1. Introduction
Since its discovery, antibiotics have become essential in successfully combating infectious diseases to improve human health [
1,
2]. In dentistry, antibiotics are selectively used for orofacial infections of odontogenic and non-odontogenic origins such as dental abscess, pulpal necrosis, periodontal diseases, dental caries, dental trauma, adenoiditis, otomastoiditis, and in prophylaxis [
2,
3,
4,
5]. Penicillin beta-lactam, Amoxicillin along with Clindamycin, are the most widely prescribed oral antibiotic agents in dentistry, prophylactically and for therapeutic use, for their broad-spectrum antimicrobial activity of the former and as an alternate to penicillin allergic responses for the latter [
1,
6,
7]. Along with its health benefits, however, the overprescription of antibiotics is a concern for clinicians and for patients. An analysis of outpatient prescription data from British Columbia showed the dental antibiotic prescription rate increasing by 71.6 % between the years 1996 to 2013 [
6]. Similar UK and US studies also found that over 65% of antibiotic prescriptions were issued when there was no evidence of spreading infection, and 73% to 85% of antibiotic prescriptions of penicillin beta-lactam and lincosamides for adults and children outpatient population were either unnecessary or untimely. [
8,
9,
10,
11].
Microorganisms, such as actinomyces, streptococci, and enterococci faecalis (
E. faecalis), that produce dental biofilms, complex communities of microorganisms composed of bacteria and fungi that adhere to surfaces forming a protective matrix of extracellular substances, often diminish the effectiveness of antibiotics, increase their resistance, and contribute to the development of endodontic and periodontal diseases ultimately causing failures of their therapies [
12,
13]. In teeth that are treated with root canal therapy, 77% to 90% of recurring infections and subsequent treatment failures are linked to
E. faecalis [
14,
15,
16]. Furthermore,
E. faecalis is commonly recovered in teeth that were treated in multiple visits [
12,
17], likely due to its ability to form biofilms, persist in saliva, survive in nutrient-free environments, resist many antibiotics, and remain dormant as a facultative anaerobe [
18,
19,
20]. In addition to root canal infections, enterococcal bacteria are also associated with endocarditis, bacteremia, urinary tract infections, intra-abdominal infections, and prostatitis [
21,
22]. Increased natural defense mechanisms acquired by the microorganisms, along with overprescription and increased prophylactic use of antibiotics, have elevated global concern for the emergence of antibiotic-resistant microorganisms and increased incidences of antibiotics-related secondary health issues such as dysbiosis, clostridium difficile infection, resistant urinary tract infection, and methicillin-resistant Staphylococcus aureus infections [
6,
23,
24,
25,
26].
Essential oils (EOs) and their anti-inflammatory, antifungal, antimicrobial, and antibacterial properties are well elucidated [
27,
28,
29,
30,
31]. Overall, EOs display increased sensitivity to Gram-positive bacteria, and their antimicrobial responses are mediated in part by ATP and inhibition of ATPases, disruption of membrane permeability, and inhibition of biofilm synthesis on the microbes [
32,
33,
34,
35]. In vitro studies report significant antibacterial effects of thyme, clove, sage, peppermint, lavender, cinnamon EOs and their chemical components of thymol, eugenol, thujone, menthol, linalool and cinnamaldehyde, on caries-causing bacteria such as streptococci and lactobacilli spp. [
36,
37]. Among the phenylpropanoid EOs, thymol, eugenol, menthol, and cinnamaldehyde report potent antimicrobial properties in MIC (minimum inhibitory concentration), MBC (minimum bactericidal concentration), disk diffusion, and mouth rinse tests [
38,
39,
40,
41]. Moreover, combination of cinnamon, lavender, peppermint, oregano, and thyme EOs with antibiotics (β-lactam, penicillin, cephalosporin, and aminoglycoside) report enhanced and synergistic antimicrobial effects compared to when tested individually [
42,
43,
44,
45,
46]. The enhanced antimicrobial benefits of combining antibiotics with EOs could further be examined as a novel strategy to lower the concentrations and use of antibiotics to mitigate the proliferation of antibiotic resistant bacteria.
In this study, we assessed the synergistic effect of cinnamon and clove essential oils when combined with penicillin and aminoglycoside antibiotics in inhibiting the growth of Enterococcus faecalis in disk diffusion test.
2. Materials and Methods
Essential Oils and Antibiotics
Cinnamon (Cinnamomum Zeylanicum; bark) and clove (Eugenia Caryophyllus) essential oils (EOs) were purchased from Now Pure Essential Oils (Bloomingdale, IL). According to the GCMS data by the manufacturer, the main chemical component of the cinnamon and clove EOs was trans-cinnamaldehyde and eugenol, respectively. The EOs were diluted in DMSO (20% v/v) to make a stock concentration of 10% for the cinnamon (CM) and 50% for the clove (CL) EOs. For the experiments, the CM oil was further diluted and tested at 5%, 2.5%, and 1.25%, and the CL oil was tested at 50% and 25% concentration.
The antibiotics Gentamicin (20 mg/ml), Streptomycin (50 mg/ml), Ampicillin (100mg/mL), and Kanamycin (50 mg/mL) were purchased from Fisher Scientific (Waltham, MA). The antibiotics were diluted in distilled water and tested at the following concentrations: gentamicin at 1.5 mg/ml, streptomycin at 2.5 mg/ml, ampicillin at 5 mg/ml, and kanamycin at 2.5 mg/ml.
Bacterial Strain and Culture Conditions
E. faecalis ATCC 29212 and Bile Esculin Azide Agar (BEA) were purchased from Fisher Scientific (Waltham, MA). The E. faecalis was grown on BEA plates under aerobic condition (5% CO2, 37℃). For the experiments, the E. faecalis-inoculated BEA plates were incubated for 24hr after the treatment condition and the size of zone of inhibitions was quantitated.
Disk Diffusion Test and the of Zones of Inhibition
The antibacterial effects of EOs and the antibiotics were quantitated using the disk diffusion test. Briefly, sterile diffusion disks were placed in a test tube containing the treatment condition, vortexed for 30 sec, and placed on non-overlapping regions of BEA agar plates that were inoculated with E. faecalis. The plates were incubated at 37℃ for 24hr, and the zone of inhibition (ZOI) was measured from the smallest clearing region around the disk using a ruler. The ZOI was measured to the closest 1mm scale (
Figure 1).
Antibacterial effects of cinnamon and clove EOs
The baseline antibacterial effects of cinnamon (CM5%, CM2.5%, CM1.25%) and clove (CL50%, CL25%) EOs on E. faecalis were tested using the disk diffusion test as described above (n=6/group). The size of ZOI was measured after 24hr incubation period.
Effects of Antibiotics on E. faecalis
The baseline antibacterial effects of gentamicin (1.5mg/ml), streptomycin (2.5mg/ml), ampicillin (5mg/ml), and kanamycin (2.5mg/ml) were tested using the disk diffusion test as described above (n=4/antibiotic). The size of ZOI was measured after 24hr incubation period.
Synergistic antibacterial effects of antibiotics combined with EOs
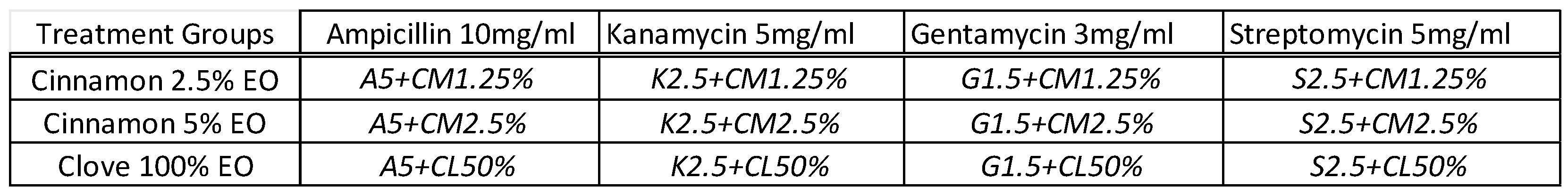
Next, the synergistic antibacterial effects of antibiotics when combined with the EOs were examined. Briefly, the stock concentrations of cinnamon and clove EOs were combined and mixed with each of the four antibiotics to generate twelve different treatment groups (
Table 1). For example, ampicillin 10mg/ml solution was mixed with an equal volume of cinnamon 2.5% solution to make a A5+CM1.25% solution. For this experiment, the final concentrations tested for CM oils were at 2.5% and 1.25%, and for CL oil was at 50%.
The test tubes were vortexed to thoroughly mix the solutions, and then diffusion disks (n=8/condition) were immersed into the mixture for 30 sec. The disks were placed on the BEA plates inoculated with E. faecalis, incubated at 37℃ for 24hr and the zones of inhibition were measured. The diffusion disks immersed in DMSO (20% v/v) were used as control.
Statistical Analysis
Statistical analyses were performed using a One-way ANOVA followed by a Tukey’s HSD post hoc test (GraphPad, La Jolla, CA). All results were considered statistically significant at p<0.05.
3. Results
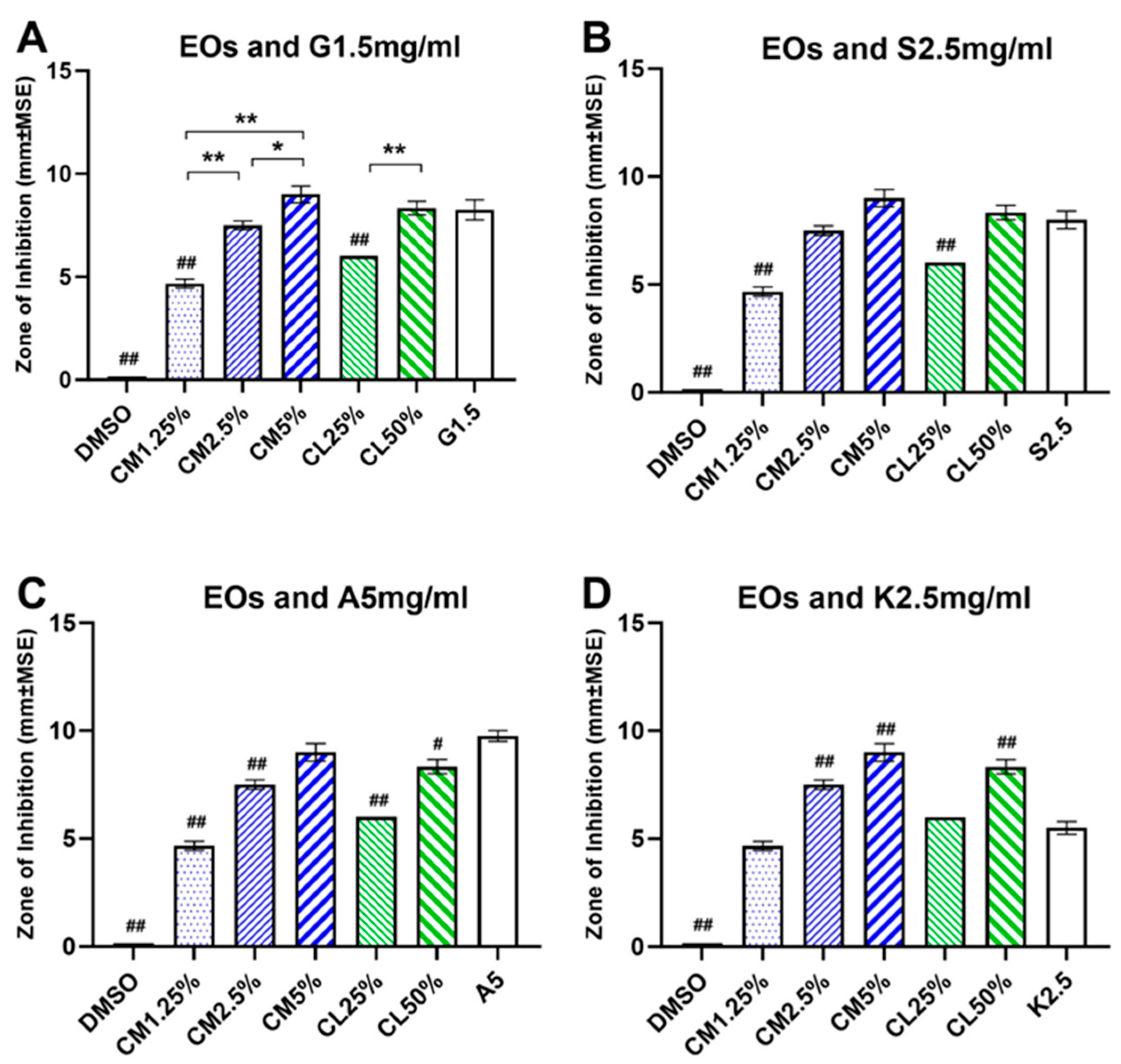
3.1. Antibacterial effects of cinnamon and clove EOs
The antibacterial efficacies of EOs on
E. faecalis were examined at 5% to 1.25% for CM oil and at 50% to 25% for CL oil in disk diffusion test. The cinnamon and clove EOs showed a concentration-dependent growth inhibition of
E. faecalis at all tested concentrations compared to control (F (5, 22) = 202.3, p<0.0001,
Figure 2A). At high concentrations, the CM5% (9.00±0.40 mm) and the CL50% (8.34±0.33 mm) were most effective in inhibiting the growth of
E. faecalis and produced the largest ZOI. At intermediate concentrations, the CM2.5% (7.50±0.22 mm) and CL25% (6.00±0.00 mm) showed medium inhibitory growth responses, while at low concentration, CM1.25% (4.67±0.21 mm) showed the least inhibitory response against the growth of
E. faecalis. The diffusion disks immersed in control DMEM solution did not produce any inhibitory responses. The Tukey post-hoc test showed significant paired differences between the cinnamon EOs where the CM5% had significantly larger ZOI than the CM2.5% and CM1.25%, and the ZOI of CM2.5% was significantly larger than CM1.25% (
Figure 2A; CM5% vs. CM2.5%, p<0.001; CM1,25% vs. CM2.5% or CM5%, p<0.0001). Similar statistical response was observed between the clove EOs where the CL50% had significantly larger ZOI than the CL25% (
Figure 2A; CL50% vs. CL25%, p<0.0001).
3.2. Antibacterial effects of antibiotics
The effectiveness of antibiotics on inhibiting the growth of
E. faecalis were evaluated. The baseline ZOI for gentamicin (1.5 mg/ml), streptomycin (2.5 mg/ml), ampicillin (5mg/ml) and kanamycin (2.5mg/ml) were 8.25+0.47mm, 8.00+0.40mm, 9.75±0.25mm and 5.50±0.28mm, respectively (
Figure 2). Ampicillin 5mg/ml was most effective against
E. faecalis, while gentamicin 1.5mg/ml and streptomycin 2.5mg/ml showed intermediate inhibitory growth responses against
E. faecalis. Kanamycin 2.5mg/ml had the least antibacterial effect among the antibiotics tested (F (3, 12) = 22.92, p<0.0001).
3.3. Comparison between Antibiotics and EOs on inhibiting E. faecalis
All antibiotics, except for kanamycin, were more effective than the low concentrations of EOs in inhibiting the growth of
E. faecalis (F (9, 34) = 107.3, p<0.0001). However, at intermediate and high concentrations, the EOs were as effective or almost as effective as the antibiotics (
Figure 2). Gentamicin 1.5mg/ml (F (6, 25) = 139.8, p<0.0001) and streptomycin 2.5mg/ml (F (6, 25) = 151.3, p<0.0001) had significantly larger ZOI than CM1.25% and CL25% but were statistically indifferent to CM2.5%, CM5% and CL50% in inhibiting
E. faecalis (
Figure 2A, B). Ampicillin 5mg/ml had significantly larger ZOI than CM1.25%, CM2.5%, CL25% and CL50% (F (6, 25) = 208.0, p<0.0001), but was not statistically different from CM5% (
Figure 2C). For kanamycin 2.5mg/ml, the medium and high concentrations of EOs were more effective than kanamycin. The ZOI of kanamycin was similar to CM1.25% and CL25%, but the ZOI of CM2.5%, CM5% and CL50% were significantly larger than kanamycin (F (6, 25) = 162.2, p<0.0001,
Figure 2D).
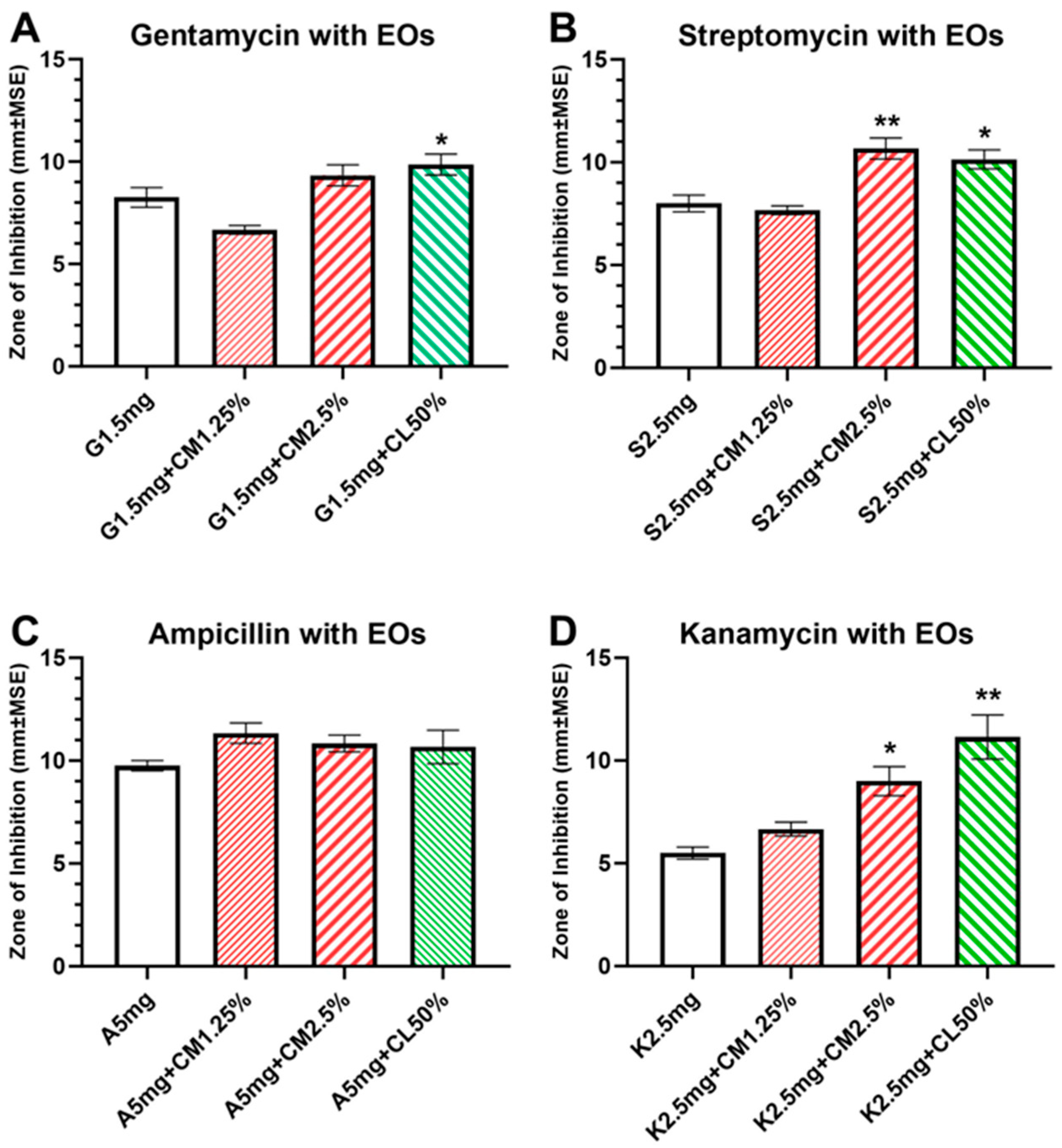
3.4. Combined Antibacterial effects of antibiotics with EOs
There were enhanced and synergistic antibacterial effects when antibiotics were combined with EOs, and the combined solution significantly increased the ZOI against
E. faecalis compared to antibiotics alone (
Figure 3). These synergistic effects were observed mainly with antibiotics combined with high concentrations of EOs (CM2.5% and CL50%). Such enhanced combinational effects were not observed with low concentrations of EOs.
For gentamicin 1.5mg/ml combined with EOs, there was a significant difference among treatment groups (F (3, 19) = 14.48, p<0.0001;
Figure 3A) and a significant increase in ZOI of G1.5+CL50% group (9.85±0.50mm, p=0.0004) compared to gentamicin alone. For streptomycin 2.5mg/ml combined with EOs, there was a significant difference among treatment groups (F (3, 19) = 18.22, p<0.0001;
Figure 3B), and significant increases in ZOI were observed in S2.5+CM2.5% (10.67±0.21, p=0.0005) and S2.5+CL50% (10.14±0.45mm, p=0.0035) groups when compared to streptomycin alone. For kanamycin 2.5mg/ml combined with EOs, there was a significant difference among treatment groups (F (3, 18) = 11.54, p=0.0002;
Figure 3D), and significant increases in ZOI were observed in K2.5+CM2.5% (9.00±0.31, p=0.03) and K2.5+CL50% (11.14±1.07mm, p=0.0004) groups compared to kanamycin alone. For ampicillin 5mg/ml, there was no statistical significance among treatment groups when combined with EOs (F (3, 18) = 2.994, p=0.0581). There was a noticeable increase in the ZOI for A5+CM1.25% group compared to ampicillin alone, albeit non-significant.
4. Discussion
The increased rate of prescription for antibiotics in recent decades, their prophylactic use and subsequent rise of antibiotic-resistant pathogens are changing prescription protocols for the use of antibiotics in medical and dental clinical settings and in search for alternate therapeutic medicinal compounds such as the essential oils. In dentistry, endodontic diseases of periapical and intraradicular infections and their root canal treatments are becoming increasingly difficult to manage due to E. faecalis, a facultative aerobe that forms biofilms, survives in low nutrient environment and can resist antibiotics, in the root canal space. The EOs, with their anti-inflammatory, antifungal, antimicrobial, and antibacterial properties, have been shown to enhance the antimicrobial effects against E. faecalis when combined with antibiotics and antiseptics. Given this, the examination of synergistic effects of antibiotics with EOs becomes highly relevant. In this study, we investigated the growth-inhibiting effects of penicillin and aminoglycoside antibiotics when combined with cinnamon and clove oils in E. faecalis.
All concentrations of cinnamon and clove EOs were effective in inhibiting the growth of
E. faecalis in a dose-dependent manner. The cinnamon EO produced a greater antimicrobial effect at a much lower concentration than clove EO. For clove EO, the ZOI of CL25% was 7.5±0.2mm and increased by 139% when the concentration of clove EO was increased to CL50%. For cinnamon EO, increasing the oil concentration by twofold from 1.25% to 2.5% and 5% also increased the size of ZOI to 160% and 193%, respectively. Our data support previous reports showing 1% to 10% of cinnamon and 50% of clove EOs were effective in inhibiting the growth of
E. faecalis to almost 100% within 15min of exposure and with the cinnamon EO, the inhibitory effect was maintained for up to 10 days [
47,
48,
49]. Marcoux et.al (2020) reported that cinnamon EO (MIC 1.56 ug/ml) was equally effective on
E. faecalis embedded in biofilm, killing over 90% within 15min, and outperforming chlorhexidine wash which only showed 31% effectiveness.
For the antibiotics examined in the present study, ampicillin and gentamicin had the greatest inhibitory effect in inhibiting the growth of
E. faecalis with 9.75±0.25mm and 8.25±0.47mm of ZOI, respectively, whereas kanamycin showed the least effect with 5.50±0.28mm. Our data are in line with a previous report where the MIC for ampicillin, gentamicin, and streptomycin were in a similar range of 8-14 ug/ml (MIC for kanamycin was 32 ug/ml) on antimicrobial test against
E. faecalis, and that gentamicin was much more effective than kanamycin on in vivo E. coli meningitis bacteremia test [
50,
51,
52].
There were enhanced and synergistic antimicrobial effects against E. faecalis when antibiotics were combined with cinnamon and clove EOs, except for ampicillin. Overall, clove EO was more effective than cinnamon EO in increasing the antimicrobial effects. The increased effects of clove EO were observed when combined with gentamicin, streptomycin, and kanamycin, whereas cinnamon EO showed equally strong antimicrobial synergistic effect when combined with streptomycin and kanamycin only. For streptomycin and kanamycin, combining with CM2.5% and CL50% produced significantly enhanced antimicrobial effects compared to use of antibiotics alone. For streptomycin, the antimicrobial effects increased to 133% and 122% when combined with CM2.5% and CL50%, respectively. The synergistic effect was much more pronounced for kanamycin where the antimicrobial effect increased by 157% and 191% when combined with CM2.5% and CL50%, respectively. For gentamicin, which already showed a high ZOI when used alone, only combining with clove EO was effective in increasing the antimicrobial effect against E. faecalis. Ampicillin, which had the highest antimicrobial effect by itself, did not show any further increase when combined with either of the EOs.
Acquisition of antibiotic resistance by
E. faecalis are reported to be associated in part with its ability to synthesize β-lactamase, incorporate aminoglycoside-resistant genes aac(6’)-Ie-aph(2”)-Ia and aph(2’)-Ib, upregulate expression of low-affinity penicillin-binding protein Pbp5, and the presence of ATP-binding cassette multidrug efflux pump EfrAB [
53,
54,
55,
56,
57]. These adaptations, along with its ability to survive in biologically inhospitable environments, make
E. faecalis an ideal candidate to thrive and persist at sites in and around endodontic infections. The antimicrobial and antibacterials properties observed in EOs, such as cinnamon and clove oils, involve disruption of bacterial genes, non-specific permeabilization of the cell membrane, inhibition of transmembrane proton motif force and ATPase, and via anti-quorum sensing effects [
32]. Our
in vitro data shows that the enhanced antimicrobial effects observed against
E. faecalis by combining the antibiotics with cinnamon and clove EOs, presumably by interfering with the antibiotic-resistant cellular mechanisms, may be a suitable and practical approach to reduce the prevalence and incidence of persistent dental infections and treatment failures. Exploration of such strategies in in vivo and clinical studies to assess the efficacy, safety, and duration of their effects should be examined in future studies.
5. Conclusions
In conclusion, our study reports on the enhanced effectiveness of combining essential oils (EOs) with antibiotics in inhibiting the growth of E. faecalis, a pathogen associated with persistent dental infections and treatment failures. Our results showed that both cinnamon and clove EOs exhibited dose-dependent growth-inhibiting effects on E. faecalis, with cinnamon EO displaying superior efficacy at lower concentrations. Moreover, synergistic antimicrobial effects were observed when 2.5% cinnamon and 50% clove EOs were combined with gentamicin, streptomycin, and kanamycin. Ampicillin combined with EOs did not show synergistic effect. The antimicrobial properties of EOs by disruption of bacterial genes and cell membrane permeabilization may offer as novel strategies to combat antibiotics-resilient pathogen such as E. faecalis. Future research should explore these strategies in in vivo and clinical settings to assess their safety, efficacy, and duration of action.
Author Contributions
Conceptualization, P.L., T.D., and T.Y.; methodology, P.L., T.Y.; software, J.L.; validation, P.L., T.D. and T.Y.; formal analysis, J.L.; investigation, S.J.; resources, T.D. and T.Y.; data curation, T.Y.; writing—original draft preparation, J.L.; writing—review and editing, J.L., T.N.; visualization, J.L.; supervision, T.Y.; project administration, T.D. and T.Y.; funding acquisition, T.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by LECOM Seed Grant 23-121
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stein, K.; Farmer, J.; Singhal, S.; Marra, F.; Sutherland, S.; Quiñonez, C. The Use and Misuse of Antibiotics in Dentistry: A Scoping Review. J Am Dent Assoc 2018, 149, 869–884.e5. [Google Scholar] [CrossRef]
- Epstein, J.B.; Chong, S.; Le, N.D. A Survey of Antibiotic Use in Dentistry. J Am Dent Assoc 2000, 131, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.H.; Ha-Phuoc, A.-K.; Mohr, C. Treatment of Odontogenic Abscesses: Comparison of Primary and Secondary Removal of the Odontogenic Focus and Antibiotic Therapy. Oral Maxillofac Surg 2020, 24, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bogacz, M.; Morawiec, T.; Śmieszek-Wilczewska, J.; Janowska-Bogacz, K.; Bubiłek-Bogacz, A.; Rój, R.; Pinocy, K.; Mertas, A. Evaluation of Drug Susceptibility of Microorganisms in Odontogenic Inflammations and Dental Surgery Procedures Performed on an Outpatient Basis. Biomed Res Int 2019, 2019, 2010453. [Google Scholar] [CrossRef]
- Warnke, P.H.; Becker, S.T.; Springer, I.N.G.; Haerle, F.; Ullmann, U.; Russo, P.A.J.; Wiltfang, J.; Fickenscher, H.; Schubert, S. Penicillin Compared with Other Advanced Broad Spectrum Antibiotics Regarding Antibacterial Activity against Oral Pathogens Isolated from Odontogenic Abscesses. J Craniomaxillofac Surg 2008, 36, 462–467. [Google Scholar] [CrossRef]
- Marra, F.; George, D.; Chong, M.; Sutherland, S.; Patrick, D.M. Antibiotic Prescribing by Dentists Has Increased: Why? J Am Dent Assoc 2016, 147, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Yan, C.H.; Hubbard, C.; Calip, G.S.; Sharp, L.K.; Evans, C.T.; Rowan, S.; McGregor, J.C.; Gross, A.E.; Hershow, R.C.; et al. Changes in Antibiotic Prescribing by Dentists in the United States, 2012-2019. Infect Control Hosp Epidemiol 2023, 1–6. [Google Scholar] [CrossRef]
- Hicks, L.A.; Bartoces, M.G.; Roberts, R.M.; Suda, K.J.; Hunkler, R.J.; Taylor, T.H.; Schrag, S.J. US Outpatient Antibiotic Prescribing Variation According to Geography, Patient Population, and Provider Specialty in 2011. Clin Infect Dis 2015, 60, 1308–1316. [Google Scholar] [CrossRef]
- Gross, A.E.; Hanna, D.; Rowan, S.A.; Bleasdale, S.C.; Suda, K.J. Successful Implementation of an Antibiotic Stewardship Program in an Academic Dental Practice. Open Forum Infect Dis 2019, 6, ofz067. [Google Scholar] [CrossRef]
- Suda, K.J.; Henschel, H.; Patel, U.; Fitzpatrick, M.A.; Evans, C.T. Use of Antibiotic Prophylaxis for Tooth Extractions, Dental Implants, and Periodontal Surgical Procedures. Open Forum Infect Dis 2018, 5, ofx250. [Google Scholar] [CrossRef]
- Cope, A.L.; Francis, N.A.; Wood, F.; Chestnutt, I.G. Antibiotic Prescribing in UK General Dental Practice: A Cross-Sectional Study. Community Dent Oral Epidemiol 2016, 44, 145–153. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus Faecalis: Its Role in Root Canal Treatment Failure and Current Concepts in Retreatment. J Endod 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Duggan, J.M.; Sedgley, C.M. Biofilm Formation of Oral and Endodontic Enterococcus Faecalis. J Endod 2007, 33, 815–818. [Google Scholar] [CrossRef]
- Pinheiro, E.T.; Gomes, B.P.F.A.; Ferraz, C.C.R.; Sousa, E.L.R.; Teixeira, F.B.; Souza-Filho, F.J. Microorganisms from Canals of Root-Filled Teeth with Periapical Lesions. Int Endod J 2003, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rôças, I.N. Polymerase Chain Reaction-Based Analysis of Microorganisms Associated with Failed Endodontic Treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004, 97, 85–94. [Google Scholar] [CrossRef]
- Rôças, I.N.; Siqueira, J.F.; Santos, K.R.N. Association of Enterococcus Faecalis with Different Forms of Periradicular Diseases. J Endod 2004, 30, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, J.; Peng, Z. Correlation between Enterococcus Faecalis and Persistent Intraradicular Infection Compared with Primary Intraradicular Infection: A Systematic Review. J Endod 2015, 41, 1207–1213. [Google Scholar] [CrossRef]
- Pinheiro, E.T.; Gomes, B.P.F.A.; Ferraz, C.C.R.; Teixeira, F.B.; Zaia, A.A.; Souza Filho, F.J. Evaluation of Root Canal Microorganisms Isolated from Teeth with Endodontic Failure and Their Antimicrobial Susceptibility. Oral Microbiol Immunol 2003, 18, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Dornelles-Morgental, R.; Guerreiro-Tanomaru, J.M.; de Faria-Júnior, N.B.; Hungaro-Duarte, M.A.; Kuga, M.C.; Tanomaru-Filho, M. Antibacterial Efficacy of Endodontic Irrigating Solutions and Their Combinations in Root Canals Contaminated with Enterococcus Faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011, 112, 396–400. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Zhang, C.-F.; Chu, C.-H.; Zhu, X.-F. Prevalence of Enterococcus Faecalis in Saliva and Filled Root Canals of Teeth Associated with Apical Periodontitis. Int J Oral Sci 2012, 4, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical Presentation, Etiology, and Outcome of Infective Endocarditis in the 21st Century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Cornia, P.B.; Takahashi, T.A.; Lipsky, B.A. The Microbiology of Bacteriuria in Men: A 5-Year Study at a Veterans’ Affairs Hospital. Diagn Microbiol Infect Dis 2006, 56, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Koppen, L.; Suda, K.J.; Rowan, S.; McGregor, J.; Evans, C.T. Dentists’ Prescribing of Antibiotics and Opioids to Medicare Part D Beneficiaries: Medications of High Impact to Public Health. J Am Dent Assoc 2018, 149, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.J.; Calip, G.S.; Zhou, J.; Rowan, S.; Gross, A.E.; Hershow, R.C.; Perez, R.I.; McGregor, J.C.; Evans, C.T. Assessment of the Appropriateness of Antibiotic Prescriptions for Infection Prophylaxis Before Dental Procedures, 2011 to 2015. JAMA Netw Open 2019, 2, e193909. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial Resistance: Risk Associated with Antibiotic Overuse and Initiatives to Reduce the Problem. Ther Adv Drug Saf 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T. V Antimicrobial Activity of Essential Oils and Other Plant Extracts. J Appl Microbiol 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In Vitro Antibacterial Activity of Some Plant Essential Oils. BMC Complement Altern Med 2006, 6, 39. [Google Scholar] [CrossRef]
- An, B.-S.; Kang, J.-H.; Yang, H.; Jung, E.-M.; Kang, H.-S.; Choi, I.-G.; Park, M.-J.; Jeung, E.-B. Anti-Inflammatory Effects of Essential Oils from Chamaecyparis Obtusa via the Cyclooxygenase-2 Pathway in Rats. Mol Med Rep 2013, 8, 255–259. [Google Scholar] [CrossRef]
- MARUZZELLA, J.C.; HENRY, P.A. The in Vitro Antibacterial Activity of Essential Oils and Oil Combinations. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1958, 47, 294–296. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front Microbiol 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Bersani, C.; Comi, G. Antimicrobial Activity of the Essential Oils of Thymus Vulgaris L. Measured Using a Bioimpedometric Method. J Food Prot 1999, 62, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.E.; McElmeel, L.; Wiederhold, N.P. In Vitro Activity of Essential Oils Against Gram-Positive and Gram-Negative Clinical Isolates, Including Carbapenem-Resistant Enterobacteriaceae. Open Forum Infect Dis 2019, 6, ofz502. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob Agents Chemother 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Shapiro, S.; Meier, A.; Guggenheim, B. The Antimicrobial Activity of Essential Oils and Essential Oil Components towards Oral Bacteria. Oral Microbiol Immunol 1994, 9, 202–208. [Google Scholar] [CrossRef]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint. Crit Rev Microbiol 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Łysakowska, M.; Ciećwierz, J.; Denys, P.; Kowalczyk, E. Antibacterial Activity of Thyme and Lavender Essential Oils. Med Chem 2011, 7, 674–689. [Google Scholar] [CrossRef]
- Filoche, S.K.; Soma, K.; Sissons, C.H. Antimicrobial Effects of Essential Oils in Combination with Chlorhexidine Digluconate. Oral Microbiol Immunol 2005, 20, 221–225. [Google Scholar] [CrossRef]
- Fine, D.H.; Furgang, D.; Sinatra, K.; Charles, C.; McGuire, A.; Kumar, L.D. In Vivo Antimicrobial Effectiveness of an Essential Oil-Containing Mouth Rinse 12 h after a Single Use and 14 Days’ Use. J Clin Periodontol 2005, 32, 335–340. [Google Scholar] [CrossRef]
- Fadli, M.; Saad, A.; Sayadi, S.; Chevalier, J.; Mezrioui, N.-E.; Pagès, J.-M.; Hassani, L. Antibacterial Activity of Thymus Maroccanus and Thymus Broussonetii Essential Oils against Nosocomial Infection - Bacteria and Their Synergistic Potential with Antibiotics. Phytomedicine 2012, 19, 464–471. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit Rev Microbiol 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Lim, S.H.E.; Hu, C.P.; Yiap, B.C. Combination of Essential Oils and Antibiotics Reduce Antibiotic Resistance in Plasmid-Conferred Multidrug Resistant Bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Hu, J.; Liu, Z.; Zeng, Z. Antibacterial Effect of Oregano Essential Oil Alone and in Combination with Antibiotics against Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli. FEMS Immunol Med Microbiol 2008, 53, 190–194. [Google Scholar] [CrossRef]
- Marcoux, E.; Lagha, A. Ben; Gauthier, P.; Grenier, D. Antimicrobial Activities of Natural Plant Compounds against Endodontic Pathogens and Biocompatibility with Human Gingival Fibroblasts. Arch Oral Biol 2020, 116, 104734. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Duhan, J.; Tewari, S.; Sangwan, P.; Yadav, A.; Singh, G.; Juneja, R.; Saini, H. Comparative Evaluation of Antimicrobial Efficacy of Syzygium Aromaticum, Ocimum Sanctum and Cinnamomum Zeylanicum Plant Extracts against Enterococcus Faecalis: A Preliminary Study. Int Endod J 2013, 46, 775–783. [Google Scholar] [CrossRef]
- Ali, I.A.A.; Cheung, B.P.K.; Matinlinna, J.; Lévesque, C.M.; Neelakantan, P. Trans-Cinnamaldehyde Potently Kills Enterococcus Faecalis Biofilm Cells and Prevents Biofilm Recovery. Microb Pathog 2020, 149, 104482. [Google Scholar] [CrossRef]
- Nagy-Bota, M.C.; Man, A.; Santacroce, L.; Brinzaniuc, K.; Pap, Z.; Pacurar, M.; Pribac, M.; Ciurea, C.N.; Pintea-Simon, I.A.; Kovacs, M. Essential Oils as Alternatives for Root-Canal Treatment and Infection Control against Enterococcus Faecalis—A Preliminary Study. Applied Sciences 2021, 11, 1422. [Google Scholar] [CrossRef]
- Kim, K.S. Comparison of Gentamicin and Kanamycin Alone and in Combination with Ampicillin in Experimental Escherichia Coli Bacteremia and Meningitis. Pediatr Res 1985, 19, 1152–1155. [Google Scholar] [CrossRef]
- EUCAST-ESCMID Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. Clinical Microbiology and Infection 2003, 9, ix–xv. [CrossRef]
- Khan, A.; Miller, W.R.; Axell-House, D.; Munita, J.M.; Arias, C.A. Antimicrobial Susceptibility Testing for Enterococci. J Clin Microbiol 2022, 60, e0084321. [Google Scholar] [CrossRef]
- Coudron, P.E.; Markowitz, S.M.; Wong, E.S. Isolation of a Beta-Lactamase-Producing, Aminoglycoside-Resistant Strain of Enterococcus Faecium. Antimicrob Agents Chemother 1992, 36, 1125–1126. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R.; Dutka-Malen, S.; Brisson-Noël, A.; Molinas, C.; Derlot, E.; Arthur, M.; Duval, J.; Courvalin, P. Resistance of Enterococci to Aminoglycosides and Glycopeptides. Clin Infect Dis 1992, 15, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.W.; Zervos, M.J.; Lerner, S.A.; Thal, L.A.; Donabedian, S.M.; Jaworski, D.D.; Tsai, S.; Shaw, K.J.; Clewell, D.B. A Novel Gentamicin Resistance Gene in Enterococcus. Antimicrob Agents Chemother 1997, 41, 511–514. [Google Scholar] [CrossRef]
- Kristich, C.J.; Rice, L.B.; Arias, C.A. 2014.
- Lee, E.-W.; Huda, M.N.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. EfrAB, an ABC Multidrug Efflux Pump in Enterococcus Faecalis. Antimicrob Agents Chemother 2003, 47, 3733–3738. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).