Submitted:
04 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract

Keywords:
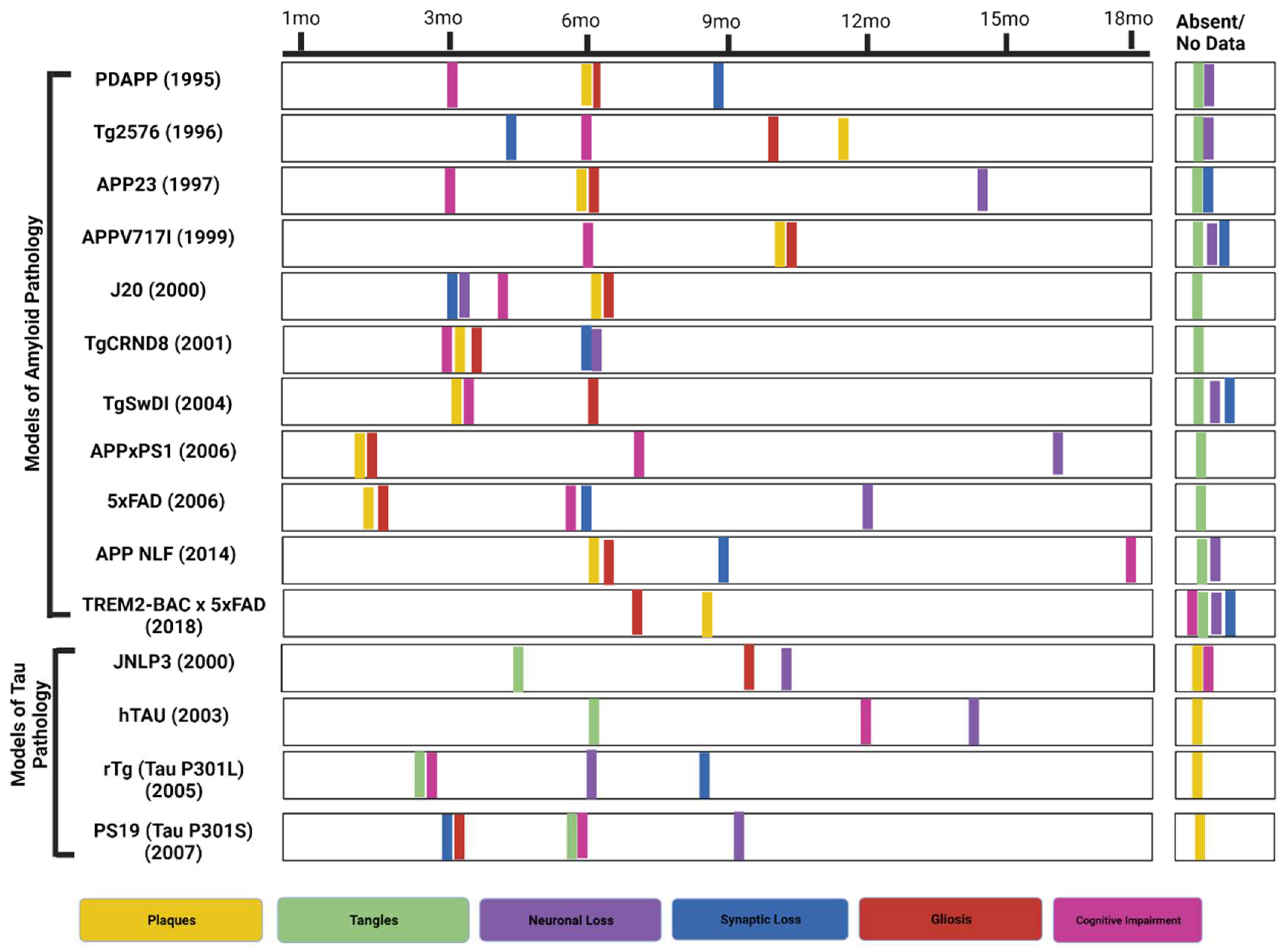
1. Introduction
| Model | Mutation | Model Background | Aβ Pathology | Other Pathologies and Impairments | References |
| Tg2576 | APP KM670/671NL (Swedish) | C57BL/6 x SJL | Plaques by 11-13 months | Synaptic loss by 4.5 monthsCognitive impairment by 6 months | [27], [33,34,35] |
| PDAPP | APP V717F (Indiana) | C57BL6 x DBA2 | Plaques at 6 months | Cognitive impairments at 3 monthsSynaptic loss by 8-9 monthsGliosis by 6 months | [28],[31], [29], [27] |
| 5xFAD (B6SJL) | APP KM670/671NL (Swedish), APP I716V (Florida), APP V717I (London), PSEN1 M146L, PSEN1 L286V | C57BL/6 x SJL | Plaques by 1.5 months | Gliosis at 2 monthsSynaptic and Neuronal loss at 4 and 6 monthsCognitive impairments by 4-5 months. | [27], [26] |
| 5xFAD (C57BL6) | APP KM670/671NL (Swedish), APP I716V (Florida), APP V717I (London), PSEN1 M146L, PSEN1 L286V | C57BL6 | Plaques by 1.5-2 months | Cognitive impairments between 3-6 months Synaptic and Neuronal loss at 6 and 12 months | [60], [59], [69], [62] |
| APPxPS1 | APP K670_M671delinsNL (Swedish) and PSEN1 L166P | C57BL/6 | Plaques by 1.5 months (cortex) and 3-4 months (hippocampus) | Cognitive impairment at 7 monthsGliosis at approx. 1.5 monthsSynaptic loss at 2-3 monthsNeuronal loss at 17 months | [63], [67], [64], [66], [65] |
| TREM2- BAC x 5xFAD | APP KM670/671NL (Swedish), APP I716V (Florida), APP V717I (London), PSEN1 M146L, PSEN1 L286V | TREM2-BAC: FVB/NJ;5xFAD: C57BL/6 x SJL | Plaques by 8.5 months | Neuronal and synaptic loss no data reportedGliosis by 7 months | [70], [71], [72], [73], [26] |
| APPNL-F | APP KM670/671NL (Swedish), APP I716F (Iberian) | C57BL/6 | Plaques by 6 months | Synaptic loss by 9-12 monthsGliosis in 6 months | [26], [68] |
| APPV717I | APP V717I (London) | C57BL/6 x FVB/N | Plaques by 10 months | Spatial memory deficits by 6 monthsCAA (15 months)Microhemorrhages (25-30 months) | [44], [43] |
| J20 | APP K670_M671delinsNL (Swedish), APP V717F (Indiana) | C57BL/6 | Plaques by 5-7 months | Neuronal loss, synaptic loss by 3 months, Cognitive impairments by 4 monthsTangles absent | [45], [46], [48], [47], [26] |
| TgCRND8 | APP K670_M671delinsNL (Swedish), APP V717F (Indiana) | Hybrid C3H/He-C57BL/6 | Plaques by 3 months | Cognitive impairment and gliosis by 3 months Tangles absent | [50], [51], [49], [52], [26] |
| Tg-SwDI | APP K670_M671delinsNL (Swedish), APP E693Q (Dutch), APP D694N (Iowa) | C57BL/6 | Plaques by 3 months | Cognitive impairment by 3 months,Gliosis by 6 months,Tangles absent | [26], [53], [54] |
| Model | Mutation | Model Background | Tau Pathology | Other Pathology | References |
|---|---|---|---|---|---|
| JNPL3 | MAPT P301L | C57BL/6, DBA/2, SW mixed background | NFTs develop from 4.5-6.5 months | Aβ plaques absentNeuronal loss at 9-10 monthsAstrogliosis by 10 months | [81], [82], [84], [83] |
| hTau | M.A.P.T.: knock out; M.A.P.T.: Transgenic | Cross between SW and B6D2F1 | Hyperphosphorylated tau at 6 months | Aβ plaques absentCognitive deficits at 12 monthsNeuronal death observed at 14 months | [87], [88], [89] |
| rTg (tau P301L) | MAPT P301L | Produced by crossing 129S6 (activator) to F.V.B. (responder) | Pretangles at 2.5 months. Argyrophilic tangle-like inclusions in the cortex by 4 months and in the hippocampus by 5.5 months | Aβ plaques absentNeuronal loss was observed at different periodsSynaptic loss at 8-9 monthsMotor impairments by 6 monthsMemory impairments by 2.5-4 months | [87], [88], [96], [26] |
| PS19 (tau P301S)- | MAPT P301S | (C57BL/6 x C3H)F1 | NFTs develop at 6 months | Cognitive deficits at 6 monthsSynaptic loss and gliosis at 3 monthsNeuronal loss at 9-12 months | [26], [99] |
2. Behavioral Studies
3. Fluid Biomarkers
4. Neuro Imaging in Murine Models
5. Conclusion
Author Contributions
Conflicts of Interest
References
- Cummings, J.L. , et al., The costs of developing treatments for Alzheimer's disease: A retrospective exploration. Alzheimers Dement 2022, 18, 469–477. [Google Scholar] [CrossRef]
- Poon, C.H. , et al., Rodent models of amyloid-beta feature of alzheimer’s disease: Development and potential treatment implications. Aging and disease 2020, 11, 1235. [Google Scholar] [CrossRef] [PubMed]
- Therriault, J. , et al., Biomarker modeling of Alzheimer’s disease using PET-based Braak staging. Nature Aging, 2022, 1-10.
- DeTure, M.A. and D.W. Dickson, The neuropathological diagnosis of Alzheimer’s disease. Molecular neurodegeneration 2019, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R. , et al., The development of amyloid β protein deposits in the aged brain. Science of aging knowledge environment 2006, 2006, re1–re1. [Google Scholar] [CrossRef]
- Jankowsky, J.L. and H. Zheng, Practical considerations for choosing a mouse model of Alzheimer’s disease. Molecular neurodegeneration 2017, 12, 1–22. [Google Scholar] [CrossRef]
- Wattmo, C. and A.K. Wallin, Early- versus late-onset Alzheimer's disease in clinical practice: cognitive and global outcomes over 3 years. Alzheimers Res Ther 2017, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Verghese, P.B., J. M. Castellano, and D.M. Holtzman, Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 2011, 10, 241–252. [Google Scholar] [CrossRef]
- Kim, J., J. M. Basak, and D.M. Holtzman, The role of apolipoprotein E in Alzheimer's disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef]
- Vassar, R. , BACE1: the beta-secretase enzyme in Alzheimer's disease. J Mol Neurosci 2004, 23, 105–114. [Google Scholar] [CrossRef]
- Drummond, E. and T. Wisniewski, Alzheimer’s disease: experimental models and reality. Acta Neuropathologica 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Cole, S.L. and R. Vassar, The Alzheimer's disease beta-secretase enzyme, BACE1. Mol Neurodegener 2007, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H. , et al., The beta-Secretase BACE1 in Alzheimer's Disease. Biol Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I. , et al. , Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci 1999, 14, 419–427. [Google Scholar] [PubMed]
- Sinha, S. , et al., Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 1999, 402, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R. , et al., Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Lin, X. , et al., Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc Natl Acad Sci U S A 2000, 97, 1456–1460. [Google Scholar] [CrossRef]
- Goate, A. , et al., Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 1991, 349, 704–706. [Google Scholar] [CrossRef]
- Petit, D. , et al., Aβ profiles generated by Alzheimer’s disease causing PSEN1 variants determine the pathogenicity of the mutation and predict age at disease onset. Molecular Psychiatry 2022, 27, 2821–2832. [Google Scholar] [CrossRef]
- Hardy, J. and D. Allsop, Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Selkoe, D.J. and J. Hardy, The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Mandelkow, E.-M. and E. Mandelkow, Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harbor perspectives in medicine 2012, 2, a006247. [Google Scholar] [CrossRef]
- Gendron, T.F. and L. Petrucelli, The role of tau in neurodegeneration. Molecular neurodegeneration 2009, 4, 1–19. [Google Scholar] [CrossRef]
- Ribeiro, F.M. , et al. , Animal models of neurodegenerative diseases. Revista brasileira de psiquiatria (Sao Paulo, Brazil : 1999) 2013, 35 (Suppl 2), S82–91. [Google Scholar] [CrossRef]
- KoSIK, K.S., C. L. Joachim, and D.J. Selkoe, Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proceedings of the National Academy of Sciences 1986, 83, 4044–4048. [Google Scholar] [CrossRef] [PubMed]
- Research Models Alzheimer's Disease. Available from: https://www.alzforum.org/research-models/alzheimers-disease.
- Puzzo, D. , et al., Rodent models for Alzheimer's disease drug discovery. Expert opinion on drug discovery 2015, 10, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Games, D. , et al., Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 1995, 373, 523–527. [Google Scholar] [CrossRef]
- Masliah, E. , et al., Comparison of Neurodegenerative Pathology in Transgenic Mice Overexpressing V717F \upbeta{}{-}{A}myloid Precursor Protein and Alzheimer's Disease. The Journal of Neuroscience 1996, 16, 5795–5811. [Google Scholar] [CrossRef] [PubMed]
- Chen, G. , et al., A learning deficit related to age and \upbeta{}{-}{a}myloid plaques in a mouse model of Alzheimer\textquotesingles disease. Nature 2000, 408, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Dodart, J.-C. , et al., Neuroanatomical Abnormalities in Behaviorally Characterized APPV717F Transgenic Mice. Neurobiology of Disease 2000, 7, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.F. , et al., Amyloid deposition in the hippocampus and entorhinal cortex: Quantitative analysis of a transgenic mouse model. Proceedings of the National Academy of Sciences 2003, 100, 4837–4842. [Google Scholar] [CrossRef]
- Hsiao, K. , et al., Correlative Memory Deficits, A$\upbeta$ Elevation, and Amyloid Plaques in Transgenic Mice. Science 1996, 274, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L. , et al., Impaired Spine Stability Underlies Plaque-Related Spine Loss in an Alzheimer\textquotesingles Disease Mouse Model. The American Journal of Pathology 2007, 171, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- IRIZARRY, M.I.C.H.A.E.L.C. , et al., {APPSW} Transgenic Mice Develop Age-related A$\upbeta$ Deposits and Neuropil Abnormalities, but no Neuronal Loss in {CA}1. Journal of Neuropathology and Experimental Neurology 1997, 56, 965–973. [Google Scholar] [CrossRef] [PubMed]
- King, D.L. and G.W. Arendash, Behavioral characterization of the Tg2576 transgenic model of Alzheimer\textquotesingles disease through 19 months. Physiology {\&}amp$\mathsemicolon$ Behavior 2002, 75, 627–642. [Google Scholar]
- Sturchler-Pierrat, C. , et al., Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A 1997, 94, 13287–13292. [Google Scholar] [CrossRef]
- Thal, D.R., W. S. Griffin, and H. Braak, Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer's disease. J Cell Mol Med 2008, 12, 1848–1862. [Google Scholar] [CrossRef]
- Reuter, B. , et al., Statin Therapy and the Development of Cerebral Amyloid Angiopathy--A Rodent in Vivo Approach. Int J Mol Sci 2016, 17. [Google Scholar]
- Fryer, J.D. , et al., Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci 2005, 25, 2803–2810. [Google Scholar] [CrossRef]
- Meyer, E.P. , et al., Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer's disease. Proc Natl Acad Sci U S A 2008, 105, 3587–3592. [Google Scholar] [CrossRef]
- Nehra, G., B. Bauer, and A.M. Hartz, Blood-brain barrier leakage in Alzheimer’s disease: from discovery to clinical relevance. Pharmacology & Therapeutics.
- Caroni, P. , Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods 1997, 71, 3–9. [Google Scholar] [CrossRef]
- Lloyd, G.M. , et al., Prominent amyloid plaque pathology and cerebral amyloid angiopathy in APP V717I (London) carrier - phenotypic variability in autosomal dominant Alzheimer's disease. Acta Neuropathol Commun 2020, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Mucke, L. , et al., High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 2000, 20, 4050–4058. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H. , et al., Global changes of phospholipids identified by MALDI imaging mass spectrometry in a mouse model of Alzheimer's disease. J Lipid Res 2016, 57, 36–45. [Google Scholar] [CrossRef]
- Ameen-Ali, K.E. , et al., The Time Course of Recognition Memory Impairment and Glial Pathology in the hAPP-J20 Mouse Model of Alzheimer's Disease. J Alzheimers Dis 2019, 68, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Shabir, O. , et al., Enhanced Cerebral Blood Volume under Normobaric Hyperoxia in the J20-hAPP Mouse Model of Alzheimer's Disease. Sci Rep 2020, 10, 7518. [Google Scholar] [CrossRef]
- Chishti, M.A. , et al., Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem 2001, 276, 21562–21570. [Google Scholar] [CrossRef]
- Boy, J. , et al., Expression mapping of tetracycline-responsive prion protein promoter: digital atlasing for generating cell-specific disease models. Neuroimage 2006, 33, 449–462. [Google Scholar] [CrossRef]
- Cortes-Canteli, M. , et al., Fibrin deposited in the Alzheimer's disease brain promotes neuronal degeneration. Neurobiol Aging 2015, 36, 608–617. [Google Scholar] [CrossRef]
- Brautigam, H. , et al., The isotropic fractionator provides evidence for differential loss of hippocampal neurons in two mouse models of Alzheimer's disease. Mol Neurodegener 2012, 7, 58. [Google Scholar] [CrossRef]
- Davis, J. , et al., Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem 2004, 279, 20296–20306. [Google Scholar] [CrossRef]
- Davis, J. , et al., Deficient cerebral clearance of vasculotropic mutant Dutch/Iowa Double A beta in human A betaPP transgenic mice. Neurobiol Aging 2006, 27, 946–954. [Google Scholar] [CrossRef]
- Choi, S. , et al., Asymmetric dimethylarginine exacerbates cognitive dysfunction associated with cerebrovascular pathology. FASEB J 2020, 34, 6808–6823. [Google Scholar] [CrossRef]
- Searcy, J.L. , et al., Impact of age on the cerebrovascular proteomes of wild-type and Tg-SwDI mice. PLoS One 2014, 9, e89970. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H. , et al., Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. Journal of Neuroscience 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Forner, S. , et al., Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer’s disease. Scientific data 2021, 8, 270. [Google Scholar] [CrossRef]
- Jawhar, S. , et al., Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal A$\upbeta$ aggregation in the 5XFAD mouse model of Alzheimer{\textquotesingle}s disease. Neurobiology of Aging 2012, 33, 196–e29. [Google Scholar] [CrossRef]
- Oakley, H. , et al., Intraneuronal beta-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer\textquotesingles Disease Mutations: Potential Factors in Amyloid Plaque Formation. Journal of Neuroscience 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Oblak, A.L. , et al., Comprehensive evaluation of the 5XFAD mouse model for preclinical testing applications: a MODEL-AD study. Frontiers in aging neuroscience 2021, 13, 713726. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.-H. , et al., Axonal and myelinic pathology in 5xFAD Alzheimer's mouse spinal cord. PLOS ONE 2017, 12, e0188218. [Google Scholar] [CrossRef]
- Radde, R. , et al., Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep 2006, 7, 940–946. [Google Scholar] [CrossRef]
- Bittner, T. , et al., Amyloid plaque formation precedes dendritic spine loss. Acta Neuropathol 2012, 124, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Serneels, L. , et al., gamma-Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer's disease. Science 2009, 324, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Gengler, S., A. Hamilton, and C. Holscher, Synaptic plasticity in the hippocampus of a APP/PS1 mouse model of Alzheimer's disease is impaired in old but not young mice. PLoS One 2010, 5, e9764. [Google Scholar] [CrossRef] [PubMed]
- Rupp, N.J. , et al., Early onset amyloid lesions lead to severe neuritic abnormalities and local, but not global neuron loss in APPPS1 transgenic mice. Neurobiol Aging 2011, 32, 2324–e1. [Google Scholar] [CrossRef]
- Saito, T. , et al., Single App knock-in mouse models of Alzheimer's disease. Nat Neurosci 2014, 17, 661–663. [Google Scholar] [CrossRef]
- Forner, S. , et al., Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer's disease. Scientific Data 2021, 8. [Google Scholar] [CrossRef]
- Udeochu, J., F. A. Sayed, and L. Gan, TREM2 and Amyloid Beta: A Love-Hate Relationship. Neuron 2018, 97, 991–993. [Google Scholar] [CrossRef]
- Lee, C.Y.D. , et al., Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Pathological Phenotypes in Alzheimer's Disease Models. Neuron 2018, 97, 1032–1048. [Google Scholar] [CrossRef]
- Poon, C.H. , et al., Rodent Models of Amyloid-Beta Feature of Alzheimer's Disease: Development and Potential Treatment Implications. Aging and disease 2020, 11, 1235. [Google Scholar] [CrossRef]
- Song, W.M. , et al., Humanized TREM2 mice reveal microglia-intrinsic and -extrinsic effects of R47H polymorphism. Journal of Experimental Medicine 2018, 215, 745–760. [Google Scholar] [CrossRef]
- Barthelemy, N.R. , et al., Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer's disease. J Exp Med 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M. and E.D. Roberson, Mouse models of Alzheimer's disease. Brain Res Bull 2012, 88, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R. , et al., Animal models in the study of Alzheimer's disease and Parkinson's disease: A historical perspective. Animal models and experimental medicine 2022, 5, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Probst, A. , et al., Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathologica 2000, 99, 469–481. [Google Scholar] [CrossRef] [PubMed]
- LaFerla, F.M. and K.N. Green, Animal Models of Alzheimer Disease. Cold Spring Harbor Perspectives in Medicine 2012, 2, a006320–a006320. [Google Scholar] [CrossRef]
- Goedert, M., D. S. Eisenberg, and R.A. Crowther, Propagation of Tau Aggregates and Neurodegeneration. Annual review of neuroscience 2017, 40, 189–210. [Google Scholar] [CrossRef]
- Dawson, T.M., T. E. Golde, and C. Lagier-Tourenne, Animal models of neurodegenerative diseases. Nature neuroscience 2018, 21, 1370–1379. [Google Scholar] [CrossRef]
- Lewis, J. , et al., Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nature genetics 2000, 25, 402–405. [Google Scholar] [CrossRef]
- Hutton, M. , Missense and splice site mutations in tau associated with FTDP-17: multiple pathogenic mechanisms. Neurology 2001, 56(11 Suppl 4), S21–5. [Google Scholar] [CrossRef]
- Duff, K. , et al., Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiology of disease 2000, 7, 87–98. [Google Scholar] [CrossRef]
- Hutton, M. , et al., Analysis of tauopathies with transgenic mice. Trends Mol Med 2001, 7, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Andorfer, C. , et al., Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. Journal of Neurochemistry 2003, 86, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Andorfer, C. , et al., Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. The Journal of neuroscience : the official journal of the Society for Neuroscience 2005, 25, 5446–5454. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, M. , et al. , Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). The Journal of neuroscience : the official journal of the Society for Neuroscience 2005, 25, 10637–10647. [Google Scholar]
- Santacruz, K. , et al. , Tau suppression in a neurodegenerative mouse model improves memory function. Science (New York, N.Y.) 2005, 309, 476–481. [Google Scholar]
- Tucker, K.L., M. Meyer, and Y.A. Barde, Neurotrophins are required for nerve growth during development. Nature neuroscience 2001, 4, 29–37. [Google Scholar] [CrossRef]
- Neddens, J. , et al., Constant Levels of Tau Phosphorylation in the Brain of htau Mice. Front Mol Neurosci 2020, 13, 136. [Google Scholar] [CrossRef]
- Wenger, K. , et al., Common mouse models of tauopathy reflect early but not late human disease. Mol Neurodegener 2023, 18, 10. [Google Scholar] [CrossRef]
- Billings, L.M. , et al., Intraneuronal Aβ causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 2005, 45, 675–688. [Google Scholar] [CrossRef]
- Jankowsky, J.L. and H. Zheng, Practical considerations for choosing a mouse model of Alzheimer's disease. Mol Neurodegener 2017, 12, 89. [Google Scholar] [CrossRef]
- Yue, M. , et al., Sex difference in pathology and memory decline in rTg4510 mouse model of tauopathy. Neurobiol Aging 2011, 32, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Song, L. , et al., Analysis of tau post-translational modifications in rTg4510 mice, a model of tau pathology. Mol Neurodegener 2015, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Helboe, L. , et al., Early depletion of CA1 neurons and late neurodegeneration in a mouse tauopathy model. Brain Res 2017, 1665, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Hoover, B.R. , et al., Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef]
- Gamache, J. , et al., Factors other than hTau overexpression that contribute to tauopathy-like phenotype in rTg4510 mice. Nat Commun 2019, 10, 2479. [Google Scholar] [CrossRef]
- Yoshiyama, Y. , et al., Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef]
- Oddo, S. , et al., Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging 2003, 24, 1063–1070. [Google Scholar] [CrossRef]
- Rodriguez, J.J. , et al., Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS One 2008, 3, e2935. [Google Scholar] [CrossRef]
- Do, T.M. , et al. , Age-Dependent Regulation of the Blood-Brain Barrier Influx/Efflux Equilibrium of Amyloid-beta Peptide in a Mouse Model of Alzheimer's Disease (3xTg-AD). J Alzheimers Dis 2016, 49, 287–300. [Google Scholar]
- Origlia, N. , et al., MAPK, beta-amyloid and synaptic dysfunction: the role of RAGE. Expert Rev Neurother 2009, 9, 1635–1645. [Google Scholar] [CrossRef]
- Padmanabhan, P. and J. Götz, Clinical relevance of animal models in aging-related dementia research. Nature Aging 2023, 3, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Badea, A. , et al., Identifying Vulnerable Brain Networks in Mouse Models of Genetic Risk Factors for Late Onset Alzheimer's Disease. Front Neuroinform 2019, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Bagyinszky, E. , et al., Role of inflammatory molecules in the Alzheimer's disease progression and diagnosis. J Neurol Sci 2017, 376, 242–254. [Google Scholar] [CrossRef]
- Reiman, E.M. , et al., Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nature Communications 2020, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.K., K. Uchida, and H. Nakayama, White matter myelin loss in the brains of aged dogs. Experimental Gerontology 2012, 47, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. and R.W. Mahley, Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiol Dis 2014, 72 Pt A, 3–12. [Google Scholar] [CrossRef]
- Liu, C.C. , et al., Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Bales, K.R. , The value and limitations of transgenic mouse models used in drug discovery for Alzheimer's disease: an update. Expert Opin Drug Discov 2012, 7, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Shineman, D.W. , et al., Accelerating drug discovery for Alzheimer's disease: best practices for preclinical animal studies. Alzheimers Res Ther 2011, 3, 28. [Google Scholar] [CrossRef]
- Oblak, A.L. , et al., Model organism development and evaluation for late-onset Alzheimer's disease: MODEL-AD. Alzheimers Dement (N Y) 2020, 6, e12110. [Google Scholar] [CrossRef]
- Yagi, H. , et al., Age-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in recent memory. Brain Res 1988, 474, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Tanisawa, K. , et al., Exome sequencing of senescence-accelerated mice (SAM) reveals deleterious mutations in degenerative disease-causing genes. BMC genomics 2013, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.A. , et al., Association of cerebrovascular dysfunction with the development of Alzheimer’s disease-like pathology in OXYS rats. BMC genomics 2018, 19, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, N. , et al., Senescence-accelerated OXYS rats: A genetic model of premature aging and age-related diseases. Advances in Gerontology 2014, 4, 294–298. [Google Scholar] [CrossRef]
- Liu, B., J. Liu, and J.S. Shi, SAMP8 Mice as a Model of Age-Related Cognition Decline with Underlying Mechanisms in Alzheimer's Disease. J Alzheimers Dis 2020, 75, 385–395. [Google Scholar] [CrossRef]
- Kepchia, D. , et al., The Alzheimer's disease drug candidate J147 decreases blood plasma fatty acid levels via modulation of AMPK/ACC1 signaling in the liver. Biomed Pharmacother 2022, 147, 112648. [Google Scholar] [CrossRef]
- Codony, S. , et al., Discovery and In Vivo Proof of Concept of a Highly Potent Dual Inhibitor of Soluble Epoxide Hydrolase and Acetylcholinesterase for the Treatment of Alzheimer's Disease. J Med Chem 2022, 65, 4909–4925. [Google Scholar] [CrossRef]
- Kolosova, N.G. , et al., [The senescence-accelerated oxys rats--a genetic model of premature aging and age-dependent degenerative diseases]. Adv Gerontol 2014, 27, 336–340. [Google Scholar]
- Stefanova, N.A. , et al., Association of cerebrovascular dysfunction with the development of Alzheimer's disease-like pathology in OXYS rats. BMC Genomics 2018, 19 (Suppl 3). [Google Scholar] [CrossRef]
- Hurley, M.J. , et al., The long-lived Octodon degus as a rodent drug discovery model for Alzheimer's and other age-related diseases. Pharmacology & therapeutics 2018, 188, 36–44. [Google Scholar]
- Inestrosa, N.C. , et al., Human-like rodent amyloid-beta-peptide determines Alzheimer pathology in aged wild-type Octodon degu. Neurobiology of aging 2005, 26, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N. , et al., Accelerating Alzheimer's research through 'natural' animal models. Curr Opin Psychiatry 2015, 28, 155–164. [Google Scholar] [CrossRef]
- Steffen, J. , et al. , Revisiting rodent models: Octodon degus as Alzheimer's disease model? Acta neuropathologica communications 2016, 4, 91. [Google Scholar] [PubMed]
- Vitek, M.P. , et al., Translational animal models for Alzheimer\textquotesingles disease: An Alzheimer\textquotesingles Association Business Consortium Think Tank. Alzheimer{\textquotesingle}s {\&}amp$\mathsemicolon$ Dementia: Translational Research {\&}amp$\mathsemicolon$ Clinical Interventions 2020, 6. [Google Scholar]
- Bruce-Keller, A.J. , et al., Cognitive impairment in humanized APP\texttimesPS1 mice is linked to A\upbeta{}{1}{–}42 and NOX activation. Neurobiology of Disease 2011, 44, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. , et al., Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiology of disease 2007, 27, 301–311. [Google Scholar] [CrossRef]
- Xu, W. , et al., Cerebral microvascular rather than parenchymal amyloid-β protein pathology promotes early cognitive impairment in transgenic mice. Journal of Alzheimer's Disease 2014, 38, 621–632. [Google Scholar] [CrossRef]
- Fisher, E.M.C. and D.M. Bannerman, Mouse models of neurodegeneration: Know your question, know your mouse. Science translational medicine 2019, 11. [Google Scholar] [CrossRef]
- Sabbagh, J.J., J. W. Kinney, and J.L. Cummings, Animal systems in the development of treatments for Alzheimer's disease: challenges, methods, and implications. Neurobiol Aging 2013, 34, 169–183. [Google Scholar] [CrossRef]
- Cotman, C.W. and E. Head, The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. Journal of Alzheimer's disease : JAD 2008, 15, 685–707. [Google Scholar] [CrossRef]
- Head, E. , A canine model of human aging and Alzheimer's disease. Biochim Biophys Acta 2013, 1832, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.L., T. M. Ballard, and C. Glavis-Bloom, Animal paradigms to assess cognition with translation to humans. Handbook of experimental pharmacology 2015, 228, 27–57. [Google Scholar] [PubMed]
- Perez, S.E. , et al., Alzheimer's disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla). Journal of Comparative Neurology 2013, 521, 4318–4338. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.E. , et al., Early Alzheimer's disease-type pathology in the frontal cortex of wild mountain gorillas (Gorilla beringei beringei). Neurobiol Aging 2016, 39, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Edler, M.K. , et al., Aged chimpanzees exhibit pathologic hallmarks of Alzheimer's disease. Neurobiol Aging 2017, 59, 107–120. [Google Scholar] [CrossRef]
- Oikawa, N., N. Kimura, and K. Yanagisawa, Alzheimer-type tau pathology in advanced aged nonhuman primate brains harboring substantial amyloid deposition. Brain Res 2010, 1315, 137–149. [Google Scholar] [CrossRef]
- Gearing, M. , et al., Neuropathology and apolipoprotein E profile of aged chimpanzees: implications for Alzheimer disease. Proc Natl Acad Sci U S A 1994, 91, 9382–9386. [Google Scholar] [CrossRef]
- Gearing, M. , et al., beta-Amyloid (A beta) deposition in the brains of aged orangutans. Neurobiol Aging 1997, 18, 139–146. [Google Scholar] [CrossRef]
- Heuer, E. , et al., Nonhuman primate models of Alzheimer-like cerebral proteopathy. Current pharmaceutical design 2012, 18, 1159–1169. [Google Scholar] [CrossRef]
- Kitt Cheryl, A. , et al., Evidence for Cholinergic Neurites in Senile Plaques. Science 1984, 226, 1443–1445. [Google Scholar] [CrossRef]
- Mufson, E.J. , et al., Apolipoprotein E-immunoreactivity in aged rhesus monkey cortex: Colocalization with amyloid plaques. Neurobiology of Aging 1994, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Didier, E.S. , et al., Contributions of Nonhuman Primates to Research on Aging. Veterinary pathology 2016, 53, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Callejas, J.D., E. Fuchs, and C. Perez-Cruz, Evidence of Tau Hyperphosphorylation and Dystrophic Microglia in the Common Marmoset. Frontiers in Aging Neuroscience 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- hAβ-KI Alzforum. Available from: https://www.alzforum.org/research-models/hav-ki.
- Baglietto-Vargas, D. , et al., Generation of a humanized Aβ expressing mouse demonstrating aspects of Alzheimer's disease-like pathology. Nat Commun 2021, 12, 2421. [Google Scholar] [CrossRef]
- Serrano-Pozo, A. , et al., Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 2011, 1, a006189. [Google Scholar] [CrossRef]
- Squire, L.R., B. Knowlton, and G. Musen, The structure and organization of memory. Annu Rev Psychol 1993, 44, 453–495. [Google Scholar] [CrossRef]
- Bussière, T. , et al., Stereologic assessment of the total cortical volume occupied by amyloid deposits and its relationship with cognitive status in aging and Alzheimer’s disease. Neuroscience 2002, 112, 75–91. [Google Scholar] [CrossRef]
- Wang, L. , et al., Abnormalities of hippocampal surface structure in very mild dementia of the Alzheimer type. Neuroimage 2006, 30, 52–60. [Google Scholar] [CrossRef]
- Puzzo, D. , et al., Behavioral assays with mouse models of Alzheimer's disease: practical considerations and guidelines. Biochem Pharmacol 2014, 88, 450–467. [Google Scholar] [CrossRef]
- Clark, R.E. and L.R. Squire, Similarity in form and function of the hippocampus in rodents, monkeys, and humans. Proceedings of the National Academy of Sciences 2013, 110 (Suppl. S2), 10365–10370. [Google Scholar] [CrossRef]
- Watrous, A.J. , et al., A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus 2013, 23, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.J. , et al., Cerebrospinal fluid tau levels are associated with abnormal neuronal plasticity markers in Alzheimer’s disease. Molecular Neurodegeneration 2022, 17, 27. [Google Scholar] [CrossRef]
- An, S.S.A. and J.P. Hulme, Plasma amyloid-beta oligomer and phosphorylated tau: diagnostic tools for progressive Alzheimer’s disease. Neural Regeneration Research 2023, 18, 2391–2392. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. , et al., Dual-Modal NIR-Fluorophore Conjugated Magnetic Nanoparticle for Imaging Amyloid-beta Species In Vivo. Small 2018, 14, e1800901. [Google Scholar] [CrossRef]
- Peng, C. , et al., Versatile fluorescent probes for near-infrared imaging of amyloid-β species in Alzheimer's disease mouse model. Journal of Materials Chemistry B 2019, 7, 1986–1995. [Google Scholar] [CrossRef]
- Jack, C.R., Jr. , et al., Magnetic resonance imaging of Alzheimer's pathology in the brains of living transgenic mice: a new tool in Alzheimer's disease research. Neuroscientist 2007, 13, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J. , et al., Evaluation of the neuroprotective effect of taurine in Alzheimer’s disease using functional molecular imaging. Scientific Reports 2020, 10, 15551. [Google Scholar] [CrossRef] [PubMed]
- Chiquita, S. , et al., A longitudinal multimodal in vivo molecular imaging study of the 3xTg-AD mouse model shows progressive early hippocampal and taurine loss. Hum Mol Genet 2019, 28, 2174–2188. [Google Scholar] [CrossRef]
- Guell-Bosch, J. , et al., Progression of Alzheimer's disease and effect of scFv-h3D6 immunotherapy in the 3xTg-AD mouse model: An in vivo longitudinal study using Magnetic Resonance Imaging and Spectroscopy. NMR Biomed 2020, 33, e4263. [Google Scholar] [CrossRef]
- Mlynarik, V. , et al., Proton and phosphorus magnetic resonance spectroscopy of a mouse model of Alzheimer's disease. J Alzheimers Dis 2012, 31 (Suppl 3), S87–99. [Google Scholar] [CrossRef]
- Benavides, F. , et al., Genetic quality assurance and genetic monitoring of laboratory mice and rats: FELASA Working Group Report. Lab Anim 2020, 54, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Mekada, K. , et al., Development of SNP markers for C57BL/6N-derived mouse inbred strains. Exp Anim 2015, 64, 91–100. [Google Scholar] [CrossRef]
- Shinohara, M. , et al., Regional distribution of synaptic markers and APP correlate with distinct clinicopathological features in sporadic and familial Alzheimer's disease. Brain 2014, 137 Pt 5, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, R.M. , et al., Tau aggregation and its interplay with amyloid-beta. Acta Neuropathol 2015, 129, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Hyde, L.A. , et al., Studies to investigate the in vivo therapeutic window of the γ-secretase inhibitor N2-[(2S)-2-(3, 5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-oxo-6, 7-dihydro-5H-dibenzo [b, d] azepin-7-yl]-L-alaninamide (LY411, 575) in the CRND8 mouse. Journal of Pharmacology and Experimental Therapeutics 2006, 319, 1133–1143. [Google Scholar]
- Head, E. , et al., A Two-Year Study with Fibrillar β-Amyloid (Aβ) Immunization in Aged Canines: Effects on Cognitive Function and Brain Aβ. J. Neurosci. 2008, 28, 3555. [Google Scholar] [CrossRef]
- Davis, P.R. , et al., Aβ vaccination in combination with behavioral enrichment in aged beagles: effects on cognition, Aβ, and microhemorrhages. Neurobiology of aging 2017, 49, 86–99. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





