Submitted:
12 September 2023
Posted:
13 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction and Epidemiology: Atrial Fibrillation in Cancer Patients
2. Risk factor and pathogenesis of atrial fibrillation in cancer patients
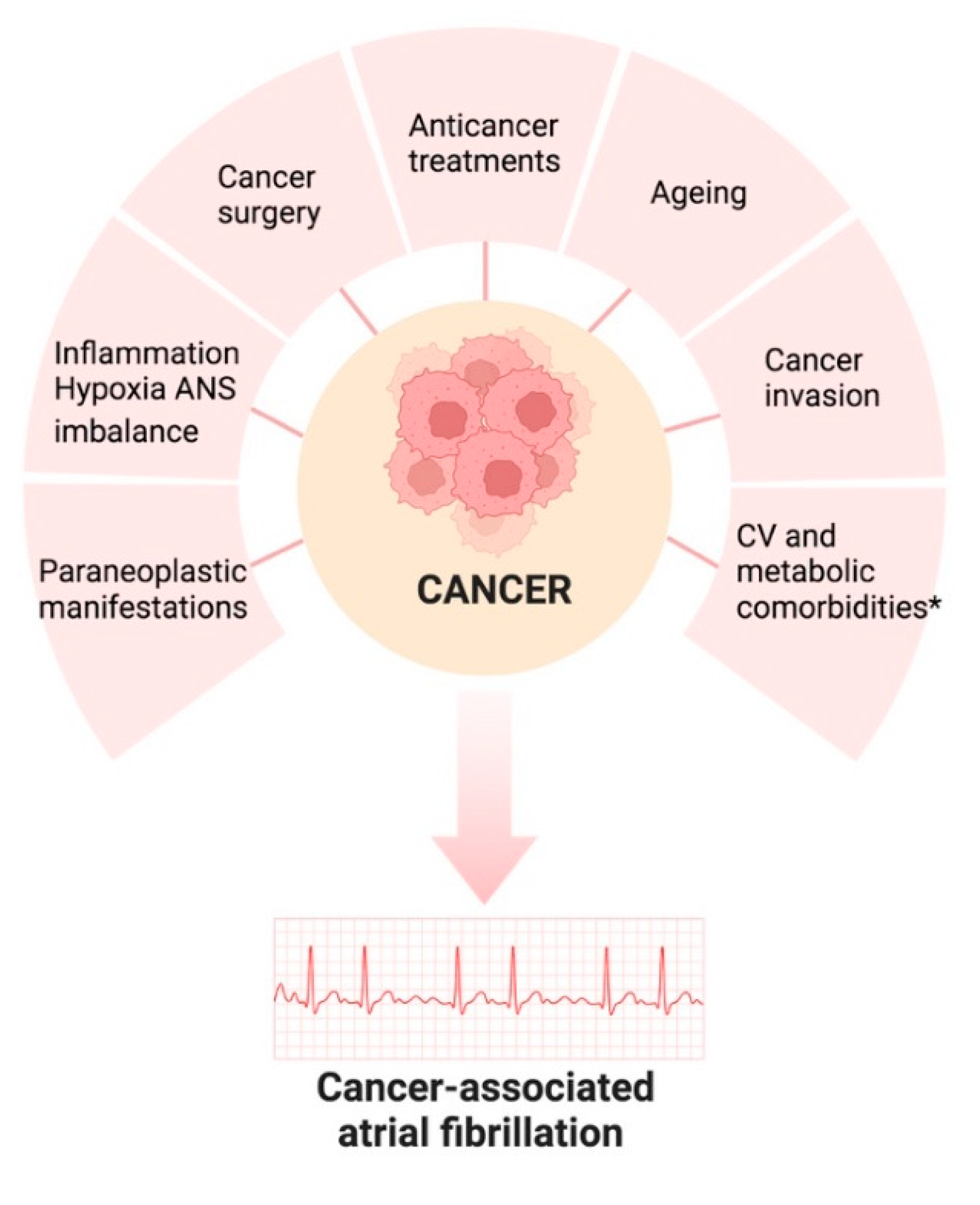
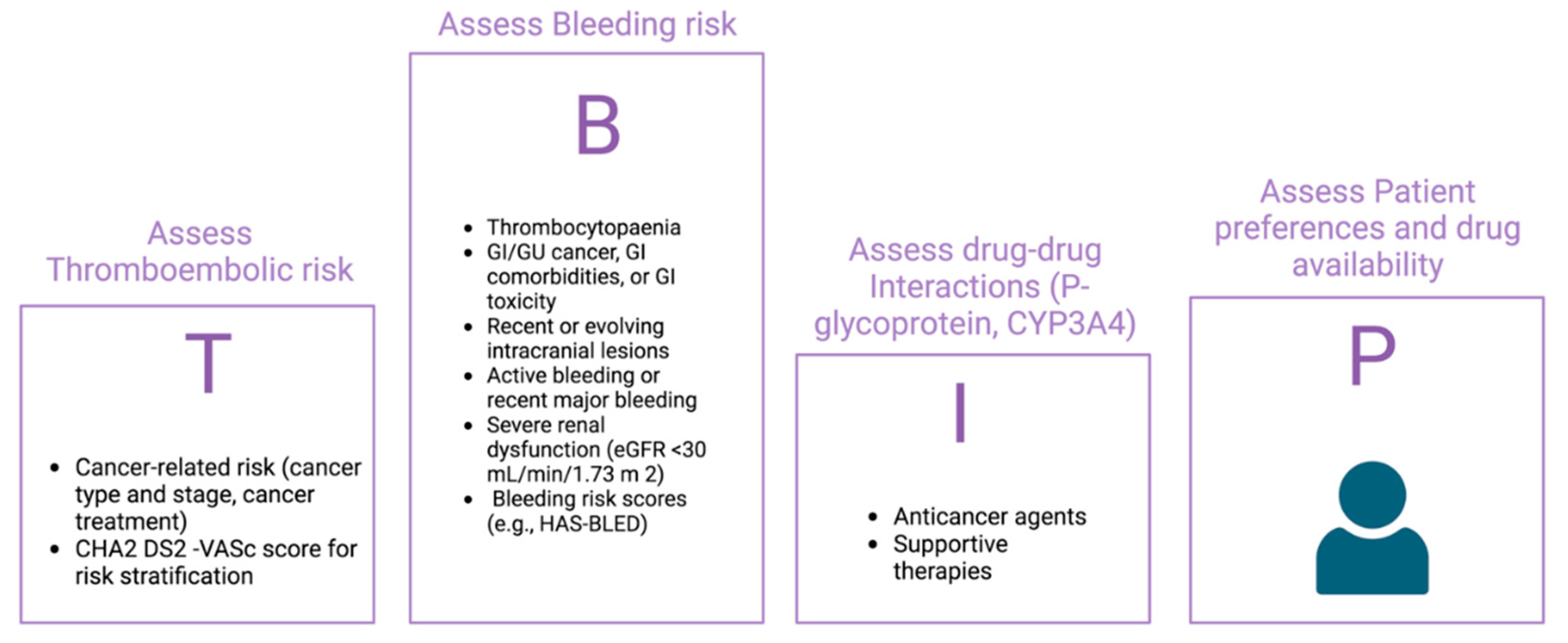
4. Management of Atrial Fibrillation in the setting of Cancer
4.1. Rate and Rhythm control
4.2. Anticoagulant treatment:
| Points | Condition | |
|---|---|---|
| H -Hypertension | 1 | Systolic blood pressure > 160 mmHg |
| A -Abnormal liver or renal function | 1 each | Abnormal renal function: dialysis, creatinine > 2.3 mg/dL,transplantation. Abnormale liver function: chornic hepatitis, cirrhosis, bilirubin > 2 ULN, ALT > 3 ULN |
| S -Stroke | 1 | Previous history, particularly lacunar |
| B - Bleeding | 1 | Recent bleed, anemia, etc |
| L -Labile INR | 1 | Unstable/high INR or TTR < 60% |
| E - Eldery | 1 | Age > 65 year, extreme frailty |
| D -Drugs or Alcohol | 1 each | Prior Alcohol or Drug Usage History (≥ 8 drinks/week) Drugs: concomitant antiplatelet, or NSAID use, etc.. |
| Points | ||
|---|---|---|
| H –Hepatic or Renal disease | 1 each | |
| E – Ethanol Abuse | 1 | |
|
M-Malignancy History |
1 | |
|
O-Older (Age > 75) |
1 | |
|
R-Reduced Platelet Count or Function |
1 | Includes aspirin use, any thrombocytopenia or blood dyscrasia, like hemophilia. |
|
R- Rebleeding Risk |
2 | |
|
H- Hypertension (Uncontrolled) |
1 | |
| A-Anemia | 1 | Hgb <13 g/dL for Men; Hgb <12 g/dL for Women |
| G-Genetic Factors | 1 | CYP 2C9 single-nucleotide polymorphisms |
|
E-Excessive Fall Risk |
1 | |
| S-Stroke Hystory | 1 |
- a) Choice of anticoagulant therapy.
5. Conclusion
Conflicts of Interest
References
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH; ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022 Nov 1;43(41):4229-4361. [CrossRef]
- Madnick DL, Fradley MG. Atrial Fibrillation and Cancer Patients: Mechanisms and Management. Curr Cardiol Rep. 2022 Oct;24(10):1517-1527. [CrossRef]
- Boriani G, Menna P, Morgagni R, Minotti G, Vitolo M. Ibrutinib and Bruton's tyrosine kinase inhibitors in chronic lymphocytic leukemia: focus on atrial fibrillation and ventricular tachyarrhythmias/sudden cardiac death. Chemotherapy. 2022 Nov 10. [CrossRef]
- O'Neal WT, Lakoski SG, Qureshi W, Judd SE, Howard G, Howard VJ, Cushman M, Soliman EZ. Relation between cancer and atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke Study). Am J Cardiol. 2015 Apr 15;115(8):1090-4. [CrossRef]
- Ay C, Grilz E, Nopp S, Moik F, Königsbrügge O, Klimek P, Thurner S, Posch F, Pabinger I. Atrial fibrillation and cancer: prevalence and relative risk from a nationwide study. Res Pract Thromb Haemost. 2022 Dec 23;7(1):100026. [CrossRef]
- Han H, Chen L, Lin Z, Wei X, Guo W, Yu Y, Wu C, Cao Y, He J. Prevalence, trends, and outcomes of atrial fibrillation in hospitalized patients with metastatic cancer: findings from a national sample. Cancer Med. 2021 Aug;10(16):5661-5670. [CrossRef]
- Guha A, Fradley MG, Dent SF, Weintraub NL, Lustberg MB, Alonso A, Addison D. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-Medicare analysis. Eur Heart J. 2022 Jan 31;43(4):300-312. [CrossRef]
- Yun JP, Choi EK, Han KD, Park SH, Jung JH, Park SH, Ahn HJ, Lim JH, Lee SR, Oh S. Risk of Atrial Fibrillation According to Cancer Type: A Nationwide Population-Based Study. JACC CardioOncol. 2021 Jun 15;3(2):221-232. [CrossRef]
- Fabiani I, Colombo A, Bacchiani G, Cipolla CM, Cardinale DM. Incidence, Management, Prevention and Outcome of Post-Operative Atrial Fibrillation in Thoracic Surgical Oncology. J Clin Med. 2019 Dec 23;9(1):37. [CrossRef]
- Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014 Mar 18;63(10):945-53. [CrossRef]
- López-Fernández T, Martín-García A, Roldán Rabadán I, Mitroi C, Mazón Ramos P, Díez-Villanueva P, Escobar Cervantes C, Alonso Martín C, Alonso Salinas GL, Arenas M, Arrarte Esteban VI, Ayala de La Peña F, Castro Fernández A, García Pardo H, García-Sanz R, González Porras JR, López de Sá E, Lozano T, Marco Vera P, Martínez Marín V, Mesa Rubio D, Montero Á, Oristrell G, Pérez de Prado A, Velasco Del Castillo S, Virizuela Echaburu JA, Zatarain-Nicolás E, Anguita Sánchez M, Tamargo Menéndez J; Expert reviewers. Atrial Fibrillation in Active Cancer Patients: Expert Position Paper and Recommendations. Rev Esp Cardiol. 2019 Sep;72(9):749-759. English, Spanish. [CrossRef]
- Buza V, Rajagopalan B, Curtis AB. Cancer Treatment-Induced Arrhythmias: Focus on Chemotherapy and Targeted Therapies. Circ Arrhythm Electrophysiol. 2017 Aug;10(8):e005443. [CrossRef]
- Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015 Apr;12(4):230-43. [CrossRef]
- Cheng WL, Kao YH, Chen SA, Chen YJ. Pathophysiology of cancer therapy-provoked atrial fibrillation. Int J Cardiol. 2016 Sep 15;219:186-94.
- Yao, C. , Veleva T., Scott L., Jr., et al. Enhanced cardiomyocyte NLRP3 inflammasome signalling promotes atrial fibrillation. Circulation. 2018;138:2227-2242).
- Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018 Aug;80:50-64. Epub 2017 Jun 3. [CrossRef] [PubMed]
- Rudolph V, Andrié RP, Rudolph TK, Friedrichs K, Klinke A, Hirsch-Hoffmann B, Schwoerer AP, Lau D, Fu X, Klingel K, Sydow K, Didié M, Seniuk A, von Leitner EC, Szoecs K, Schrickel JW, Treede H, Wenzel U, Lewalter T, Nickenig G, Zimmermann WH, Meinertz T, Böger RH, Reichenspurner H, Freeman BA, Eschenhagen T, Ehmke H, Hazen SL, Willems S, Baldus S. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med. 2010 Apr;16(4):470-4.
- Leiva O, AbdelHameid D, Connors JM, Cannon CP, Bhatt DL. Common Pathophysiology in Cancer, Atrial Fibrillation, Atherosclerosis, and Thrombosis: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2021 Nov 16;3(5):619-634.
- Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, Mahmood SS, Barac A, Groarke JD, Hayek SS, Dani S, Venesy D, Patten R, Nohria A. Ibrutinib-Associated Atrial Fibrillation. JACC Clin Electrophysiol. 2018 Dec;4(12):1491-1500.
- Yang T, Yang P, Roden DM, Darbar D. Novel KCNA5 mutation implicates tyrosine kinase signaling in human atrial fibrillation. Heart Rhythm. 2010 Sep;7(9):1246-52.
- Yang X, Li X, Yuan M, et al Anticancer therapy-induced atrial fibrillation: electrophysiology and related mechanisms. Front Pharmacol. 2018;9:1058.
- Alexandre, J. , Moslehi, J. J., Bersell, K. R., Funck-Brentano, C., Roden, D. M., & Salem, J. E. (2018). Anticancer drug-induced cardiac rhythm disorders: Current knowledge and basic underlying mechanisms. Pharmacol Ther, 189, 89–103.
- Onaitis M, D'Amico T, Zhao Y, O'Brien S, Harpole D. Risk factors for atrial fibrillation after lung cancer surgery: analysis of the Society of Thoracic Surgeons general thoracic surgery database. Ann Thorac Surg. 2010 Aug;90(2):368-74.
- Kumar M, Lopetegui-Lia N, Malouf CA, Almnajam M, Coll PP, Kim AS. Atrial fibrillation in older adults with cancer. J Geriatr Cardiol. 2022 Jan 28;19(1):1-8.
- 25. Suter TM, Ewer MS Cancer drugs and the heart: importance and management. Eur Heart J. 2013;34:1102–1111. [CrossRef]
- 26. Butany J, Leong SW, Carmichael K, Komeda M. A 30-year analysis of cardiac neoplasms at autopsy. Can J Cardiol.
- Gibson TM, Li Z, Green DM, Armstrong GT, Mulrooney DA, Srivastava D, Bhakta N, Ness KK, Hudson MM, Robison LL. Blood Pressure Status in Adult Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. Cancer Epidemiol Biomarkers Prev. 2017 Dec;26(12):1705-1713.
- Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B; ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021 Sep 7;42(34):3227-3337.
- 29. Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol. 2007;60(1):27-34.
- Tsang, T. S. et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin.
- Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. S M Vaziri, M G Larson, E J Benjamin and D Levy.
- Seko Y, Kato T, Haruna T, Izumi T, Miyamoto S, Nakane E, Inoko M. Association between atrial fibrillation, atrial enlargement, and left ventricular geometric remodeling. Sci Rep. 2018 Apr 23;8(1):6366. PMID: 29686287; PMCID: PMC5913256. [CrossRef]
- Rosenberg MA, Gottdiener JS, Heckbert SR, Mukamal KJ. Echocardiographic diastolic parameters and risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012 Apr;33(7):904-12. Epub 2011 Oct 11. PMID: 21990265; PMCID: PMC3345546. [CrossRef]
- Left Atrial Strain and the Risk of Atrial Arrhythmias From Extended Ambulatory Cardiac Monitoring: MESA. Matthew P. Huber, MD MS; Jay A. Pandit, MD; Paul N. Jensen, PhD; Kerri L. Wiggins, MS; Ravi B. Patel, MD, MS; Benjamin H. Freed, MD; Alain G. Bertoni, MD, MPH; Sanjiv J. Shah, MD; Susan R. Heckbert , MD, PhD; James S. Floyd, MD, MS.
- Müller P, Weijs B, Bemelmans NMAA, Mügge A, Eckardt L, Crijns HJGM, Bax JJ, Linz D, den Uijl DW. Echocardiography-derived total atrial conduction time (PA-TDI duration): risk stratification and guidance in atrial fibrillation management. Clin Res Cardiol. 2021 Nov;110(11):1734-1742. Epub 2021 Aug 28. PMID: 34453577; PMCID: PMC8563556. [CrossRef]
- 8.Sieweke JT, Hagemus J, Biber S, Berliner D, Grosse GM, Schallhorn S, Pfeffer TJ, Derda AA, Neuser J, Bauersachs J and Bavendiek U (2022) Echocardiographic Parameters to Predict Atrial Fibrillation in Clinical Routine—The EAHsy-AF Risk Score. Front. Cardiovasc. Med. 9:851474. [CrossRef]
- Predictive value of preoperative tissue Doppler echocardiographic analysis for postoperative atrial fibrillation after pulmonary resection for lung cancerTakashi Nojiri, MD,a Hajime Maeda, MD, PhD,a Yukiyasu Takeuchi, MD, PhD,a Yasunobu Funakoshi, MD, PhD,a Ryoji Maekura, MD, PhD,b Kazuhiro Yamamoto, MD, PhD,c and Meinoshin Okumura, MD, PhDd.
- Anile M, Telha V, Diso D, De Giacomo T, Sciomer S, Rendina EA, et al. Left atrial size predicts the onset of atrial fibrillation after major pulmonary resections. Eur J Cardiothorac Surg. 2012;41:1094–7.9.Nojiri T, Maeda H, Takeuchi Y, Funakoshi Y, Maekura R, Yamamoto K, et al. Predictive value of preoperative tissue Doppler echocardiographic analysis for postoperative atrial fibrillation after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2010;140:764–8.
- .
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021 Feb 1;42(5):373-498. Erratum in: Eur Heart J. 2021 Feb 1;42(5):507. Erratum in: Eur Heart J. 2021 Feb 1;42(5):546-547. Erratum in: Eur Heart J. 2021 Oct 21;42(40):4194. [CrossRef]
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH; ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022 Nov 1;43(41):4229-4361.
- Heist EK, Mansour M, Ruskin JN. Rate control in atrial fibrillation: targets, methods, resynchronization considerations. Circulation. 2011 Dec 13;124(24):2746-55.
- Echt DS, Ruskin JN. Use of Flecainide for the Treatment of Atrial Fibrillation. Am J Cardiol. 2020 Apr 1;125(7):1123-1133.
- Hammann F, Gotta V, Conen K, Medinger M, Cesana P, Rochlitz C, Taegtmeyer AB. Pharmacokinetic interaction between taxanes and amiodarone leading to severe toxicity. Br J Clin Pharmacol. 2017 Apr;83(4):927-930.
- Su VY, Hu YW, Chou KT, Ou SM, Lee YC, Lin EY, Chen TJ, Tzeng CH, Liu CJ. Amiodarone and the risk of cancer: a nationwide population-based study. Cancer. 2013 May 1;119(9):1699-705.
- Tamargo J, Caballero R, Delpón E. Cancer chemotherapy and cardiac arrhythmias: a review. Drug Saf. 2015 Feb;38(2):129-52.
- Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J. 2013 Apr;34(15):1102-11.
- Ganatra S, Abraham S, Kumar A, Parikh R, Patel R, Khadke S, Kumar A, Liu V, Diaz ANR, Neilan TG, Martin D, Hook B, Dani SS, Asnani A, Nohria A. Efficacy and safety of catheter ablation for atrial fibrillation in patients with history of cancer. Cardiooncology. 2023 Apr 5;9(1):19. [CrossRef]
- Kanmanthareddy A, Vallakati A, Reddy Yeruva M, Dixit S, DI Biase L, Mansour M, Boolani H, Gunda S, Bunch TJ, Day JD, Ruskin JN, Buddam A, Koripalli S, Bommana S, Natale A, Lakkireddy D. Pulmonary vein isolation for atrial fibrillation in the postpneumonectomy population: a feasibility, safety, and outcomes study. J Cardiovasc Electrophysiol. 2015 Apr;26(4):385-389.
- Chatterjee NA, Upadhyay GA, Ellenbogen KA, McAlister FA, Choudhry NK, Singh JP. Atrioventricular nodal ablation in atrial fibrillation: a meta-analysis and systematic review. Circ Arrhythm Electrophysiol. 2012 Feb;5(1):68-76.
- Leader A, Mendelson Cohen N, Afek S, Jaschek R, Frajman A, Itzhaki Ben Zadok O, Raanani P, Lishner M, Spectre G. Arterial Thromboembolism in Patients With AF and CHA2DS2-VASc Score 0-2 With and Without Cancer. JACC CardioOncol. 2023 Jan 17;5(2):174-185. [CrossRef]
- Pastori D, Marang A, Bisson A, Menichelli D, Herbert J, Lip GYH, Fauchier L. Thromboembolism, mortality, and bleeding in 2,435,541 atrial fibrillation patients with and without cancer: A nationwide cohort study. Cancer. 2021 Jun 15;127(12):2122-2129. [CrossRef]
- 53. Patell R, Gutierrez A, Rybicki L, Khorana AA Usefulness of CHADS2 and CHA2DS2-VASc scores for stroke prediction in patients with cancer and atrial fibrillation. Am J Cardiol. 2186. [CrossRef]
- O'Neal WT, Claxton JS, Sandesara PB, MacLehose RF, Chen LY, Bengtson LGS, Chamberlain AM, Norby FL, Lutsey PL, Alonso A. Provider Specialty, Anticoagulation, and Stroke Risk in Patients With Atrial Fibrillation and Cancer. J Am Coll Cardiol. 2018 Oct 16;72(16):1913-1922. [CrossRef]
- Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR 2 HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in nonwarfarin anticoagulated atrial fibrillation patients. J Am Coll Cardiol 2013;61:386–7.
- Farmakis D, Papakotoulas P, Angelopoulou E, Bischiniotis T, Giannakoulas G, Kliridis P, Richter D, Paraskevaidis I. Anticoagulation for atrial fibrillation in active cancer. Oncol Lett. 2022 Apr;23(4):124. [CrossRef]
- Angelini DE, Radivoyevitch T, McCrae KR, Khorana AA. Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am J Hematol. 2019 Jul;94(7):780-785. [CrossRef]
- Delluc A, Wang TF, Yap ES, Ay C, Schaefer J, Carrier M, Noble S. Anticoagulation of cancer patients with non-valvular atrial fibrillation receiving chemotherapy: Guidance from the SSC of the ISTH. J Thromb Haemost. 2019 Aug;17(8):1247-1252. [CrossRef]
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH; ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022 Nov 1;43(41):4229-4361. [CrossRef]
- Chen ST, Hellkamp AS, Becker RC, Berkowitz SD, Breithardt G, Fox KAA, Hacke W, Halperin JL, Hankey GJ, Mahaffey KW, Nessel CC, Piccini JP, Singer DE, Patel MR, Melloni C. Efficacy and safety of rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation and a history of cancer: observations from ROCKET AF. Eur Heart J Qual Care Clin Outcomes. 2019 Apr 1;5(2):145-152. [CrossRef]
- Melloni C, Dunning A, Granger CB, Thomas L, Khouri MG, Garcia DA, Hylek EM, Hanna M, Wallentin L, Gersh BJ, Douglas PS, Alexander JH, Lopes RD. Efficacy and Safety of Apixaban Versus Warfarin in Patients with Atrial Fibrillation and a History of Cancer: Insights from the ARISTOTLE Trial. Am J Med. 2017 Dec;130(12):1440-1448.e1. [CrossRef]
- Fanola CL, Ruff CT, Murphy SA, Jin J, Duggal A, Babilonia NA, Sritara P, Mercuri MF, Kamphuisen PW, Antman EM, Braunwald E, Giugliano RP. Efficacy and Safety of Edoxaban in Patients With Active Malignancy and Atrial Fibrillation: Analysis of the ENGAGE AF - TIMI 48 Trial. J Am Heart Assoc. 2018 Aug 21;7(16):e008987. [CrossRef]
- Sawant AC, Kumar A, Mccray W, Tetewsky S, Parone L, Sridhara S, Prakash MPH, Tse G, Liu T, Kanwar N, Bhardwaj A, Khan S, Manion C, Lahoti A, Pershad A, Elkin P, Corbelli J. Superior safety of direct oral anticoagulants compared to Warfarin in patients with atrial fibrillation and underlying cancer: a national veterans affairs database study. J Geriatr Cardiol. 2019 Sep;16(9):706-709. [CrossRef]
- Shah S, Norby FL, Datta YH, Lutsey PL, MacLehose RF, Chen LY, Alonso A. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018 Feb 13;2(3):200-209. [CrossRef]
- Deitelzweig S, Keshishian AV, Zhang Y, Kang A, Dhamane AD, Luo X, Klem C, Ferri M, Jiang J, Yuce H, Lip GYH. Effectiveness and Safety of Oral Anticoagulants Among Nonvalvular Atrial Fibrillation Patients With Active Cancer. JACC CardioOncol. 2021 Sep 21;3(3):411-424. [CrossRef]
- Mariani MV, Magnocavallo M, Straito M, Piro A, Severino P, Iannucci G, Chimenti C, Mancone M, Rocca DGD, Forleo GB, Fedele F, Lavalle C. Direct oral anticoagulants versus vitamin K antagonists in patients with atrial fibrillation and cancer a meta-analysis. J Thromb Thrombolysis. 2021 Feb;51(2):419-429. [CrossRef]
- Yang P, Zhu D, Xu X, Shen W, Wang C, Jiang Y, Xu G, Wu Q. Efficacy and safety of oral anticoagulants in atrial fibrillation patients with cancer-a network meta-analysis. Heart Fail Rev. 2020 Sep;25(5):823-831. [CrossRef]
- Potter AS, Patel A, Khawaja M, Chen C, Zheng H, Kaczmarek J, Gao F, Karimzad K, Song J, Koutroumpakis E, Khalaf S, Iliescu C, Deswal A, Palaskas NL. Outcomes by Class of Anticoagulant Use for Nonvalvular Atrial Fibrillation in Patients With Active Cancer. JACC CardioOncol. 2022 Sep 20;4(3):341-350. [CrossRef]
- Mehta HB, An H, Ardeshirrouhanifard S, Raji MA, Alexander GC, Segal JB. Comparative Effectiveness and Safety of Direct Oral Anticoagulants Versus Warfarin Among Adults With Cancer and Atrial Fibrillation. Circ Cardiovasc Qual Outcomes. 2022 Dec;15(12):e008951. [CrossRef]
- Ardeshirrouhanifard S, An H, Goyal RK, Raji MA, Segal JB, Alexander GC, Mehta HB. Use of oral anticoagulants among individuals with cancer and atrial fibrillation in the United States, 2010-2016. Pharmacotherapy. 2022 May;42(5):375-386. [CrossRef]
- Carbone A, Bottino R, D'Andrea A, Russo V. Direct Oral Anticoagulants for Stroke Prevention in Special Populations: Beyond the Clinical Trials. Biomedicines. 2023 Jan 4;11(1):131. [CrossRef]
- Mosarla RC, Vaduganathan M, Qamar A, Moslehi J, Piazza G, Giugliano RP. Anticoagulation Strategies in Patients With Cancer: JACC Review Topic of the Week. J Am Coll Cardiol. 2019 Mar 26;73(11):1336-1349. [CrossRef]
- Peixoto de Miranda ÉJF, Takahashi T, Iwamoto F, Yamashiro S, Samano E, Macedo AVS, Ramacciotti E. Drug-Drug Interactions of 257 Antineoplastic and Supportive Care Agents With 7 Anticoagulants: A Comprehensive Review of Interactions and Mechanisms. Clin Appl Thromb Hemost. 2020 Jan-Dec;26:1076029620936325. [CrossRef]
- Wu VC, Wang CL, Huang YT, Lan WC, Wu M, Kuo CF, Chen SW, Chu PH, Wen MS, Kuo CC, Chang SH. Novel Oral Anticoagulant versus Warfarin in Cancer Patients with Atrial Fibrillation: An 8-Year Population-Based Cohort Study. J Cancer. 2020 Jan 1;11(1):92-99. PMID: 31892976; PMCID: PMC6930400. [CrossRef]
- Yasui T, Shioyama W, Oboshi M, Oka T, Fujita M. Oral Anticoagulants in Japanese Patients with Atrial Fibrillation and Active Cancer. Intern Med. 2019 Jul 1;58(13):1845-1849. [CrossRef]
- Kim K, Lee YJ, Kim TH, Uhm JS, Pak HN, Lee MH, Joung B. Effect of Non-vitamin K Antagonist Oral Anticoagulants in Atrial Fibrillation Patients with Newly Diagnosed Cancer. Korean Circ J. 2018 May;48(5):406-417. [CrossRef]
- Ording AG, Horváth-Puhó E, Adelborg K, Pedersen L, Prandoni P, Sørensen HT. Thromboembolic and bleeding complications during oral anticoagulation therapy in cancer patients with atrial fibrillation: a Danish nationwide population-based cohort study. Cancer Med. 2017 Jun;6(6):1165-1172. [CrossRef]
- Tran E, Ledbetter LE. A retrospective evaluation of direct oral anticoagulant (DOAC) management strategies in patients with cancer on active chemotherapy. J Thromb Thrombolysis. 2023 May;55(4):721-728. [CrossRef]
- Parrini I, Lucà F, Rao CM, Parise G, Micali LR, Musumeci G, La Meir M, Colivicchi F, Gulizia MM, Gelsomino S. Superiority of Direct Oral Anticoagulants over Vitamin K Antagonists in Oncological Patients with Atrial Fibrillation: Analysis of Efficacy and Safety Outcomes. J Clin Med. 2022 Sep 27;11(19):5712. PMID: 36233581; PMCID: PMC9572823. [CrossRef]
- Liu F, Xu Z, Luo J, Yu P, Ma J, Yuan P, Zhu W. Effectiveness and Safety of DOACs vs. VKAs in AF Patients With Cancer: Evidence From Randomized Clinical Trials and Observational Studies. Front Cardiovasc Med. 2021 Nov 5;8:766377. PMID: 34805320; PMCID: PMC8602680. [CrossRef]
| Publication year | Trial/reference | Type of evidence | Prospective/retreospective | Number of patients | Drug | Summary of evidences |
|---|---|---|---|---|---|---|
| 2019 | ROCKET-AF (58) | Sub-group analysis of RCT | Prospecitve | 640 | Rivaroxaban | No efficacy and safety differences. Increased risk of bleeding |
| 2017 | ARISTOTLE (59) |
Sub-group analysis of RCT | Prospecitve | 1,236 | Apixaban | Similar efficacy in preventing stroke and systemic embolism. No increase in major bleedings |
| 2018 | ENGAGE AF- TIMI 48 (60) |
Sub-group analysis of RCT | Prospecitve | 1,153 | Edoxaban | Similar efficacy and safety |
| 2018 | Shah S, et al. (63) | Administrative analysis | Retrospective | 16,096 | Various NOACs | lower or similar rates of bleeding and stroke and a lower rate of incident VTE |
| 2022 | Potter AS, et al. (67) | Single-center database analysis | Retrospective | 1,133 | Various NOACs | Similar risks for cerebrovascular accident, gastrointestinal bleeding, and intracranial hemorrhage |
| 2020 | Wu VC, et al. (73) | Administrative analysis | Retrospective | 336 | Various NOACs | reduced IS/SE, major bleeding, and ICH compared to warfarin. |
| 2019 | Yasui T, et al. (74) | Single-center database analysis | Retrospective | 127 | Various NOACs | Similar rates of IS, SE and major bleeding |
| 2018 | Kim K, et al. (75) |
Single-center database analysis | Retrospective | 388 | Various NOACs | NOACs associated with lower incidences of IS/SE, major bleeding and all-cause mortality |
| 2017 | Ording AG, et al. (76) | Administrative analysis | Retrospective | 1809 | Various NOACs | Similar risks of SE or bleeding in patients with and without cancer |
| 2021 | Mariani MV, et al. (65) | Meta-analysis | Prospective/Retrospective | 46,424 | Various NOACs | NOACs associated with reduction of thromboembolic events and major bleeding |
| 2023 | Tran E, et al. (77) | Single-center database analysis | Retrospective | 58 | Various NOACs | Evidence for management issues during chemotherapy |
| 2022 | Parrini I, et al. (78) | Meta-analysis | Prospective/Retrospective | 228,497 | Various NOACs | NOACs showed better efficacy and safety outcomes than warfarin |
| 2021 | Liu F, et al. (79) | Meta-analysis | Prospective/Retrospective | 248,218 | Various NOACs | Reduction in SE, VTE, intracranial and GI bleeding. Same risk of IS, MI, CV death, all-cause death, major bleeding, major or NMCR bleeding. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





