Submitted:
05 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
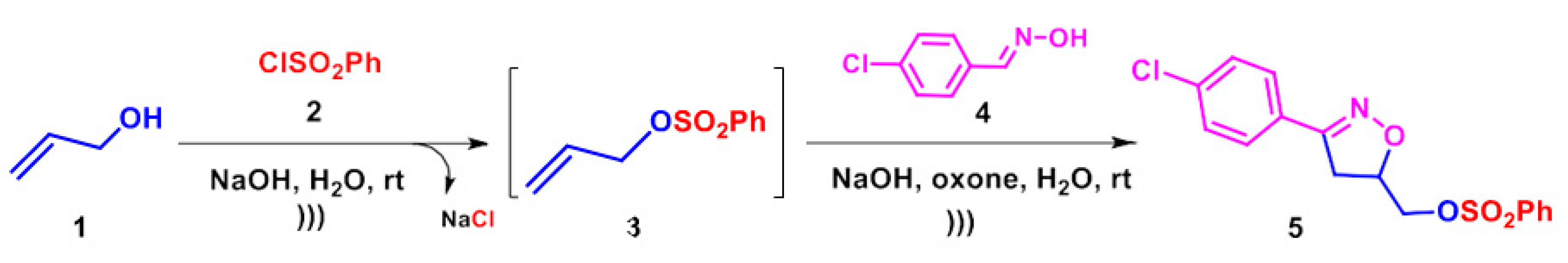
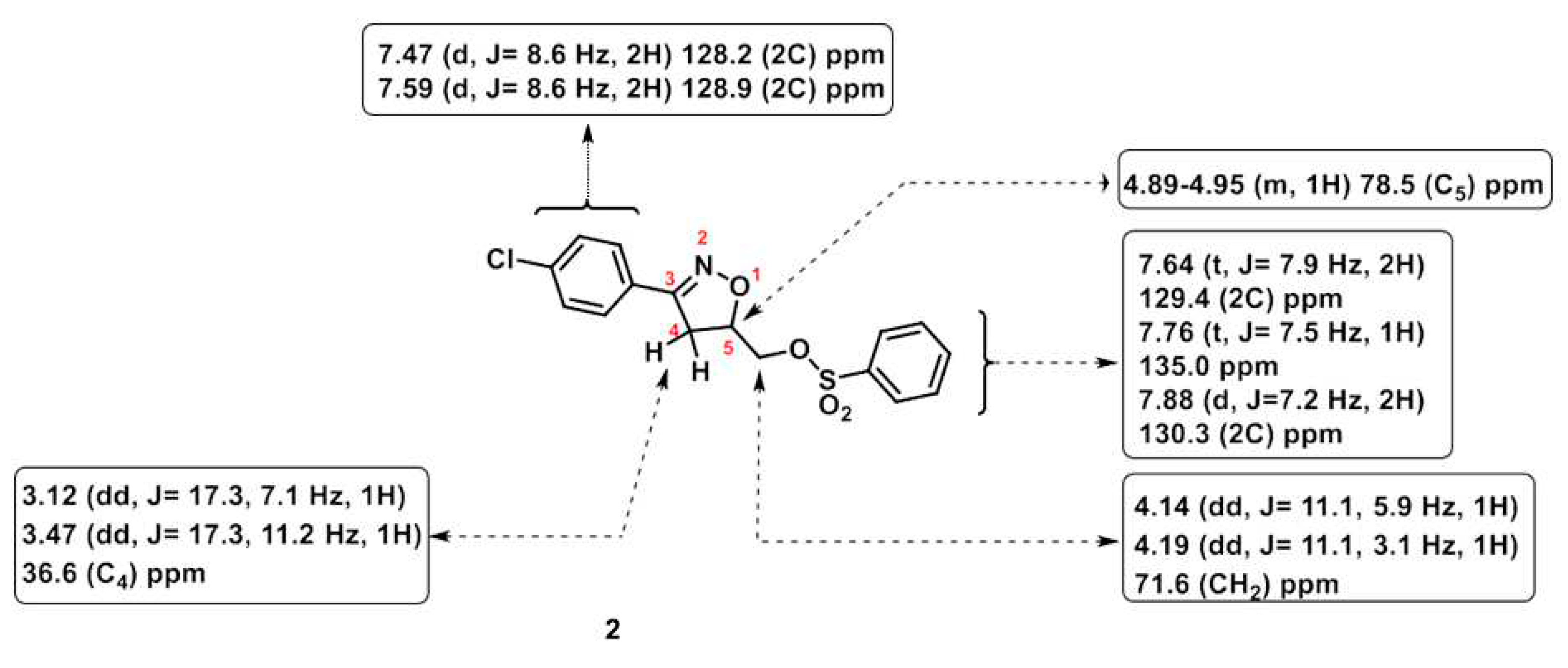
2.1. Synthesis
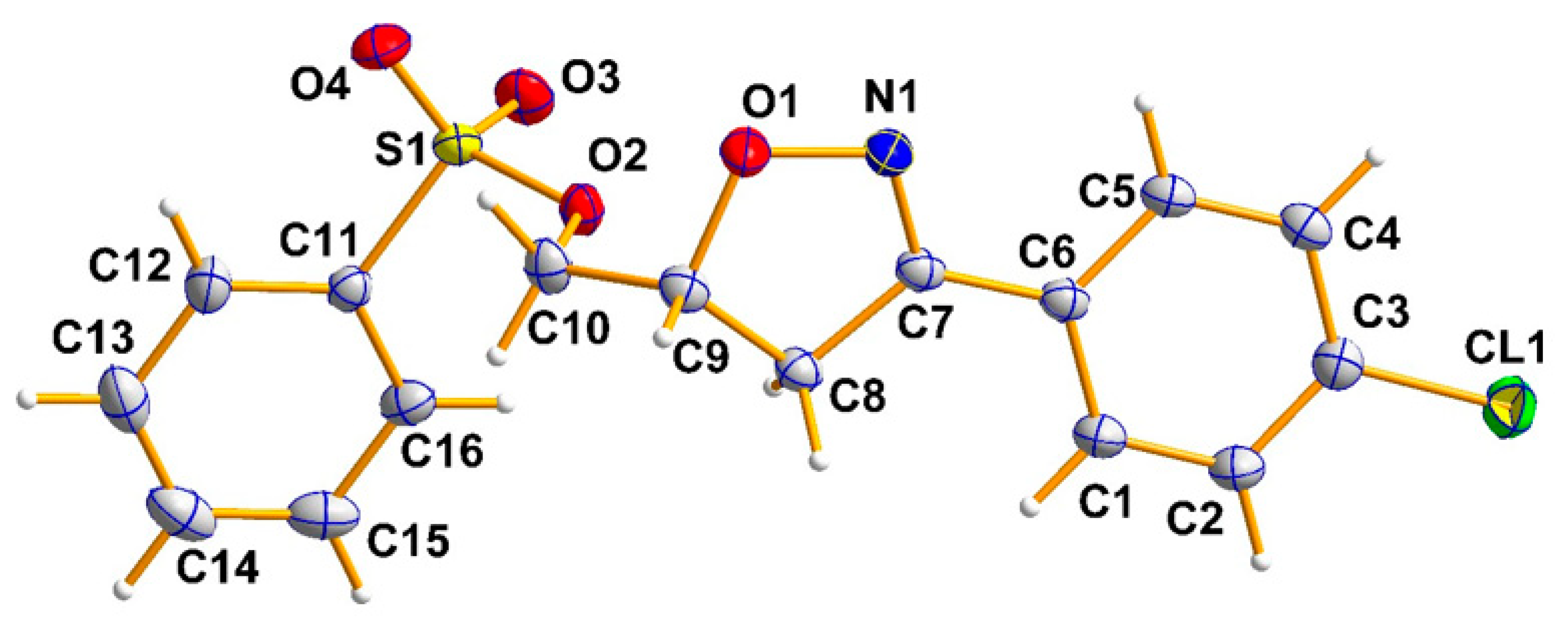
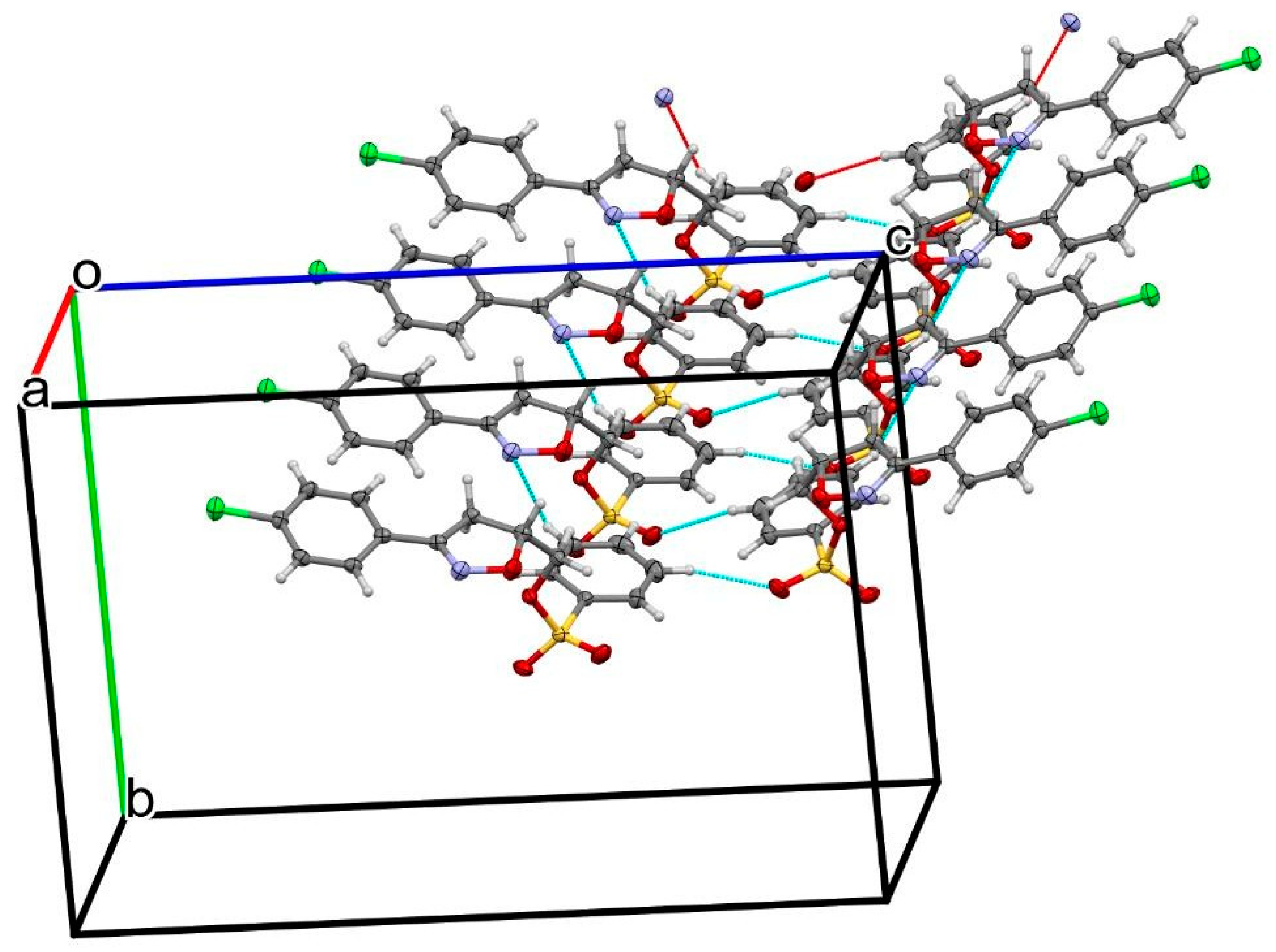
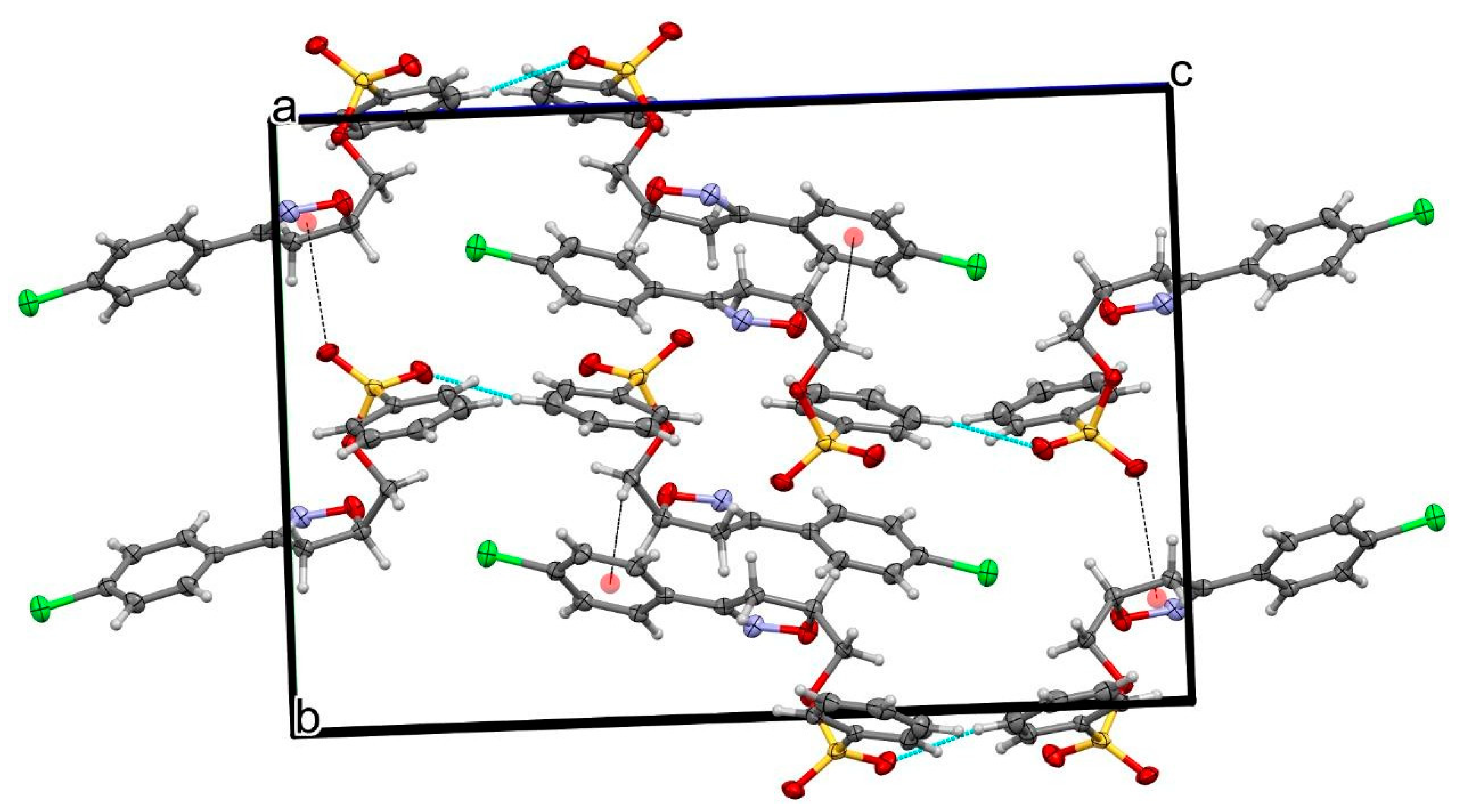
2.2. X-ray Analysis
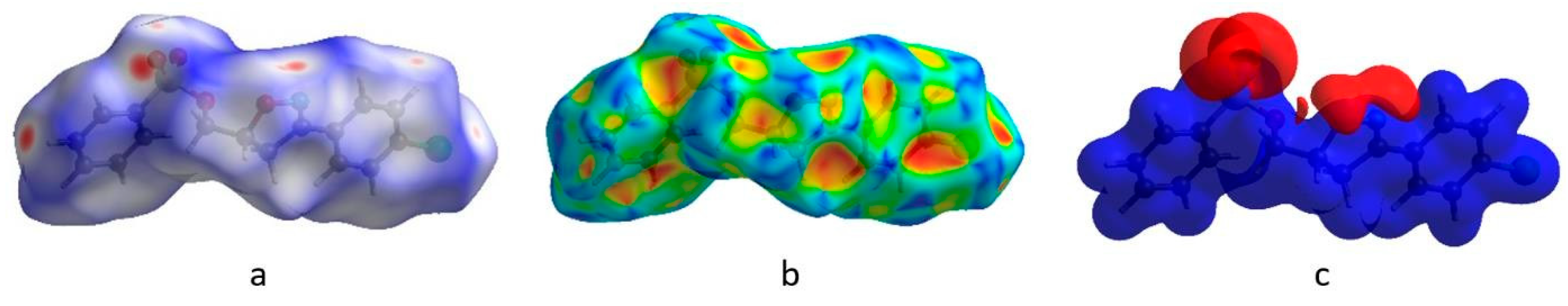
2.3. Theoretical Calculation Details
3. Experimental Section
3.1. Materials and Methods
3.2. Preparation of Compound 5
3.3. X-ray Crystal Structure Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aarjane, M.; Slassi, S.; Ghaleb, A.; Tazi, B.; Amine, A. Synthesis, biological evaluation, molecular docking and in silico ADMET screening studies of novel isoxazoline derivatives from acridone. Arab. J. Chem. 2021, 14, 103057–103069. [Google Scholar] [CrossRef]
- Kudryavtseva, T.N.; Lamanov, A.Y.; Sysoev, P.I.; Klimova, L.G. Synthesis and antibacterial activity of new acridone derivatives containing an isoxazoline fragment. Russ. J. Gen. Chem. 2020, 90, 45–49. [Google Scholar] [CrossRef]
- Chalkha, M.; Nour, H.; Chebbac, K.; Nakkabi, A.; Bahsis, L.; Bakhouch, M.; Akhazzane, M.; Bourass, M.; Chtita, S.; Bin Jardan, A.Y.; et al. Synthesis, Characterization, DFT Mechanistic Study, Antimicrobial Activity, Molecular Modeling, and ADMET Properties of Novel Pyrazole-isoxazoline Hybrids. ACS omega 2022, 7, 46731–46744. [Google Scholar] [CrossRef]
- Rasool, J.U.; Sawhney, G.; Shaikh, M.; Nalli, Y.; Madishetti, S.; Ahmed, Z.; Ali, A. Site selective synthesis and anti-inflammatory evaluation of Spiro-isoxazoline stitched adducts of arteannuin. B. Bioorg. Chem. 2021, 117, 105408–105417. [Google Scholar] [CrossRef] [PubMed]
- Shaik, A.; Bhandare, R.R.; Palleapati, K.; Nissankararao, S.; Kancharlapalli, V.; Shaik, S. Antimicrobial, antioxidant, and anticancer activities of some novel isoxazole ring containing chalcone and dihydropyrazole derivatives. Mol. 2020, 25, 1047–057. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.C.; Vesga, L.C.; Mendez-Sánchez, S.C.; Romero Bohórquez, A.R. Synthesis and anticancer activity of new tetrahydroquinoline hybrid derivatives tethered to isoxazoline moiety. Med. Chem. Res. 2020, 29, 675–689. [Google Scholar] [CrossRef]
- Goyard, D.; Kónya, B.; Chajistamatiou, A.S.; Chrysina, E.D.; Leroy, J.; Balzarin, S.; Maurel, P. Glucose-derived spiro- 276 isoxazolines are anti-hyperglycemic agents against type 2 diabetes through glycogen phosphorylase inhibition. Eur. J. Med. 277 Chem. 2016, 108, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Suk, D.H.; Cho, N.C.; Bhattarai, D.; Kang, S.B.; Kim, Y.; Keum, G. Synthesis and biological evaluation of isooxazoline derivatives as potent M1 muscarinic acetylcholine receptor agonists. Bioorg. Med. Chem. Lett. 2015, 25, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guan, A.; Wu, Q.; Cui, D.; Liu, C. Design, synthesis and herbicidal evaluation of novel uracil derivatives containing an isoxazoline moiety. Pest Manag. Sci. 2020, 76, 3395–3402. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Feng, D.; Li, F.; Luo, Y.; He, S.; Dong, Y.; Hu, D. Design, Synthesis, and Insecticidal Activity of Novel Isoxazoline Compounds That Contain Meta-diamides against Fall Armyworm (Spodoptera frugiperda). J. Agric. Food Chem. 2023, 71, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, H.; Hu, Y.; Li, X.; Gao, Y.; Ma, Z.; Lei, P. Structural Diversity Design, Synthesis, and Insecticidal Activity Analysis of Ester-Containing Isoxazoline Derivatives Acting on the GABA Receptor. J. Agric. Food Chem. 2023, 71, 3184–3191. [Google Scholar] [CrossRef]
- Mahmoudi, A.E.; Fegrouche, R.; Tachallait, H.; Lumaret, J.P.; Arshad, S.; Karrouchi, K.; Bougrin, K. Green Synthesis, Characterization, and Biochemical Impacts of New Bioactive Isoxazoline-sulfonamides as Potential Insecticidal Agents against the Sphodroxia Maroccana Ley. Pest Manag. Sci. 2023. [CrossRef] [PubMed]
- Shan, X.; Lv, M.; Wang, J.; Qin, Y.; Xu, H. Acaricidal and insecticidal efficacy of new esters derivatives of a natural coumarinosthole. Ind. Crops Prod. 2022, 182, 114855–114862. [Google Scholar] [CrossRef]
- Krishna, P. Chemoselective synthesis of 5-Amino-7-Bromoquinolin-8-Yl Sulfonate derivatives and their antimicrobial evaluation. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 685–690. [Google Scholar] [CrossRef]
- Kanabar, D.; Farrales, P.; Gnanamony, M.; Almasri, J.; Abo-Ali, E.M.; Otmankel, Y.; Shaha, H.; Nguyen, D.; Menyewia, M.E.; Dukhande, V.V.; D’Souzac, A.; Muth, A. Structural modification of the aryl sulfonate ester of cjoc42 for enhanced gankyrin binding and anti-cancer activity. Bioorganic Med. Chem. Lett. 2020, 30, 126889–126893. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Yang, Z.; Hu, X.; Wen, Y. Synthesis, antibacterial and insecticidal activities of novel capsaicin derivatives containing a sulfonic acid esters moiety. Front. Chem. 2022, 10, 929050–929057. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, G.; Zhang, Y.; Feng, T.; Xu, M.; Xu, H. Design, synthesis and evaluation of novel isoxazolines/oxime sulfonates of 2′(2′, 6′)-(di) chloropodophyllotoxins as insecticidal agents. Sci. Rep. 2016, 6, 33062–33073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, H.; Hu, Y.; Li, X.; Gao, Y.; Ma, Z.; Lei, P. Structural Diversity Design, Synthesis, and Insecticidal Activity Analysis of Ester-Containing Isoxazoline Derivatives Acting on the GABA Receptor. J. Agric. Food Chem. 2023, 71, 3184–3191. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Ouyang, L.; Jin, Q.; Zhang, J.; Luo, R. Recent advances in the oxime-participating synthesis of isoxazolines. Org. Biomol. Chem. 2020, 18, 4709–4716. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, J.; Li, C.; Lin, L.; Xu, Y. Progress in the Synthesis of Isoxazoline Derivatives by Cycloylation of AllyOxime. Chinese J. Org. Chem. 2020, 40, 2742. [Google Scholar] [CrossRef]
- Rane, D.; Sibi, M. Recent advances in nitrile oxide cycloadditions. Synthesis of isoxazolines. Curr. Org. Synth. 2011, 8, 616–627. [Google Scholar] [CrossRef]
- Lei, X.; Jalla, A.; Abou Shama, M.A.; Stafford, J.M.; Cao, B. Chromatography-free and eco-friendly synthesis of aryl tosylates and mesylates. Synthesis 2015, 2578–2585. [Google Scholar] [CrossRef]
- Alaoui, S.; Driowya, M.; Demange, L.; Benhida, R.; Bougrin, K. Ultrasound-assisted facile one-pot sequential synthesis of novel sulfonamide-isoxazoles using cerium (IV) ammonium nitrate (CAN) as an efficient oxidant in aqueous medium. Ultrason Sonochem. 2018, 40, 289–297. [Google Scholar] [CrossRef]
- Talha, A.; Favreau, C.; Bourgoin, M.; Robert, G.; Auberger, P.; Ammari, L.E.; Saadi, M.; Benhida, R.; Martin, A.R.; Bougrin, K. Ultrasound-assisted one-pot three-component synthesis of new isoxazolines bearing sulfonamides and their evaluation against hematological malignancies. Ultrason. Sonochem. 2021, 78, 105748–105759. [Google Scholar] [CrossRef]
- El Mahmoudi, A.; Chkirate, K.; Tachallait, H.; Van Meervelt, L.; Bougrin, K. 2-((3-(4-Methoxyphenyl)-4,5-dihydroisoxazol-5-yl)methyl)benzo[d]isothiazol-3(2H)-one1,1-dioxide. Molbank 2022, M1488. [Google Scholar] [CrossRef]
- El Mahmoudi, A.; Chkirate, K.; Mokhi, L.; Mague, J.T.; Bougrin, K. 2-(N-allylsulfamoyl)-N-propylbenzamide. Molbank 2023, M1678. [Google Scholar] [CrossRef]
- Thari, F.Z.; Tachallait, H.; El Alaoui, N.E.; Talha, A.; Arshad, S.; Álvarez, E.; Karrouchi, K.; Bougrin, K. Ultrasound-assisted one-pot green synthesis of new N-substituted-5-arylidene-thiazolidine-2, 4-dione-isoxazoline derivatives using NaCl/Oxone/Na3PO4 in aqueous media. Ultrason. Sonochem. 2020, 68, 105222–105251. [Google Scholar] [CrossRef] [PubMed]
- Bruker. APEX4, SAINT & SHELXTL, Bruker AXS LLC, Madison, WI. 2021.
- Sheldrick, G.M. SHELXT - Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D. Spackman, M.A. CrystalExplorer17. The University of Western Australia 2017.
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W. et al. D.J. GAUSSIAN09. Revision A.02. Gaussian Inc, Wallingford, CT, USA 2009.
- Chkirate, K.; Essassi, E.M. Pyrazole and Benzimidazole Derivatives: Chelating Properties Towards Metals Ions and their Applications. Curr. Org. Chem. 2022, 26, 1735–1766. [Google Scholar] [CrossRef]
- Faraj, I.; Oubella, A.; Chkirate, K.; Al Mamari, K.; Hökelek, T.; Mague, J.T.; El Ghayati, L.; Sebbar, N.K.; Essassi, E.M. Crystal 339 structure, Hirshfeld surface analysis and DFT calculations of (E)-3-[1-(2-hydroxyphenylanilino)ethylidene]-6-methylpyran-2,4- 340 dione. Acta Cryst. E 2022, 78, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Putz, H. DIAMOND. Crystal Impact GbR, Bonn, Germany. 2012.
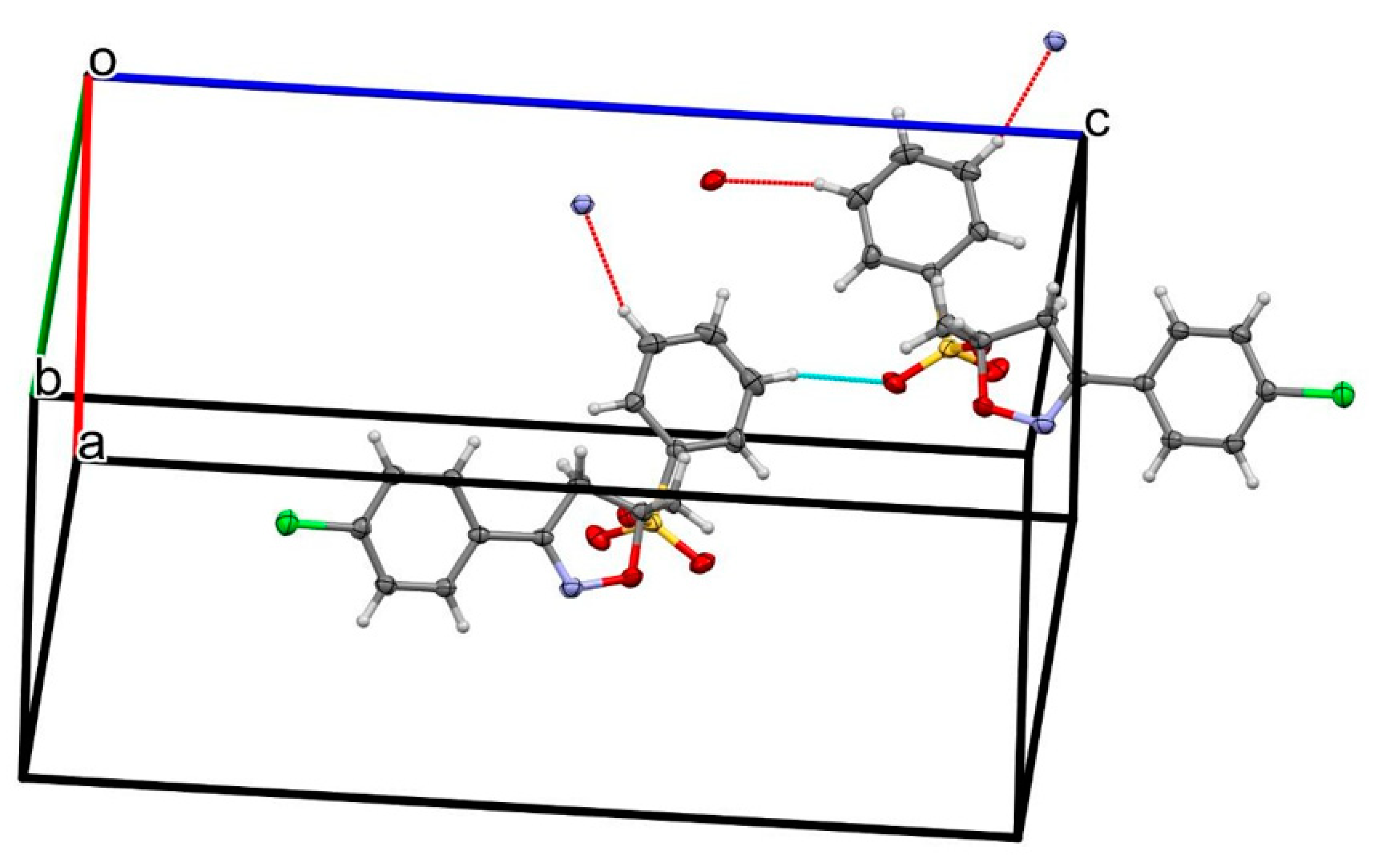
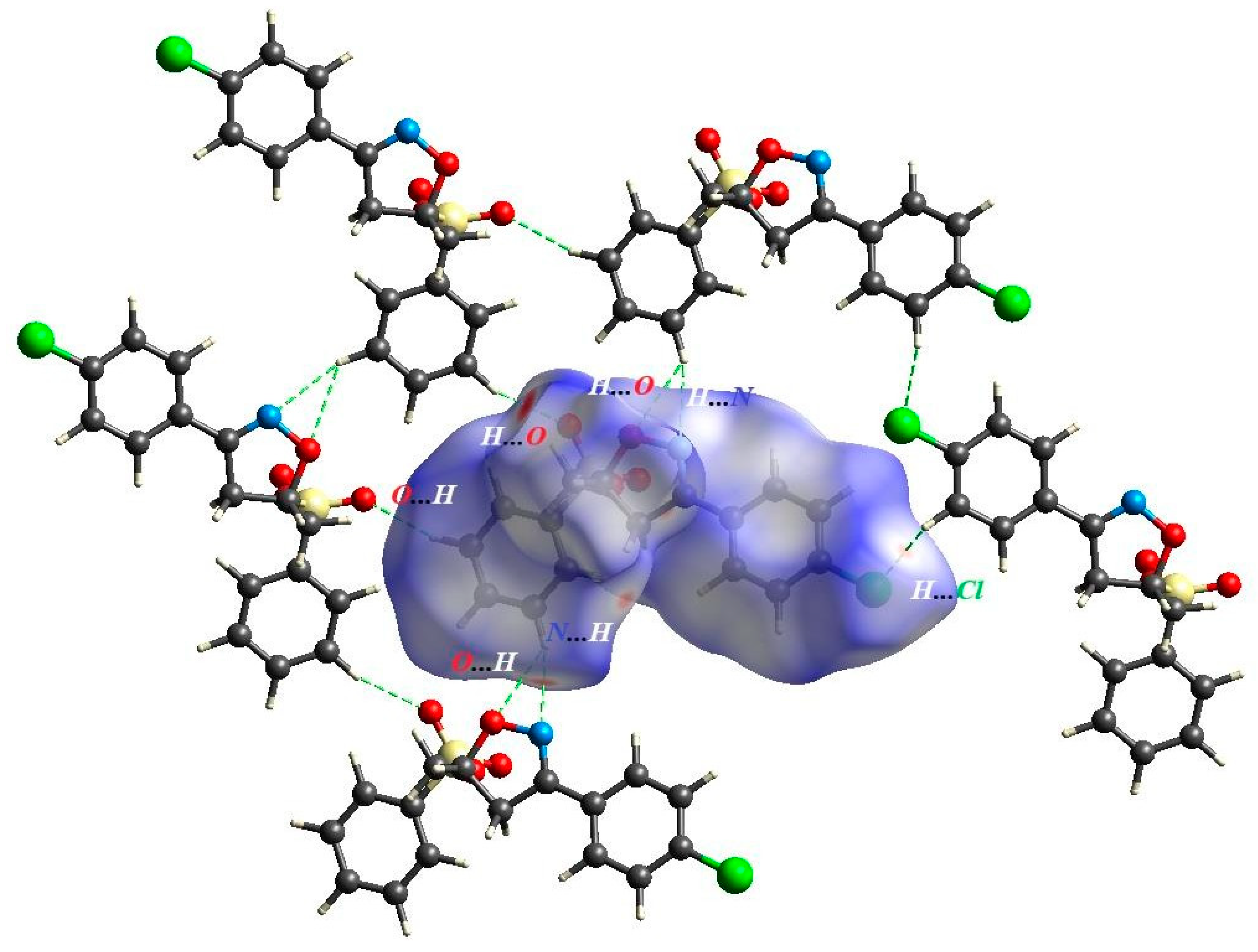
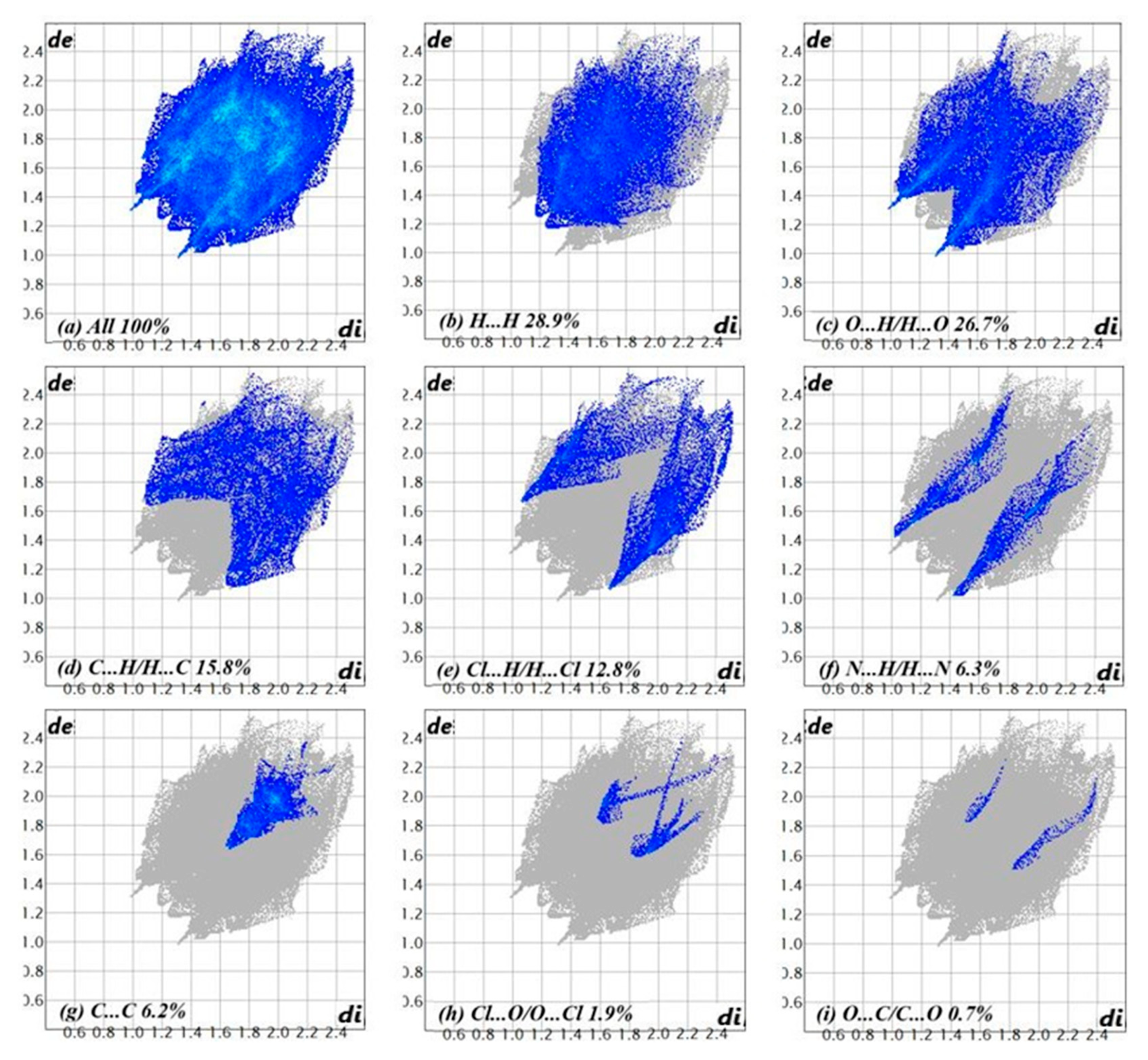
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| C2—H2···Cl1i | 0.95 | 2.84 | 3.5624 (11) | 133.2 |
| C4—H4···O4ii | 0.95 | 2.59 | 3.2807 (12) | 129.5 |
| C16—H16···O2iii | 0.95 | 2.61 | 3.4229 (12) | 144.1 |
| C16—H16···O3iii | 0.95 | 2.61 | 3.4416 (13) | 146.5 |
| X-ray | B3LYP/6–311G+(d,p) | |
|---|---|---|
| S1-O4 | 1.4318(8) | 1.4564 |
| S1-O3 | 1.4304(8) | 1.4566 |
| S1-O2 | 1.5791(7) | 1.6509 |
| S1-C11 | 1.7534(9) | 1.7867 |
| C10-O2 | 1.4573(11) | 1.4522 |
| C9-O1 | 1.4609(12) | 1.4564 |
| N1-O1 | 1.4126(11) | 1.3954 |
| N1-C7 | 1.2845(12) | 1.2827 |
| C3-Cl1 | 1.7445(10) | 1.7562 |
| S1-C11-C12 | 120.00(7) | 118.8948 |
| S1-C11-C16 | 118.24(7) | 118.9752 |
| C11-S1-O4 | 109.17(5) | 110.1399 |
| C11-S1-O3 | 110.00(5) | 109.829 |
| O4-S1-O3 | 119.42(5) | 120.2536 |
| O4-S1-O2 | 109.46(5) | 108.1129 |
| S1-O2-C10 | 117.55(6) | 116.2247 |
| C10-C9-O1 | 108.18(8) | 106.9904 |
| C9-O1-N1 | 109.36(7) | 109.628 |
| O1-N1-C7 | 109.72(8) | 110.3487 |
| N1-C7-C8 | 114.14(8) | 113.2056 |
| N1-C7-C6 | 121.07(8) | 121.4667 |
| C4-C3-Cl1 | 119.91(7) | 119.4282 |
| C2-C3-Cl1 | 118.44(8) | 119.5688 |
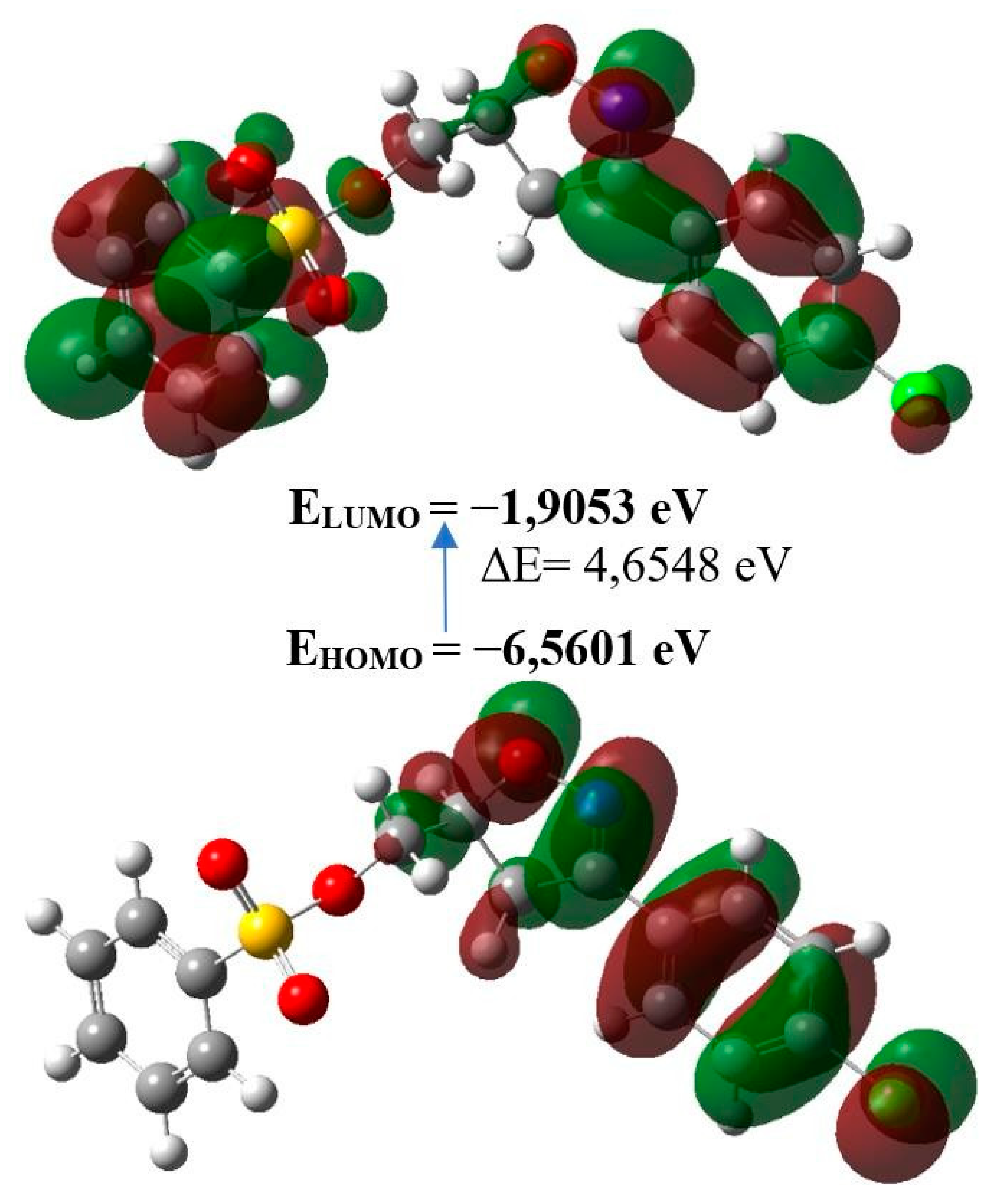
| Molecular Energy | Title Product |
|---|---|
| Total Energy TE (eV) | -49861,0176 |
| EHOMO (eV) | -6,5601 |
| ELUMO (eV) | -1,9053 |
| Gap, ΔE (eV) | 4,6548 |
| Dipole moment, µ (Debye) | 6.4787 |
| Ionization potential, I (eV) | 6,5601 |
| Electron affinity, A | 1,9053 |
| Electronegativity, χ | 4,2327 |
| Hardness, η | 2,3274 |
| Electrophilicity, index ω | 3,8489 |
| Softness, σ | 0,4297 |
| Transfer of a fraction of an electron, ΔN | 0,5945 |
| Crystal data | |
|---|---|
| CCDC Number | 2288360 |
| Empirical formula | C16H14ClNO4S |
| Formula weight | 351.79 |
| Temperature/K | 150 |
| Crystal system and Space group | Orthorhombic, Pbca |
| a, b, c (Å) | 9.5722 (11), 15.0133 (17), 21.978 (2) |
| α, β, γ (°) | 90, 90, 90 |
| Volume (Å3) | 3158.5 (6) |
| Z | 8 |
| Radiation type | Mo Kα |
| µ (mm−1) | 0.39 |
| Crystal size (mm) | 0.30 × 0.25 × 0.16 |
| Data collection | |
| Diffractometer | diffractometer Bruker D8 QUEST PHOTON 3 |
| Absorption correction | Numerical mu Calculated SADABS [40] |
| Tmin, Tmax | 0.89, 0.94 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 68386, 5453, 4943 |
| Rint | 0.032 |
| (sin θ/λ)max (Å−1) | 0.748 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.032, 0.093, 1.07 |
| No. of reflections | 5453 |
| No. of parameters | 208 |
| No. of restraints | 0 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.41, −0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





