1. Introduction
Several pieces of evidence indicate that moving tactile stimuli induce specific cortical activity. For example, the hMT+/V5 and inferior parietal cortex contributed to the perception of the moving tactile stimuli [
1,
2]. Transcranial magnetic stimulation over the primary sensory cortex or V5/hMT+ reduced the accuracy of discriminating the direction of the moving tactile stimuli to the fingertip [
3]. Accordingly, the moving tactile stimuli may influence the motor process differently from the static stimulus. Indeed, the body in quiet stance swayed laterally in accordance with the phase of the moving tactile stimuli that mimicked the tactile sensation of the sole during gait [
4]. Accordingly, the moving tactile stimuli influence postural control in quiet stance.
The center of pressure (COP) moves to the initial swing leg side and backward immediately before the gait initiation, called S1 period [
5,
6,
7,
8,
9]. The COP displacement in the S1 period represents anticipatory postural adjustment (APA) of the gait initiation [
5,
6,
7,
9]. The COP displacement in the S1 period was small in patients with Parkinson’s disease, and has been considered to be one possible cause of the freezing of gait [
10,
11,
12]. Repetitive cutaneous cues over the left hip abductor increased the COP displacement in the S1 period [
13]. Rhythmic lateral weight shifting, rhythmic auditory cues or rhythmic arm swing increased the COP displacement in the S1 period [
14]. Those previous findings indicate that rhythmic stimuli enhance the APA before the gait initiation. The moving tactile stimuli to the sole are a kind of rhythmic stimuli. Therefore, the moving tactile stimuli to the sole may enhance the APA before the gait initiation (Hypothesis 1).
Rhythmic lateral shifting of the body increased the APA amplitude before the gait initiation [
14]. Rhythmic lateral shifting of the body causes the rhythmic change in the tactile sensation of the sole in the medial-lateral axis. Thus, laterally moving tactile stimuli may mimic the tactile sensation of the rhythmic lateral weight shifting over the feet. If this view is true, the moving tactile stimuli must enhance the APA as during the rhythmic lateral shifting of the body. If the APA is enhanced by the laterally moving tactile stimuli to the sole in healthy humans, it must be evidence suggesting that the moving tactile stimuli is potentially useful for improving freezing of gait in patients with Parkinson’s disease.
The COP displacement in the S1 period for the gait initiation in response to a cue was greater than that for the initiating gait at their own preferred time [
6,
12,
13]. This means that the APA is greater when one initiates gait in response to an external cue. Accordingly, humans may use a particular phase of the laterally moving tactile stimuli as a trigger to initiate gait for enhancing the APA (Hypothesis 2). If this hypothesis is true, the APA must onset at a specific phase of the moving tactile stimuli, and the APA amplitude must be greater when the laterally moving tactile stimuli are provided during gait initiation.
Monaural auditory cue to the ear contralateral to the preferred initial swing leg changed the probability of selecting the initial swing leg of gait initiation [
15]. One possible explanation for this finding is that the selection process of the initial swing leg is influenced by the asymmetrical sensory input. Laterally moving tactile stimuli to the sole may also play a role for this asymmetrical sensory input. If this view is true, the laterally moving tactile stimuli to the sole influences the selection process of the initial swing leg of the gait initiation (Hypothesis 3). We examined those three hypotheses in the present study.
2. Materials and Methods
2.1. Participants
Thirteen healthy males aged 36.2 ± 2.5 years participated in this study. The number of the participants was determined based on number of the participants in a previous study that found significant effect of the moving tactile stimuli (n = 13) [
2]. All participants had no history of neurological or musculoskeletal diseases. There are gender differences in physical characteristics [
16] and motor performance [
17]. Thus, to exclude variability in motor performance caused by gender difference, only males were recruited. According to the Waterloo dominant foot test [
18,
19], two participants were left-footed but the others were the right-footed. The overview of the study was explained by a document, and written informed consent was obtained from all participants. The methods of the study were in accordance with the Declaration of Helsinki. The experimental design was approved by the ethics committee of Osaka Prefecture University.
2.2. Apparatus
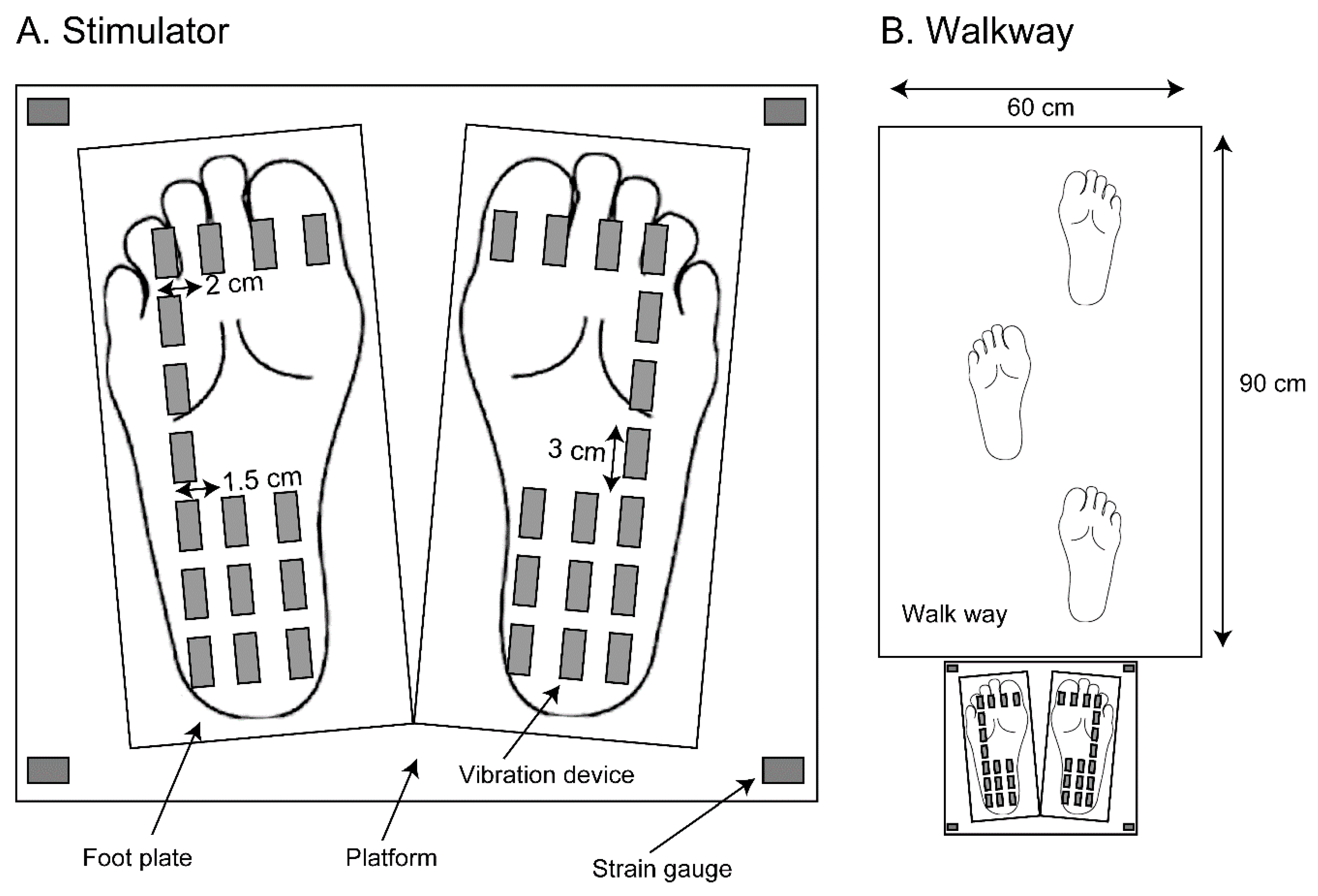
A moving tactile stimulator (S-20008; Takei Kiki, Tokyo), which provided vibration stimuli to the sole, was placed over the ground (
Figure 1A). A foot plate was placed under the participant’s each foot while the participant maintained quiet stance. Sixteen vibration devices were placed over each foot plate. The frequency of the vibration in each vibration device was 100 Hz. Strain gauges measuring the COP were placed on a rectangle platform under the foot plates. Each one of the strain gauges was placed at each corner of the rectangle platform, and the strain of the platform at each corner was measured. Using data from those four strain gauges, the COP was calculated. A walk way (width 60 cm, length 90 cm) was placed in front of this stimulator (
Figure 1B).
2.3. Procedure
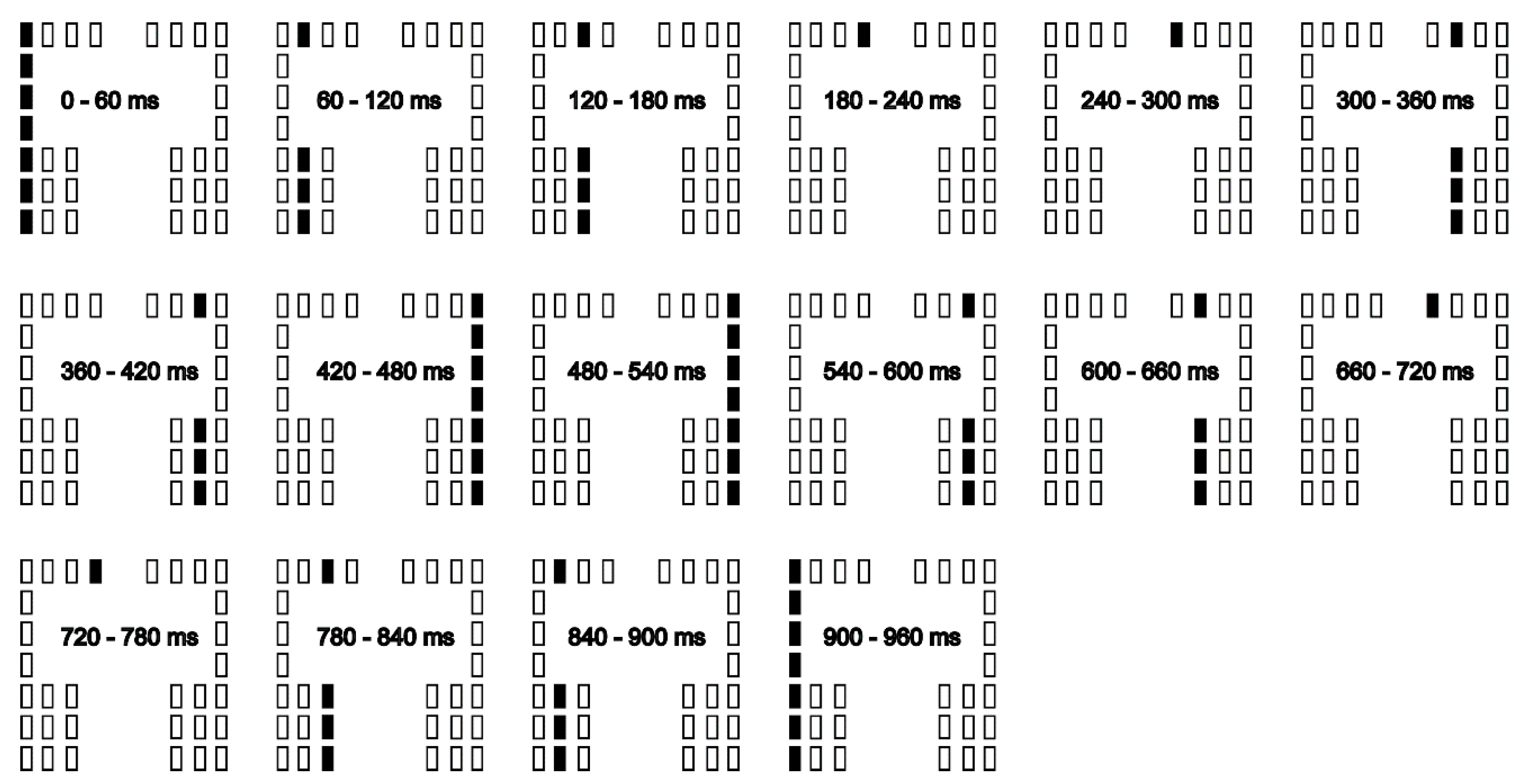
The participants maintained quiet stance on the foot plates. Each trial lasted for 15 s. A sound cue was provided at the beginning of the trial, and the participants initiated gait at their preferred time within the time window of each trial, and made three steps forward to the end of the walk-way with their preferred velocity. The initial swing leg of the gait initiation was freely chosen by the participants in each trial. One of two conditions, gait initiation with (stimulation condition) and without stimuli to the sole (non-stimulation condition), was assigned in each trial. In the stimulation condition, the moving tactile stimuli were given throughout the time window of the trial. The duration of each vibratory stimulus provided by the devices was 60 ms. The cycle duration of the moving tactile stimuli was 960 ms mimicking the cycle duration of the gait cycle, which is approximately 1 s [
20]. The loci of the stimuli moved from the left to right most position of the sole and then moved from the right to left most position of that with 960 ms of one stimuli cycle, and this cycle was repeated 16 times (
Figure 2). Thus, the stimuli lasted for 15 s. The stimuli were not given in the no-stimulation condition. Twenty trials were conducted in each condition. Taken together, 40 trials were conducted. The condition was randomly assigned in each trial.
2.4. Data analysis
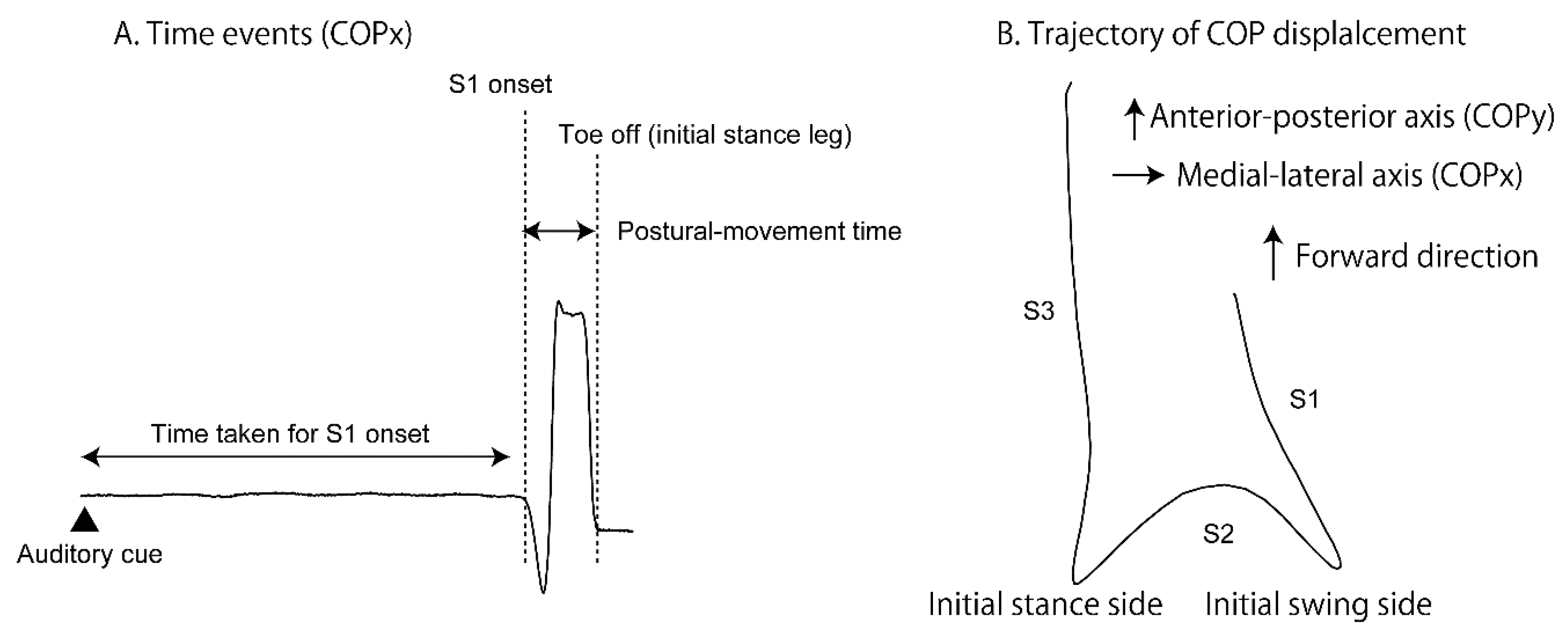
The COP displacement during the gait initiation is composed of three periods (S1, S2 and S3 periods; [
8];
Figure 3B). The S1 period of the COP displacement was considered to be the APA [
5,
6,
7,
8,
9]. The onset of the COP displacement was considered to be the onset of the S1 period. The first peak of the COPx displacement was considered to be the offset of the S1 and onset of the S2 period. The second peak of the COPy displacement was considered to be the offset of the S2 and onset of the S3 period. The offset of the COP displacement was considered to be the offset of the S3 period. The duration and amplitude of the COP displacement in each period were calculated. The probability of the initial swing side of the gait initiation was calculated.
Time events of the COP are presented in
Figure 3A. The duration between the start cue and S1 period onset (time taken for the S1 onset) was calculated. The time at the toe off of the initial stance leg was defined as the moment at which the COPx displacement was ended. The duration between the onset of the S1 period and toe off of the initial stance leg (postural-movement time) was calculated.
The mean and standard deviation (SD) of the COP position 0 - 100 ms before the S1 period onset was calculated. The SD of the COP represents the amount of the body sway, and mean COP represents the body position. The z-score of the mean COP was calculated as following. Mean COP averaged across the trials in both conditions (40 trials) was subtracted from mean COP in each trial. Then, the subtracted each COP was divided by SD of mean COP across the trials in both conditions (40 trials).
There were 16 phases of the moving tactile stimuli (
Figure 2). The moving tactile stimuli from the left to right most position of the sole consisted eight phases. The moving tactile stimuli from right to left most position of the sole also consisted eight phases. We categorized each trial into one of the 16 stimuli phases in which the S1 period onset corresponded to the time at the phase. Then, the number of the trials categorized in each stimuli phase was counted.
Paired t-test was conducted to examine the difference in means between the stimulation and non-stimulation conditions. Repeated measures two-way ANOVA was conducted to test the main effect of the direction and phase of the stimuli on the number of the trials categorized in each phase of the stimuli. The result of Greenhouse–Geisser’s correction was reported whenever Mauchly’s test of sphericity was significant. Bonferroni’s test was conducted for post-hoc test. The alpha level was 0.05. All the statistical analyses were carried out using Excel Tokei ver. 3.20 (Social Survey Research Information, Tokyo). All the data in Results were expressed as the mean and standard error of mean.
3. Results
3.1. COP before S1 onset
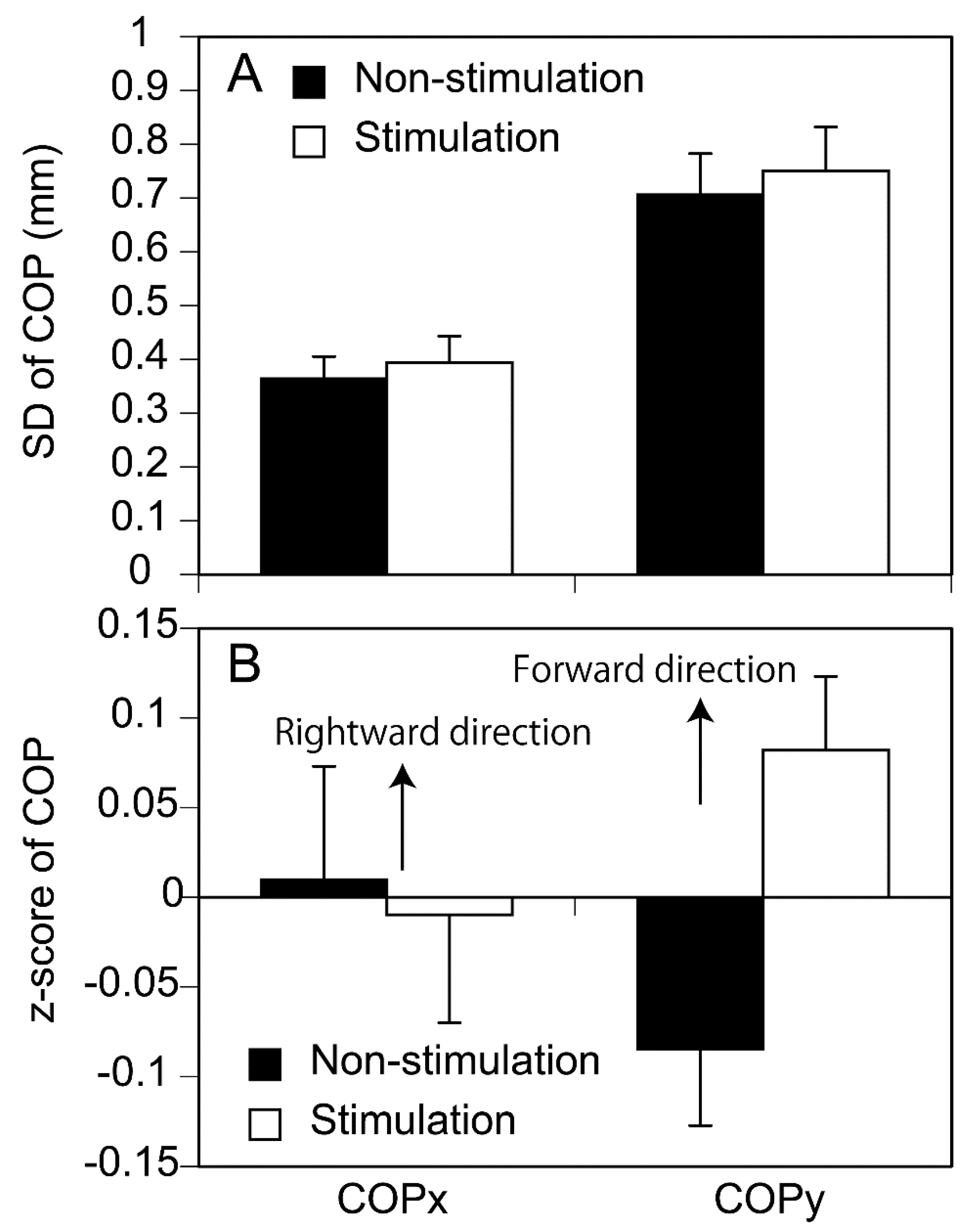
SD of the COP immediately before the S1 period onset is shown in
Figure 4A. There was no significant difference in SD of the COP between the stimulation and non-stimulation conditions either in the anterior-posterior (p = 0.507) or medial-lateral axis (p = 0.210). The z-score of the mean COP is shown in
Figure 4B. There was no significant difference in mean COP between the conditions either in the anterior-posterior (p = 0.071) or medial-lateral axis (p = 0.876).
3.2. S1 onset
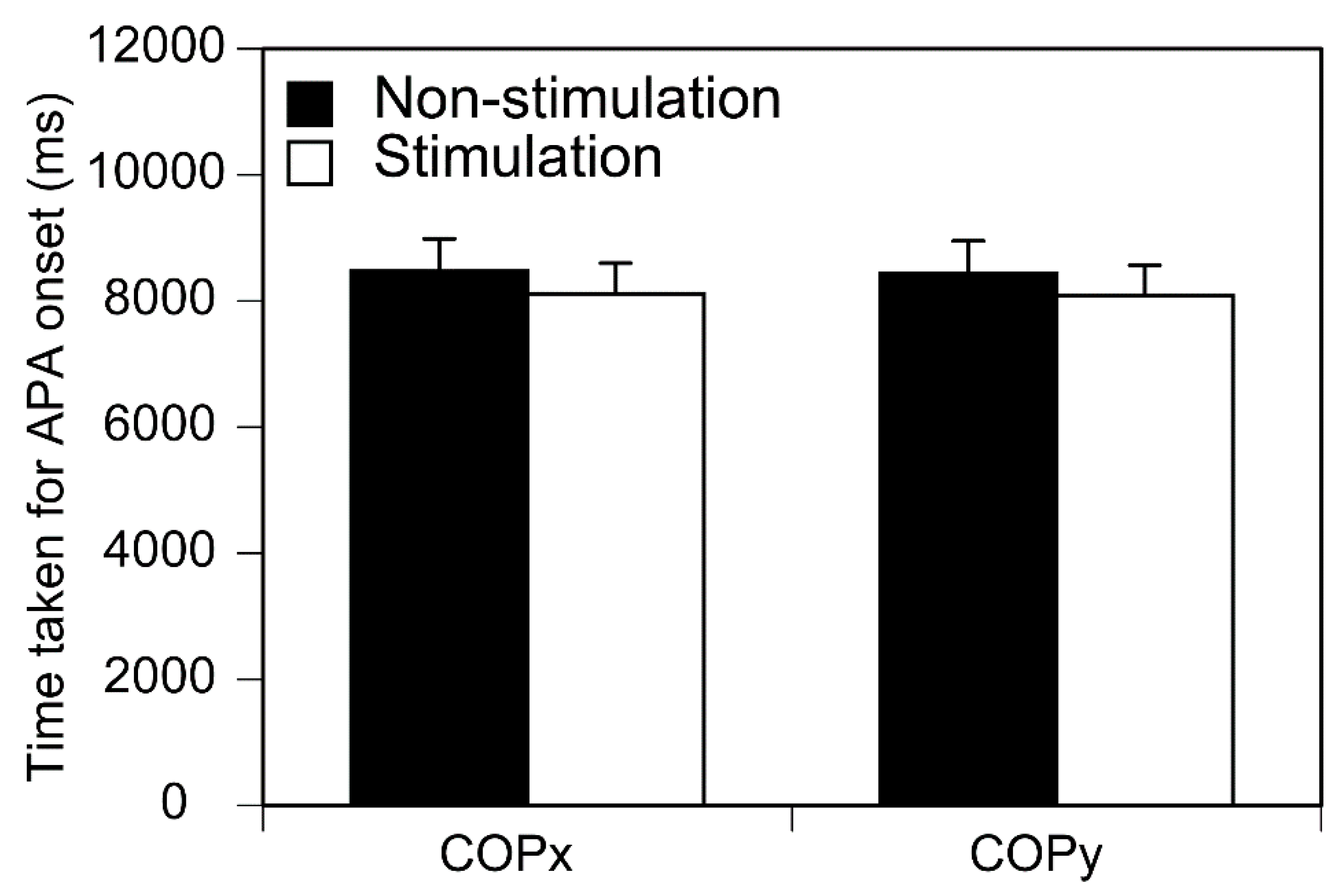
Time taken for the S1 period onset is shown in
Figure 5. There was no significant difference in the time taken for the S1 period onset between the two conditions either in the anterior-posterior (p = 0.241) or medial-lateral axis (p = 0.246). There was no significant main effect for the direction [F (1, 12) = 0.823 P = 0.382] or phase [F (7, 84) = 1.135 p = 0.349] of the stimuli on the number of the trials in which the S1 period onset categorized in each stimuli phase. There was no significant interaction between those two main effects [F (7, 84) = 0.835 p = 0.561].
3.3. COP displacement
The duration and amplitude of the COP displacement in the S1 period are shown in
Figure 6A and D. The amplitude of the COP displacement in the stimulation condition was significantly smaller than that in the non-stimulation condition either in the anterior-posterior (COPy, p = 0.047) or medial-lateral axis (COPx, p = 0.030). The duration of the COP displacement in the stimulation condition was significantly longer than that in the non-stimulation condition in the medial-lateral axis (p = 0.014), but was not significantly different between the two conditions in the anterior-posterior axis (p = 0.224).
The duration and amplitude of the COP displacement in the S2 period are shown in
Figure 6B and E. The amplitude of the COP displacement in the stimulation condition was significantly smaller than that in the non-stimulation condition in the medial-lateral axis (COPx, p = 0.004), but that was not significantly different between the two conditions in the anterior-posterior axis (COPy, p = 0.986). The duration of the COP displacement in the stimulation condition was not significantly different between the two conditions either in the anterior-posterior (COPy, p = 0.116) or medial-lateral axis (COPx, p = 0.148).
The duration and amplitude of the COP displacement in the S3 period is shown in
Figure 6C and F. The amplitude of the COP displacement in the stimulation condition was not significantly different between the two conditions either in the anterior-posterior (COPy, p = 0.317) or medial-lateral axis (COPx, p = 0.309). The duration of the COP displacement in the stimulation condition was not significantly different between the two conditions either in the anterior-posterior (COPy, p = 0.416) or medial-lateral axis (COPx, p = 0.548).
3.4. Postural-movement time
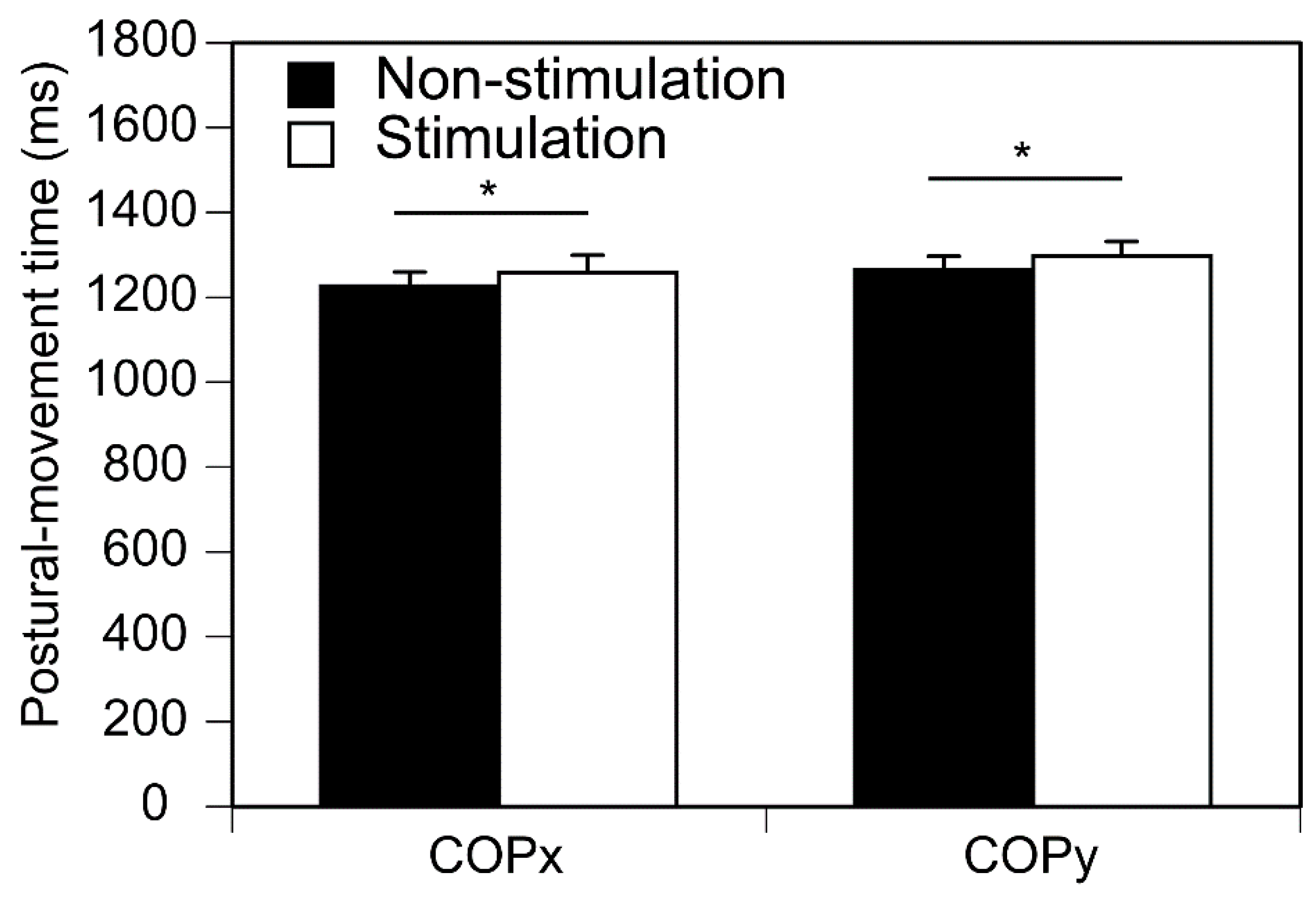
The postural-movement time is shown in
Figure 7. The time in the stimulation condition was longer than that in the non-stimulation condition both in the medial-lateral (COPx, p = 0.033) and anterior-posterior axes (COPy, p = 0.048).
3.5. Selecting initial swing leg
The participants initiated gait with the right leg in most of the trials both in the left- and right-footed participants. The participants initiated gait with the left leg with probability of 0.007 ± 0.007 in the stimulation condition, and was 0.004 ± 0.004 in the non-stimulation condition. There was no significant difference in the probability of the gait initiation with the left leg between the conditions (p = 0.690).
4. Discussion
4.1. APA amplitude
The COP displacement in the S1 was greater when the rhythmic lateral shifting of the body was performed before the gait initiation [
14]. Rhythmic tactile sensation of the sole occurs during the lateral shifting of the body in stance. Thus, one possible explanation for this previous finding is that rhythmic tactile sensation of the sole enhances the APA before the gait initiation. Accordingly, we hypothesized that the moving tactile stimuli to the sole, that induces the rhythmic tactile sensation of the sole, enhance the APA before the gait initiation (Hypothesis 1). In spite of this hypothesis, the COP displacement in the S1 period was decreased by the moving tactile stimuli to the sole. Thus, Hypothesis 1 was not supported.
The postural-movement time was extended when the moving tactile stimuli were provided to the sole. The prolonged postural-movement time reflects slowing down of the gait initiation. The COP displacement in the S1 period increased as the velocity of the gait initiation increased [
9,
13]. The COP displacement in the S1 period was interacted with the step movement of the gait initiation [
21]. Accordingly, the decrease in the APA amplitude induced by the moving tactile stimuli is likely caused by the slowing down of the gait initiation.
Likely explanation for the slowing down of the gait initiation induced by the moving tactile stimuli to the sole is that the moving tactile stimulation to the sole masked the tactile sensation of the sole produced by the weight bearing over the feet. On the one hand, the tactile sensation of the sole occurs when humans maintain the stance because of weight bearing over the feet. On the other hand, the moving tactile stimuli are artificial stimulation, and the spatio-temporal pattern of the stimuli is not related to the tactile sensation of the sole produced by the weight bearing over the feet in stance. Accordingly, the moving tactile stimuli must have functioned as noise for the tactile sensation of the sole produced by the weight bearing over the feet in stance.
It has been shown that masking tactile sensation of the sole changes the body sway in quiet stance. For example, the COP velocity and displacement in quiet stance was increased when the tactile sensation of the sole was masked with the ischemic nerve block [
22]. Acceleration of the lumber deviation in quiet stance was greater in patients with diabetic peripheral neuropathy who had impairment of tactile sensation [
23]. Masking tactile sensation induced by the static tactile stimulus to the sole changed the body sway in quiet stance [
24]. The APA occurs at the quiet stance position before the gait initiation, and thus, the postural control in stance contributes to the APA before the gait initiation. Accordingly, the decrease in the size of the COP displacement in the S1 period and slowing down of the gait initiation induced by the moving tactile stimuli to the sole may be explained by the view that the moving tactile stimuli masked the tactile sensation of the sole, causing the change in the postural control process, leading to the slowing down of the gait initiation and negative impact on the APA.
However, this view must be handled with caution. If the slowing down of the gait initiation is caused the masking of the tactile sensation of the sole, the body sway in stance immediately before the gait initiation must be influenced by the moving tactile stimuli as occurring during masking tactile sensation of the sole in quiet stance in previous studies [
22,
23,
24]. In the present study, as conflicting with the previous findings, the SD of the COP displacement before the S1 period was not significantly different between the conditions, as consistent with a previous finding that no significant change in the amount of the body sway was induced by the moving tactile stimuli to the sole in quiet stance [
4]. Accordingly, there is still unresolved question to confirm the view that the decrease in the APA amplitude and the slowing down of the gait initiation are mediated by the mechanism underlying the effect of the masking tactile sensation of the sole in quiet stance. Further studies are needed to determine whether the decrease in the APA amplitude and slowing down of the gait initiation induced by the moving tactile stimuli is caused by masking tactile sensation of the sole.
4.2. S2 period
The medial-lateral displacement of the COP in the S2 period was decreased by the moving tactile stimuli. The S2 period is the moment where the body weight shifts from the initial swing leg to the initial stance leg [
8]. The COP displacement in the S1 period is for shifting the body to the initial stance leg, which corresponds to the COP displacement in the S2 period [
25,
26]. Thus, the present finding is explained by the view that the decrease in the COP displacement in the S2 period is due to insufficient preparation of the weight shifting represented by the small COP displacement in the S1 period.
4.3. Trigger of gait initiation
The COP displacement in the S1 period when initiating gait in response to a cue was greater than that when initiating gait at their own preferred time [
6,
12,
13]. This means that the APA is greater when humans initiate gait in response to an external cue. Accordingly, we hypothesized that humans may use a particular phase of the laterally moving tactile stimuli as a trigger to initiate gait (Hypothesis 2). If this hypothesis is true, the COP displacement in the S1 period must onset at a particular phase of the laterally moving tactile stimuli, and the APA must be greater when the moving tactile stimuli are provided. The onset of the S1 period was not specific to a particular phase or direction of the laterally moving tactile stimuli, and the S1 amplitude was not significantly different between the two conditions. Thus, Hypothesis 2 was not supported. The moving tactile stimuli were continuously provided throughout a trial. Thus, the negative finding may be explained by the view that the participants did not perceive the stimuli at each stimuli phase, but perceived the moving stimuli as a sequence of the stimuli across the phases.
4.4. Selecting initial swing leg
Monaural auditory cue changed the probability of selecting the initial swing leg of gait initiation [
15]. This indicates that the selection process of the initial swing side is influenced by the asymmetrical sensory input. Accordingly, laterally moving tactile stimuli to the sole in the present study may play a role for this asymmetrical sensory input. Based on this view, we hypothesized that the laterally moving tactile stimuli to the sole influence the selection of the initial swing leg side of the gait initiation (Hypothesis 3). The laterally moving tactile stimuli to the sole did not affect the selection of the initial swing leg. This means that laterally moving tactile sensation does not influence the selection process of the leg during gait initiation; Hypothesis 3 was not supported.
5. Conclusions
The moving tactile stimuli to the sole decreased the APA amplitude before the gait initiation, and made slower gait initiation. Those findings are explained by the view that the laterally moving tactile stimuli to the sole slows down the gait initiation due to masking tactile sensation of the sole, and this reduces the amplitude of the APA.
Author Contributions
Conceptualization, K.H.; methodology, K.H.; investigation, H.K., H.O., T.K., R.T., N.H., S.F., M.M., K.H.; writing—original draft preparation, K.H.; writing—review and editing, H.K.; supervision, K.H.; project administration, K.H.; funding acquisition, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number JP20K11187.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Osaka Prefecture University (approval number; 2021-116).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the participants to publish this paper.
Data Availability Statement
The data is unavailable due to ethical restrictions.
Conflicts of Interest
There is no conflict of interest.
References
- Summers, I.R.; Francis, S.T.; Bowtell, R.W.; McGlone, F.P.; Clemence, M. A functional-magnetic-resonance-imaging investigation of cortical activation from moving vibrotactile stimuli on the fingertip. J. Acoust. Soc. Am. 2009, 125, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Wacker, E.; Spitzer, B.; Lützkendorf, R.; Bernarding, J.; Blankenburg, F. Tactile Motion and Pattern Processing Assessed with High-Field fMRI. PLOS ONE 2011, 6, e24860. [Google Scholar] [CrossRef]
- Amemiya, T.; Beck, B.; Walsh, V.; Gomi, H.; Haggard, P. Visual area V5/hMT+ contributes to perception of tactile motion direction: a TMS study. Scientific Reports 2017, 7, 40937. [Google Scholar] [CrossRef] [PubMed]
- Sawaguchi, Y.; Kawasaki, T.; Hiraoka, K. Effect of Moving Tactile Stimuli to Mimic Altered Weight Distribution During Gait on Quiet Stance Body Sway. Percept. Mot. Ski. 2023. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.A.; Hagy, J.L.; White, V.; Liddell, D. The initiation of gait. J. Bone Jt. Surg. 1979, 61, 232–239. [Google Scholar] [CrossRef]
- Burleigh-Jacobs, A.; Horak, F.B.; Nutt, J.G.; Obeso, J.A. Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Movement Disorders 1997, 12, 206–215. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, W.E.; Maki, B.E. The control of lateral stability during rapid stepping reactions evoked by antero-posterior perturbation: does anticipatory control play a role? Gait Posture 1999, 9, 190–198. [Google Scholar] [CrossRef]
- Hass, C.J.; Gregor, R.J.; Waddell, D.E.; Oliver, A.; Smith, D.W.; Fleming, R.P.; Wolf, S.L. The influence of Tai Chi training on the center of pressure trajectory during gait initiation in older adults. Arch. Phys. Med. Rehabilitation 2004, 85, 1593–1598. [Google Scholar] [CrossRef]
- Caderby, T.; Yiou, E.; Peyrot, N.; Begon, M.; Dalleau, G. Influence of gait speed on the control of mediolateral dynamic stability during gait initiation. J. Biomech. 2014, 47, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Halliday, S.E.; Winter, D.A.; Frank, J.S.; Patla, A.E.; Prince, F. The initiation of gait in young, elderly, and Parkinson’s disease subjects. Gait Posture 1998, 8, 8–14. [Google Scholar] [CrossRef]
- Carpinella, I.; Crenna, P.; Calabrese, E.; Rabuffetti, M.; Mazzoleni, P.; Nemni, R.; Ferrarin, M. Locomotor function in the early stage of Parkinson’s disease. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2007, 15, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Fukumoto, T.; Takatori, K.; Nagino, K.; Hiraoka, K. Variable Initial Swing Side and Prolonged Double Limb Support Represent Abnormalities of the First Three Steps of Gait Initiation in Patients with Parkinson’s Disease with Freezing of Gait. Front. Neurol. 2011, 2, 85. [Google Scholar] [CrossRef] [PubMed]
- Dibble, L.E.; Nicholson, D.E.; Shultz, B.; MacWilliams, B.A.; Marcus, R.L.; Moncur, C. Sensory cueing effects on maximal speed gait initiation in persons with Parkinson’s disease and healthy elders. Gait Posture 2004, 19, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Kunimura, H.; Oda, H.; Kawasaki, T.; Sawaguchi, Y. Rhythmic movement and rhythmic auditory cues enhance anticipatory postural adjustment of gait initiation. Somatosens. Mot. Res. 2020, 37, 213–221. [Google Scholar] [CrossRef]
- Hiraoka, K.; Ae, M.; Ogura, N.; Sano, C.; Shiomi, K.; Morita, Y.; Yokoyama, H.; Iwata, Y.; Jono, Y.; Nomura, Y.; et al. Monaural Auditory Cue Affects the Process of Choosing the Initial Swing Leg in Gait Initiation. J. Mot. Behav. 2015, 47, 522–526. [Google Scholar] [CrossRef]
- Hamill, P.V. 1977. NCHS growth curves for children: Birth-18 years, United States (No. 165). US Department of Health, Education, and Welfare, Public Health Service, National Center for Health Statistics.
- Thomas, J.R.; French, K.E. Gender differences across age in motor performance: A meta-analysis. Psychological Bulletin 1985, 98, 260. [Google Scholar] [CrossRef]
- Elias, L.J.; Bryden, M.; Bulman-Fleming, M. Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia 1998, 36, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zverev, Y.P. Spatial parameters of walking gait and footedness. Ann. Hum. Biol. 2006, 33, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Perry, J., & Burnfield, J. M. (2010). Gait analysis. Normal and pathological function 2nd ed. California: Slack.
- Mille, M.-L.; Simoneau, M.; Rogers, M.W. Postural dependence of human locomotion during gait initiation. J. Neurophysiol. 2014, 112, 3095–3103. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Lin, S.-I. Sensitivity of plantar cutaneous sensation and postural stability. Clin. Biomech. 2008, 23, 493–499. [Google Scholar] [CrossRef]
- Turcot, K.; Allet, L.; Golay, A.; Hoffmeyer, P.; Armand, S. Investigation of standing balance in diabetic patients with and without peripheral neuropathy using accelerometers. Clin. Biomech. 2009, 24, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Sawaguchi, Y.; Kawasaki, T.; Oda, H.; Kunimura, H.; Hiraoka, K. Contribution of vision and tactile sensation on body sway during quiet stance. J. Phys. Ther. Sci. 2022, 34, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Yiou, E.; Do, M.C. Effects of medio-lateral postural perturbation induced by voluntary arm raising on the biomechanical organization of rapid step initiation. Motor Control 2011, 15, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Yiou, E.; Caderby, T.; Delafontaine, A.; Fourcade, P.; Honeine, J.-L. Balance control during gait initiation: State-of-the-art and research perspectives. World J. Orthop. 2017, 8, 815–828. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).