1. Introduction
The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains a global emergency situation, with more than 6 million deaths reported so far [
1]. The pediatric population develop mostly mild or moderate symptoms in SARS-CoV-2 infections, but there is evidence of severe complications such as the multisystem inflammatory syndrome (MIS-C) and long-term sequelae ("long COVID") [
2,
3]. Children also play an important role in the transmission of the disease to other vulnerable groups, such as people over 65 years [
4].
With more than 13 billion doses administrated so far, vaccination against COVID-19 in the adult population has been extended worldwide, but only 32.6 % of the population in low income countries has received at least one vaccine dose [
5]. Several vaccines have been approved for children by local and global agencies; mRNA-based vaccines and virus-inactivated vaccines have been the most applied in the pediatric population over 5 years-old [
6]. Vaccines based on protein subunits are very attractive for childhood vaccination due to their safety and efficacy as demonstrated through millions of doses administered in children against typical childhood infectious diseases like pneumonia or meningitis [
7]. SOBERANA
® 02 and SOBERANA
® Plus are two protein subunit vaccines against SARS-CoV-2 produced by the Finlay Institute of Vaccines in Havana, Cuba. SOBERANA
® 02 (or FINLAY-FR-2) is based on the chemical conjugation of the SARS-CoV-2 recombinant receptor binding domain (RBD) to tetanus toxoid [
8]; SOBERANA
® Plus (or FINLAY-FR-1A) is based on the recombinant RBD-dimer [
9]. The heterologous three-dose combination of two doses of SOBERANA
® 02 28 days apart followed by one dose of SOBERANA
® Plus have demonstrated its safety and immunogenicity in adults 19-80 y/o [
10,
11,
12] and in children 3-18 y/o [
13]. This scheme prevented COVID-19 disease in adults with an efficacy of 92% protection against symptomatic disease [
10].
A phase I/II clinical trial in 3-18 y/o children measured the humoral immune response as well as cytokines secretion induced by this heterologous scheme [
13]. In the present study, we characterize in detail the humoral and cellular response by measuring specific B and T cell subpopulations in a subset of children vaccinated with the heterologous scheme during the clinical trial compared to children recovered from mild symptomatic COVID-19 after natural infection with SARS-CoV-2.
2. Materials and Methods
2.1. Subjects and ethics
A subgroup of children between 5-11 years of age (n=15), included in a phase I/II clinical trial conducted at the “Juan Manuel Marquez” Pediatric Hospital, were randomly selected from this study [
13] (trial registry:
https://rpcec.sld.cu/trials/RPCEC00000374-En). They were vaccinated every 28 days with the heterologous scheme of two doses of SOBERANA
® 02 and a third dose with SOBERANA
® Plus. The study was conducted during October 2021 with a prevalence of delta variant and before the appearance of omicron [
14].
Additionally, 10 children aged 4-11 y/o, who all had suffered from mild symptomatic COVID-19 45 - 60 days before blood draw (as control of natural immunity to SARS-CoV-2), were recruited in a specialized follow-up medical consultation for convalescent children, at the same hospital where the clinical trial was conducted. These children recovered from COVID-19 were studied as reference for immunity caused by natural infection; they were not participants in the clinical trial. Their parents signed an informed consent for including their children in this study and the results were informed to them.
During recruitment, the medical investigators provided to the parents, both orally and written, all information about the vaccine and potential risks and benefits.
2.2. Blood sampling and peripheral blood mononuclear cells isolation
Blood samples were taken 14 days after the last (third) dose of the SOBERANA® 02/SOBERANA® Plus heterologous scheme for vaccinated children, and 45-60 days after the infection diagnosis (by RT-PCR for SARS-CoV-2) for the control group (children recovered of the symptomatic mild COVID-19 disease).
Blood samples from individuals were collected by venipuncture in EDTA-K3 coated tubes Vacutainer™ (Greiner Bio-One). Peripheral blood mononuclear cells (PBMCs) were isolated by differential centrifugation in a gradient of Ficoll-Paque™ Plus (GE-Healthcare) following manufacturer’s guidelines. Briefly, anti-coagulated PBMCs were carefully layered over Ficoll and centrifuged at 400 xg during 20 min at room temperature. After centrifugation, the plasma fraction was removed for antibody determinations and stored at -80 °C until use. The PBMC-rich layer was carefully removed, washed multiple times, centrifuged to remove platelets and stored in a cryopreservation medium with heat-inactivated fetal bovine serum (FBS, Gibco) and 10% DMSO (Sigma-Aldrich) in liquid nitrogen until use.
2.3. Antibody determinations
Plasma samples were tested for S-specific IgG response using the quantitative electrochemiluminescence immunoassay Elecsys
® Anti SARS-CoV-2 S test on the Cobas e411 Analyzer (Roche Diagnostics) under manufacturer´s guidelines. The manufacturer specific U/mL of the Elecsys
® Anti-SARS-CoV-2 S assay can be considered equivalent to the Binding Arbitrary Units (BAU/mL) of the first WHO International Standard for anti-SARS-CoV-2 immunoglobulin. The inhibitory capacity of the antibodies for blocking the RBD-hACE2 interaction was assessed with a competitive ELISA as recently described [
13]. RBD-specific IgA was determined in plasma using the bead-based multiplex assay, LEGENDplex SARS-CoV-2 Serological IgA Panel (2-plex) Spike (S1) (Biolegend) following the manufacturer´s instructions.
2.4. Sample staining and multicolor flow cytometry
PBMCs were characterized by multiparametric flow cytometry for B and T cells subpopulations. The FITC anti-human CD19 (HIB19, BD Pharmingen™), PE-Cy7 anti-human IgD (IA6-2, BioLegend™), BV421 anti human CD27 (O323, BioLegend™), V500 anti-human CD45 (HI30, BD Horizon™) and BV605 anti-human CD3 (OKT3¸ BioLegend™) were used to identify total B cell populations. T helper polarization markers were assessed with a FITC anti-human CD3 (SK7, BD Biosciences), PE anti-human CD183(CXCR3) (1C6/CXCR3, BD Pharmigen™), PE-Cy7 anti-human CD196(CCR6) (G034E3, BioLegend), APC anti-human CD4 (RPA-T4, BD Pharmigen™), APC-H7 anti-human CD8 (SK1, BD Pharmingen™), V500 anti-human CD45 (HI30, BD Horizon™) and BV605 anti-human CD194 (CCR4) (1G1, BD Biosciences) antibody cocktail. Representative gating strategies are shown in Figure S2 and Figure S3.
Specific B cell analysis was assessed with by double tetramer staining using RBD specific B cell kit (Miltenyi-Biotec, 130-128-032), following the manufacturer´s instructions. Data were adquired on MACSQuant16 Flow Cytometer (Miltenyi-Biotec) and analyzed with FlowJo software (FlowJo LLC). Live singlets CD19+ lymphocytes were gated based on 7AAD fluorescence and specific B cells. Cells incubated with streptavidin PE and PEVio770 alone were used as negative controls. IgG+, IgM+ and IgA+ specific memory B cells were defined as CD27+ CD19+ on tetramer+ B cells.
2.5. IFN-γ secretion assay for evaluation of t cell specific response
PBMCs were thawed and their viability was determined upon trypan blue staining in a hemocytometer. Cells were adjusted to 1 x 107cells/mL in X-Vivo 15 (Lonza) supplemented with 1 mM sodium pyruvate (Sigma-Aldrich), 1 mM L-glutamine (Sigma-Aldrich) and 1% penicillin/streptomycin (Sigma-Aldrich) and either left untreated or stimulated with 1 µg/mL of a SARS-CoV-2 S1 peptide pool (PepTivator® SARS-CoV-2 Spike Protein Peptide Pool Miltenyi-Biotec) consisting mainly of 15-mer sequences with 11 amino acids overlap covering the immunodominant sequence domains of the spike glycoprotein (“S1”) of SARS-CoV-2 ) or left untreated. After 16-18 hours at 37°C in 5% CO2, the culture supernatants were collected and stored at -80°C, whereas the cells were evaluated with an IFN-γ secretion kit assay (Miltenyi-Biotec) designed for the detection and analysis of viable IFN-γ-secreting leukocytes, following the manufacturer´s recommendations. Briefly, the cells were stained for 5 min on ice with the capture anti-human IFN-γ antibody and incubated for 45 min at 37 °C under slow continuous rotation followed by washing and by staining with anti-human IFN-γ-APC (Miltenyi-Biotec) for 10 min on ice. In order to dissect the cell populations secreting IFNγ and their activation and memory status, the cells were also stained with PE anti-human CCR7 (150503, BD Pharmingen™), PerCP/Cy5.5 anti-human CD4 (RPA-T4, BioLegend™), PE-Cy 7 anti-human CD45RA (HI100, BD Pharmingen™), APC-H7 anti-human CD8 (SK1, BD Pharmingen™), V500 anti-human CD45 (HI30, BD Horizon™) and BV605 anti-human CD3 (OKT3¸ BioLegend™) antibodies. The cells were then acquired on a LSR Fortessa flow cytometer (BD Biosciences) and data analyzed with FACS Diva and FlowJo software (BD Biosciences). Representative gating strategies are shown in Figure S1.
2.6. Determination of cytokine release
For the determination of released cytokines, the supernatants of cell cultures stimulated with PepTivator Peptide Pools were analyzed using a LEGENDplex CD8/NK cytokine-profile 13-plex kit (Biolegend) according to the manufacturer’s instructions. Cytokine levels were normalized by removing the values from unstimulated controls. Data were analyzed with the LEGENDplex v8.0 Software (Biolegend).
2.7. Statistics
GraphPad Prism 9 (Graphpad Software, San Diego, CA, USA) was used for the statistical analysis. Data were described using geometric means and medians with 95% confidence intervals. Non paired samples were analyzed with non-parametric Mann-Whitney test.
3. Results
3.1. Characterization of the study cohort
Children aged 4-11 years old, who recovered from COVID-19 (n=10) or vaccinated with the heterologous scheme SOBERANA
® 02/SOBERANA
® Plus (n=15) were recruited in order to analyze in depth their humoral and cellular immune responses. Their demographic characteristics are presented in
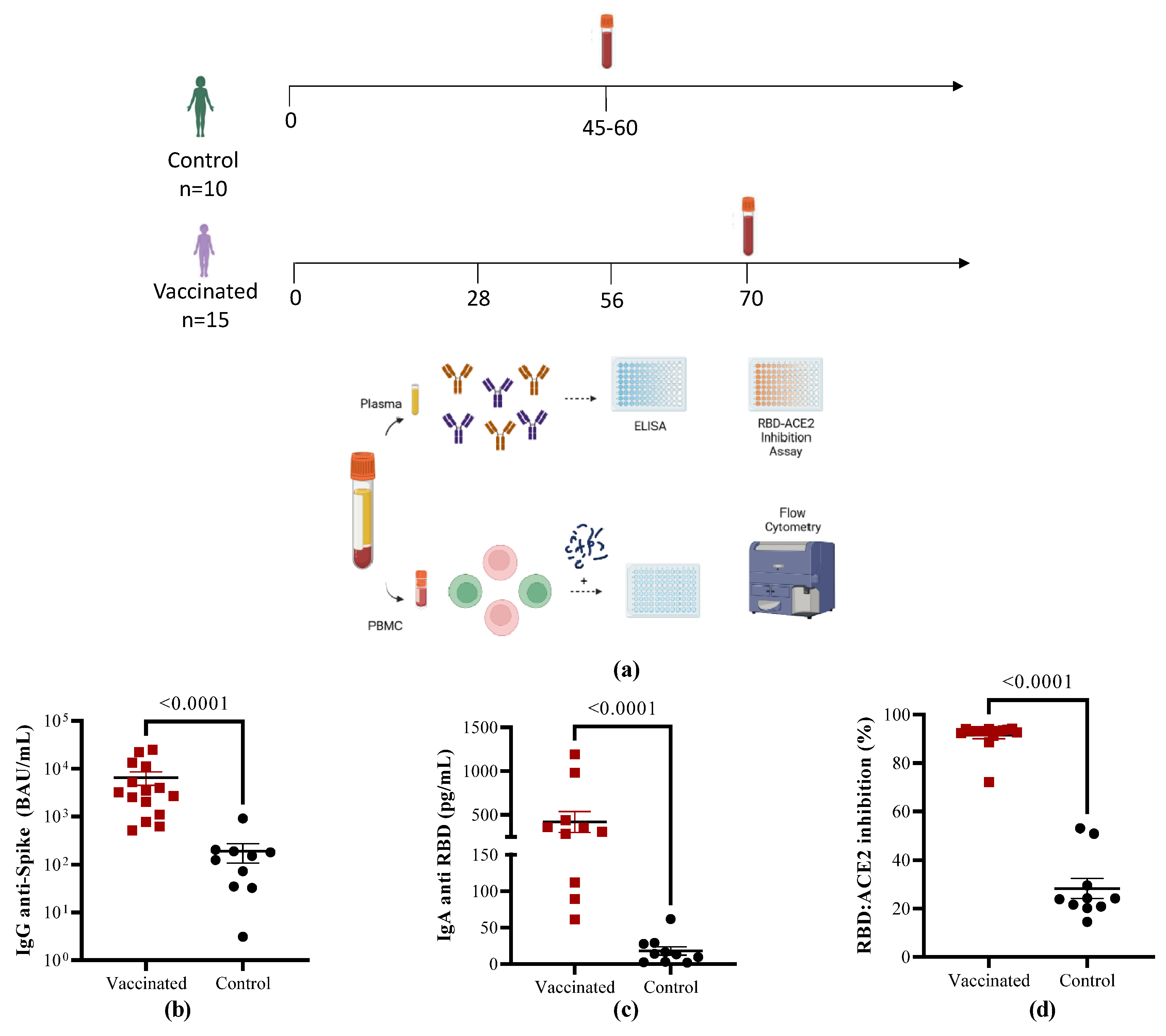
Table 1. The study design is summarized in
Figure 1A.
3.2. Antibody immune responses in vaccinated children compared to COVID-19 recovered children
To characterize the antibody response, anti-Spike IgG levels were determined with an electrochemiluminescence assay calibrated with international reference serum. The children vaccinated with SOBERANA
® 02/SOBERANA
® Plus had higher IgG antibody titers against RBD compared to COVID-19 convalescent children (
Figure 1B, p < 0.0001). In line with the anti-RBD IgG response, the levels of anti-RBD IgA were also higher in response to vaccination than after natural infection (
Figure 1C, p < 0.0001). The neutralizing capacity of antibodies for blocking the binding of the RBD to its receptor was evaluated using a competitive immunoassay, which measures the percentage of inhibition of RBD-hACE2 interaction. As shown in
Figure 1D, all the vaccinated individuals performed better than the children who recovered from disease (p < 0.0001).
3.3. Total and RBD-specific B cells and T helper populations in vaccinated and COVID-19 recovered children
In order to characterize functional memory B cell response, we first analyze the frequency of total B cells subpopulations on PBMCs from vaccinated and COVID recovered children using multiparametric flow cytometry. The gating strategy shown in Figure Supp S1 and S3. As result, no significant differences were found between vaccinated and recovered children regarding the frequency of total naïve (IgD+CD27-), exhausted (IgD-CD27-), and pre-switched B cells (IgD+CD27+), switched memory plasmablast (CD24-CD38+) (
Figure 2SA-E) and transitional naïve B cells (CD24+CD38+) (
Figure 2SF), but significant higher levels (p=0,0044) of switched memory B cells (IgD-CD27+) were found in vaccinated children (
Figure 2SD).
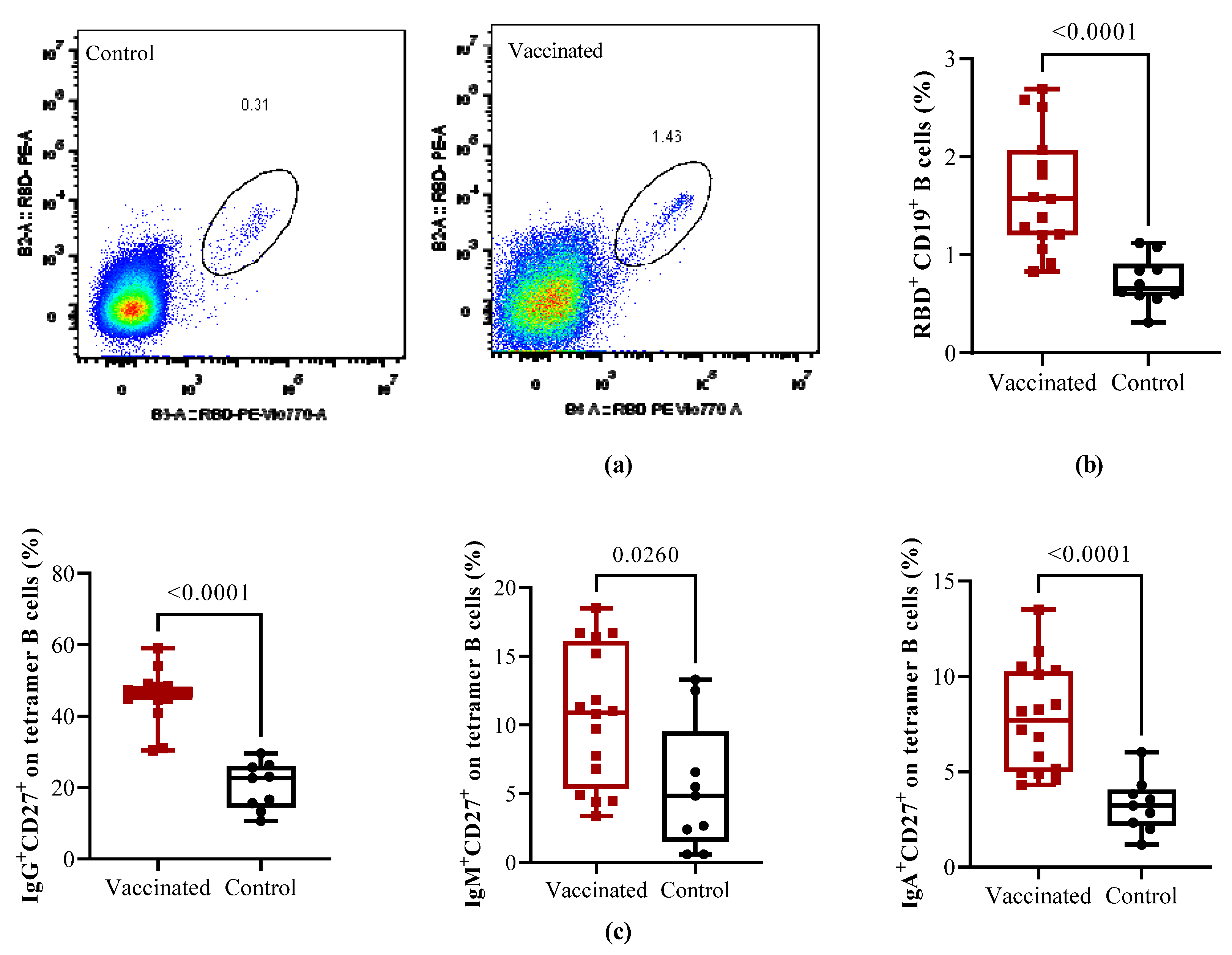
Cells from the same subjects were analyzed in order to evaluate the magnitude of RBD-specific memory B cells with a double tetramer staining approach measured by flow cytometry. Representative flow cytometry pseudocolor plots of RBD-specific are shown in
Figure 2A. Compared to recovered children, in vaccinated individuals, total RBD-specific circulating CD19+ B cells were significantly higher (p < 0.0001). Further analyses of the specific CD27+ memory B cells subsets shown that in comparison with naturally infected controls the frequencies of isotype memory subpopulations IgG
+CD27
+ (p < 0.0001), IgM
+CD27
+ (p < 0.0260) and IgA
+CD27
+ B cells (p < 0.0001) cells were significantly higher in vaccinated children.
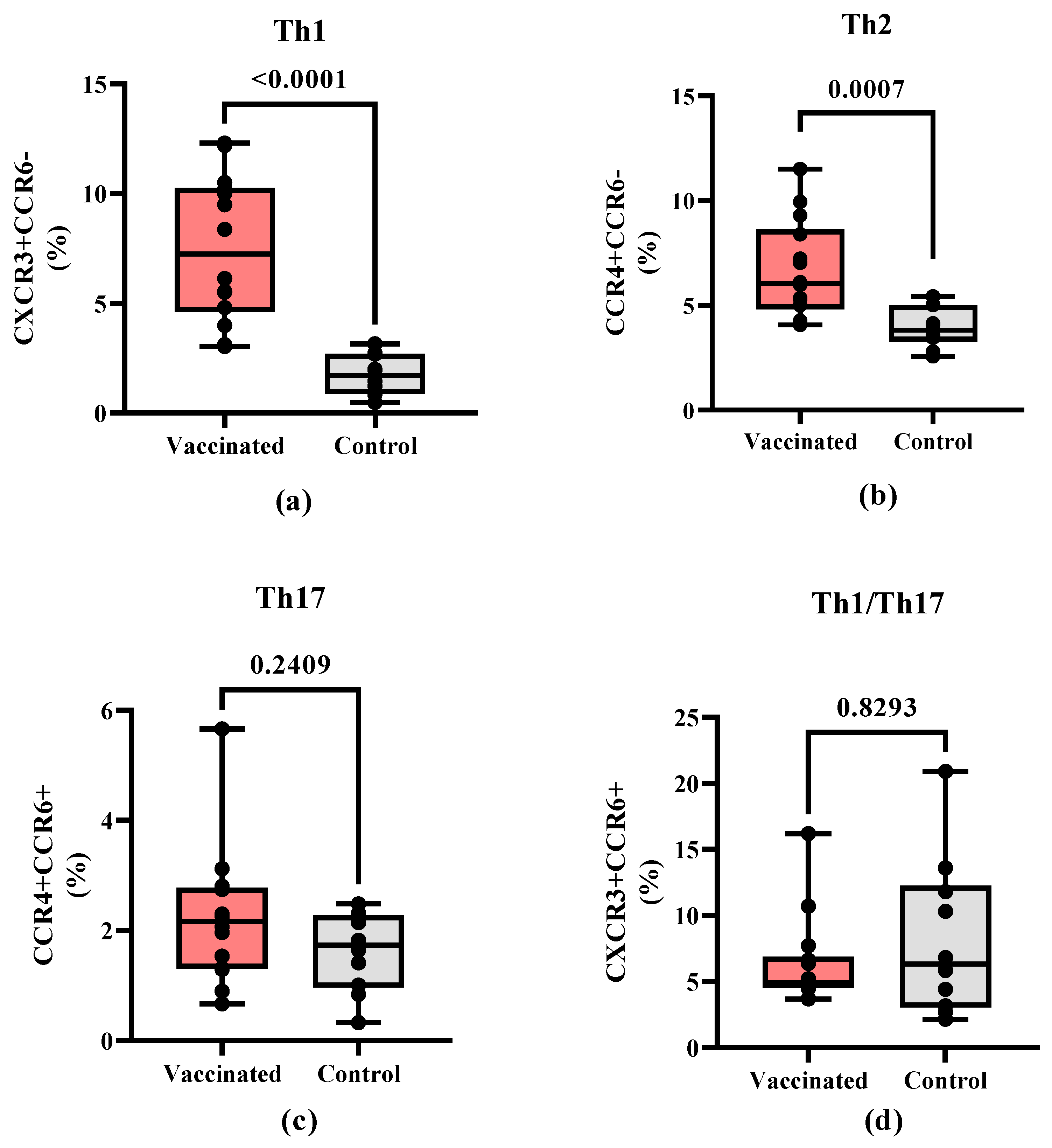
In addition, CD4+ T cells subpopulations were further characterized to compare the frequencies of the different T helper cell polarization profiles. Th1-like (CD4+CXCR3+CCR6-, p < 0.0001) cells (
Figure 3A) and Th2-like (CD4+CCR4+CCR6-, p=0,0007) cells (
Figure 3B) showed a higher percentage of cells in vaccinated children. In contrast, no statistically significant differences were found in Th17-like (CD4+CCR4+CCR6+) (
Figure 3C) and Th1/Th17-like subpopulations (CD4+CXCR3+CCR6+) between both groups (
Figure 3D).
3.4. Functional properties of T cells after antigen-specific in vitro stimulation
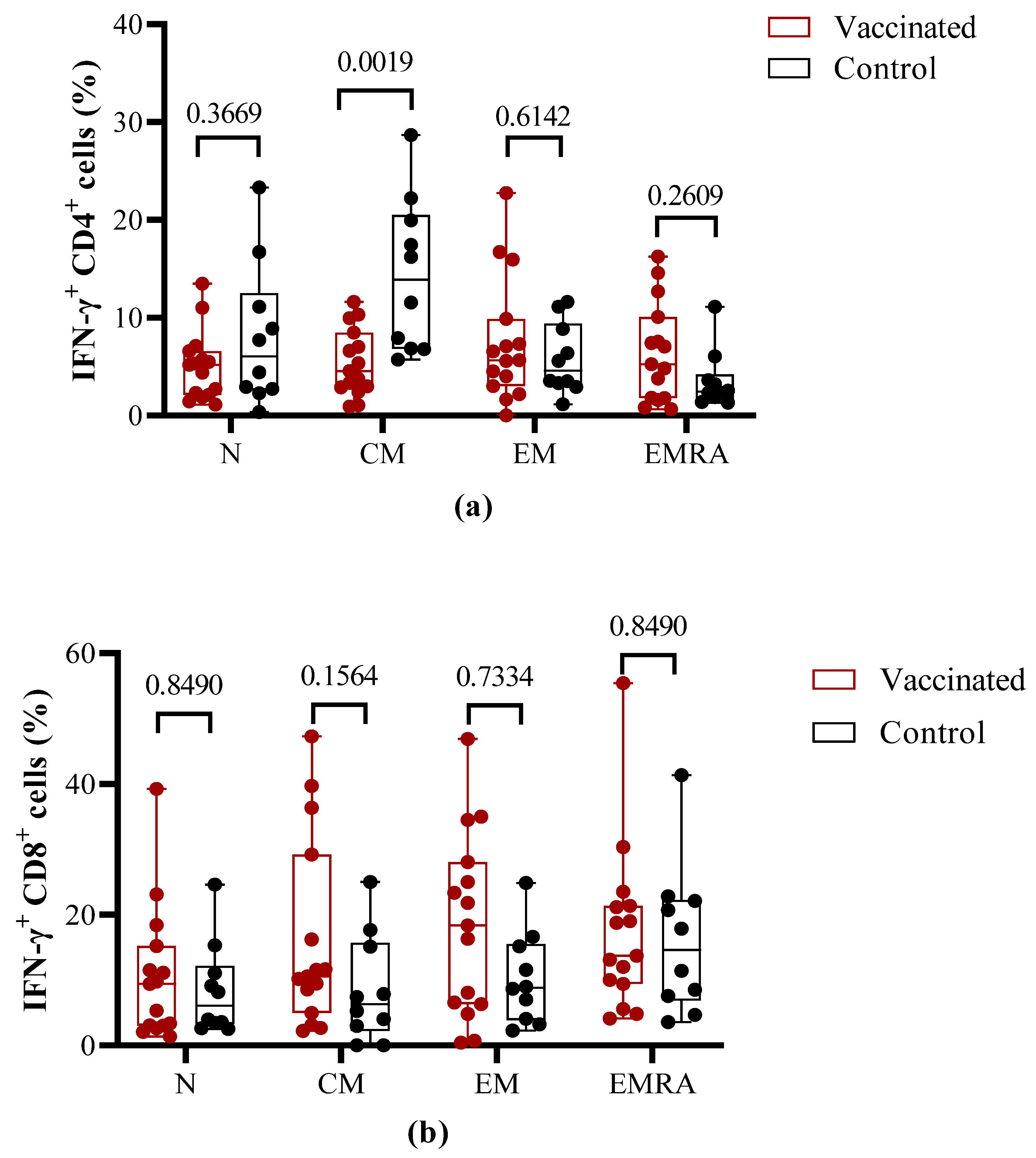
To compare the effector properties of T cells induced by vaccination versus natural infection, PBMCs were stimulated
in vitro with the S1 peptide pool and then evaluated by flow cytometry for IFN-γ release and expression of T cell memory markers. Within the CD4+ T cells populations, no statistical differences on IFN-γ release were found for naïve effector memory (EM) and terminally differentiated effector memory (EMRA) between vaccinated and COVID-19 recovered children. In contrast, in the central memory (CM) CD4+ T cell subpopulation, a higher IFN-γ secretion (p=0.0019) was detected in COVID-19 recovered children (
Figure 4A).
The percentage of activated IFN-γ secreting CD8+ central memory (CM) and effector memory (EM) T cells did not show a statistically significant difference (p > 0.05) between vaccinated and COVID-19 recovered children. Nevertheless, the relative percent is higher for CD8+ (CM) and CD8+ (EM) in the vaccinated individuals in comparison to recovered children. The percentage of IFN-γ secreting CD8+ (EMRA) and CD8+(naïve) cells did not show statistical differences with controls, but vaccinated children show higher values for both populations (
Figure 4B).
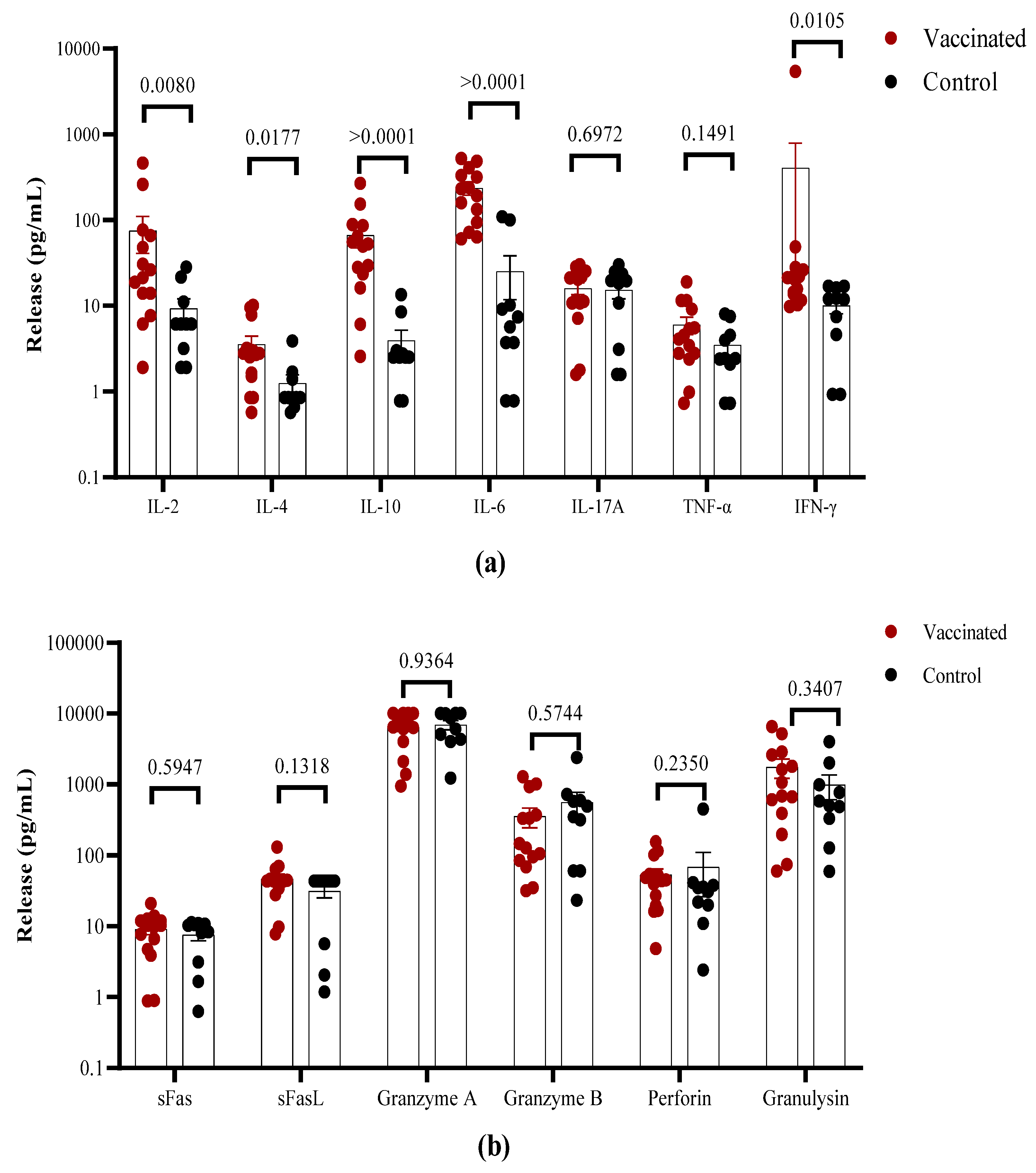
The overall cytokine profile produced in response to stimulation with the S1 peptide pool was analyzed in the cell culture supernatants using a multiplex assay. Higher levels of IFN-γ and IL-2, but not of TNF-α were found in vaccinated children in comparison to convalescent individuals (
Figure 5A). The higher IFN-γ levels in the T cell supernatants from vaccinated children are consistent with the higher frequency of responding cells. In contrast, higher concentrations of IL-10 and IL-6 were found in the supernatants of T cells from vaccinated in comparison to recovered children. In general, low levels of IL-4 production were found in both groups with values <10 pg/mL, but higher levels of this cytokine were detected in vaccinated individuals. No differences were detected in IL-17A release (
Figure 5A). Furthermore, higher levels of the cytotoxicity-associated molecules perforin, sFas/sFasL, granulysin as well as granzyme A and B were detected, but there existed no differences between vaccinated and convalescent children (
Figure 5B).
4. Discussion
After three years of COVID pandemic, vaccination against SARS-CoV-2 in pediatric populations has so far not been extended worldwide. Two doses of SOBERANA
® 02 in heterologous scheme with a third SOBERANA
® Plus has shown an excellent safety profile and immunogenicity in children aged 3-18 years [
13]. This heterologous vaccination scheme is characterized by high levels of anti-RBD antibodies with neutralizing capacity against VOCs (including BA.1) and induces IFN-secreting T cells [
13]. These results supported the emergency use authorization and their use in a massive vaccination campaign covering more than 96% of the pediatric population aged 2-18 years in Cuba [
13,
15]. The novelty of this study lies in the comprehensive characterization of SARS-CoV-2 specific humoral and cellular immune responses in children vaccinated with SOBERANA
® 02 and SOBERANA
® Plus heterologous scheme compare to children who have recovered from symptomatic COVID-19. The study also provides new evidences on the characterization of humoral response to this vaccination with respect to the previous findings [
13].
Our study demonstrated a higher Spike-specific IgG antibody response in vaccinated compared to COVID-19 recovered children as was found previously for adults vaccinated with mRNA vaccine [
16]. Also, the concentrations of Spike-specific IgG antibodies elicited after vaccination with the heterologous scheme of SOBERANA
® 02 and SOBERANA
® Plus are in accordance with others reports in children and adolescents after different vaccination schemes [
17,
18]. Since the humoral response can decay over time in recovered children and adolescents [
19,
20], the need for a booster immunization in children post natural infection was suggested [
9].
Taking into account that IgA antibodies predominated in the acute phase of the COVID-19 disease [
21], serum RBD specific IgA antibody levels were determined in vaccinated children and compared to the COVID-19 recovered. In accordance with the Spike-specific IgG values, vaccination also induced higher RBD-specific IgA concentrations.
Cellular response differs between vaccinated and recovered children. The percentage of total circulating B cells in vaccinated children differed from that of COVID-19 convalescents children with a higher percentage of switched memory B cells (CD19+CD27+IgD-). No significant difference in naïve, pre-switched and exhausted B cells between both groups was found. In accordance with antibody concentration and the levels of total switched memory B cells, RBD+ IgG, IgM and IgA memory subpopulation shown significant differences between both groups with higher values induced after vaccination. These results significantly correlated with specific antibodies for both isotypes. The association between antibodies, neutralizing capacity and specific circulating memory B cells have been previously described by other researchers after infection and vaccination with mRNA vaccines [
22,
23]. Also, it has been reported that the switch of the memory B cell compartment remains elevated after vaccination with SARS-CoV-2 mRNA vaccines [
24,
25]. The high level of RBD-specific IgA antibodies and the detection of an IgA memory B cell population suggest a protection not only at systemic level, also in the mucosal compartment by the migration of these cells. This phenomenon has been described and discussed before after vaccination with the BNT162b2 vaccine [
26]. In addition, a role of IgA antibodies induced by vaccination in the breakdown infection was demonstrated [
27].
The generation of an immunological memory is the key for vaccine success. Both memory B and T cells contribute to the maintenance of the immune response over time. In this study we found that both next to the RBD-specific antibody response, the relative percentage of the memory B cell compartment was increased in vaccinated individuals suggesting a qualitative and quantitative higher humoral immune response induced by vaccination with SOBERANA® 02/ SOBERANA® Plus than after natural infection.
In addition to antibodies, both CD4+ T helper (Th) cells and CD8+ cytotoxic T lymphocyte (CTL)-specific responses are key factors against viral infections, like COVID-19 [
28]. Furthermore, each CD4+ Th cell subpopulation has different functions and their balance is critical for the control of pathogen infections. We found a higher percentage of Th1 and Th2 CD4+ T cells in vaccinated children compared to convalescent individuals. This result is in accordance with previous findings after vaccination with SOBERANA
® 02 and SOBERANA
® Plus in children in a phase I/II clinical trial as determined by IFN-γ and IL-4 ELISpot assay [
13]. Taken together, these results indicate a mixed post-vaccination response profile that would have implications not only for the coordination of the T cell-mediated response, especially of CTL, but also for the coordination of the humoral response necessary for viral neutralization. Indeed, a decrease in the percentage of CD4+ cells with a Th1 and Th17 phenotype was detected in patients with COVID-19 [
29].
Previous studies showed that the CD8+ specific cytotoxic T cell response is critical in the protection against SARS-CoV-2 infection [
30]. To evaluate the functionality of these T cell subpopulations, the IFN-γ release in different memory subpopulations was compared after
in vitro stimulation with S1 peptides. The analysis showed a predominant effector memory phenotype in individuals vaccinated with SOBERANA
® 02/SOBERANA
® Plus scheme, which was comparable to results obtained after vaccination with mRNA-1273 and ChAdOx1 vaccines in children and adolescents [
17]. These results are also in accordance with previous data showing that COVID-recovered children mostly exhibit a CD4+ and CD8+ effector memory phenotype [
31,
32]. Thus, the heterologous scheme presented here is comparable in term effector memory capacity to that of natural infection.
In addition to the IFN-γ release, the level of different cytokines was determined. Despite differences between assays the concentration of IFN-γ in the supernatant of Th1-related cytokines is in line with the higher percentage of activated memory T cells. Dowell
et al. also reported that after mRNA-1273 and ChAdOx vaccination, children develop response cell Th1 [
17].
Despite our interesting results, this study has some limitations, which in particular comprise the small number of participants, which did not allow an age-related analysis. However, our study provides new insights regarding anti-RBD-specific IgA antibodies, characterizes the phenotype of circulating B and T cells and the specific IFN-γ secretion of CD4+ and CD8+ T cells after in vitro stimulation with S1 peptides induced by the heterologous SOBERANA® 02/SOBERANA® Plus scheme in children aged 5-11 years and provides new evidences of the T-cells and memory response in younger children. The heterologous scheme can induce a coordinated humoral and cellular memory responses against COVID-19 in the pediatric population, which is qualitatively superior to the response generated by the natural infection. Taken together with the safety profile of these vaccines in the pediatric population, the heterologous SOBERANA® 02/SOBERANA® Plus scheme as an excellent alternative for COVID-19 vaccination in children is suggested.
Author Contributions
RPN, DGR and BS conceived the study. RPN, CM, LRN, AM, MGF and IPA performed antibody determination, T and B immune phenotyping, T cell stimulation assays and cytokine analysis. RPG, YRD, BPM, MRG, AGH, YVB, DGR, VVB coordinate the study and sample acquisition. RPN, CM, DGR and BS writing the original draft which was critically revised by all authors. DR, YVB, DGR, VVB, BS were responsible of study supervision, project administration and funding. All authors contributed to the article and approved the submitted version.
Funding
The work was sponsored by the DAAD Germany (Project GLACIER 57592717) (BS, DR, RPN, LMR) and by a grant of Saxony-Anhalt (BS). The study was also funded by the National Funds for Sciences and Technology from the Ministry of Science, Technology, and Environment (FONCI-CITMA-Cuba, contract 2020-20).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of “Juan Manuel Marquez” Pediatric Hospital and endorsed by the Cuban National Pediatric Group. The Cuban National Regulatory Agency (Centre for State Control of Medicines and Medical Devices, CECMED) approved the trial (June10th, 2021, Authorization Reference: 05.010.21BA).
Informed Consent Statement
During recruitment, the medical investigators provided to the parents, both orally and written, all information about the vaccine and potential risks and benefits. Both parents signed the informed consent for including their children in the trial. The decision to participate was not remunerated. The children recovered from COVID-19 were studied as reference for immunity caused by natural infection; they were not participants in the clinical trial. Their parents signed an informed consent for including their children in this study and the results were informed to them. Trial registry: RPCEC00000374 (Cuban Public Registry of Clinical Trials and WHO International Clinical Registry Trials Platform) [
33].
Data Availability Statement
Data available upon request.
Acknowledgments
We would especially thank to the parents and children who participated in this study. We thank Dr. Lila Castellanos for scientific advice and corrections. We would like to thank Maria Heise for excellent secretarial help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McVernon, J.; Liberman, J. WHO keeps covid-19 a public health emergency of international concern. BMJ 2023, 380, p504. [CrossRef]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. New England Journal of Medicine 2020, 382, 1663-1665. [CrossRef]
- Yasuhara, J.; Kuno, T.; Takagi, H.; Sumitomo, N. Clinical characteristics of COVID-19 in children: A systematic review. Pediatric Pulmonology 2020, 55, 2565-2575. [CrossRef]
- Chua, G.T.; Wong, J.S.C.; Lam, I.; Ho, P.P.K.; Chan, W.H.; Yau, F.Y.S.; Rosa Duque, J.S.; Ho, A.C.C.; Siu, K.K.; Cheung, T.W.Y.; et al. Clinical Characteristics and Transmission of COVID-19 in Children and Youths During 3 Waves of Outbreaks in Hong Kong. JAMA Network Open 2021, 4, e218824-e218824. [CrossRef]
- http://ourworldindata.org/covid-vaccinations. Availabe online: (accessed on 30 August 2023).
- Tian, Y.; Chen, L.; Shi, Y. Safety, Efficacy, and Immunogenicity of Varying Types of COVID-19 Vaccines in Children Younger Than 18 Years: An Update of Systematic Review and Meta-Analysis. Vaccines 2023, 11, 87. [CrossRef]
- Frenkel, L.D. The global burden of vaccine-preventable infectious diseases in children less than 5 years of age: Implications for COVID-19 vaccination. How can we do better? In Proceedings of Allergy and Asthma Proceedings; pp. 378-385. [CrossRef]
- Valdes-Balbin, Y.; Santana-Mederos, D.; Quintero, L.; Fernández, S.; Rodriguez, L.; Sanchez Ramirez, B.; Perez-Nicado, R.; Acosta, C.; Méndez, Y.; Ricardo, M.G.; et al. SARS-CoV-2 RBD-Tetanus Toxoid Conjugate Vaccine Induces a Strong Neutralizing Immunity in Preclinical Studies. ACS Chemical Biology 2021, 16, 1223-1233. [CrossRef]
- Ochoa-Azze, R.; Chang-Monteagudo, A.; Climent-Ruiz, Y.; Macías-Abraham, C.; Valenzuela-Silva, C.; de los Ángeles García-García, M.; Jerez-Barceló, Y.; Triana-Marrero, Y.; Ruiz-Villegas, L.; Dairon Rodríguez-Prieto, L.; et al. Safety and immunogenicity of the FINLAY-FR-1A vaccine in COVID-19 convalescent participants: An open-label phase 2a and double-blind, randomised, placebo-controlled, phase 2b, seamless, clinical trial. The Lancet Respiratory Medicine 2022, 10, 785-795. [CrossRef]
- Toledo-Romaní, M.E.; García-Carmenate, M.; Valenzuela-Silva, C.; Baldoquín-Rodríguez, W.; Martínez-Pérez, M.; Rodríguez-González, M.; Paredes-Moreno, B.; Mendoza-Hernández, I.; González-Mujica Romero, R.; Samón-Tabio, O.; et al. Safety and efficacy of the two doses conjugated protein-based SOBERANA-02 COVID-19 vaccine and of a heterologous three-dose combination with SOBERANA-Plus: A double-blind, randomised, placebo-controlled phase 3 clinical trial. The Lancet Regional Health – Americas 2023, 18. [CrossRef]
- Toledo-Romani, M.E.; García-Carmenate, M.; Verdecia-Sánchez, L.; Pérez-Rodríguez, S.; Rodriguez-González, M.; Valenzuela-Silva, C.; Paredes-Moreno, B.; Sanchez-Ramirez, B.; González-Mugica, R.; Hernández-Garcia, T.; et al. Safety and immunogenicity of anti-SARS-CoV-2 heterologous scheme with SOBERANA 02 and SOBERANA Plus vaccines: Phase IIb clinical trial in adults. Med 2022, 3, 760-773.e765. [CrossRef]
- Toledo-Romaní, M.E.; Verdecia-Sánchez, L.; Rodríguez-González, M.; Rodríguez-Noda, L.; Valenzuela-Silva, C.; Paredes-Moreno, B.; Sánchez-Ramírez, B.; Pérez-Nicado, R.; González-Mugica, R.; Hernández-García, T.; et al. Safety and immunogenicity of anti-SARS CoV-2 vaccine SOBERANA 02 in homologous or heterologous scheme: Open label phase I and phase IIa clinical trials. Vaccine 2022, 40, 4220-4230. [CrossRef]
- Puga-Gómez, R.; Ricardo-Delgado, Y.; Rojas-Iriarte, C.; Céspedes-Henriquez, L.; Piedra-Bello, M.; Vega-Mendoza, D.; Pérez, N.P.; Paredes-Moreno, B.; Rodríguez-González, M.; Valenzuela-Silva, C.; et al. Open-label phase I/II clinical trial of SARS-CoV-2 receptor binding domain-tetanus toxoid conjugate vaccine (FINLAY-FR-2) in combination with receptor binding domain-protein vaccine (FINLAY-FR-1A) in children. International Journal of Infectious Diseases 2023, 126, 164-173. [CrossRef]
- Eugenia-Toledo-Romani, M.; Valenzuela-Silva, C.; Montero-Diaz, M.; Iniguez-Rojas, L.; Rodriguez-González, M.; Martinez-Cabrera, M.; Puga-Gómez, R.; German-Almeida, A.; Fernandez-Castillo, S.; Climent-Ruiz, Y.; et al. Real-World Effectiveness of the OBERANA02 and SOBERANA-Plus Vaccine Combination in Children 2 to 11 Years of Age during the SARS CoV-2 Omicron Wave in Cuba: A Regression Discontinuity Study. SSRN: 2023. [CrossRef]
- Osterholm, M.T.; Rabadán-Diehl, C.; Anzinger, J.; Bottazzi, M.E.; Christie-Samuels, C.; Erondu, N.; Marrazzo, J.; Milan, S.; Quashie, P.K.; Schwaab, T.; et al. EXECUTIVE SUMMARY Insights from Cuba's COVID-19 Vaccine Enterprise: Report from a High Level Fact-Finding Delegation to Cuba. MEDICC Rev 2022, 24, 109-128. [CrossRef]
- Lagousi, T.; Routsias, J.; Mavrouli, M.; Papadatou, I.; Geropeppa, M.; Spoulou, V. Comparative Characterization of Human Antibody Response Induced by BNT162b2 Vaccination vs. SARS-CoV-2 Wild-Type Infection. Vaccines 2022, 10, 1210. [CrossRef]
- Dowell, A.C.; Powell, A.A.; Davis, C.; Scott, S.; Logan, N.; Willett, B.J.; Bruton, R.; Ayodele, M.; Jinks, E.; Gunn, J.; et al. mRNA or ChAd0x1 COVID-19 Vaccination of Adolescents Induces Robust Antibody and Cellular Responses With Continued Recognition of Omicron Following mRNA-1273. Frontiers in Immunology 2022, 13. [CrossRef]
- Soto, J.A.; Melo-González, F.; Gutierrez-Vera, C.; Schultz, B.M.; Berríos-Rojas, R.V.; Rivera-Pérez, D.; Piña-Iturbe, A.; Hoppe-Elsholz, G.; Duarte, L.F.; Vázquez, Y.; et al. Inactivated Vaccine-Induced SARS-CoV-2 Variant-Specific Immunity in Children. mBio 2022. e01311-01322. [CrossRef]
- Tsang, H.W.; Chua, G.T.; To, K.K.W.; Wong, J.S.C.; Tu, W.; Kwok, J.S.Y.; Wong, W.H.S.; Wang, X.; Zhang, Y.; Rosa Duque, J.S.; et al. Assessment of SARS-CoV-2 Immunity in Convalescent Children and Adolescents. Frontiers in Immunology 2021, 12. [CrossRef]
- Sieber, J.; Mayer, M.; Schmidthaler, K.; Kopanja, S.; Camp, J.V.; Popovitsch, A.; Dwivedi, V.; Hoz, J.; Schoof, A.; Weseslindtner, L.; et al. Long-Lived Immunity in SARS-CoV-2-Recovered Children and Its Neutralizing Capacity Against Omicron. Frontiers in Immunology 2022, 13. [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Science Translational Medicine 2021, 13, eabd2223. [CrossRef]
- Kared, H.; Wolf, A.-S.; Alirezaylavasani, A.; Ravussin, A.; Solum, G.; Tran, T.T.; Lund-Johansen, F.; Vaage, J.T.; Nissen-Meyer, L.S.; Nygaard, U.C.; et al. Immune responses in Omicron SARS-CoV-2 breakthrough infection in vaccinated adults. Nature Communications 2022, 13, 4165. [CrossRef]
- Perico, L.; Todeschini, M.; Casiraghi, F.; Mister, M.; Pezzotta, A.; Peracchi, T.; Tomasoni, S.; Trionfini, P.; Benigni, A.; Remuzzi, G. Long-term adaptive response in COVID-19 vaccine recipients and the effect of a booster dose. Frontiers in Immunology 2023, 14. [CrossRef]
- José-Cascón, M.S.; de la Varga-Martínez, R.; Campos-Caro, A.; Rodríguez, C. Dynamics of B-Cell Responses after SARS-CoV-2 Vaccination in Spain. Vaccines 2022, 10, 1615. [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination. Science Immunology 2021, 6, eabi6950. [CrossRef]
- Piano Mortari, E.; Russo, C.; Vinci, M.R.; Terreri, S.; Fernandez Salinas, A.; Piccioni, L.; Alteri, C.; Colagrossi, L.; Coltella, L.; Ranno, S.; et al. Highly Specific Memory B Cells Generation after the 2nd Dose of BNT162b2 Vaccine Compensate for the Decline of Serum Antibodies and Absence of Mucosal IgA. Cells 2021, 10, 2541. [CrossRef]
- Kaku, C.I.; Bergeron, A.J.; Ahlm, C.; Normark, J.; Sakharkar, M.; Forsell, M.N.E.; Walker, L.M. Recall of preexisting cross-reactive B cell memory after Omicron BA.1 breakthrough infection. Science Immunology 2022, 7, eabq3511. [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Frontiers in Immunology 2020, 11. [CrossRef]
- Gil-Etayo, F.J.; Suàrez-Fernández, P.; Cabrera-Marante, O.; Arroyo, D.; Garcinuño, S.; Naranjo, L.; Pleguezuelo, D.E.; Allende, L.M.; Mancebo, E.; Lalueza, A.; et al. T-Helper Cell Subset Response Is a Determining Factor in COVID-19 Progression. Frontiers in Cellular and Infection Microbiology 2021, 11. [CrossRef]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630-634. [CrossRef]
- Cohen, C.A.; Li, A.P.Y.; Hachim, A.; Hui, D.S.C.; Kwan, M.Y.W.; Tsang, O.T.Y.; Chiu, S.S.; Chan, W.H.; Yau, Y.S.; Kavian, N.; et al. SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nature Communications 2021, 12, 4678. [CrossRef]
- Conway, S.R.; Lazarski, C.A.; Field, N.E.; Jensen-Wachspress, M.; Lang, H.; Kankate, V.; Durkee-Shock, J.; Kinoshita, H.; Suslovic, W.; Webber, K.; et al. SARS-CoV-2-Specific T Cell Responses Are Stronger in Children With Multisystem Inflammatory Syndrome Compared to Children With Uncomplicated SARS-CoV-2 Infection. Frontiers in Immunology 2022, 12. [CrossRef]
- International register clinical trials. Identifier RPCEC00000374. Phase I–II study, sequential during phase I, open-label, adaptive and multicenter to evaluate the safety, reactogenicity and immunogenicity of a heterologous two-dose schedule of the prophylactic anti-SARS- CoV-2 vaccine candidate, FINLAY-FR- 2 and a dose of FINLAY-FR-1A. 2021.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).