Submitted:
05 September 2023
Posted:
07 September 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
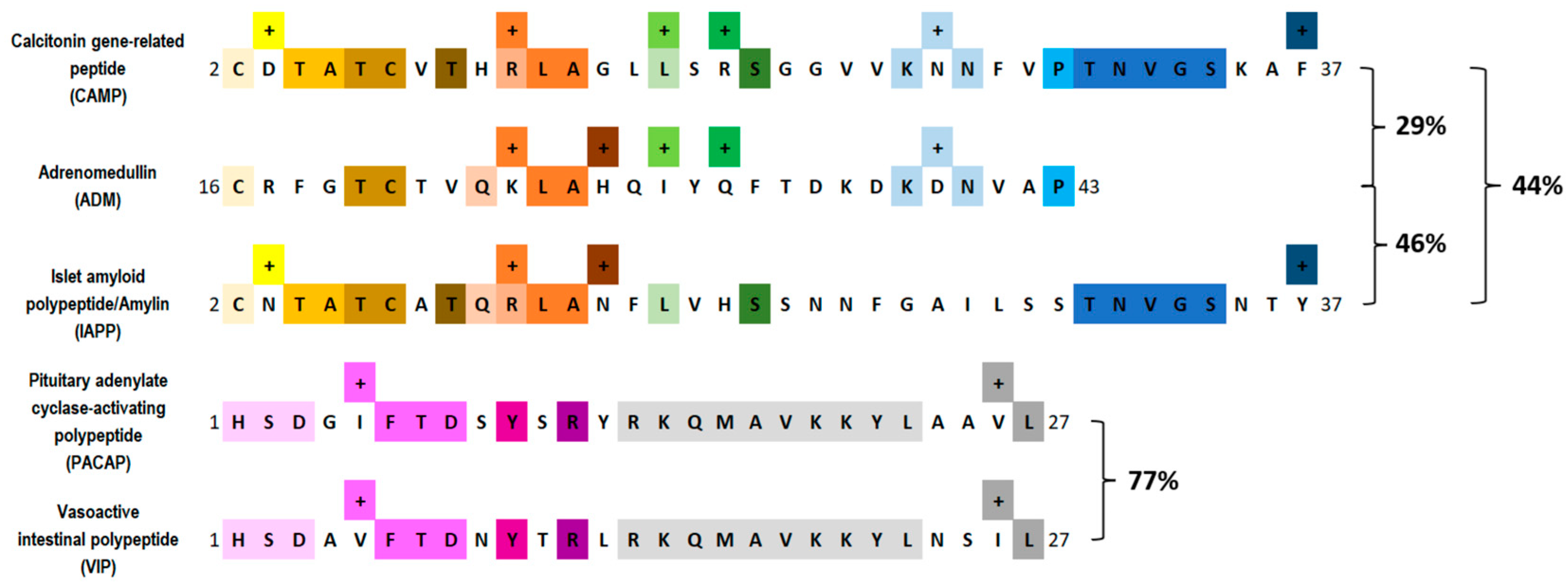
2. Pituitary Adenylate Cyclase–Activating Peptide
2.1. Background
2.2. Receptor and Signaling Mechanisms of PACAP
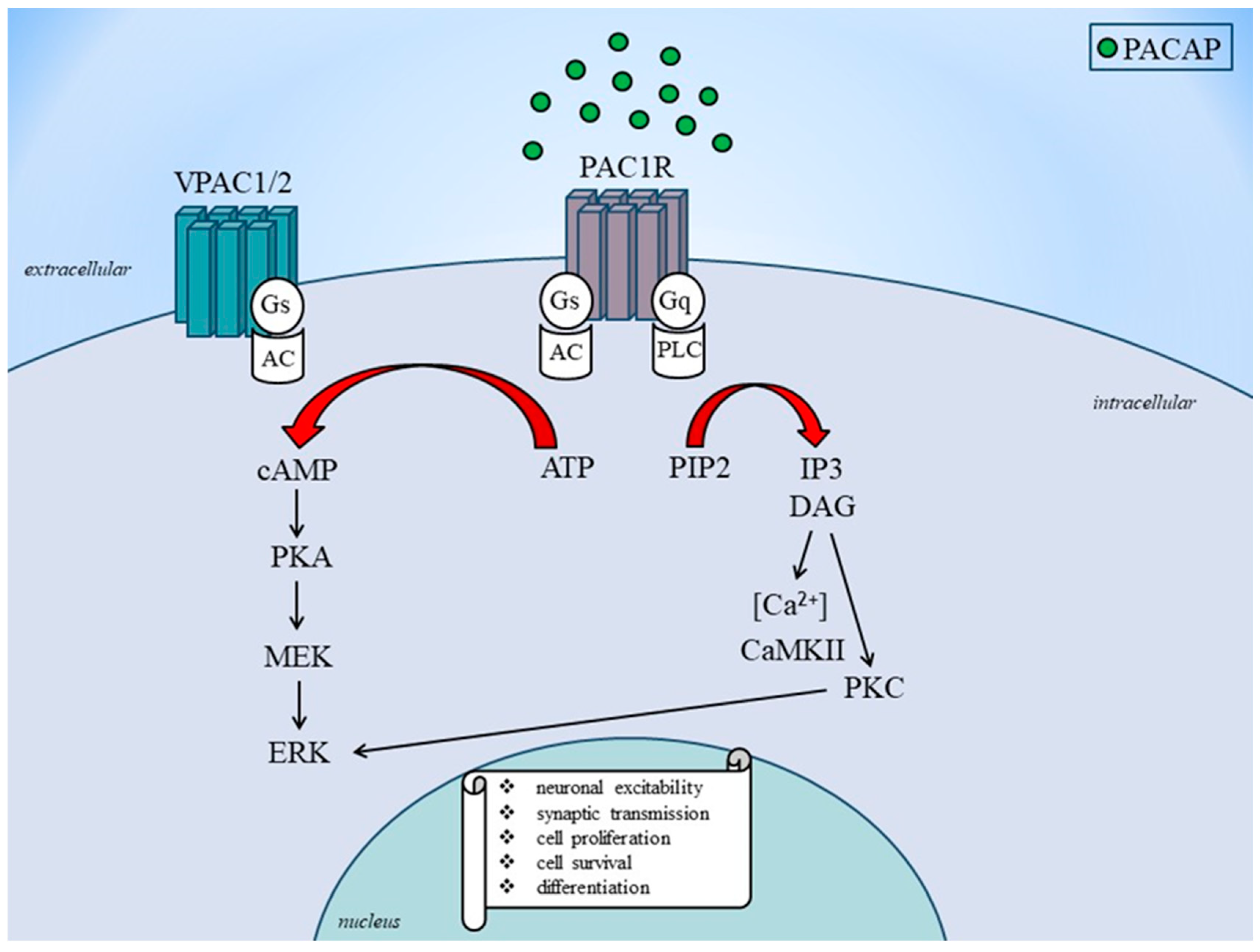
2.3. Role of PACAP in Migraine
2.4. Preclinical Studies
2.5. Clinical Studies
| ClinicalTrials.gov Identifier | Monoclonal antibody | Target | Status | Ref. |
|---|---|---|---|---|
| NCT03238781 | AMG 301 | receptor | No benefit over placebo for migraine prevention | [150,151] |
| NCT05133323 | Lu AG09222 | ligand | No results posted | [152,153] |
| NCT04498910 | LY3451838 | receptor | No results posted | [154] |
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | adenylate cyclase |
| ADM | adrenomedullin |
| cAMP | cyclic adenosine monophosphate |
| CGRP | calcitonin gene-related peptide |
| DAG | diacylglycerol |
| ERK | extracellular signal-regulated kinase |
| GPCR | G protein-coupled receptors |
| IAPP | islet amyloid polypeptide/amylin |
| mAbs | monoclonal antibodies |
| MAPK | mitogen-activated protein kinase |
| MCA | middle cerebral artery |
| MEK: | mitogen-activated protein kinase kinase |
| PAC1 | pituitary adenylate cyclase-activating Peptide 1 |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| PACAP1–38 | 38-amino acid form of PACAP |
| PACAP1–27 | 27-amino acid form of PACAP |
| PACAP6-38 | 6–38-amino acid form of PACAP |
| PIP2 | phosphatidyl inositol 4,5-bisphosphate |
| PKA | protein kinase A |
| PKC | activates protein kinase C |
| PRP | PACAP-related peptide |
| TNC | trigeminal nucleus caudalis |
| TS | trigeminovascular system |
| VPAC | vasoactive intestinal peptide receptor |
| VIP | vasoactive intestinal polypeptide |
References
- Pescador Ruschel, M.A.; De Jesus, O. Migraine Headache; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK560787/ (accessed on 28 August 2023).
- Amiri, P.; Kazeminasab, S.; Nejadghaderi, S.A.; Mohammadinasab, R.; Pourfathi, H.; Araj-Khodaei. M.; Sullman, M.J.M.; Kolahi, A.A.; Safiri, S. Migraine: A Review on Its History, Global Epidemiology, Risk Factors, and Comorbidities. Front. Neurol. 2022, 12, 800605. [Google Scholar] [CrossRef]
- Ferrari, M.D. , Goadsby, P.J.; Burstein, R.; Kurth, T.; Ayata, C.; Charles, A.; Ashina, M.; van den Maagdenberg, A.M.J.M.; Dodick, D.W. Migraine. Nat Rev Dis Primers. 2022, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W. A Phase-by-Phase Review of Migraine Pathophysiology. Headache 2018, 58 (Suppl. 1), 4–16. [Google Scholar] [CrossRef] [PubMed]
- Schankin, C.J.; Viana, M.; Goadsby, P.J. Persistent and Repetitive Visual Disturbances in Migraine: A Review. Headache 2017, 57, 1–16. [Google Scholar] [CrossRef]
- Gasparini, C.F.; Sutherland, H.G.; Griffiths, L.R. Studies on the pathophysiology and genetic basis of migraine. Curr. Genomics 2013, 14, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Asoom, L.I.A.; Sunni, A.A.; Rafique, N.; Latif, R.; Saif, S.A.; Almandil, N.B.; Almohazey, D.; AbdulAzeez, S.; Borgio, J.F. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed. Pharmacother. 2021, 139, 111557. [Google Scholar] [CrossRef]
- Buse, D.C.; Rupnow, M.F.; Lipton, R.B. Assessing and managing all aspects of migraine: Migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin. Proc. 2009, 84, 422–435. [Google Scholar] [CrossRef]
- Gupta, J.; Gaurkar, S.S. Migraine: An Underestimated Neurological Condition Affecting Billions. Cureus 2022, 14, e28347. [Google Scholar] [CrossRef]
- Villar-Martinez, M.D.; Goadsby, P.J. Pathophysiology and Therapy of Associated Features of Migraine. Cells 2022, 11, 2767. [Google Scholar] [CrossRef] [PubMed]
- Durham, P.L. Calcitonin gene-related peptide (CGRP) and migraine. Headache 2006, 46 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef]
- Waschek, J.A.; Baca, S.M.; Akerman, S. PACAP and migraine headache: Immunomodulation of neural circuits in autonomic ganglia and brain parenchyma. J. Headache Pain 2018, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Pellesi, L.; Al-Karagholi, M.A.; De Icco, R.; Coskun H, Elbahi FA, Lopez-Lopez, C. ; Snellman, J.; Hannibal, J.; Amin, F.M.; Ashina, M. Effect of Vasoactive Intestinal Polypeptide on Development of Migraine Headaches: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2118543. [Google Scholar] [CrossRef] [PubMed]
- Ghanizada, H.; Al-Karagholi, M.A.; Walker, C.S.; Arngrim, N.; Rees, T.; Petersen, J.; Siow, A.; Mørch-Rasmussen, M.; Tan, S.; O'Carroll, S.J.; et al. Amylin Analog Pramlintide Induces Migraine-like Attacks in Patients. Ann. Neurol. 2021, 89, 1157–1171. [Google Scholar] [CrossRef]
- May, A.; Goadsby, P.J. Substance P receptor antagonists in the therapy of migraine. Expert Opin. Investig. Drugs 2001, 10, 673–678. [Google Scholar] [CrossRef]
- Petersen, K.A.; Birk, S.; Kitamura, K.; Olesen, J. Effect of adrenomedullin on the cerebral circulation: Relevance to primary headache disorders. Cephalalgia 2009, 29, 23–30. [Google Scholar] [CrossRef]
- Hay, D.L.; Garelja, M.L.; Poyner, D.R.; Walker, C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 2018, 175, 3–17. [Google Scholar] [CrossRef]
- Poyner, D.R.; Hay, D.L. Secretin family (Class B) G protein-coupled receptors - from molecular to clinical perspectives. Br. J. Pharmacol. 2012, 166, 1–3. [Google Scholar] [CrossRef]
- Edvinsson, L.; Grell, A.S.; Warfvinge, K. Expression of the CGRP Family of Neuropeptides and their Receptors in the Trigeminal Ganglion. J. Mol. Neurosci. 2020, 70, 930–944. [Google Scholar] [CrossRef]
- Dux, M.; Vogler, B.; Kuhn, A.; Mackenzie, K.D.; Stratton, J.; Messlinger, K. The Anti-CGRP Antibody Fremanezumab Lowers CGRP Release from Rat Dura Mater and Meningeal Blood Flow. Cells 2022, 11, 1768. [Google Scholar] [CrossRef] [PubMed]
- Pavelic, A.R.; Wöber, C.; Riederer, F.; Zebenholzer, K. Monoclonal Antibodies against Calcitonin Gene-Related Peptide for Migraine Prophylaxis: A Systematic Review of Real-World Data. Cells 2023, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Körtési, T.; Spekker, E.; Vécsei, L. Exploring the Tryptophan Metabolic Pathways in Migraine-Related Mechanisms. Cells 2022, 11, 3795. [Google Scholar] [CrossRef]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the Trigeminal System in Migraine. Headache 2019, 59, 659–681. [Google Scholar] [CrossRef]
- Edvinsson, L.; Tajti, J.; Szalárdy, L.; Vécsei, L. PACAP and its role in primary headaches. J. Headache Pain 2018, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Körtési, T.; Tuka, B.; Nyári, A.; Vécsei, L.; Tajti, J. The effect of orofacial complete Freund's adjuvant treatment on the expression of migraine-related molecules. J. Headache Pain 2019, 20, 43. [Google Scholar] [CrossRef]
- Russo, A.F. Calcitonin gene-related peptide (CGRP): A new target for migraine. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 533–552. [Google Scholar] [CrossRef]
- Kaiser, E.A.; Russo, A.F. CGRP and migraine: Could PACAP play a role too? Neuropeptides 2013, 47, 451–461. [Google Scholar] [CrossRef]
- Kuburas, A.; Mason, B.N.; Hing, B.; Wattiez, A.S.; Reis, A.S.; Sowers, L.P.; Moldovan Loomis, C.; Garcia-Martinez, L.F.; Russo, A.F. PACAP Induces Light Aversion in Mice by an Inheritable Mechanism Independent of CGRP. J. Neurosci. 2021, 26, 41–4697. [Google Scholar] [CrossRef]
- Russo, A.F. CGRP as a neuropeptide in migraine: Lessons from mice. Br. J. Clin Pharmacol. 2015, 80, 403–414. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988, 23, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Tuka, B.; Helyes, Z.; Markovics, A.; Bagoly, T.; Szolcsányi, J.; Szabó, N.; Tóth, E.; Kincses, Z.T.; Vécsei, L.; Tajti, J. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 2013, 33, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Lassen, L.H.; Haderslev, P.A.; Jacobsen, V.B.; Iversen, H.K.; Sperling, B.; Olesen, J. CGRP may play a causative role in migraine. Cephalalgia 2002, 22, 54–61. [Google Scholar] [CrossRef]
- Schytz, H.W. , Birk, S.; Wienecke, T.; Kruuse, C.; Olesen, J.; Ashina, M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 2009, 132 Pt 1, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Vollesen, A.L.H.; Amin, F.M.; Ashina, M. Targeted Pituitary Adenylate Cyclase-Activating Peptide Therapies for Migraine. Neurotherapeutics 2018, 15, 371–376. [Google Scholar] [CrossRef]
- Ghanizada, H.; Al-Karagholi, M.A.; Arngrim, N.; Olesen, J.; Ashina, M. PACAP27 induces migraine-like attacks in migraine patients. Cephalalgia 2020, 40, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Cernuda-Morollón, E.; Martínez-Camblor, P.; Alvarez, R.; Larrosa, D.; Ramón, C.; Pascual, J. Increased VIP levels in peripheral blood outside migraine attacks as a potential biomarker of cranial parasympathetic activation in chronic migraine. Cephalalgia 2015, 35, 310–316. [Google Scholar] [CrossRef]
- Hery, M.; Faudon, M.; Hery, F. Effect of vasoactive intestinal peptide on serotonin release in the suprachiasmatic area of the rat. Modulation by oestradiol. Peptides 1984, 5, 313–317. [Google Scholar] [CrossRef]
- Pellesi, L.; Al-Karagholi, M.A.; De Icco, R.; Chaudhry, B.A.; Lopez, C.L.; Snellman, J.; Hannibal, J.; Amin, F.M.; Ashina, M. Plasma Levels of CGRP During a 2-h Infusion of VIP in Healthy Volunteers and Patients With Migraine: An Exploratory Study. Front. Neurol. 2022, 13, 871176. [Google Scholar] [CrossRef]
- De la Fuente, M.; Delgado, M.; Gomariz, R.P. VIP modulation of immune cell functions. Adv. Neuroimmunol. 1996, 6, 75–91. [Google Scholar] [CrossRef]
- Hoffmann, J.; Baca, S.M.; Akerman, S. Neurovascular mechanisms of migraine and cluster headache. J. Cereb. Blood. Flow Metab. 2019, 39, 573–594. [Google Scholar] [CrossRef]
- Ocheretyaner, E.R.; Kofman, M.; Quattrocchi, E. Calcitonin gene-related peptide (CGRP) receptor antagonists for the acute treatment of migraines in adults. Drugs Context. 2022, 11, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Song, J.; You, C. Efficacy and Safety of Monoclonal Antibody Against Calcitonin Gene-Related Peptide or Its Receptor for Migraine: A Systematic Review and Network Meta-analysis. Front. Pharmacol. 2021, 12, 649143. [Google Scholar] [CrossRef]
- Berger, A.A.; Winnick, A.; Popovsky, D.; Kaneb, A.; Berardino, K.; Kaye, A.M.; Cornett, E.M.; Kaye, A.D.; Viswanath, O.; Urits, I. Lasmiditan for the Treatment of Migraines With or Without Aura in Adults. Psychopharmacol. Bull. 2020, 50, 163–188. [Google Scholar] [PubMed]
- Rissardo, J.P.; Caprara, A.L.F. Gepants for Acute and Preventive Migraine Treatment: A Narrative Review. Brain Sci. 2022, 12, 1612. [Google Scholar] [CrossRef]
- Ibekwe, A.; Perras, C.; Mierzwinski-Urban, M.; Monoclonal Antibodies to Prevent Migraine Headaches. In: CADTH Issues in Emerging Health Technologies. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, Ottawa, Canada, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538376/ (accessed on 28 August 2023).
- Nie, L.; Sun, K.; Gong, Z.; Li, H.; Quinn, J.P.; Wang, M. Src Family Kinases Facilitate the Crosstalk between CGRP and Cytokines in Sensitizing Trigeminal Ganglion via Transmitting CGRP Receptor/PKA Pathway. Cells 2022, 11, 3498. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Francavilla, M.; Zanaboni, A.M.; Tassorelli, C. Antagonism of CGRP Receptor: Central and Peripheral Mechanisms and Mediators in an Animal Model of Chronic Migraine. Cells 2022, 11, 3092. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, T.; Deligianni, C.I.; Karagiorgis, G.; Daponte, A.; Breza, M.; Mitsikostas, D.D. Monoclonal Antibodies Targeting CGRP: From Clinical Studies to Real-World Evidence—What Do We Know So Far? Pharmaceuticals 2021, 14, 700. [Google Scholar] [CrossRef]
- Pellesi, L.; Guerzoni, S.; Pini, L.A. Spotlight on Anti-CGRP Monoclonal Antibodies in Migraine: The Clinical Evidence to Date. Clin. Pharmacol. Drug Dev. 2017, 6, 534–547. [Google Scholar] [CrossRef]
- Nguyen, J.L.; Munshi, K.; Peasah, S.K.; Swart, E.C.S.; Kohli, M.; Henderson, R.; Good, C.B. Trends in utilization and costs of migraine medications, 2017-2020. J. Headache Pain 2022, 23, 111. [Google Scholar] [CrossRef]
- Haanes, K.A. , Edvinsson, L.; Sams, A. Understanding side-effects of anti-CGRP and anti-CGRP receptor antibodies. J. Headache Pain 2020, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Telegdy, G.; Adamik, A.; Tanaka, M.; Schally, A.V. Effects of the LHRH antagonist Cetrorelix on affective and cognitive functions in rats. Regul. Pept. 2010, 159, 142–147. [Google Scholar] [CrossRef]
- Tanaka, M.; Schally, A.V.; Telegdy, G. Neurotransmission of the antidepressant-like effects of the growth hormone-releasing hormone antagonist MZ-4-71. Behav. Brain Res. 2012, 228, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Telegdy, G. Neurotransmissions of antidepressant-like effects of neuromedin U-23 in mice. Behav. Brain Res. 2014, 259, 196–199. [Google Scholar] [CrossRef]
- Tanaka, M.; Csabafi, K.; Telegdy, G. Neurotransmissions of antidepressant-like effects of kisspeptin-13. Regul. Pept. 2013, 180, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Telegdy, G.; Tanaka, M.; Schally, A.V. Effects of the growth hormone-releasing hormone (GH-RH) antagonist on brain functions in mice. Behav. Brain Res. 2011, 224, 155–158. [Google Scholar] [CrossRef]
- Rákosi, K.; Masaru, T.; Zarándia, M.; Telegdy, G.; Tóth, G.K. Short analogs and mimetics of human urocortin 3 display antidepressant effects in vivo. Peptides 2014, 62, 59–66. [Google Scholar] [CrossRef]
- Tran, K.N.; Nguyen, N.P.K.; Nguyen, L.T.H.; Shin, H.-M.; Yang, I.-J. Screening for Neuroprotective and Rapid Antidepressant-like Effects of 20 Essential Oils. Biomedicines 2023, 11, 1248. [Google Scholar] [CrossRef]
- Tanaka, M.; Kádár, K.; Tóth, G.; Telegdy, G. Antidepressant-like effects of urocortin 3 fragments. Brain Res. Bull. 2011, 84, 414–418. [Google Scholar] [CrossRef]
- Baliellas, D.E.M.; Barros, M.P.; Vardaris, C.V.; Guariroba, M.; Poppe, S.C.; Martins, M.F.; Pereira, Á.A.F.; Bondan, E.F. Propentofylline Improves Thiol-Based Antioxidant Defenses and Limits Lipid Peroxidation following Gliotoxic Injury in the Rat Brainstem. Biomedicines 2023, 11, 1652. [Google Scholar] [CrossRef]
- Montanari, M.; Imbriani, P.; Bonsi, P.; Martella, G.; Peppe, A. Beyond the Microbiota: Understanding the Role of the Enteric Nervous System in Parkinson’s Disease from Mice to Human. Biomedicines 2023, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Garifulin, R.; Davleeva, M.; Izmailov, A.; Fadeev, F.; Markosyan, V.; Shevchenko, R.; Minyazeva, I.; Minekayev, T.; Lavrov, I.; Islamov, R. Evaluation of the Autologous Genetically Enriched Leucoconcentrate on the Lumbar Spinal Cord Morpho-Functional Recovery in a Mini Pig with Thoracic Spine Contusion Injury. Biomedicines 2023, 11, 1331. [Google Scholar] [CrossRef]
- Bueno, C.R.d.S.; Tonin, M.C.C.; Buchaim, D.V.; Barraviera, B.; Ferreira Junior, R.S.; Santos, P.S.d.S.; Reis, C.H.B.; Pastori, C.M.; Pereira, E.d.S.B.M.; Nogueira, D.M.B.; et al. Morphofunctional Improvement of the Facial Nerve and Muscles with Repair Using Heterologous Fibrin Biopolymer and Photobiomodulation. Pharmaceuticals 2023, 16, 653. [Google Scholar] [CrossRef] [PubMed]
- Sojka, A.; Żarowski, M.; Steinborn, B.; Hedzelek, W.; Wiśniewska-Spychała, B.; Dorocka-Bobkowska, B. Temporomandibular disorders in adolescents with headache. Adv. Clin. Exp. Med. 2018, 27, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M.; et al. The Tryptophan-Kynurenine Metabolic System Is Suppressed in Cuprizone-Induced Model of Demyelination Simulating Progressive Multiple Sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Preclinical modeling in depression and anxiety: Current challenges and future research directions. Adv. Clin. Exp. Med. 2023, 32, 505–509. [Google Scholar] [CrossRef]
- Chu, P.-C.; Huang, C.-S.; Chang, P.-K.; Chen, R.-S.; Chen, K.-T.; Hsieh, T.-H.; Liu, H.-L. Weak Ultrasound Contributes to Neuromodulatory Effects in the Rat Motor Cortex. Int. J. Mol. Sci. 2023, 24, 2578. [Google Scholar] [CrossRef]
- Gecse, K.; Édes, A.E.; Nagy, T.; Demeter, A.K.; Virág, D.; Király, M.; Dalmadi Kiss, B.; Ludányi, K.; Környei, Z.; Denes, A.; et al. Citalopram Neuroendocrine Challenge Shows Altered Tryptophan and Kynurenine Metabolism in Migraine. Cells 2022, 11, 2258. [Google Scholar] [CrossRef]
- Nasini, S.; Tidei, S.; Shkodra, A.; De Gregorio, D.; Cambiaghi, M.; Comai, S. Age-Related Effects of Exogenous Melatonin on Anxiety-like Behavior in C57/B6J Mice. Biomedicines 2023, 11, 1705. [Google Scholar] [CrossRef]
- Chen, W.-C.; Wang, T.-S.; Chang, F.-Y.; Chen, P.-A.; Chen, Y.-C. ; Age, Dose, and Locomotion: Decoding Vulnerability to Ketamine in C57BL/6J and BALB/c Mice. Biomedicines 2023, 11, 1821. [Google Scholar] [CrossRef]
- Statsenko, Y.; Habuza, T.; Smetanina, D.; Simiyu, G.L.; Meribout, S.; King, F.C.; Gelovani, J.G.; Das, K.M.; Gorkom, K.N.-V.; Zaręba, K.; et al. Unraveling Lifelong Brain Morphometric Dynamics: A Protocol for Systematic Review and Meta-Analysis in Healthy Neurodevelopment and Ageing. Biomedicines 2023, 11, 1999. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Tao, Q.; Niu, X.; Zhang, M.; Gao, X.; Yang, Z.; Yu, M.; Wang, W.; Han, S. Meta-Analysis of Structural and Functional Brain Abnormalities in Cocaine Addiction. Front. Psychiatry 2022, 13, 927075. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Rassler, B.; Blinowska, K.; Kaminski, M.; Pfurtscheller, G. Analysis of Respiratory Sinus Arrhythmia and Directed Information Flow between Brain and Body Indicate Different Management Strategies of fMRI-Related Anxiety. Biomedicines 2023, 11, 1028. [Google Scholar] [CrossRef]
- Arimura, A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn. J. Physiol. 1998, 48, 301–331. [Google Scholar] [CrossRef]
- Holland, P.R.; Barloese, M.; Fahrenkrug, J. PACAP in hypothalamic regulation of sleep and circadian rhythm: Importance for headache. J. Headache Pain 2018, 19, 20. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Musumeci, G.; Reglodi, D.; D’Agata, V. Effects of PACAP on Schwann Cells: Focus on Nerve Injury. Int. J. Mol. Sci. 2020, 21, 8233. [Google Scholar] [CrossRef]
- Johnson, G.C.; May, V.; Parsons, R.L.; Hammack, S.E. Parallel signaling pathways of pituitary adenylate cyclase activating polypeptide (PACAP) regulate several intrinsic ion channels. Ann. N.Y. Acad. Sci. 2019, 1455, 105–112. [Google Scholar] [CrossRef]
- Clement, A.; Guo, S.; Jansen-Olesen, I.; Christensen, S.L. ATP-Sensitive Potassium Channels in Migraine: Translational Findings and Therapeutic Potential. Cells 2022, 11, 2406. [Google Scholar] [CrossRef]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Denes, V.; Geck, P.; Mester, A.; Gabriel, R. Pituitary Adenylate Cyclase-Activating Polypeptide: 30 Years in Research Spotlight and 600 Million Years in Service. J. Clin. Med. 2019, 8, 1488. [Google Scholar] [CrossRef]
- Hypophysis Adenylate Cyclase Activating Polypeptide. Handbook of Biologically Active Peptides (Second Edition), 2013. Available online: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/hypophysis-adenylate-cyclase-activating-polypeptide (accessed on 28 August 2023).
- Tam, J.K.; Lee, L.T.; Chow, B.K. PACAP-related peptide (PRP)--molecular evolution and potential functions. Peptides 2007, 28, 1920–1929. [Google Scholar] [CrossRef]
- Köves, K.; Szabó, E.; Kántor, O.; Heinzlmann, A.; Szabó, F.; Csáki, Á. Current State of Understanding of the Role of PACAP in the Hypothalamo-Hypophyseal Gonadotropin Functions of Mammals. Front. Endocrinol. (Lausanne) 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Seo, S.R. Neuroprotective roles of pituitary adenylate cyclase-activating polypeptide in neurodegenerative diseases. BMB Rep. 2014, 47, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Sadanandan, N.; Cozene, B.; Park, Y.J.; Farooq, J.; Kingsbury, C.; Wang, Z.J.; Moscatello, A.; Saft, M.; Cho, J.; Gonzales-Portillo, B.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide: A Potent Therapeutic Agent in Oxidative Stress. Antioxidants (Basel) 2021, 10, 354. [Google Scholar] [CrossRef]
- Waschek, J.A. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Qiu, P.; Gong, H.Z.; Chen, X.M. , Sun, Y.; Hong, A.; Ma, Y. PACAP ameliorates hepatic metabolism and inflammation through up-regulating FAIM in obesity. J. Cell Mol. Med. 2019, 23, 5970–5980. [Google Scholar] [CrossRef]
- Langer, I.; Jeandriens, J.; Couvineau, A.; Sanmukh, S.; Latek, D. Signal Transduction by VIP and PACAP Receptors. Biomedicines 2022, 10, 406. [Google Scholar] [CrossRef]
- Fizanne, L.; Sigaudo-Roussel, D.; Saumet, J.L.; Fromy, B. Evidence for the involvement of VPAC1 and VPAC2 receptors in pressure-induced vasodilatation in rodents. J. Physiol. 2004, 554 Pt 2, 519–528. [Google Scholar] [CrossRef]
- Parsons RL, May, V. PACAP-Induced PAC1 Receptor Internalization and Recruitment of Endosomal Signaling Regulate Cardiac Neuron Excitability. J. Mol Neurosci. 2019, 68, 340–347. [Google Scholar] [CrossRef]
- Bill, C.A.; Vines, C.M.; Phospholipase, C. Adv. Exp. Med. Biol. 2020, 1131, 215–242. [CrossRef]
- Barloese, M.; Chitgar, M.; Hannibal, J.; Møller, S. Pituitary adenylate cyclase-activating peptide: Potential roles in the pathophysiology and complications of cirrhosis. Liver Int. 2020, 40, 2578–2589. [Google Scholar] [CrossRef] [PubMed]
- Makhinson, M.; Chotiner, J.K.; Watson, J.B.; O'Dell, T.J. Adenylyl cyclase activation modulates activity-dependent changes in synaptic strength and Ca2+/calmodulin-dependent kinase II autophosphorylation. J. Neurosci. 1999, 19, 2500–2510. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Piper, S.J.; Zhao, P.; Miller, L.J.; Wootten, D.; Sexton, P.M. Targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors. Int. J. Mol. Sci. 2022, 23, 8069. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Nakamachi, T.; Shioda, S. Discovery of PACAP and its receptors in the brain. J. Headache Pain, 2018, 19, 28. [Google Scholar] [CrossRef]
- Vasoactive Intestinal Polypeptide Receptor 1. Methods in Enzymology. 2013. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/vasoactive-intestinal-polypeptide-receptor-1 (accessed on 28 August 2023).
- Vasoactive Intestinal Polypeptide Receptor. Autonomic Neuroscience. 2007. Available online: https://www.sciencedirect.com/topics/neuroscience/vasoactive-intestinal-polypeptide-receptor (accessed on 28 August 2023).
- May, V.; Buttolph, T.R.; Girard, B.M.; Clason, T.A.; Parsons, R.L. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am. J. Physiol. Cell Physiol. 2014, 306, C1068–C1079. [Google Scholar] [CrossRef] [PubMed]
- Hou,X. ; Yang, D.; Yang, G.; Li. M.; Zhang, J.; Zhang, J.; Zhang, Y.; Liu, Y. Therapeutic potential of vasoactive intestinal peptide and its receptor VPAC2 in type 2 diabetes. Front. Endocrinol. 2022, 13, 984198. [Google Scholar] [CrossRef]
- Sundrum, T.; Walker, C.S. Pituitary adenylate cyclase-activating polypeptide receptors in the trigeminovascular system: Implications for migraine. Br. J. Pharmacol. 2018, 175, 4109–4120. [Google Scholar] [CrossRef]
- Liu, J.; Wang, G.; Dan, Y.; Liu, X. CGRP and PACAP-38 play an important role in diagnosing pediatric migraine. J. Headache Pain 2022, 23, 68. [Google Scholar] [CrossRef]
- Schytz, H.W.; Olesen, J.; Ashina, M. The PACAP receptor: A novel target for migraine treatment. Neurotherapeutics 2010, 7, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Kuburas, A.; Russo, A.F. Shared and independent roles of CGRP and PACAP in migraine pathophysiology. J. Headache Pain 2023, 24, 34. [Google Scholar] [CrossRef] [PubMed]
- Ernstsen, C.; Christensen, S.L.; Rasmussen, R.H.; Nielsen, B.S.; Jansen-Olesen, I.; Olesen, J.; Kristensen, D.M. The PACAP pathway is independent of CGRP in mouse models of migraine: Possible new drug target? Brain 2022, 145, 2450–2460. [Google Scholar] [CrossRef]
- Christensen, C.E.; Ashina, M.; Amin, F.M. Calcitonin Gene-Related Peptide (CGRP) and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Migraine Pathogenesis. Pharmaceuticals 2022, 15, 1189. [Google Scholar] [CrossRef]
- Anapindi, K.D.B.; Yang, N.; Romanova, E.V.; Rubakhin, S.S.; Tipton, A.; Dripps, I.; Sheets, Z.; Sweedler, J.V.; Pradhan, A.A. PACAP and Other Neuropeptide Targets Link Chronic Migraine and Opioid-induced Hyperalgesia in Mouse Models. Mol. Cell Proteomics 2019, 18, 2447–2458. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, M.; Iannone, L.F.; Orologio, I.; Tessitore, A.; Tedeschi, G.; Geppetti, P.; Russo, A. Migraine Treatment: Towards New Pharmacological Targets. Int. J. Mol. Sci. 2023, 24, 12268. [Google Scholar] [CrossRef] [PubMed]
- Pellesi, L.; Chaudhry, B.A.; Vollesen, A.L.H.; Snoer, A.H.; Baumann, K.; Skov, P.S.; Jensen, R.H.; Ashina, M. PACAP38- and VIP-induced cluster headache attacks are not associated with changes of plasma CGRP or markers of mast cell activation. Cephalalgia 2022, 42, 687–695. [Google Scholar] [CrossRef]
- Rasmussen, N.B.; Deligianni, C.; Christensen, C.E.; Karlsson, W.K.; Al-Khazali, H.M.; Van de Casteele, T.; Granhall, C.; Amin, F.M.; Ashina, M. The effect of Lu AG09222 on PACAP38- and VIP-induced vasodilation, heart rate increase, and headache in healthy subjects: An interventional, randomized, double-blind, parallel-group, placebo-controlled study. J. Headache Pain 2023, 24, 60. [Google Scholar] [CrossRef]
- Adenylate Cyclase. Adenylate cyclases are enzymes that catalyze the conversion of ATP to cAMP and pyrophosphate. From: The Senses: A Comprehensive Reference. 2008. https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/adenylate-cyclase. 2008. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/adenylate-cyclase (accessed on 29 August 2023).
- Roberts, R.E. The extracellular signal-regulated kinase (ERK) pathway: A potential therapeutic target in hypertension. J. Exp. Pharmacol. 2012, 4, 77–83. [Google Scholar] [CrossRef]
- Lund, A.M.; Hannibal, J. Localization of the neuropeptides pituitary adenylate cyclase-activating polypeptide, vasoactive intestinal peptide, and their receptors in the basal brain blood vessels and trigeminal ganglion of the mouse CNS; an immunohistochemical study. Front. Neuroanat. 2022, 16, 991403. [Google Scholar] [CrossRef]
- Ivic, I.; Balasko, M.; Fulop, B.D.; Hashimoto, H.; Toth, G.; Tamas, A.; Juhasz, T.; Koller, A.; Reglodi, D.; Solymár, M. VPAC1 receptors play a dominant role in PACAP-induced vasorelaxation in female mice. PLoS ONE 2019, 14, e0211433. [Google Scholar] [CrossRef] [PubMed]
- VIP and PACAP receptors - IUPHAR/BPS Guide to PHARMACOLOGY.". Available online: https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=67 (accessed on 29 August 2023).
- Datki, Z.; Sinka, R. Translational biomedicine-oriented exploratory research on bioactive rotifer-specific biopolymers. Adv. Clin. Exp. Med. 2022, 31, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Palotai, M.; Telegdy, G.; Tanaka, M.; Bagosi, Z.; Jászberényi, M. Neuropeptide AF induces anxiety-like and antidepressant-like behavior in mice. Behav. Brain Res. 2014, 274, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Lieb, A.; Thaler, G.; Fogli, B.; Trovato, O.; Posch, M.A.; Kaserer, T.; Zangrandi, L. Functional Characterization of Spinocerebellar Ataxia Associated Dynorphin A Mutant Peptides. Biomedicines 2021, 9, 1882. [Google Scholar] [CrossRef]
- Skobeleva, K.; Shalygin, A.; Mikhaylova, E.; Guzhova, I.; Ryazantseva, M.; Kaznacheyeva, E. The STIM1/2-Regulated Calcium Homeostasis Is Impaired in Hippocampal Neurons of the 5xFAD Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 14810. [Google Scholar] [CrossRef]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan–Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef]
- Tanaka, M.; Telegdy, G. Involvement of adrenergic and serotonergic receptors in antidepressant-like effect of urocortin 3 in a modified forced swimming test in mice. Brain Res. Bull. 2008, 77, 301–305. [Google Scholar] [CrossRef]
- Tanaka, M.; Spekker, E.; Szabó, Á.; Polyák, H.; Vécsei, L. Modelling the neurodevelopmental pathogenesis in neuropsychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents-in celebration of 80th birthday of Professor Peter Riederer. J. Neural. Transm. (Vienna) 2022, 129, 627–642. [Google Scholar] [CrossRef]
- Reducha, P.V.; Edvinsson, L.; Haanes, K.A. Could Experimental Inflammation Provide Better Understanding of Migraines? Cells 2022, 11, 2444. [Google Scholar] [CrossRef]
- Ojala, J.; Tooke, K.; Hsiang, H.; Girard, B.M.; May, V.; Vizzard, M.A. PACAP/PAC1 Expression and Function in Micturition Pathways. J. Mol. Neurosci. 2019, 68, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Tamas, A.; Reglodi, D.; Farkas, O.; Kovesdi, E.; Pal, J.; Povlishock, J.T.; Schwarcz, A.; Czeiter, E.; Szanto, Z.; Doczi, T.; et al. Effect of PACAP in central and peripheral nerve injuries. Int. J. Mol. Sci. 2012, 13, 8430–8448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Y.; Yang, L.; Wang, Y.; Xiao, Z. PACAP6-38 improves nitroglycerin-induced central sensitization by modulating synaptic plasticity at the trigeminal nucleus caudalis in a male rat model of chronic migraine. J. Headache Pain 2023, 24, 66. [Google Scholar] [CrossRef] [PubMed]
- Takács-Lovász K, Kun J, Aczél T. PACAP-38 Induces Transcriptomic Changes in Rat Trigeminal Ganglion Cells Related to Neuroinflammation and Altered Mitochondrial Function Presumably via PAC1/VPAC2 Receptor-Independent Mechanism. Int. J. Mol. Sci. 2022, 23, 2120. [Google Scholar] [CrossRef]
- Frederiksen, S.D.; Haanes, K.A.; Warfvinge, K.; Edvinsson, L. Perivascular neurotransmitters: Regulation of cerebral blood flow and role in primary headaches. J. Cereb. Blood Flow Metab. 2019, 39, 610–632. [Google Scholar] [CrossRef]
- Markovics, A.; Kormos, V.; Gaszner, B.; Lashgarara, A.; Szoke, E.; Sandor, K.; Szabadfi, K.; Tuka, B.; Tajti, J.; Szolcsanyi, J.; et al. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol. Dis. 2012, 45, 633–644. [Google Scholar] [CrossRef]
- Edvinsson, L. PACAP and its receptors in migraine pathophysiology: Commentary on Walker. Br. J. Pharmacol. 2015, 172, 4782–4784. [Google Scholar] [CrossRef]
- Saposnik, G.; Montalban, X.; Selchen, D.; Terzaghi, M.A.; Bakdache, F.; Montoya, A.; Fruns, M.; Caceres, F.; Oh, J. Therapeutic Inertia in Multiple Sclerosis Care: A Study of Canadian Neurologists. Front. Neurol. 2018, 9, 781. [Google Scholar] [CrossRef]
- Harding, S.D.; Armstrong, J.F.; Faccenda, E.; Southan, C.; Alexander, S.P.H.; Davenport, A.P.; Pawson, A.J.; Spedding, M.; Davies, J.A.; NC-IUPHAR. (2021) The IUPHAR/BPS guide to PHARMACOLOGY in 2022: Curating pharmacology for COVID-19, malaria and antibacterials. Nucl. Acids Res. 2022, 50, D1282–D1294. [Google Scholar] [CrossRef]
- Beebe, X.; Darczak, D.; Davis-Taber, R.A.; Uchic, M.E.; Scott, V.E.; Jarvis, M.F.; Stewart, A.O. Discovery and SAR of hydrazide antagonists of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor type 1 (PAC1-R). Bioorg. Med. Chem. Lett. 2008, 18, 2162–2166. [Google Scholar] [CrossRef] [PubMed]
- Laburthe, M.; Couvineau, A.; Tan, V. Class II G protein-coupled receptors for VIP and PACAP: Structure, models of activation and pharmacology. Peptides 2007, 28, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Tanaka, M. , Szabó, Á., Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines. 2022, 10, 76. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A. Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef]
- Guo, S.; Vollesen, A.L.; Hansen, R.D.; Esserlind, A.L.; Amin, F.M.; Christensen, A.F.; Olesen, J.; Ashina, M. Part I: Pituitary adenylate cyclase-activating polypeptide-38 induced migraine-like attacks in patients with and without familial aggregation of migraine. Cephalalgia 2017, 37, 125–135. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov; PACAP Induced Migraine Attacks in Patients With High and Low Genetic Load. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02158221 (accessed on 29 August 2023).
- Togha, M.; Ghorbani, Z.; Ramazi, S.; Zavvari, F.; Karimzadeh, F. Evaluation of Serum Levels of Transient Receptor Potential Cation Channel Subfamily V Member 1, Vasoactive Intestinal Polypeptide, and Pituitary Adenylate Cyclase-Activating Polypeptide in Chronic and Episodic Migraine: The Possible Role in Migraine Transformation. Front. Neurol. 2021, 12, 770980. [Google Scholar] [CrossRef]
- Körtési, T.; Tuka, B.; Tajti, J.; Bagoly, T.; Fülöp, F.; Helyes, Z.; Vécsei, L. Kynurenic Acid Inhibits the Electrical Stimulation Induced Elevated Pituitary Adenylate Cyclase-Activating Polypeptide Expression in the TNC. Front. Neurol. 2018, 8, 745. [Google Scholar] [CrossRef]
- Guo, S.; Vollesen, A.L.; Hansen, Y.B.; Frandsen, E.; Andersen, M.R.; Amin, F.M.; Fahrenkrug, J.; Olesen, J.; Ashina, M. Part II: Biochemical changes after pituitary adenylate cyclase-activating polypeptide-38 infusion in migraine patients. Cephalalgia 2017, 37, 136–147. [Google Scholar] [CrossRef]
- Amin, F.M.; Asghar, M.S.; Guo, S.; Hougaard, A.; Hansen, A.E.; Schytz, H.W.; van der Geest, R.J.; de Koning, P.J.; Larsson, H.B.; Olesen, J.; et al. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia 2012, 32, 140–149. [Google Scholar] [CrossRef]
- Maasz, G.; Zrinyi, Z.; Reglodi, D.; Petrovics, D.; Rivnyak, A.; Kiss, T.; Jungling, A.; Tamas, A.; Pirger, Z. Pituitary adenylate cyclase-activating polypeptide (PACAP) has a neuroprotective function in dopamine-based neurodegeneration in rat and snail parkinsonian models. Dis. Model Mech. 2017, 10, 127–139. [Google Scholar] [CrossRef]
- Rubio-Beltrán, E.; Correnti, E.; Deen, M.; Kamm, K.; Kelderman, T.; Papetti, L.; Vigneri, S.; MaassenVanDenBrink, A.; Edvinsson, L.; European Headache Federation School of Advanced Studies (EHF-SAS). PACAP38 and PAC1 receptor blockade: A new target for headache? J. Headache Pain 2018, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Doležil, D.; Bonner, J.H.; Zhou, L.; Klatt, J.; Picard, H.; Mikol, D.D. A phase 2, randomized, double-blind, placebo-controlled trial of AMG 301, a pituitary adenylate cyclase-activating polypeptide PAC1 receptor monoclonal antibody for migraine prevention. Cephalalgia 2021, 41, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Study to Evaluate the Efficacy and Safety of AMG 301 in Migraine Prevention. 2020. Available online: https://ClinicalTrials.gov/show/NCT03238781 (accessed on 4 September 2023).
- Lundbeck News Room: Lundbeck announced the start of a phase II clinical study to assess Lu AG09222 for migraine prevention. Available online: https://newsroom.lundbeckus.com/news-release/2021/lundbeck-announced-start-of-phase-ii-clinical-study-for-migraine-prevention (accessed on 29 August 2023).
- A Study With Lu AG09222 in Adults With Migraine Who Have Not Been Helped by Prior Preventive Treatments 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05133323 (accessed on 4 September 2023).
- A Study of LY3451838 in Participants With Migraine 2023. Available online: https://ClinicalTrials.gov/show/NCT04498910 (accessed on 4 September 2023).
- ClinicalTrials.gov; The Effects of a Long-lasting Infusion of Vasoactive Intestinal Peptide (VIP) in Episodic Migraine Patients. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04260035 (accessed on 29 August 2023).
- Tanaka, M.; Szabó, Á.; Vécsei, L. Integrating Armchair, Bench, and Bedside Research for Behavioral Neurology and Neuropsychiatry: Editorial. Biomed. 2022, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, A.J.; Finner-Prével, M.; Sudar, F.P.; Langer, F.H.; Keskin, F.; Gebel, A.; Zweerings, J.; Mathiak, K. The Interplay between Vitamin D, Exposure of Anticholinergic Antipsychotics and Cognition in Schizophrenia. Biomedicines 2022, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Mariqueo, L.; Giménez-Llort, L. Impact of Behavioral Assessment and Re-Test as Functional Trainings That Modify Survival, Anxiety and Functional Profile (Physical Endurance and Motor Learning) of Old Male and Female 3xTg-AD Mice and NTg Mice with Normal Aging. Biomedicines 2022, 10, 973. [Google Scholar] [CrossRef]
- Lee, E.C.; Hong, D.-Y.; Lee, D.-H.; Park, S.-W.; Lee, J.Y.; Jeong, J.H.; Kim, E.-Y.; Chung, H.-M.; Hong, K.-S.; Park, S.-P.; et al. Inflammation and Rho-Associated Protein Kinase-Induced Brain Changes in Vascular Dementia. Biomedicines 2022, 10, 446. [Google Scholar] [CrossRef]
- Simonato, M.; Dall’Acqua, S.; Zilli, C.; Sut, S.; Tenconi, R.; Gallo, N.; Sfriso, P.; Sartori, L.; Cavallin, F.; Fiocco, U.; et al. Tryptophan Metabolites, Cytokines, and Fatty Acid Binding Protein 2 in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines 2021, 9, 1724. [Google Scholar] [CrossRef]
- Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Belozertseva, I.V.; Tamkovich, N.V.; Baranov, K.O.; Kudryavtseva, N.N. Chronic Lithium Treatment Affects Anxious Behaviors and theExpression of Serotonergic Genes in Midbrain Raphe Nuclei of Defeated Male Mice. Biomedicines 2021, 9, 1293. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef]
- Vila-Merkle, H.; González-Martínez, A.; Campos-Jiménez, R.; Martínez-Ricós, J.; Teruel-Martí, V.; Blasco-Serra, A.; Lloret, A.; Celada, P.; Cervera-Ferri, A. The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders. Biomedicines 2021, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Santana-Santana, M.; Bayascas, J.-R.; Giménez-Llort, L. Fine-Tuning the PI3K/Akt Signaling Pathway Intensity by Sex and Genotype-Load: Sex-Dependent Homozygotic Threshold for Somatic Growth but Feminization of Anxious Phenotype in Middle-Aged PDK1 K465E Knock-In and Heterozygous Mice. Biomedicines 2021, 9, 747. [Google Scholar] [CrossRef]
- Muntsant, A.; Giménez-Llort, L. Genotype Load Modulates Amyloid Burden and Anxiety-Like Patterns in Male 3xTg-AD Survivors despite Similar Neuro-Immunoendocrine, Synaptic and Cognitive Impairments. Biomedicines 2021, 9, 715. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Marin-Pardo, D.; Marazuela, P.; Hernández-Guillamón, M. Survival Bias and Crosstalk between Chronological and Behavioral Age: Age- and Genotype-Sensitivity Tests Define Behavioral Signatures in Middle-Aged, Old, and Long-Lived Mice with Normal and AD-Associated Aging. Biomedicines 2021, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Watanabe, E.; Fukuchi, M. Psychiatric Neural Networks and Precision Therapeutics by Machine Learning. Biomedicines 2021, 9, 403. [Google Scholar] [CrossRef]
- Caruso, G.; Godos, J.; Castellano, S.; Micek, A.; Murabito, P.; Galvano, F.; Ferri, R.; Grosso, G.; Caraci, F. The Therapeutic Potential of Carnosine/Anserine Supplementation against Cognitive Decline: A Systematic Review with Meta-Analysis. Biomedicines 2021, 9, 253. [Google Scholar] [CrossRef]
- Correia, B.S.B.; Nani, J.V.; Waladares Ricardo, R.; Stanisic, D.; Costa, T.B.B.C.; Hayashi, M.A.F.; Tasic, L. Effects of Psychostimulants and Antipsychotics on Serum Lipids in an Animal Model for Schizophrenia. Biomedicines 2021, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Ikonnikova, A.; Anisimova, A.; Galkin, S.; Gunchenko, A.; Abdukhalikova, Z.; Filippova, M.; Surzhikov, S.; Selyaeva, L.; Shershov, V.; Zasedatelev, A.; et al. Genetic Association Study and Machine Learning to Investigate Differences in Platelet Reactivity in Patients with Acute Ischemic Stroke Treated with Aspirin. Biomedicines 2022, 10, 2564. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Miranda, O.; Qi, X.; Kofler, J.; Sweet, R.A.; Wang, L. Unveiling the Enigma: Exploring Risk Factors and Mechanisms for Psychotic Symptoms in Alzheimer’s Disease through Electronic Medical Records with Deep Learning Models. Pharmaceuticals 2023, 16, 911. [Google Scholar] [CrossRef] [PubMed]
- Parolini, F.; Goethel, M.; Becker, K.; Fernandes, C.; Fernandes, R.J.; Ervilha, U.F.; Santos, R.; Vilas-Boas, J.P. Breaking Barriers: Artificial Intelligence Interpreting the Interplay between Mental Illness and Pain as Defined by the International Association for the Study of Pain. Biomedicines 2023, 11, 2042. [Google Scholar] [CrossRef]
- Tanaka, M.; Diano, M.; Battaglia, S. Editorial: Insights into structural and functional organization of the brain: Evidence from neuroimaging and non-invasive brain stimulation techniques. Front. Psychiatry. 2023, 14, 1225755. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Soga, T.; Ahemad, N.; Bhuvanendran, S.; Parhar, I. Kisspeptin-10 Rescues Cholinergic Differentiated SHSY-5Y Cells from α-Synuclein-Induced Toxicity In Vitro. Int. J. Mol. Sci. 2022, 23, 5193. [Google Scholar] [CrossRef] [PubMed]
- Okanda Nyatega, C.; Qiang, L.; Jajere Adamu, M.; Bello Kawuwa, H. Altered striatal functional connectivity and structural dysconnectivity in individuals with bipolar disorder: A resting state magnetic resonance imaging study. Front. Psychiatry. 2022, 13, 1054380. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, Y.; Hong, Y.; Huo, J.; Chang, T.; Wang, H.; Huang, Y.; Li, W.; Zhang, Y. Altered brain activities in mesocorticolimbic pathway in primary dysmenorrhea patients of long-term menstrual pain. Front. Neurosci. 2023, 17, 1098573. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, B.; Wang, H.; Zeng, Y.; Xin, J.; Li, X. The non-linear correlation between the volume of cerebral white matter lesions and incidence of bipolar disorder: A secondary analysis of data from a cross-sectional study. Front. Psychiatry 2023, 14, 1149663. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, R.; DeSouza, J.F.X.; Shen, Y.; Zhang, H.; Zhu, C.; Huang, P.; Wang, C. Differential responses from the left postcentral gyrus, right middle frontal gyrus, and precuneus to meal ingestion in patients with functional dyspepsia. Front. Psychiatry 2023, 14, 1184797. [Google Scholar] [CrossRef]
- Adamu, M.J.; Qiang, L.; Nyatega, C.O.; Younis, A.; Kawuwa, H.B.; Jabire, A.H.; Saminu, S. Unraveling the pathophysiology of schizophrenia: Insights from structural magnetic resonance imaging studies. Front. Psychiatry 2023, 14, 1188603. [Google Scholar] [CrossRef]
- Vuralli, D.; Ayata, C.; Bolay, H. Cognitive dysfunction and migraine. J. Headache Pain 2018, 19, 109. [Google Scholar] [CrossRef]
- Minen, M.T.; Begasse De Dhaem, O.; Kroon Van Diest, A.; Powers, S.; Schwedt, T.J.; Lipton, R.; Silbersweig, D. Migraine and its psychiatric comorbidities. J. Neurol. Neurosurg. Psychiatry 2016, 87, 741–749. [Google Scholar] [CrossRef]
- Tanaka, M.; Chen, C. Editorial: Towards a mechanistic understanding of depression, anxiety, and their comorbidity: Perspectives from cognitive neuroscience. Front. Behav. Neurosci. 2023, 17, 1268156. [Google Scholar] [CrossRef]
- Gonzalez-Escamilla, G.; Dörfel, D.; Becke, M.; Trefz, J.; Bonanno, G.A.; Groppa, S. Associating Flexible Regulation of Emotional Expression With Psychopathological Symptoms. Front. Behav. Neurosci. 2022, 16, 924305. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. The Influence of Vicarious Fear-Learning in "Infecting" Reactive Action Inhibition. Front. Behav. Neurosci. 2022, 16, 946263. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. Stopping in (e)motion: Reactive action inhibition when facing valence-independent emotional stimuli. Front Behav. Neurosci. 2022, 16, 998714. [Google Scholar] [CrossRef] [PubMed]
- Ironside, M.; DeVille, D.C.; Kuplicki, R.T.; Burrows, K.P.; Smith, R.; Teed, A.R.; Paulus, M.P.; Khalsa, S.S. The unique face of comorbid anxiety and depression: Increased interoceptive fearfulness and reactivity. Front. Behav. Neurosci. 2023, 16, 1083357. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Comorbid depression and anxiety: Integration of insights from attachment theory and cognitive neuroscience, and their implications for research and treatment. Front. Behav. Neurosci. 2022, 16, 1104928. [Google Scholar] [CrossRef]
- Vila-Merkle, H.; González-Martínez, A.; Campos-Jiménez, R.; Martínez-Ricós, J.; Teruel-Martí, V.; Lloret, A.; Blasco-Serra, A.; Cervera-Ferri, A. Sex differences in amygdalohippocampal oscillations and neuronal activation in a rodent anxiety model and in response to infralimbic deep brain stimulation. Front. Behav. Neurosci. 2023, 17, 1122163. [Google Scholar] [CrossRef]
- Panov, G.; Panova, P. Obsessive-compulsive symptoms in patient with schizophrenia: The influence of disorganized symptoms, duration of schizophrenia, and drug resistance. Front. Psychiatry 2023, 14, 1120974. [Google Scholar] [CrossRef]
- Hakamata, Y.; Hori, H.; Mizukami, S.; Izawa, S.; Yoshida, F.; Moriguchi, Y.; Hanakawa, T.; Inoue, Y.; Tagaya, H. Blunted diurnal interleukin-6 rhythm is associated with amygdala emotional hyporeactivity and depression: A modulating role of gene-stressor interactions. Front. Psychiatry 2023, 14, 1196235. [Google Scholar] [CrossRef] [PubMed]
- Fraile-Ramos, J.; Garrit, A.; Reig-Vilallonga, J.; Giménez-Llort, L. Hepatic Oxi-Inflammation and Neophobia as Potential Liver–Brain Axis Targets for Alzheimer’s Disease and Aging, with Strong Sensitivity to Sex, Isolation, and Obesity. Cells 2023, 12, 1517. [Google Scholar] [CrossRef]
- Sobolewska-Nowak, J.; Wachowska, K.; Nowak, A.; Orzechowska, A.; Szulc, A.; Płaza, O.; Gałecki, P. Exploring the Heart–Mind Connection: Unraveling the Shared Pathways between Depression and Cardiovascular Diseases. Biomedicines 2023, 11, 1903. [Google Scholar] [CrossRef]
- Festa, F.; Medori, S.; Macrì, M. Move Your Body, Boost Your Brain: The Positive Impact of Physical Activity on Cognition across All Age Groups. Biomedicines 2023, 11, 1765. [Google Scholar] [CrossRef]
- Spekker, E.; Bohár, Z.; Fejes-Szabó, A.; Szűcs, M.; Vécsei, L.; Párdutz, Á. Estradiol Treatment Enhances Behavioral and Molecular Changes Induced by Repetitive Trigeminal Activation in a Rat Model of Migraine. Biomedicines 2022, 10, 3175. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, S.P.; Kim, D.; Kim, W.J. Gut Dysbiosis: A New Avenue for Stroke Prevention and Therapeutics. Biomedicines 2023, 11, 2352. [Google Scholar] [CrossRef]
- Montanari, M.; Imbriani, P.; Bonsi, P.; Martella, G.; Peppe, A. Beyond the Microbiota: Understanding the Role of the Enteric Nervous System in Parkinson’s Disease from Mice to Human. Biomedicines 2023, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Corbelli, I.; de Tommaso, M.; Di Lorenzo, C.; Di Lorenzo, G.; Di Renzo, A. , Filippi, M.; Jannini, T.B.; Messina, R.; Parisi, P.; et al. Pathophysiological Bases of Comorbidity in Migraine. Front. Hum. Neurosci. 2021, 15, 640574. [Google Scholar] [CrossRef]
- Schwedt, T.J.; Vargas, B. Neurostimulation for Treatment of Migraine and Cluster Headache. Pain Med. 2015, 16, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study. Biomedicines 2023, 11, 2219. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, D.K.L.; Mahboob, A.; Zhai, K.; Paul, P.; Kammen, A.; Lee, D.J.; Yousef, M.S.; Chaari, A. Deep Brain Stimulation beyond the Clinic: Navigating the Future of Parkinson’s and Alzheimer’s Disease Therapy. Cells 2023, 12, 1478. [Google Scholar] [CrossRef]
- Chu, P.-C.; Huang, C.-S.; Chang, P.-K.; Chen, R.-S.; Chen, K.-T.; Hsieh, T.-H.; Liu, H.-L. Weak Ultrasound Contributes to Neuromodulatory Effects in the Rat Motor Cortex. Int. J. Mol. Sci. 2023, 24, 2578. [Google Scholar] [CrossRef]
- Adeel, M.; Chen, C.-C.; Lin, B.-S.; Chen, H.-C.; Liou, J.-C.; Li, Y.-T.; Peng, C.-W. Safety of Special Waveform of Transcranial Electrical Stimulation (TES): In Vivo Assessment. Int. J. Mol. Sci. 2022, 23, 6850. [Google Scholar] [CrossRef]
- Battaglia, S.; Schmidt, A.; Hassel, S.; Tanaka, M. Editorial: Case reports in neuroimaging and stimulation. Front. Psychiatry 2023, 14, 1264669. [Google Scholar] [CrossRef]
- Chang, C.H.; Wang, W.L.; Shieh, Y.H.; Peng, H.Y.; Ho, C.S.; Tsai, H.C. Case Report: Low-Frequency Repetitive Transcranial Magnetic Stimulation to Dorsolateral Prefrontal Cortex and Auditory Cortex in a Patient With Tinnitus and Depression. Front. Psychiatry 2022, 13, 847618. [Google Scholar] [CrossRef]
- Zakia, H.; Iskandar, S. Case report: Depressive disorder with peripartum onset camouflages suspected intracranial tuberculoma. Front. Psychiatry 2022, 13, 932635. [Google Scholar] [CrossRef] [PubMed]
- Nyatega, C.O.; Qiang, L.; Adamu, M.J.; Kawuwa, H.B. Gray matter, white matter and cerebrospinal fluid abnormalities in Parkinson's disease: A voxel-based morphometry study. Front Psychiatry 2022, 13, 1027907. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, J.; Wieczorek, T.; Fila-Witecka, K.; Smarzewska, K. ; Weiser, A, Piotrowski P, Tabakow, P. Various neuromodulation methods including Deep Brain Stimulation of the medial forebrain bundle combined with psychopharmacotherapy of treatment-resistant depression-Case report. Front. Psychiatry 2023, 13, 1068054. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xie, X.; Xie, J.; Tian, S.; Du, X.; Feng, H.; Zhang, H. Early-onset Alzheimer's disease with depression as the first symptom: A case report with literature review. Front. Psychiatry 2023, 14, 1192562. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, S.H.; Han, C.; Jeong, H.G.; Lee, M.S.; Kim, J. Antidepressant-induced mania in panic disorder: A single-case study of clinical and functional connectivity characteristics. Front. Psychiatry 2023, 14, 1205126. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, Y.; Deng, G.; Fang, J.; Qiu, C. Transient splenial lesion syndrome in bipolar-II disorder: A case report highlighting reversible brain changes during hypomanic episodes. Front. Psychiatry 2023, 14, 1219592. [Google Scholar] [CrossRef]
- Statsenko, Y.; Habuza, T.; Smetanina, D.; Simiyu, G.L.; Meribout, S.; King, F.C.; Gelovani, J.G.; Das, K.M.; Gorkom, K.N.-V.; Zaręba, K.; et al. Unraveling Lifelong Brain Morphometric Dynamics: A Protocol for Systematic Review and Meta-Analysis in Healthy Neurodevelopment and Ageing. Biomedicines 2023, 11, 1999. [Google Scholar] [CrossRef]
- Gazerani, P. Human Brain Organoids in Migraine Research: Pathogenesis and Drug Development. Int. J. Mol. Sci. 2023, 24, 3113. [Google Scholar] [CrossRef]
- Jalink, P.; Caiazzo, M. Brain Organoids: Filling the Need for a Human Model of Neurological Disorder. Biology 2021, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Song, H.; Ming, G.L. Modeling neurological disorders using brain organoids. Semin. Cell Dev. Biol. 2021, 111, 4–14. [Google Scholar] [CrossRef] [PubMed]
| Peptides | Receptors |
|---|---|
| CGRP | CLR |
| PACAP1–38 | >>PAC1, <VPAC1, <VPAC2 |
| PACAP6-38 | ? |
| PACAP1–27 | >PAC1, <VPAC1, <VPAC2 |
| PRP | ? |
| VIP | >VPAC1, >VPAC2, <PAC1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





