results observed in 36 instances. Notably, 55 patients were identified as displaying positive (red) ZedScan results. Importantly, no adverse events linked to the use of ZedScan were reported by any of the patients. The pivotal metrics of sensitivity, specificity, positive predictive value, and negative predictive value were calculated by aligning the outcomes of colposcopy and ZedScan examinations with histopathological findings, thus providing a comprehensive assessment of their diagnostic efficacy.
Intriguingly, the agreement rate between ZedScan results and biopsy outcomes was notably high at 79.2%, underpinned by a statistically significant kappa coefficient of 0.71 (p<0.001). Similarly, the concordance between colposcopy results and biopsy findings reached an agreement rate of 74.3%, accompanied by a substantial kappa coefficient of 0.71 (p<0.001). Furthermore, the alignment rate between ZedScan and colposcopy findings was 65.3%, marked by a kappa coefficient of 0.58 (p<0.001).
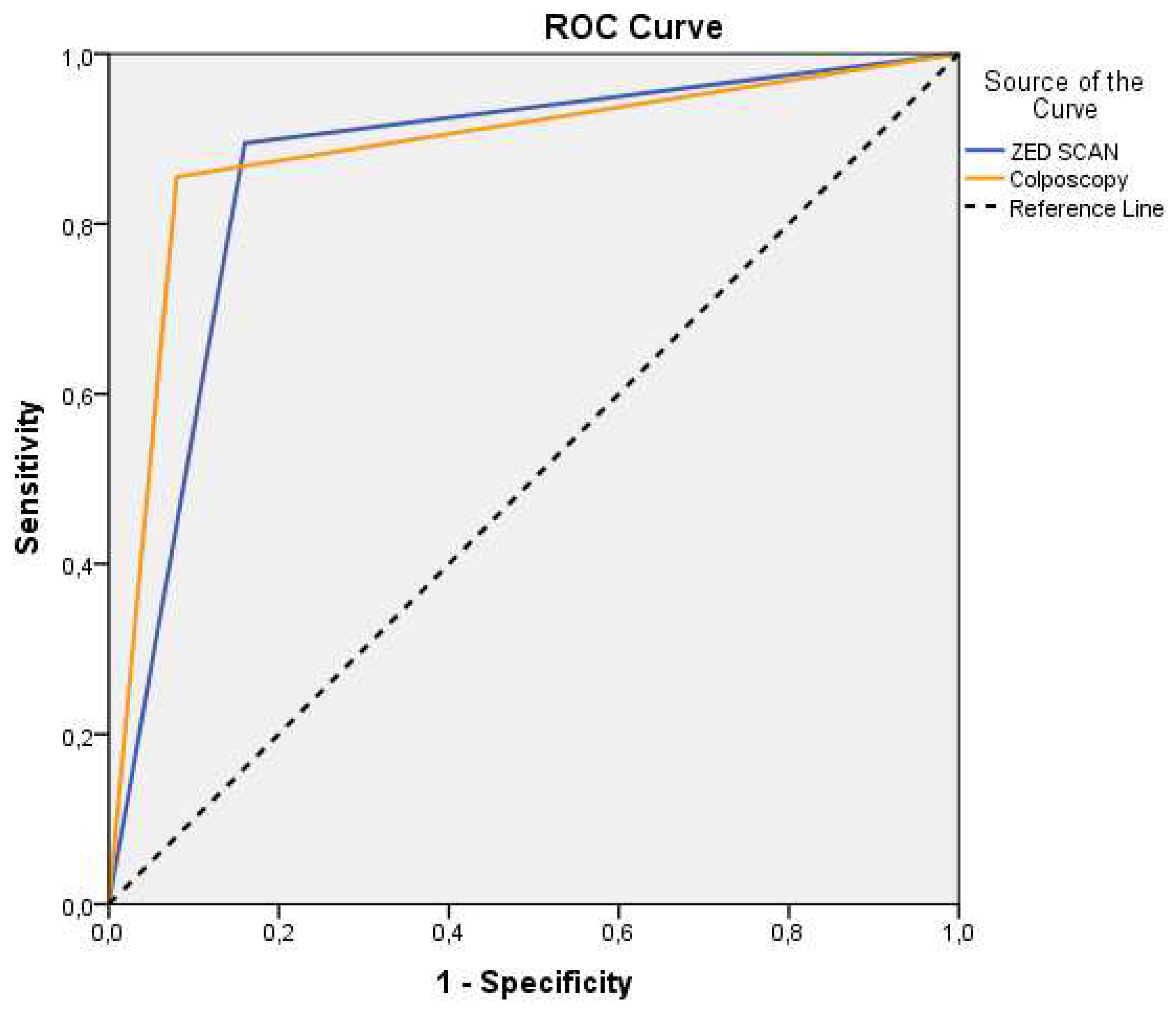
Regarding the diagnostic capabilities of ZedScan, its sensitivity rate stood at 89.5%, indicating its effectiveness in identifying true positive cases. Correspondingly, its specificity rate was calculated at 84%, underscoring its ability to accurately identify true negative cases. Furthermore, the positive prognostic value (PPV) of ZedScan reached 94.4%, while its negative prognostic value (NPV) was 72.4% in predicting biopsy outcomes. Notably, the area under the curve (AUC) for ZedScan stood at 0.87 (
Figure 3), significantly surpassing the baseline of 0.5 (p<0.001), thereby emphasizing its diagnostic robustness.
In parallel, the diagnostic capabilities of colposcopy were revealed, showcasing a sensitivity rate of 85.5% and a specificity rate of 92%. Its positive prognostic value reached 97%, while the negative prognostic value was determined to be 67.6% for predicting biopsy outcomes. The AUC for colposcopy was 0.89 (
Figure 2), firmly establishing its diagnostic prowess and superiority over the baseline (p<0.001). Finally, the comparative assessment of ZedScan and colposcopy's prognostic capabilities yielded intriguing insights. The comparison of AUCs yielded a p-value of 0.741, indicating no statistically significant difference between their diagnostic abilities.
Individual statistical analysis was also performed for all risk factors identified in the individual patient recall history. Except for the use of the number of lifetime sexual partners, which was statistically significant (p = 0.029 <= 0.05), most ZedScan parameters were not statistically significant. Smoking (p = 0.069 > 0.05), prophylactics (p = 0.134 > 0.05), age (p = 0.376 > 0.05), number of children (p = 0.765 > 0.05), and HPV vaccination (p = 0.067 > 0.05) all had no statistical significance.
Even if statistical crosstabulation illustrates the common distribution between the pairs of variables under consideration, the chi-square test cannot be deemed sufficiently reliable because many cells in the table require low expected frequencies. Given that the values of all variables under consideration are ordinal, we used Spearman's (rho) non-parametric correlation coefficient, with values close to +1 indicating strong positive correlation and values close to -1 indicating strong negative correlation. The number of lifetime sexual partners (p = 0.018) is the only statistically significant variable for the two approaches with a substantial positive correlation (p = 0.85), which can be verified by the existing bibliography that it corresponds with the emergence of CIN2+. The association can be verified for women who have had more than three partners in their lives. All of the other variables' correlation coefficients were between 0.4 and 0.7, indicating a moderate correlation, or less than 0.5, indicating a low correlation.
In conclusion, this study analyzed the diagnostic capabilities of ZedScan and colposcopy in a carefully selected patient cohort with low-grade cervical intraepithelial lesion. The thorough evaluation of agreement rates, sensitivity, specificity, positive and negative predictive values, and AUCs collectively delineated the robustness of these diagnostic tools, offering invaluable insights into their potential applications in clinical practice.From the analysis of the demographic characteristics of the sample, only the number of sexual partners is related to the occurrence of CIN 2+, while the rest of the observations follow the data we receive from the international literature.
4. Discussion
It is clear that for the early prevention of cervical cancer, the detection of HSIL/CIN2+ lesions and the proper management of these patients are necessary so that they do not develop into invasive diseases17. Until now, both international guidelines and national protocols use colposcopy with acetic acid and subsequent histological examination of the suspicious tissue as the gold standard of diagnosis. However, the international scientific community faces the challenge of creating new diagnostic tools, which will have the ability to detect cervical lesions during colposcopy with a high positive predictive value. Thus, patients will benefit by diagnosing lesions before they become visible with acetic acid and without waiting for histological results.
Another thing that should seriously be taken under consideration, is the subjective nature of the diagnosis made through colposcopy. It is known that the decision whether to send a non-histological tissue examination is a decision of the doctor performing the colposcopy. Several studies have found no changes in overall colposcopy performance between more and less experienced colposcopists, but substantial variations in PPV and sensitivity32-34. Junior colposcopists exhibited higher sensitivity but a lower PPV than senior colposcopists. This translates to a greater number of biopsies retrieved clinically. This, in its turn, results in increased sensitivity at the expense of decreased PPV35. Wei B. et al36, on the other hand, found that senior colposcopists had a much greater incidence of detecting HGSIL lesions in terms of accuracy, specificity, and sensitivity.
The use of Zedscan gives quite promising results that allow us to look forward to its complementary use in the future as an adjunct to classic colposcopy. Data from the literature are limited to 5 published studies, as the use of this specific tool began in 2017 17,18,38-42. Three of these are from a single location with a small number of patients, and they investigate whether using ZedScan in conjunction with colposcopy improves sensitivity in finding high-grade lesions at the expense of a slight rise in the amount of biopsies.17,38,40. One study specifically addressed the sensitivity of Zedscan in the detection of lesions associated with HPV infection, which did not show a statistically significant sensitivity of the method for these lesions. Finally, Tidy et al in 2022 completed a large multicenter study of 5257 participants confirming that simultaneous colposcopy and ZEDscan increase the detection of HSIL and reduce the number of biopsies performed18.
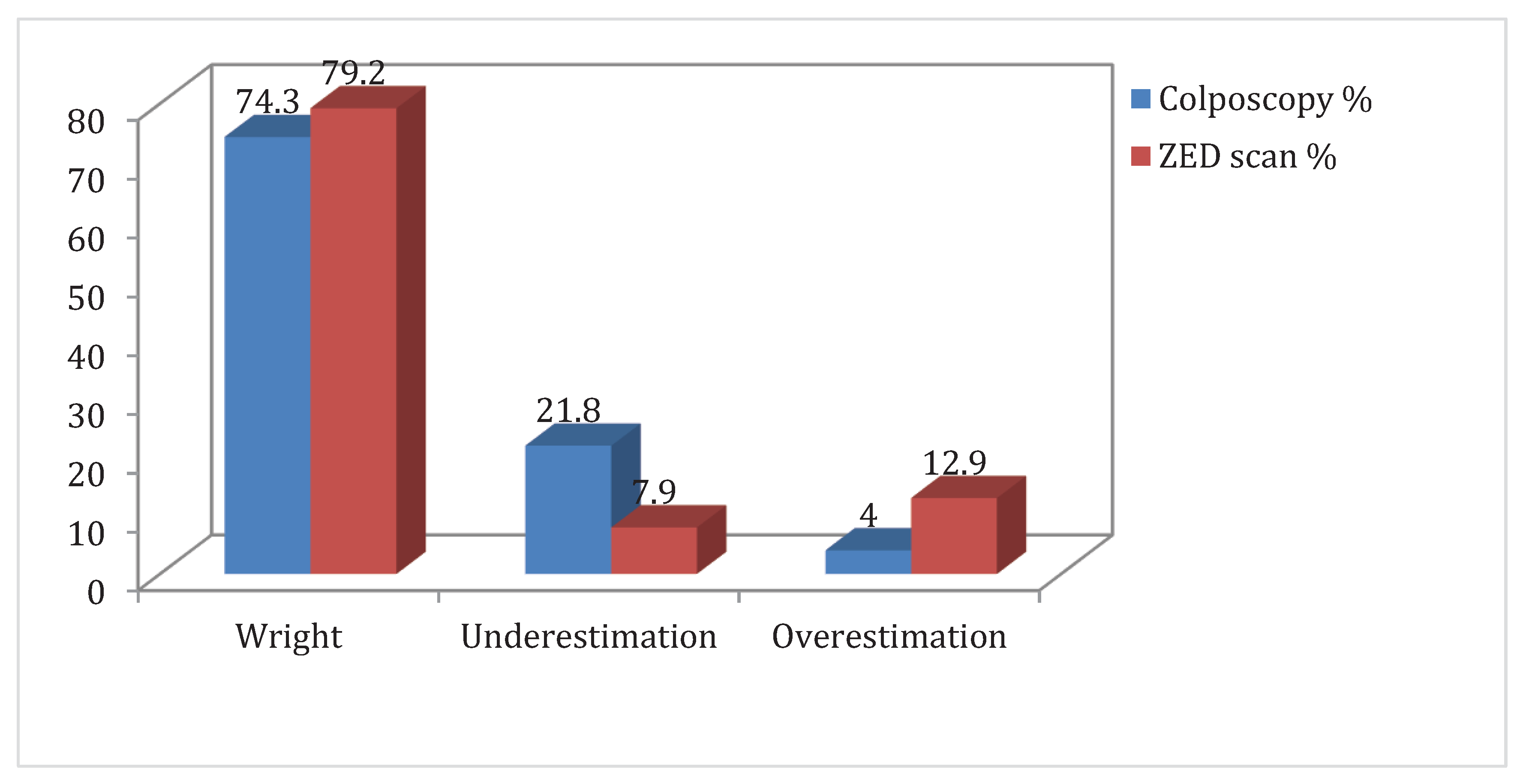
Colposcopy alone had a specificity rate of 92% and a sensitivity rate of 85.5% for detecting high-grade lesions in this investigation, which is slightly greater than what is described in the literature29-31. Moreover, the negative prognostic value (NPV) was 67.6% while the positive prognostic value (PPV) was 97% for the prediction of biopsy results. On the other hand, ZedScan exhibited a sensitivity rate of 89.5% and a specificity rate of 84%, while PPV was 94.4% and NPV 72.4% for the prediction of biopsy results. Furthermore, the overestimation rate was 4% for colposcopy and 12.9% for ZED scan, and on the other hand, the underestimation rate was 21.8% for colposcopy and 7.9% for ZedScan. Last, the proportion of false positive results was 16% for ZedScan and 8% for colposcopy. Interestingly, ZedScan exhibited similar high rates of sensitivity and specificity, while colposcopy exhibited lower sensitivity and higher specificity, data with no statistical importance (p>0.05). In conclusion, in agreement with the literature, we conclude that ZedScan sensitivity and NPV are greater than simple colposcopy when performed by a junior colposcopist.
After analyzing the data from our sample, no statistically significant relationship emerged with any of the literature-known risk factors for causing uterine cancer, apart from the number of sexual partners. More specifically, the number of women who mentioned more than three partners during their lifetime had a clear correlation with the appearance of the disease. We also discovered that the relationship between sexual partners, invasive cervical carcinoma and non-malignant cervical disease is non-linear. The risk for both malignant and non-malignant disease is a bit higher but relatively stable for women with 4 to 7 sexual partners43.
In closing, we would like to summarize that colposcopy is a technique of daily practice for the diagnosis of HGSIL. It is a subjective method, dependent on the experience of the operator and many times unnecessary biopsies are performed to confirm the final diagnosis. According to our own conclusions, but also in agreement with the international literature, the diagnostic tool ZedScan has a higher sensitivity and NPV for HSIL detection. These observations also extend to junior colposcopists. It is, therefore, clear that more clinical studies are needed to confirm its reinforcing role in early colposcopic diagnosis and without histological confirmation to integrate it into daily clinical practice.
Author Contributions
Conceptualization, Georgios Panagakis and Ioannis Papapanagiotou; methodology, Adamantia Kontogeorgi and Nikolaos Thomakos; software, Adamantia Kontogeorgi and Charalampos Theofanakis; validation, Paraskevi Tsetsa, Nikolaos Thomakos and Dimitrios Chaidopoulos; formal analysis, Alexandros Rodolakis; investigation, Georgios Panagakis and Ioannis Papapanagiotou; resources, Nikolaos Thomakos; data curation, Paraskevi Tsetsa and Alexandros Rodolakis; writing—original draft preparation, Georgios Panagakis and Adamantia Kontogeorgi; writing—review and editing, Ioannis Papapanagiotou; visualization, Georgios Panagakis; supervision, Nikolaos Thomakos and Dimitrios Chaidopoulos; project administration, Georgios Panagakis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mattiuzzi C, Lippi G Cancer statistics: a comparison between World Health Organization (WHO) and Global Burden of Disease (GBD) Eur J Public Health. 2020;30:1026–7. [CrossRef]
- https://www.who.int/news-room/fact-sheets/detail/cervical-cancer.
- zur Hausen H Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989;49:4677–81.
- Walboomers JM, Jacobs MV, Manos MM, et al Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. [CrossRef]
- Herfs M, Yamamoto Y, Laury A, Wang X, Nucci MR, McLaughlin-Drubin ME, Münger K, Feldman S, McKeon FD, Xian W, Crum CP. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):10516-21. Epub 2012 Jun 11. [CrossRef] [PubMed] [PubMed Central]
- Marina OC, Sanders CK, Mourant JR. Effects of acetic acid on light scattering from cells. J Biomed Opt. 2012 Aug;17(8):085002-1. [CrossRef] [PubMed] [PubMed Central]
- Yim EK, Park JS. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005 Dec;37(6):319-24. Epub 2005 Dec 31. [CrossRef] [PubMed] [PubMed Central]
- Wentzensen N, Walker JL, Gold MA, Smith KM, Zuna RE, Mathews C, et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J Clin Oncol 2015;33:83–9. [CrossRef]
- Pretorius RG, Zhang W-H, Belinson JL, Huang M-N, Wu L-Y, Zhang X, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol 2004;191:430–4. [CrossRef]
- Van der Marel J, Rodriguez A, Del Pino M, van Baars R, Jenkins D, van de Sandt MM, et al. The value of endocervical curettage in addition to biopsies in women referred to colposcopy. J Low Genit Tract Dis 2015;19:282–7. [CrossRef]
- Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FAR, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015;125:330–7.
- Porras C, Wentzensen N, Rodrı ́guez AC, Morales J, Burk RD, Alfaro M, et al. Switch from cytology-based to HPV-based cervical screening: implications for colposcopy. Int J Cancer 2012;130:1879–87.
- Nayar R, Wilbur DC. The Pap Test and Bethesda 2014. ‘‘The reports of my demise have been greatly exaggerated.’’ (after a quotation from Mark Twain). Acta Cytol 2015;59:121–32.
- Brown BH, Tidy JA, Boston K, Blackett AD, Smallwood RH, Sharp F. Relation between tissue structure and imposed electrical current flow in cervical neoplasia. Lancet 2000;355:892–5. [CrossRef]
- Sauvaget C, Fayette JM, Muwonge R, Wesley R, Sankaranarayanan R. Accuracy of visual inspection with acetic acid for cervical cancer screening. Int J Gynaecol Obstet. 2011 Apr;113(1):14-24. Epub 2011 Jan 22. [CrossRef] [PubMed]
- Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budu kh AM, Hingmire S, Malvi SG, Thorat R, Kothari A, Chinoy R, Kelkar R, Kane S, Desai S, Keskar VR, Rajeshwarkar R, Panse N, Dinshaw KA. HPV screening for cervical cancer in rural India. N Engl J Med. 2009 Apr 2;360(14):1385-94. [CrossRef] [PubMed]
- C. Muszynski, E. Dupont, B. Vaysse, et al. The impact of using electrical impedance spectroscopy (ZedScan) on the performance of colposcopy in diagnosing high grade squamous lesions of the cervix. J Gynecol Obstet Hum Reprod (2017). [CrossRef]
- JA Tidy, BH Brown, TJ Healey et al. Accuracy of detection of high-grade cervical intraepithelial neoplasia using electrical impedance spectroscopy with colposcopy. BJOG 2013; 120:400-411. [CrossRef]
- A. Pathiraja, R.A. Weerakkody, A.C. von Roon et al. The clinical application of electrical impedance technology in the detection of malignant neoplasms: a systematic review. J Transl Med 2020; 18:227. [CrossRef]
- Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998 Apr;91(4):626-31. [CrossRef] [PubMed]
- Braun RP, Mangana J, Goldinger S, French L, Dummer R, Marghoob AA. Electrical Impedance Spectroscopy in Skin Cancer Diagnosis. Dermatol Clin. 2017 Oct;35(4):489-493. Epub 2017 Aug 7. [CrossRef] [PubMed]
- Balasubramani L, Brown BH, Healey J, Tidy JA. The detection of cervical intraepithelial neoplasia by electrical impedance spectroscopy: the effects of acetic acid and tissue homogeneity. Gynecol Oncol. 2009 Nov;115(2):267-71. Epub 2009 Sep 9. [CrossRef] [PubMed]
- Kim HW, Yun J, Lee JZ, Shin DG, Lee JH. Evaluation of Electrical Impedance Spectroscopy-on-a-Needle as a Novel Tool to Determine Optimal Surgical Margin in Partial Nephrectomy. Adv Healthc Mater. 2017 Sep;6(18). Epub 2017 Jul 11. [CrossRef] [PubMed]
- Brown BH, Tidy JA, Boston K, Blackett AD, Smallwood RH, Sharp F. Relation between tissue structure and imposed electrical current flow in cervical neoplasia. Lancet. 2000 Mar 11;355(9207):892-5. [CrossRef] [PubMed]
- Brown BH, Milnes P, Abdul S, Tidy JA. Detection of cervical intraepithelial neoplasia using impedance spectroscopy: a prospective study. BJOG. 2005 Jun;112(6):802-6. [CrossRef] [PubMed]
- Balasubramani L, Brown BH, Healey J, Tidy JA. The detection of cervical intraepithelial neoplasia by electrical impedance spectroscopy: the effects of acetic acid and tissue homogeneity. Gynecol Oncol 2009;115:267–71. [CrossRef]
- Abdul S, Brown BH, Milnes P, Tidy JA. The use of electrical impedance spectroscopy in the detection of cervical intraepithelial neoplasia. Int J Gyne- col Cancer 2006;16:1823–32.
- Brown BH, Milnes P, Abdul S, Tidy JA. Detection of cervical intraepithelial neoplasia using impedance spectroscopy: a prospective study. BJOG 2005;112:802–6. [CrossRef]
- Traige Study (ALTS) ASCUS-LSIL Group. A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol 2003;188:1393–400. [CrossRef]
- Bekkers RL, van de Nieuwenhof HP, Neesham DE, Hendriks JH, Tan J, Quinn MA. Does experience in colposcopy improve identification of high-grade abnor- malities? Eur J Obstet Gynecol Reprod Biol 2008;141:75–8. [CrossRef]
- Cantor SB, Ca ́rdenas-Turanzas M, Cox DD, Atkinson EN, Nogueras-Gonzalez GM, Beck JR, et al. Accuracy of colposcopy in the diagnostic setting compared with the screening setting. Obstet Gynecol 2008;111:7–14. [CrossRef]
- Sheshadri V, O’Connor D. The agreement of colposcopic grading as compared to directed biopsy results. Journal of Lower Genital Tract Disease 1999;3(3):150–4 (ref type: Journal (full)). [CrossRef]
- Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol 2006;108(2):264–72. [CrossRef]
- Massad LS, Collins YC. Strength of correlations between colposcopic impression and biopsy histology. Gynecol Oncol 2003;89(3):424–8. [CrossRef]
- Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta- analysis. Obstet Gynecol 1998;91(4):626–31. [CrossRef]
- Namkha Dorji, Sangay Tshering, Sonam Choden, et al. BMC Cancer. 2022; 22: 930.
- Tidy JA, Brown BH. Increased detection of high grade CIN, when using electrical impedance spectroscopy as an adjunct to routine colposcopy, is maintained when used across international boundaries: Prospective data from nine European countries. Eur J Obstet Gynecol Reprod Biol. 2022 Aug;275:41-45. [CrossRef]
- Nikolaos Tsampazis, Eleftherios Vavoulidis, Chrysoula Margioula Siarkou et al. Diagnostic comparison of electrical impedance spectroscopy with colposcopy and HPV mRNA-testing in the prediction of CIN2+ women in Greece. J Obstet Gynaecol Res 2023 Apr;49(4):1222-1229. [CrossRef]
- Homola W, Fuchs T, Baranski P, Zimmer A, Zimmer M, Pomorski M. Use of electrical impedance spectroscopy as an adjunct to colposcopy in a pathway of cervical intraepithelial neoplasia diagnostics. Ginekol Pol. 2019;90(11):628-632. [CrossRef] [PubMed]
- Macdonald MC, Brown BH, Lyon RE, Healey TJ, Palmer JE, Tidy JA. Influence of high risk HPV genotype on colposcopic performance: A large prospective study demonstrates improved detection of disease with ZedScan I, particularly in non-HPV 16 patients. Eur J Obstet Gynecol Reprod Biol. 2017 Apr;211:194-198. Epub 2017 Feb 20. [CrossRef] [PubMed]
- C. Muszynski, E. Dupont, B. Vaysse, et al. The impact of using electrical impedance spectroscopy (ZedScan) on the performance of colposcopy in diagnosing high grade squamous lesions of the cervix. J Gynecol Obstet Hum Reprod (2017). [CrossRef]
- Macdonald MC, Brown BH, Lyon RE, Healey TJ, Palmer JE, Tidy JA. Influence of high risk HPV genotype on colposcopic performance: A large prospective study demonstrates improved detection of disease with ZedScan I, particularly in non-HPV 16 patients. Eur J Obstet Gynecol Reprod Biol. 2017 Apr;211:194-198. Epub 2017 Feb 20. [CrossRef] [PubMed]
- Liu ZC, Liu WD, Liu YH, Ye XH, Chen SD. Multiple Sexual Partners as a Potential Independent Risk Factor for Cervical Cancer: a Meta-analysis of Epidemiological Studies. Asian Pac J Cancer Prev. 2015;16(9):3893-900. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).