1. Introduction
Medical literature defines narcolepsy as a rare and lifelong neurological disorder that affects the brain’s ability to control the sleep-wake cycle. Narcolepsy is a complex disorder that is thought to have a genetic component, but the specific genetic causes are not yet fully understood. People with narcolepsy experience excessive daytime sleepiness and difficulty staying awake during the day, as well as disruptions in their normal sleep patterns at night. According to the International Classification of Sleep Disorders, Third Edition (ICSD-3, 2014) and Orphanet (European reference portal for information on rare diseases and orphan drugs), narcolepsy term is not recognized as only one disease, but a group of diseases based on the symptoms’ characterization and different underlying causes (ORPHA:619284). So, there are two forms of narcolepsy. Type 1 narcolepsy (NT1 or T1N) was previously called narcolepsy with cataplexy. Type 2 narcolepsy (NT2 or T2N) is also known as narcolepsy without cataplexy. [
1,
2] Narcolepsy may have a significant impact on a person’s daily life affecting the ability to work, study, and participate in daily activities and social life.
The literature on this topic is constantly evolving. As of 2022, the PubMed and Scopus databases have included 316 and 402 studies, respectively, on various aspects of narcolepsy. Despite this, there is still much unknown about the condition.
Our literature review, conducted using validated search strategies and inclusion/exclusion criteria, aimed to provide a comprehensive and up-to-date overview of the current knowledge on narcolepsy. As a complex disorder that may involve multiple factors, further research is necessary to fully understand its causes and develop more effective treatments. The goal of our review is to increase awareness about narcolepsy and encourage a multidisciplinary approach and further research among professionals.
2. Development of the first knowledge of the narcolepsy – a timeline
In 1880, J.B.E. Gélineau, a French neurologist, presented his clinical case report describing narcolepsy as a distinct entity. Gélineau used a combination of Greek words (narkē + lepsis) to coin the disease. Narkē means numbness, drowsiness, and the meaning of lepsis is” attack” [
3]. A few years earlier, in 1877, K.F.O. Westphal, a German psychiatrist, described the first familial clinical case of narcoleptic sleep attacks, and cataleptic attacks. Both Westphal and Gelineau have described the symptoms of narcolepsy and cataplexy. Later in 1902, Lowenfeld proposed the cataplexy term for sudden atonia triggered by emotions.[
4] Over time more cases of narcolepsy were reported.[
3] In 1934, L.E. Daniels, a fellow in Neurology at Mayo Foundation, reviewed in detail the published literature about the aetiology, symptoms, and course of narcolepsy.[
5,
6] Due to his insightful review on the topic and further studies of Yoss and Dali (1957), it was possible to establish the criteria for the diagnosis of the narcoleptic syndrome (”the clinical tetrad”): daytime
sleepiness, cataplexy, sleep paralysis, and
hypnagogic hallucinations.[
7] Another landmark event is the discovery of REM sleep (rapid eyes movements). In 1953, E. Aserinsky and N. Kleitman discovered the REM sleep, an important moment for the birth of modern sleep research. In 1960, Vogel noted that patients with narcolepsy had early onset of REM sleep on their electroencephalograms. At the First International Symposium on Narcolepsy in 1975, the symptom of disturbed nocturnal sleep was added to the clinical diagnostic criteria for narcolepsy.[
8]
3. Information about narcolepsy in different population
Prevalence, and incidence, both are important measures in the field of public health and epidemiology. They can play an important role in raising awareness about the narcolepsy identifying the high-risk groups and providing a better understanding of the burden of the disease on the population.
Prevalence of narcolepsy, as the total number of cases of a disease present in a population at a specific point in time, may be under-reported due to lack of awareness and misdiagnosis/undiagnosed. Narcolepsy shares many features with other sleep disorders, such as insomnia, sleep apnoea, and restless legs syndrome, as well as with other medical conditions, such as depression, fibromyalgia, and chronic fatigue syndrome. In addition, some studies have reported higher prevalence of narcolepsy in young populations in comparison with older populations. Thus, the actual number of people affected by the condition may be higher.
The overall prevalence of narcolepsy is estimated to be around 0.02 – 0.05% of general population. Longstreth et al. (2007) reported a high prevalence for NT1 estimated between 25 – 50 per 100,000 people, compared with NT2 estimated between 20 - 34 per 100,000 individuals. [
9,
10] However, other studies may report slightly different figures (
Table 1) depending on their sample size, population, methods used to collect data, and diagnostic criteria (case detection rates) or genetic and environmental factors.
Although narcolepsy can be noted with the same symptoms in both men and women, some studies reported different prevalence depending on gender. For example, prevalence is equally common in males and females [
11], it is more common among men [
19,
20], or a greater prevalence in females than men. [
26,
28]
The incidence of narcolepsy is a measure of the number of new cases of the disorder that develop in a given population over a specific period, typically expressed as a rate per 100,000 people per year. Studies have estimated the incidence of narcolepsy to be around 0.74 cases per 100,000 person-years [
9] or higher, such as 1.37 per 100,000 persons per year [
25]. Scheer et al. found a greater incidence (NT1 +NT2) than most previous published studies, such as 7.67 per 100,000 persons per year.[
26] However, it's important to note that the exact incidence rate may vary depending on the population being studied and the diagnostic criteria used. In this context, it should be mentioned that some studies highlighted an increase of incidence following the Pandemrix vaccine which was used in the 2009-2010 H1N1 influenza pandemic. Several studies have shown that the incidence of narcolepsy in children and adolescents may have risen after the H1N1 influenza pandemic. These studies suggest that the H1N1 influenza vaccination may have played a role in the increased risk of narcolepsy in individuals who received the Pandemrix H1N1 vaccine. [
29,
30,
31,
32,
33,
34]. There have been some studies that have not found a significant increase in the incidence of narcolepsy following H1N1 vaccination. [
35,
36,
37] The exact cause of this increased risk is not fully understood, but it is important to note that these studies were observational and further research is needed to confirm the association between the H1N1 influenza pandemic and the incidence of narcolepsy and to understand the underlying mechanisms.[
38]
4. Age of onset
Age of onset varies from childhood to the fifth decade (usually between the ages of 15 and 25), with a peak in the second decade. [
39] According to Dauvilliers et al. (2001), narcolepsy has a bimodal distribution in age of onset, with a highest peak at about 15 years and a second less pronounce peak at about 35 years, suggesting that age of onset is genetically determined.[
40] In rare cases the signs may be visible as early as 2-3 years of age or later, in adults over 50. It's important for people who suspect they may have narcolepsy to seek the evaluation of a sleep specialist, as early diagnosis and treatment can help to improve quality of life and prevent complications.
5. Causes and risk factors of narcolepsy
Narcolepsy is a disorder that is thought to be caused by a combination of genetic, autoimmune, and environmental factors. Some causes and risk factors for narcolepsy may include:
5.1. Hypocretin (orexin) deficiency
The role of hypocretin in the development of narcolepsy has been well established through research on animal models and studies on human patients. Studies on mice that lack the ability to produce orexin have shown that these animals display symptoms of narcolepsy, including cataplexy and excessive daytime sleepiness.[
41,
42] Additionally, studies on human narcoleptic patients have revealed that there is a deficiency of orexin in the cerebrospinal fluid (CSF) of these individuals, further supporting the idea that a dysfunction of orexin signalling plays a key role in the pathophysiology of narcolepsy.[
43]
Thannickal et al. found that the number of hypocretin-making neurons was significantly reduced in the brains of individuals with narcolepsy type 1 compared to controls. This suggests that a loss of hypocretin neurons is a key feature of narcolepsy.[
44] Peyron et al. investigated the involvement of hypocretins in narcolepsy by examining six narcoleptic brains through histopathology, and by conducting mutation screenings of Hcrt, Hcrtr1, and Hcrtr2 in 74 patients with different human leukocyte antigen and family history statuses. One patient with an early onset narcolepsy with cataplexy has been reported to have a heterozygous mutation in the HCRT gene (hypocretin, also known as orexin) on chromosome 17q21.2. [
45]
Valko et al. investigated the relationship between histamine-containing neurons and narcolepsy. The researchers found a significant increase in the number of histaminergic tuberomammillary neurons (TMN) in the hypothalamus of individuals with narcolepsy compared to controls. The authors hypothesized that the increase in histaminergic TMN could be a compensatory mechanism in response to the loss of orexin (hypocretin)-containing neurons in the hypothalamus, which is a characteristic feature of narcolepsy. They suggested that the histamine-containing neurons may play a role in maintaining wakefulness and regulating sleep-wake cycles. [
46] Another independent study conducted by John and colleagues aimed to investigate whether there are any changes in histamine cells in individuals with human narcolepsy with cataplexy. The study found that the number of histamine-containing neurons was significantly increased in the hypothalamus of individuals with narcolepsy with cataplexy compared to controls. The authors suggested that the increase in histamine-containing neurons may be a compensatory mechanism in response to the loss of orexin (hypocretin)-containing neurons in the hypothalamus, which is a hallmark feature of narcolepsy with cataplexy.[
47] In summary, while there may be an increase in histamine cells in people with narcolepsy with cataplexy, it is not the primary cause of the disorder and is instead a secondary effect of the loss of hypocretin cells.
5.2. Loci associated with susceptibility to narcolepsy.
The HCRT gene produces two neuropeptides, hypocretin-1 and hypocretin-2 (also known as orexin-A and orexin-B), which are primarily produced by a group of neurons in the hypothalamus called the hypothalamic orexinergic neurons. They bind to two receptors in the brain, Hcrtr1 and Hcrtr2 (hypocretin receptor1 and hypocretin receptor2), which are predominantly expressed in regions associated with sleep and wake regulation. Additional loci associated with susceptibility to narcolepsy have been mapped to chromosomes 4p13-q21, 21q11.2, 22q13, 14q11, and 19p13.2. NRCLP7 (MIM 614250) is caused by mutation in the MOG gene (Myelin Oligodendrocyte Glycoprotein: MIM 159465) on chromosome 6p22.1. [
48] (
Table 2)
Although there is an increased risk for first-degree relatives of individuals with narcolepsy-cataplexy to develop the condition, familial cases are uncommon. The concordance rate for monozygotic twins is only partially significant at 25-31%, indicating that genetics may play a partial role in the development of narcolepsy.[
49] However, most cases of narcolepsy-cataplexy do not have a familial history, occurring at random rather than being inherited.
Rare cases of narcolepsy are autosomal dominant. Winkelmann et al. investigated members of four families with autosomal dominant cerebellar ataxia, deafness, and narcolepsy (ADCA-DN) (MIM 604121). The authors postulated that the DNMT1 (DNA-methyltransferase 1) mutations may result in abnormal gene expression or silencing neuronal cells. The authors also noted that DNMT1 is expressed in immune cells, which may play a role in narcolepsy. [
50] Changes in global DNA methylation patterns are associated not only with ADCA-DN but also with narcolepsy. Furthermore, low orexin A levels in the CSF are commonly seen in both ADCA-DN and narcolepsy patients. Therefore, these two diseases may have a shared pathogenesis. The DNA methylation changes may contribute to decreased orexin A levels in the CSF or destruction of orexin neurons. [
51]
Resistance to narcolepsy is associated with minor alleles of a SNP (single nucleotide polymorphism) and a marker in the NLC1A gene (MIM 610259) on chromosome 21q22. This means that individuals who carry these variations are less likely to develop narcolepsy. [
52]
5.3. Variations in the specific human leukocyte antigen (HLA) gene
There is strong evidence to suggest that genetics plays a role in the development of narcolepsy. Studies have shown that individuals with narcolepsy are more likely to have certain genetic variations, particularly in the HLA (human leukocyte antigen) region of chromosome 6p21. [
53] Hor et al. reported that the HLA haplotype DRB5*0101-DRB1*1501-DQA1*0102-DQB1*0602 is found in nearly 100% of individuals with NT1 of European descent, however, it is also present in 15-25% of the general population. Other reported findings are consistent with only about 40% to 50% of NT-2 patients carry DQB1*06:02. This means that while the haplotype is necessary for the development of narcolepsy, it alone is not enough to cause the disorder. [
54,
55] HLA variations are associated with the immune system and may also play a role in the development of autoimmune disorders. Thus, a possible autoimmune aetiology of narcolepsy is suggested, specifically involving the destruction of hypocretin/orexin-producing neurons in the hypothalamus. [
55] However, no clear evidence of an autoimmune disorder or immune system activation has been found in human narcoleptics. [
44]
Other studies have found that narcolepsy may be caused by an autoimmune response against certain cells in the brain that produce the neurotransmitter hypocretin.[
56] So, the body's immune system attacks and destroys the orexin-producing neurons in the hypothalamus. This is supported by the fact that narcolepsy is often associated with other autoimmune conditions such as celiac disease, rheumatoid arthritis, and Sjogren's syndrome.[
57] Kornum et al. reported P2RY11(purinergic receptor P2Y located on 19p13.2) as an important regulator of immune cell survival, with possible implications in narcolepsy and other autoimmune diseases.[
58] Hallmayer et al. reported a SNP in the gene encoding T-cell receptor alpha (TCRA on chromosome 14q11.2) that plays a role in the immune response and has been found to double the risk of NT1.[
59] Later, it has been reported that immune system cells known as T cells in people with narcolepsy may be linked to an abnormal immune response triggered by CD4+ and CD8+ T cells. [
60,
61]
Dysfunction of the HCRT gene, either through genetic mutations or autoimmune-mediated destruction of the orexinergic neurons, has been implicated in the development of narcolepsy, and other symptoms related to sleep fragmentation. Moreover, alterations in the hypocretin/orexin system have been associated with other sleep disorders, such as insomnia, and metabolic disorders, such as obesity and diabetes. [
62,
63,
64,
65]
5.4. Environmental factors
In addition to genetic and autoimmune causes, environmental factors (environmental tiggers, infections, toxins, or psychological stress) may also play a role in the development of narcolepsy in individuals who have a genetic predisposition to the disorder. [
33,
66]
5.5. Brain injuries
Secondary narcolepsy is a type of narcolepsy that is caused by damage to the hypothalamus region of the brain. The damage can be a result of different conditions, such as traumatic brain injuries, strokes, brain tumours, or other similar conditions that affect the hypothalamus. While it is not a common occurrence, individuals with hypothalamus damage are at risk of developing narcolepsy consequently. [
67]
The cause of narcolepsy type 2 is still not fully understood. but it is thought that hypocretin cell loss may be a common feature of both types of narcolepsies, despite the absence of cataplexy in the former. [
68] Bauman-Vogel et al. show that narcolepsy type 2 does exist, but it is less common than narcolepsy type 1. The authors emphasize that it is important to carefully eliminate any other potential causes of excessive sleepiness, if possible, by using a combination of 2-week actigraphy and polysomnography.[
69]
These studies could have significant implications for developing new therapeutic strategies and finding a cure for narcolepsy. However, some cases of narcolepsy do not have a clear or direct cause. Thus, there may be different subtypes of narcolepsy with varying underlying causes, and more research is necessary to fully understand the genetic and neurobiological mechanisms that underlie both NT1 and NT2.
6. Functional changes in narcolepsy
Narcolepsy affects the brain, specifically the hypothalamus. The hypothalamus plays a key role in regulating many of the body's essential functions, including sleep and wakefulness, hunger, thirst, and body temperature. In narcolepsy, there is a dysfunction in the hypothalamus that leads to an imbalance in the brain chemicals that regulate sleep and wakefulness. Specifically, there is a loss of the hypothalamic neurons that produce hypocretin, which leads to the main symptoms of narcolepsy, including excessive daytime sleepiness, cataplexy, sleep paralysis, and hypnagogic hallucinations.
One of the main functional changes in narcolepsy is the disruption of the normal sleep-wake cycle. This means excessive daytime sleepiness, which can greatly impair a person's ability to function in daily life because of a dysfunction of the neurological processes involved in keeping people awake and helping them fall asleep.
Other functional changes may include difficulty staying awake during important activities, such as driving or working, as well as problems with memory and concentration.
Another functional change in narcolepsy is the loss of muscle tone, called cataplexy, which can occur during periods of strong emotion and can lead to sudden collapses. Cataplexy can lead to falls and injuries. Sleep paralysis and vivid hallucinations can be distressing and disruptive to sleep. In summary, the main functional changes in narcolepsy include:
Excessive daytime sleepiness (EDS): People with narcolepsy often feel drowsy and fatigued during the day, regardless of how much sleep they get at night.
Cataplexy: This is a sudden loss of muscle tone that can cause a person to collapse or become weak. It is usually triggered by strong emotions such as laughter or anger.
Sleep attacks: Narcoleptics may experience sudden and irresistible urges to sleep during the day.
Sleep paralysis: This is a temporary inability to move or speak while falling asleep or waking up.
Hallucinations: Hypnagogic hallucinations, which occur as one is falling asleep, and hypnopompic hallucinations, which occur as one is waking up, can occur with narcolepsy.
7. Symptoms of narcolepsy
As a chronic disease, the symptoms of narcolepsy usually have a slow development, but there are also cases with an acute evolution, in only a few weeks. The main possible symptoms are daily sleepiness (with an important social implication as well, the patient can be considered as lazy or even impolite by someone who does not know the context), the sleep attacks (that can occur at any time, without previous signs), cataplexy, sleep paralysis (when falling asleep or immediately after waking up, the patient cannot move or speak), but some other problems as well, such as hallucinations, headache, nightmares or depression.[
70]
The adults do often have comorbidities or various medical treatments that can interfere with the narcoleptic symptoms. They usually tend to rationalize what is happening to them, believing that the other diagnoses that they know to have been the cause of the problems. In comparison, the symptoms of narcolepsy in children and teenagers are harder to be catalogued than those from adults, due to high clinical variations. Mainly, the great number of sleeping hours is remarked, unusual for the respective age, as well as the recurrence of the short episodes when the child falls asleep, during daytime. Also, the hyperactivity, the irritability, the aggressivity and the mood disorders represent symptoms that might appear because of narcolepsy. Consequently, the greatest concerns should consist in the susceptibility of the youngsters to consume illegal or harmful drugs, as well as the alteration of their academic performances or social interactions. [
71]
Considering that the youngsters have a less developed immunity and tend to neglect the protective measures against various environmental pathogens, in antithesis with the adults, the respiratory infections with influenza or streptococcus may also be an important historical element, capable of triggering type 1 narcolepsy, the cataplexic one. Cataplexy, meaning sudden, short loss of muscular tone with the falling of the patient, eventually, has been largely described by Postiglione et al., including the “cataplexic face”, complex motor disfunctions, hyperkinesia. The specific face consists of ptosis, opened mouth and tongue protrusion, everything in a hypotonic context, all these representing the mixed type of cataplexy. There can also occur the active type, with hyperkinesia and twitches that are amplified by strong emotions, as well as the negative one, that is emotionally independent. [
71]
Whilst the adults’ symptoms might lose their historical impact due to other existent pathological conditions, at young person’s there is also the risk of considering some of the signs as normal behaviour, typical for a certain age, for example the REM alterations or the hallucinations due to sleep to be seen as simple nightmares. Early puberty and obesity, also the results of narcolepsy, may be wrongly seen as the actual cause of important sleep disorders. [
71]
Defining the clear distinction between a normal, physiological sleep and uncontrolled episodes of sleep is crucial. As a principle, the narcoleptic episodes do appear in some moments and circumstances in which the child is not expected to leave the wakefulness state, their self-intending to have some energic activity. For example, it is absolutely expected from a child laying on the sofa in a calm environment to fall asleep, but if that happens when they are on their feet, talking to another person, then there are some serious questions to be answered. In this regard, it is reported that school is an environment that brings the symptoms to existence, so it is important that the personnel of the institution to be alerted, in case of suspicion. [
72]
It is important to mention that the signs of this disease appear in more than half of the cases before the individual turns 18 years old and they might modify as time passes. The diagnosis may suffer delays since the symptoms can be initially neglected, but the long-term consequences of this fact might be extremely serious. As mentioned earlier, a relevant sign can be the suddenly increased number of sleeping hours a day. Furthermore, the fear that children have in answering the physician’s questions must be considered. They can feel intimidated by the situation or there can be the fact that many of them do not have enough discernment to observe that there is something unusual about their bodies. Therefore, an important part of the management belongs to the parents, who should be aware of their children’s sleeping program and be aware of any spontaneous change of it. It is obvious the fact that the person who is responsible to report the pathological situation is different to the suffering one constitutes an important negative element in managing narcolepsy in children, a problem that does not occur in narcoleptic adults. [
72]
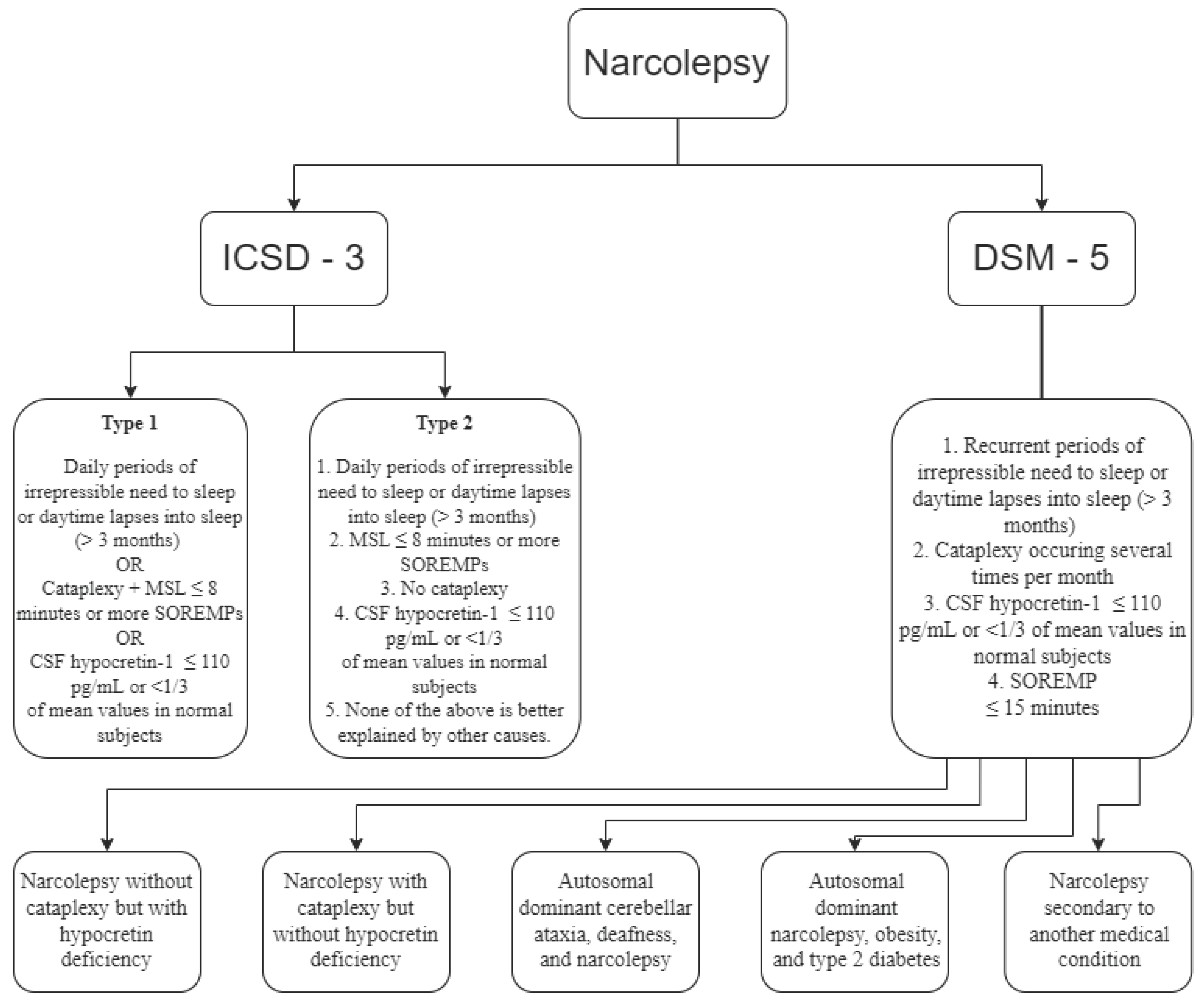
8. Classification and Diagnosis
Narcolepsy is classified by the International Classification of Sleep Disorders - Third Edition (ICSD-3) as part of the Central Disorders of Hypersomnolence section, under two separate disorders [
88]:
Narcolepsy Type 1
Narcolepsy type 2
The Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5) classifies narcolepsy under the sleep-wake disorders section, which requires the specifications of one of the following types [
80]:
Narcolepsy without cataplexy but with hypocretin deficiency
Narcolepsy with cataplexy but without hypocretin deficiency
Autosomal dominant cerebellar ataxia, deafness, and narcolepsy
Autosomal dominant narcolepsy, obesity, and type 2 diabetes
Narcolepsy secondary to another medical condition
Following ICSD-3, narcolepsy type 1, with cataplexy, is precipitated by strong, generally pleasant emotions, and type 2, which rarely presents cataplexy. Cataplexy is defined as more than one episode of a sudden loss of muscle tone of the antigravitational muscles, with retained consciousness, usually less than two minutes. [
77,
88]. Excessive sleepiness, which is the fundamental sign of narcolepsy [
78], associated with cataplexy is pathognomonic for narcolepsy [
74]. Further classification of narcolepsy uses the following criteria:
Type 1: excessive daytime sleepiness, irrepressible need to sleep or lapses into sleep (for at least three months), low levels of hypocretin (also known as orexin, a neuropeptide with a role in the regulation of arousal and sleep states) [
75,
76,
77], cataplexy and specific paraclinical features [
78,
88]. Note that cataplexy may manifest itself years after the onset of excessive sleepiness. [
89]
Other, less specific signs are weight gain, disturbed sleep, hypnagogic or hypnopompic hallucinations, and sleep paralysis [
74,
78]. Rapid weight gain is associated with higher levels of sleepiness, which is seen in younger patients [
79].
Type 2 (rarely childhood-onset): excessive daytime sleepiness, irrepressible need to sleep or lapses into sleep (for at least three months), sometimes improvement or disappearance of symptoms or change in phenotype to idiopathic hypersomnia, normal hypocretin levels, and specific paraclinical features and rarely cataplexy [
78,
88].
Both types of narcolepsies are most common in patients between the ages of 10 and 30 [
78].
Regarding the orexin level, some studies have questioned the diagnostic importance of the substance, as some patients who have significantly low levels of hypocretin do not present symptoms of cataplexy [
80].
The diagnosis is based on the objective examination, along with laboratory tests [
81]:
Nocturnal Polysomnogram (NPSG), which records electroencephalogram, eye movement, electrocardiogram, and pulse oximetry during sleep [
95].
Multiple Sleep Latency Test (MSLT), which measures the time from the moment lights are out to the first epoch of any stage of sleep [
96]
Additionally, with the short nocturnal rapid eye movement sleep latency (REML), calculated within NPSG, comes a more accurate narcolepsy diagnosis [
82]. We can also test for HLA haplotype and predisposition genes to identify the patients that are at risk of developing narcolepsy, in the context of familial cases.
The sleep logs should be obtained, to calculate the sleep-wake schedule before the laboratory tests. NPSG with a sleep period (lasting for at least 360 minutes) must precede MSLT, within a 1.5-3-hour interval after the NPSG. In the MSLT the patient is given 5 opportunities to sleep at 2-hour intervals during wake times [
73,
88,
99]. If no sleep is detected, the sleep latency is recorded as 20 minutes. Sleep-onset rapid eye movement periods (SOREMPs), measured on MSLT, are very common in narcolepsy but are not exclusively present in this disorder [
97]. The number SOREMPs increases as the sleep latency decreases [
98]. Most patients have more than two SOREMPs, but they are not linked to cataplexy [
97].
The diagnosis of narcolepsy is based on the orexin levels in the cerebrospinal fluid or sleep-onset rapid eye movement periods (SOREMPs), cataplexy and excessive daytime sleepiness for at least three months (
Figure 1). [
73,
88,
93]
The differential diagnosis is made with pathologies such as Niemann-Pick (may give cataplexy), brain trauma and other disorders with hypersomnia. [
83,
84,
85,
88]
9. Comorbidities
Patients with narcolepsy associate psychiatric, cardiovascular, metabolic disorders, respiratory diseases, or sleep disorders, such as sleep apnoea, and insomnia. [
91,
92]. Depressive, bipolar and anxiety disorders can be present alongside narcolepsy. Schizophrenia rarely co-occurs with narcolepsy. Weight gain may also be present [
88,
93].
10. Treatments
In 1935, American neurologist, dr. Prinzmetal and his colleague, dr. Bloomberg, published an article describing their use of the drug benzedrine (amphetamine), a sympathomimetic related to ephedrine and epinephrine, in the treatment of narcolepsy and it was widely used for several years. This was the first drug specifically developed for treatment of excessive daytime sleepiness and sleep attacks. The drug works by increasing the level of dopamine and norepinephrine in the brain, which help to improve wakefulness and reduce the symptoms of narcolepsy, such as excessive daytime sleepiness and sleep attacks. [
100] However, the treatment had many side effects, such as irritability, headache, nervousness, palpitations, insomnia, and less often orofacial dyskinesia, anorexia, nausea, excessive sweating, and psychosis. So, the treatment for narcolepsy was still challenging. Treatment guidelines for narcolepsy were first published in Europe in 2006 [
101] and in the United States in 2007 [
102]. As time passed, newer and more effective medications having fewer side effects were developed.
10.1. Non-pharmacological treatment
The treatment for narcolepsy is divided into two: non-pharmacological and pharmacological [
103]. First, the non-pharmacological treatment for narcolepsy consists of a good sleeping pattern in suitable environment [
104,
106]. The brief naps prescribed by the GP (general practitioner) can improve the productivity during the day [
103]. The GP can decide with short naps that fits the patient’s daily routine [
105]. It is recommended a strict bedtime routine with an emphasis on going to bed and waking up at the same time every day [
105,
107]. The GPs also recommend relaxation before bed: listening to some smooth music or having a warm bath before bedtime [
103]. Another recommendation is avoiding caffeine 3-6 hours before bedtime, as it produces sleep disturbances [
103,
104]. Large meals before bed are also not recommended [
103]. Furthermore, patients should avoid any types of sedatives or alcohol, as they increase sleepiness [
100]. Cardiovascular fitness is recommended for a decrease in daytime sleepiness, frequency of cataplexic episodes [
107] and avoiding obesity because people with narcolepsy tend to be overweight. One study showed that patients with narcolepsy have a lower basal metabolic rate than the average person [
108].
10.2. Pharmacological treatment
10.2.1. Treatment for excessive daytime sleepiness
Secondly, the pharmacological treatment for narcolepsy is prescribed in the more serious forms of this disease. The treatment is strictly symptomatic, addressing excessive daytime sleepiness and cataplexy, but it is not entirely effective and does not cover all the symptoms. For most patients, the treatment should be taken for the entirety of their life [
110]. The first drug used to ameliorate the symptoms of this disease was found in 1935, benzedrine (beta-phenylisopropylamine), which is a sympathomimetic related to ephedrine and epinephrine. It has a more stimulating effect on the central nervous system than adrenaline and could help the patient stay awake during the day [
109,
110].
In current practice, the first-line treatments for extreme daytime sleepiness include modafinil, armodafinil, pitolisant, which are central nervous system stimulants [
105,
107] and sodium oxybate, which is a central nervous system depressant [
119]. The side effects are weight loss, irritability, stomach aches, insomnia, nervousness, nausea, and headaches [
103].
One of the stimulants most often used is modafinil, which selectively activates wake-generating regions in the hypothalamus, thus decreasing the extreme daytime sleepiness [
110,
111]. Modafinil is linked to arrhythmias and increased blood pressure, thus monitoring of the cardiovascular system during the day is needed [
104]. Sodium oxybate can improve the sleep quality and ameliorate the sudden loss of muscular control [
103]. The common side effects for this drug include blurred vision, weight loss, vomiting, bedwetting, diarrhoea, headaches, dizziness, and nausea [
105]. Alternatively, there are second and third-line treatments for patients who do not respond well to the most used drugs [
110]. Methylphenidate is a type of amphetamine used as a stimulant [
100]. In addition, another drug used is solriamfetol, which is a dopamine and norepinephrine reuptake inhibitor, independent of orexin, with comparable effects to modafinil [
100,
117]. Further trials are required to determine if the immune-based therapies could be able to stop the destruction of the orexin neurons [
98].
10.2.2. Treatment for cataplexy
The first-line treatment for cataplexy includes sodium oxybate, venlafaxine and pitolisant. Sodium oxybate activates the gamma-hydroxybutyric acid B-subtype (GABAb) receptor, specifically from the hypothalamus and basal ganglia, but it is thought to have properties beyond this mechanism of action [
99,
112]. Daytime drowsiness is the most common side effect of this drug [
113], alongside bedwetting and sleep walking [
114]. Pitolisant is an inverse agonist of the histamine H3 receptor, with side effects such as headache, insomnia, and anxiety [
115,
116]. Alternatively, there are second-line treatments for patients who do not respond well to the most used drugs [
107]. These include antidepressants such as selective serotonin reuptake inhibitors (SSRIs): fluoxetine and citalopram and the tricyclic antidepressant clomipramine [
101]. They are also used to treat symptoms such asleep paralysis, hallucinations, and loss of muscle control [
103]. The mechanism of action of these drugs is uncertain and it is thought that they work by altering the levels of certain neurochemicals and reducing the amount of REM sleep [
108]. The side effects include sexual dysfunction, insomnia, drowsiness, dizziness, constipation, blurred vision, and anxiety [
109].
10.3. Treatments for special narcoleptic groups
There is insufficient data regarding the safety of antinarcoleptic drugs taken during pregnancy, conceiving or breastfeeding. A survey that questioned experts showed that they recommend discontinuing the medication [
109]. If a woman wants to take modafinil when she is pregnant, it is safe to discuss the medication with the GP [
104].
Treatment for children with narcolepsy is like the one in adults, with adjustment of the dosage. A cardiovascular evaluation is recommended before administering these types of stimulants [
100,
110].
Several studies have reported noteworthy improvements in sleepiness and other symptoms of narcolepsy, indicating the potential effectiveness of orexin gene therapy in mouse models. While more research is needed to optimize this approach, AAV-orexin could potentially become a valuable treatment option for individuals with narcolepsy in the future. [
124,
125]
11. Prevention
There is currently no known way to prevent narcolepsy, as the cause of the disorder is not fully understood. However, maintaining a healthy lifestyle, including getting enough sleep, regular exercise, and managing stress, may help to alleviate some of the symptoms of narcolepsy. Additionally, medications and other treatment options are available to help manage the symptoms and improve the quality of life. In familial narcolepsy, genetic counselling is indicated. By using remote patient monitoring, healthcare providers can work with patients to optimize their treatment and minimize the risk of complications.
11. Prognosis
Overall, the prognosis for children and adults with narcolepsy is generally good (normal life span) with proper and lifelong treatment and management, but it can vary depending on the severity of the disease.
12. Daily life
People with narcolepsy are impacted not only by the diagnosis itself but more importantly, by the symptoms of the disease. Mild symptoms include sleepiness, mental fogginess, and poor memory, but others are more debilitating to one’s life: hallucinations or cataplexy. The most common areas that are impacted are one’s self-esteem, quality of life and social relationships, as it is sometimes hard for the patients to stay awake during social activities or hallucinate during interpersonal interactions. In addition, the state of the patients after waking up may be grumpiness or confusion. An article that interviewed a patient mentioned the state of vulnerability of falling asleep in public [
90,
126]. It is necessary for the family and friends, along with oneself to manage the impacts of narcolepsy, through education (especially about the risk of driving accidents), communication and social flexibility or by finding support [
127]. It is important for people with narcolepsy to work with a sleep specialist to develop an individualised treatment plan that will help them manage their symptoms and improve their quality of life. Because of the nature of the trigger of cataplexy (pleasant emotions), patients may avoid activities they enjoy. [
128] It is significant as well to note that narcolepsy may have an impact on the whole family, not only the affected person. Family members may need to adjust in their daily routine, and they may also have to provide emotional and practical support to the affected person.
13. Limitations
The authors limit included studies to those published in English and focused on the prevalence, causes, symptoms, diagnosis, and treatment of the narcolepsy.
References
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed, American Academy of Sleep Medicine, 2014.
- Orphanet: Narcolepsy (ORPHA:619284). Available online: https://www.orpha.net/consor/cgibin/Disease_Search.php?lng=EN&data_id=10493&disease=Rare-sleep-disorder&search=Disease_Search_Simple (accessed on 4 January 2023).
- Schenck CH, Bassetti CL, Arnulf I, Mignot E. English translations of the first clinical reports on narcolepsy and cataplexy by Westphal and Gélineau in the late 19th century, with commentary. J Clin Sleep Med. 2007, 3, 301–11.
- Mignot, E.J.M. History of narcolepsy at Stanford University. Immunol. Res. 2014, 58, 315–339. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Huang, Y.-S.; Lin, C.-M. Narcolepsy syndrome: a new view at the beginning of the second millennium. 2006; 59. [Google Scholar] [CrossRef]
- Daniels, L.E. , Narcolepsy. Medicine 1934, 13, 1–22. [Google Scholar] [CrossRef]
- Yoss, R.E. and Daly D. , Criteria for the diagnosis of the narcoleptic syndrome, Proceedings of the staff meetings of the Mayo Clinic 1957, 32, 320–328. [Google Scholar] [PubMed]
- Fromherz, S.; Mignot, E. Narcolepsy research: past, present, and future perspectives. Arch. Ital. de Biol. 2004, 142. [Google Scholar]
- Longstreth, W.; Koepsell, T.D.; Ton, T.G.; Hendrickson, A.F.; van Belle, G. The Epidemiology of Narcolepsy. Sleep 2007, 30, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Kornum, B.R.; Knudsen, S.; Ollila, H.M.; Pizza, F.; Jennum, P.J.; Dauvilliers, Y.; Overeem, S. Narcolepsy. Nat. Rev. Dis. Prim. 2017, 3, 16100. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Priest, R.G.; Zulley, J.; Smirne, S.; Paiva, T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology 2002, 58, 1826–1833. [Google Scholar] [CrossRef]
- Heier, M.S.; Evsiukova, T.; Wilson, J.; Abdelnoor, M.; Hublin, C.; Ervik, S. Prevalence of narcolepsy with cataplexy in Norway. Acta Neurol. Scand. 2009, 120, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Hublin, C.; Kaprio, J.; Partinen, M.; Koskenvuo, M.; Heikkila, K.; Koskimies, S.; Guilleminault, C. The prevalence of narcolepsy: An epidemiological study of the Finnish Twin Cohort. Ann. Neurol. 1994, 35, 709–716. [Google Scholar] [CrossRef]
- Doherty, L.; Crowe, C.; Sweeney, B. National narcolepsy survey. Ir. Med J. 2010, 103. [Google Scholar]
- Roth, B. In: Narcolepsy and Hypersomnia. Broughton R, translator. Chapter 10. London: 1980.
- Tió, E.; Gaig, C.; Giner-Soriano, M.; Romero, O.; Jurado, M.-J.; Sansa, G.; Pujol, M.; Sans, O.; Álvarez-Guerrico, I.; Caballol, N.; et al. The prevalence of narcolepsy in Catalunya (Spain). J. Sleep Res. 2017, 27, e12640. [Google Scholar] [CrossRef]
- Lavie P, Peled R. Letter to the Editor: Narcolepsy is a Rare Disease in Israel. Sleep. 1987, 10, 608–609.
- Tashiro T, Kanbayashi T, Iijima S, et al. An epidemiological study of narcolepsy in Japanese (abstract). J Sleep Res. 1992, 1, 228.
- Imanishi, A.; Kamada, Y.; Shibata, K.; Sakata, Y.; Munakata, H.; Ishii, M. Prevalence, incidence, and medications of narcolepsy in Japan: a descriptive observational study using a health insurance claims database. Sleep Biol. Rhythm. 2022, 20, 585–594. [Google Scholar] [CrossRef]
- Park, H.R.; Song, P.; Lee, S.-Y. ; on behalf of Epidemiology Committee of Korean Sleep Research National Estimates of Narcolepsy in Korea. J. Clin. Neurol. 2023, 19, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Wing, Y.-K.; Li, R.H.-Y.; Lam, C.-W.; Ho, C.K.-W.; Fong, S.Y.-Y.; Leung, T. The prevalence of narcolepsy among Chinese in Hong Kong. Ann. Neurol. 2002, 51, 578–584. [Google Scholar] [CrossRef]
- Han, F.; Chen, E.; Wei, H.; Dong, X.; He, Q.; Ding, D.; Strohl, K.P. Childhood Narcolepsy in North China. Sleep 2001, 24, 321–324. [Google Scholar] [CrossRef]
- Solomon, P. Narcolepsy in Negroes. Dis Nerv Sys. 1945, 6, 179–183. [Google Scholar]
- Dement WC, Carskadon M, Ley R. The prevalence of narcolepsy II. Sleep Res. 1973, 2, 147.
- Silber, M.H.; Krahn, L.E.; Olson, E.J.; Pankratz, V.S. The Epidemiology of Narcolepsy in Olmsted County, Minnesota: A Population-Based Study. Sleep 2002, 25, 197–202. [Google Scholar] [CrossRef]
- Scheer, D.; Schwartz, S.W.; Parr, M.; Zgibor, J.; Sanchez-Anguiano, A.; Rajaram, L. Prevalence and incidence of narcolepsy in a US health care claims database, 2008–2010. Sleep 2019, 42. [Google Scholar] [CrossRef]
- Acquavella J, Mehra R, Bron M, Suomi JM-H, Hess GP. Prevalence of narcolepsy, other sleep disorders, and diagnostic tests from 2013–2016: insured patients actively seeking care. J Clin Sleep Med. 2020, 16, 1255–1263.
- Al Rajeh, S.; Bademosi, O.; Ismail, H.; Awada, A.; Dawodu, A.; Al-Freihi, H.; Assuhaimi, S.; Borollosi, M.; Al-Shammasi, S. A Community Survey of Neurological Disorders in Saudi Arabia: The Thugbah Study. Neuroepidemiology 1993, 12, 164–178. [Google Scholar] [CrossRef]
- Partinen M, Saarenpää-Heikkilä O, Ilveskoski I, Hublin C, Linna M, Olsén P, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One. 2012, 7, e33723.
- Heier, M.; Gautvik, K.; Wannag, E.; Bronder, K.; Midtlyng, E.; Kamaleri, Y.; Storsaeter, J. Incidence of narcolepsy in Norwegian children and adolescents after vaccination against H1N1 influenza A. Sleep Med. 2013, 14, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Szakács A, Darin N, Hallböök T. Increased childhood incidence of narcolepsy in Western Sweden after H1N1 influenza vaccination. Neurology. 2013, 80, 1315–21.
- Wijnans, L.; Lecomte, C.; de Vries, C.; Weibel, D.; Sammon, C.; Hviid, A.; Svanström, H.; Mølgaard-Nielsen, D.; Heijbel, H.; Dahlström, L.A.; et al. The incidence of narcolepsy in Europe: Before, during, and after the influenza A(H1N1)pdm09 pandemic and vaccination campaigns. Vaccine 2013, 31, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Lin, L.; Warby, S.C.; Faraco, J.; Li, J.; Dong, S.X.; An, P.; Zhao, L.; Wang, L.H.; Li, Q.Y.; et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in china. Ann. Neurol. 2011, 70, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhuang, J.; Stone, W.S.; Zhang, L.; Zhao, Z.; Wang, Z.; Yang, Y.; Li, X.; Zhao, X.; Zhao, Z. Symptoms and occurrences of narcolepsy: a retrospective study of 162 patients during a 10-year period in Eastern China. Sleep Med. 2014, 15, 607–613. [Google Scholar] [CrossRef]
- Choe, Y.J.; Bae, G.-R.; Lee, D.-H. No association between influenza A(H1N1)pdm09 vaccination and narcolepsy in South Korea: An ecological study. Vaccine 2012, 30, 7439–7442. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.; Weintraub, E.; Vellozzi, C.; DeStefano, F.; Datalink, O.B.O.T.V.S. Narcolepsy and influenza A(H1N1) pandemic 2009 vaccination in the United States. Neurology 2014, 83, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Weibel, D.; Sturkenboom, M.; Black, S.; de Ridder, M.; Dodd, C.; Bonhoeffer, J.; Vanrolleghem, A.; van der Maas, N.; Lammers, G.J.; Overeem, S.; et al. Narcolepsy and adjuvanted pandemic influenza A (H1N1) 2009 vaccines – Multi-country assessment. Vaccine 2018, 36, 6202–6211. [Google Scholar] [CrossRef] [PubMed]
- Spruyt, K. Narcolepsy Presentation in Diverse Populations: an Update. Curr. Sleep Med. Rep. 2020, 6, 239–250. [Google Scholar] [CrossRef]
- Avidan, AY. Sleep and its Disorders Chapter 101 in Bradley and Daroff’s Neurology in Clinical Practice.2022 Eight Edition, Elsevier., 1664 -1744.
- Dauvilliers, Y.; Montplaisir, J.; Molinari, N.; Carlander, B.; Ondze, B.; Besset, A.; Billiard, M. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001, 57, 2029–2033. [Google Scholar] [CrossRef]
- Chemelli, R.M.; Willie, J.T.; Sinton, C.M.; Elmquist, J.K.; Scammell, T.; Lee, C.; Richardson, J.A.; Williams, S.; Xiong, Y.; Kisanuki, Y.; et al. Narcolepsy in orexin Knockout Mice: Molecular Genetics of Sleep Regulation. Cell 1999, 98, 437–451. [Google Scholar] [CrossRef]
- Behn, C.G.D.; Klerman, E.B.; Mochizuki, T.; Shih-Chieh, L.; Scammell, T.E. Abnormal Sleep/Wake Dynamics in Orexin Knockout Mice. Sleep 2010, 33, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Mignot, E.; Lammers, G.J.; Ripley, B.; Okun, M.; Nevsimalova, S.; Overeem, S.; Vankova, J.; Black, J.; Harsh, J.; Bassetti, C.; et al. The Role of Cerebrospinal Fluid Hypocretin Measurement in the Diagnosis of Narcolepsy and Other Hypersomnias. Arch. Neurol. 2002, 59, 1553–1562. [Google Scholar] [CrossRef]
- Thannickal, T, Moore C, Nienhuis RY, Ramanathan R, Gulyani L, Aldrich S, M et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron, 2000, 27, 469–474.
- Peyron, C.; Faraco, J.; Rogers, W.; Ripley, B.; Overeem, S.; Charnay, Y.; Nevsimalova, S.; Aldrich, M.; Reynolds, D.; Albin, R.; et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 2000, 6, 991–997. [Google Scholar] [CrossRef]
- Valko, P.O.; Gavrilov, Y.V.; Yamamoto, M.; Reddy, H.; Haybaeck, J.; Mignot, E.; Baumann, C.R.; Scammell, T.E. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann. Neurol. 2013, 74, 794–804. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Thannickal, T.C.; McGregor, R.; Ramanathan, L.; Ohtsu, H.; Nishino, S.; Sakai, N.; Yamanaka, A.; Stone, C.; Cornford, M.; et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann. Neurol. 2013, 74, 786–793. [Google Scholar] [CrossRef]
- Narcolepsy 1: Available online: https://www.omim.org/entry/161400?
- Mignot, E. Genetic and familial aspects of narcolepsy. Neurology 1998, 50, S16–S22. [Google Scholar] [CrossRef]
- Winkelmann, J.; Lin, L.; Schormair, B.; Kornum, B.R.; Faraco, J.; Plazzi, G.; Melberg, A.; Cornelio, F.; Urban, A.E.; Pizza, F.; et al. Mutations in DNMT1 cause autosomal dominant cerebellar ataxia, deafness and narcolepsy. Hum. Mol. Genet. 2012, 21, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, T.; Tokunaga, K. Genetics of narcolepsy. Hum. Genome Var. 2019, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Tamiya, G.; Oka, A.; Hohjoh, H.; Juji, T.; Ebisawa, T.; Honda, Y.; Inoko, H.; Tokunaga, K. Genomewide Association Analysis of Human Narcolepsy and a New Resistance Gene. Am. J. Hum. Genet. 2006, 79, 252–263. [Google Scholar] [CrossRef]
- Lin L, Hungs M, Mignot E. Narcolepsy and the HLA region. Journal of neuroimmunology 2001, 117, 9–20.
- Hor, H.; Kutalik, Z.; Dauvilliers, Y.; Valsesia, A.; Lammers, G.J.; Donjacour, C.E.H.M.; Iranzo, A.; Santamaria, J.; Adrados, R.P.; Vicario, J.L.; et al. Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nat. Genet. 2010, 42, 786–789. [Google Scholar] [CrossRef]
- Mignot, E.; Lin, L.; Rogers, W.; Honda, Y.; Qiu, X.; Lin, X.; Okun, M.; Hohjoh, H.; Miki, T.; Hsu, S.H.; et al. Complex HLA-DR and -DQ Interactions Confer Risk of Narcolepsy-Cataplexy in Three Ethnic Groups. Am. J. Hum. Genet. 2001, 68, 686–699. [Google Scholar] [CrossRef]
- Yong, E. Narcolepsy confirmed as autoimmune disease. Nature 2013. [Google Scholar] [CrossRef]
- Martinez-Orozco, F.J.; Vicario, J.L.; De Andres, C.; Fernandez-Arquero, M.; Peraita-Adrados, R. Comorbidity of Narcolepsy Type 1 With Autoimmune Diseases and Other Immunopathological Disorders: A Case-Control Study. J. Clin. Med. Res. 2016, 8, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kornum, B.R.; Kawashima, M.; Faraco, J.; Lin, L.; Rico, T.J.; Hesselson, S.; Axtell, R.C.; Kuipers, H.; Weiner, K.; Hamacher, A.; et al. Common variants in P2RY11 are associated with narcolepsy. Nat. Genet. 2010, 43, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hallmayer, J. , Faraco, J. , Lin, L., Hesselson, S., Winkelmann, J., Kawashima, M., Mayer, G., Plazzi, G., Nevsimalova, S., Bourgin, P., Hong, S.-C., Honda, Y., and 36 others. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nature Genet. 2009, 41, 708–711. [Google Scholar]
- Latorre, D.; Kallweit, U.; Armentani, E.; Foglierini, M.; Mele, F.; Cassotta, A.; Jovic, S.; Jarrossay, D.; Mathis, J.; Zellini, F.; et al. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature 2018, 562, 63–68. [Google Scholar] [CrossRef]
- Pedersen, N.W.; Holm, A.; Kristensen, N.P.; Bjerregaard, A.-M.; Bentzen, A.K.; Marquard, A.M.; Tamhane, T.; Burgdorf, K.S.; Ullum, H.; Jennum, P.; et al. CD8+ T cells from patients with narcolepsy and healthy controls recognize hypocretin neuron-specific antigens. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- WebMD - Narcolepsy, vs. Insomnia: What's the Difference? Available online:. Available online: https://www.webmd.com/sleep-disorders/narcolepsy-insomnia-difference (accessed on April 26, 2023).
- Roberts, HJ. The syndrome of narcolepsy and diabetogenic (“functional”) hyperinsulinism, with special reference to obesity, diabetes, idiopathic oedema, cerebral dysrhythmias, and multiple sclerosis (200 patients). J Am Geriatr Soc (1964) 12:926–976.
- Dahmen, N.; Bierbrauer, J.; Kasten, M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur. Arch. Psychiatry Clin. Neurosci. 2001, 251, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Straat, M.E.; Schinkelshoek, M.S.; Fronczek, R.; Lammers, G.J.; Rensen, P.C.N.; Boon, M.R. Role of Brown Adipose Tissue in Adiposity Associated With Narcolepsy Type 1. Front. Endocrinol. 2020, 11, 145. [Google Scholar] [CrossRef]
- Billiard M, Orellana C. Chapter 40 Environmental Factors in Narcolepsy in Narcolepsy and Hypersomnia edited by Bassetti C, Billiard M, Mignot E, 1st Edition, CRC Press, 2007.
- Viola-Saltzman, M.; Musleh, C. Traumatic brain injury-induced sleep disorders. Neuropsychiatr. Dis. Treat. 2016, ume 12, 339–348. [Google Scholar] [CrossRef]
- Thannickal, T.C.; Nienhuis, R.; Siegel, J.M. Localized Loss of Hypocretin (Orexin) Cells in Narcolepsy Without Cataplexy. Sleep 2009, 32, 993–998. [Google Scholar] [CrossRef]
- Baumann-Vogel, H.; Schreckenbauer, L.; Valko, P.O.; Werth, E.; Baumann, C.R. Narcolepsy type 2: A rare, yet existing entity. J. Sleep Res. 2020, 30, e13203. [Google Scholar] [CrossRef]
- The National Health Service: Narcolepsy. Available online: https://www.nhs.uk/conditions/narcolepsy/symptoms/ (accessed on 10 January 2023).
- Morse AM, Narcolepsy in Children and Adults: A Guide to Improved Recognition, Diagnosis and Management. Available online: https://www.mendeley.com/catalogue/2a501988-2588-338f-83be-50c64f067366/ (accessed on 10 January 2023).
- Wang, G.; Benmedjahed, K.; Lambert, J.; Evans, C.J.; Hwang, S.; Black, J.; Johns, M.W. Assessing narcolepsy with cataplexy in children and adolescents: development of a cataplexy diary and the ESS-CHAD. Nat. Sci. Sleep 2017, ume 9, 201–211. [Google Scholar] [CrossRef]
- Sateia, M. J. International Classification of Sleep Disorders-Third Edition. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Babiker, M.O.; Prasad, M. Narcolepsy in Children: A Diagnostic and Management Approach. Pediatr. Neurol. 2015, 52, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-B.; de Lecea, L. The hypocretin (orexin) system: from a neural circuitry perspective. Neuropharmacology 2020, 167, 107993. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, I.; Howard, R.S.; Kopelman, M.D.; Sharief, M.K.; Williams, A.J. The Hypocretin/Orexin System. J. R. Soc. Med. 2002, 95, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Nishino, S.; Sakurai, E.; Nevsimalova, S.; Yoshida, Y.; Watanabe, T.; Yanai, K.; Mignot, E. Decreased CSF Histamine in Narcolepsy With and Without Low CSF Hypocretin-1 in Comparison to Healthy Controls. Sleep 2009, 32, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Orphanet: Narcolepsy type 1 (ORPHA:2073). Available online: https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=EN&data_id=3637&Disease_Disease_Search_diseaseGroup=narcolepsy&Disease_Disease_Search_diseaseType=Pat&Disease (accessed on 14 January 2023).
- Zhang, M.; Thieux, M.; Inocente, C.O.; Vieux, N.; Arvis, L.; Villanueva, C.; Lin, J.; Plancoulaine, S.; Guyon, A.; Franco, P. Characterization of rapid weight gain phenotype in children with narcolepsy. CNS Neurosci. Ther. 2022, 28, 829–841. [Google Scholar] [CrossRef]
- Andlauer, O.; Moore, H.; Hong, S.-C.; Dauvilliers, Y.; Kanbayashi, T.; Nishino, S.; Han, F.; Silber, M.H.; Rico, T.; Einen, M.; et al. Predictors of Hypocretin (Orexin) Deficiency in Narcolepsy Without Cataplexy. Sleep 2012, 35, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Narcolepsy fact sheet. Available online: https://www.ninds.nih.gov/health-information/patient-caregiver-education/fact-sheets/narcolepsy-fact-sheet#3201_6, (accessed on 14 January 2023).
- Andlauer, O.; Moore, H.; Jouhier, L.; Drake, C.; Peppard, P.E.; Han, F.; Hong, S.-C.; Poli, F.; Plazzi, G.; O’hara, R.; et al. Nocturnal Rapid Eye Movement Sleep Latency for Identifying Patients With Narcolepsy/Hypocretin Deficiency. JAMA Neurol. 2013, 70, 891–902. [Google Scholar] [CrossRef]
- Maeda M, Tamaoka A, Hayashi A, Mizusawa H, Shoji S. [A case of HLA-DR2, DQw1 negative post-traumatic narcolepsy] Rinsho Shinkeigaku = Clinical Neurology. 1995, 35, 811–813. [PubMed]
- Smit, L.S.; Lammers, G.J.; Catsman-Berrevoets, C.E. Cataplexy Leading to the Diagnosis of Niemann-Pick Disease Type C. Pediatr. Neurol. 2006, 35, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, I.O.; Peacock, K.W.; Williams, A.J. Posttraumatic Narcolepsy — Two Case Reports and a Mini Review. Sleep Med. 2005, 01, 153–156. [Google Scholar] [CrossRef]
- Available online:. Available online: https://healthysleep.med.harvard.edu/narcolepsy/living-with-narcolepsy/dailylife (accessed on 14 January 2023).
- Available online:. Available online: https://emedicine.medscape.com/article/1188433-overview#a6 (accessed on 16 January 2023).
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. A: Darien, IL, 2014.
- Ruoff, C.; Rye, D. The ICSD-3 and DSM-5 guidelines for diagnosing narcolepsy: clinical relevance and practicality. Curr. Med Res. Opin. 2016, 32, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- My life with narcolepsy – Available online:. Available online: https://sleepeducation.org/life-with-narcolepsy/ (accessed on 16 January 2023).
- Krahn, L.E.; Zee, P.C.; Thorpy, M.J. Current Understanding of Narcolepsy 1 and its Comorbidities: What Clinicians Need to Know. Adv. Ther. 2021, 39, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-H.; Chin, W.-C.; Huang, Y.-S.; Wang, C.-H. Pediatric Narcolepsy—A Practical Review. Children 2022, 9, 974. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Sleep-wake disorders. In: Diagnostic and Statistical Manual of Mental Disorders, 5th edn. Washington, DC: American Psychiatric Association; 2013. p. 361–422.
- Ahmed, I.; Thorpy, M. Clinical Features, Diagnosis and Treatment of Narcolepsy. Clin. Chest Med. 2010, 31, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Rundo, J.V.; Downey, R. Polysomnography. 160. [CrossRef]
- Berry, R.B. Subjective and Objective Measures of Daytime Sleepiness. 2011. [Google Scholar] [CrossRef]
- Arand D, Bonnet M, Hurwitz T, et al: A review by the MSLT and MWT Task Force of the Standards of Practice Committee of the AASM. The clinical use of the MSLT and MWT. Sleep 2005;28:123–144.
- Amira, S.A.; Johnson, T.S.; Logowitz, N.B. Diagnosis of Narcolepsy Using the Multiple Sleep Latency Test: Analysis of Current Laboratory Criteria. Sleep 1985, 8, 325–331. [Google Scholar] [CrossRef]
- Berry, R.B. Polysomnography, Portable Monitoring, and Actigraphy. 2011. [Google Scholar] [CrossRef]
- Prinzmetal, M.; Bloomberg, W. THE USE OF BENZEDRINE FOR THE TREATMENT OF NARCOLEPSY. J. Am. Med Assoc. 1935, 105, 2051. [Google Scholar] [CrossRef]
- Billiard, M.; Bassetti, C.; Dauvilliers, Y.; Dolenc-Groselj, L.; Lammers, G.J.; Mayer, G.; Pollmächer, T.; Reading, P.; Sonka, K. EFNS guidelines on management of narcolepsy. Eur. J. Neurol. 2006, 13, 1035–1048. [Google Scholar] [CrossRef]
- Morgenthaler TI, Kapur VK, Brown TM, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007, 30, 1705–1711.
- https://www.nhs.
- Golden, E.C.; Lipford, M.C. Narcolepsy: Diagnosis and management. Clevel. Clin. J. Med. 2018, 85, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.A.; Adamantidis, A.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Lammers, G.J.; et al. Narcolepsy — clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef]
- Bassetti, C.L.A.; Kallweit, U.; Vignatelli, L.; Plazzi, G.; Lecendreux, M.; Baldin, E.; Dolenc-Groselj, L.; Jennum, P.; Khatami, R.; Manconi, M.; et al. European guideline and expert statements on the management of narcolepsy in adults and children. J. Sleep Res. 2021, 30, e13387. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, C.; Pizza, F.; Antelmi, E.; Folli, M.C.; Plazzi, G. Narcolepsy treatment: pharmacological and behavioral strategies in adults and children. Sleep Breath. 2019, 24, 615–627. [Google Scholar] [CrossRef]
- Mignot, E.J.M. A Practical Guide to the Therapy of Narcolepsy and Hypersomnia Syndromes. Neurotherapeutics 2012, 9, 739–752. [Google Scholar] [CrossRef]
- Barateau, L.; Dauvilliers, Y. Recent advances in treatment for narcolepsy. Ther. Adv. Neurol. Disord. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Barateau, L.; Lopez, R.; Dauvilliers, Y. Treatment Options for Narcolepsy. CNS Drugs 2016, 30, 369–379. [Google Scholar] [CrossRef]
- Drake, C.; Roehrs, T.; Shambroom, J.; Roth, T. Caffeine Effects on Sleep Taken 0, 3, or 6 Hours before Going to Bed. Sleep Med. 2013, 9, 1195–1200. [Google Scholar] [CrossRef]
- https://www.nhs.uk/conditions/narcolepsy/treatment/#:~:text=Stimulants,taken%20as%20tablets%20every%20morning. 106.https://www.mayoclinic.org/diseases-conditions/narcolepsy/diagnosis-treatment/drc-20375503#:~:text=Stimulants.,habit%2Dforming%20as%20older%20stimulants.
- Matoulek, M.; Tuka, V.; Fialová, M.; Nevšímalová, S.; Šonka, K. Cardiovascular fitness in narcolepsy is inversely related to sleepiness and the number of cataplexy episodes. Sleep Med. 2017, 34, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Chabas, D.; Foulon, C.; Gonzalez, J.; Nasr, M.; Lyon-Caen, O.; Willer, J.-C.; Derenne, J.-P.; Arnulf, I. Eating Disorder and Metabolism in Narcoleptic Patients. Sleep 2007, 30, 1267–1273. [Google Scholar] [CrossRef]
- Thorpy, M.; Zhao, C.G.; Dauvilliers, Y. Management of narcolepsy during pregnancy. Sleep Med. 2013, 14, 367–376. [Google Scholar] [CrossRef]
- Scammell, T.E.; Estabrooke, I.V.; McCarthy, M.T.; Chemelli, R.M.; Yanagisawa, M.; Miller, M.S.; Saper, C.B. Hypothalamic Arousal Regions Are Activated during Modafinil-Induced Wakefulness. J. Neurosci. 2000, 20, 8620–8628. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, M.; Escriu, J.M.; Stokan, O. The effects of gamma-hydroxybutyrate on sleep. Biol. Psychiatry 1977, 12. [Google Scholar]
- Mamelak, M.; Scharf, M.B.; Woods, M. Treatment of Narcolepsy with γ-Hydroxybutyrate. A Review of Clinical and Sleep Laboratory Findings. Sleep 1986, 9, 285–289. [Google Scholar] [CrossRef]
- https://www.mayoclinic. 2006.
- Syed, Y.Y. Pitolisant: First Global Approval. Drugs 2016, 76, 1313–1318. [Google Scholar] [CrossRef]
- Lamb, Y.N. Pitolisant: A Review in Narcolepsy with or without Cataplexy. CNS Drugs 2020, 34, 207–218. [Google Scholar] [CrossRef] [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Solriamfetol. [Updated 2021 Aug 18]. Available online: https://www.ncbi.nlm.nih. 5737.
- Dominguez A, Soca Gallego L, Parmar M. Sodium Oxybate. [Updated 2023 Jan 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available online: https://www.ncbi.nlm.nih. 5622.
- Kantor, S.; Mochizuki, T.; Lops, S.N.; Ko, B.; Clain, E.; Clark, E.; Yamamoto, M.; Scammell, T.E. Orexin Gene Therapy Restores the Timing and Maintenance of Wakefulness in Narcoleptic Mice. Sleep 2013, 36, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Blanco-Centurion, C.; Konadhode, R.; Begum, S.; Pelluru, D.; Gerashchenko, D.; Sakurai, T.; Yanagisawa, M.; Pol, A.N.v.D.; Shiromani, P.J. Orexin Gene Transfer into Zona Incerta Neurons Suppresses Muscle Paralysis in Narcoleptic Mice. J. Neurosci. 2011, 31, 6028–6040. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, C.; Fante, C.; Filardi, M.; Folli, M.C.; Brazzi, F.; Pizza, F.; D’anselmo, A.; Ingravallo, F.; Antelmi, E.; Plazzi, G. Can a Peer Support the Process of Self-Management in Narcolepsy? A Qualitative Narrative Analysis of a Narcoleptic Patient. Front. Psychol. 2020, 11, 1353. [Google Scholar] [CrossRef]
- Daily life – Available online:. Available online: https://sleep.hms.harvard.edu/education-training/public-education/sleep-and-health-education-program/sleep-health-education-5 (accessed on 26 January 2023).
- Schiappa, C.; Scarpelli, S.; D’atri, A.; Gorgoni, M.; De Gennaro, L. Narcolepsy and emotional experience: a review of the literature. Behav. Brain Funct. 2018, 14, 1–11. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).