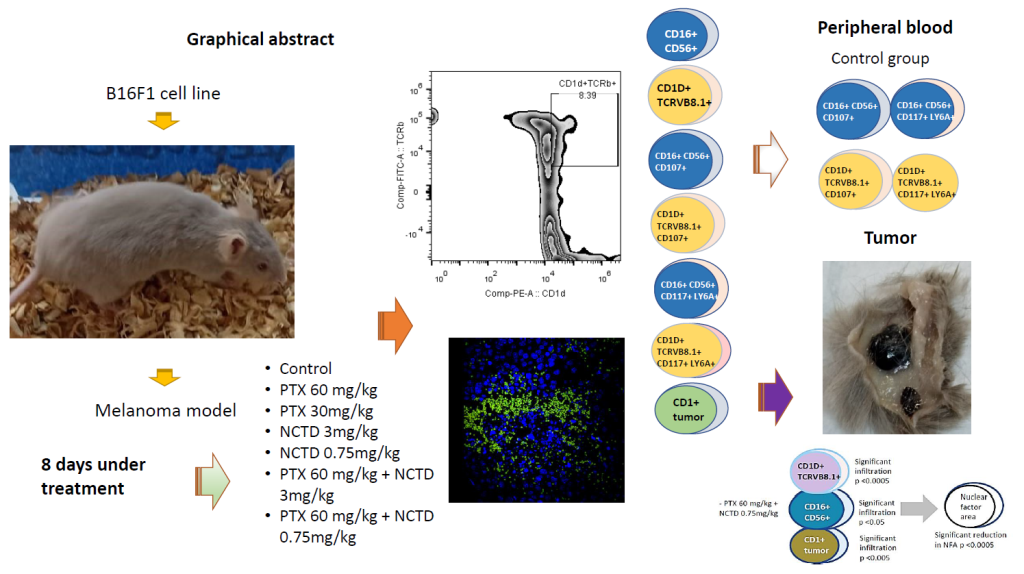
1. Introduction
Using mechanisms to increase the migration and infiltration of NK and iNKT cells in different neoplasms, including melanoma, is a promising approach for treating these diseases—particularly the innate immune system cells and cytotoxic cells. Natural killer (NK) cells are an essential part of the innate immune system; they are necessary for initiating and augmenting adaptive immune responses, with a critical role in antitumor immune surveillance [
1,
2,
3,
4]. Invariant natural killer T (iNKT) cells are NK cells with invariant αβ chains that possess the innate T cell receptor (TCR) able to recognize lipid antigens presented by CD1d. NK and iNKT cells exhibit potent antitumor activity through a cytotoxic response, e.g., they exert direct tumor lysis, recruitment, and activation of other innate and adaptive immune cells by initiating a Th1 cytokine cascade. They also regulate immunosuppressive cells in the tumor microenvironment [
5,
6,
7,
8,
9]. However, the functionality of these cells will depend on their activation and state of maturation.
For the treatment of melanoma, our team work demonstrated encouraging prognostic results in a murine melanoma model treated with pentoxifylline (PTX), α-galactosylceramide and their combinations, and specimens subjected to drug treatments and radiotherapy [
10]. The PTX is a semi-synthetic methylxanthine derivative and has a potential role in combination therapy against cancer. Its antineoplastic effect has been described as increased susceptibility to radiation therapy and chemotherapy in different neoplasms, including melanoma. PTX protects against systemic and local side effects of chemotherapy and radiotherapy [
11]. The way to study the immune response is varied, and one way to do it is by studying the intratumoral inflammatory infiltrate. In this context, melanoma's tumor-infiltrating lymphocytes (TILs) are associated with a favorable prognosis and have been interpreted as an indicator of a host immune response against the tumor [
12]. Similarly, the mitotic rate has been used to predict aggressive behavior in various neoplasms. In melanoma, the mitotic rate has been found to have independent prognostic value in agreement with the National Comprehensive Cancer Network criterion for indicating selective sentinel node biopsy [
13]. At the same time, the nuclear factor area (NFA) can be an early indicator of cell morphological changes preceding or during apoptosis [
10,
14]. found that in the melanoma model treated with PTX, a reduction in the rate of mitosis of tumor cells and an increase in leukocyte infiltrate and necroapoptosis; however, it remains to be shown whether the infiltrating leukocytes are NK or iNKT cells and whether they are active.
Another exciting substance for the adjuvant or neoadjuvant treatment of melanoma is norcantharidin (NCTD) [
15]. Its antitumor effect has been extensively investigated in different types of malignant tumors. NCTD exerts an antineoplastic effect by inhibiting tumor cell reproduction, inducing apoptosis and autophagy, restraining migration and metastasis, and affecting immunity and angiogenesis [
16]. However, it has been documented that the NCTD markedly inhibits lymphoproliferation
in vitro under ConA or LPS stimulation. The overhead suggests that this compound strongly suppresses lymphocyte activation
in vitro [
17]. Notwithstanding the above, it is necessary to verify whether NCTD can inhibit NK and iNKT cells in a murine melanoma model and reduce their recruitment at the specific site of the tumor for their potential use as neoadjuvant or adjuvant therapy for melanoma treatment.
Despite the importance of recruitment, the degree of maturation of natural killer cells, and their activation for the control of neoplasms, there is a lack of information on whether adjuvant and neoadjuvant treatments can modify these critical parameters for melanoma control. The current study assessed the effect of PTX, NCTD, and their mixtures on NK cells, iNKT, on the degree of maturation, their activation in peripheral blood, and their recruitment to the tumor site, the mitotic rate and nuclear factor area in a murine melanoma model.
4. Discussion
NK and iNKT cells contribute to the immune response against cancer [
19]. Their innate ability to detect tumor cells makes them critical cells in antitumor immune surveillance through the release of a potent cytotoxic response, which is why they have become,
per se, a therapeutic target against melanoma [
20]. In this context, our study is one of the first to evaluate the effects of pentoxifylline (PTX) and norcantharidin (NCTD) as a therapeutic targets against melanoma, evaluating the presence, activation, and degree of maturity of NK and iNKT cells in peripheral blood, as well as their infiltration in the tumor. Both pentoxifylline (PTX) and norcantharidin (NCTD) have shown a significant antitumor effect in melanoma and other types of cancer. In the case of PTX, there is low toxicity, and for NCTD, safe doses have been established in mice without exerting irreversible cytotoxic effects [
18]. Within several mechanisms of action, the migration of lymphocytes in solid tumors, including melanoma, stands out. Our work group demonstrated that PTX increased the leukocyte infiltration into the tumor mass (line B16-F1 melanoma cell) related to a good prognosis [
10]. On the other hand, NCTD treatment can decrease the number of tumor-infiltrating Tregs and increase the number of CD4+ and CD8+ T cells in prostate cancer cells [
21]. Considering these findings and the background described above, it was hypothesized that PTX and NCTD influence the number and recruitment of NK and iNKT cells in a mouse melanoma model.
Interestingly, the number of NK and iNKT cells in their different phenotypes in peripheral blood evaluated by flow cytometry did not present significant numerical changes in any treatment group. This finding suggests that NK and iNKT cells are depleted in circulation as they are recruited into the tumor mass. However, the Pearson correlation analysis showed that, in the murine model of melanoma, in addition to the recruitment of natural killer cells to the tumor site, a series of relatively complex events occur, both in specimens without drug treatment and in those subjected to different drug schemes with PTX, NCTD, and their mixtures, something that also depends on the test doses.
In specimens with melanoma tumors, NK cells (CD16+CD56+) and iNKT cells (CD1D+TCRvB8.1) from peripheral blood are active (CD107a+) and preserve immature markers such as Sca-1+, in addition to the transcription factor/immaturity c-kit (CD117+). The lysosome-associated membrane glycoprotein 1 (LAMP-1 or CD107a) has been described as a marker of the functional activity of NK cells associated with the secretion of lytic granules containing molecules such as perforin and granzymes [
22]. This fact could be a favorable indication of combating the disease [
23,
24,
25]. However, something exciting in the mouse melanoma model is that the activated natural killer cells require or are c-kit+/Sca-1+, suggesting that these markers of immaturity are involved in natural killer cell proliferation, survival, and activity. On the other hand, T-cell acute lymphocytic leukemia protein 1 "SCF" or "SCL," also known as c-kit ligand, promotes the growth of lineage-specific hematopoietic precursor cells, synergizes with hematopoietic growth factors such as erythropoietin, granulocyte colony-stimulating factor (G-CSF), and granulocyte/macrophage–colony-stimulating factor (GM-CSF) [
26]. C-kit possesses a cytoplasmic basic-helix-loop-helix heterodimer through its HLH domain and bridging proteins. The helix-loop-helix heterodimer (SCL, most probably coupled with bHLH E12/E47) DNA-binding domain acts as a transcription factor [
25,
27]. In the specific case of NK cells, it promotes the survival of peripheral CD16+CD56+c-kit+Sca-1+ cells, and the absence of c-kit signaling has also been shown to reduce the generation of NK cells from fetal liver precursors [
28]. Based on the present study's findings, it is feasible to propose that c-kit functions as a transcription factor for the activation of NK and iNKT cells in a mouse melanoma model, coupled with the role of Sca-1. Although stem cell antigen 1 (Sca-1, also named Ly-6A/E) has been best characterized as a marker of hematopoietic stem cells, in the context of NK cells, it serves as a marker of early, nonselective NK cell activation that is associated with increased IFN-γ production, thereby promoting apoptosis and cytolysis of target tumor cells [
29,
30]. Furthermore, the expression of Sca-1 in NK cells may be involved in the terminal differentiation of NK cells under the stimulus of IL-12, coupled with the high cytotoxicity and high potential to produce IFN-γ that pre-NK cells present [
25], that is, those that preserve primitiveness markers.
Also, in the melanoma model without drug treatment, it was observed that the leukocyte infiltrates in the tumor area assessed at 10x were inversely related to peripheral blood NK cells. These findings suggest that other lymphocytes with cytotoxic capacity, such as CD+8 T cells, infiltrate the tumor of mice without pharmacological treatment trying to control this disease. The recruitment of lymphocytes has also been previously documented in a mouse model of melanoma treated with PTX and α-galactosylceramide [
10]. Tumor-infiltrating lymphocytes in patients with melanoma are inversely related to sentinel lymph node positivity and directly correlated with a statistically significant improvement in both melanoma-specific survival time and recurrence-free of disease [
21].
Enigmatically, tumor cells' nuclear factor area (NFA) was inversely related to peripheral blood NK cells expressing immaturity factors such as Sca-1 and c-kit in the melanoma control group. NFA can be an early indicator of cell morphological changes that occur during apoptosis, and a quantitative measure of apoptosis can be obtained through image analysis [
14], as performed in the current study. Although these immaturity markers give NK cells a higher cytotoxic capacity due to the production of IFN-γ and the release of granzymes and perforins [
25,
29,
30], probably the growth of tumor cells interferes with the lack of maturation of NK cells. On the subject, it has been shown that in multiple neoplasms, including breast cancer, colon cancer, and melanoma cell lines, the growth of these malignant cells can interrupt the functional maturation of NK cells and impair the antitumor capacity of NK cells [
31]. The previous report and the findings of this study suggest that B16F1 cells impede the maturation of NK cells at the bone marrow level as a side effect of their growth in animals with melanoma without drug treatment. In addition, NK cells are not significantly recruited to the tumor site under this condition.
There are no previous results on this particular subject. However, from the murine melanoma model without drug treatment, it was possible to demonstrate the relevance of transcription factors such as c-kit/Sca-1 for activating peripheral blood NK cells and iNKT despite other cytotoxic lymphocytes predominating at the tumor site.
As in the control group, in the specimens treated with PTX at 60 mg/kg, active peripheral blood NK cells expressed c-kit+Sca-1+. This finding reinforces the hypothesis that these immaturity markers favor its activation through LAMP-1, also probably regulated by c-kit. Likewise, it was observed that PTX at high doses can "orient" the response of natural killer cells so that both cell subpopulations remain active and correlated. Interestingly, and as documented in specimens without drug treatment, immaturity markers (c-kit+/Sca-1+) are required for iNKT activation and correlated with active NK in peripheral blood. However, in the treatment with PTX at 60 mg/kg, it was found that the number of mitotic bodies in the tumor cells was directly related to the number of NK cells in peripheral blood. In patients, tumor mitotic body count is a significant predictor, which has the potential to improve the accuracy of melanoma staging further, as well as more rigidly define melanoma risk categories independent of the survival of patients with primary cutaneous melanoma [
12,
32,
33].
In the same way, in the specimens treated with PTX at 30 mg/kg, the tumor mitotic body count in the melanoma cells presented a direct relationship with the lymphocytic infiltrate. The statistical associations in the present study seem paradoxical; however, it is possible to emphasize the following. Tumor-infiltrating lymphocytes (TILs) comprise effector T lymphocytes, regulatory T lymphocytes, NK cells, dendritic cells, and macrophages. Nevertheless, its distribution and activation status may be variable and modulate clinical outcomes [
34]. In addition, melanoma tumor cells can employ different immune evasion strategies, such as downregulating MHC class I molecules to avoid recognizing CD8+ T cells [
35]. They release IL-8, IL-10, TGF-β1, and VEGF to limit the normal maturation of debris cells [
36]
. Likewise, these cells interfere with the activation of inhibitory immune checkpoints such as programmed cell death-1 (PD-1), cytotoxic T lymphocyte antigen-4 (CTLA-4), B and T lymphocyte attenuator (BTLA), and T-cell immunoglobulin and mucin domain-3 (TIM-3) [
37]. In the context of NK cells, the mechanism of inhibition elicited by melanoma cells on NK cells probably consists of TGF-β release, an increase in MHC I expression, and the antitumor effect of Tregs that limits the availability of IL-2 performed by Tregs [
66] for NK cells to exert their cytotoxic effects. In brief, the findings of this study suggest that, under this treatment scheme (PTX at 60 and 30 mg/kg), other cell populations with cytotoxic capacity are those that participate against the tumor, in addition to the effect of the drug itself [
10]. Such is the case of iNKT, as demonstrated in the present study and previous works [
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40].
Peripheral blood iNKT cells from mice given the low dose of NCTD (0.75 mg/kg) were active, and displayed markers of immaturity/activation (Sca-1+/c-kit+), even though in the higher dose (3.0 mg/kg NCTD), the activation of NK cells was also related to these markers of immaturity, as observed in the control group. This statistical relationship again corroborates the importance of c-kit as a transcription factor, probably due to the induction of conformational change and functionality of SCL/bHLH E12/E47 DNA-binding domain involved in hematopoietic development [
27,
41]. While the expression of Sca-1 increases the cytotoxic potential of natural killer cells [
25]. These results contrast with those found
in vitro where NCTD was shown to suppress lymphocyte activation [
17], something that is very likely to occur
in vitro since NCTD is a potent inhibitor of protein phosphatase 1, 2A, 2B, and 5, involved in the maintenance of many cellular functions [
42].
Interestingly, in mice treated with NCTD at 3.0 mg/k, the lymphocyte count in the tumor mass was closely related to peripheral blood CD1D+TCRvB8.1+CD107a+ cells and also to this same subpopulation expressing Sca-1+CD117+. The migration of NK cells and other immune cells in melanoma has been studied through different approaches. Previous studies reveal that NK cell accumulation in tumors depends on the expression of the CXCR3 ligand, CXCL10, in tumor tissue [
43]. However, B16F10 cells with low expression of CCL5 display chemotactic activity and induce the migration of various subsets of immune cells to inflammatory sites through three different chemokine receptors: CCR1, CCR3, and CCR5 [
43]. In patients, higher CCL5 levels correlate with significantly longer survival [
44]. Another factor that could influence this response is the multifunctional protein acting through the receptor ChemR23/CMKLR1 named chemerin (ChemR23), present in B16 cells. This biomolecule has antiangiogenic properties also linked to the induction of necrosis [
45]. ChemR23 likewise mediates migration, recruitment of plasmacytoid dendritic cells and ChemR23-dependent NK cells and further suppresses melanoma growth [
46]. Infiltration of cytotoxic NK cells and iNKT cells into tumors is a favorable prognostic marker for various cancers, including melanoma [
20]. Therefore, treatment with NCTD at a dose of 3.0 mg/kg, in addition to inducing a cytotoxic pharmacological effect on tumor cells [
15], may favor the migration of iNKT cells to the tumor site. This finding is probably through chemokine receptors [
43] and other proteins [
45,
46], something to be demonstrated and that is outside the scope of this work. Nevertheless, here it was found that a significant part of the tumor-infiltrating lymphocytes is active iNKT cells (CD107a+) presenting immature markers (Sca-1/c-kit+) in mice treated with NCTD at 3.0 mg/kg. Interestingly, tumor-infiltrated iNKT cells were inversely related to tumor-infiltrated areas. This finding suggests the presence of chemotactic factors that allow their accumulation at specific tumor sites. About the topic, chemokines are closely associated with iNKT cell maturation and localization, specifically CCL3 and CCL4, accompanied by their CXCR3 ligands (CXCL9-11), and are secreted by activated dendritic cells. These chemokines attract iNKT cells to chemokine/ligand sites [
47]. CCL4 has also been found to induce distinct chemotaxis in different iNKT subsets, particularly attracting cells expressing CCR5. Likewise, CXCR6 is closely related to the localization of iNKT cells due to its interaction with CXCL16 [
48]. Also, interleukins activate iNKT cells by binding to receptors on the cell surface. Some interleukins such as IL-2, 12, 15, 18, 21, 27, and 33 can activate iNKT cells [
49]. Specifically, IL-27 modulates IL-12 secretion from dendritic cells, indirectly enhancing the maintenance and recruitment of iNKT cells [
50,
51]. Furthermore, some lipid antigens, such as α-galactosylceramide synthesized by the intestinal microbiota, and derivatives, such as α-galacturonosylceramide [
10,
52,
53,
54] are presented by dendritic cells to iNKT cells through CD1d for their proliferation at specific sites. These reports and our results indicate that iNKT cells are confined to specific sites in the melanoma of NCTD-treated mice at 3.0 mg/kg (p≤0.05) and at 0.75 mg/kg (p≤0.01), where they are likely to be most highly expressed some chemokines and their ligands. Additionally, in the treatment with NCTD at 0.75 mg/kg, the total area infiltrated by lymphocytes was negatively related to the mitosis rate of the tumor cells. This finding corroborates that cytotoxic cell recruited to the tumor site, such as CD8+ T cells and iNKT cells, contribute to the depletion of tumor cells and the effect of the drugs themselves, as previously discussed. Briefly, NCTD alone in the murine melanoma model activates peripheral blood NK and iNKT cells depending on the presence of transcription/activation factors and the dose. iNKT cells migrate to the tumor site and are active. In addition, the statistical relationships with the total area of infiltrated leukocytes suggest that iNKTs are located in specific tumor sites.
The current study has tested for the first time the effect of PTX in combination with NCTD in a mouse model of melanoma is capable of inducing cell death, decreasing the rate of mitosis, and improving antitumor immunity through the recruitment of NK cells and iNKT by tumor site. Through Pearson's correlation analysis, it was found that in peripheral blood, NK cells are active (CD107a+) expressing Sca-1+/c-kit+ (p≤0.01) in both drug combinations. At the same time, concerning the control group, the NK cells infiltrated in the tumor reached a significant maximum (p≤0.001) in the treatment of PTX at 60 mg/kg + NCTD at 3.0 mg/kg. Similarly, iNKT cells in the tumor were negatively correlated (p≤0.05) with peripheral blood NK cells in PTX 60 mg/kg + NCTD 3.0 mg/kg treatment, demonstrated by Pearson's correlations. These findings in the specimens treated with the combination of PTX and NCTD corroborate the migration of these peripheral blood natural killer cells to the tumor site to control this neoplasm together with other cytotoxic cells, as has been reported for other types of cancer, including melanoma [
38,
39,
40]. Histopathological analysis revealed that NCTD in combination with PTX (PTX 60 mg/kg + NCTD 3 mg/kg and PTX 60 mg/kg + NCTD 0.75 mg/kg) increased the number of infiltrating lymphocytic cells in the tumor mass regarding the control group (p≤0.01 and p<0.05, respectively). Interestingly, in the PTX 60 mg/kg + NCTD 3.0 mg/kg treatment, iNKT cells present at the tumor site were inversely related to peripheral blood NK cells (p≤0.01), which may be an indication of their recruitment to the tumor site, and they are active cells (CD107a+) corroborating their presence with the statistical correlations that they keep with the total lymphocyte count (p≤0.05) demonstrated using correlations.
Infiltration of cytotoxic NK cells and iNKT cells into tumors is a favorable prognostic marker for various cancers, including melanoma [
20]. Furthermore, previous research has shown that NK cells isolated from an active site of inflammation possess enhanced functional capabilities [
55,
56]. While in patients with melanoma, tumor-infiltrating lymphocytes confer more remarkable survival and prolong disease-free lifetime [
21]. The response mediated by the recruitment and functioning of NK and iNKT cells on the tumor by administering NCTD and PTX was corroborated by the mitotic index and the nuclear area factor (sensitive predictor of the early apoptotic effect of anticancer therapy). The two combined treatment groups (PTX 60 mg/kg + NCTD 3.0 mg/kg and PTX 60 mg/kg + NCTD 0.75 mg/kg) showed a lower presence of mitotic bodies compared to the control group (p≤0.01 and p≤0.05, respectively).
As previously discussed, the mitotic body count is a significant independent predictor of survival in patients with primary cutaneous melanoma [
12,
32]. Therefore, the highly significant inverse correlations (r2=-1) between the mitotic bodies and the infiltrated lymphocyte count (40x) in the treatment of PTX at 60 mg/kg + NCTD at 3.0 mg/kg demonstrated that cytotoxic cells participate in the fate of the tumor; the greater the number of infiltrated lymphocytes, the lower the replication rate of the tumor cells. In the same sense, in the treatment of PTX at 60 mg/kg + NCTD at 0.75 mg/kg, a significant correlation (p≤0.01) was observed between iNKT cells expressing Sca-1+/c-kit+ with the lymphocytic infiltrate. (40x). This finding indicates that these cells are part of the immune arsenal against melanoma in mice treated with this combination of drugs. In the PTX 60 mg/kg + NCTD 3.0 mg/kg dosing scheme, the NFA of the tumor cells was increased (p≤0.01) concerning the control; however, it did not correlate with NK or iNKT cells.
In contrast, with PTX at 60 mg/kg + NCTD at 0.75 mg/kg, NFA had a direct correlation (p≤0.01) with peripheral blood iNKT. These findings suggest that combining both drugs induces this damage preceding tumor cell apoptosis. On the subject, PTX has been shown to induce apoptosis in A375 and MeWo human melanoma cell lines [
57,
58], in squamous cell carcinoma [
59] and in a mouse model of melanoma induced with B16-F1 cells [
10]. While NCTD exerts this same damage in human melanoma A375 cells [
60,
61] and in WM35, and 1205Lu melanoma cell lines, as well as in a transgenic mouse model in which expression of BRAFV600E [
62]. In the same way, the two groups of combined treatment showed a greater NFA concerning the control group with statistical significance. Due to the characteristic changes in nuclear morphology during apoptosis, morphological features can be used as indicators of activation of programmed cell death. A low NFA has been reported as an early sign of cell death [
63,
64,
65]. However, the participation of peripheral blood iNKTs in NFA induction is ruled out since the induction of cytotoxic damage induced by these cells occurs by direct contact with the target cell. However, it remains to be demonstrated whether the INF-γ produced by these cells and released into the bloodstream under the corresponding treatment scheme (PTX at 60 mg/kg + NCTD at 0.75 mg/kg) would be capable of reaching the tumor microenvironment and modulate apoptosis.
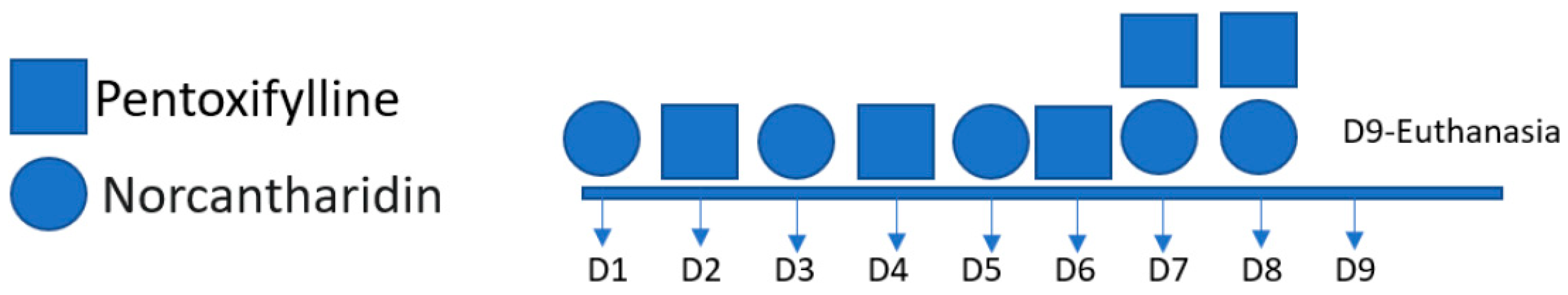
Figure 1.
The figure shows the days of drug administration; pentoxifylline was administered on days 2, 4, 6, 7, and 8; norcantharidin was administered on days 1, 3, 5, 7 and 8.
Figure 1.
The figure shows the days of drug administration; pentoxifylline was administered on days 2, 4, 6, 7, and 8; norcantharidin was administered on days 1, 3, 5, 7 and 8.
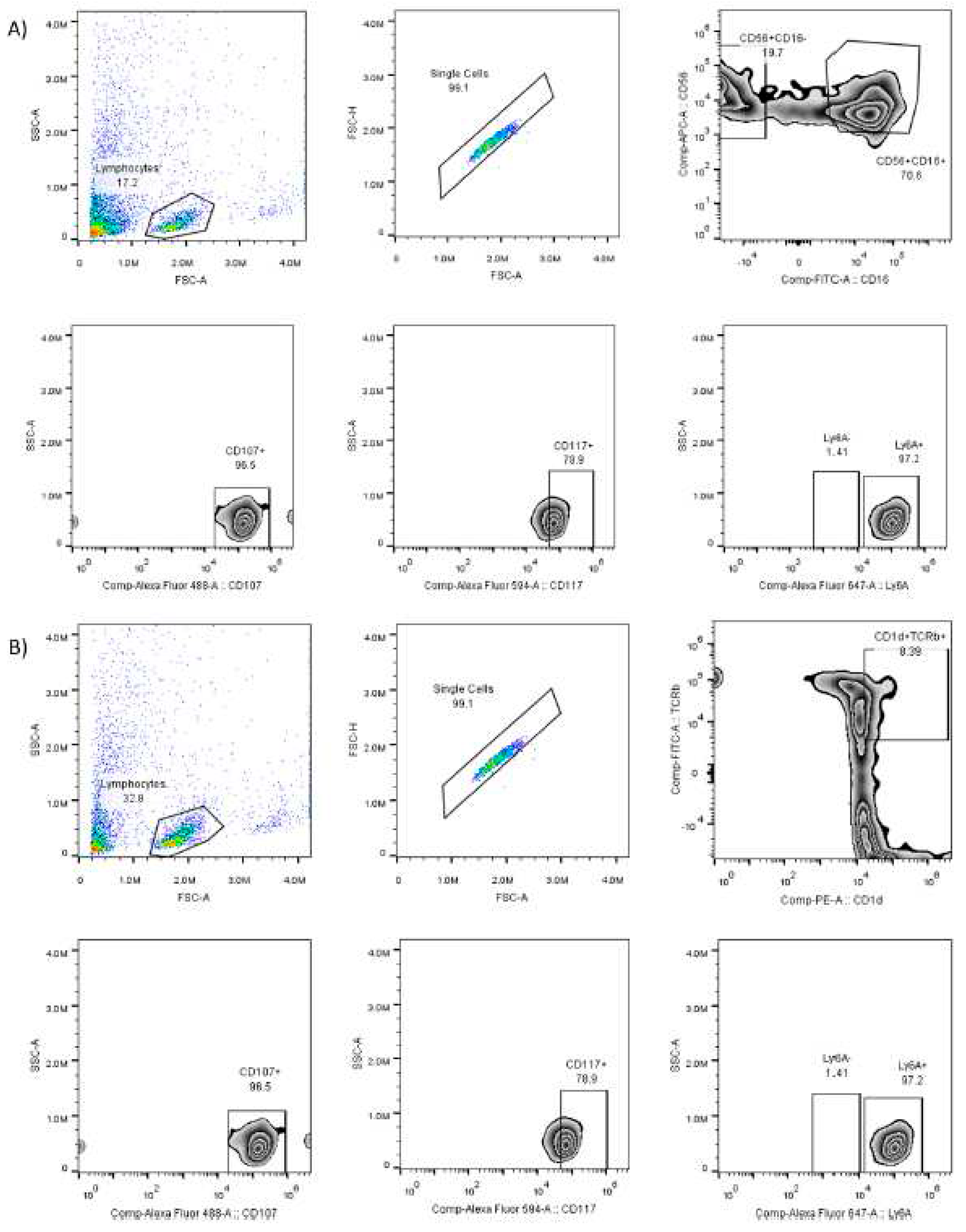
Figure 2.
Gating strategy to identify subpopulations of natural killer cells. Panel A) for NK cells shows from left to right the number of events recorded, number of lymphocytes, number of NK cells (CD56+ and CD16+ positivity), number of active NK cells (CD107+), number of NK cells with the marker c-kit (CD117+), number of NK cells with the Sca-1 marker (Ly6A+). Panel B) for iNKT cells shows from left to right the number of events recorded, number of lymphocytes, number of iNKT cells (CD1D+ and TCRVB8.1+ positivity), number of active iNKT cells (CD107+), number of iNKT cells with c-kit marker (CD117+), number of iNKT cells with Sca-1 marker (Ly6A+).
Figure 2.
Gating strategy to identify subpopulations of natural killer cells. Panel A) for NK cells shows from left to right the number of events recorded, number of lymphocytes, number of NK cells (CD56+ and CD16+ positivity), number of active NK cells (CD107+), number of NK cells with the marker c-kit (CD117+), number of NK cells with the Sca-1 marker (Ly6A+). Panel B) for iNKT cells shows from left to right the number of events recorded, number of lymphocytes, number of iNKT cells (CD1D+ and TCRVB8.1+ positivity), number of active iNKT cells (CD107+), number of iNKT cells with c-kit marker (CD117+), number of iNKT cells with Sca-1 marker (Ly6A+).
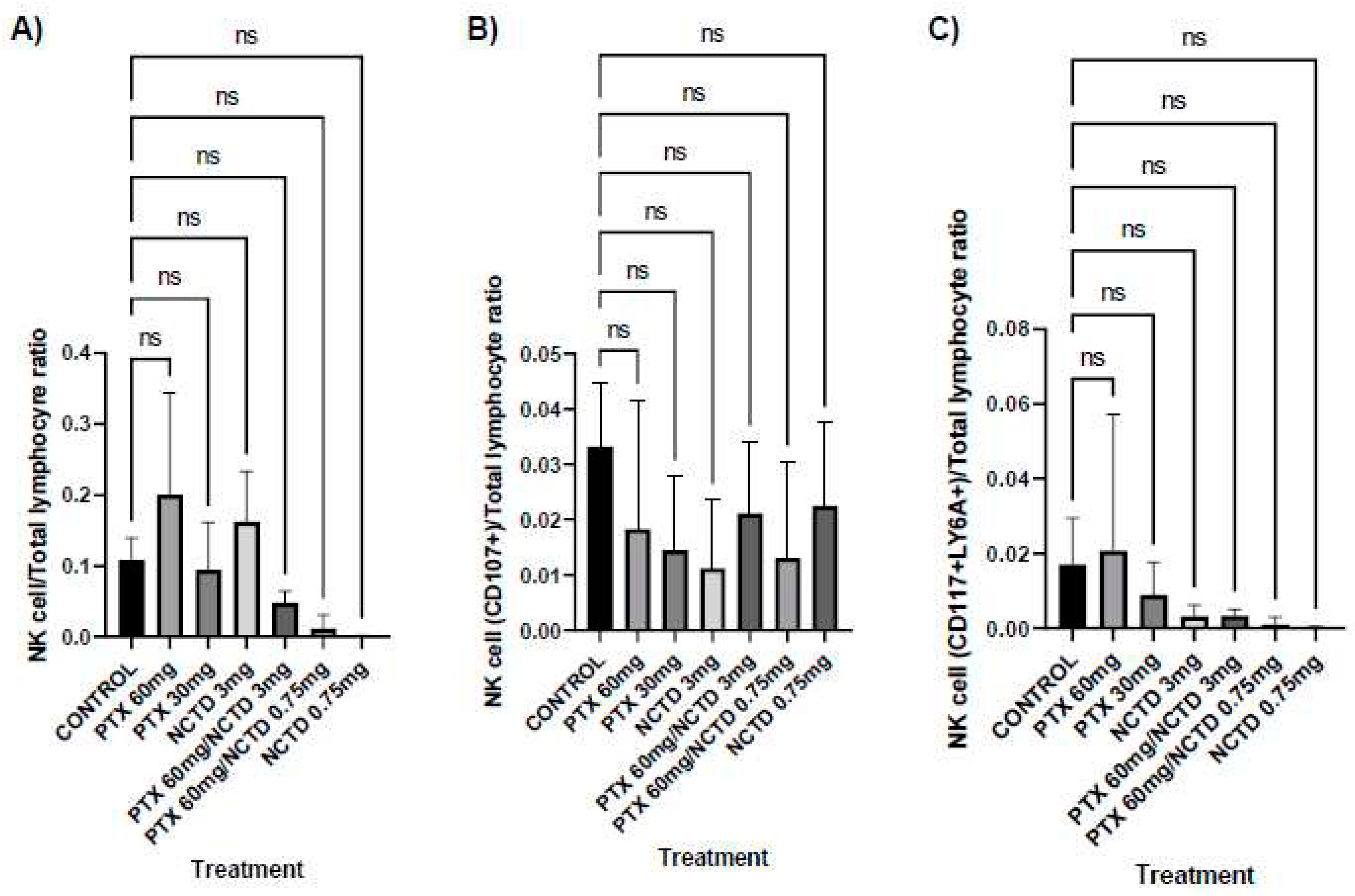
Figure 3.
The phenotype of Peripheral NK Cells. A) Proportion of NK cells concerning the number of total lymphocytes. B) Proportion of NK cells (CD107+) to the number of total lymphocytes. C) Proportion of NK cells (CD117+LY6A+). Statistical differences to the control, p≤0.05, p≤0.01, p≤0.001.
Figure 3.
The phenotype of Peripheral NK Cells. A) Proportion of NK cells concerning the number of total lymphocytes. B) Proportion of NK cells (CD107+) to the number of total lymphocytes. C) Proportion of NK cells (CD117+LY6A+). Statistical differences to the control, p≤0.05, p≤0.01, p≤0.001.
Figure 4.
The phenotype of Peripheral iNKT Cells. A) Proportion of iNKT cells (CD1D+TCRVB8.1+) concerning the number of total lymphocytes. B) Proportion of iNKT cells (CD107+) to the number of total lymphocytes. C) Proportion of iNKT cells (CD117+LY6A+) about the number of total lymphocytes. Statistical differences concerning the control, p≤0.05, p≤0.01, p≤0.001.
Figure 4.
The phenotype of Peripheral iNKT Cells. A) Proportion of iNKT cells (CD1D+TCRVB8.1+) concerning the number of total lymphocytes. B) Proportion of iNKT cells (CD107+) to the number of total lymphocytes. C) Proportion of iNKT cells (CD117+LY6A+) about the number of total lymphocytes. Statistical differences concerning the control, p≤0.05, p≤0.01, p≤0.001.
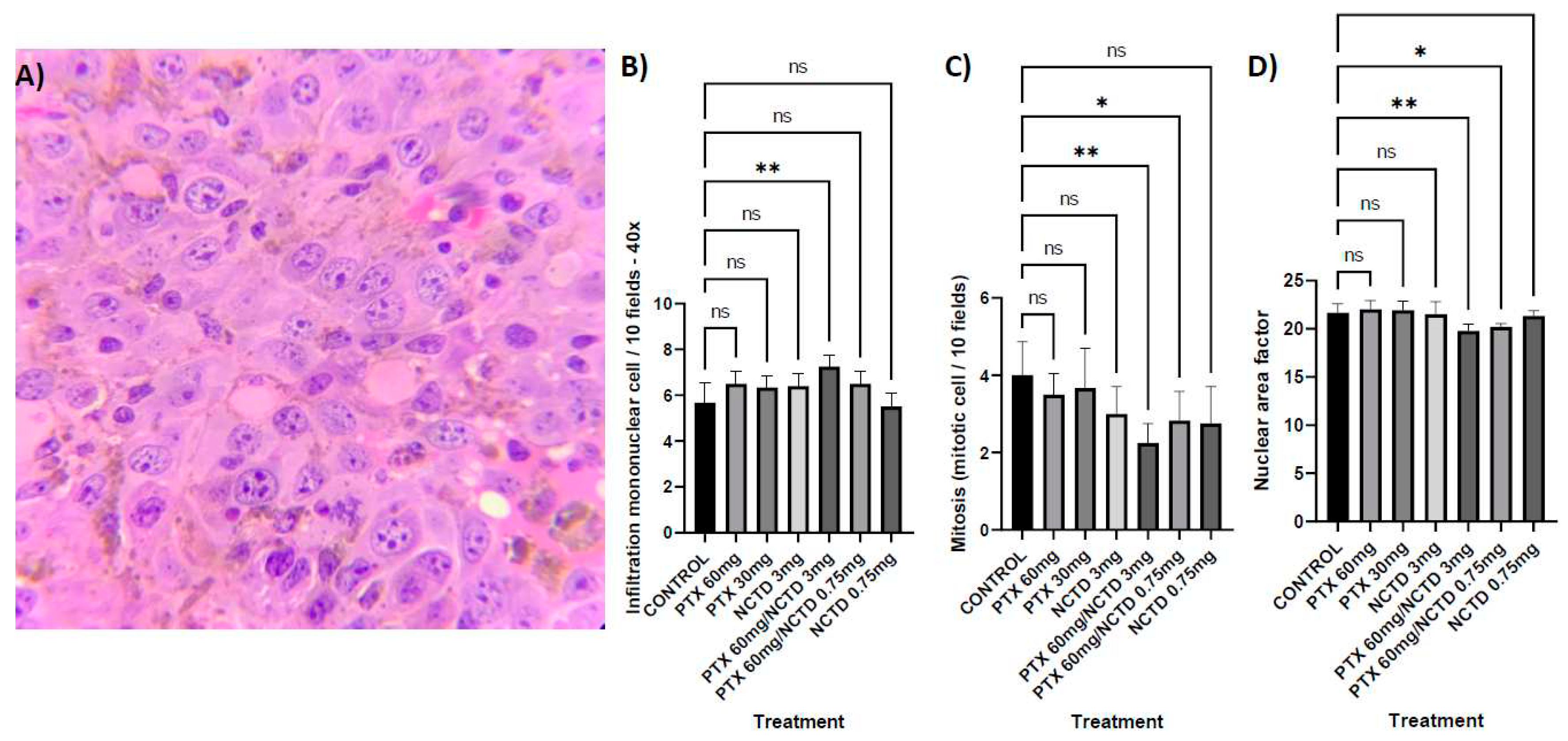
Figure 5.
Histopathological analysis. A) Infiltration of mononuclear cells, the treatment group with PTX 60 mg/kg in combination with NCTD 3 mg/kg (v.p.), showed a more significant infiltration of mononuclear cells concerning the control group with statistical significance (p≤0.01). B) Mitotic figures, the treatment groups with PTX 60 mg/kg in combination with NCTD 3 mg/kg and PTX 60 mg/kg in combination with NCTD 0.75 mg/kg showed a lower presence of mitotic bodies to the control group with statistical significance (p≤0.01 and p≤0.05, respectively). C) Nuclear factor area, the treatment groups with PTX 60 mg/kg in combination with NCTD 3 mg/kg and PTX 60 mg/kg in combination with NCTD 0.75 mg/kg showed a higher nuclear area factor concerning the group control with statistical significance (p≤0.001 and p≤0.05, respectively).
Figure 5.
Histopathological analysis. A) Infiltration of mononuclear cells, the treatment group with PTX 60 mg/kg in combination with NCTD 3 mg/kg (v.p.), showed a more significant infiltration of mononuclear cells concerning the control group with statistical significance (p≤0.01). B) Mitotic figures, the treatment groups with PTX 60 mg/kg in combination with NCTD 3 mg/kg and PTX 60 mg/kg in combination with NCTD 0.75 mg/kg showed a lower presence of mitotic bodies to the control group with statistical significance (p≤0.01 and p≤0.05, respectively). C) Nuclear factor area, the treatment groups with PTX 60 mg/kg in combination with NCTD 3 mg/kg and PTX 60 mg/kg in combination with NCTD 0.75 mg/kg showed a higher nuclear area factor concerning the group control with statistical significance (p≤0.001 and p≤0.05, respectively).
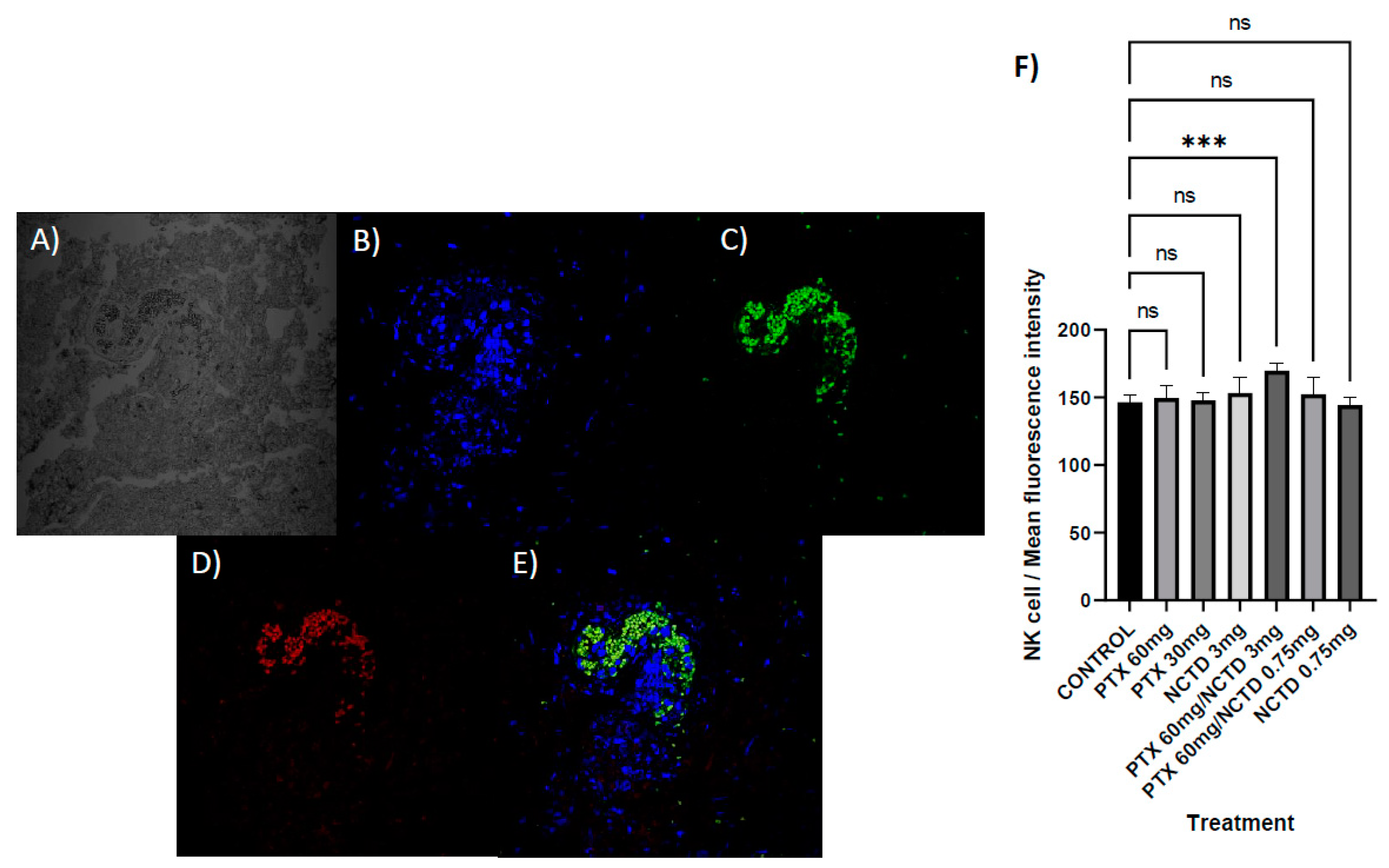
Figure 6.
Localization of NK cells in melanoma sections, A) Without fluorescence. B) DAPI-stained nuclei. C) CD16/32. D) CD56. E) Merged. F) Immunofluorescence microscopy analysis, intratumoral lymphocytic infiltration by NK cells (CD16+CD56+) with statistical significance for the treatment group with PTX 60 mg/kg in combination with NCTD 3 mg/kg, (p 0.0004). No statistically significant difference was detected in the rest of the treatments.
Figure 6.
Localization of NK cells in melanoma sections, A) Without fluorescence. B) DAPI-stained nuclei. C) CD16/32. D) CD56. E) Merged. F) Immunofluorescence microscopy analysis, intratumoral lymphocytic infiltration by NK cells (CD16+CD56+) with statistical significance for the treatment group with PTX 60 mg/kg in combination with NCTD 3 mg/kg, (p 0.0004). No statistically significant difference was detected in the rest of the treatments.
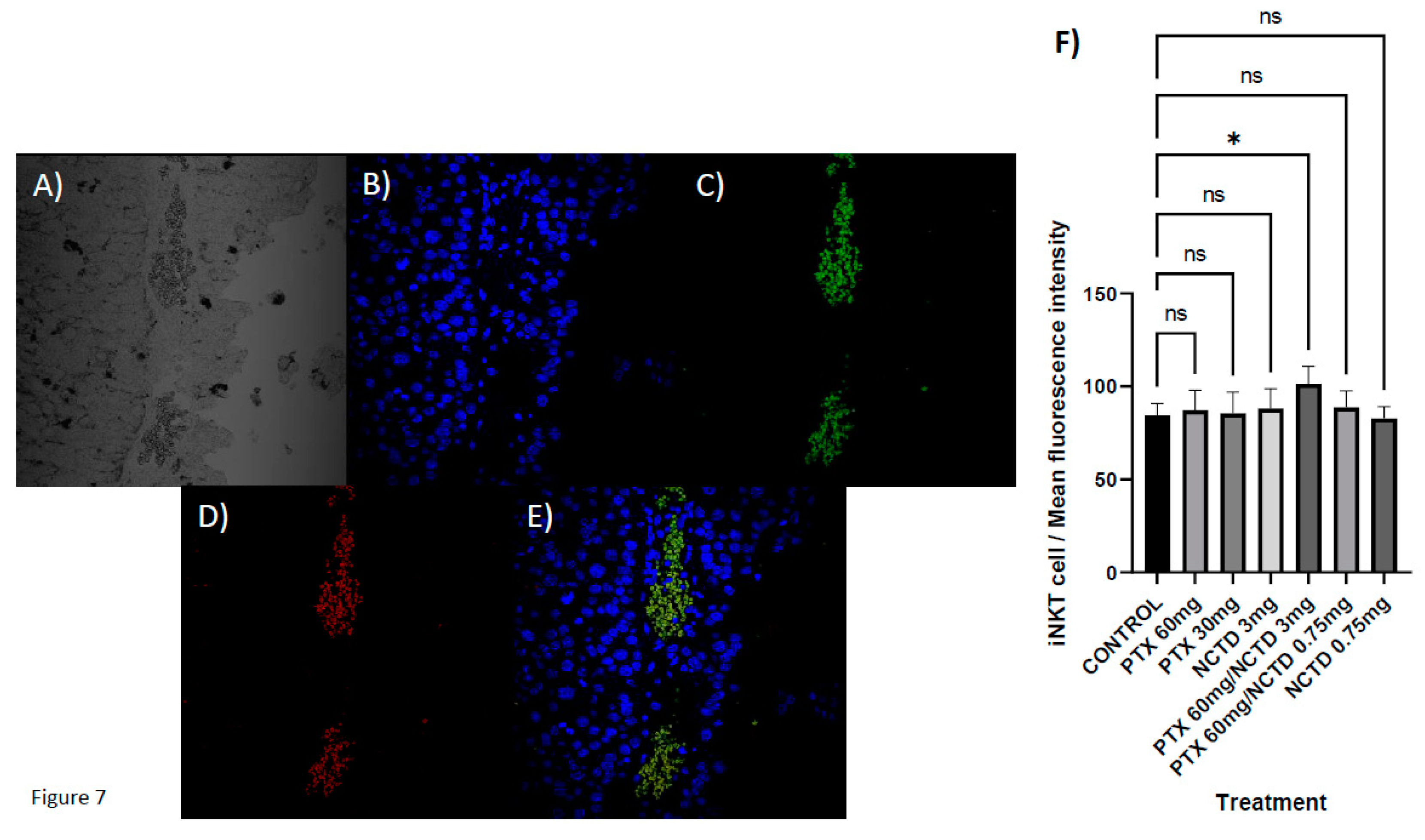
Figure 7.
Localization of iNKT cells in melanoma sections. A) No fluorescence. B) DAPI-stained nuclei. C) TCRVB8.1. D) CD1D. E) Merged. F) Immunofluorescence microscopy analysis, intratumoral lymphocytic infiltration by iNKT cells (CD1D+TCRVB8.1+) with statistical significance for the treatment group with PTX 60 mg/kg in combination with NCTD 3 mg/kg, (p 0.0231). No statistically significant difference was detected in the rest of the treatments.
Figure 7.
Localization of iNKT cells in melanoma sections. A) No fluorescence. B) DAPI-stained nuclei. C) TCRVB8.1. D) CD1D. E) Merged. F) Immunofluorescence microscopy analysis, intratumoral lymphocytic infiltration by iNKT cells (CD1D+TCRVB8.1+) with statistical significance for the treatment group with PTX 60 mg/kg in combination with NCTD 3 mg/kg, (p 0.0231). No statistically significant difference was detected in the rest of the treatments.
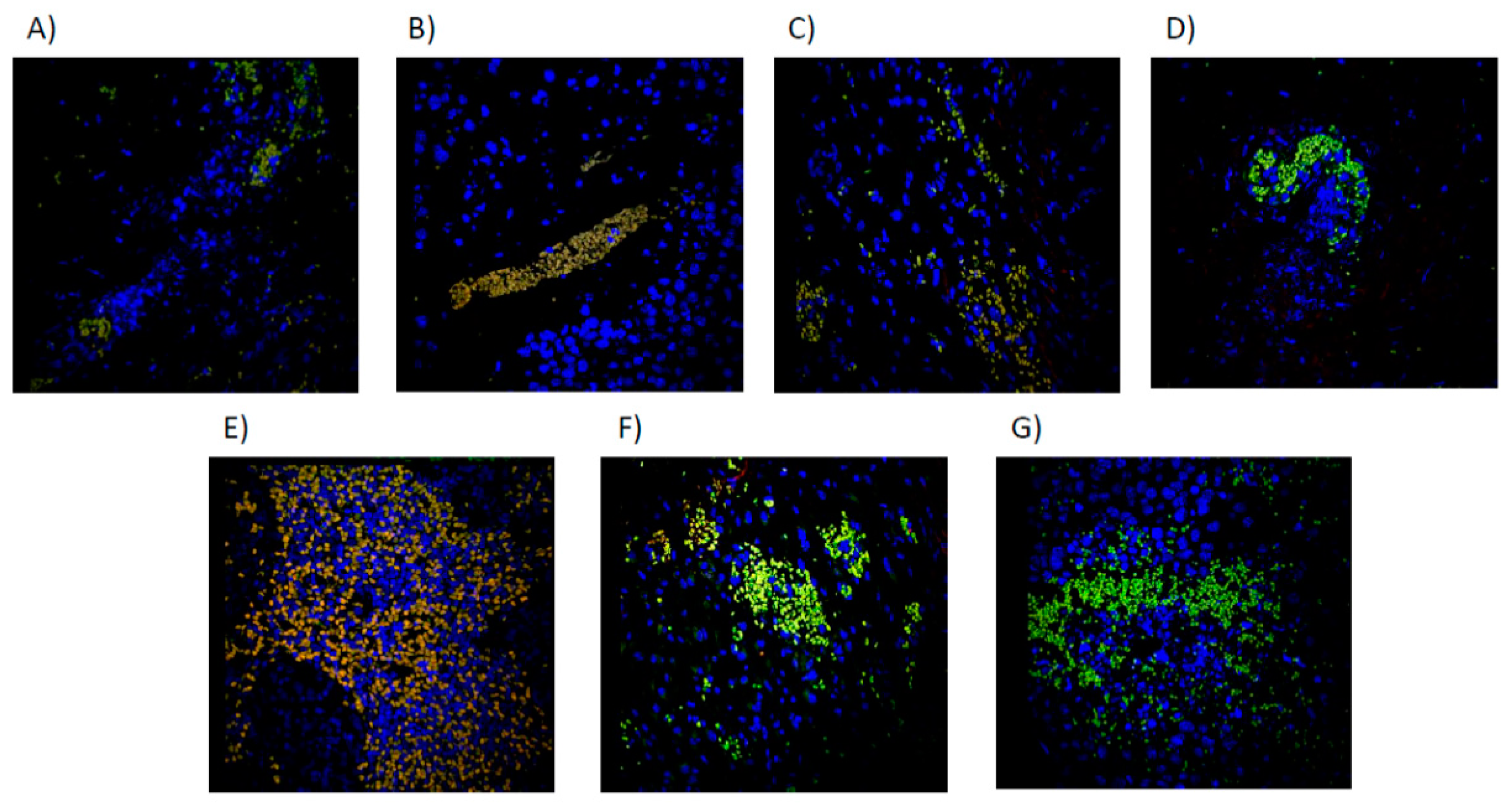
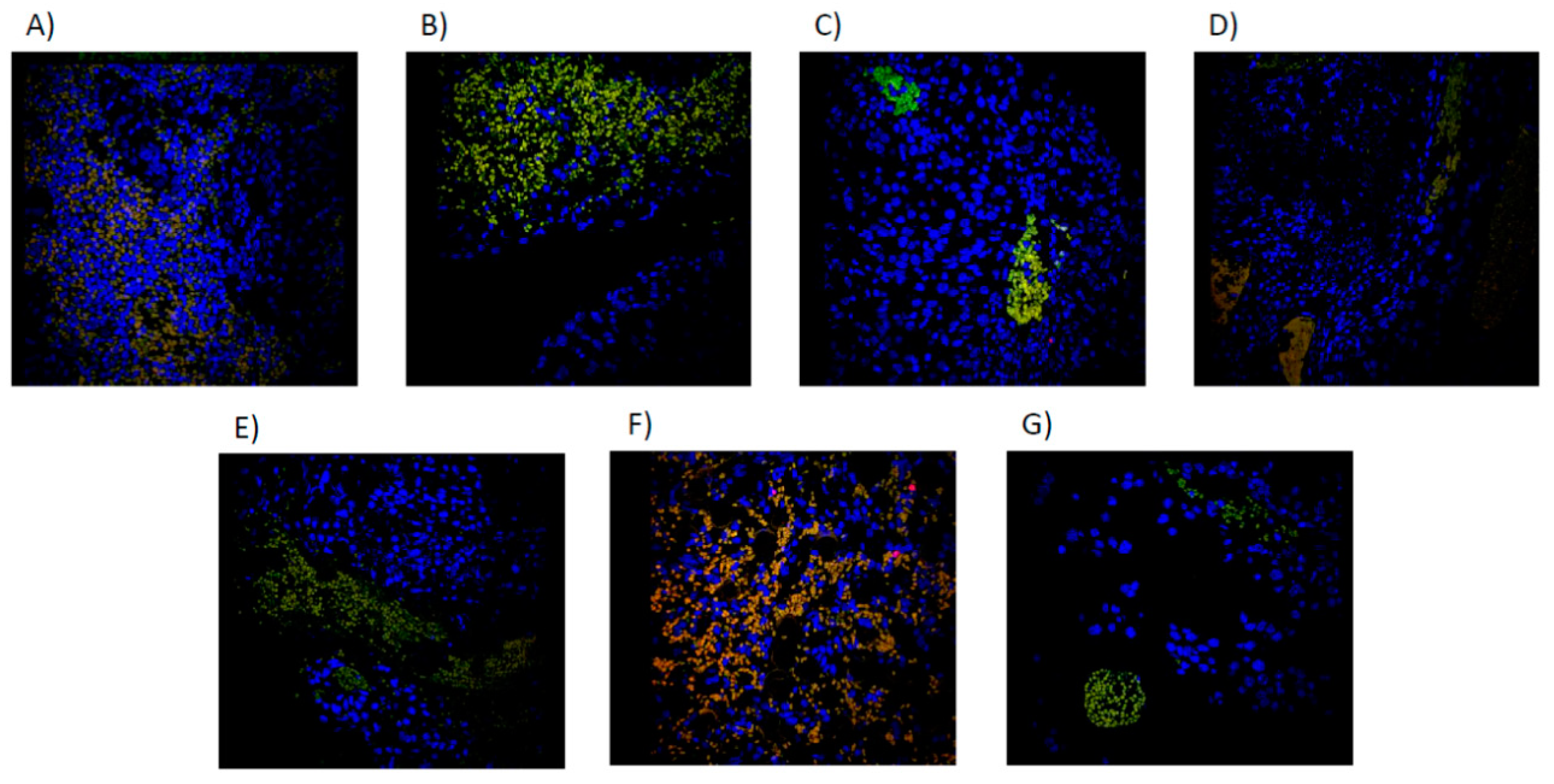
Figure 8.
Immunofluorescence microscopy image of Merged NK cells (CD16+/CD56+/DAPI+) by treatment group. A): CONTROL, sterile saline solution. B) PTX 60 mg/kg. C) PTX 30 mg/kg. D) NCTD 3mg/kg. E) PTX 60 mg/kg + NCTD 3 mg/kg. F) PTX 60mg + NCTD 0.75mg/kg. G) NCTD 0.75 mg/kg.
Figure 8.
Immunofluorescence microscopy image of Merged NK cells (CD16+/CD56+/DAPI+) by treatment group. A): CONTROL, sterile saline solution. B) PTX 60 mg/kg. C) PTX 30 mg/kg. D) NCTD 3mg/kg. E) PTX 60 mg/kg + NCTD 3 mg/kg. F) PTX 60mg + NCTD 0.75mg/kg. G) NCTD 0.75 mg/kg.
Figure 9.
Immunofluorescence microscopy image of iNKT cells in Merged (CD1D+/TCRVB8.1+/DAPI+) by treatment group. A): CONTROL, sterile saline solution. B) PTX 60 mg/kg. C) PTX 30 mg/kg. D) NCTD 3mg/kg. E) PTX 60 mg/kg + NCTD 3 mg/kg. F) PTX 60mg + NCTD 0.75mg/kg. G) NCTD 0.75 mg/kg.
Figure 9.
Immunofluorescence microscopy image of iNKT cells in Merged (CD1D+/TCRVB8.1+/DAPI+) by treatment group. A): CONTROL, sterile saline solution. B) PTX 60 mg/kg. C) PTX 30 mg/kg. D) NCTD 3mg/kg. E) PTX 60 mg/kg + NCTD 3 mg/kg. F) PTX 60mg + NCTD 0.75mg/kg. G) NCTD 0.75 mg/kg.
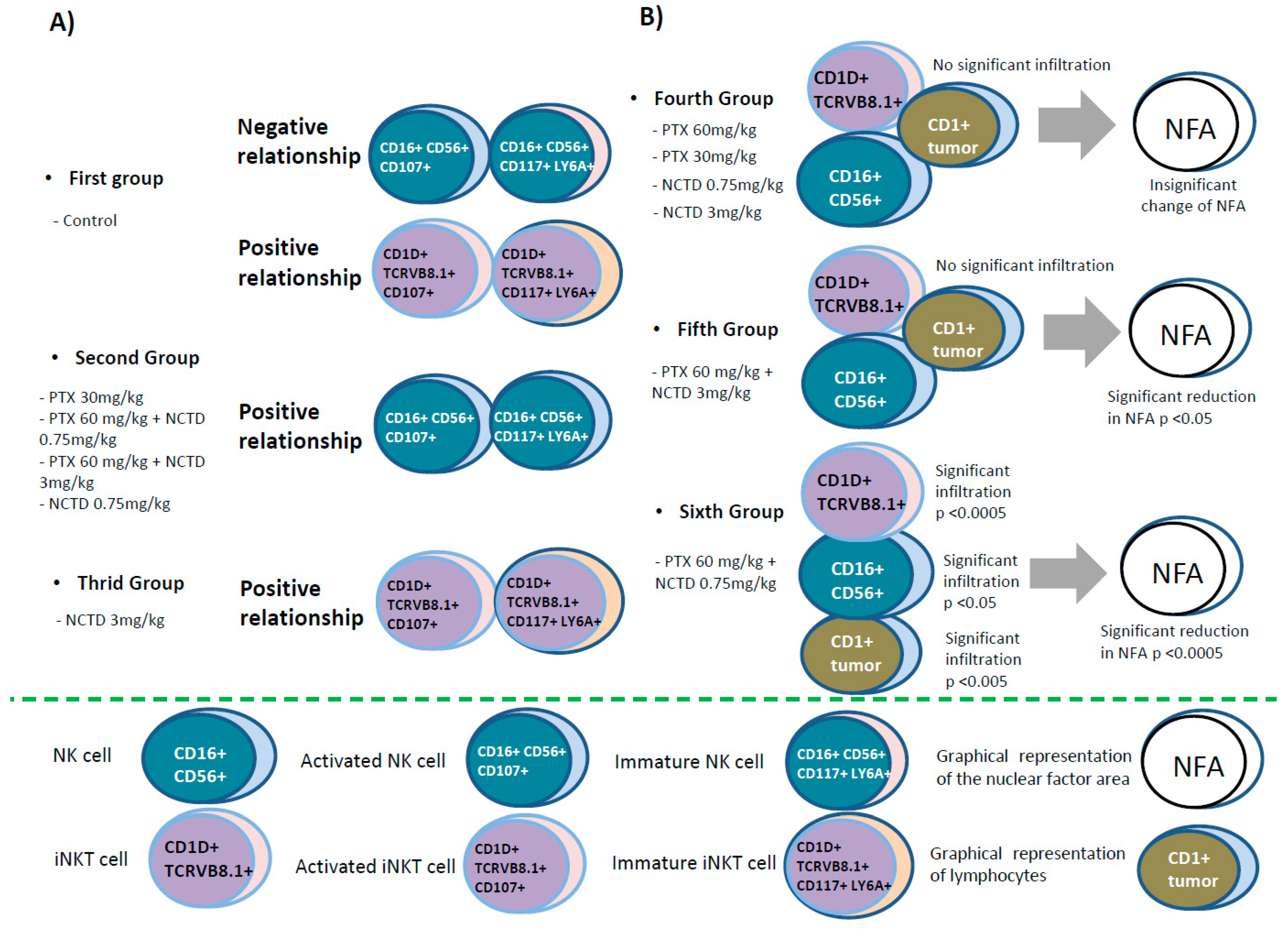
Figure 10.
Graphic representation of the correlations of the natural killer cell subpopulations (NK and iNKT) in peripheral blood and at the tumor site in a mouse melanoma model treated with pentoxifylline (PTX), norcantharidin (NCTD) and their mixtures. A) Peripheral blood, according to the evaluation by flow cytometry, the following distribution groups of the subpopulations of NK and iNKT cells were formed. B) Melanoma tumor infiltration of NK and iNKT cells by Confocal microscope.
Figure 10.
Graphic representation of the correlations of the natural killer cell subpopulations (NK and iNKT) in peripheral blood and at the tumor site in a mouse melanoma model treated with pentoxifylline (PTX), norcantharidin (NCTD) and their mixtures. A) Peripheral blood, according to the evaluation by flow cytometry, the following distribution groups of the subpopulations of NK and iNKT cells were formed. B) Melanoma tumor infiltration of NK and iNKT cells by Confocal microscope.
Table 1.
Pearson correlation analysis shows significant correlations of natural killer NK (CD16+CD56+) and iNKT (CD1D+TCRvB8.1+) cells, either in an activated state (CD107a+) or presenting immaturity/activation markers (c -kit+/Lya6A+) in a mouse model of melanoma. The relationships of these cells in peripheral blood or infiltrates at the tumor site concerning some variables analyzed in the tumor are presented.
Table 1.
Pearson correlation analysis shows significant correlations of natural killer NK (CD16+CD56+) and iNKT (CD1D+TCRvB8.1+) cells, either in an activated state (CD107a+) or presenting immaturity/activation markers (c -kit+/Lya6A+) in a mouse model of melanoma. The relationships of these cells in peripheral blood or infiltrates at the tumor site concerning some variables analyzed in the tumor are presented.
| Treatment |
Correlated Variables |
r2 |
p Value |
| Control |
NKc-kit/Lya6A |
NK107a |
-0.668 |
p≤0.05 |
| iNKT |
iNKT107a |
0.890 |
p≤0.01 |
| iNKT |
iNKT/c-kit/LyA6A |
0.982 |
p≤0.001 |
| iNKTc-kit/LyA6A |
iNKT107a |
0.796 |
p≤0.05 |
| NKc-kit/Lya6A |
NFA |
-0.704 |
p≤0.05 |
| PTX 60 mg/kg |
NKc-kit/LyA6A |
NK107a |
0.957 |
p≤0.01 |
| NK107a |
iNKT107a |
0.929 |
p≤0.01 |
NK107a
NKc-kit/Lya6A |
iNKTc-kit/Lya6A
iNKT107a |
0.885
0.849 |
p≤0.05
p≤0.05 |
| NKc-kit/Lya6A |
iNKTc-kit/Lya6A |
0.977 |
p≤0.001 |
| NK |
Mitosis |
0.895 |
p≤0.05 |
| PTX 30 mg/kg |
NKc-kit/Lya6A |
NK107a |
0.878 |
p≤0.05 |
| L.I. 40x |
Mitosis |
1.0 |
p≤0.001 |
| NCTD 3.0 mg/kg |
iNKTc-kit/Lya6A |
iNKT107a |
0.970 |
p≤0.01 |
| iNKT107a |
L.C. 10x |
0.875 |
p≤0.05 |
| iNKTc-kit/LyA6A |
L.C. 10x |
0.870 |
p≤0.05 |
| iNKT tumor |
Area |
-0.983 |
p≤0.01 |
| NCTD 0.75 mg/kg |
NK |
NK107a |
0.987 |
p≤0.05 |
| NKc-kit/Lya6A |
NK107a |
0.967 |
p≤0.05 |
| NK tumor |
Mitosis |
0.972 |
p≤0.05 |
| NK |
iNKT tumor |
0.959 |
p≤0.05 |
| NK107a |
iNKT tumor |
0.943 |
p≤0.05 |
| Mitosis |
Area |
-0.993 |
p≤0.01 |
| NK tumor |
Area |
-0.992 |
p≤0.01 |
| PTX 60 mg/kg + NCTD 3.0 mg/kg |
NKc-kit/Lya6A |
NK107a |
0.991 |
p≤0.01 |
| Mitosis |
L.I. 40x |
-1.0 |
p≤0.001 |
| NK |
iNKT tumor |
-0.949 |
p≤0.05 |
| iNKT107a |
L.C 10x |
0.944 |
p≤0.05 |
| PTX 60mg/kg + NCTD 0.75 mg/kg |
NK |
NK107a |
0.97 |
p≤0.001 |
| NK |
NKc-kit/Lya6A |
0.994 |
p≤0.001 |
| NKc-kit/Lya6A |
NK107a |
0.945 |
p≤0.01 |
| iNKTc-kit/Lya6A |
L.I. 40x |
0.941 |
p≤0.01 |
| iNKT |
NFA |
0.886 |
p≤0.05 |
| iNKT |
NK tumor |
0.828 |
p≤0.05 |
| NK tumor |
L.I. 40x |
-0.857 |
p≤0.05 |
| iNKTc-kit/Lya6A |
iNKT tumor |
0.837 |
p≤0.05 |
| L.C 10x |
Area |
0.874 |
p≤0.05 |