Submitted:
12 September 2023
Posted:
13 September 2023
You are already at the latest version
Abstract
Keywords:
Introductıon
II. Cancer immunology and immunotherapy
III. Immunotherapy in GI cancers: Cellular treatment systems
a. Cancer vaccines: rationale, limitations and potential combinations
Dendritic cell vaccines
Cancer vaccines and combined strategies to improve immunogenic activity in GI cancers
Cancer vaccines for esophageal and gastric cancer: clinical evidence
Cancer vaccines for colorectal cancer: clinical evidence
b. Adoptive cell therapy: cytokine-induced killer cells as an immunotherapy approach in GI cancers
c. Combined immunotherapy approaches: Rationale and preclinical evidence
d. Clinical applications of combined cellular immunotherapy in GI cancer: Dendritic cell vaccines and cytokine-induced killer cells
IV. Case reports
Case 1
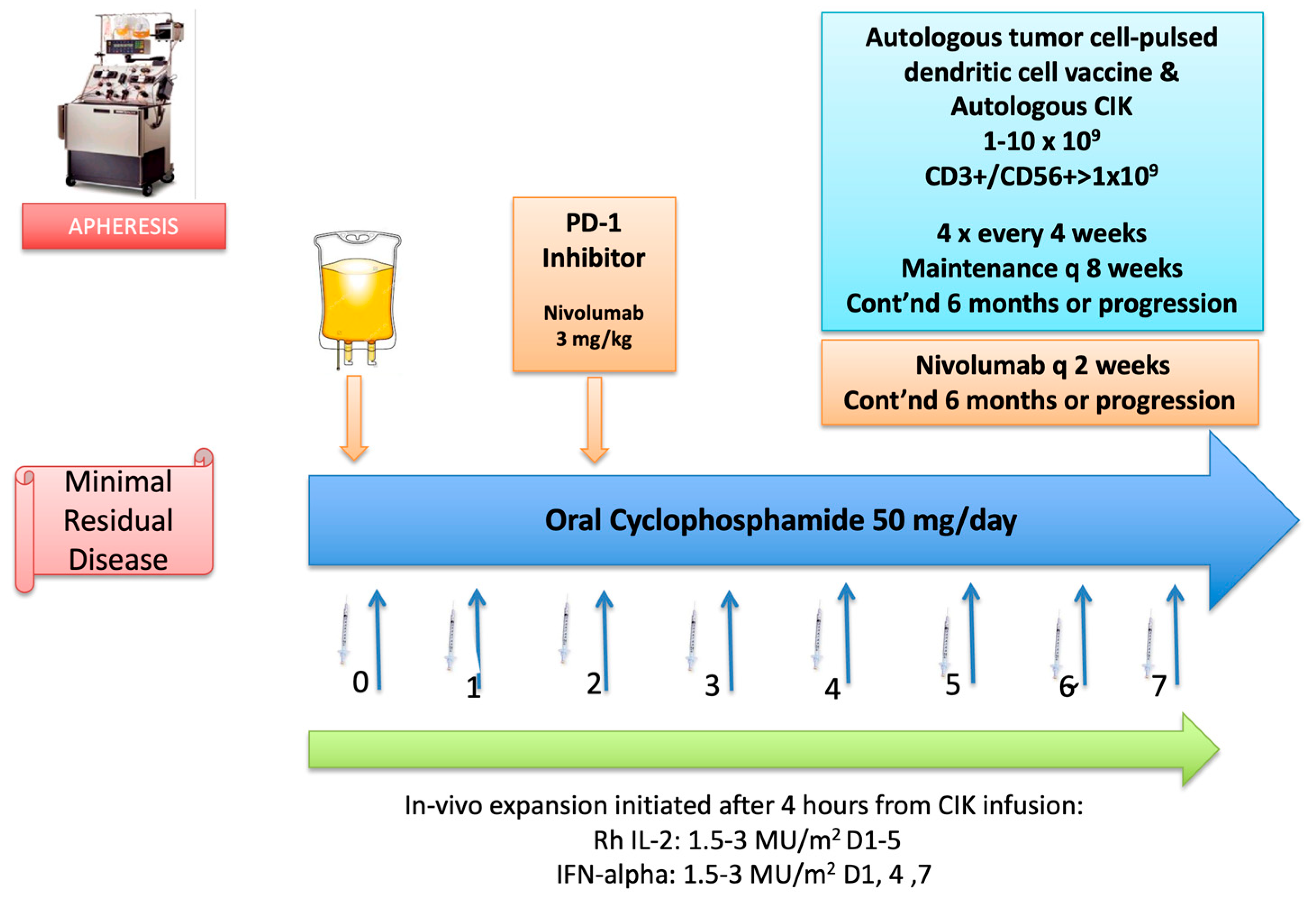
Case 2

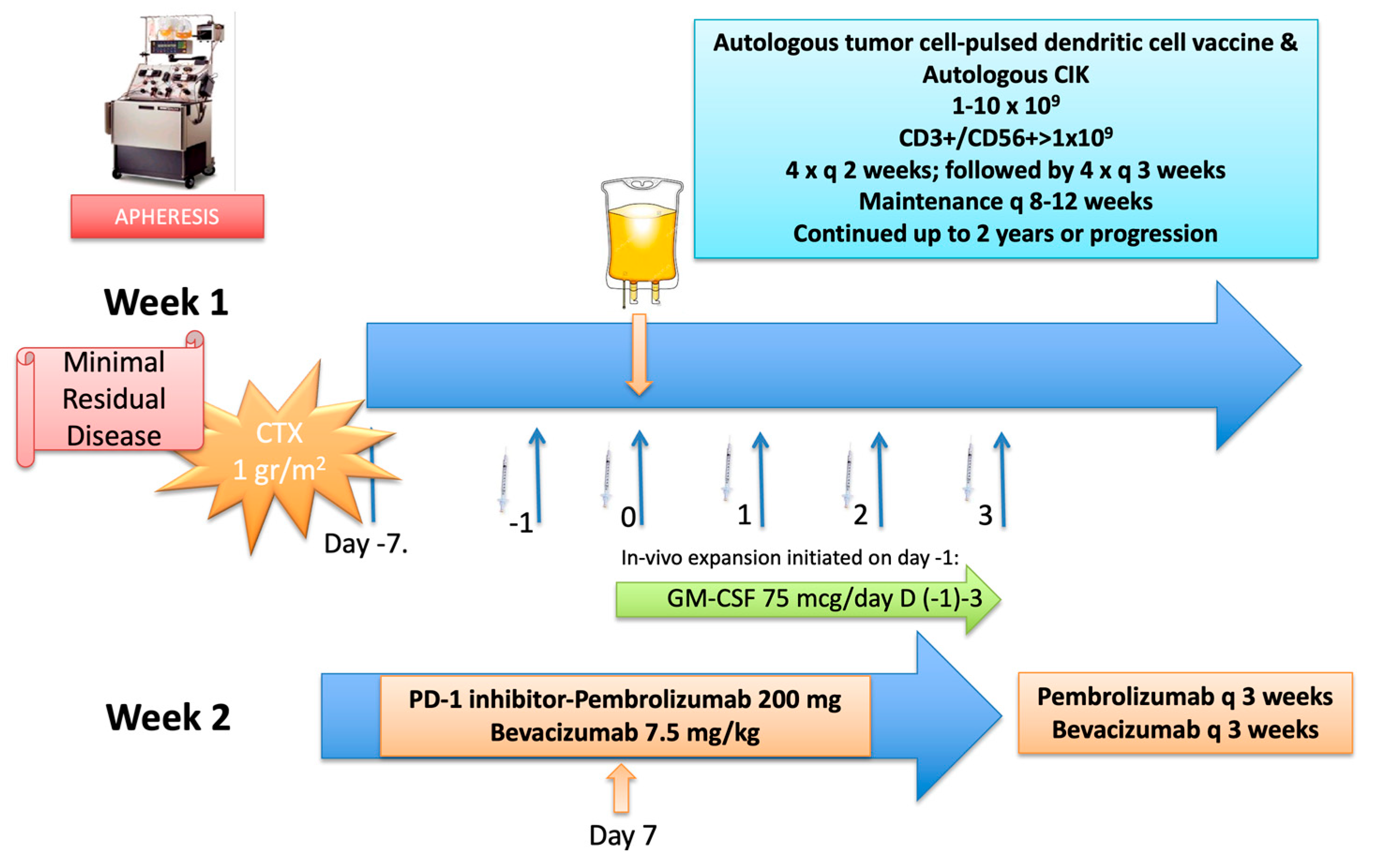
Case 3
Methods
V. Future prospects and conclusion
Author Contributions
Conflicts of Interest
Informed Consent; Institutional and Central Review Board Approval
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021, 3, 27–40. [Google Scholar] [CrossRef]
- Rha, S.Y.; Wyrwicz, L.S.; Weber, P.E.Y.; Bai, Y.; Ryu, M.H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.K.; et al. VP1-2023: Pembrolizumab (pembro) plus chemotherapy (chemo) as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Phase III KEYNOTE-859 study. Annals of Oncology 2023, 34, 319–320. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Randon, G.; Di Bartolomeo, M.; Luciani, A.; Chao, J.; Smyth, E.; Petrelli, F. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open 2021, 6, 100036. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jager, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- A Diaz, L.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Taefehshokr, S.; Parhizkar, A.; Hayati, S.; Mousapour, M.; Mahmoudpour, A.; Eleid, L.; Rahmanpour, D.; Fattahi, S.; Shabani, H.; Taefehshokr, N. Cancer immunotherapy: Challenges and limitations. Pathol. - Res. Pr. 2022, 229, 153723. [Google Scholar] [CrossRef] [PubMed]
- Gerard, C.; Delyon, J.; Wicky, A.; Homicsko, K.; Cuendet, M.A.; Michielin, O. Turning tumors from cold to inflamed to improve immunotherapy response. Cancer Treat. Rev. 2021, 101, 102227. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Jeon, J.W.; Sievers, C.; Allen, C.T. Antigen processing and presentation in cancer immunotherapy. J Immunother Cancer. 2020, 8, e001111. [Google Scholar] [CrossRef]
- O’keeffe, M.; Mok, W.H.; Radford, K.J. Human dendritic cell subsets and function in health and disease. Cell. Mol. Life Sci. 2015, 72, 4309–4325. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Nussenzweig, M.C. Origin and development of dendritic cells. Immunol. Rev. 2010, 234, 45–54. [Google Scholar] [CrossRef]
- Peterson, E.E.; Barry, K.C. The Natural Killer–Dendritic Cell Immune Axis in Anti-Cancer Immunity and Immunotherapy. Front. Immunol. 2021, 11, 621254. [Google Scholar] [CrossRef] [PubMed]
- Constantino, J.; Gomes, C.; Falcão, A.; Neves, B.M.; Cruz, M.T. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol. Res. 2017, 65, 798–810. [Google Scholar] [CrossRef]
- van de Laar, L.; Buitenhuis, M.; Wensveen, F.M.; Janssen, H.L.; Coffer, P.J.; Woltman, A.M. Human CD34-Derived Myeloid Dendritic Cell Development Requires Intact Phosphatidylinositol 3-Kinase–Protein Kinase B–Mammalian Target of Rapamycin Signaling. J. Immunol. 2010, 184, 6600–6611. [Google Scholar] [CrossRef]
- Lee, J.; Breton, G.; Oliveira, T.Y.K.; Zhou, Y.J.; Aljoufi, A.; Puhr, S.; Cameron, M.J.; Sékaly, R.-P.; Nussenzweig, M.C.; Liu, K. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J. Exp. Med. 2015, 212, 385–399. [Google Scholar] [CrossRef]
- Naik, S.H.; Sathe, P.; Park, H.-Y.; Metcalf, D.; I Proietto, A.; Dakic, A.; Carotta, S.; O'Keeffe, M.; Bahlo, M.; Papenfuss, A.; et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 2007, 8, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F.; Kofod-Olsen, E.; Agger, R. Activation of dendritic cells by targeted DNA: a potential addition to the armamentarium for anti-cancer immunotherapy. Cancer Immunol. Immunother. 2019, 68, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Saban, D.R. The Chemokine Receptor CCR7 Expressed by Dendritic Cells: A Key Player in Corneal and Ocular Surface Inflammation. Ocul. Surf. 2014, 12, 87–99. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, R.; Wang, X.; Hu, K.; Huang, L.; Lu, M.; HU, Q. CCL19 and CCR7 Expression, Signaling Pathways, and Adjuvant Functions in Viral Infection and Prevention. Front Cell Dev Biol 2019, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Anari, F.; Ramamurthy, C.; Zibelman, M. Impact of tumor microenvironment composition on therapeutic responses and clinical outcomes in cancer. Futur. Oncol. 2018, 14, 1409–1421. [Google Scholar] [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.-T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef]
- Li, J.; Byrne, K.T.; Yan, F.; Yamazoe, T.; Chen, Z.; Baslan, T.; Richman, L.P.; Lin, J.H.; Sun, Y.H.; Rech, A.J.; et al. Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy. Immunity. 2018, 49, 178–193. [Google Scholar] [CrossRef]
- Gerard, C.; Delyon, J.; Wicky, A.; Homicsko, K.; Cuendet, M.A.; Michielin, O. Turning tumors from cold to inflamed to improve immunotherapy response. Cancer Treat. Rev. 2021, 101, 102227. [Google Scholar] [CrossRef]
- Ito, F.; Chang, A.E. Cancer immunotherapy: current status and future directions. Surg Oncol Clin N Am. 2013, 22, 765–83. [Google Scholar] [CrossRef]
- Ayana, R.; Kumar, A.R.; Devan, A.R.; Nair, B.; Vinod, B.S.; Nath, L.R. Harnessing the immune system against cancer: current immunotherapy approaches and therapeutic targets. Molecular Biology Reports 2021, 48, 8075–8095. [Google Scholar]
- Stephan, K.; Matthias, I.; Sebastian, K. Advances in cancer immunotherapy 2019 – latest trends. Exp Clin Cancer Res 2019, 38, 1–11. [Google Scholar]
- Marshall, H.T.; Djamgoz, M.B.A. Immuno-Oncology: Emerging targets and combination therapies. Front Oncol. 2018, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016, 13, 273–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cao, Y.J. Adoptive Cell Therapy Targeting Neoantigens: A Frontier for Cancer Research. Front Immunol. 2020, 11, 176. [Google Scholar] [CrossRef]
- Coley, W.B. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am. J. Med. Sci. 1893, 105, 487–511. [Google Scholar] [CrossRef]
- Parish, C.R. Cancer immunotherapy: the past, the present and the future. Immunol Cell Biol 2003, 81, 106–13. [Google Scholar] [CrossRef]
- Fritah, H.G.; Rovelli, R.; Chiang, C.L.-L.; Kandalaft, L.E. The current clinical landscape of personalized cancer vaccines. Cancer Treat. Rev. 2022, 106, 102383. [Google Scholar] [CrossRef]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: the next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; et al. Faculty Opinions recommendation of Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. Journal of clinical oncology 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Fritsch, E.F.; Burkhardt, U.E.; Hacohen, N.; Wu, C.J. Personal Neoantigen Cancer Vaccines: A Road Not Fully Paved. Cancer Immunol. Res. 2020, 8, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Constantino, J.; Gomes, C.; Falcão, A.; Cruz, M.T.; Neves, B.M. Antitumor dendritic cell–based vaccines: lessons from 20 years of clinical trials and future perspectives. Transl. Res. 2016, 168, 74–95. [Google Scholar] [CrossRef]
- Chudnovskiy, A.; Pasqual, G.; Victora, G.D. Studying interactions between dendritic cells and T cells in vivo. Curr. Opin. Immunol. 2019, 58, 24–30. [Google Scholar] [CrossRef]
- Zanna, M.Y.; Yasmin, A.R.; Omar, A.R.; Arshad, S.S.; Mariatulqabtiah, A.R.; Nur-Fazila, S.H.; Mahiza, I.N. Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. Int. J. Mol. Sci. 2021, 22, 8044. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.S.; Waithman, J.; Bedoui, S.; Jones, C.M.; Villadangos, J.A.; Zhan, Y.; Lew, A.M.; Shortman, K.; Heath, W.R.; Carbone, F.R. Migratory Dendritic Cells Transfer Antigen to a Lymph Node-Resident Dendritic Cell Population for Efficient CTL Priming. Immunity 2006, 25, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, N.; Riol-Blanco, L.; Rodríguez-Fernández, J.L. The Multiple Personalities of the Chemokine Receptor CCR7 in Dendritic Cells. J. Immunol. 2006, 176, 5153–5159. [Google Scholar] [CrossRef]
- Filin, I.Y.; Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A.; Solovyeva, V.V. Recent Advances in Experimental Dendritic Cell Vaccines for Cancer. Front. Oncol. 2021, 11, 730824. [Google Scholar] [CrossRef]
- Gardner, A.; Ruffell, B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef]
- Hsu, F.J.; Benike, C.; Fagnoni, F.; Liles, T.M.; Czerwinski, D.; Taidi, B.; Engleman, E.G.; Levy, R. Vaccination of patients with B–cell lymphoma using autologous antigen–pulsed dendritic cells. Nat. Med. 1996, 2, 52–58. [Google Scholar] [CrossRef]
- Dashti, A.; Ebrahimi, M.; Hadjati, J.; Memarnejadian, A.; Moazzeni, S.M. Dendritic cell based immunotherapy using tumor stem cells mediates potent antitumor immune responses. Cancer Lett. 2016, 374, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.R.; Hildebrand, W.H.; Mardis, E.R.; Linette, G.P. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef]
- Jeng, L.B.; Liao, L.Y.; Shih, F.Y.; Teng, CF. Dendritic-Cell-Vaccine-Based Immunotherapy for Hepatocellular Carcinoma: Clinical Trials and Recent Preclinical Studies. Cancers (Basel) 2022, 14, 4380. [Google Scholar] [CrossRef]
- Ju, H.; Xing, W.; Yang, J.; Zheng, Y.; Jia, X.; Zhang, B.; Ren, H. An effective cytokine adjuvant vaccine induces autologous T-cell response against colon cancer in an animal model. BMC Immunol. 2016, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Brivio, F.; Fumagalli, L.; Di Fede, G.; Brera, G. Enhancement of the efficacy of chemotherapy with oxaliplatin plus 5-fluorouracil by pretreatment with IL-2 subcutaneous immunotherapy in metastatic colorectal cancer patients with lymphocytopenia prior to therapy. Vivo 2005, 19, 1077–80. [Google Scholar]
- Borden, E.C. Interferons α and β in cancer: therapeutic opportunities from new insights. Nat Rev Drug Discov. 2019, 18, 219–234. [Google Scholar] [CrossRef]
- Spaapen, R.M.; Leung, M.Y.K.; Fuertes, M.B.; Kline, J.P.; Zhang, L.; Zheng, Y.; Fu, Y.-X.; Luo, X.; Cohen, K.S.; Gajewski, T.F. Therapeutic Activity of High-Dose Intratumoral IFN-β Requires Direct Effect on the Tumor Vasculature. J. Immunol. 2014, 193, 4254–4260. [Google Scholar] [CrossRef]
- Anderson, A.C. Tim-3: An Emerging Target in the Cancer Immunotherapy Landscape. Cancer Immunol. Res. 2014, 2, 393–398. [Google Scholar] [CrossRef]
- Shen, R.; Postow, M.A.; Adamow, M.; Arora, A.; Hannum, M.; Maher, C.; Wong, P.; Curran, M.A.; Hollmann, T.J.; Jia, L.; et al. LAG-3 expression on peripheral blood cells identifies patients with poorer outcomes after immune checkpoint blockade. Sci. Transl. Med. 2021, 13, eabf5107. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Zhang, H.; Ye, J.; Moore, C.; Lu, C.; Fang, Y.; Fu, Y.X.; Li, B. Concurrent delivery of immune checkpoint blockade modulates T cell dynamics to enhance neoantigen vaccine-generated antitumor immunity. Nat Cancer. 2022, 3, 437–452. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Hu, Y.; Yang, Z.; Li, J.; Liu, X.; Deng, L.; Wang, Y.; Zhang, X.; Jiang, T.; Lu, X. Targeting PD-1 and Tim-3 Pathways to Reverse CD8 T-Cell Exhaustion and Enhance Ex Vivo T-Cell Responses to Autologous Dendritic/Tumor Vaccines. J Immunother. 2016, 39, 171–80. [Google Scholar] [CrossRef]
- Vanmeerbeek, I.; Sprooten, J.; De Ruysscher, D.; Tejpar, S.; Vandenberghe, P.; Fucikova, J.; Spisek, R.; Zitvogel, L.; Kroemer, G.; Galluzzi, L.; et al. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. OncoImmunology 2020, 9, 1703449. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Lenz, H.J.; Marshall, J.; Singh, D.; Garett, C.; Cripps, C.; Moore, M.; von Mehren, M.; Dalfen, R.; Heim, W.J.; et al. Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res. 2008, 14, 4843–4849. [Google Scholar] [CrossRef]
- Weihrauch, M.R.; Ansén, S.; Jurkiewicz, E.; Geisen, C.; Xia, Z.; Anderson, K.S.; Gracien, E.; Schmidt, M.; Wittig, B.; Diehl, V.; et al. Phase I/II combined chemoimmunotherapy with carcinoembryonic antigen-derived HLA-A2-restricted CAP-1 peptide and irinotecan, 5-fluorouracil, and leucovorin in patients with primary metastatic colorectal cancer. Clin Cancer Res. 2005, 11, 5993–6001. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: the next generation. Cell. 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ji, Y.R.; Le, Y.M. Crosstalk between angiogenesis and immune regulation in the tumor microenvironment. Arch. Pharm. Res. 2022, 45, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Hodi, F.S. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin Cancer Res. 2019, 25, 5449–5457. [Google Scholar] [CrossRef] [PubMed]
- Osada, T.; Chong, G.; Tansik, R.; Hong, T.; Spector, N.; Kumar, R.; Hurwitz, H.I.; Dev, I.; Nixon, A.B.; Lyerly, H.K.; et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol. Immunother. 2008, 57, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Terme, M.; Pernot, S.; Marcheteau, E.; Sandoval, F.; Benhamouda, N.; Colussi, O.; Dubreuil, O.; Carpentier, A.F.; Tartour, E.; Taieb, J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013, 73, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Sadanaga, N.; Nagashima, H.; Mashino, K.; Tahara, K.; Yamaguchi, H.; Ohta, M.; Fujie, T.; Tanaka, F.; Inoue, H.; Takesako, K.; et al. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin. Cancer Res. 2001, 7, 2277–2284. [Google Scholar]
- Galetto, A.; Contarini, M.; Sapino, A.; Cassoni, P.; Consalvo, E.; Forno, S.; Pezzi, C.; Barnaba, V.; Mussa, A.; Matera, L. Ex Vivo Host Response to Gastrointestinal Cancer Cells Presented by Autologous Dendritic Cells. J. Surg. Res. 2001, 100, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Takahashi, A.; Sugai, H.; Fujii, H.; Choudhury, A.R.; Kiessling, R.; Matsumoto, Y. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002, 8, 3394–400. [Google Scholar] [PubMed]
- Higashihara, Y.; Kato, J.; Nagahara, A.; Izumi, K.; Konishi, M.; Kodani, T.; Serizawa, N.; Osada, T.; Watanabe, S. Phase I clinical trial of peptide vaccination with URLC10 and VEGFR1 epitope peptides in patients with advanced gastric cancer. Int J Oncol. 2014, 44, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, U.; Garner-Spitzer, E.; Chao, Y.; Maglakelidze, M.; Bulat, I.; Dechaphunkul, A.; Arpornwirat, W.; Charoentum, C.; Yen, C.J.; Yau, T.C.; et al. Clinical and Immunologic Responses to a B-Cell Epitope Vaccine in Patients with HER2/neu-Overexpressing Advanced Gastric Cancer-Results from Phase Ib Trial IMU.ACS.001. Clin Cancer Res. 2021, 27, 3649–3660. [Google Scholar] [CrossRef]
- Ajani, J.A.; Hecht, J.R.; Ho, L.; Baker, J.; Oortgiesen, M.; Eduljee, A.; Michaeli, D. An open-label, multinational, multicenter study of G17DT vaccination combined with cisplatin and 5-fluorouracil in patients with untreated, advanced gastric or gastroesophageal cancer : The GC4 study. Cancer 2006, 106, 1908–1916. [Google Scholar] [CrossRef]
- Ogasawara, M. Dendritic cell vaccine-based immunotherapy in combination with salvage chemotherapy for patients with advanced or relapsed gastric cancer. Ann. Oncol. 2018, 29, P075. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Sugimura, K.; Miyata, H.; Omori, T.; Nakano, H.; Mochizuki, C.; Shimizu, K.; Saito, H.; Ashida, K.; Honjyo, S.; Nakamura, Y.; Yano, M. A Pilot Study of Post-Operative Adjuvant Vaccine for Advanced Gastric Cancer. Yonago Acta Med. 2017, 60, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yuan, Y.; Chen, C.; Lin, J.; Ma, Q.; Liu, G.; Gao, Y.; Huang, Y.; Chen, L.; Chen, L.Z.; et al. Durable complete response to neoantigen-loaded dendritic-cell vaccine following anti-PD-1 therapy in metastatic gastric cancer. NPJ Precis Oncol. 2022, 6, 34. [Google Scholar] [CrossRef]
- Lesterhuis, W.J.; de Vries, I.J.M.; Schuurhuis, D.H.; Boullart, A.C.I.; Jacobs, J.F.M.; de Boer, A.J.; Scharenborg, N.M.; Brouwer, H.M.H.; van de Rakt, M.W.M.M.; Figdor, C.G.; et al. Vaccination of colorectal cancer patients with CEA-loaded dendritic cells: antigen-specific T cell responses in DTH skin tests. Ann. Oncol. 2006, 17, 974–980. [Google Scholar] [CrossRef]
- Morse, M.A.; Chaudhry, A.; Gabitzsch, E.S.; Hobeika, A.C.; Osada, T.; Clay, T.M.; Amalfitano, A.; Burnett, B.K.; Devi, G.R.; Hsu, D.S.; et al. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer Immunol. Immunother. 2013, 62, 1293–1301. [Google Scholar] [CrossRef]
- Kimura, T.; McKolanis, J.R.; Dzubinski, L.A.; Islam, K.; Potter, D.M.; Salazar, A.M.; Schoen, R.E.; Finn, O.J. MUC1 Vaccine for Individuals with Advanced Adenoma of the Colon: A Cancer Immunoprevention Feasibility Study. Cancer Prev. Res. 2013, 6, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Niedzwiecki, D.; Marshall, J.L.; Garrett, C.; Chang, D.Z.; Aklilu, M.; Crocenzi, T.S.; Cole, D.J.; Dessureault, S.; Hobeika, A.C.; et al. A randomized phase II study of immunization with dendritic cells modified with pox vectors encoding CEA and MUC1 compared with the same pox vectors plus GM-CSF for resected metastatic colorectal cancer. Ann Surg. 2013, 258, 879–86. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, C.L.; Martinenaite, E.; Kjeldsen, J.W.; Holmstroem, R.B.; Mørk, S.K.; Pedersen, A.W.; Ehrnrooth, E.; Andersen, M.H.; Svane, I.M. Arginase-1 targeting peptide vaccine in patients with metastatic solid tumors – A phase I trial. Front. Immunol. 2022, 13, 1023023. [Google Scholar] [CrossRef]
- E Rahma, O.; Hamilton, J.M.; Wojtowicz, M.; Dakheel, O.; Bernstein, S.; Liewehr, D.J.; Steinberg, S.M.; Khleif, S.N. The immunological and clinical effects of mutated ras peptide vaccine in combination with IL-2, GM-CSF, or both in patients with solid tumors. J. Transl. Med. 2014, 12, 55–55. [Google Scholar] [CrossRef]
- Barve, V.; Adams, N.; Stanbery, L.; Manning, L.; Horvath, S.; Wallraven, G.; Bognar, E.; Barve, M.; Nemunaitis, J. Case Report: Marked Survival Advantage of Two Colorectal Cancer Patients with Liver Metastases Treated with Vigil and FOLFOX-6. Vaccines 2021, 9, 1201. [Google Scholar] [CrossRef]
- Hubbard, J.M.; Tőke, E.R.; Moretto, R.; Graham, R.P.; Youssoufian, H.; Lőrincz, O.; Molnár, L.; Csiszovszki, Z.; Mitchell, J.L.; Wessling, J.; et al. Safety and Activity of PolyPEPI1018 Combined with Maintenance Therapy in Metastatic Colorectal Cancer: an Open-Label, Multicenter, Phase Ib Study. Clin Cancer Res. 2022, 28, 2818–2829. [Google Scholar] [CrossRef]
- Yarchoan, M.; Huang, C.Y.; Zhu, Q.; Ferguson, A.K.; Durham, J.N.; Anders, R.A.; Thompson, E.D.; Rozich, N.S.; Thomas, D.L., 2nd; Nauroth, J.M.; et al. A phase 2 study of GVAX colon vaccine with cyclophosphamide and pembrolizumab in patients with mismatch repair proficient advanced colorectal cancer. Cancer Med. 2020, 9, 1485–1494. [Google Scholar] [CrossRef]
- Español-Rego, M.; Fernández-Martos, C.; Elez, E.; Foguet, C.; Pedrosa, L.; Rodríguez, N.; Ruiz-Casado, A.; Pineda, E.; Cid, J.; Cabezón, R.; et al. A Phase I-II multicenter trial with Avelumab plus autologous dendritic cell vaccine in pre-treated mismatch repair-proficient (MSS) metastatic colorectal cancer patients; GEMCAD 1602 study. Cancer Immunol. Immunother. 2023, 72, 827–840. [Google Scholar] [CrossRef]
- Palmer, C.D.; Rappaport, A.R.; Davis, M.J.; Hart, M.G.; Scallan, C.D.; Hong, S.-J.; Gitlin, L.; Kraemer, L.D.; Kounlavouth, S.; Yang, A.; et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: phase 1 trial interim results. Nat. Med. 2022, 28, 1619–1629. [Google Scholar] [CrossRef]
- Redman, J.M.; Tsai, Y.-T.; A Weinberg, B.; Donahue, R.N.; Gandhy, S.; E Gatti-Mays, M.; Sater, H.A.; Bilusic, M.; Cordes, L.M.; Steinberg, S.M.; et al. A Randomized Phase II Trial of mFOLFOX6 + Bevacizumab Alone or with AdCEA Vaccine + Avelumab Immunotherapy for Untreated Metastatic Colorectal Cancer. Oncol. 2022, 27, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; Hughes, M.S.; Phan, G.Q.; Citrin, D.E.; Restifo, N.P.; Robbins, P.F.; Wunderlich, J.R.; et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011, 17, 4550–4557. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Mi, Y.; Guo, N.; Xu, H.; Xu, L.; Gou, X.; Jin, W. Cytokine-Induced Killer Cells As Pharmacological Tools for Cancer Immunotherapy. Front Immunol. 2017, 8, 774. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yu, Z.; Wu, Y.; Du, T.; Chen, S.; Meng, F.; Su, N.; Ma, Y.; Li, X.; Sun, S.; Zhang, G. Cell-based immunotherapy with cytokine-induced killer (CIK) cells: From preparation and testing to clinical application. Hum Vaccin Immunother. 2017, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, L.; Zhang, Q.; He, X.; Yin, Y.; Gu, Y.; Guo, R.; Lu, K.; Liu, L.; Liu, P.; Shu, Y. Effects of cytokine-induced killer cell treatment in colorectal cancer patients: a retrospective study. Biomed Pharmacother. 2014, 68, 715–720. [Google Scholar] [CrossRef]
- Xu, Y.C.; Xu, Q.; Li, J.J.; Gu, X.F.; Lin, X.L.; Sun, L.; Lu, H.M.; Tang, L.; Ma, Y.; Lu, Z.; Wang, H.X. Chemotherapy with or without autologous cytokine-induced killer cell transfusion as the first-line treatment for stage IV gastrointestinal cancer: a phase II clinical trial. J Cancer Res Clin Oncol. 2016, 142, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Y.; Yu, J.; Wei, F.; Cao, S.; Zhang, X.; Dong, N.; Li, H.; Ren, X. Autologous Cytokine-Induced Killer Cells Improves Overall Survival of Metastatic Colorectal Cancer Patients: Results From a Phase II Clinical Trial. Clin Colorectal Cancer. 2016, 15, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, H.; Li, Y.; Bai, J.; Liu, L.; Liu, Y.; Qu, Y.; Qu, X. Efficacy of postoperative adjuvant transfusion of cytokine-induced killer cells combined with chemotherapy in patients with colorectal cancer. Cancer Immunol Immunother. 2013, 62, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yao, M.; Fan, H.; Song, L.; Sun, J.; Zhou, Z.; Du, Y.; Lu, K.; Li, T.; Yin, A.; Xu, J.; Wei, S. Effects of Autologous Cytokine-Induced Killer Cells Infusion in Colorectal Cancer Patients: A Prospective Study. Cancer Biother Radiopharm. 2017, 32, 221–226. [Google Scholar] [CrossRef]
- Pan, Q.Z.; Zhao, J.J.; Yang, C.P.; Zhou, Y.Q.; Lin, J.Z.; Tang, Y.; Gu, J.M.; Wang, Q.J.; Li, Y.Q.; He, J.; et al. Efficacy of adjuvant cytokine-induced killer cell immunotherapy in patients with colorectal cancer after radical resection. Oncoimmunology 2020, 9, 1752563. [Google Scholar] [CrossRef]
- Li, C.M.Y.; Tomita, Y.; Dhakal, B.; Li, R.; Li, J.; Drew, P.; Price, T.; Smith, E.; Maddern, G.J.; Fenix, K.A. Use of cytokine-induced killer cell therapy in patients with colorectal cancer: a systematic review and meta-analysis. J. Immunother. Cancer 2023, 11, e006764. [Google Scholar] [CrossRef]
- Rettinger, E.; Kuçi, S.; Naumann, I.; Becker, P.; Kreyenberg, H.; Anzaghe, M.; Willasch, A.; Koehl, U.; Bug, G.; Ruthardt, M.; et al. The cytotoxic potential of interleukin-15-stimulated cytokine-induced killer cells against leukemia cells. Cytotherapy 2012, 14, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Wang, J.; Lao, X.; Wang, J.; Li, L.; Li, S.; Zhang, J.; Dong, Y.; Chang, A.E.; Li, Q.; Li, S. Interleukin-6 inhibits regulatory T cells and improves the proliferation and cytotoxic activity of cytokine-induced killer cells. J Immunother. 2012, 35, 337–343. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Q.; Xu, K.; Shan, J.; Shen, J.; Liu, L.; Xu, Y.; Xia, F.; Bie, P.; Zhang, X.; et al. Combined therapy with cytokine-induced killer cells and oncolytic adenovirus expressing IL-12 induce enhanced antitumor activity in liver tumor model. PLoS One. 2012, 7, e44802. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liang, W.; Li, Z.; Xu, Y.; Chen, L. Interleukin-15-transferred cytokine-induced killer cells elevated anti-tumor activity in a gastric tumor-bearing nude mice model. Cell Biol Int. 2016, 40, 204–13. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, E.; Merlini, A.; D'Ambrosio, L.; Cerviere, I.; Berrino, E.; Marchiò, C.; Giraudo, L.; Basiricò, M.; Massa, A.; Donini, C.; et al. Integrated Antitumor Activities of Cellular Immunotherapy with CIK Lymphocytes and Interferons against KIT/PDGFRA Wild Type GIST. Int J Mol Sci. 2022, 23, 10368. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakazawa, T.; Nakamura, M.; Nishimura, F.; Matsuda, R.; Omoto, K.; Shida, Y.; Murakami, T.; Nakagawa, I.; Motoyama, Y.; et al. Ex vivo-expanded highly purified natural killer cells in combination with temozolomide induce antitumor effects in human glioblastoma cells in vitro. PLOS ONE 2019, 14, e0212455. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, H.; Zhao, L.; Song, Y.; Gao, Q. Combination of sorafenib and cytokine-induced killer cells in metastatic renal cell carcinoma: a potential regimen. Immunotherapy. 2017, 9, 629–635. [Google Scholar] [CrossRef]
- Capellero, S.; Erriquez, J.; Melano, C.; Mesiano, G.; Genta, S.; Pisacane, A.; Mittica, G.; Ghisoni, E.; Olivero, M.; Di Renzo, M.F.; et al. Preclinical immunotherapy with Cytokine-Induced Killer lymphocytes against epithelial ovarian cancer. Sci. Rep. 2020, 10, 6478. [Google Scholar] [CrossRef]
- Wang, C.; Yan, J.; Yin, P.; Gui, L.; Ji, L.; Ma, B.; Gao, W.Q. β-Catenin inhibition shapes tumor immunity and synergizes with immunotherapy in colorectal cancer. Oncoimmunology. 2020, 9, 1809947. [Google Scholar] [CrossRef]
- Dai, C.; Lin, F.; Geng, R.; Ge, X.; Tang, W.; Chang, J.; Wu, Z.; Liu, X.; Lin, Y.; Zhang, Z.; Li, J. Implication of combined PD-L1/PD-1 blockade with cytokine-induced killer cells as a synergistic immunotherapy for gastrointestinal cancer. Oncotarget 2016, 7, 10332–10344. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Feng, F.; Chen, C.; Zeng, H.; Wen, S.; Xu, X.; He, J.; Li, J. Programmed cell death protein-1/programmed death-ligand 1 blockade enhances the antitumor efficacy of adoptive cell therapy against non-small cell lung cancer. J Thorac Dis. 2018, 10, 6711–6721. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sharma, A.; Wu, X.; Weiher, H.; Skowasch, D.; Essler, M.; Schmidt-Wolf, I.G.H. A Combination of Cytokine-Induced Killer Cells With PD-1 Blockade and ALK Inhibitor Showed Substantial Intrinsic Variability Across Non-Small Cell Lung Cancer Cell Lines. Front Oncol. 2022, 12, 713476. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Hamilton, C.A.; Cheung, M.K.; Karimi, M.; Baker, J.; Gall, J.M.; Schulz, S.; Thorne, S.H.; Teng, N.N.; Contag, C.H.; et al. Enhanced Killing of Primary Ovarian Cancer by Retargeting Autologous Cytokine-Induced Killer Cells with Bispecific Antibodies: A Preclinical Study. Clin. Cancer Res. 2006, 12, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, M.; Li, L.; Li, H.; Yazaki, P.J.; Swiderski, P.; Shively, J.E. T-cell surface generation of dual bivalent, bispecific T-cell engaging, RNA duplex cross-linked antibodies (dbBiTERs) for re-directed tumor cell lysis. Biotechnol. J. 2022, 17, e2100389. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, Y.G.; Kim, J.S.; Park, E.J.; Kim, B.; Park, K.H.; Kang, J.S.; Hong, J.T.; Kim, Y.; Han, S.B. Cytokine-induced killer cells interact with tumor lysate-pulsed dendritic cells via CCR5 signaling. Cancer Lett. 2016, 378, 142–149. [Google Scholar] [CrossRef]
- Cao, J.; Chen, C.; Wang, Y.; Chen, X.; Chen, Z.; Luo, X. Influence of autologous dendritic cells on cytokine-induced killer cell proliferation, cell phenotype and antitumor activity in vitro. Oncol Lett. 2016, 12, 2033–2037. [Google Scholar] [CrossRef]
- Jung, N.C.; Lee, J.H.; Choi, H.J.; Hwang, S.U.; Song, J.Y.; Seo, H.G.; Choi, J.; Jung, S.Y.; Han, S.G.; Lim, D.S. Dendritic Cell Immunotherapy Combined with Cytokine-Induced Killer Cells Effectively Suppresses Established Hepatocellular Carcinomas in Mice. Immunol Invest. 2016, 45, 553–565. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, Y.; Zhao, Y.; Li, L.; Sun, P.; Liu, H.; Fan, Q.; Liang, K.; Liang, W.; Sun, H.; Du, X.; Li, R. Combination of E2F-1 promoter-regulated oncolytic adenovirus and cytokine-induced killer cells enhances the antitumor effects in an orthotopic rectal cancer model. Tumour Biol. 2014, 35, 1113–1122. [Google Scholar] [CrossRef]
- Liu, F.R.; Bai, S.; Feng, Q.; Pan, X.Y.; Song, S.L.; Fang, H. Anti-colorectal cancer effects of anti-p21Ras scFv delivered by the recombinant adenovirus KGHV500 and cytokine-induced killer cells. BMC Cancer. 2018, 18, 1087. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, P.B.; Feng, Q.; Pan, X.Y.; Song, S.L.; Cui, J.; Yang, J.L. Cytokine-induced killer cells carrying recombinant oncolytic adenovirus expressing p21Ras scFv inhibited liver cancer. J Cancer. 2021, 12, 2768–2776. [Google Scholar] [CrossRef]
- Niu, J.; Ren, Y.; Zhang, T.; Yang, X.; Zhu, W.; Zhu, H.; Li, J.; Li, J.; Pang, Y. Retrospective comparative study of the effects of dendritic cell vaccine and cytokine-induced killer cell immunotherapy with that of chemotherapy alone and in combination for colorectal cancer. Biomed Res Int. 2014, 2014, 214727. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, X.; Li, J.; Ren, Y.; Zhang, T.; Zhang, C.; Zhang, J.; Li, J.; Pang, Y. Immune response, safety, and survival and quality of life outcomes for advanced colorectal cancer patients treated with dendritic cell vaccine and cytokine-induced killer cell therapy. Biomed Res Int. 2014, 2014, 603871. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, C.; Xie, X.; Zhao, P.; Wei, X.; Sun, W.; Liu, H.C.; Alexandrou, A.T.; Jones, J.; Zhao, R.; Li, J.J. Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PLoS One. 2014, 9, e93886. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Song, C.; Chuo, D.Y.; Zhang, H.; Zhao, J. Clinical effects of autologous dendritic cells combined with cytokine-induced killer cells followed by chemotherapy in treating patients with advanced colorectal cancer: a prospective study. Tumour Biol. 2016, 37, 4367–4372. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Huang, L.; Chen, L.; Lin, X.; Chen, L.; Zheng, Q. Effect of dendritic cell-cytokine-induced killer cells in patients with advanced colorectal cancer combined with first-line treatment. World J. Surg. Oncol. 2017, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Qin, W.; Feng, H.; Song, D.; Yang, X.; Zhang, J. Analysis of the Clinical Efficacy of Dendritic Cell -cytokine Induced Killer Cell-based Adoptive Immunotherapy for Colorectal Cancer. Immunol Invest. 2021, 50, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Yin, Y.; Hu, Y.; Lu, Y.; Zou, H.; Lu, G.; Wang, Q.; Lian, J.; Gao, J.; Shen, X. Tumor RNA-loaded nanoliposomes increases the anti-tumor immune response in colorectal cancer. Drug Deliv. 2021, 28, 1548–1561. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Sun, M.; Cui, Y.; Xing, J.; Teng, L.; Xi, Z.; Yang, Z. Exosomes as smart drug delivery vehicles for cancer immunotherapy. Front Immunol. 2023, 13, 1093607. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, S.; Zhao, J.; Chen, H.; Si, X.; Huang, Z.; Yu, Z.; Song, W.; Tang, Z.; Chen, X. Mannan-decorated pathogen-like polymeric nanoparticles as nanovaccine carriers for eliciting superior anticancer immunity. Biomaterials. 2022, 284, 121489. [Google Scholar] [CrossRef]
- Gabel, M.; Knauss, A.; Fischer, D.; Neurath, M.F.; Weigmann, B. Surface Design Options in Polymer- and Lipid-Based siRNA Nanoparticles Using Antibodies. Int. J. Mol. Sci. 2022, 23, 13929. [Google Scholar] [CrossRef]
- Salomon, R.; Rotem, H.; Katzenelenbogen, Y.; Weiner, A.; Cohen Saban, N.; Feferman, T.; Amit, I.; Dahan, R. Bispecific antibodies increase the therapeutic window of CD40 agonists through selective dendritic cell targeting. Nat Cancer. 2022, 3, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Breitbach, C.J.; Lee, J.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Moon, A.; Mun, J.H.; Sommermann, E.M.; Maruri Avidal, L.; Patt, R.; Pelusio, A.; Burke, J.; Hwang, T.H.; Kirn, D.; Park, Y.S. Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer. Mol Ther. 2015, 23, 1532–40. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Salazar, R.; Duran, I.; Osman-Garcia, I.; Paz-Ares, L.; Bozada, J.M.; Boni, V.; Blanc, C.; Seymour, L.; Beadle, J.; et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J. Immunother. Cancer 2017, 5, 71. [Google Scholar] [CrossRef]
- Parakrama, R.; Fogel, E.; Chandy, C.; Augustine, T.; Coffey, M.; Tesfa, L.; Goel, S.; Maitra, R. Immune characterization of metastatic colorectal cancer patients post reovirus administration. BMC Cancer 2020, 20, 569. [Google Scholar] [CrossRef]
- Jonker, D.J.; Tang, P.A.; Kennecke, H.; Welch, S.A.; Cripps, M.C.; Asmis, T.; Chalchal, H.; Tomiak, A.; Lim, H.; Ko, Y.J.; Chen, E.X.; Alcindor, T.; Goffin, J.R.; Korpanty, G.J.; Feilotter, H.; Tsao, M.S.; Theis, A.; Tu, D.; Seymour, L. A Randomized Phase II Study of FOLFOX6/Bevacizumab With or Without Pelareorep in Patients With Metastatic Colorectal Cancer: IND 210, a Canadian Cancer Trials Group Trial. Clin Colorectal Cancer. 2018, 17, 231–239.e7. [Google Scholar] [CrossRef] [PubMed]
- Monge, C.; Xie, C.; Myojin, Y.; Coffman, K.; Hrones, D.M.; Wang, S.; Hernandez, J.M.; Wood, B.J.; Levy, E.B.; Juburi, I.; Hewitt, S.M.; Kleiner, D.E.; Steinberg, S.M.; Figg, W.D.; Redd, B.; Homan, P.; Cam, M.; Ruf, B.; Duffy, A.G.; Greten, TF. Phase I/II study of PexaVec in combination with immune checkpoint inhibition in refractory metastatic colorectal cancer. J Immunother Cancer. 2023, 11, e005640. [Google Scholar] [CrossRef]
- Fakih, M.; Harb, W.; Mahadevan, D.; Babiker, H.; Berlin, J.; Lillie, T.; Krige, D.; Carter, J.; Cox, C.; Patel, M.; et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev, in combination with nivolumab, in patients with advanced/metastatic epithelial cancer: a phase I clinical trial (SPICE). J. Immunother. Cancer 2023, 11, e006561. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Zhang, J.V.; Wu, Y.; Wang, L.; Chen, X.; Ji, D.; Zhou, G.G. Enhancing Therapeutic Efficacy of Oncolytic Herpes Simplex Virus with MEK Inhibitor Trametinib in Some BRAF or KRAS-Mutated Colorectal or Lung Carcinoma Models. Viruses. 2021, 13, 1758. [Google Scholar] [CrossRef]
- Maus, M.V. A decade of CAR T cell evolution. Nat Cancer. 2022, 3, 270–271. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Jiang, Q.; Jiang, H.; Hu, L.J.; Zhao, T.; Yu, X.X.; Huang, X.J. Expanded clinical-grade membrane-bound IL-21/4-1BBL NK cell products exhibit activity against acute myeloid leukemia in vivo. Eur J Immunol. 2020, 50, 1374–1385. [Google Scholar] [CrossRef]
- Mani, R.; Rajgolikar, G.; Nunes, J.; Zapolnik, K.; Wasmuth, R.; Mo, X.; Byrd, J.C.; Lee, D.A.; Muthusamy, N.; Vasu, S. Fc-engineered anti-CD33 monoclonal antibody potentiates cytotoxicity of membrane-bound interleukin-21 expanded natural killer cells in acute myeloid leukemia. Cytotherapy. 2020, 22, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Mailankody, S.; Devlin, S.M.; Landa, J.; Nath, K.; Diamonte, C.; Carstens, E.J.; Russo, D.; Auclair, R.; Fitzgerald, L.; Cadzin, B.; et al. GPRC5D-Targeted CAR T Cells for Myeloma. New Engl. J. Med. 2022, 387, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Guzman, G.; Reed, M.R.; Bielamowicz, K.; Koss, B.; Rodriguez, A. CAR-T Therapies in Solid Tumors: Opportunities and Challenges. Curr. Oncol. Rep. 2023, 25, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Abken, H. TRUCKs: the fourth generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef]
- Feng, Z.; He, X.; Zhang, X.; Wu, Y.; Xing, B.; Knowles, A.; Shan, Q.; Miller, S.; Hojnacki, T.; Ma, J.; Katona, B.W.; Gade, T.P.F.; Schrader, J.; Metz, D.C.; June, C.H.; Hua, X. Potent suppression of neuroendocrine tumors and gastrointestinal cancers by CDH17CAR T cells without toxicity to normal tissues. Nat Cancer. 2022, 3, 581–594. [Google Scholar] [CrossRef]
- Magee, M.S.; Abraham, T.S.; Baybutt, T.R.; Flickinger, J.C. Jr; Ridge, N.A.; Marszalowicz, G.P.; Prajapati, P.; Hersperger, A.R.; Waldman, S.A.; Snook, A.E. Human GUCY2C-Targeted Chimeric Antigen Receptor (CAR)-Expressing T Cells Eliminate Colorectal Cancer Metastases. Cancer Immunol Res. 2018, 6, 509–516. [Google Scholar] [CrossRef]
- Sureban, S.M.; Berahovich, R.; Zhou, H.; Xu, S.; Wu, L.; Ding, K.; May, R.; Qu, D.; Bannerman-Menson, E.; Golubovskaya, V.; Houchen, C.W. DCLK1 Monoclonal Antibody-Based CAR-T Cells as a Novel Treatment Strategy against Human Colorectal Cancers. Cancers (Basel). 2019, 12, 54. [Google Scholar] [CrossRef]
- Olivera, I.; Bolaños, E.; Gonzalez-Gomariz, J.; Hervas-Stubbs, S.; Mariño, K.V.; Luri-Rey, C.; Etxeberria, I.; Cirella, A.; Egea, J.; Glez-Vaz, J.; Garasa, S.; Alvarez, M.; Eguren-Santamaria, I.; Guedan, S.; Sanmamed, M.F.; Berraondo, P.; Rabinovich, G.A.; Teijeira, A.; Melero, I. mRNAs encoding IL-12 and a decoy-resistant variant of IL-18 synergize to engineer T cells for efficacious intratumoral adoptive immunotherapy. Cell Rep Med. 2023, 4, 100978. [Google Scholar] [CrossRef]
- Milone, M.C.; Xu, J.; Chen, S.J.; Collins, M.A.; Zhou, J.; Powell, D.J. Jr; Melenhorst, J.J. Engineering enhanced CAR T-cells for improved cancer therapy. Nat Cancer. 2021, 2, 780–793. [Google Scholar] [CrossRef]
- Drougkas, K.; Karampinos, K.; Karavolias, I.; Koumprentziotis, I.A.; Ploumaki, I.; Triantafyllou, E.; Trontzas, I.; Kotteas, E. Comprehensive clinical evaluation of CAR-T cell immunotherapy for solid tumors: a path moving forward or a dead end? J Cancer Res Clin Oncol. 2023, 149, 2709–2734. [Google Scholar] [CrossRef]
- Kim, R.D.; Prenen, H.; Rottey, S.; Kim, D.W.; Flament, A.; Lehmann, F.; Van Cutsem, E. KEYNOTE-B79 phase 1b trial to evaluate the allogeneic CAR T-cells CYAD-101 and pembrolizumab in refractory metastatic colorectal cancer patients. J. Clin. Oncol. 2022, 40, TPS227–TPS227. [Google Scholar] [CrossRef]
| Vaccine type | Intervention/ neoantigen | Adjuvant / Combined Tx | Stage | Design | Outome measures | Status | Phase | Reference |
|---|---|---|---|---|---|---|---|---|
| B-cell & Monocyte | BVAC-B (Her-2) |
none | IV | Her2 (+) GC, 0,4,8, 12 wks | Establish MTD, AE | completed | Ib | NCT03425773 Cellid Co, Ltd. |
| Allogeneic | K562-GM | Cyclophosphamide Celecoxib |
NED/MRD Primary site |
Esophagus & Mediastinal after standard Tx;6 vaccines q4 wks | AE, humoral immune response | Terminated (futility) | Ib | NCT01143545 NCI |
| mRNA | personalized | none | IV | Esophageal, GC, CRC; 4 vaccines SC |
AE, DCR, PFS, TTP, OS | Unknown | Ib | NCT03468244 Chanhai Hospital, China |
| B-cell epitope | Her-2 | CT (Cisplatin-FU/Capecitabine) |
IV | Her2 (+) GC/GE D1,14,28 vaccine vs CT + vaccine |
AE, ORR, humoral immune response | Ongoing | Ib | NCT05315830 Bengbu Med College, China |
| MVB-BN non-replicating viral |
TAEK-VAC-Herby encoding Her-2, CD40L, TF Brachyury |
Trastuzumab Pertuzumab |
IV | Her2 (+) GC/GE, Breast, Chordoma 3 vaccines q3 wks |
DLT | Ongoing | Ib/II | NCT04246671 Bavarian Nordic |
| T-cell receptor | KK-LC1 neoantigen HLA-A-01:01 restricted |
2 x prior CT & IL-2 4 days & Cyclophosphamide (D-6, -5) + Fludarabine (D-6—2) |
IV | KK-LC1 (+) GC, breast, lung cancer 1 vaccine infusion |
DLT | Ongoing | I | NCT05035407 NCI |
| mRNA | PGV-002 Personalized mRNA |
PD-1/PD-L1 (expansion phase) | IV | GC, esophageal, liver cancer refractory to standard CT | AE, ORR, MTD, PFS | Ongoing | I | NCT05192460 Chinese Academy of MMS NeoCura |
| Peptide | Personalized | Pembrolizumab, Cyclophosphamide (D-3), GM-CSF (D 1,4,8, 15) q 3 wks | III -IV | GC/GEJ, breast, NSCLC, HCC, Merkel, GU | AE, ORR, feasibility, humoral immune response | Ongoing | I | NCT05269381 Mayo Clinic |
| Peptide (Da VINci) |
OTSGC-A24 Multiple epitope |
Nivolumab, Ipilimumab | IV | GC; refractory | AE, ORR, humoral immune response | Ongoing | Ib | NCT03784040 Natl. Uni Hospital, Singapore |
| Peptide | iNEO-Vac-P01 Personalized |
GM-CSF | II-III maintenance |
Esophageal, resectable, after resection & (neo)/adj CT+PD-1 7 vaccines D1-82 |
AE, RFS, OS, QoL | Ongoing | I | ZheJiang Uni; Hangzhou Neoantigen Ther Co, Ltd |
| mRNA | Personalized | none | IIIC -IV | Esophageal ca; NSCLC Failure of standard Tx |
AE, ORR, TTP, PFS | Ongoing | NA | NCT03908671 Zhengzhou Uni; Stemirna Ther |
| Adenoviral Self-replicating mRNA |
GRT-C901 & R902 (predicted multiple epitopes) |
Nivolumab Ipilimumab |
IV | GE/G ≤ 1 prior CT |
AE, ORR, dose finding | Ongoing | Ib | NCT03639714 Gritstone Bio, Inc |
| Vaccine type | Intervention/ neoantigen | Adjuvant / Combined Tx | Stage | Design | Outome measures | Status | Phase | Reference |
|---|---|---|---|---|---|---|---|---|
| Adenoviral Self-replicating mRNA |
GRT-C901 & R902 (predicted multiple epitopes) |
Nivolumab Ipilimumab |
IV | CRC-MSS; GC/GEJ; NSCLC; GU1 prior CT | AE, ORR, dose finding, Humoral immune response |
Completed | Ib/II | NCT03639714 Gritstone Bio, Inc |
| Adenoviral Self-replicating mRNA |
GRT-C901 & R902 (predicted multiple epitopes) |
Atezolizumab Ipilimumab |
II-III; MRD (ctDNA (+)) | CRC-MSS; Resected, after adj CT; 6 x vaccine 2x Ipilimumab; 13x Atezolizumab q4 wks |
AE, ORR (ctDNA); RFS, OS) | Terminated; reprioritization | II | NCT05456165 Gritstone Bio, Inc |
| Dendritic cell | Personalized Antigen pulsed DC |
mFOLFOX6 | IV | Untreated mCRC; CT vs CT+vac Vaccination in cycles 1-3; 7-9 |
PFS, OS, ORR | Unknown | III | NCT02503150 Second MMU, China |
| Adenovirus | QUILT-2.004 Ad-CEA |
Avelumab CT (FOLFOX) Bevacizumab |
IV | Untreated mCRC-MSS; CT (FOLFOX+Bevacizumab) vs CT+ Avelumab + vaccine x 12 | PFS | Terminated; Futility on interim analysis |
II | NCT03050814 NCI |
| Viral (modified vaccinia Ankara- Bavarian Nordic) | CV 301 MVA-BN CEA/MUC1 & Fowlpox booster |
Bifunctional fusion protein composed of IgG1 PD-L1 & TGF-beta; IL-15 fusion protein IL-12 |
IV | mCRC; small bowel ca. Triple vs quadruple Tx (± IL12) |
AE, ORR; PFS; OS | Completed | II | NCT04491955 NCI |
| Autologous | Cryovax; Personalized autologous tumor |
Bioengineered allogenic immunecells (AlloStim) | IV | >2 previous lines of CT; Vaccine x6 over 10 wks |
DLT, QoL, Humoral immune response; ORR |
Completed | II | NCT02380443 Immunovative Therapies Ltd. |
| Allogenic engineered | GVAX whole tumor cell engineered to secrete GM-CSF | Cyc Guadecitabine |
IV | mCRC; stable on 1-2nd Line CT; Vaccine x 1 q 4 wks |
AE; TIL; PFS | Completed | I | NCT01966289 Sydney Kimmel Cancer Center |
| Peptide | PolyPEPI1018 Six sythetic CTA neoantigens |
TAS 102 | IV | mCRC-MSS; prior CTVaccine q2 weeks x 7 doses | AE, PFS, ORR; OS | Completed | I | NCT05130060 Mayo clinic |
| mRNA | mRNA 5671/V941-001 | Pembrolizumab | IV | mCRC- KRASm-MSS; NSCLC; Pancreas Vaccine q 3 wks 9 doses vs Vaccine + Pembrolizumab |
AE, DLT | Completed | I | NCT03948763 Merck Sharp & Dohme LLC |
| Synthetic peptide | Her-2 & CEA HLA A2 / A3 restricted |
GM-CSF Tetanus toxioid Montanide ISA 51 |
II-IV | CRC | AE; T cell response in lymph node | Terminated Slow accrual |
I | NCT00091286 University of Virginia |
| Alphavirus replicon particles | AVX 701 CEA |
none | III | CRC; After adjuvant CT Vaccine x4 q 3 wks |
AE; Humoral immune response | Completed | I | NCT01890213 Duke University |
| Inactivated virus | Influenza vaccine | none | Early, operable | CRC; intratumoral injection x 1 | AE, local immune responses | Completed | II | NCT04591379 Zealand University |
| Plasmid DNA | MYPHISMO Tet-MYB |
BGB-A317 (Anti-PD-1 IgG4 mab) | IV | mCRC, ACC; refractory; Vaccine x 6 q 7d |
AE, ORR; CBR, PFS | Completed | I | NCT03287427 Peter Mc Callum Cancer Centre, Au |
| Adenoviral Self-replicating mRNA |
GRANITE-GRT-C901 & R902 (predicted multiple epitopes) |
Atezolizumab Ipilimumab 5-FU Bevacizumab |
IV | mCRC; maintenace following 1st L CT (FOLFOX-Bevacizumab) | Molecular response; PFS |
Ongoing | II/III | NCT05141721 Gritstone Bio; Inc |
| Liposomal | StimVax; MUC-1 |
AlloStim; Allogenic immune cells |
IV | mCRC-MSS; >2 previous lines of CT; Vaccine 3 cycles (x 5/wk q 6 wks) |
AE, OS | Ongoing | IIb | NCT04444622 Immunovative Ther, LTD |
| Dendritic cell | Personalized neoantigen | Nivolumab | IV; MRD | mCRC; resected liver met; HCC Vaccine x 10 doses q 2wks |
RFS, Humoral immune response | Ongoing | II | NCT04912765 Natl Cancer Centre, Singapore |
| Peptide | Personalized synthetic neoantigen | Pembrolizumab Imoquimod Sotigolimab |
mCRC; any line; Pancreas ca; Vaccine x 7-11 dose q 2 wks |
AE, Feasibility, ORR; PFS; OS | Ongoing | I | NCT02600949 MD Anderson Cancer Center |
|
| Chimeric recombinant protein; Recombinant viral |
KISIMA-01; ATP-128-Three neoantigens+ TLR agonist; VSV-GP128-booster |
Ezabenlimab (anti-PD-1) | IV | mCRC-MSS; 1st Line CT; refractory; liver-only |
AE, PFS; ORR; MTD; RFS |
Ongoing | I/II | NCT04046445 Amal Ther. |
| Peptide | KRAS peptide | Nivolumab Ipilimumab |
IV | mCRC; pancreas; >2 previous lines of CT; Vaccine x 3 q 7 d; x 5 boosters q 8 wks |
AE; humoral immune response; DFS; ORR; PFS; OS | Ongoing | I/II | NCT04117087 Sydney Kimmel Cancer Center |
| Peptide | PolyPEPI1018 7 peptide noeantigens; Montanide |
Atezolizumab | IV | mCRC-MSS >2 previous lines of CT; |
AE, ORR; PFS; OS; Humoral immune response |
Ongoing | II | NCT05243862 Treos Bio Ltd. |
| Yeast cell particles |
PalloV-CRC; Allogenic tumor cells delivered on yeast cell particles |
none | I-IV | CRC prior to surgery Vaccine x 4 q 4 wks |
AE; humoral immune response | Ongoing | I | NCT03827967 Cancer Insight, LLC |
| Dendritic cell | COREVAX-1; Dendritic cells pulsed with autologous tumor cells |
IL-2 (D3-7) | IV; MRD | mCRC; Following resection | AE; humoral immune response; RFS; OS |
Ongoing | II | NCT02919644 Instituto Scientifico Romagnolo per lo Studio e la cura dei Tumori |
| Peptide | CLAUDE; EO2040; TAA-CD8 / CD4 T cell epitopes |
Montanide Nivolumab |
II-IV; MRD (ct-DNA (+) | mCRC; Following resection & standard Tx | ORR at 6 months; AE; DFS; OS | Ongoing | II | NCT05350501 Enterome |
| Lipid conjugated oligonucleotide & peptide | ELI-002; KRAS / NRAS |
none | MRD; ct-DNA (+) | mCRC; pancreatic; NSCLC; Vaccine x 4 q 7 d; x 8 boosters/4 wks |
Ct-DNA clearance; RFS; OS | Ongoing | I/II | NCT05726864 Elicio Ther |
| Adenovirus | Tri-AD5 Trivalent CEA/MUC-1/Brachyury | nogapendekin alfa inbakicept (IL-15 agonist-fusion protein) | Prevention | Lynch Syndrome; colon adenomas & CRC NED; Vaccine x 4 (wks 0,4,8,52) |
Cumulative incidence of adenomas; extracolonic carcinomas; humoral immune responses | Ongoing | IIb | NCT05419011 NCI |
| Adenovirus | Nous-209; Gad-209-FSP priming; MVA-209-FSP booster |
None | Prevention | gLynch Syndrome; NED after non-sporadic MMRd malignant tumors; Vaccine D1 / booster wk 8 |
AE; humoral immune responses; response in colorectal adenomas; incidence of Lynch-associated carcinomas | Ongoing | I/II | NCT05078866 NCI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




