1. Introduction
Modern cardiac surgery and cardiac anesthesia are developing intensively, more and more surgeries are provided by using mini-invasive or non-invasive (endovascular) technologies. However, congenital heart defects (CHD) correction in children usually demands significant (valuable) surgery aggression and using of cardiopulmonary bypass (CPB). These procedures have risk of children`s brain damage because of many harmful, factors, first of all because of CPB. CPB may has direct effects (such an embolism, hypoxia, changes of hemodynamics, failing of cerebral circulation self-regulation) and indirect through the system inflammation response (SIR) initiation because of blood contacting with the extracorporeal circuit, hemolysis, and temperature violation [1-4].
We must take into account that regulation in the brain is the special cell interaction process that demands to create isolated microenvironment to achieve their complete functioning. For this, there is such phenomenon as the blood-brain barrier and its components exist and its functional unit is called the neurovascular unit (NVU). NVU consists of micro-vessels associated with astrocytes connected to neurons and their axons. There are also special transport proteins in blood-brain barrier (BBB) for selective transport of substances from the blood plasma to neurons. All of them provide coordinated brain function due to intercellular communication and metabolic conjugation of the central nervous system cells [
5]. Cerebral damage leads to the microglia and astrocytes activation and consequential production of proinflammatory mediators in the brain [
6]. These mediators cause BBB injury and then induce cell death and gliosis [
7]. Moreover, when BBB integrity fails, there are not only local but also system cytokines, which can also affect the NVU. Those cytokines are often present in patient’s blood after cardiac surgery. [
8] They stimulate expression of adhesion molecules; potentiate adhesion and extravasation of neutrophils and monocytes into ischemic tissues [
9]. Moreover, local chemokines production increase WBC extravasation in these tissues [
10]. All of these processes occur in the NVU, inducing specific pathological changes in cerebral tissues with their subsequent dysfunction.
Children`s brain, especially in the first-years, has a lot of specific features: it goes through the active neuronal differentiation and synaptogenesis, glial cells active growth and myelination; it is high-hydrophilic and has high metabolism rate [11, 12]. All of these qualities make child brain vulnerable for any pathogens. That cause the high rate of post- operative cognitive disorders. For example, post-operative delirium frequency after CHD correction reaches 57% [
13]. It is also notable that we still don’t know much about how any damage or dysfunction in childhood affects further child`s progress and growth. Now we have studies which prove the lack of the cognitive function in children who underwent cardiac surgery the year before [
14].
Thus, the search for opportunities to prevent cerebral damage during the correction of congenital heart defects in children remains actual. One of the perspective ways to achieve it is to restrict blood transfusion in perioperative period. This strategy based on the thought that donor’s blood components initiate system inflammation response syndrome (SIRS) in a patient, which performed as neuroinflammation with consequential injury of the whole NVU (i.e., the complex of neurons, astrocytes and endothelial cells) [15, 16]. Therefore, we should understand that refrain from RBC-containing donor blood components using can lead us to the risk of hemic hypoxia because of hemodilution during CPB [
17]. For this reason, we have an important question: what is more dangerous for the NVU - hypoxia when we limit the transfusion or SIRS effects when we use it? This is the answer that we could not get from the clinical trial because it is dangerous for the patient. However, we have a decision for that problem: we can explore hypoxia and SIRS effects on the NVU cellular model in vitro [
18], what we did set as the purpose of our research.
2. Materials and Methods
We had the collection of serum from 78 pediatric patients - from 1 month to 6,5 years old with body mass from 3,3 to 21,5 kg, all of them undergo surgical correction of septal CHD using CPB on the Research Institute for Complex Issues of Cardiovascular Diseases base. In each sample, we evaluated IL-6 level as one of the main SIRS markers [
19]. We chose three serum samples from our collection (with the lowest and the highest concentration of IL-6) to the in vitro stage - they have the highest and the lowest level of SIRS presence as it all. Frozen serum was delivered to the Krasnoyarsk State Medical University in proper temperature chain sequence.
The model of brain injury during cardiac surgeries was invented based on the NVU cell model, which can be cultivated under different conditions that mimic the factors of the intraoperative period. This model was created in the laboratory of Krasnoyarsk State Medical University.
2.1. In vitro stage of experiment
Getting primary cells cultures of the brain in vitro
We used primary cultures of endothelium cells, astrocytes and cerebral neurons, which we got from rats of Wistar line. Animals lived in cages with the free access to water and food. Temperature was constant - 21±1°С. The light cycle was 12 h day / 12 h night. Our tests on animals were performed according to the humanity principles declared in European society directive (2010/63/EC). General animals number n=10.
We needed several stages to get the cell model of NVU in vitro:
Extraction of cerebral endothelial cells
Extraction and cultivation of neurospheres.
Extraction of astrocytes and neurons from these neurospheres we got earlier using directional differentiating in astrocytes and neurons.
Forming the NVU model in vitro. To get it we put the mix of neurons and endothelial cells on the bottom of culture plate wells. Then we set culture inserts (Corning-Costar USA) and put astrocytes at the top of them. We cultivated this mix of cells in the liquid containing DMEM with FBS, glutamine and antibiotics on 37°C with 5% CO2 [
19]
2.2. Experiment design
After formation the NBU cell model, we provided the main experiment.
In the aim to reveal the IL-6 effects on the NVU cell model in vitro, we added serum samples (with minimal and maximal levels of IL-6) in the cultural liquid.
Then we formed groups depending on the IL-6 concentration.
The «Control» group - the intact model of NVU.
The «Minimum» group - samples with the lowest IL-6 level in the culture.
The «Maximum» group - samples with the highest IL-6 level in culture.
Number of repeats in serum incubation groups - 10.
According to the hypoxic incubation NVU the following groups were formed:
Control - standard conditions of cultivation - N2-75%, O2-20%, CO2-5.
Hypoxia 1: N2-99 %, O2-1 %, CO2, t-37 С°.
Hypoxia 2: N2-98 %, O2-2 %, CO2, t-37 С°.
Hypoxia 3: N2-97 %, O2-3 %, CO2, t-37 С°.
Hypoxia 4: N2-96 %, O2-4 %, CO2, t-37 С°.
Number of repeats in serum incubation groups - 5.
Duration of hypoxia action - 30 minutes.
Temperature level - normal (37,0o С)
2.3. Evaluation of NVU functionality
To assess the effects of hypoxia and the serum on the cell culture we evaluate the next parameters: transendothelial resistance (TER) and permeability of endothelial layer in NVU model as the BBB functionality rate.
We registered TER level in cell model in vitro after 1, 2, 4 and 24 hours of cultivation. Direct measurement of transendothelial electric potential we made with epithelial voltmeter EVOM2 using the electrode STX2 (World Precision Instruments, USA).
The fluorescent dye Lucifer Yellow (LY) was added to the cultural environment in the final concentration 50мcМ to estimate permeability. After 60 and 90 minutes, the medium was collected from the lower compartment of the wells; the optical density of the solution was measured on the spectrofluorometer-spectrophotometer SM 2203 (“SOLAR”, Belarus). We estimated relative permeability according to the change in the LY concentration in experimental groups compared to the control group.
2.4. Statistical processing
Statistical data processing was made by using the BioStat Pro 5.9.8 program. The data are presented in the form of median (Me), upper (Q1) and lower quartiles (Q3) since most of the data has a distribution different from the normal one (Shapiro-Wilk criterion, p<0.05). Comparative analysis of quantitative variables was made using the Mann-Witney criterion. The differences at p<0.03 were considered as statistically significant.
3. Results
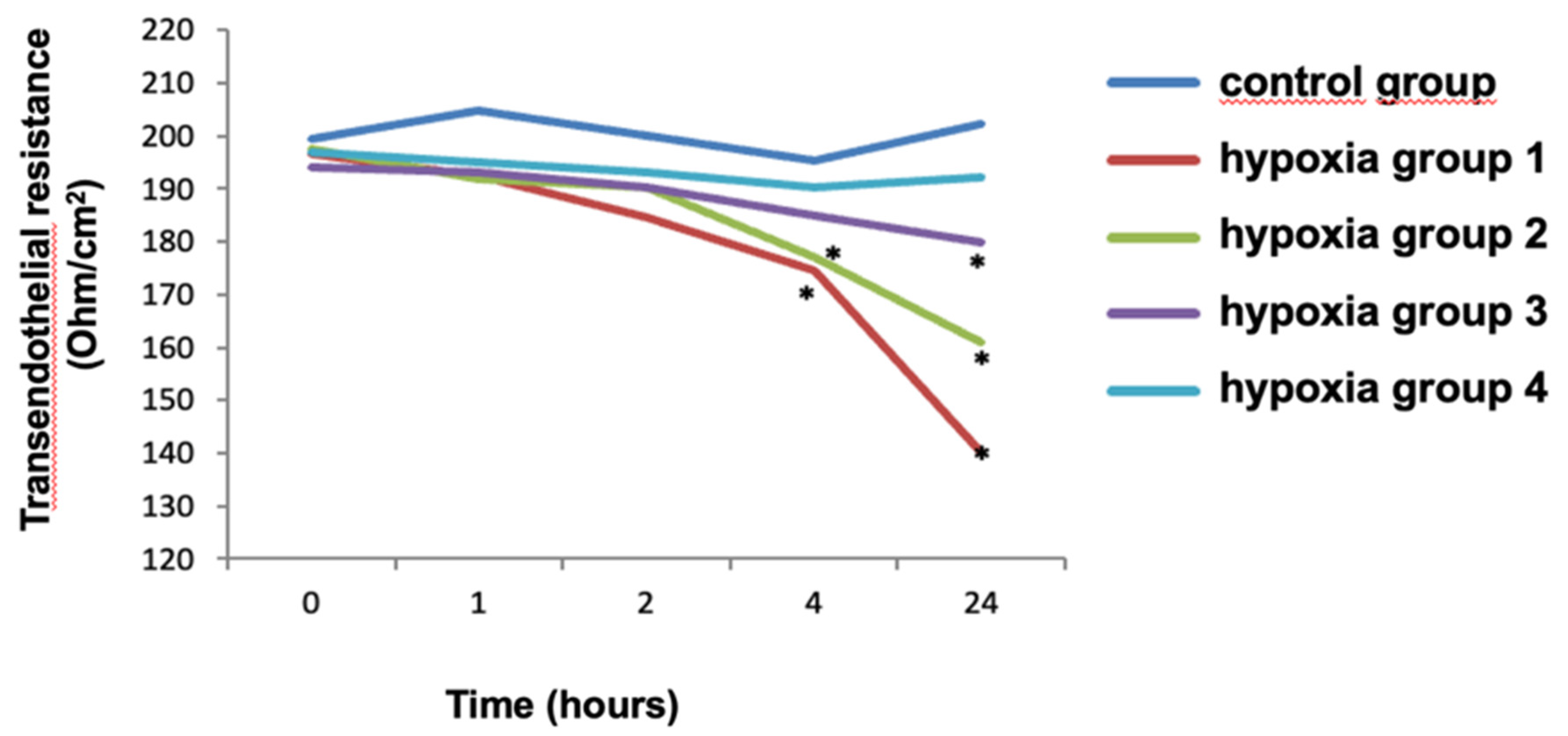
When we estimate hypoxia effects on the TER index, we learnt that statistically significant changes began in 4 hours after exposure. So, in Hypoxia 1 and Hypoxia 2 groups in 4 hours after exposure the TER indicator decreased by 30% in group 1, 20% in group 2 and 10% in group 3 compared with the control group (p<0,03).
Table 1.
TER values during cell incubation under hypoxic conditions.
Table 1.
TER values during cell incubation under hypoxic conditions.
| TER |
CG |
HG 1 |
HG 2 |
HG 3 |
HG 4 |
| 0 hours |
199,5
[197,25 − 201,50] |
197,0
[196,75 −
199,25]
(p=0,15325) |
197,5
[196,25 − 199,25]
(p=0,33156) |
192,5
[190,75 −
195,0]
(p=0,04071) |
195,0
[194,75 − 196,50]
(p=0,11689) |
| 1 hours |
204,25
[202,75 − 206,0] |
191,0
[189,75 −
193,0]
(p=0,03) |
191,0
[189,50 − 192,5]
(p=0,031) |
191,0
[189,75 − 193,25]
(p=0,033) |
193,0
[191,50 −
195,0]
(p=0,034) |
| 2 hours |
200,0
[196,75 − 203,50] |
185,0
[182,25 − 187,25]
(p=0,054) |
191,5
[188,75 − 194,75]
(p=0,04) |
192,0
[187,50 −
195,0]
(p=0,066) |
193,0
[190,75 − 194,50]
(p=0,04) |
| 4 hours |
194,5
[193 −
195,75] |
175,5
[174,5 − 176,75]
(p=0,01046) |
177,0
[175,75 − 178,25]
(p=0,01519) |
183,5
[182,0 − 185,5]
(p=0,03291) |
189,0
[187,5 −
191,25]
(p=0,14405) |
| 24 hours |
203,0
[200,75 −
205,5] |
141,0
[138,75 −
143,0]
(p=0,01046) |
162,0
[160,0 −
164,0]
(p=0,02092) |
179,5
[175,0 −
183,25]
(p=0,01519) |
192,0
[191,0 −
192,75]
(p=0,05314) |
Figure 1.
The effect of hypoxia on the transendothelial resistance index compared with the control group. Note. * − Statistical significance, p <0.03; Mann–Whitney U test.
Figure 1.
The effect of hypoxia on the transendothelial resistance index compared with the control group. Note. * − Statistical significance, p <0.03; Mann–Whitney U test.
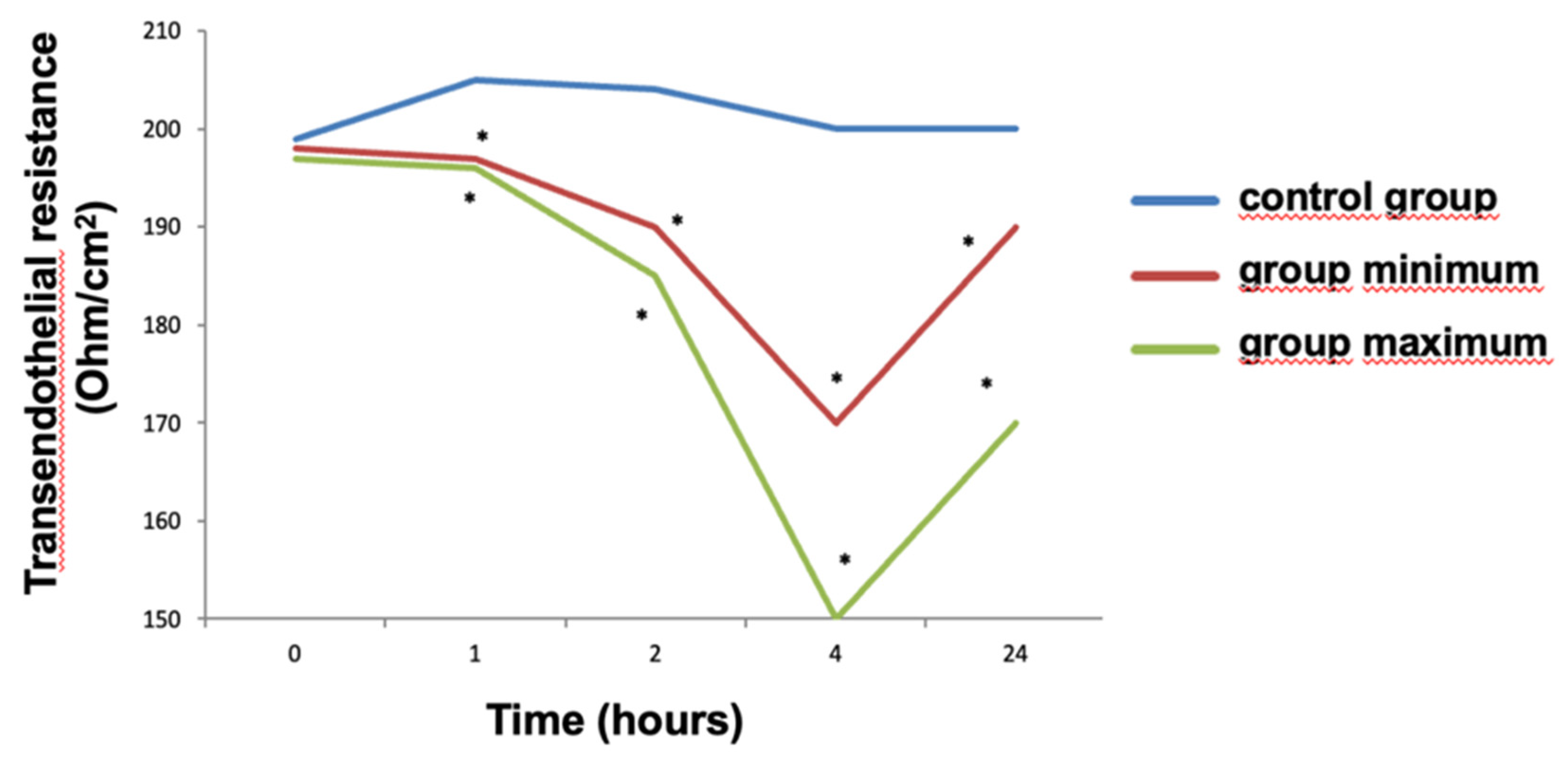
When we evaluated the effect of IL-6 on the transendothelial resistance index, we found that after 2 hours the TER level in the "Minimum" group significantly decreased by 5%, and in the "Maximum" group by 7.5% compared to the control (
Figure 2). In 4 hours after the effect, the TER indicator decreased, compared with the control, by 15% in the "Minimum" group and by 25% in the "Maximum" group. By 24 hours after exposure, the level of TER increased, but remained statistically significantly low in both the "Maximum" and "Minimum" groups. At the same time, there was a statistically significant difference in TER levels between the "Minimum" and "Maximum" groups at the following time points: 2 hours (p= 0.00640), 4 hours (p=0.00017), 24 hours (p= 0.00016).
Table 2.
TES indicators during incubation with blood serum.
Table 2.
TES indicators during incubation with blood serum.
| TER |
CG |
GMin |
GMax |
| 0 hours |
198,0
[197,0 − 200,0] |
199,0
[197,0 − 199,0]
(p=0,25) |
197,0
[195,0 − 198,0]
(p=0,073) |
| 1 hours |
206,0
[205,0 − 207,0] |
197,0
[196,0 − 198,0]
(p=0,00027) |
196,0
[196,0 − 197,0]
(p=0,00016) |
| 2 hours |
203,0
[202,0 − 204,0] |
190,0
[188,0 − 192,0]
(p= 0,00017) |
185,0
[185,0 − 188,0]
(p= 0,00016) |
| 4 hours |
200,0
[196,0 − 203,0] |
168,0
[168,0 − 170,0]
(p= 0,00016) |
151,0
[148,0 − 152,0]
(p= 0,00017) |
| 24 hours |
199,0
[198,0 − 202,0] |
190,0
[188,0 − 192,0]
(p= 0,00020) |
169,0
[169,0 − 172,0]
(p= 0,00031) |
Because the transendothelial resistance is an indicator for the endothelium functional condition in the blood-brain barrier, and the changes of TER were revealed, we explored the permeability for the LY dye.
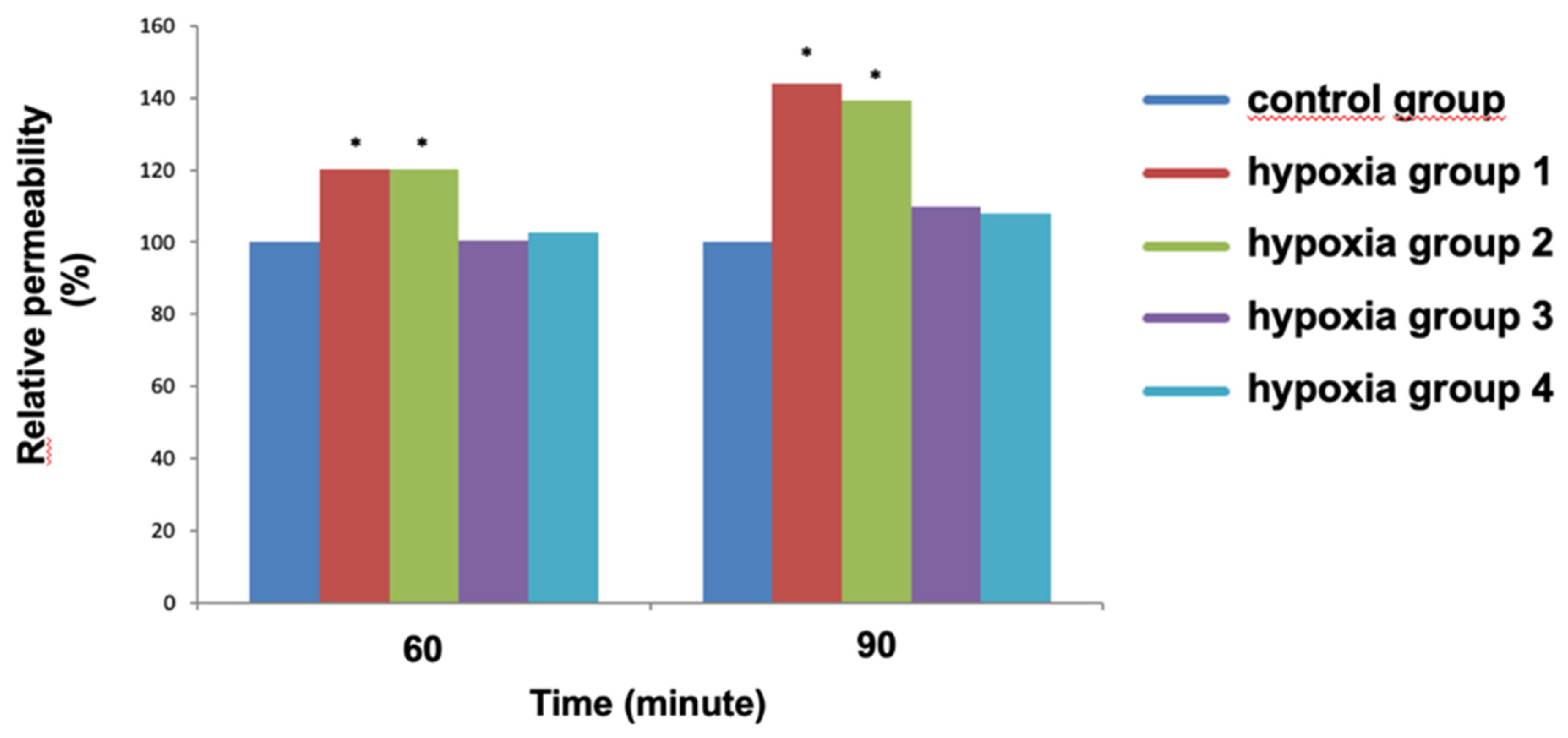
The effect of short-time hypoxia on the permeability to LY was registered after 60 minutes in the Hypoxia 1 and Hypoxia 2 groups compared to the control (
Figure 3). So, after 1 hour, the permeability increased by 20% in both groups and at the same time did not change statistically significant in the groups Hypoxia 3 and Hypoxia 4. In 90 minutes after exposure, permeability increased by 45% in the Hypoxia 1 group and by 40% in the Hypoxia 2 group compared to the control group. No statistically significant changes were found in groups Hypoxia 3 and Hypoxia 4.
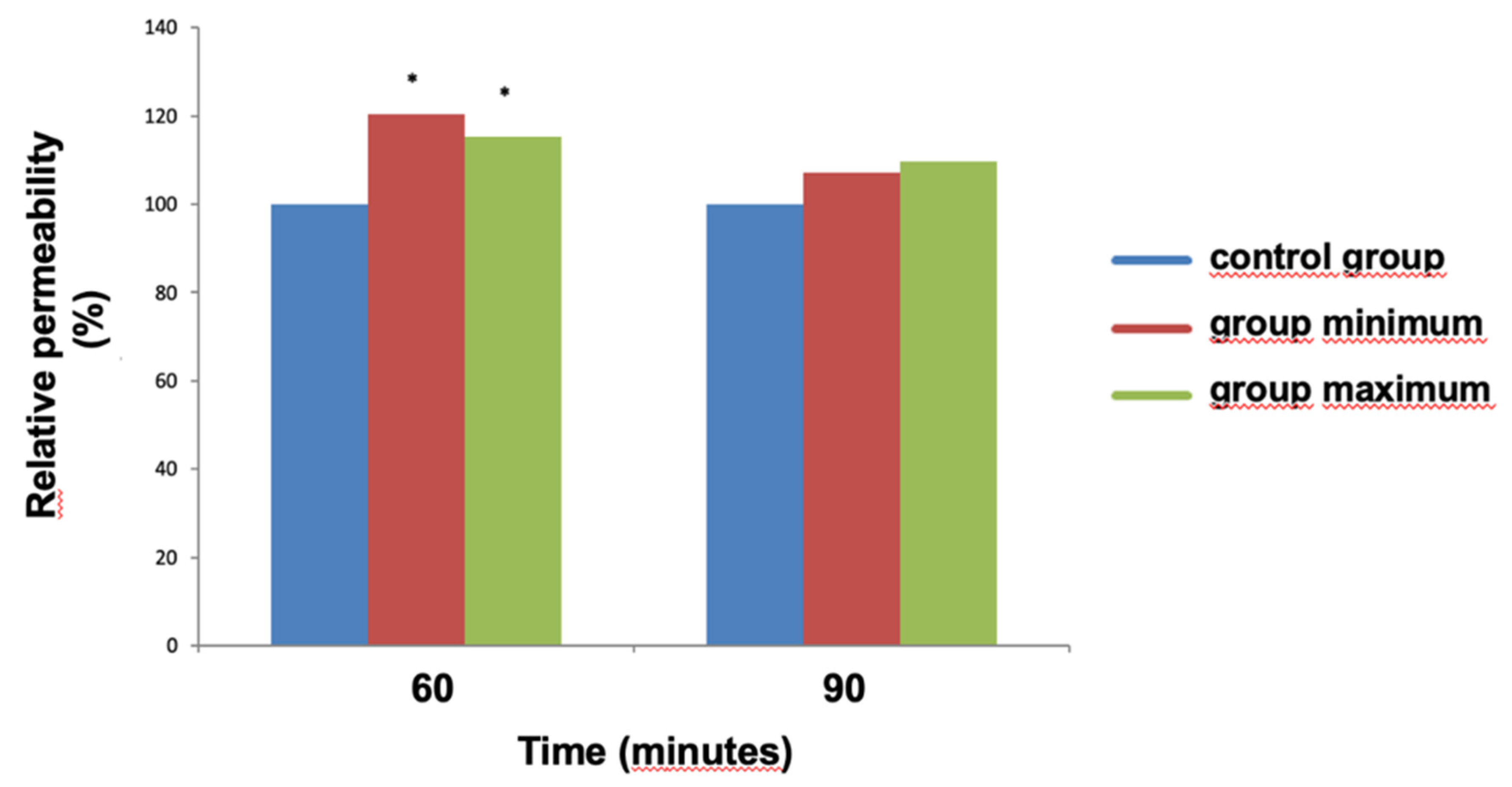
The effects of patient's blood serum on permeability are shown in
Figure 4. After 60 minutes of exposure, an increase in relative permeability by 15-20% was registered. It is interesting that after 90 minutes, the permeability in both experimental groups did not differ from the control group.
Table 3.
Indicators of relative permeability during cell incubation under hypoxic conditions.
Table 3.
Indicators of relative permeability during cell incubation under hypoxic conditions.
| TER |
CG |
HG 1 |
HG 2 |
HG 3 |
HG 4 |
| 60 minutes |
99,5
[98,0 − 101,5] |
121,0
[117,5 − 124,25] (p=0,01008) |
119,5
[118,5 − 120,75]
(p=0,0140 |
100,5
[99,5 − 101,25]
(p=0,44084) |
101,0
[100,75 − 102,5]
(p=0,37185) |
| 90 minutes |
99,5
[99,0 − 100,0] |
146,0
[143,75 − 148,0]
(p=0,00971) |
139,0
[137,5 − 141,0]
(p=0,01942) |
110,0
[107,75 − 112,0]
(p=0,03228) |
107,0
[106,0 − 109,0]
(p=0,05095) |
Table 4.
Relative permeability indices during incubation of cells with blood serum.
Table 4.
Relative permeability indices during incubation of cells with blood serum.
| TER |
CG |
GMin |
GMax |
| 60 minutes |
99,5
[98,75 − 100,5] |
119,5
[118,75 − 121,0]
(p=0,01046) |
114,5
[114,0 − 115,25]
(p=0,01470) |
| 90 minutes |
99,5,0
[99,0 − 100,5] |
106,5
[105,75 − 107,25]
(p=0,05314) |
109,5
[108,75 − 110,5]
(p=0,03291) |
4. Discussion
The effects of hypoxia on the NVU proved that even short-term hypoxia affects the TER level. The effects of the blood serum of patients also changes TES, while the changes compared to hypoxia are stronger and more rapid, but shorter.
We confirmed the suggestion of a rapid but short-term and possibly reversible effect of the serum also when we measured permeability for the LY dye. At the same time, exposure to minimal hypoxia with an oxygen content in the environment of 4% did not damage the NVU.
In our previous investigation, we showed SIRS and cerebral damage markers dynamics during cardiac surgery in children with same including criteria [
21].
In our previous research, we showed the SIRS and cerebral damage changings markers during the correction of CHD among children with similar inclusion criteria [
21].
These markers both SIRS and cerebral damage had their maximum levels right after the bypass was stopped phase with the followed sharp decrease in 16 hours after surgery, sometimes to their initial (preoperative) level.
Considering the fact that the effect of systemic inflammation was rapid and brief in the experiment with the NVU, we can say that during operations of this type the main influence on the brain was made by factors that initiate SIRS. One of these factors is the transfusion itself [
16]. At the same time, the fact that the cell culture did not change with minimal hypoxia proves the safety of our decision to refuse transfusion, as well as the safety of hemodilution that occurs during this. Many clinical cases proved that our initial suggestion was correct [22, 23].
The data we obtained correlate with the results of another experiment on rats, in which they studied the effect of cardiopulmonary bypass on them. As a result, they received a rapid increase in the concentration of inflammatory markers and impaired BBB permeability [
24]. It is worth noting that in this study, the value of BBB permeability reached a minimum in 3 days after the bypass, but in our study, we measured TER only in the first 24 hours. In addition, they found changes associated with hypoxia in the brains of rats. These changes appeared 2 hours after the bypass and continued for another 7 days slowly decreasing. Considering these data, in the future we plan to increase the time of observation of the NVU culture in order to collect the maximum amount of information. Another study conducted on pigs who underwent the bypass also confirmed the importance of diagnosing hypoxic brain damage. The authors of the study used magnetic resonance imaging and revealed the damages specific for brain hypoxia after a procedure of bypass lasted 3.5 hours [
25].
5. Conclusions
The study we performed on the NVU cellular model showed that 39-minutes hypoxia does not have a notable effect on brain cells until the minimum oxygen concentration is reached. When the oxygen level in the medium was 3% or less, significant changes in the functional state of NVU cells have occurred, and persisted 24 hours after exposure of hypoxia. Exposure of the cytokine-containing patient’s serum leads to the specific NVU damage: stronger, but shorter. Thus, a preliminary conclusion can be drawn: the strategy of avoiding intraoperative transfusion as a method of limiting SIRS and hemic hypoxia, as its consequence, is more likely to have a less destructive effect on the patient's brain.
At the same time, the use of transfusion, even considering the maintenance of a normal oxygen delivery and consumption in the brain can still lead to its damage due to the increased level of systemic inflammation.
Author Contributions
Conceptualization, A.A.I.; Methodology, A.A.I.; Validation, E.G.; Formal analysis, A.A.I and D.G.B.; Investigation, A.V.M..; Data curation, A.A.I.; Writing—original draft, A.A.I., E.G.; Project administration, E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the scientific grant of the Russian Science Foundation №. 22-15-00258 « Investigation of damage markers and methods of neurovascular unit protection in pediatric patients in cardiac surgery», https://rscf.ru/project/22-15-00258/.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Research Institute for Complex Issues of Cardiovascular Diseases (protocol code 20, 20, November 2018).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CHD |
Congenital heart defects |
| CPB |
cardiopulmonary bypass |
| NVU |
neurovascular unit |
| SIR |
Systemic Inflammatory Response |
| BBB |
blood-brain barrier |
| TER |
transendothelial resistance |
| LY |
Lucifer Yellow |
| SIRS |
Systemic Inflammatory Response Syndrome |
References
- Guenther U, Theuerkauf N, Frommann I, Brimmers K, Malik R, Stori, S., Scheidemann, M., Putensen, C., Popp, J. Predisposing and precipitating factors of delirium after cardiac surgery. A prospective observational cohort study. Annals of Surgery. 2013; 257: 1160–1167. [CrossRef]
- Hirata, Y. Cardiopulmonary bypass for pediatric cardiac surgery. General Thoracic and Cardiovascular Surgery. 2018; 66 (2): 65-70. [CrossRef]
- Engelman R, Baker RA, Likosky DS, Grigore A, Dickinson TA, Shore-Lesserson, L., Hammon JW. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: Clinical Practice Guidelines for Cardiopulmonary Bypass--Temperature Management During Cardiopulmonary Bypass. J Cardiothorac Vasc Anesth. 2015 Aug;29(4):1104-13. [CrossRef]
- Spilka JM, O'Halloran CP, Marino BS, Brady KM. Perspective on Cerebral Autoregulation Monitoring in Neonatal Cardiac Surgery Requiring Cardiopulmonary Bypass. Front Neurol. 2021 Oct 5;12:74018. [CrossRef]
- Torb. ett BE, Baird, A., Eliceiri BP. Understanding the rules of the road: Proteomic approaches to interrogate the blood brain barrier. Frontiers in Neuroscience. 2015; 4; (9): 70. [CrossRef]
- Brough, D., Denes, A. Interleukin-1α and brain inflammation. IUBMB Life. 2015 May;67(5):323-30. [CrossRef]
- Kaushal, V., Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. Journal of Neuroscience. 2008; 28 (9): 2221-2230. [CrossRef]
- Naruka V, Salmasi MY, Arjomandi Rad A, Marczin N, Lazopoulos G, Moscarelli M, Casula, R., Athanasiou, T. Use of Cytokine Filters During Cardiopulmonary Bypass: Systematic Review and Meta-Analysis. Heart Lung Circ. 2022 Nov;31(11):1493-1503. [CrossRef]
- Christov A, Ottman JT, Grammas, P. Vascular inflammatory, oxidative and protease-based processes: Implications for neuronal cell death in Alzheimer's disease. Neurological Research. 2004; 26 (5): 540-546. [CrossRef]
- Minami, M., Satoh, M. Chemokines and their receptors in the brain: Pathophysiological roles in ischemic brain injury. Life Sciences. 2003; 5; 74(2-3): 321-7. [CrossRef]
- Hansen TG. Anesthesia-related neurotoxicity and the developing animal brain is not a significant problem in children. Paediatric anaesthesia. 2015; 25(1): 65-72. [CrossRef]
- Jevtovic-Todorovic, V. General Anesthetics and Neurotoxicity: How Much Do We Know?. Anesthesiology clinics. 2016; 34(3): 439-451. [CrossRef]
- Alvarez RV, Palmer C, Czaja AS, Peyton C, Silver G, Traube, Mourani PM, Kaufman, J. Delirium is a Common and Early Finding in Patients in the Pediatric Cardiac Intensive Care Unit. The Journal of pediatrics. 2018; 195: 206-212. [CrossRef]
- Gunn JK, Beca J, Hunt RW, Goldsworthy M, Brizard CP, Finucane K, Donath S, Shekerdemian LS. Perioperative risk factors for impaired neurodevelopment after cardiac surgery in early infancy. Arch Dis Child. 2016 Nov;101(11):1010-1016. [CrossRef]
- Jufar AH, Lankadeva YR, May CN, Cochrane AD, Marino B, Bellomo R, Evans RG. Renal and Cerebral Hypoxia and Inflammation During Cardiopulmonary Bypass. Compr Physiol. 2021 Dec 29;12(1):2799-2834. [CrossRef]
- Ferraris VA, Ballert EQ, Mahan, A. The relationship between intraoperative blood transfusion and postoperative systemic inflammatory response syndrome. The American Journal of Surgery. 2013; 205 (4): 457-465. [CrossRef]
- Migeot C, Ma I, El Arid JM, Soulé N, Garnier E, Neville, P., Lefort, B. Factors associated with red blood cells transfusion during first bloodless priming cardiac surgery in children. Arch Pediatr. 2022 Jul;29(5):370-375. [CrossRef]
- Ivkin, A.A., Grigoriev, E.V., Morgun, A.V. Substantiation of protection of a neurovascular unit in the clinical model of cardiopulmonary bypass. Complex Issues of Cardiovascular Diseases. 2022;11(4): 177-183. [CrossRef]
- Jekarl DW, Lee SY, Lee J, Park YJ, Kim Y, Park JH, Wee JH, Choi SP. Procalcitonin as a diagnostic marker and IL-6 as a prognostic marker for sepsis. Diagn Microbiol Infect Dis. 2013 Apr;75(4):342-7. [CrossRef]
- Khilazheva, E.D., Boytsova, E.B., Pozhilenkova, E.A., Solonchuk Yu.R., Salmina, A.B. Obtaining a three-cell model of a neurovascular unit in vitro. Cell Tiss. Biol. 2015; 9. (6): 447-451. [CrossRef]
- Ivkin AA, Grigoriev, E., Sinitskaya AV. Refraining from Packed Red Blood Cells in Cardiopulmonary Bypass Priming as a Method of Neuroprotection in Pediatric Cardiac Surgery. J Clin Med. 2023 Feb 12;12(4):1465. [CrossRef]
- Hao, X., Wei, W. Severe low cerebral oximetry in difficult cardiopulmonary bypass weaning of low body-weight infant: A case report and literature review. BMC Anesthesiol. 2020 Jun 27;20(1):159. [CrossRef]
- Wloch A, Boettcher W, Sinzobahamvya N, Cho MY, Redlin M, Dähnert, I., Photiadis, J. Bloodless priming of the cardiopulmonary bypass circuit: Determinants of successful transfusion-free operation in neonates and infants with a maximum body weight of 7 kg. Cardiol Young. 2018 Oct;28(10):1141-1147. [CrossRef]
- Liu T, Deng R, Wang X, Liu P, Xiao QX, Liu Q, Zhang, Y. Mechanisms of hypoxia in the hippocampal CA3 region in postoperative cognitive dysfunction after cardiopulmonary bypass. J Cardiothorac Surg. 2022 May 7;17(1):106. [CrossRef]
- Mutch WA, Ryner LN, Kozlowski P, Scarth G, Warrian RK, Lefevre GR, Wong TG, Thiessen DB, Girling LG, Doiron L, McCudden C, Saunders JK. Cerebral hypoxia during cardiopulmonary bypass: A magnetic resonance imaging study. Ann Thorac Surg. 1997 Sep;64(3):695-701. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).