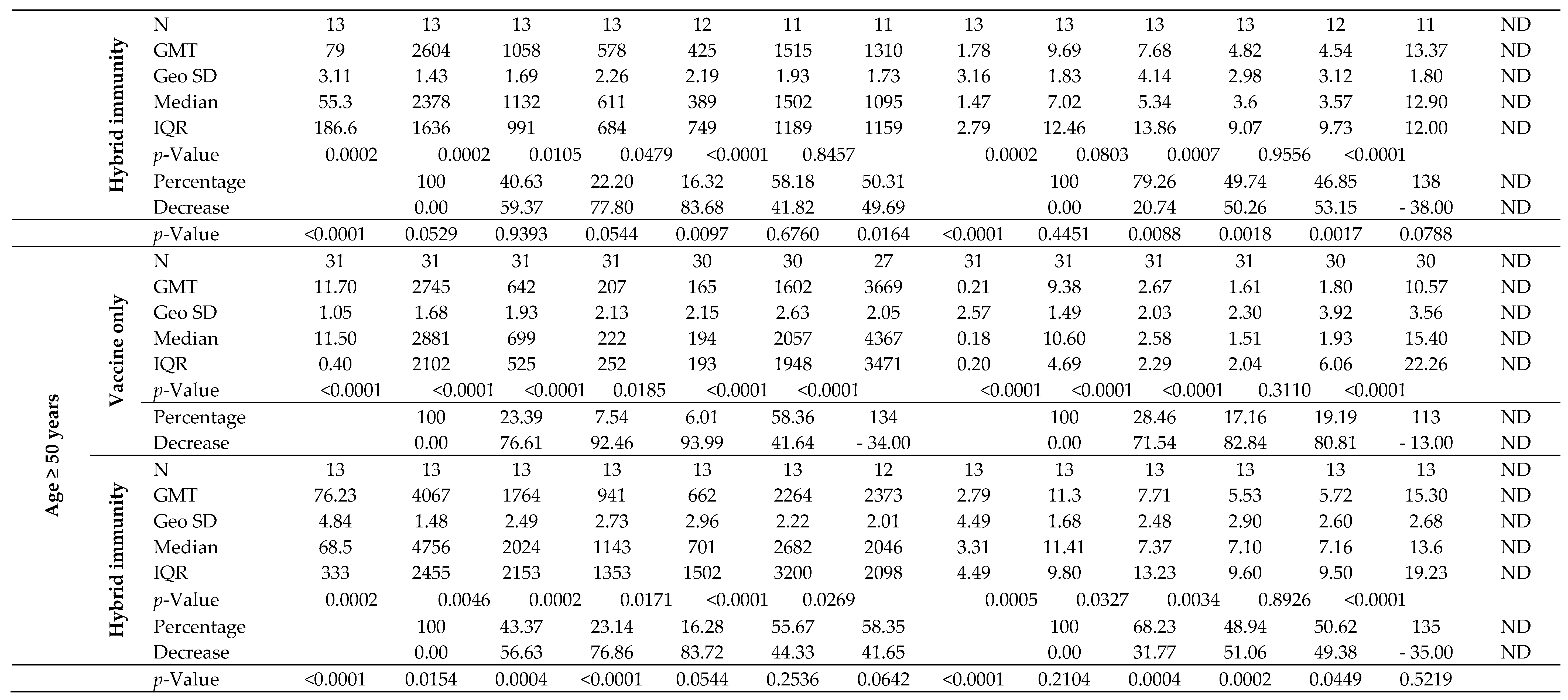1. Introduction
The global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) resulted in pandemic status for the illness by the World Health Organisation (WHO) [
1]. Since the first identification of SARS-CoV-2 in December 2019, the WHO has recorded more than 770 million confirmed cases of COVID-19, including almost 7 million deaths [
2]. Vaccination is one of the most effective and cost-efficient public health interventions to prevent infectious diseases [
3]. There are several COVID-19 vaccines validated for use by the WHO. The mass vaccination programme started in early December 2020, and the number of vaccine doses currently administered is more than 13 billion [
2,
4].
The first COVID-19 vaccine recommended by the WHO was the BNT162b2 (Comirnaty ®, Pfizer, Philadelphia, PA, USA and BioNTech, Mainz, Germany), which consists of a nucleoside-modified mRNA encoding spike (S) protein, specific to the Wuhan-Hu-1 strain isolated in China during the first outbreak in late 2019, formulated in lipid nanoparticles [
4,
5]. Transient expression of the S antigen induces neutralising antibodies and cellular immune responses providing defence from COVID-19.5 Data obtained from clinical trials have shown that a two-dose scheme of the vaccine, administered 21 days apart, offered 86% and 95% protection against infection and severe disease, respectively [
4,
6,
7]. The emergence of SARS-CoV-2 mutants, carrying changes mainly in the S protein, led to increased transmission and/or infectivity of the virus and reduced vaccine efficacy, resulting in infections of vaccinated individuals [
8,
9,
10,
11]. In addition, several studies have shown that the antibody levels decline markedly in six months following primary vaccination, which may also contribute to an increase in breakthrough infections [
12,
13,
14,
15,
16]. Booster doses were aimed at enhancing the immune response to provide long-term protection against COVID-19, including that caused by the variants of concern (VOCs) [
6,
7,
16,
17]. For Alpha, Beta, Gamma and Delta variants, the effectiveness of the vaccine in preventing infection and severe disease remained similar as the assumed efficacy of the ancestral strain, while for Omicron sub-lineages (including BA.1, BA.2, BA.5) it amounts to 44% and 72%, respectively [
7]. Furthermore, vaccination after recovery from SARS-CoV-2 infection resulting a 'hybrid' immunity was found to significantly increases the strength of the humoral response [
7,
18,
19,
20,
21].
Vaccine effectiveness, in contrast to vaccine efficacy assessed in clinical trials, is based on a reduction in the risk of infection/disease among vaccinated individuals in real life. This can be influenced by many factors, including internal host factors (e.g. age, gender, genetics, co-morbidities), external host factors (e.g. pre-existing immunity, microbiota, past infections), environmental factors (e.g. geographical location, season, family size) and behavioural factors (e.g. smoking, alcohol consumption, exercise, rest/sleep). In addition, factors related to the kind of vaccine (product type, adjuvant, dose) and factors associated to administration (schedule, site, route, time of vaccination, concurrent other vaccines and medicines) are also important [
22,
23]. Despite the high vaccine effectiveness, some people have concerns about receiving the COVID-19 vaccine related to its safety and side effects [
24,
25,
26]. Therefore, a better understanding of adverse reactions of individual vaccines allows for more informed decisions about vaccination.
The VAERS and EudraVigilance vaccine safety systems (maintained by the Food and Drug Administration and the European Medicines Agency, respectively) were established to report vaccine adverse reactions, including monitoring the safety profile of COVID-19 vaccines administered to the public [
27,
28]. The most commonly recognised side effects within seven days of each vaccine dose consisted injection site redness and swelling, headache, myalgia and fatigue. Severe reactions with facial paralysis, anaphylaxis and cerebral venous sinus thrombosis were rare and occurred at similar rates in vaccinated and unvaccinated individuals [
24,
29]. Most research on vaccine side effects has focused on clinical trials or pre- and post-intervention investigations. Over time, more observational studies have emerged, and these have provided data on the safety of COVID-19 vaccine in real-world settings [
26,
30].
The aim of the study was a long-term monitoring (total of seven checkpoints in almost two years) of the humoral immune response to BNT162b2 vaccine (two primary doses and two boosters) and SARS-CoV-2 infection (including mainly breakthrough infections) of a well-characterized relatively invariable patient group. In addition, adverse effects reported after the primary vaccine doses were defined.
2. Materials and Methods
2.1. Study design, data collection and cohort characteristics
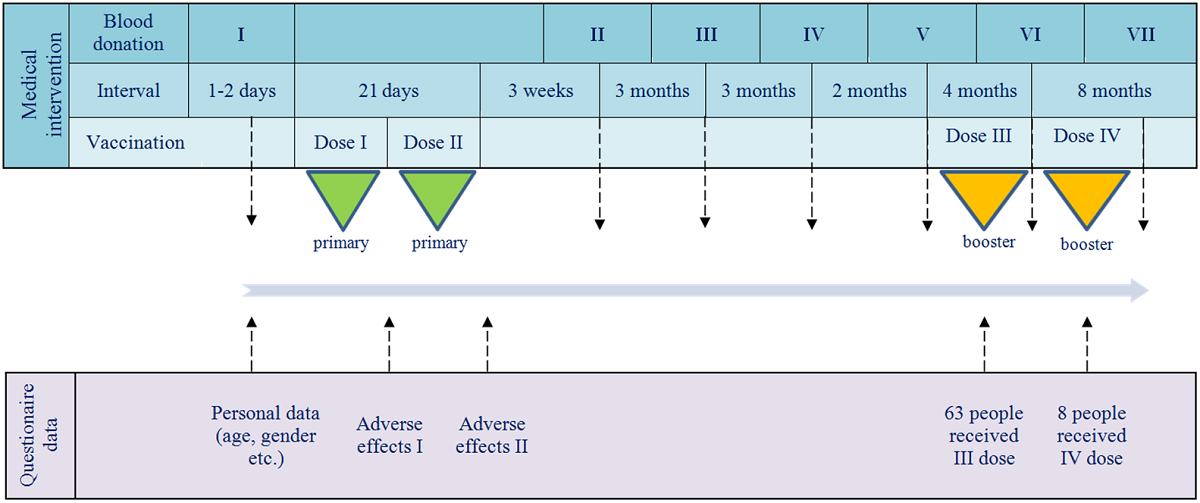
The investigation was designed to monitoring the humoral response in IgG and IgA classes of anti-SARS-CoV-2 antibodies. It was carried out as part of routine diagnostics in the medical laboratory of the National Medicines Institute (NMI) and was attended by NMI workers. All study participants received primary doses of BNT162b2 and some also were administered booster dose(s) of this vaccine. The timeline of vaccine administrations and blood donation checkpoints, as well as the conducted questionnaires, is shown in
Figure 1.
Information collected from the questionnaires consisted of patient characteristics (age, gender, body weight/growth and chronic diseases), a history of SARS-CoV-2 infection and the occurrence of vaccine side effects. Data analysis including patient characteristics and antibody kinetics was performed for the total cohort and by type of acquired immunity (‘vaccine only’ or ‘hybrid’), as well as by age above/below 50 years. The correlation of seroconversions with the presence of chronic disease or body mass index (BMI) was also investigated.
2.2. Serum collection and anti-SARS-CoV-2 antibody tests
Blood donations were performed according to the timeline shown in
Figure 1. Serum samples, obtained by whole blood centrifugation, were stored at -20oC pending antibody testing. To assess the humoral response, IgG and IgA antibodies were quantified by enzyme linked immunosorbent assay (ELISA) using an Infinite® M1000 PRO instrument (Tecan Trading AG, Männedor, Switzerland). The IgG and IgA antibodies against S protein (corresponding to IgG-S1 and IgA-S1) and IgG antibodies against nucleocapsid (N) protein (IgG-NCP) were tested with three commercial tests, Anti-SARS-CoV-2 QuantiVac-ELISA (IgG), Anti-SARS-CoV-2 ELISA (IgA) and Anti-SARS-CoV-2-NCP ELISA (IgG) (EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany), respectively. The obtained results were interpreted according to the manufacturer's guidelines. Due to the low levels of IgA-S-1, their determination was waived at the seventh checkpoint.
2.3. Statistical analysis
The results were analysed with GraphPad Prism v 8.0.1 (GraphPad Software, San Diego, CA, USA). Various assays, including the two-tailed Wilcoxon matched-pairs signed rank test, Mann-Whitney test or Chi-square test, were used in calculating the p-value at alpha < 0.05.
3. Results
3.1. Participant characteristics
A total of 91 participants were included in the study and their characteristics are shown in
Table 1. Until the third blood donation, the number of patients remained unchanged. However, for the kinetic analyses, the fallowing checkpoints were represented by 90, 86, 85 and 76 subjects, respectively.
3.2. Antibody kinetics
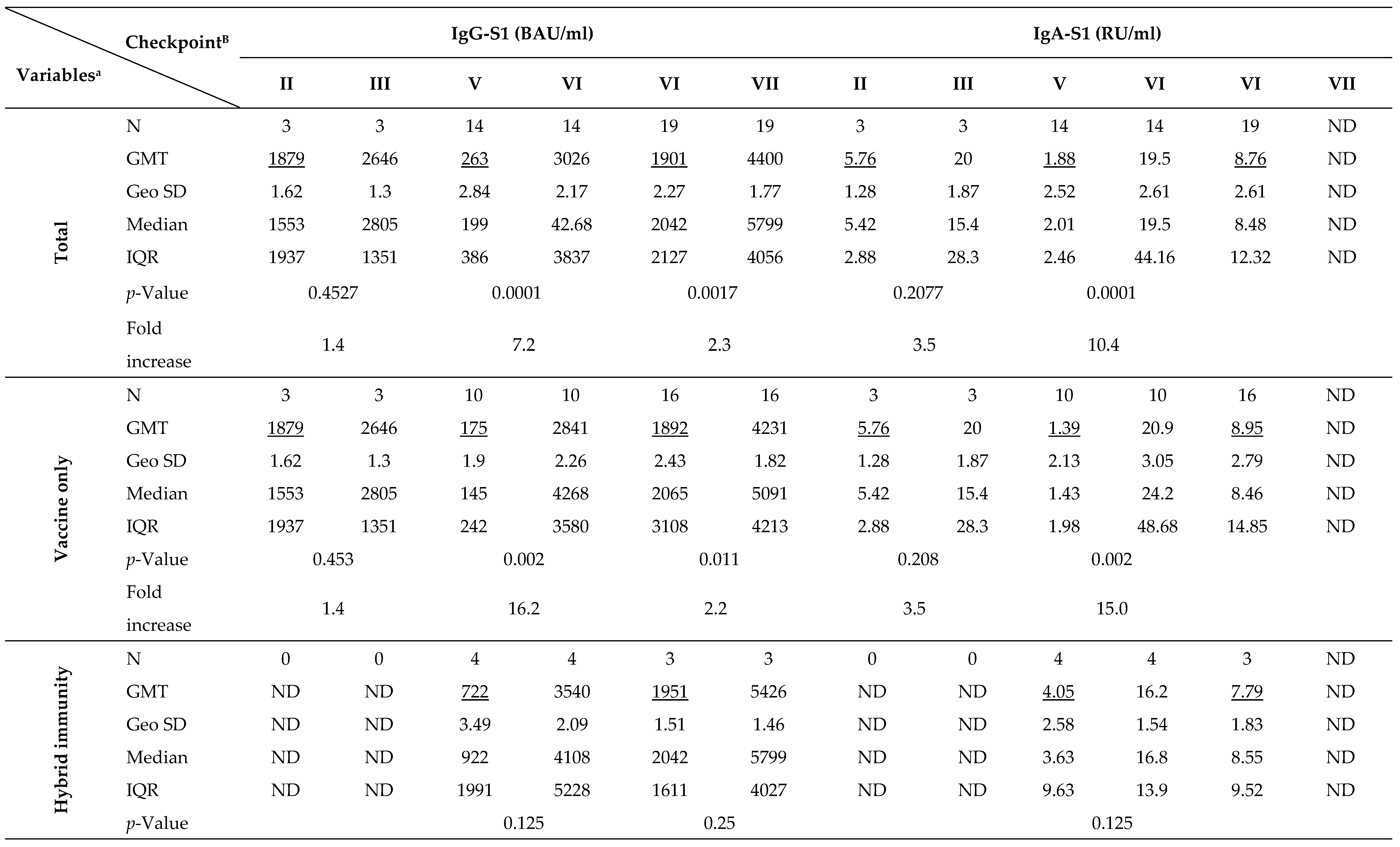
The kinetics of IgG-S1 and IgA-S1 antibodies, for the total cohort and by type of immunity, as well as by age above/below 50 years, are shown in
Table 2. To track trends in antibody levels after primary vaccine doses in intact systems (with vaccine-induced immunity only), three individuals who contracted SARS-CoV-2 infection between the second and third blood donations were excluded from the analysis. In addition, one of these subjects finished participation in the study after the fourth checkpoint. Antibody levels for the sixth and seventh blood donations include all participants tested at these checkpoints, except those previously ruled out.
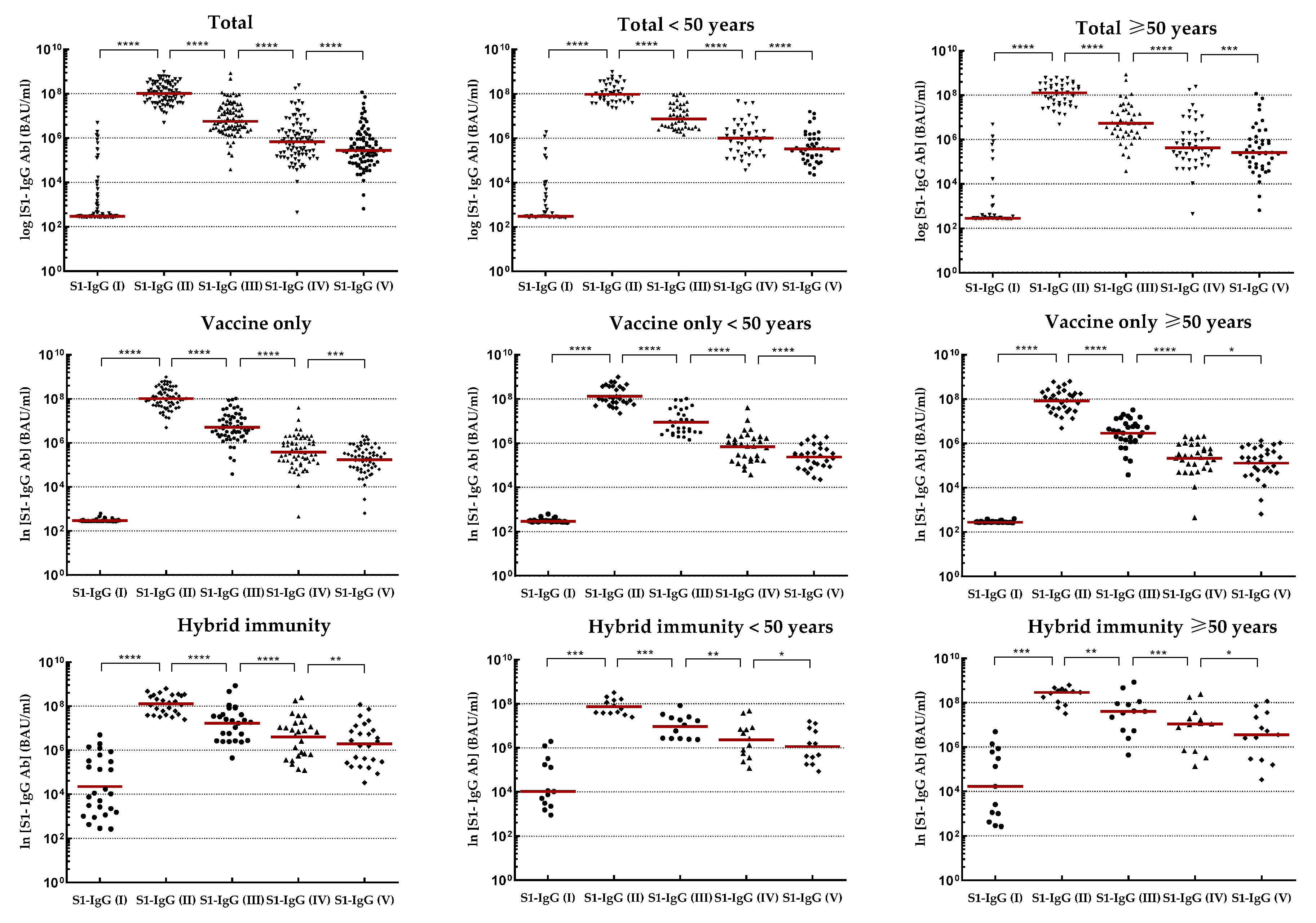
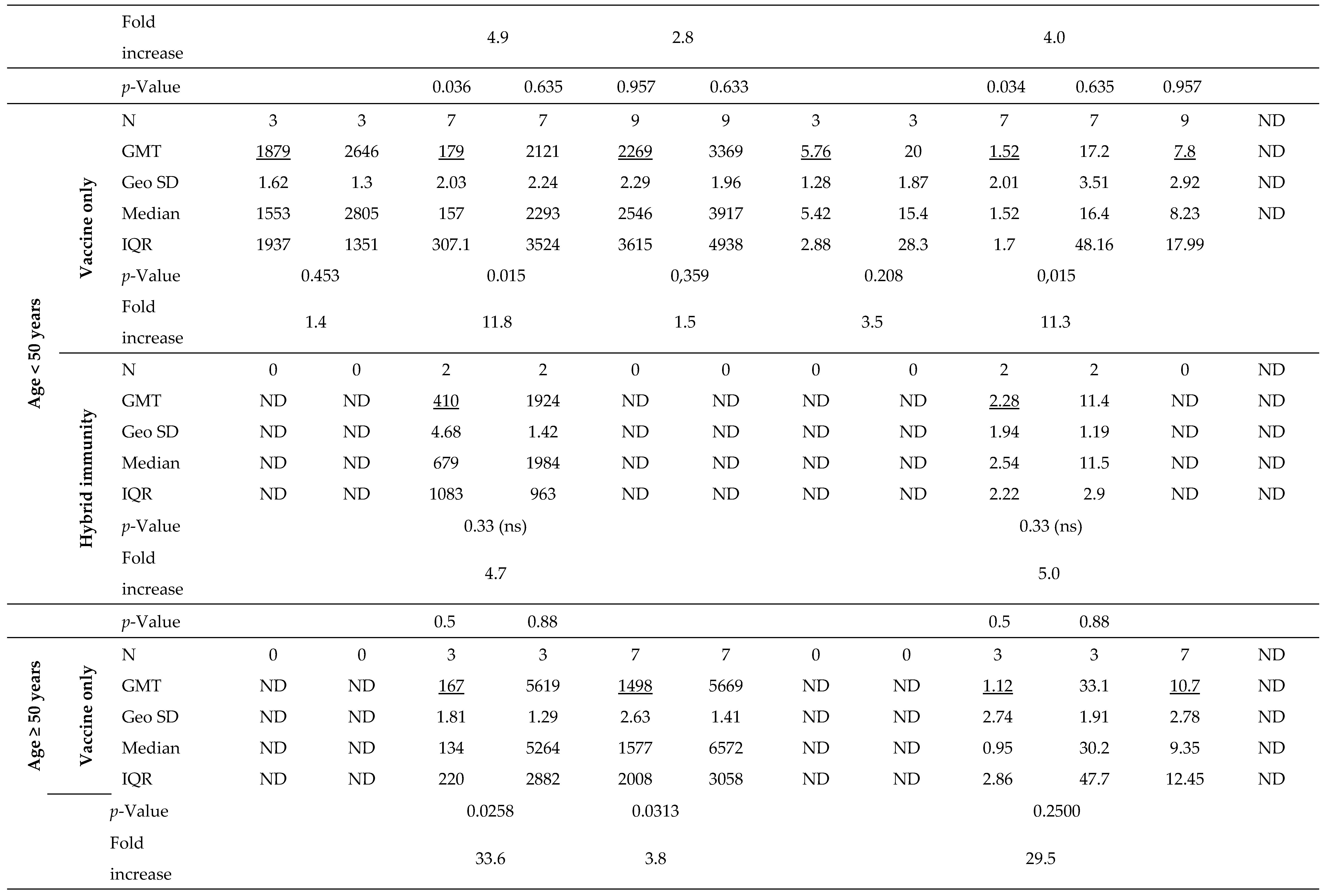
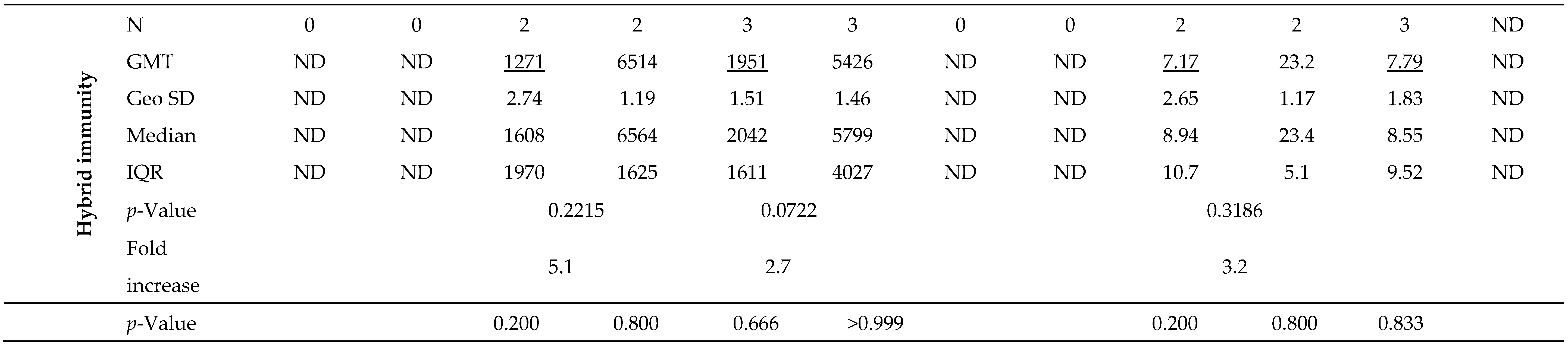
Joining the study, 26 individuals possessed a recovered status (positive results for IgG-NCP in the first blood donation) with higher antibody levels for IgG-S1 and IgA-S1 classes (7- and 10-fold, respectively) than in uninfected subjects. Nevertheless, antibody levels at the second checkpoint for the total cohort were only slightly higher with ‘hybrid’ immunity than with ‘vaccine only’ status (p=0.6659). Strong immunogenicity of the vaccine (increase in IgG-S1 antibody titters by almost 150-fold and IgA-S1 by more than 20-fold) was observed in all study cohorts. Despite the differences in mean levels of antibody, they were not statistically significant in individuals under 50 years old in the ‘vaccine only’ and ‘hybrid’ immunity groups. In contrast, a statistically significant difference (p=0.0154) in antibody levels between groups with different immunization statuses was observed in participants over 50 years old.
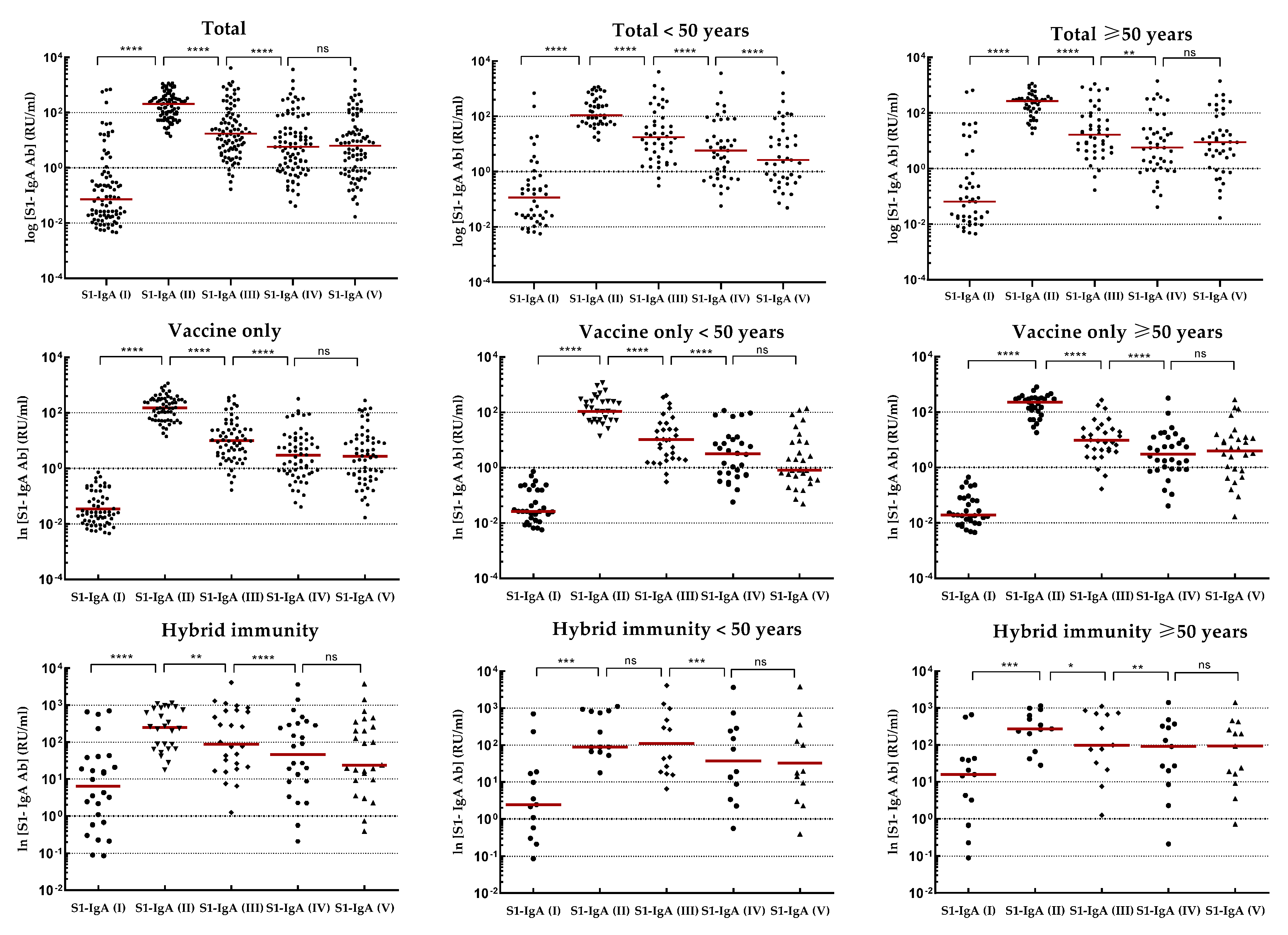
At the following checkpoints (up to the fifth blood donation), a systematic decrease in antibody levels from baseline values obtained at the second checkpoint was observed in all studied groups (
Figure 2 and
Figure 3). The decline in antibodies of individuals with 'vaccine only' status was higher than in those with 'hybrid' immunity by about 13% and 30% in IgG-S1 and IgA-S1, respectively. At the fifth checkpoint, the IgG-S1 and IgA-S1 antibody levels decreased by about 90% and 80%, respectively. Between the fifth and sixth checkpoint, 63 participants received the third dose of the vaccine. Despite the fact that 20 individuals did not accept the first booster, the average levels of antibody at the sixth blood donation increased compared to the fifth checkpoint in all studied groups. The growing trend in antibody levels remained until the seventh blood donation, with eight subjects receiving the second booster before this checkpoint.
No correlation was observed between chronic disease or BMI of participants and the seroconversions or duration of antibody persistence (data not shown).
3.3. Breakthrough infection
All SARS-CoV-2 infections were confirmed by RT-qPCRs or antigen tests, assays that we described previously [
31,
32]. Immune breakthrough levels were determined as IgG-S1 and IgA-S1 antibody levels obtained after vaccination (primary doses and boosters) and prior to positive IgG-NCP antibody levels. As infections occurred in the interval between checkpoints, the real breakthrough levels were the same or less than those assigned. In the ‘hybrid’ immune status group, breakthrough levels in IgG-S1 and IgA-S1 classes were identified for individuals with positive and by rising IgG-NCP levels after vaccination relative to previously established IgG-NCP levels. Consecutive positive results for IgG-NCP characterized by a decreasing trend were not included in the analysis and they were considered a single infections. The results of antibody breakthrough levels, for the total cohort and by age of individuals within type of immunity are shown in
Table 3.
After primary vaccination, 34 individuals (37.4% of all participants) presented with breakthrough infections. Two subjects were infected twice. In seven cases (19.4% of breakthrough infections), they had full-blown course of the disease with 1-3 days of fever, muscle aches, sore throat, cough or gastrointestinal symptoms. The others mostly complained of a sore throat and/or headache. Overall, breakthrough infections were found four times more often in individuals with ‘vaccine only’ status than with ‘hybrid’ immunity. They were also more common in those under 50 years old (n=21; 58.3%) than in the elderly (n=15; 41.7%).
The first three breakthrough infections occurred in just under four months after receiving the primary vaccine doses. Despite an increase in average antibody levels after infection (1.4- and 3.5-fold for IgG-S1 and IgA-S1, respectively), there was no significant immune enhancement (p>0.05). All patients with breakthrough infections between the fifth and seventh checkpoints received a booster vaccine dose, so the increases in antibody levels at the sixth and seventh blood donations are the results of both the booster and the infection. Average antibody levels at the sixth checkpoint were significantly higher than those observed at the fifth by 11.5- and 10.4-times for IgG-S1 and IgA-S1, respectively (p=0.0001). Also at the last sampling, the mean level of IgG-S1 antibodies increased compared to the previous checkpoint (p=0.0017).
3.4. Persistence of anti-SARS-CoV-2 antibodies over time
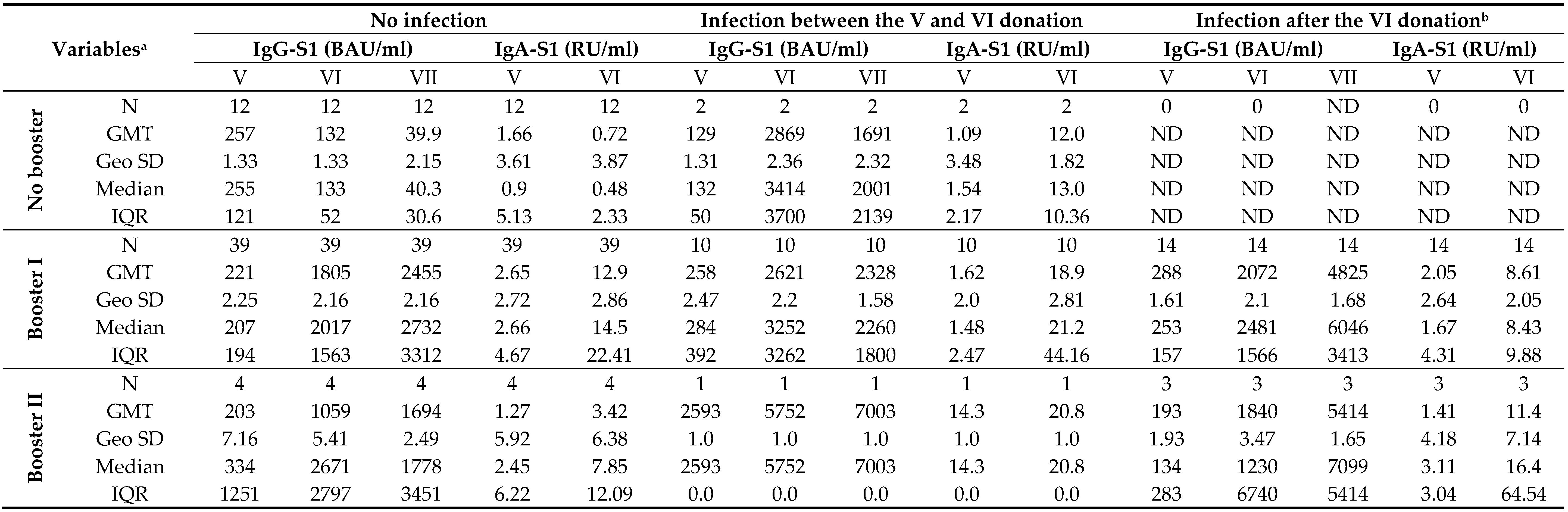
The temporal correlation of anti-SARS-CoV-2 antibody levels with the number of received vaccine doses in recovered (excluded infection before primary vaccination) and uninfected individuals is shown in
Table 4, although it should be noted that some groups were represented by only a few participants.
Twenty months after primary vaccination, an almost complete decrease in both IgG-S1 and IgA-S1 was observed in individuals who remained uninfected and did not receive any reminder dose. In contrast, adoption of the booster(s) by such subjects increased antibody levels by around 8-fold in both classes. On the other hand, although infection initially increased antibody levels (20- and 11-times for IgG-S1 and for IgA-S1, respectively), they were found to decrease by as many as half at the next checkpoint. As in uninfected individuals, the admission of booster dose(s) by recovered patients resulted in increase in both classes of antibodies.
3.5. Vaccine safety assessment
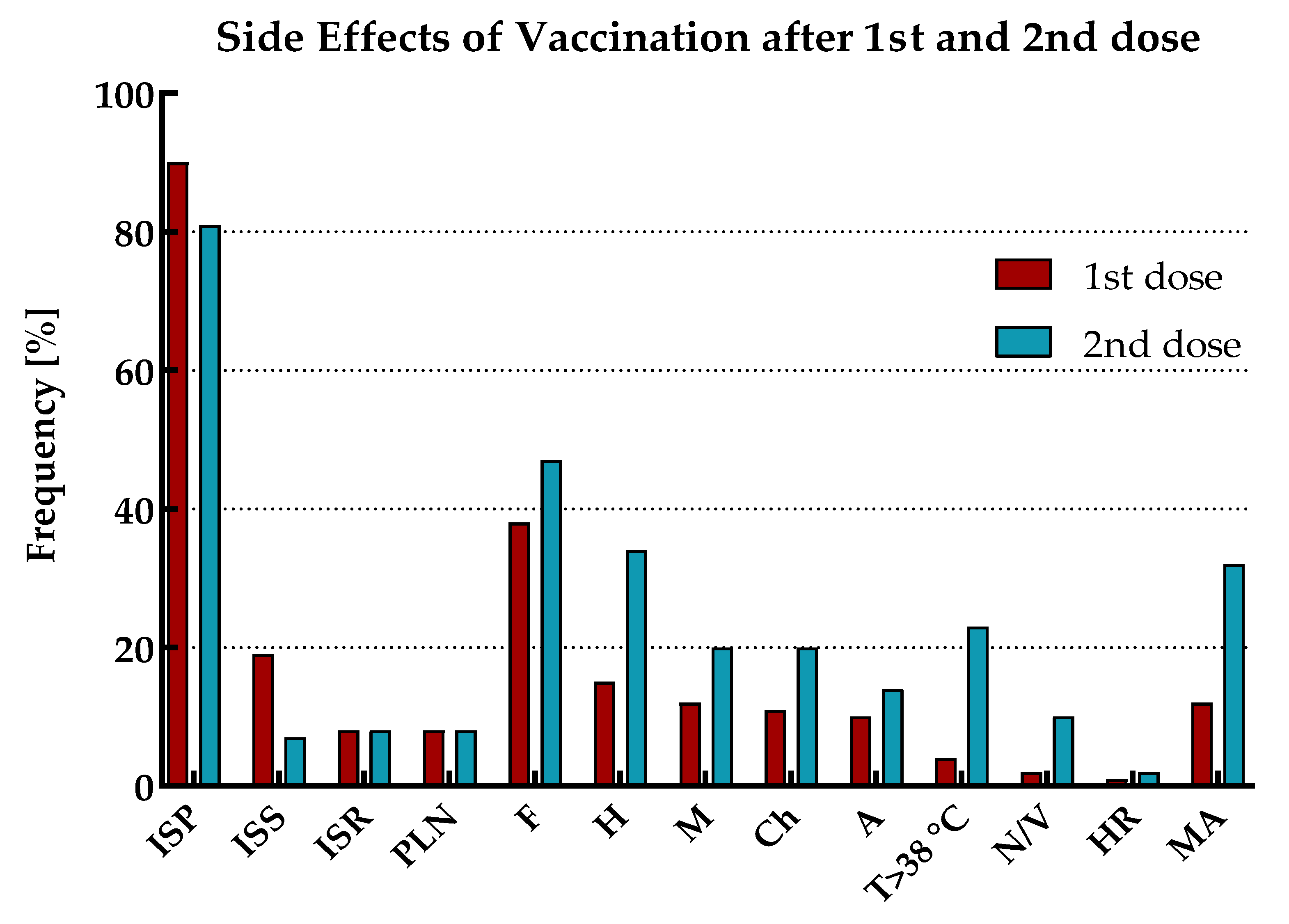
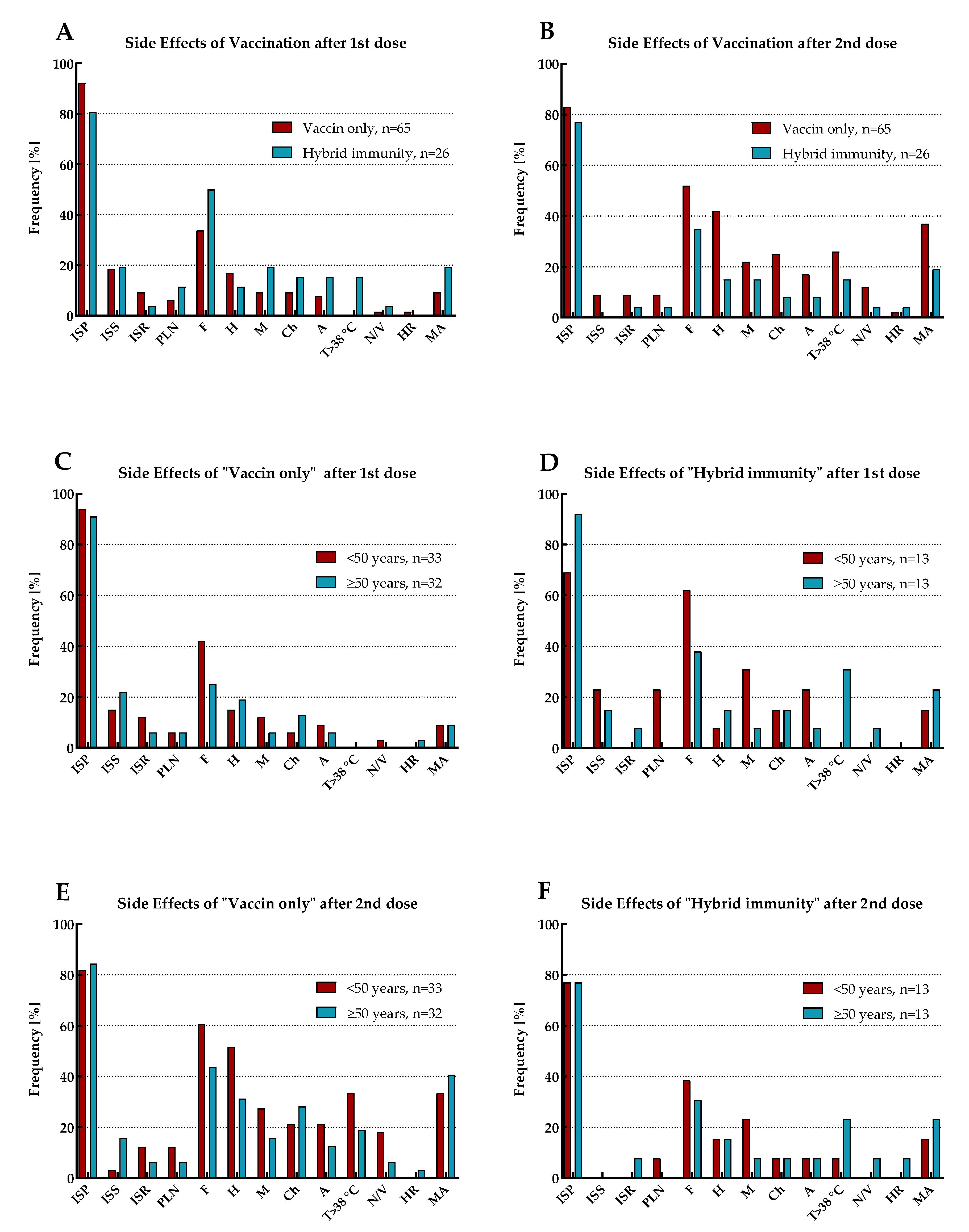
The reported side effects of vaccination (SEV) included injection site pain (ISP), injection site swelling (ISS), injection site redness (ISR), pain/enlargement of lymph nodes (PLN), fatigue (F), headache (H), myalgia (M), chills (Ch), arthralgia (A), temperature over 38oC (T>38oC), nausea or vomiting (N/V) and hypersensitivity reaction (HR). The prevalence of SEV with mean duration and declaration of medication admission (MA) for the total cohort and by immune status (including the age range of study participants), separately for the first and second vaccine doses, is shown in
Figure 4 and
Figure 5, and
Table 5.
SEV occurred after both doses of vaccination in all participants. Except for ISP and ISS, the vast majority of adverse reactions for the total cohort were more frequently observed after the second dose of vaccination than the first. Moreover, MA (antipyretics only) was also more often reported in this period.
The most common symptoms after the first vaccine dose were ISP, followed by F and ISS. However, as many as half of those with ‘hybrid’ immunity suffered from F, while in the group of individuals with ‘vaccine only’ status, only every third. In addition, in both these populations, this symptom occurred more frequently in patients under 50 years old. As previously, after the second dose of vaccination, the most common SEV for the total cohort were ISP and F. However, after this dose, the next most frequent adverse reaction was H. Both F and H were most prevalent in individuals under 50 years old with 'vaccine only' immunity than in the elderly or those with ‘hybrid’ status.
4. Discussion
Although there are no recommendations for routine testing of anti-SARS-CoV-2 antibody levels, monitoring the immune response after vaccination and/or illness is one of the key steps in studies of vaccine efficacy and/or population immunity. The benefits of such investigations include the assessment of baseline seroprevalence of SARS-CoV-2 infection in unvaccinated individuals, early identification of poor- or non-responders to vaccination, and timely detection of more rapid decline in anti-SARS-CoV-2 antibody levels. Such knowledge is crucial for making rational decisions about booster doses and managing a possible next wave of the COVID-19 pandemic [
33].
In Poland, the mass vaccination programme started on 27 December 2020 and, in its initial phase, targeted health care workers (HCWs), most of whom received two primary doses by the end of March 2021 [
34]. In this study, a group of 91 HCWs vaccinated with BNT162b2 were followed up for almost two years considering antibody levels before vaccination (baseline seroprevalence determination), after primary vaccination (between four and six checkpoints for participants without or with a booster, respectively) and after booster dose(s) (up to two checkpoints).
In contrast to previous reports from Poland presenting a very low (up to 2.4%) seroprevalence in HCWs, the current investigation demonstrated recovery status in 28.6% of the tested participants [
35]. This difference is most likely due to the fact that most of these surveys were conducted in May-August 2020 with relatively fewer SARS-CoV-2 infections in Poland at that time compared to other European countries, whereas in our study, the first testing of anti-SARS-CoV-2 antibody levels occurred in January 2021, just after the peak incidence of COVID-19 in the country in October-November 2020 [
2,
8,
36]. The timing of the first examination, scheduled up to two days before the first vaccine dose, allowed comparison of the immunogenicity induced by the infection with that of the vaccine. Vaccination was found to be more immunogenic (around 40- and 5-times in IgG and IgA antibody classes, respectively) than illness which is consistent with earlier reports [
20,
35,
37,
38]. Over time, a systematic decrease in antibody levels was observed compared to baseline values obtained after the primary vaccination. The decline in antibodies of individuals with 'vaccine only' status was higher than in those with 'hybrid' immunity. Our observations are in line with studies from other groups, so it seems that full vaccination of convalescents is completely justified, and that vaccination of people without previous COVID-19 disease with booster doses is even necessary [
20,
37,
39]. In contrast to the susceptibility to SARS-CoV-2 infection, no correlation was found between the presence of chronic diseases or the BMI value of study participants with the induction and duration of antibody persistence. The immune response in patients with chronic diseases depends on the type of co-morbidity and may remain the same as in healthy individuals, or it may be retarded or severely disabled [
40,
41]. On the other hand, conclusions regarding the correlation of BMI and neutralising antibody titres vary between reports [
12,
42]. Therefore, considering that vaccination primarily prevents the severe outcomes of COVID-19, it seems that it should be performed regardless of patient characteristics, unless there are obvious obstacles to it. Overall, after eight months, antibody levels in study participants decreased by about 90%, only to fall back to pre-vaccination levels after 20 months in those who did not receive any booster. As the antibody response gradually weakens after the primary vaccination, this contributes to an increase in breakthrough infections. SARS-CoV-2 infections were found often in individuals with ‘vaccine only’ status than with ‘hybrid’ immunity which confirms the findings of other researchers that the humoral response and vaccine effectiveness in previously infected patients is better than in those receiving only vaccine alone [
12,
20,
21,
35,
37]. Although the breakthrough infections in our study resulted in seroconversion similar to the booster, at the last checkpoint the antibody levels of those who recovered (and did not accept booster) were almost half those of participants who received a subsequent vaccine dose(s). This observation once again confirms the legitimacy of accepting a booster(s), despite the breakthrough infection [
18,
19,
20,
21].
Due to the emergence of VOCs and differences in the neutralisation levels of mutants by antibodies produced by the vaccine based on the Wuhan-Hu-1 strain, it is not possible to estimate a common level of antibodies that would determine the humoral protective response. In our study, the first patient with an Alpha variant breakthrough infection was identified just two weeks after the second baseline vaccine dose, as we previously reported [
10]. Although the expected efficacy of BNT162b2 against this variant was similar to that of the wild-type strain, in this case it proved to be unsatisfactory [
7]. Unfortunately, the level of anti-SARS-CoV-2 antibodies was not tested in this individual after the second dose of vaccine, immediately before the infection. Therefore, we could not determine whether insufficient antibodies produced after vaccination contributed to the disease. However, it should be noted that despite comorbidities that would have put the patient at risk of an unfavourable prognosis of infection, the participant presented only mild cold symptoms. Only two other individuals developed SARS-CoV-2 infections less than four months after receiving baseline vaccination doses. The other breakthrough infections were identified at a time when there were already significant declines in antibody levels. These infections were most likely due to the Omicron variant, which emerged in Poland in December 2021 [
8].
The most commonly recognized SEVs of each vaccine dose were redness and swelling at the injection site, as well as fatigue and headache. Interestingly, SEVs after the first dose of vaccination were more often reported by participants with 'hybrid' immunity than those who had not been previously infected. The opposite was found for SEVs after the second dose of vaccination, where they were more often presented by those with a ‘vaccine only’ status than by recovered individuals. Probably, the observed correlations resulted from a more violent reaction of the patients' immune systems during re-exposure (second contact) to the S protein of virus. In addition, it was found that those who had more frequent SEVs were also more likely to receive antipyretics.
Our study was conducted on a relatively small number of participants and therefore may not represent the general population. In addition, we were not able to perform gender specific analyses because the study group consisted of four times as many female as male. However, this allowed the monitoring of a relatively invariable patient group for almost two years. An important aspect of our investigation is also the demonstration of the persistence of anti-SARS-CoV-2 antibodies over time depending on the number of vaccine doses received and the course of infection (no infection and primary vaccination only, no infection and booster(s) vaccination, infection and primary vaccination only, infection and booster(s) vaccination).
5. Conclusions
We demonstrated the strong immunogenicity of the BNT162b2 vaccine. Vaccination is highly effective in preventing the most severe outcomes of COVID-19 and should be performed regardless of prior infection. Over time, a systematic decrease in antibody levels was observed compared to baseline values obtained after primary vaccination. Booster doses significantly enhance anti-SARS-CoV-2 antibody levels and, in contrast to those obtained by breakthrough infection, they remain longer.
Author Contributions
Conceptualization, JW and IK; methodology, JW and IK; statistical analysis, JW; tables and figure preparation, JW and AB; investigation, JW and IK; writing—original draft preparation, JW, IK, MK and AB; writing—review and editing, JW and AB. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by internal funding from the National Medicines Institute.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and it was part of routine diagnostics in a medical laboratory. The study was a retrospective analysis of diagnostic test data with the consent of patients monitoring humoral immunity and did not require ethics committee approval. Samples used in the scientific study were analysed anonymously.
Informed Consent Statement
Written informed consent including General Data Protection Regulation (GDPR) has been obtained from all subjects involved in the study to publish this paper.
Data Availability Statement
Raw data will be available upon suitable request to the corresponding author.
Acknowledgments
We would like to thank medical analytics technician Katarzyna Wieckowska, who collected blood samples for routine diagnostics.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Coronavirus disease (COVID-19) pandemic (2019). WHO website: https://www.who.int/emergencies/diseases/novel-coronavirus-2019, access: May 12, 2023.
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. WHO website: https://covid19.who.int, access: September 7, 2023.
- World Health Organization. State of the world’s vaccines and immunization (2009). WHO website: https://apps.who.int/iris/bitstream/handle/10665/44169/9789241563864_eng.pdf?sequence=1&isAllowed=y, access: September 7, 2023.
- World Health Organization. Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing. WHO website: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BNT162b2-2021.1, access: September 7, 2023.
- European Medicines Agency. Covid-19 mRNA vaccine (Comirnaty): EU summary of product characteristics (2020). EU website: http://ec.europa.eu, access: May 12, 2023.
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020, 383, 2603–2615. [CrossRef]
- Institute for Health Metrics and Evaluation. COVID-19 model update: Omicron and waning immunity. IHME website: https://www.healthdata.org/special-analysis/omicron-and-waning-immunity, access: May 12, 2023.
- Wegrzynska, K.; Komiazyk, M.; Walory, J.; Kozinska, A.; Wasko, I.; Baraniak, A. Differentiation of SARS-CoV-2 Variants Using RT-qPCRs by Targeting Recurrent Mutation Sites: A Diagnostic Laboratory Experience from Multi-Center Regional Study, August 2020–December 2021, Poland. Int. J. Mol. Sci. 2022, 23, 9416. [CrossRef]
- Ravindra K. Gupta. Will SARS-CoV-2 variants of concern affect the promise of vaccines? Nat Rev Immunol 2021, 21, 340–341. [CrossRef]
- Komiazyk, M.; Walory, J.; Gawor, J.; Ksiazek, I.; Gromadka, R.; Baraniak, A. Case Report of COVID-19 after Full Vaccination: Viral Loads and Anti-SARS-CoV-2 Antibodies. Diagnostics 2021, 11, 1815. [CrossRef]
- Shitrit P, Zuckerman NS, Mor O, Gottesman BS, Chowers M. Nosocomial outbreak caused by the SARS-CoV-2 Delta variant in a highly vaccinated population, Israel, July 2021. Euro Surveill 2021, 26, 2100822. [CrossRef]
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med 2021, 385, e84. [CrossRef]
- Thomas SJ, Moreira Jr ED, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med 2021, 385, 1761–1773. [CrossRef]
- Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [CrossRef]
- Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, Elbaz M, Nesher L, Stein M, et al. BNT162b2 vaccine breakthrough: Clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect 2021, 27, 1652–1657. [CrossRef]
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med 2021, 385, 1393–1400. [CrossRef]
- Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021, 398, 2093–2100. [CrossRef]
- Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [CrossRef]
- Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med 2021, 385, 1474–1484. [CrossRef]
- Bates TA, McBride SK, Leier HC, Guzman G, Lyski ZL, Schoen D, et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol 2022, 7, eabn8014. [CrossRef]
- Walls AC, Sprouse KR, Bowen JE, Joshi A, Franko N, Navarro MJ, et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell 2022, 185, 872–880.e3. [CrossRef]
- Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev 2019, 32, e00084–e18. [CrossRef]
- Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol 2021, 21, 626-636. [CrossRef]
- McMurry R, Lenehan P, Awasthi S, Silvert E, Puranik A, Pawlowski C, et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med 2021, 2, 965–978.e5. [CrossRef]
- Kirzinger A, Kearney A, Hamel L, Brodie M. KFF/The Washington Post Frontline Health Care Workers Survey (2021). KFF website: https://www.kff.org/report-section/kff-washington-post-frontline-health-care-workers-survey-vaccine-intentions, access: May 12, 2023.
- U.S. Food and Drug Administration. Vaccine Adverse Events. FDA website: https://www.fda.gov/vaccines-blood-biologics/report-problem-center-biologics-evaluation-research/vaccine-adverse-events, access: May 12, 2023.
- Schäfer I, Oltrogge JH, Nestoriuc Y, et al. Expectations and Prior Experiences Associated With Adverse Effects of COVID-19 Vaccination. JAMA Netw Open 2023, 6, e234732. [CrossRef]
- European Medicines Agency. Human regulatory, EudraVigilance. EMA website: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance, access: Mai 12, 2023.
- Centers for Disease Control and Prevention (CDC). Possible Side Effects After Getting a COVID-19 Vaccine. CDC website: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html, access: May 12, 2023.
- Voulgaridi, I.; Sarrou, S.; Dadouli, A.; Peristeri, A.-M.; Nasika, A.; Onoufriadis, I.; Kyritsi, M.A.; Anagnostopoulos, L.; Theodoridou, A.; Avakian, I.; et al. Intensity of Humoral Immune Responses, Adverse Reactions, and Post-Vaccination Morbidity after Adenovirus Vector-Based and mRNA Anti-COVID-19 Vaccines. Vaccines 2022, 10, 1268. [CrossRef]
- Komiazyk, M.; Walory, J.; Kozinska, A.; Wasko, I.; Baraniak, A. Impact of the Nucleic Acid Extraction Method and the RT-qPCR Assay on SARS-CoV-2 Detection in Low-Viral Samples. Diagnostics 2021, 11, 2247. [CrossRef]
- Wegrzynska, K.; Walory, J.; Charkiewicz, R.; Lewandowska, M.A.; Wasko, I.; Kozinska, A.; Majewski, P.; Baraniak, A. Clinical Validation of GenBody COVID-19 Ag, Nasal and Nasopharyngeal Rapid Antigen Tests for Detection of SARS-CoV-2 in European Adult Population. Biomedicines 2023, 11, 493. [CrossRef]
- Lippi, G.; Henry, B.M.; Plebani, M. Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How? Diagnostics 2021, 11, 941. [CrossRef]
- Service of the Republic of Poland. Szczepienie Przeciwko COVID-19. Narodowy Program Szczepień Przeciw COVID-19. Gov.pl website: https://www.gov.pl/web/szczepimysie/narodowy-program-szczepien-przeciw-covid-19, access: May 12, 2023.
- Lorent, D.; Nowak, R.; Luwański, D.; Pisarska-Krawczyk, M.; Figlerowicz, M.; Zmora, P. The Longitudinal Analysis on the Anti-SARS-CoV-2 Antibodies among Healthcare Workers in Poland—Before and after BNT126b2 mRNA COVID-19 Vaccination. Vaccines 2022, 10, 1576. [CrossRef]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. WHO website: https://covid19.who.int/region/euro/country/pl, access: May 12, 2023.
- Tretyn, A.; Szczepanek, J.; Skorupa, M.; Jarkiewicz-Tretyn, J.; Sandomierz, D.; Dejewska, J.; Ciechanowska, K.; Jarkiewicz-Tretyn, A.; Koper, W.; Pałgan, K. Differences in the Concentration of Anti-SARS-CoV-2 IgG Antibodies Post-COVID-19 Recovery or Post-Vaccination. Cells 2021, 10, 1952. [CrossRef]
- Röltgen K, Nielsen SCA, Silva O, Younes SF, Zaslavsky M, Costales C, et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040.e14. [CrossRef]
- Ali H, Alahmad B, Al-Shammari AA, Alterki A, Hammad M, Cherian P, et al. Previous COVID-19 Infection and Antibody Levels After Vaccination. Front Public Health 2021, 9, 778243. [CrossRef]
- Cantarelli C, Angeletti A, Perin L, Russo LS, Sabiu G, Podestà MA, et al. Immune responses to SARS-CoV-2 in dialysis and kidney transplantation. Clin Kidney J 2022, 15, 1816–1828. [CrossRef]
- Cossiga V, Capasso M, Guarino M, Loperto I, Brusa S, Cutolo FM, et al. Safety and Immunogenicity of Anti-SARS-CoV-2 Booster Dose in Patients with Chronic Liver Disease. J Clin Med 2023,12, 2281. [CrossRef]
- Bates, J.T.; Farmer, A.P.; Bierdeman, M.A.; Ederer, D.R.; Carney, L.S.; Montgomery, D.D.; Lirette, S.T.; Marshall, G.D. IgG Antibody Response to the Pfizer BNT162b2 SARS-CoV-2 Vaccine in Healthcare Workers with Healthy Weight, Overweight, and Obesity. Vaccines 2022, 10, 512. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).