Submitted:
08 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Inclusion criteria
2.2. Data collection
2.3. Ultrasound evaluation
3. Results
4. Discussion
5. Conclusion
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Condous, G.; Khalid, A.; Okaro, E.; Bourne, T. Should we be examining the ovaries in pregnancy? Prevalence and natural history of adnexal pathology detected at first-trimester sonography. Ultrasound Obstet. Gynecol. 2004, 24, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, Y.H.; Lee, M.Y.; Ko, H.S.; Oh, S.Y.; Seol, H.J.; Kim, J.W.; Ahn, K.H.; Na, S.; Seong, W.J.; Kim, H.S.; Park, C.W.; Park, J.S.; Jun, J.K.; Won, H.S.; Kim, M.Y.; Hwang, H.S.; Lee, S.M. Ultrasonographic evaluation of ovarian mass for predicting malignancy in pregnant women. Gynecologic Oncology 2021, 163, 385–391. [Google Scholar] [CrossRef]
- Hill, L.M.; Connors-Beatty, D.J.; Nowak, A.; Tush, B. The role of ultrasonography in the detection and management of adnexal masses during the second and third trimesters of pregnancy. Am J Obstet Gynecol 1998, 179, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Yazbek, J.; Salim, R.; Woelfer, B.; Aslam, N.; Lee, C.T.; Jurkovic, D. The value of ultrasound visualization of the ovaries during the routine 11–14 weeks nuchal translucency scan. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 132, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Hogston, P.; Lilford, R.J. Ultrasound study of ovarian cysts in pregnancy: prevalence and significance. BJOG: Int. J. Obstet. Gynaecol. 1986, 93, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, L.M.; Klebba, P.K.; Gray, D.L.; Mutch, D.G. Predictors of persistence of adnexal masses in pregnancy. Obstet Gynecol 1999, 93, 585–589. [Google Scholar]
- Moro, F.; Poma, C.B.; Zannoni, G.F.; Urbinati, A.V.; Pasciuto, T.; Ludovisi, M.; Moruzzi, M.C.; Carinelli, S.; Franchi, D.; Scambia, G.; et al. Imaging in gynecological disease (12): clinical and ultrasound features of invasive and non-invasive malignant serous ovarian tumors. Ultrasound Obstet. Gynecol. 2017, 50, 788–799. [Google Scholar] [CrossRef]
- Ludovisi, M.; Foo, X.; Mainenti, S.; Testa, A.C.; Arora, R.; Jurkovic, D. Ultrasound diagnosis of serous surface papillary borderline ovarian tumor: A case series with a review of the literature. J. Clin. Ultrasound 2015, 43, 573–577. [Google Scholar] [CrossRef]
- Fagotti, A.; Ludovisi, M.; De Blasis, I.; Virgilio, B.; Di Legge, A.; Mascilini, F.; Moruzzi, M.; Giansiracusa, C.; Fanfani, F.; Tropeano, G.; et al. The sonographic prediction of invasive carcinoma in unilocular-solid ovarian cysts in premenopausal patients: a pilot study. Hum. Reprod. 2012, 27, 2676–2683. [Google Scholar] [CrossRef]
- Valentin, L. Use of morphology to characterize and manage common adnexal masses. Best Pr. Res. Clin. Obstet. Gynaecol. 2004, 18, 71–89. [Google Scholar] [CrossRef]
- Hoover, K.; Jenkins, T.R. Evaluation and management of adnexal mass in pregnancy. American Journal of Obstetrics & Gynecology august 2011, 97–102. [Google Scholar]
- Fruscio, R.; De Daan, J.; Van Calsteren, K.; Verheecke, M.; Mhallem, M.; Amant, F. Ovarian cancer in pregnancy. Best Practice & Research Clinical Obstetrics and Gynaecology 2017, 41, 108–117. [Google Scholar]
- Aggarwal, P.; Kehoe, S. Ovarian tumours in pregnancy: A literature review. Eur J Obstet Gynecol Reprod Biol 2011, 155, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ferrazzi, E.; Ameye, L.; Konstantinovic, M.L.; Van Calster, B.; Collins, W.P.; Vergote, I.; Van Huffel, S.; et al. Logistic Regression Model to Distinguish Between the Benign and Malignant Adnexal Mass Before Surgery: A Multicenter Study by the International Ovarian Tumor Analysis Group. J. Clin. Oncol. 2005, 23, 8794–8801. [Google Scholar] [CrossRef]
- D’ambrosio, V.; Brunelli, R.; Musacchio, L.; Del Negro, V.; Vena, F.; Boccuzzi, G.; Boccherini, C.; Di Donato, V.; Piccioni, M.G.; Panici, P.B.; et al. Adnexal masses in pregnancy: an updated review on diagnosis and treatment. Tumori J. 2020, 107, 12–16. [Google Scholar] [CrossRef]
- Valentin, L. Prospective cross-validation of Doppler ultrasound examination and gray-scale ultrasound imaging for discrimination of benign and malignant pelvic masses. Ultrasound Obstet. Gynecol. 1999, 14, 273–283. [Google Scholar] [CrossRef]
- Froyman, W.; Landolfo, C.; De Cock, B.; Wynants, L.; Sladkevicius, P.; Testa, A.C.; Van Holsbeke, C.; Domali, E.; Fruscio, R.; Epstein, E.; et al. Risk of complications in patients with conservatively managed ovarian tumours (IOTA5): a 2-year interim analysis of a multicentre, prospective, cohort study. Lancet Oncol. 2019, 20, 448–458. [Google Scholar] [CrossRef]
- Timmerman, D.; Ameye, L.; Fischerova, D.; Epstein, E.; Melis, G.B.; Guerriero, S.; Van Holsbeke, C.; Savelli, L.; Fruscio, R.; Lissoni, A.A.; Testa, A.C.; Veldman, J.; Vergote, I.; Van Huffel, S.; Bourne, T.; Valentin, L. Simple ultrasound rules to distinguish between benign and malignant adnexal masses be-fore surgery: Prospective validation by IOTA group. BMJ 2010, 341, c6839. [Google Scholar] [CrossRef]
- Nunes, N.; Ambler, G.; Foo, X.; Naftalin, J.; Widschwendter, M.; Jurkovic, D. Use of IOTA simple rules for diagnosis of ovarian cancer: meta-analysis. Ultrasound Obstet. Gynecol. 2014, 44, 503–514. [Google Scholar] [CrossRef]
- Van Calster, B.; Van Hoorde, K.; Valentin, L.; Testa, A.C.; Fischerova, D.; Van Holsbeke, C.; Savelli, L.; Franchi, D.; Epstein, E.; Kaijser, J.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ 2014, 349, g5920. [Google Scholar] [CrossRef]
- Timmerman, D.; Van Calster, B.; Testa, A.; Savelli, L.; Fischerova, D.; Froyman, W.; Wynants, L.; Van Holsbeke, C.; Epstein, E.; Franchi, D.; et al. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am. J. Obstet. Gynecol. 2016, 214, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.J.; Kim, T.J.; Lee, J.E.; Kwon, Y.S.; Kim, H.J.; Lee, I.H.; Lim, K.T.; Lee, K.H.; Shim, J.U.; Mok, J.E. Risk of torsion and malignancy by adnexal mass size in pregnant women. Acta Obstet Gynecol Scand 2011, 90, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Mascilini, F.; Moruzzi, C.; Giansiracusa, C.; Guastafierro, F.; Savelli, L.; De Meis, L.; Epstein, E.; Timor-Tritsch I., E.; Mailath-Pokorny, M.; Ercoli, A.; Exacoustos, C.; Benacerraf B., R.; Valentin, L.; Testa, A.C. Imaging in gynecological disease. 10: Clinical and ultrasound characteristics of decidualized endometriomas surgically removed during pregnancy. Ultrasound Obstet Gynecol 2014, 44, 354–360. [Google Scholar] [CrossRef]
- Timmerman, D.; Valentin, L.; Bourne, T.H.; Collins, W.P.; Verrelst, H.; Vergote, I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.C.; Mascilini, F.; Quagliozzi, L.; Moro, F.; Bolomini, G.; Mirandola, M.T.; Moruzzi, M.C.; Scambia, G.; Fagotti, A. Management of ovarian masses in pregnancy: patient selection for interventional treatment. Int. J. Gynecol. Cancer 2020, 31, 899–906. [Google Scholar] [CrossRef]
- Whitecar, P.; Turner, S.; Higby, K. Adnexal masses in pregnancy: A review of 130 cases undergoing surgical management. Am. J. Obstet. Gynecol. 1999, 181, 19–24. [Google Scholar] [CrossRef]
- Giuntoli, R.L.; Vang, R.S.; Bristow, R.E. Evaluation and Management of Adnexal Masses During Pregnancy. Clin. Obstet. Gynecol. 2006, 49, 492–505. [Google Scholar] [CrossRef]
- Kaijser, J.; Vandecaveye, V.; Deroose, C.M.; Rockall, A.; Thomassin-Naggara, I.; Bourne, T.; Timmerman, D. Imaging techniques for the pre-surgical diagnosis of adnexal tumours. Best Pr. Res. Clin. Obstet. Gynaecol. 2014, 28, 683–695. [Google Scholar] [CrossRef]
- Valentin, L.; Hagen, B.; Tingulstad, S.; Eik-Nes, S. Comparison of ‘pattern recognition’ and logistic regression models for discrimination between benign and malignant pelvic masses: a prospective cross validation. Ultrasound Obstet. Gynecol. 2001, 18, 357–365. [Google Scholar] [CrossRef]
- Timmerman, D. The use of mathematical models to evaluate pelvic masses; can they beat an expert operator? Best Pract Res Clin Obstet Gynaecol 2004, 18, 91–104. [Google Scholar] [CrossRef]
- Meys, E.; Kaijser, J.; Kruitwagen, R.; Slangen, B.; Van Calster, B.; Aertgeerts, B.; Verbakel, J.; Timmerman, D.; Van Gorp, T. Subjective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2016, 58, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Schwärzler, P.; Collins, W.P.; Claerhout, F.; Coenen, M.; Amant, F.; Vergote, I.; Bourne, T.H. Subjective assessment of adnexal masses with the use of ultrasonography: an analysis of interobserver variability and experience. Ultrasound Obstet. Gynecol. 1999, 13, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Yazbek, J.; Raju, S.K.; Ben-Nagi, J.; Holland, T.K.; Hillaby, K.; Jurkovic, D. Effect of quality of gynaecological ultrasonography on management of patients with suspected ovarian cancer: a randomised controlled trial. Lancet Oncol. 2008, 9, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Czekierdowski, A.; Stachowicz, N.; Smole ´n, A.; Kluz, T.; Łozi ´nski, T.; Miturski, A.; Kraczkowski, J. Sonographic Assessment of Complex Ultrasound Morphology Adnexal Tumors in Pregnant Women with the Use of IOTA Simple Rules Risk and ADNEX Scoring Systems. Diagnostics 2021, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Moro, F.; Mascilini, F.; Pasciuto, T.; Leombroni, M.; Destri, M.L.; De Blasis, I.; Garofalo, S.; Scambia, G.; Testa, A.C. Ultrasound features and clinical outcome of patients with malignant ovarian masses diagnosed during pregnancy: experience of a gynecological oncology ultrasound center. Int. J. Gynecol. Cancer 2019, 29, 1182–1194. [Google Scholar] [CrossRef]
- Pateman, K.; Moro, F.; Mavrelos, D.; Foo, X.; Hoo, W.L.; Jurkovic, D. Natural history of ovarian endometrioma in pregnancy. BMC Womens Health 2014, 14, 128. [Google Scholar] [CrossRef]
- Di Legge, A.; Pollastri, P.; Mancari, R.; Ludovisi, M.; Mascilini, F.; Franchi, D.; Jurkovic, D.; Coccia M., E.; Timmerman, D.; Scambia, G.; Testa, A.C.; Valentin, L. Clinical and ultrasound characteristics of surgically removed adnexal lesions with largest diameter ≤ 2.5 cm: A pictorial essay. Ultrasound Obstet Gynecol 2017, 50, 648–656. [Google Scholar] [CrossRef]
- Bruno, M.; Capanna, G.; Di Florio, C.; Sollima, L.; Guido, M.; Ludovisi, M. Sonographic characteristics of ovarian Leydig cell tumor. Ultrasound Obstet. Gynecol. 2023, 62, 441–442. [Google Scholar] [CrossRef]
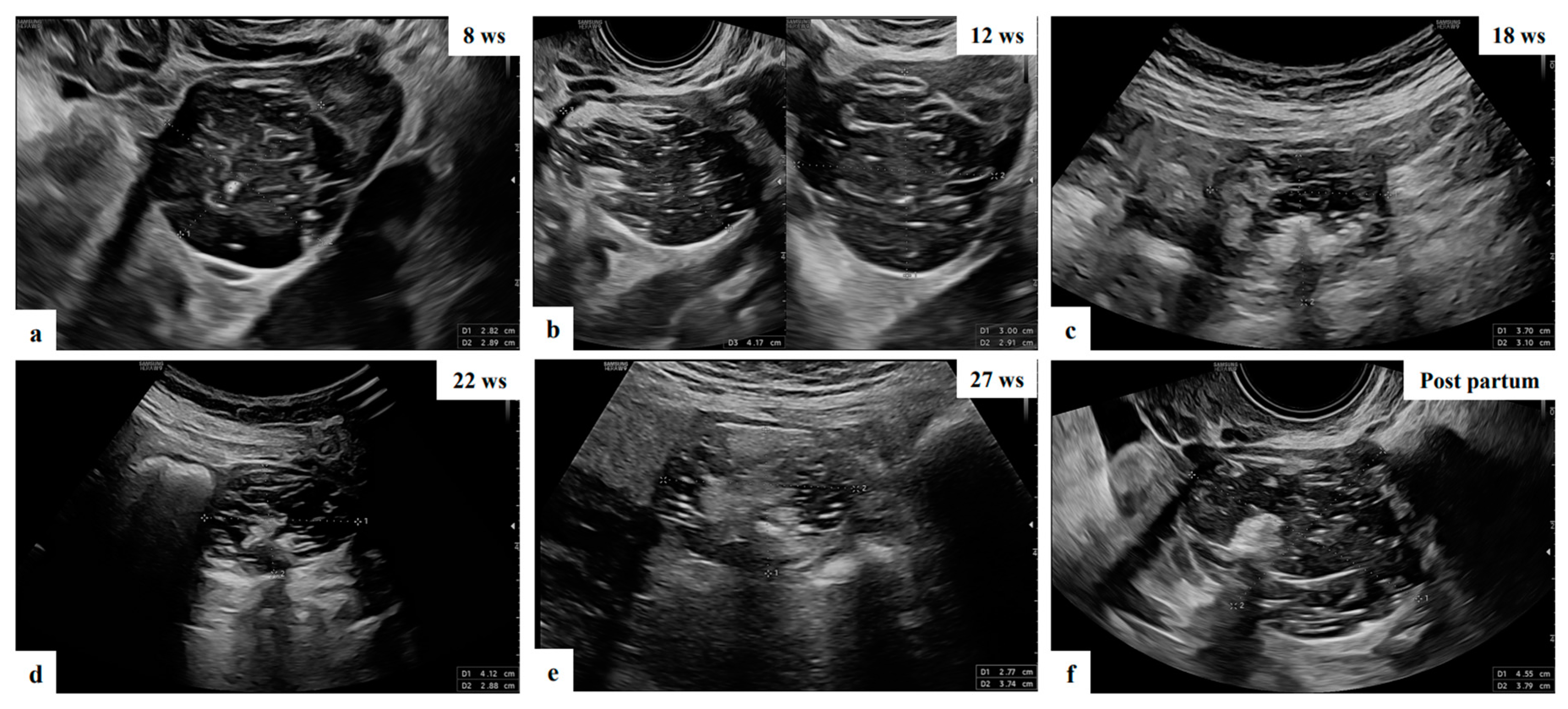
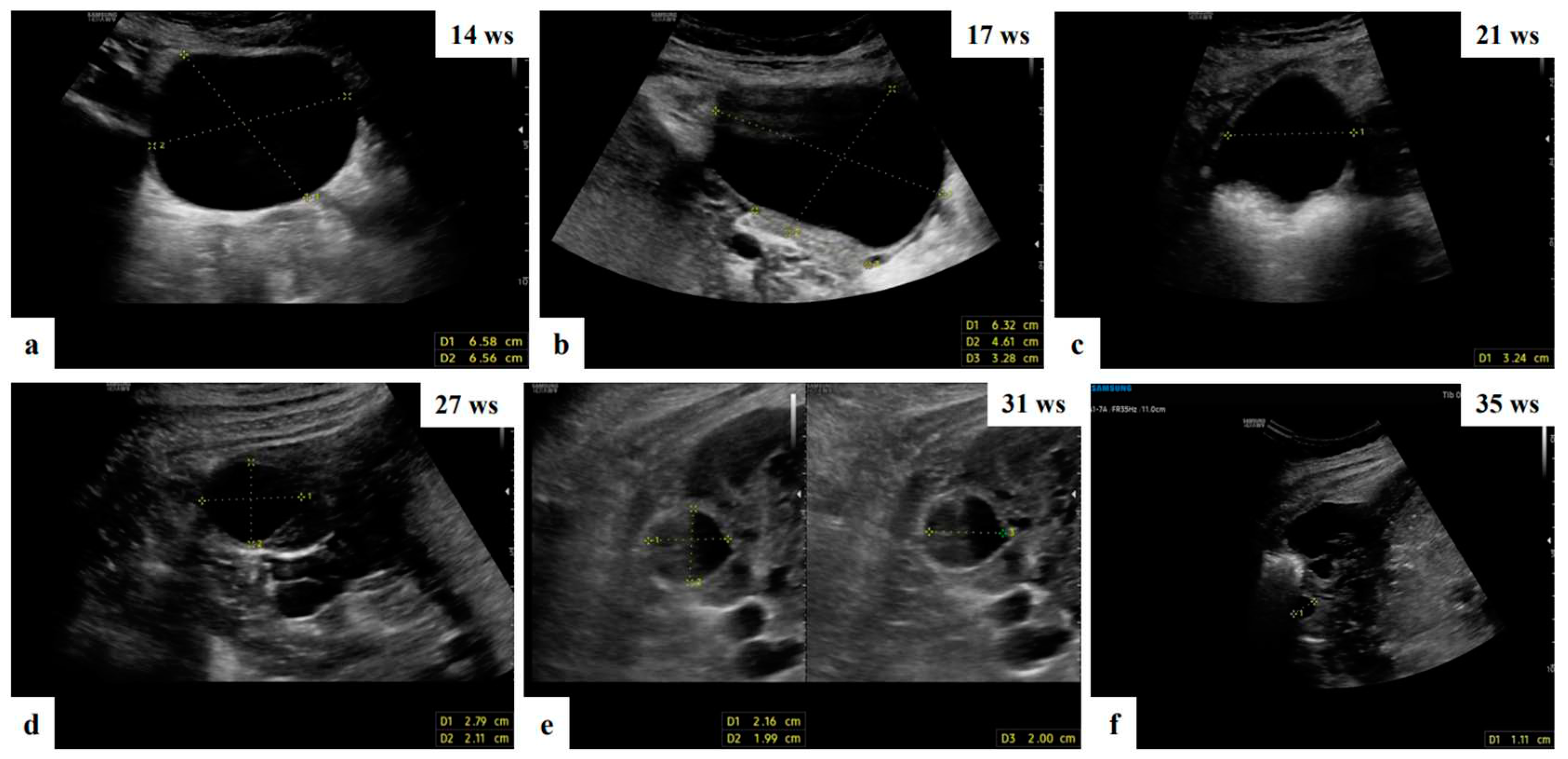

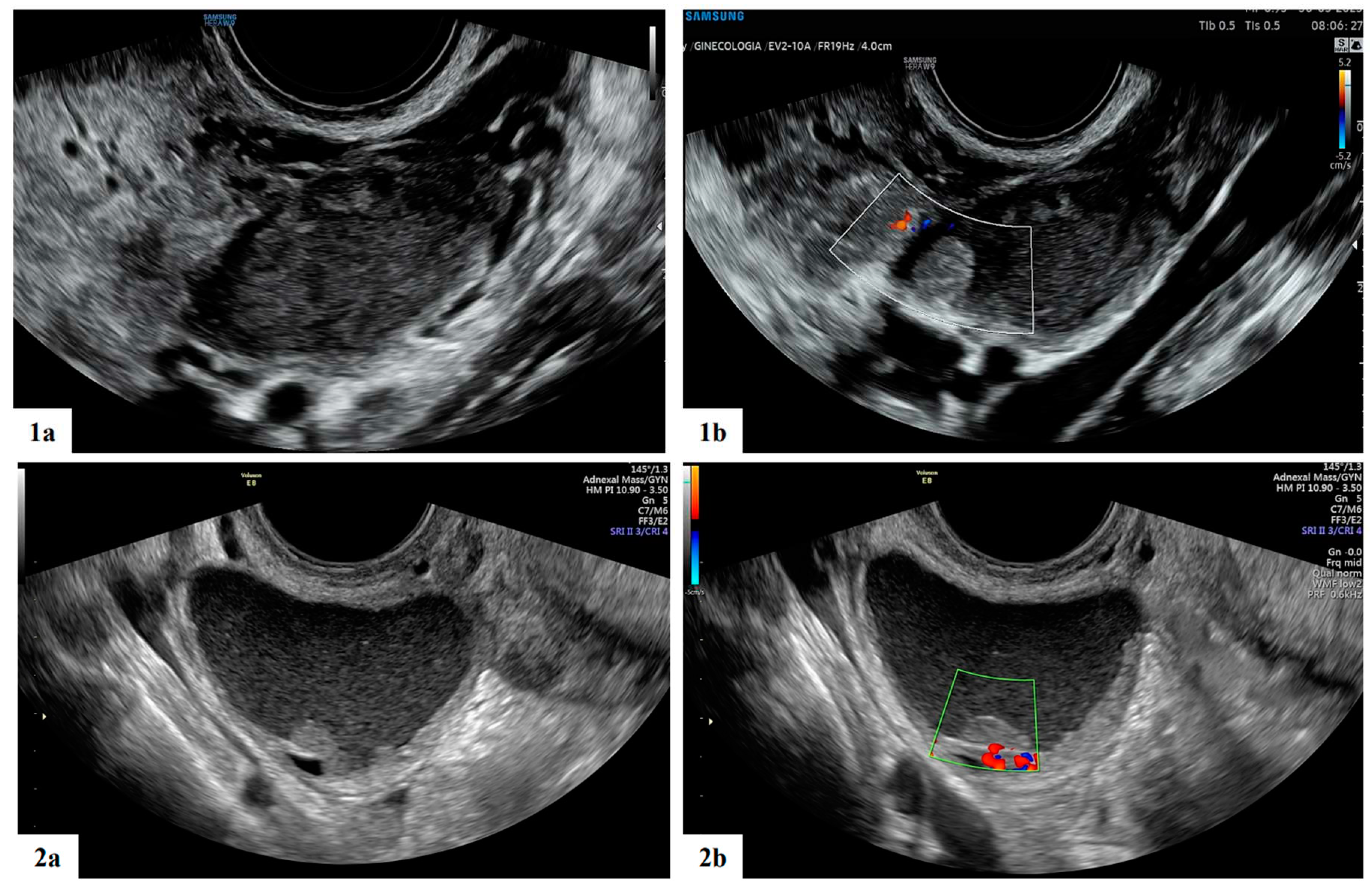
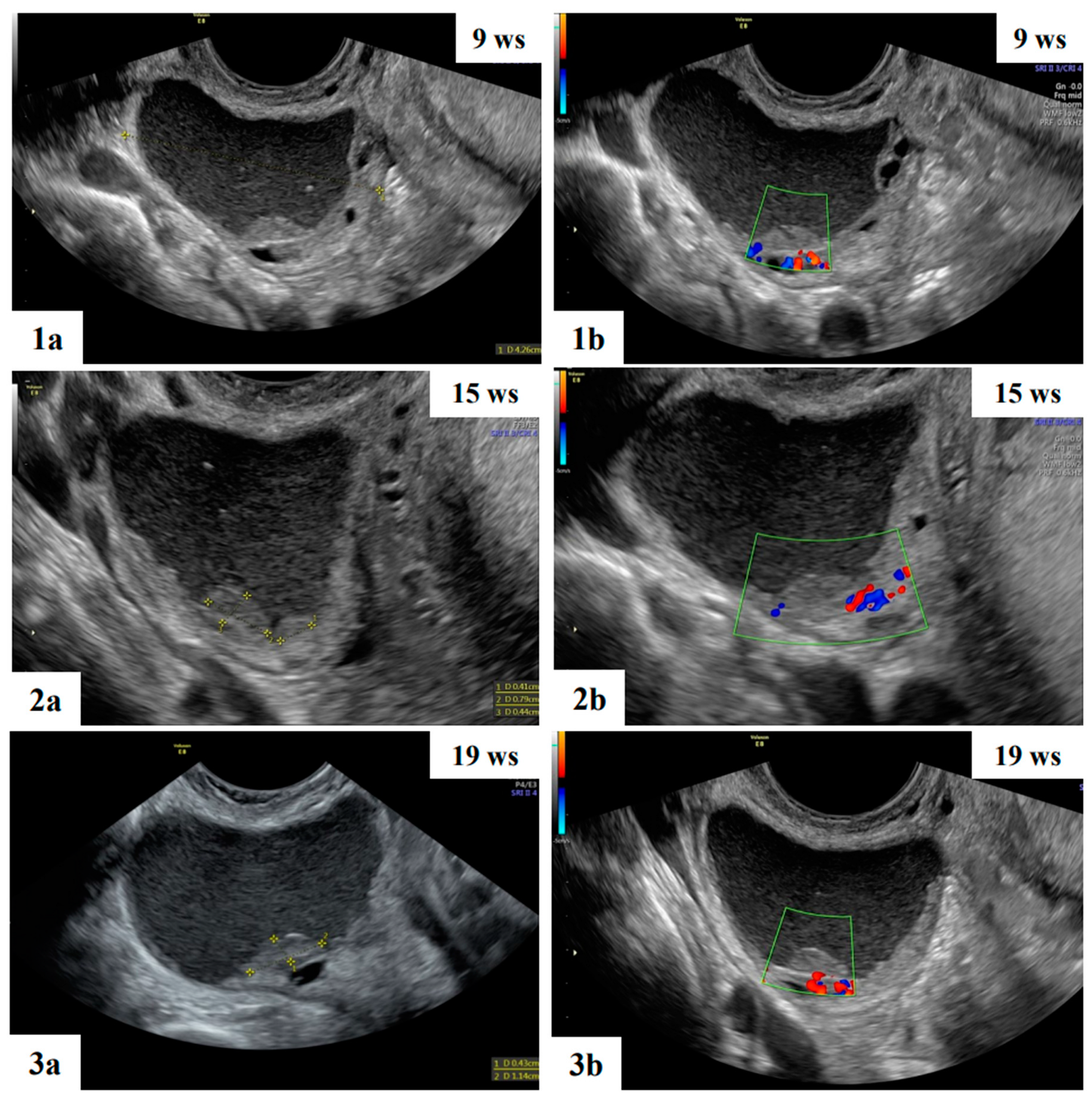
| Characteristics | Value |
|---|---|
| Number of cases | 17 |
| Nulliparous | 15 (88.2) |
| Ultrasound diagnosis before pregnancy | 5 (29.4) |
| Age at diagnosis (years) | 33 (23-39) |
| Gestational age at diagnosis (weeks) | 10 (6-21) |
| Gestational age at last ultrasound examination (weeks) | 26 (9-39) |
| Gestational age at delivery* | 39 (27-41) |
| Delivery Spontaneous delivery Cesarean section Ongoing pregnancy |
6 (35.3)5 (29.4)6 (35.3) |
| Management Follow up Surgery after delivery Spontaneous resolution |
9 (52.9)4 (23.5)4 (23.5) |
| Histological diagnosis** Teratoma Serous cystoadenofibroma Corpus luteum |
2 (50)1 (25)1 (25) |
| Ultrasound characteristics | All (n=17) |
|---|---|
| Bilateral mass | 1 |
| Maximum diameter of lesion (mm) (range) | 46 (18-121) |
| Type of tumor Unilocular Unilocular solid Multilocular Multilocular solid Solid |
9 4 3 0 1 |
| Cyst content echogenicity Anechoic Low level Ground glass Mixed Hemorrhagic Not applicable (solid mass) |
6 1 3 5 1 1 |
| Color score 1 2 3 4 |
14 2 1 0 |
| Maximum diameter of largest solid component (mm) (range) | 14 (8-38) |
| Presence of papillary projections | 4 |
| Number of papillary projections 1 2 3 |
1/4 2/4 1/4 |
| Papillation contour Irregular Smooth |
1/4 3/4 |
| Papillation flow Present Absent |
2/4 2/4 |
| Heigh of the largest papillary projection (mm) (range) | 7.5 (3-13) |
| Presence of acoustic shadow | 3 |
| Presence of crescent sign | 13 |
| Diagnosis based on subjective assessment Benign Borderline Malignant |
17 0 0 |
| Specific diagnosis suggested by the original examiner Teratoma Functional cyst Decidualized endometrioma Paraovarian cyst Endometrioma Fibroma Cystoadenofibroma Sactosalpinx Corpus luteum |
5 3 2 2 1 1 1 1 1 |
| Patients | Gestational age at delivery |
Singleton/ twin pregnancy |
Type of delivery |
Indication for cesarean section | Perineal lacerations |
Blood loss during delivery(cc) |
APGAR score | Fetal weight(gr) |
|---|---|---|---|---|---|---|---|---|
| 1 | 27 | Singleton | Caesarean section and cystectomy |
pPROM, breech presentation | - | 500 | 7/8 | 870 |
| 2 | 40 | Singleton | Vaginal delivery |
- | Episiotomy and I grade laceration | 700 | 8/9 | 3500 |
| 3 | 38 | Singleton | Vaginal delivery |
- | - | 150 | 8/9 | 3450 |
| 4 | 41 | Singleton | Caesarian delivery |
Abnormal CTG | - | 500 | 9/10 | 3185 |
| 5 | 38 | Singleton | Vaginal delivery |
- | I gradelaceration | 100 | 8/10 | 3070 |
| 6 | 39 | Singleton | Caesarian delivery |
- | II grade laceration | 300 | 9/9 | 2880 |
| 7 | 40 | Singleton | Vaginal delivery |
Abnormal CTG | - | 300 | 9/10 | 3525 |
| 8 | 40 | Singleton | Caesarian delivery |
- | Episiotomy | 500 | 9/10 | 3300 |
| 9 | 38 | Singleton | Vaginal delivery |
Fetalmalformation | - | 500 | 7/9 | 3280 |
| 10 | 40 | Singleton | Caesarian delivery |
- | Episiotomy | 200 | 9/10 | 3280 |
| 11 | 36 | Singleton | Caesarean section and unilateral salpingo- oophorectomy |
Myomapraevia | - | 600 | 8/9 | 2850 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





