Submitted:
04 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Leishmaniasis and Chagas disease
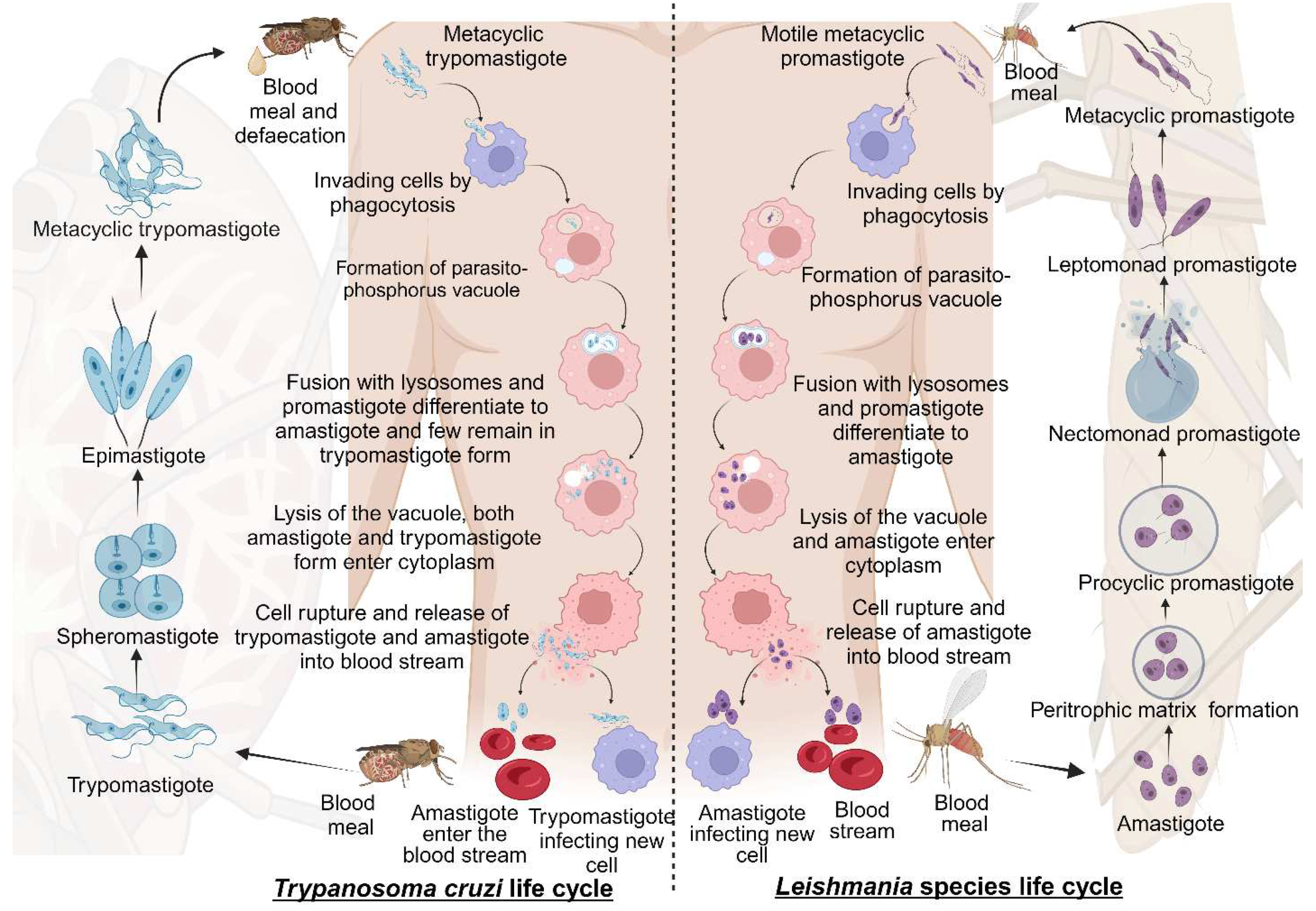
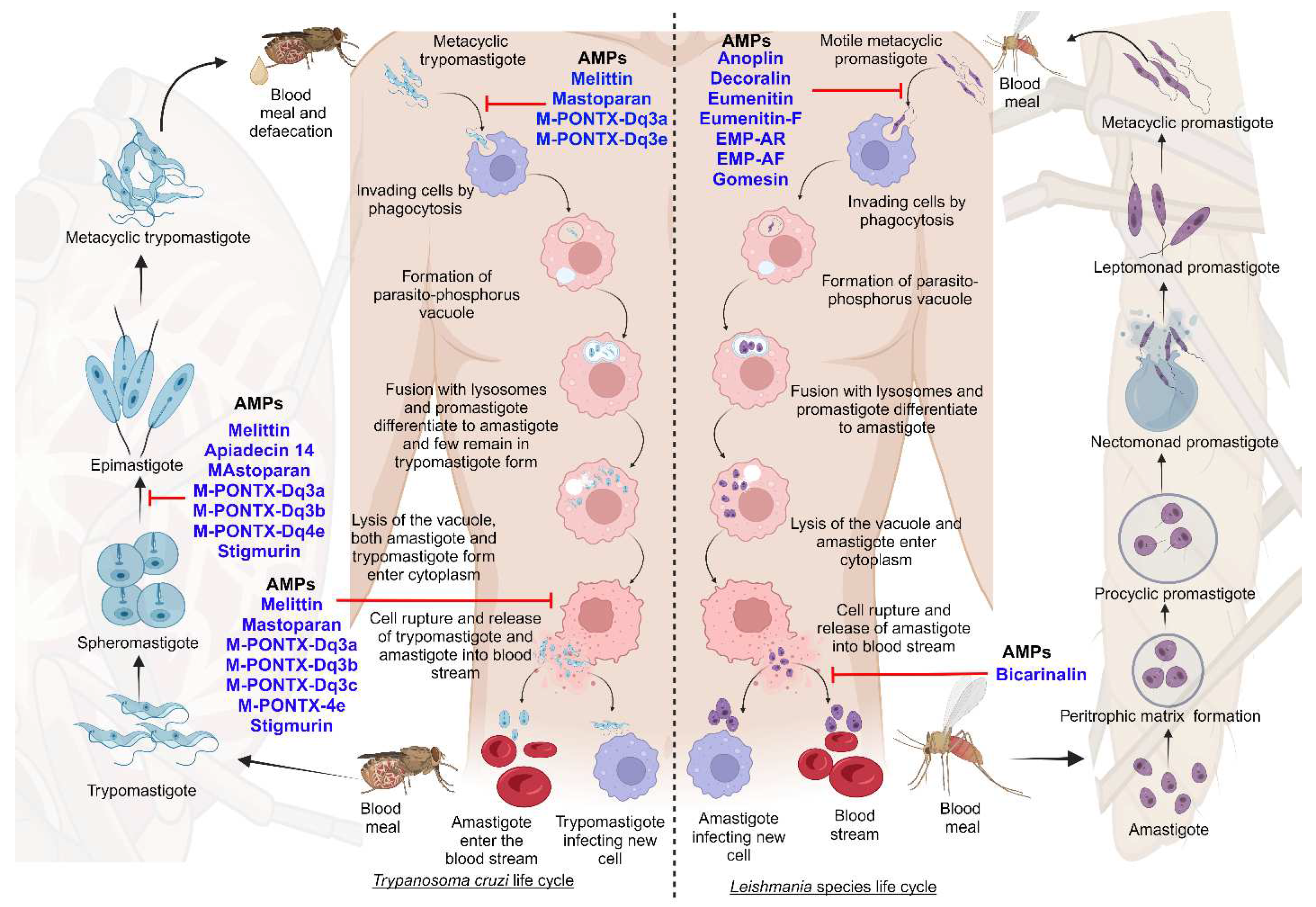
2.1. Life cycle of Trypanosomatida
2.1.1. Life cycle of Leishmania species
2.1.2. Life cycle of Trypanosoma cruzi
2.2. Epidemiology of Trypanosomatids
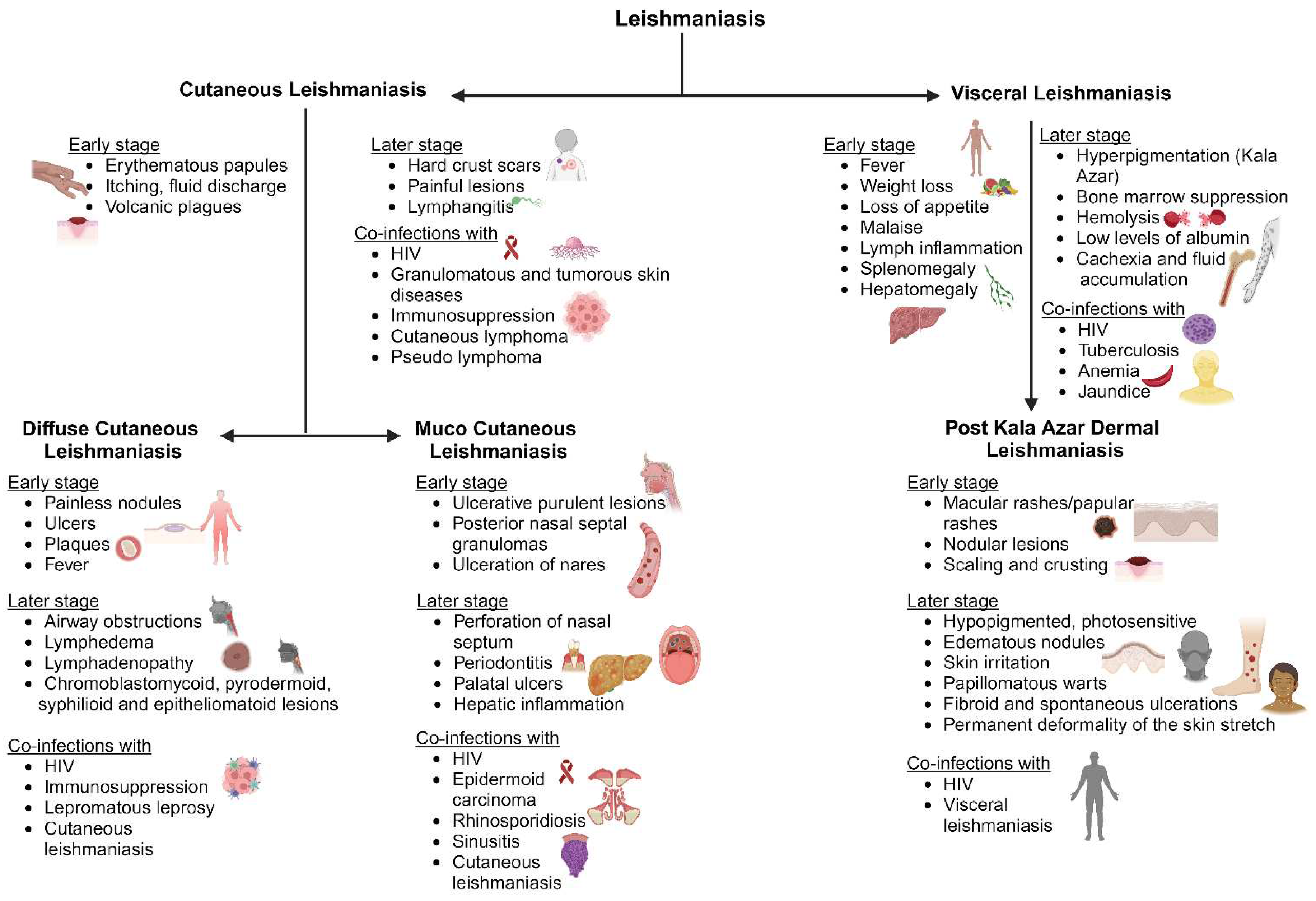
2.3. Clinical complications and conditions of leishmaniasis and Chagas disease
2.3.1. Leishmaniasis
2.3.1.1. Cutaneous leishmaniasis (CL)
2.3.1.2. Diffuse cutaneous leishmaniasis (DCL)
2.3.1.3. Mucocutaneous leishmaniasis (ML)
2.3.1.4. Visceral leishmaniasis (VL)
2.3.1.5. Post Kala Azar Dermal Leishmaniasis (PKDL)
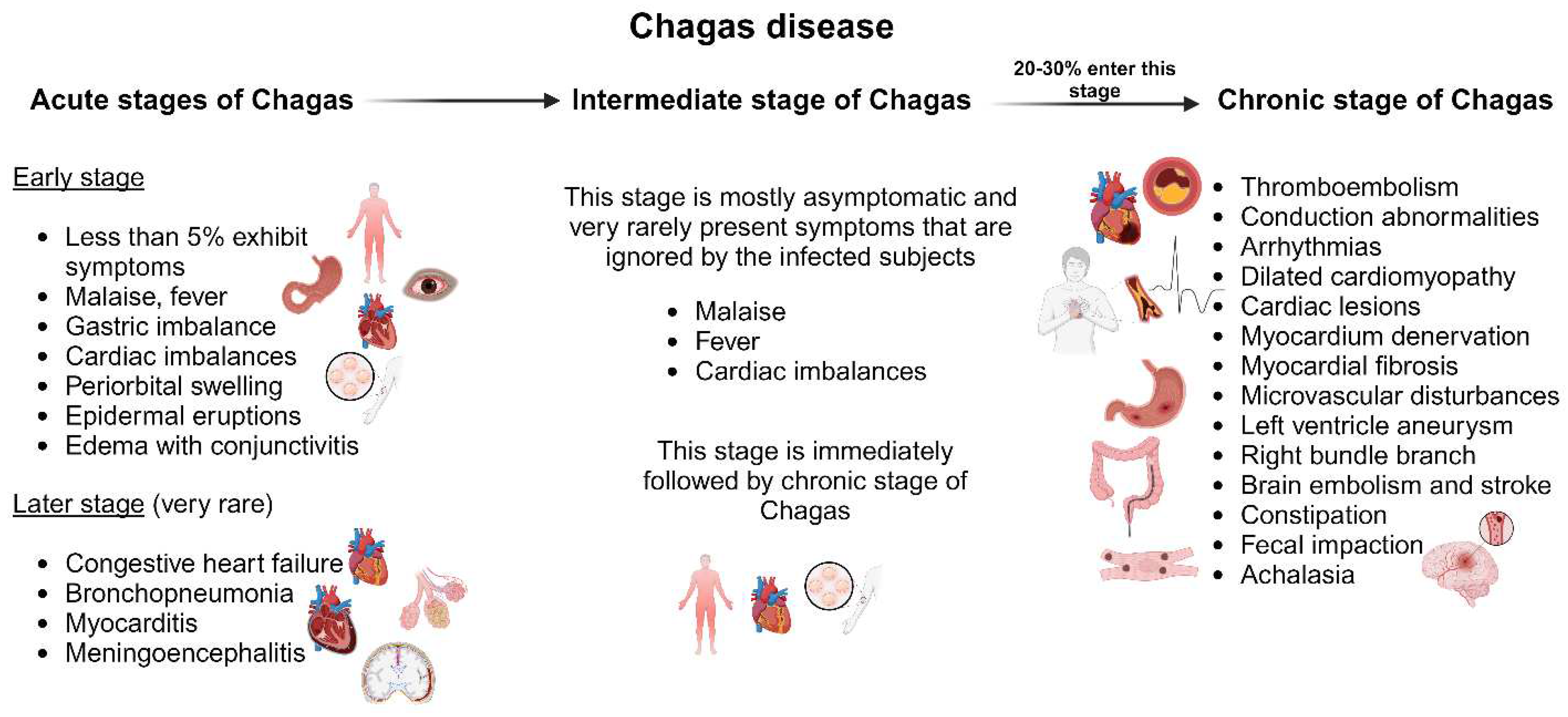
2.3.2. Chagas disease
2.3.2.1. Acute Chagas disease
2.3.2.2. Chronic Chagas disease
2.4. Pathways involved in leishmaniasis and Chagas disease
3. Drug discovery strategies and insights into current therapeutics
3.1. Approaches of drug development against leishmaniasis and Chagas disease
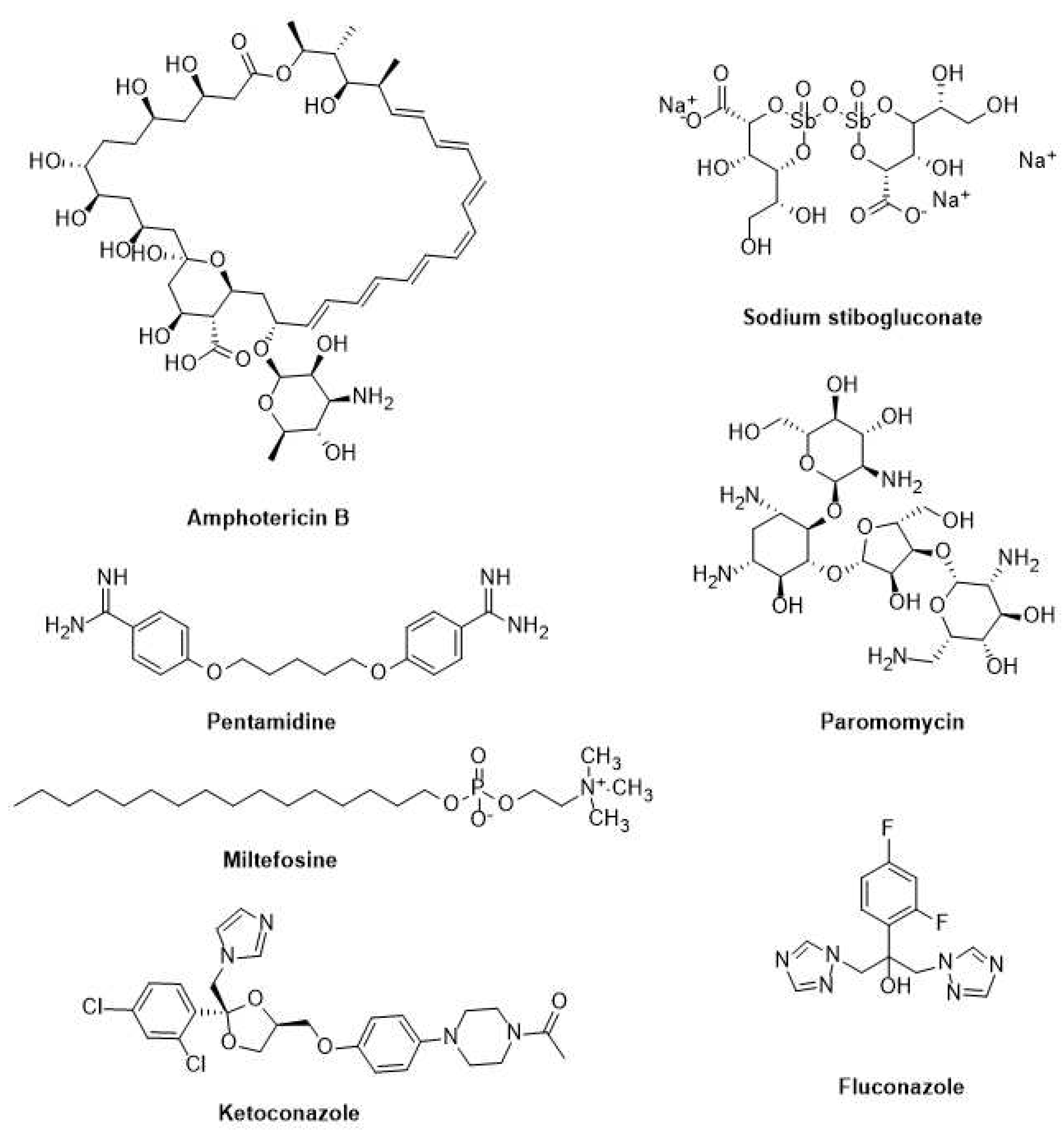
3.2. Current drugs for leishmaniasis and Chagas disease
3.2.1. Leishmaniasis
3.2.2. Chagas disease
4. Novel strategies for leishmaniasis and Chagas disease treatments
4.1. Host-directed therapy (HDT)
4.2. Multi-drug or combination therapy
4.3. Drug repurposing
4.4. Promising natural products
4.5. Nanotechnology
4.6. Nano vaccines
5. Peptide targeting for leishmaniasis and Chagas disease therapies
5.1. Peptide therapies
5.2. Anti-microbial peptides
5.2.1. Anti-microbial peptides against Chagas disease and leishmaniasis
5.3. Protein-protein interactions as drug targets in leishmaniasis and Chagas disease
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stuart, K.; Brun, R.; Croft, S.; Fairlamb, A.; Gürtler, R.E.; McKerrow, J.; Reed, S.; Tarleton, R. Kinetoplastids: related protozoan pathogens, different diseases. The Journal of clinical investigation 2008, 118, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Naula, C.; Parsons, M.; Mottram, J.C. Protein kinases as drug targets in trypanosomes and Leishmania. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics 2005, 1754, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Abadías-Granado, I.; Diago, A.; Cerro, P.; Palma-Ruiz, A.; Gilaberte, Y. Cutaneous and mucocutaneous leishmaniasis. Actas Dermo-Sifiliográficas (English Edition) 2021, 112, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kashif, M.; Srivastava, P.; Manna, P.P. Recent Advances in Chemotherapeutics for Leishmaniasis: Importance of the Cellular Biochemistry of the Parasite and Its Molecular Interaction with the Host. Pathogens 2023, 12, 706. [Google Scholar] [CrossRef]
- Assis, T.S.M.d.; Rosa, D.C.P.; Teixeira, E.d.M.; Cota, G.; Azeredo-da-Silva, A.L.F.; Werneck, G.; Rabello, A. The direct costs of treating human visceral leishmaniasis in Brazil. Revista da Sociedade Brasileira de Medicina Tropical 2017, 50, 478–482. [Google Scholar] [CrossRef]
- Alvar, J.; Arana, B. I. Appraisal of Leishmaniasis Chemotherapy, Current Status and Pipeline Strategies Chapter 1 Leishmaniasis, Impact and Therapeutic Needs 3. Drug Discovery for Leishmaniasis doi 2018, 10, 9781788010177–9781788000001. [Google Scholar]
- Rajao, M.A.; Furtado, C.; Alves, C.L.; Passos-Silva, D.G.; De Moura, M.B.; Schamber-Reis, B.L.; Kunrath-Lima, M.; Zuma, A.A.; Vieira-da-Rocha, J.P.; Garcia, J.B.F. Unveiling benznidazole's mechanism of action through overexpression of DNA repair proteins in Trypanosoma cruzi. Environmental and molecular mutagenesis 2014, 55, 309–321. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Khan, F.A.K.; Kulkarni, A.A.; Arote, R.; Patil, R.H. Antileishmanial drug discovery: Comprehensive review of the last 10 years. Rsc Advances 2015, 5, 32376–32415. [Google Scholar] [CrossRef]
- Chappuis, F.; Sundar, S.; Hailu, A.; Ghalib, H.; Rijal, S.; Peeling, R.W.; Alvar, J.; Boelaert, M. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nature reviews microbiology 2007, 5, 873–882. [Google Scholar] [CrossRef]
- Sundberg, S.A. High-throughput and ultra-high-throughput screening: solution-and cell-based approaches. Current opinion in biotechnology 2000, 11, 47–53. [Google Scholar] [CrossRef]
- Mayr, L.M.; Bojanic, D. Novel trends in high-throughput screening. Current opinion in pharmacology 2009, 9, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Canan, S.; Cao, Y.; Condroski, K.; Engkvist, O.; Itono, S.; Kaki, R.; Kimura, C.; Kogej, T.; Nagaoka, K. Collaborative virtual screening to elaborate an imidazo [1, 2-a] pyridine hit series for visceral leishmaniasis. RSC medicinal chemistry 2021, 12, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Nalini, S.; Lampa, S.; Saw, S.; Gleeson, M.P.; Ola, S.; Chanin, N. Towards reproducible computational drug discovery. Journal of Cheminformatics 2020, 12. [Google Scholar]
- Chatelain, E.; Ioset, J.-R. Phenotypic screening approaches for Chagas disease drug discovery. Expert opinion on drug discovery 2018, 13, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Zahedifard, F.; Rafati, S. Prospects for antimicrobial peptide-based immunotherapy approaches in Leishmania control. Expert review of anti-infective therapy 2018, 16, 461–469. [Google Scholar] [CrossRef]
- Rafferty, J.; Nagaraj, H. P McCloskey A, Huwaitat R, Porter S, Albadr A, et al. Peptide therapeutics and the pharmaceutical industry: barriers encountered translating from the laboratory to patients. Curr Med Chem 2016, 23, 4231–4259. [Google Scholar] [CrossRef]
- Díaz-Garrido, P.; Cárdenas-Guerra, R.E.; Martínez, I.; Poggio, S.; Rodríguez-Hernández, K.; Rivera-Santiago, L.; Ortega-López, J.; Sánchez-Esquivel, S.; Espinoza, B. Differential activity on trypanosomatid parasites of a novel recombinant defensin type 1 from the insect Triatoma (Meccus) pallidipennis. Insect Biochemistry and Molecular Biology 2021, 139, 103673. [Google Scholar] [CrossRef]
- Dostálová, A.; Volf, P. Leishmania development in sand flies: parasite-vector interactions overview. Parasites & vectors 2012, 5, 1–12. [Google Scholar]
- Killick-Kendrick, R. The biology and control of phlebotomine sand flies. Clinics in dermatology 1999, 17, 279–289. [Google Scholar] [CrossRef]
- Teixeira, D.E.; Benchimol, M.; Rodrigues, J.C.; Crepaldi, P.H.; Pimenta, P.F.; de Souza, W. The cell biology of Leishmania: how to teach using animations. PLoS pathogens 2013, 9, e1003594. [Google Scholar] [CrossRef] [PubMed]
- Sunter, J.; Gull, K. Shape, form, function and Leishmania pathogenicity: from textbook descriptions to biological understanding. Open biology 2017, 7, 170165. [Google Scholar] [CrossRef]
- Saada, E.A.; Kabututu, Z.P.; Lopez, M.; Shimogawa, M.M.; Langousis, G.; Oberholzer, M.; Riestra, A.; Jonsson, Z.O.; Wohlschlegel, J.A.; Hill, K.L. Insect stage-specific receptor adenylate cyclases are localized to distinct subdomains of the Trypanosoma brucei flagellar membrane. Eukaryotic cell 2014, 13, 1064–1076. [Google Scholar] [CrossRef]
- Secundino, N.; Eger-Mangrich, I.; Braga, E.; Santoro, M.; Pimenta, P. Lutzomyia longipalpis peritrophic matrix: formation, structure, and chemical composition. Journal of medical entomology 2005, 42, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.E.; Chance, M.; Bates, P. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitology 2002, 124, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Gossage, S.M.; Rogers, M.E.; Bates, P.A. Two separate growth phases during the development of Leishmania in sand flies: implications for understanding the life cycle. International journal for parasitology 2003, 33, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.A. Revising Leishmania’s life cycle. Nature microbiology 2018, 3, 529–530. [Google Scholar] [CrossRef]
- Martín-Escolano, J.; Marín, C.; Rosales, M.J.; Tsaousis, A.D.; Medina-Carmona, E.; Martín-Escolano, R. An updated view of the trypanosoma cruzi life cycle: Intervention points for an effective treatment. ACS Infectious Diseases 2022, 8, 1107–1115. [Google Scholar] [CrossRef]
- Onyekwelu, K.C. Life Cycle of Trypanosoma cruzi in the Invertebrate and the Vertebrate Hosts. Biology of Trypanosoma cruzi 2019, 1–19. [Google Scholar]
- Rodríguez-Bejarano, O.H.; Avendaño, C.; Patarroyo, M.A. Mechanisms associated with Trypanosoma cruzi host target cell adhesion, recognition and internalization. Life 2021, 11, 534. [Google Scholar] [CrossRef]
- Teixeira, D.E.; Benchimol, M.; Crepaldi, P.H.; de Souza, W. Interactive multimedia to teach the life cycle of Trypanosoma cruzi, the causative agent of Chagas disease. 2012.
- Andreoli, W.; Taniwaki, N.; Mortara, R. Survival of Trypanosoma cruzi metacyclic trypomastigotes within Coxiella burnetii vacuoles: differentiation and replication within an acidic milieu. Microbes and infection 2006, 8, 172–182. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. The EMBO journal 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.; Albuquerque-Cunha, J.M.; Mello, C.; Garcia, E.d.S.; Nogueira, N.; Bourguingnon, S.C.; De Souza, W.; Azambuja, P.; Gonzalez, M.S. Trypanosoma cruzi: attachment to perimicrovillar membrane glycoproteins of Rhodnius prolixus. Experimental Parasitology 2007, 116, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.S.; Ratcliffe, N.A.; Whitten, M.M.; Gonzalez, M.S.; Azambuja, P. Exploring the role of insect host factors in the dynamics of Trypanosoma cruzi–Rhodnius prolixus interactions. Journal of Insect Physiology 2007, 53, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Roldan, J.A.; Votýpka, J.; Bandi, C.; Epis, S.; Modrý, D.; Tichá, L.; Volf, P.; Otranto, D. Leishmania tarentolae: A new frontier in the epidemiology and control of the leishmaniases. Transboundary and Emerging Diseases 2022, 69, e1326–e1337. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Quintella, L.P.; Schubach, A.d.O.; Miranda, L.d.F.C.; Madeira, M.d.F.; Pimentel, M.I.F.; Vasconcellos, É.d.C.F.e.; Lyra, M.R.; Oliveira, R.d.V.C.d.; Menezes, R.C. Comparison between Colorimetric In Situ Hybridization, Histopathology, and Immunohistochemistry for the Diagnosis of New World Cutaneous Leishmaniasis in Human Skin Samples. Tropical Medicine and Infectious Disease 2022, 7, 344. [Google Scholar] [CrossRef]
- Hadermann, A.; Heeren, S.; Maes, I.; Dujardin, J.-C.; Domagalska, M.A.; Van den Broeck, F. Genome diversity of Leishmania aethiopica. Frontiers in Cellular and Infection Microbiology 2023, 13, 406. [Google Scholar] [CrossRef]
- Bezemer, J.M.; Freire-Paspuel, B.P.; Schallig, H.D.; de Vries, H.J.; Calvopiña, M. Leishmania species and clinical characteristics of Pacific and Amazon cutaneous leishmaniasis in Ecuador and determinants of health-seeking delay: a cross-sectional study. BMC Infectious Diseases 2023, 23, 395. [Google Scholar] [CrossRef]
- Preativatanyou, K.; Chinwirunsirisup, K.; Phumee, A.; Khositharattanakool, P.; Sunantaraporn, S.; Depaquit, J.; Siriyasatien, P. Species diversity of phlebotomine sand flies and sympatric occurrence of Leishmania (Mundinia) martiniquensis, Leishmania (Leishmania) donovani complex, and Trypanosoma spp. in the visceral leishmaniasis focus of southern Thailand. Acta Tropica 2023, 244, 106949. [Google Scholar] [CrossRef]
- Dinç, M.; Yalçın, T.; Çavuş, İ.; Özbilgin, A. Comparative proteomic analysis of Leishmania parasites isolated from visceral and cutaneous leishmaniasis patients. Parasitology 2022, 149, 298–305. [Google Scholar] [CrossRef]
- Gramiccia, M.; Gradoni, L. The current status of zoonotic leishmaniases and approaches to disease control. International journal for parasitology 2005, 35, 1169–1180. [Google Scholar] [CrossRef]
- Schriefer, A.; Guimarães, L.H.; Machado, P.R.; Lessa, M.; Lessa, H.A.; Lago, E.; Ritt, G.; Góes-Neto, A.; Schriefer, A.L.; Riley, L.W. Geographic clustering of leishmaniasis in northeastern Brazil. Emerging infectious diseases 2009, 15, 871. [Google Scholar] [CrossRef]
- Montalvo Alvarez, A.M.; Nodarse, J.F.; Goodridge, I.M.; Fidalgo, L.M.; Marin, M.; Van Der Auwera, G.; Dujardin, J.-C.; Velez Bernal, I.D.; Muskus, C. Differentiation of Leishmania (Viannia) panamensis and Leishmania (V.) guyanensis using Bcc I for hsp 70 PCR-RFLP. Transactions of the Royal Society of Tropical Medicine and Hygiene 2010, 104, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, O.; Simon, S.; Ginouves, M.; Prévot, G.; Couppie, P.; Demar, M.; Blaizot, R. Leishmania naiffi and lainsoni in French Guiana: Clinical features and phylogenetic variability. PLoS Neglected Tropical Diseases 2020, 14, e0008380. [Google Scholar] [CrossRef]
- Espada, C.R.; Ferreira, B.A.; Ortiz, P.A.; Uliana, S.R.; Coelho, A.C. Full nucleotide sequencing of ribosomal DNA internal transcribed spacer of Leishmania species causing cutaneous leishmaniasis in Brazil and its potential for species typing. Acta Tropica 2021, 223, 106093. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Morales, D.; Rodríguez, A.L.; Silva-Caso, W.; Suarez-Ognio, L.; Pons, M.J.; del Valle Mendoza, J. An atypical case of disseminated cutaneous leishmaniasis due to Leishmania peruviana in the valleys of Ancash-Peru. Asian Pacific journal of tropical medicine 2017, 10, 1101–1103. [Google Scholar] [CrossRef]
- Passero, L.F.D.; Carvalho, A.K.; Bordon, M.L.; Bonfim-Melo, A.; Carvalho, K.; Kallás, E.G.; Santos, B.B.; Toyama, M.H.; Paes-Leme, A.; Corbett, C.E. Proteins of Leishmania (Viannia) shawi confer protection associated with Th1 immune response and memory generation. Parasites & vectors 2012, 5, 1–10. [Google Scholar]
- Sukhumavasi, W.; Kaewamatawong, T.; Somboonpoonpol, N.; Jiratanh, M.; Wattanamethanont, J.; Kaewthamasorn, M.; Leelayoova, S.; Tiwananthagorn, S. Liver-and spleen-specific immune responses in experimental leishmania martiniquensis infection in BALB/c mice. Frontiers in Veterinary Science 2021, 8, 794024. [Google Scholar] [CrossRef]
- Supsrisunjai, C.; Kootiratrakarn, T.; Puangpet, P.; Bunnag, T.; Chaowalit, P.; Wessagowit, V. Case report: Disseminated autochthonous dermal leishmaniasis caused by Leishmania siamensis (PCM2 Trang) in a patient from central Thailand infected with human immunodeficiency virus. The American journal of tropical medicine and hygiene 2017, 96, 1160. [Google Scholar]
- Ramírez, J.D.; Hernández, C.; León, C.M.; Ayala, M.S.; Flórez, C.; González, C. Taxonomy, diversity, temporal and geographical distribution of Cutaneous Leishmaniasis in Colombia: A retrospective study. Scientific reports 2016, 6, 28266. [Google Scholar] [CrossRef]
- Franco, J.R.; Simarro, P.P.; Diarra, A.; Jannin, J.G. Epidemiology of human African trypanosomiasis. Clinical epidemiology 2014, 257–275. [Google Scholar]
- Schmunis, G.A.; Yadon, Z.E. Chagas disease: a Latin American health problem becoming a world health problem. Acta tropica 2010, 115, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kourbeli, V.; Chontzopoulou, E.; Moschovou, K.; Pavlos, D.; Mavromoustakos, T.; Papanastasiou, I.P. An overview on target-based drug design against kinetoplastid protozoan infections: Human African trypanosomiasis, Chagas disease and leishmaniases. Molecules 2021, 26, 4629. [Google Scholar] [CrossRef]
- Bilgic-Temel, A.; Murrell, D.F.; Uzun, S. Cutaneous leishmaniasis: a neglected disfiguring disease for women. International journal of women's dermatology 2019, 5, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kaluarachchi, T.J.; Campbell, P.M.; Wickremasinghe, R.; Ranasinghe, S.; Yasewardene, S.; De Silva, H.; McBain, A.J.; Weerasekera, M. Possible clinical implications and future directions of managing bacterial biofilms in cutaneous leishmaniasis wounds. Tropical Medicine and Health 2022, 50, 58. [Google Scholar] [CrossRef]
- Martínez, D.Y.; Verdonck, K.; Kaye, P.M.; Adaui, V.; Polman, K.; Llanos-Cuentas, A.; Dujardin, J.-C.; Boelaert, M. Tegumentary leishmaniasis and coinfections other than HIV. PLoS neglected tropical diseases 2018, 12, e0006125. [Google Scholar] [CrossRef]
- Meireles, C.B.; Maia, L.C.; Soares, G.C.; Teodoro, I.P.P.; Gadelha, M.d.S.V.; da Silva, C.G.L.; de Lima, M.A.P. Atypical presentations of cutaneous leishmaniasis: a systematic review. Acta Tropica 2017, 172, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: a review. F1000Research 2017, 6. [Google Scholar] [CrossRef]
- Sinha, S.; Fernández, G.; Kapila, R.; Lambert, W.C.; Schwartz, R.A. Diffuse cutaneous leishmaniasis associated with the immune reconstitution inflammatory syndrome. International journal of dermatology 2008, 47, 1263–1270. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Gomez, E.L.; Kato, H.; Martini, L.R.; Velez, L.N.; Uezato, H. Diffuse and disseminated cutaneous leishmaniasis: clinical cases experienced in Ecuador and a brief review. Tropical medicine and health 2016, 44, 1–9. [Google Scholar] [CrossRef]
- Goihman-Yahr, M. American mucocutaneous leishmaniasis. Dermatologic clinics 1994, 12, 703–712. [Google Scholar] [CrossRef]
- Vera-Izaguirre, D.S.; Vega-Memije, E.; Quintanilla-Cedillo, M.R.; Arenas, R. Leishmaniasis. A review. Dermatología Cosmética, Médica Y Quirúrgica 2006, 4, 252–260. [Google Scholar]
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in leishmaniasis. The Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- CONVIT, J.; REYES, O.; KERDEL, F. Disseminated Anergic American Leishmaniasis: Report of Three Cases of a Type Clinically Resembling Lepromatous Leprosy. AMA archives of dermatology 1957, 76, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.G.; Bilimoria, F.E.; Katare, S. Diffuse cutaneous leishmaniasis: co-infection with human immunodeficiency virus (HIV). Indian journal of dermatology, venereology and leprology 2008, 74, 641. [Google Scholar] [CrossRef]
- David, C.V.; Craft, N. Cutaneous and mucocutaneous leishmaniasis. Dermatologic therapy 2009, 22, 491–502. [Google Scholar] [CrossRef]
- Garrido-Jareño, M.; Sahuquillo-Torralba, A.; Chouman-Arcas, R.; Castro-Hernández, I.; Molina-Moreno, J.M.; Llavador-Ros, M.; Gómez-Ruiz, M.D.; López-Hontangas, J.L.; Botella-Estrada, R.; Salavert-Lleti, M. Cutaneous and mucocutaneous leishmaniasis: experience of a Mediterranean hospital. Parasites & vectors 2020, 13, 1–7. [Google Scholar]
- Ahluwalia, S.; Lawn, S.D.; Kanagalingam, J.; Grant, H.; Lockwood, D.N. Mucocutaneous leishmaniasis: an imported infection among travellers to central and South America. Bmj 2004, 329, 842–844. [Google Scholar] [CrossRef]
- Marra, F.; Chiappetta, M.C.; Vincenti, V. Ear, nose and throat manifestations of mucocutaneous Leishmaniasis: a literature review. Acta Biomed 2014, 85, 3–7. [Google Scholar]
- Bern, C. Visceral leishmaniasis: clinical manifestations and diagnosis. U: UpToDate, Post TW ur. UpToDate [Internet]. Waltham, MA: UpToDate, 2021. [Google Scholar]
- Baba, C.S.; Makharia, G.K.; Mathur, P.; Ray, R.; Gupta, S.D.; Samantaray, J. Chronic diarrhea and malabsorption caused by Leishmania donovani. Indian Journal of Gastroenterology 2006, 25, 309. [Google Scholar]
- Boukhris, I.; Azzabi, S.; Chérif, E.; Kéchaou, I.; Mahjoub, S.; Kooli, C.; Aoun, K.; Khalfallah, N. Hemophagocytosis and disseminated intravascular coagulation in visceral leishmaniasis in adults: three new cases. The Pan African Medical Journal 2015, 22, 96–96. [Google Scholar]
- Pagliano, P.; Carannante, N.; Rossi, M.; Gramiccia, M.; Gradoni, L.; Faella, F.S.; Gaeta, G.B. Visceral leishmaniasis in pregnancy: a case series and a systematic review of the literature. Journal of Antimicrobial Chemotherapy 2005, 55, 229–233. [Google Scholar] [CrossRef]
- Herrero, M.; Orfanos, G.; Argaw, D.; Mulugeta, A.; Aparicio, P.; Parreño, F.; Bernal, O.; Rubens, D.; Pedraza, J.; Lima, M.A. Natural history of a visceral leishmaniasis outbreak in highland Ethiopia. The American journal of tropical medicine and hygiene 2009, 81, 373–377. [Google Scholar] [CrossRef]
- Mueller, Y.; Mbulamberi, D.B.; Odermatt, P.; Hoffmann, A.; Loutan, L.; Chappuis, F. Risk factors for in-hospital mortality of visceral leishmaniasis patients in eastern Uganda. Tropical Medicine & International Health 2009, 14, 910–917. [Google Scholar]
- Zijlstra, E.E. Biomarkers in post-kala-azar dermal leishmaniasis. Frontiers in cellular and infection microbiology 2019, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, E.E. The immunology of post-kala-azar dermal leishmaniasis (PKDL). Parasites & vectors 2016, 9, 1–9. [Google Scholar]
- Zijlstra, E.; Musa, A.; Khalil, E.; El Hassan, I.; El-Hassan, A. Post-kala-azar dermal leishmaniasis. The Lancet infectious diseases 2003, 3, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Chatterjee, M.; Das, N.K. Post kala-azar dermal leishmaniasis: Clinical features and differential diagnosis. Indian Journal of Dermatology 2021, 66, 24. [Google Scholar]
- Suárez, C.; Nolder, D.; García-Mingo, A.; Moore, D.A.; Chiodini, P.L. Diagnosis and clinical management of Chagas disease: An increasing challenge in non-endemic areas. Research and Reports in Tropical Medicine 2022, 25–40. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Dones, W.; Morillo, C.A.; Encina, J.J.; Ribeiro, A.L.; Cardiology, C.o.C.D.o.t.I.S.o. Chagas disease: an overview of clinical and epidemiological aspects. Journal of the American College of Cardiology 2013, 62, 767–776. [Google Scholar] [CrossRef]
- Hemmige, V.; Tanowitz, H.; Sethi, A. Trypanosoma cruzi infection: a review with emphasis on cutaneous manifestations. International journal of dermatology 2012, 51, 501–508. [Google Scholar] [CrossRef]
- Teixeira, A.; Nitz, N.; Guimaro, M.; Gomes, C.; Santos-Buch, C. Chagas disease. Postgraduate medical journal 2006, 82, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. Control of Chagas disease: second report of the WHO expert committee; World Health Organization: 2002; Vol. 2.
- Malik, L.H.; Singh, G.D.; Amsterdam, E.A. The epidemiology, clinical manifestations, and management of chagas heart disease. Clinical cardiology 2015, 38, 565–569. [Google Scholar] [CrossRef] [PubMed]
- de Lourdes Higuchi, M.; Fukasawa, S.; De Brito, T.; Parzianello, L.C.; Bellotti, G.; Ramires, J.A.F. Different microcirculatory and interstitial matrix patterns in idiopathic dilated cardiomyopathy and Chagas’ disease: a three dimensional confocal microscopy study. Heart 1999, 82, 279–285. [Google Scholar] [CrossRef]
- Benvenuti, L.; Roggério, A.; Freitas, H.; Mansur, A.; Fiorelli, A.; Higuchi, M. Chronic American trypanosomiasis: parasite persistence in endomyocardial biopsies is associated with high-grade myocarditis. Annals of Tropical Medicine & Parasitology 2008, 102, 481–487. [Google Scholar]
- Marin-Neto, J.A.; Cunha-Neto, E.; Maciel, B.C.; Simões, M.V. Pathogenesis of chronic Chagas heart disease. Circulation 2007, 115, 1109–1123. [Google Scholar] [CrossRef]
- Rossi, M.A.; Tanowitz, H.B.; Malvestio, L.M.; Celes, M.R.; Campos, E.C.; Blefari, V.; Prado, C.M. Coronary microvascular disease in chronic Chagas cardiomyopathy including an overview on history, pathology, and other proposed pathogenic mechanisms. PLoS neglected tropical diseases 2010, 4, e674. [Google Scholar] [CrossRef]
- Rocha, M.O.C.; Nunes, M.C.P.; Ribeiro, A.L. Morbidity and prognostic factors in chronic chagasic cardiopathy. Memórias do Instituto Oswaldo Cruz 2009, 104, 159–166. [Google Scholar] [CrossRef]
- Rassi Jr, A.; Rassi, S.G.; Rassi, A. Sudden death in Chagas' disease. Arquivos brasileiros de cardiologia 2001, 76, 86–96. [Google Scholar] [CrossRef]
- Nunes, M.; Dones, W.; Morillo, C.; Encina, J.; Ribeiro, A. Council on Chagas disease of the InterAmerican Society of Cardiology. J Am Coll Cardiol 2013, 62, 767–776. [Google Scholar] [CrossRef]
- Tripodi, K.E.; Menendez Bravo, S.M.; Cricco, J.A. Role of heme and heme-proteins in trypanosomatid essential metabolic pathways. Enzyme research 2011, 2011. [Google Scholar] [CrossRef]
- Friggeri, L.; Hargrove, T.Y.; Rachakonda, G.; Blobaum, A.L.; Fisher, P.; De Oliveira, G.M.; Da Silva, C.F.; Soeiro, M.d.N.C.; Nes, W.D.; Lindsley, C.W. Sterol 14α-demethylase structure-based optimization of drug candidates for human infections with the protozoan Trypanosomatidae. Journal of medicinal chemistry 2018, 61, 10910–10921. [Google Scholar] [CrossRef] [PubMed]
- de Souza, W.; Rodrigues, J.C.F. Sterol biosynthesis pathway as target for anti-trypanosomatid drugs. Interdisciplinary perspectives on infectious diseases 2009, 2009. [Google Scholar] [CrossRef]
- Marathe, C.; Bradley, M.N.; Hong, C.; Lopez, F.; de Galarreta, C.M.R.; Tontonoz, P.; Castrillo, A. The arginase II gene is an anti-inflammatory target of liver X receptor in macrophages. Journal of Biological Chemistry 2006, 281, 32197–32206. [Google Scholar] [CrossRef]
- Gallardo-Soler, A.; Gómez-Nieto, C.; Campo, M.L.; Marathe, C.; Tontonoz, P.; Castrillo, A.; Corraliza, I. Arginase I induction by modified lipoproteins in macrophages: a peroxisome proliferator-activated receptor-γ/δ-mediated effect that links lipid metabolism and immunity. Molecular endocrinology 2008, 22, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Silva-Jardim, I.; Thiemann, O.H.; Anibal, F.F. Leishmaniasis and Chagas disease chemotherapy: a critical review. Journal of the Brazilian Chemical Society 2014, 25, 1810–1823. [Google Scholar] [CrossRef]
- McConville, M.J.; Naderer, T. Metabolic pathways required for the intracellular survival of Leishmania. Annual review of microbiology 2011, 6, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.; Sanchez, M.A.; Pierce, S.; Herrmann, T.; Kimblin, N.; Archie Bouwer, H.; Landfear, S.M. Molecular genetic analysis of purine nucleobase transport in Leishmania major. Molecular microbiology 2007, 64, 1228–1243. [Google Scholar] [CrossRef]
- Figueroa-Villar, J.D.; Sales, E.M. The importance of nucleoside hydrolase enzyme (NH) in studies to treatment of Leishmania: A review. Chemico-Biological Interactions 2017, 263, 18–27. [Google Scholar] [CrossRef]
- Majumder, H.K. Drug targets in kinetoplastid parasites; Springer Science & Business Media: 2008; Vol. 625.
- Berg, M.; Van der Veken, P.; Goeminne, A.; Haemers, A.; Augustyns, K. Inhibitors of the purine salvage pathway: a valuable approach for antiprotozoal chemotherapy? Current medicinal chemistry 2010, 17, 2456–2481. [Google Scholar] [CrossRef]
- Ilgoutz, S.C.; Zawadzki, J.L.; Ralton, J.E.; McConville, M.J. Evidence that free GPI glycolipids are essential for growth of Leishmania mexicana. The EMBO journal 1999, 18, 2746–2755. [Google Scholar] [CrossRef]
- Menon, A.K.; Mayor, S.; Schwarz, R.T. Biosynthesis of glycosyl-phosphatidylinositol lipids in Trypanosoma brucei: involvement of mannosyl-phosphoryldolichol as the mannose donor. The EMBO Journal 1990, 9, 4249–4258. [Google Scholar] [CrossRef]
- Horn, D. Antigenic variation in African trypanosomes. Molecular and biochemical parasitology 2014, 195, 123–129. [Google Scholar] [CrossRef]
- Alves, M.J.M.; Colli, W. Role of the gp85/trans-sialidase superfamily of glycoproteins in the interaction of Trypanosoma cruzi with host structures. Molecular Mechanisms of Parasite Invasion: Subcellular Biochemistry, 2008; 58–69. [Google Scholar]
- Giorgi, M.E.; Lederkremer, R.M.d. The glycan structure of T. cruzi mucins depends on the host. Insights on the chameleonic galactose. Molecules 2020, 25, 3913. [Google Scholar] [CrossRef]
- Barreto de Albuquerque, J.; Silva dos Santos, D.; Stein, J.V.; de Meis, J. Oral versus intragastric inoculation: Similar Pathways of Trypanosoma cruzi Experimental infection? From target tissues, parasite evasion, and immune response. Frontiers in immunology 2018, 9, 1734. [Google Scholar] [CrossRef] [PubMed]
- Vickers, T.J.; Beverley, S.M. Folate metabolic pathways in Leishmania. Essays in biochemistry 2011, 51, 63–80. [Google Scholar]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The concept of folic acid in health and disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Beltran-Hortelano, I.; Alcolea, V.; Font, M.; Pérez-Silanes, S. Examination of multiple Trypanosoma cruzi targets in a new drug discovery approach for Chagas disease. Bioorganic & medicinal chemistry 2022, 58, 116577. [Google Scholar]
- Robello, C.; Navarro, P.; Castanys, S.; Gamarro, F. A pteridine reductase gene ptr1 contiguous to a P-glycoprotein confers resistance to antifolates in Trypanosoma cruzi. Molecular and biochemical parasitology 1997, 90, 525–535. [Google Scholar] [CrossRef]
- Bello, A.R.; Nare, B.; Freedman, D.; Hardy, L.; Beverley, S.M. PTR1: a reductase mediating salvage of oxidized pteridines and methotrexate resistance in the protozoan parasite Leishmania major. Proceedings of the National Academy of Sciences 1994, 91, 11442–11446. [Google Scholar] [CrossRef] [PubMed]
- Krauth-Siegel, R.L.; Comini, M.A. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochimica et Biophysica Acta (BBA)-General Subjects 2008, 1780, 1236–1248. [Google Scholar] [CrossRef]
- Colotti, G.; Ilari, A. Polyamine metabolism in Leishmania: from arginine to trypanothione. Amino acids 2011, 40, 269–285. [Google Scholar] [CrossRef] [PubMed]
- BAILEY, S.; SMITH, K.; FAIRLAMB, A.H.; HUNTER, W.N. Substrate interactions between trypanothione reductase and N1-glutathionylspermidine disulphide at 0.28-nm resolution. European journal of biochemistry 1993, 213, 67–75. [Google Scholar] [CrossRef]
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. The EMBO journal 1998, 17, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Manta, B.; Comini, M.; Medeiros, A.; Hugo, M.; Trujillo, M.; Radi, R. Trypanothione: a unique bis-glutathionyl derivative in trypanosomatids. Biochimica et Biophysica Acta (BBA)-General Subjects 2013, 1830, 3199–3216. [Google Scholar] [CrossRef]
- Jain, S.; Sahu, U.; Kumar, A.; Khare, P. Metabolic pathways of Leishmania parasite: source of pertinent drug targets and potent drug candidates. Pharmaceutics 2022, 14, 1590. [Google Scholar] [CrossRef]
- Moreira, D.d.S.; Duarte, A.P.; Pais, F.S.M.; Silva-Pereira, R.A.d.; Romanha, A.J.; Schenkman, S.; Murta, S.M.F. Overexpression of eukaryotic initiation factor 5A (eIF5A) affects susceptibility to benznidazole in Trypanosoma cruzi populations. Memórias do Instituto Oswaldo Cruz 2018, 113, e180162. [Google Scholar] [CrossRef]
- Nakanishi, S.; Cleveland, J.L. Targeting the polyamine-hypusine circuit for the prevention and treatment of cancer. Amino acids 2016, 48, 2353–2362. [Google Scholar] [CrossRef]
- Goldman-Pinkovich, A.; Balno, C.; Strasser, R.; Zeituni-Molad, M.; Bendelak, K.; Rentsch, D.; Ephros, M.; Wiese, M.; Jardim, A.; Myler, P.J. An arginine deprivation response pathway is induced in Leishmania during macrophage invasion. PLoS pathogens 2016, 12, e1005494. [Google Scholar] [CrossRef]
- da Silva, M.F.L.; Floeter-Winter, L.M. Arginase in leishmania. Proteins and proteomics of Leishmania and Trypanosoma 2013, 103–117. [Google Scholar]
- Darlyuk, I.; Goldman, A.; Roberts, S.C.; Ullman, B.; Rentsch, D.; Zilberstein, D. Arginine homeostasis and transport in the human pathogen Leishmania donovani. Journal of biological chemistry 2009, 284, 19800–19807. [Google Scholar] [CrossRef] [PubMed]
- Maddison, J.E.; Page, S.W.; Church, D.B. Small animal clinical pharmacology; Elsevier Health Sciences: 2008; Vol. 5.
- Marchal, B.; Van Dormael, M.; Pirard, M.; Cavalli, A.; Kegels, G.; Polman, K. Neglected tropical disease (NTD) control in health systems: the interface between programmes and general health services. Acta tropica 2011, 120, S177–S185. [Google Scholar] [CrossRef]
- Moffat, J.G.; Vincent, F.; Lee, J.A.; Eder, J.; Prunotto, M. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nature reviews Drug discovery 2017, 16, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Myler, P.J. Searching the Tritryp genomes for drug targets. Drug Targets in Kinetoplastid Parasites 2008, 133–140. [Google Scholar]
- Paradela, L.S.; Wall, R.J.; Carvalho, S.; Chemi, G.; Corpas-Lopez, V.; Moynihan, E.; Bello, D.; Patterson, S.; Güther, M.L.S.; Fairlamb, A.H. Multiple unbiased approaches identify oxidosqualene cyclase as the molecular target of a promising anti-leishmanial. Cell chemical biology 2021, 28, 711–721. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.; Prema, K.; Balaji, S. Machine learning models for drug–target interactions: current knowledge and future directions. Drug Discovery Today 2020, 25, 748–756. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: new estimates of R&D costs. Journal of health economics 2016, 47, 20–33. [Google Scholar] [PubMed]
- Thomas, D.W. Clinical development success rates 2006–2015. BIO Industry Anal. 2016, 1, 16. [Google Scholar]
- Surade, S.; Blundell, T.L. Structural biology and drug discovery of difficult targets: the limits of ligandability. Chemistry & biology 2012, 19, 42–50. [Google Scholar]
- Gamo, F.-J.; Sanz, L.M.; Vidal, J.; De Cozar, C.; Alvarez, E.; Lavandera, J.-L.; Vanderwall, D.E.; Green, D.V.; Kumar, V.; Hasan, S. Thousands of chemical starting points for antimalarial lead identification. Nature 2010, 465, 305–310. [Google Scholar] [CrossRef]
- Don, R.; Ioset, J.-R. Screening strategies to identify new chemical diversity for drug development to treat kinetoplastid infections. Parasitology 2014, 141, 140–146. [Google Scholar] [CrossRef]
- Guiguemde, W.A.; Shelat, A.A.; Bouck, D.; Duffy, S.; Crowther, G.J.; Davis, P.H.; Smithson, D.C.; Connelly, M.; Clark, J.; Zhu, F. Chemical genetics of Plasmodium falciparum. Nature 2010, 465, 311–315. [Google Scholar] [CrossRef]
- De Rycker, M.; Wyllie, S.; Horn, D.; Read, K.D.; Gilbert, I.H. Anti-trypanosomatid drug discovery: progress and challenges. Nature Reviews Microbiology 2023, 21, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peinado, N.; Cortes-Serra, N.; Losada-Galvan, I.; Alonso-Vega, C.; Urbina, J.A.; Rodríguez, A.; VandeBerg, J.L.; Pinazo, M.-J.; Gascon, J.; Alonso-Padilla, J. Emerging agents for the treatment of Chagas disease: what is in the preclinical and clinical development pipeline? Expert Opinion on Investigational Drugs 2020, 29, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Mansoldo, F.R.P.; Carta, F.; Angeli, A.; Cardoso, V.d.S.; Supuran, C.T.; Vermelho, A.B. Chagas disease: perspectives on the past and present and challenges in drug discovery. Molecules 2020, 25, 5483. [Google Scholar] [CrossRef]
- Francisco, E.C.; de Almeida Junior, J.N.; Queiroz-Telles, F.; Aquino, V.R.; Mendes, A.V.A.; de Oliveira Silva, M.; Castro, P.d.T.O.e.; Guimarães, T.; Ponzio, V.; Hahn, R.C. Correlation of Trichosporon asahii genotypes with anatomical sites and antifungal susceptibility profiles: data analyses from 284 isolates collected in the last 22 years across 24 medical centers. Antimicrobial Agents and Chemotherapy 2021, 65, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Schiavuzzo, J.; Teixeira, J.; Melo, B.; Dos Santos, D.d.S.; Jorge, C.; Oliveira-Fusaro, M.; Parada, C. Muscle hyperalgesia induced by peripheral P2X3 receptors is modulated by inflammatory mediators. Neuroscience 2015, 285, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Mackey, T.K.; Liang, B.A.; Cuomo, R.; Hafen, R.; Brouwer, K.C.; Lee, D.E. Emerging and reemerging neglected tropical diseases: a review of key characteristics, risk factors, and the policy and innovation environment. Clinical microbiology reviews 2014, 27, 949–979. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, P.A.; Santos, M.M. Recent progress in the development of indole-based compounds active against malaria, trypanosomiasis and leishmaniasis. Molecules 2022, 27, 319. [Google Scholar] [CrossRef]
- Reimão, J.Q.; Scotti, M.T.; Tempone, A.G. Anti-leishmanial and anti-trypanosomal activities of 1, 4-dihydropyridines: In vitro evaluation and structure–activity relationship study. Bioorganic & medicinal chemistry 2010, 18, 8044–8053. [Google Scholar]
- Yadav, C.L.; Manar, K.K.; Yadav, M.K.; Tiwari, N.; Singh, R.K.; Drew, M.G.; Singh, N. Synthesis, crystal structures and properties of new homoleptic Ni (II)/Pd (II) β-oxodithioester chelates. Journal of Molecular Structure 2018, 1160, 488–496. [Google Scholar] [CrossRef]
- Sahu, A.; Kumar, D.; Agrawal, R.K. Antileishmanial drug discovery: synthetic methods, chemical characteristics, and biological potential of quinazolines and its derivatives. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Inflammatory and Anti-Allergy Agents) 2017, 16, 3–32. [Google Scholar]
- Charlton, R.L.; Rossi-Bergmann, B.; Denny, P.W.; Steel, P.G. Repurposing as a strategy for the discovery of new anti-leishmanials: the-state-of-the-art. Parasitology 2018, 145, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Reguera, R.M.; Pérez-Pertejo, Y.; Gutiérrez-Corbo, C.; Domínguez-Asenjo, B.; Ordóñez, C.; García-Estrada, C.; Martínez-Valladares, M.; Balaña-Fouce, R. Current and promising novel drug candidates against visceral leishmaniasis. Pure and Applied Chemistry 2019, 91, 1385–1404. [Google Scholar] [CrossRef]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi Jr, A.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. New England Journal of Medicine 2015, 373, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Junior, P.A.S.; Molina, I.; Murta, S.M.F.; Sánchez-Montalvá, A.; Salvador, F.; Corrêa-Oliveira, R.; Carneiro, C.M. Experimental and clinical treatment of Chagas disease: a review. The American journal of tropical medicine and hygiene 2017, 97, 1289. [Google Scholar] [CrossRef]
- Caldas, I.S.; Santos, E.G.; Novaes, R.D. An evaluation of benznidazole as a Chagas disease therapeutic. Expert opinion on pharmacotherapy 2019, 20, 1797–1807. [Google Scholar] [CrossRef]
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: past, present, and future prospects. Trends in parasitology 2014, 30, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E.; Scandale, I. Animal models of Chagas disease and their translational value to drug development. Expert Opinion on Drug Discovery 2020, 15, 1381–1402. [Google Scholar] [CrossRef] [PubMed]
- Vermelho, A.B.; Rodrigues, G.C.; Supuran, C.T. Why hasn’t there been more progress in new Chagas disease drug discovery? Expert Opinion on Drug Discovery 2020, 15, 145–158. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sur, D.; Karbwang, J. Childhood visceral leishmaniasis. Indian Journal of Medical Research 2006, 123, 353. [Google Scholar]
- Sundar, S.; Singh, A. Chemotherapeutics of visceral leishmaniasis: present and future developments. Parasitology 2018, 145, 481–489. [Google Scholar] [CrossRef]
- Dorlo, T.P.; Kip, A.E.; Younis, B.M.; Ellis, S.J.; Alves, F.; Beijnen, J.H.; Njenga, S.; Kirigi, G.; Hailu, A.; Olobo, J. Visceral leishmaniasis relapse hazard is linked to reduced miltefosine exposure in patients from Eastern Africa: a population pharmacokinetic/pharmacodynamic study. Journal of Antimicrobial Chemotherapy 2017, 72, 3131–3140. [Google Scholar] [CrossRef]
- Mbui, J.; Olobo, J.; Omollo, R.; Solomos, A.; Kip, A.E.; Kirigi, G.; Sagaki, P.; Kimutai, R.; Were, L.; Omollo, T. Pharmacokinetics, safety, and efficacy of an allometric miltefosine regimen for the treatment of visceral leishmaniasis in Eastern African children: an open-label, phase II clinical trial. Clinical Infectious Diseases 2019, 68, 1530–1538. [Google Scholar] [CrossRef]
- Musa, A.M.; Younis, B.; Fadlalla, A.; Royce, C.; Balasegaram, M.; Wasunna, M.; Hailu, A.; Edwards, T.; Omollo, R.; Mudawi, M. Paromomycin for the treatment of visceral leishmaniasis in Sudan: a randomized, open-label, dose-finding study. PLoS neglected tropical diseases 2010, 4, e855. [Google Scholar] [CrossRef]
- Jamil, K.M.; Haque, R.; Rahman, R.; Faiz, M.A.; Bhuiyan, A.T.M.R.H.; Kumar, A.; Hassan, S.M.; Kelly, H.; Dhalaria, P.; Kochhar, S. Effectiveness study of paromomycin IM injection (PMIM) for the treatment of visceral leishmaniasis (VL) in Bangladesh. PLoS Neglected Tropical Diseases 2015, 9, e0004118. [Google Scholar] [CrossRef] [PubMed]
- Diro, E.; Ritmeijer, K.; Boelaert, M.; Alves, F.; Mohammed, R.; Abongomera, C.; Ravinetto, R.; De Crop, M.; Fikre, H.; Adera, C. Long-term clinical outcomes in visceral leishmaniasis-HIV co-infected patients during and after pentamidine secondary prophylaxis in Ethiopia: a single-arm clinical trial Authors and affiliations. Clinical Infectious Diseases 2017. [Google Scholar]
- Rahman, A.-E.; Mabrouk, R. The Impact of First-line Nurse Managers Leadership Development Training Program on Workgroup Climate and Performance. Diss: 2017.
- Balasegaram, M.; Ritmeijer, K.; Lima, M.A.; Burza, S.; Ortiz Genovese, G.; Milani, B.; Gaspani, S.; Potet, J.; Chappuis, F. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert opinion on emerging drugs 2012, 17, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Deray, G. Amphotericin B nephrotoxicity. Journal of antimicrobial chemotherapy 2002, 49 (Suppl. 1), 37–41. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.; Boelaert, M. Leishmaniasis Lancet 392: 951–970. 2018.
- Sundar, S.; Chakravarty, J. Antimony toxicity. International journal of environmental research and public health 2010, 7, 4267–4277. [Google Scholar] [CrossRef]
- Arce, A.; Estirado, A.; Ordobas, M.; Sevilla, S.; García, N.; Moratilla, L.; De La Fuente, S.; Martínez, A.; Pérez, A.; Aránguez, E. Re-emergence of leishmaniasis in Spain: community outbreak in Madrid, Spain, 2009 to 2012. Eurosurveillance 2013, 18, 20546. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J.; Agarwal, D.; Rai, M.; Murray, H.W. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. New England Journal of Medicine 2010, 362, 504–512. [Google Scholar] [CrossRef]
- Pereira, I.R.; Vilar-Pereira, G.; Silva, A.A.d.; Lannes-Vieira, J. Severity of chronic experimental Chagas' heart disease parallels tumour necrosis factor and nitric oxide levels in the serum: models of mild and severe disease. Memórias do Instituto Oswaldo Cruz 2014, 109, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Molina, I.; Gómez i Prat, J.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. New England Journal of Medicine 2014, 370, 1899–1908. [Google Scholar] [CrossRef]
- Lima, Á.A.; Soares-Sobrinho, J.L.; Silva, J.L.; Corrêa-Júnior, R.A.; Lyra, M.A.; Santos, F.L.; Oliveira, B.G.; Hernandes, M.Z.; Rolim, L.A.; Rolim-Neto, P.J. The use of solid dispersion systems in hydrophilic carriers to increase benznidazole solubility. Journal of pharmaceutical sciences 2011, 100, 2443–2451. [Google Scholar] [CrossRef]
- Ben Beard, C. Control of Chagas Disease. Second Report of the WHO Expert Committee. WHO Technical Report Series, No. 905. Geneva: World Health Organization, 2002. vi+ 109pp. Price Sw. fr. 23/US $20.70 (in developing countries Sw. fr. 16.10). ISBN 92-4-120905.4. Royal Society of Tropical Medicine and Hygiene: 2002.
- Organization, W.H. Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected diseases; World Health Organization: 2013.
- de Mecca, M.M.; Fanelli, S.L.; Bartel, L.C.; de Castro, C.R.; Díaz, E.G.; Castro, J.A. Nifurtimox nitroreductase activity in different cellular fractions from male rat pancreas. Biochemical and ultrastructural alterations. Life sciences 2007, 81, 144–152. [Google Scholar] [CrossRef]
- Mecca, M.M.d.; Bartel, L.C.; Castro, C.R.d.; Castro, J.A. Benznidazole biotransformation in rat heart microsomal fraction without observable ultrastructural alterations: comparison to Nifurtimox-induced cardiac effects. Memorias do Instituto Oswaldo Cruz 2008, 103, 549–553. [Google Scholar] [CrossRef]
- Bhargava, P.; Singh, R. Developments in diagnosis and antileishmanial drugs. Interdisciplinary perspectives on infectious diseases 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, L.M.; Ferreira, T.C.; Gadelha, F.R.; Miguel, D.C. Challenges in drug discovery targeting TriTryp diseases with an emphasis on leishmaniasis. International Journal for Parasitology: Drugs and Drug Resistance 2018, 8, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.M.; de Oliveira Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Applied Microbiology and Biotechnology 2020, 104, 8965–8977. [Google Scholar] [CrossRef]
- Zahid, M.S.H.; Johnson, M.M.; Tokarski II, R.J.; Satoskar, A.R.; Fuchs, J.R.; Bachelder, E.M.; Ainslie, K.M. Evaluation of synergy between host and pathogen-directed therapies against intracellular Leishmania donovani. International Journal for Parasitology: Drugs and Drug Resistance 2019, 10, 125–132. [Google Scholar] [CrossRef]
- Novais, F.O.; Amorim, C.F.; Scott, P. Host-Directed Therapies for Cutaneous Leishmaniasis. Frontiers in Immunology 2021, 12, 660183. [Google Scholar] [CrossRef]
- Kumari, D.; Perveen, S.; Sharma, R.; Singh, K. Advancement in leishmaniasis diagnosis and therapeutics: An update. European Journal of Pharmacology 2021, 910, 174436. [Google Scholar] [CrossRef]
- Rassi Jr, A.; Rassi, A.; Marin-Neto, J.A. Chagas disease. The Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. The Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Parihar, S.P.; Hartley, M.-A.; Hurdayal, R.; Guler, R.; Brombacher, F. Topical simvastatin as host-directed therapy against severity of cutaneous leishmaniasis in mice. Scientific reports 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Shivam, P.; Mandal, S.; Prasanna, P.; Kumar, S.; Prasad, S.R.; Kumar, A.; Das, P.; Ali, V.; Singh, S.K. Synthesis, characterization, and mechanistic studies of a gold nanoparticle–amphotericin B covalent conjugate with enhanced antileishmanial efficacy and reduced cytotoxicity. International Journal of Nanomedicine 2019, 14, 6073. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.R.C.; Velappan, A.B.; Chellappan, D.; Debnath, J.; Mahapatra, S.K. Eugenol derived immunomodulatory molecules against visceral leishmaniasis. European journal of medicinal chemistry 2017, 139, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Tindana, P.; de Haan, F.; Amaratunga, C.; Dhorda, M.; van der Pluijm, R.W.; Dondorp, A.M.; Cheah, P.Y. Deploying triple artemisinin-based combination therapy (TACT) for malaria treatment in Africa: ethical and practical considerations. Malaria Journal 2021, 20, 1–7. [Google Scholar] [CrossRef]
- Larkins-Ford, J.; Greenstein, T.; Van, N.; Degefu, Y.N.; Olson, M.C.; Sokolov, A.; Aldridge, B.B. Systematic measurement of combination-drug landscapes to predict in vivo treatment outcomes for tuberculosis. Cell Systems 2021, 12, 1046–1063. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C. Drug repurposing: progress, challenges and recommendations. Nature reviews Drug discovery 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Breckenridge, A.; Jacob, R. Overcoming the legal and regulatory barriers to drug repurposing. Nature reviews Drug discovery 2019, 18, 1–2. [Google Scholar] [CrossRef]
- Braga, S.S. Multi-target drugs active against leishmaniasis: A paradigm of drug repurposing. European Journal of Medicinal Chemistry 2019, 183, 111660. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.H.; Neves, B.J.; Melo-Filho, C.C.; Rodrigues, J.; Silva, D.C.; Braga, R.C.; Cravo, P.V. In silico chemogenomics drug repositioning strategies for neglected tropical diseases. Current Medicinal Chemistry 2019, 26, 4355–4379. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.A.M.; Lang, K.L. Drug repositioning: concept, classification, methodology, and importance in rare/orphans and neglected diseases. Journal of Applied Pharmaceutical Science 2018, 8, 157–165. [Google Scholar]
- Jantan, I.; Bukhari, S.N.A.; Mohamed, M.A.S.; Wai, L.K.; Mesaik, M.A. The evolving role of natural products from the tropical rainforests as a replenishable source of new drug leads. Drug Discovery and Development-From Molecules to Medicine, 2005; 3–38. [Google Scholar]
- García, M.; Monzote, L. Marine products with anti-protozoal activity: a review. Current Clinical Pharmacology 2014, 9, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, R.; Jain, N. CURRENT STRATEGIES AND RECENT ADVANCES IN NANOSTRUCTURED DELIVERY SYSTEMS FOR MANAGING LEISHMANIASIS-A REVIEW. Journal of Advanced Scientific Research 2022, 13, 43–55. [Google Scholar] [CrossRef]
- Santos, S.S.; de Araujo, R.V.; Giarolla, J.; El Seoud, O.; Ferreira, E.I. Searching for drugs for Chagas disease, leishmaniasis and schistosomiasis: a review. International journal of antimicrobial agents 2020, 55, 105906. [Google Scholar] [CrossRef]
- Gharpure, S.; Ankamwar, B. Use of nanotechnology in combating coronavirus. 3 Biotech 2021, 11, 358. [Google Scholar] [CrossRef]
- Karuppusamy, C.; Venkatesan, P. Role of nanoparticles in drug delivery system: a comprehensive review. Journal of Pharmaceutical sciences and Research 2017, 9, 318. [Google Scholar]
- Kim, D.Y.; Kadam, A.; Shinde, S.; Saratale, R.G.; Patra, J.; Ghodake, G. Recent developments in nanotechnology transforming the agricultural sector: a transition replete with opportunities. Journal of the Science of Food and Agriculture 2018, 98, 849–864. [Google Scholar] [CrossRef]
- Celis-Giraldo, C.T.; López-Abán, J.; Muro, A.; Patarroyo, M.A.; Manzano-Román, R. Nanovaccines against animal pathogens: The latest findings. Vaccines 2021, 9, 988. [Google Scholar] [CrossRef]
- Javed, R.; Ghonaim, R.; Shathili, A.; Khalifa, S.A.; El-Seedi, H.R. Phytonanotechnology: A greener approach for biomedical applications. In Biogenic Nanoparticles for Cancer Theranostics, Elsevier: 2021; pp. 43-86.
- Sangar, O.S.; Patil, A.C.; Payghan, S.A. Nanoparticles: As a Nano based Drug Delivery System. 2022.
- Nafari, A.; Cheraghipour, K.; Sepahvand, M.; Shahrokhi, G.; Gabal, E.; Mahmoudvand, H. Nanoparticles: New agents toward treatment of leishmaniasis. Parasite epidemiology and control 2020, 10, e00156. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.; Kumar, P.; Kumar, S.; Rajana, V.K.; Kant, V.; Prasad, S.R.; Mohan, U.; Ravichandiran, V.; Mandal, D. Current status of nanoscale drug delivery and the future of nano-vaccine development for leishmaniasis–a review. Biomedicine & Pharmacotherapy 2021, 141, 111920. [Google Scholar]
- Athanasiou, E.; Agallou, M.; Tastsoglou, S.; Kammona, O.; Hatzigeorgiou, A.; Kiparissides, C.; Karagouni, E. A poly (lactic-co-glycolic) acid nanovaccine based on chimeric peptides from different Leishmania infantum proteins induces dendritic cells maturation and promotes peptide-specific IFNγ-producing CD8+ T cells essential for the protection against experimental visceral leishmaniasis. Frontiers in immunology 2017, 8, 684. [Google Scholar] [PubMed]
- Agallou, M.; Margaroni, M.; Athanasiou, E.; Toubanaki, D.K.; Kontonikola, K.; Karidi, K.; Kammona, O.; Kiparissides, C.; Karagouni, E. Identification of BALB/c immune markers correlated with a partial protection to Leishmania infantum after vaccination with a rationally designed multi-epitope cysteine protease a peptide-based nanovaccine. PLoS Neglected Tropical Diseases 2017, 11, e0005311. [Google Scholar] [CrossRef] [PubMed]
- Assolini, J.P.; Carloto, A.C.M.; da Silva Bortoleti, B.T.; Gonçalves, M.D.; Pellissier, F.T.; Feuser, P.E.; Cordeiro, A.P.; de Araújo, P.H.H.; Sayer, C.; Sapla, M.M.M. Nanomedicine in leishmaniasis: A promising tool for diagnosis, treatment and prevention of disease-An update overview. European Journal of Pharmacology 2022, 923, 174934. [Google Scholar] [CrossRef] [PubMed]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The current state of peptide drug discovery: back to the future? Journal of medicinal chemistry 2018, 61, 1382–1414. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduction and Targeted Therapy 2022, 7, 48. [Google Scholar] [CrossRef]
- Derda, R.; Jafari, M.R. Synthetic cross-linking of peptides: molecular linchpins for peptide cyclization. Protein and Peptide Letters 2018, 25, 1051–1075. [Google Scholar] [CrossRef]
- Kang, N.J.; Jin, H.-S.; Lee, S.-E.; Kim, H.J.; Koh, H.; Lee, D.-W. New approaches towards the discovery and evaluation of bioactive peptides from natural resources. Critical Reviews in Environmental Science and Technology 2020, 50, 72–103. [Google Scholar] [CrossRef]
- Davda, J.; Declerck, P.; Hu-Lieskovan, S.; Hickling, T.P.; Jacobs, I.A.; Chou, J.; Salek-Ardakani, S.; Kraynov, E. Immunogenicity of immunomodulatory, antibody-based, oncology therapeutics. Journal for immunotherapy of cancer 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Nishioka, K.; Hayashi, S.; Farrer, M.J.; Singleton, A.B.; Yoshino, H.; Imai, H.; Kitami, T.; Sato, K.; Kuroda, R.; Tomiyama, H. Clinical heterogeneity of α-synuclein gene duplication in Parkinson's disease. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 2006, 59, 298–309. [Google Scholar] [CrossRef]
- Weng, H.-B.; Chen, H.-X.; Wang, M.-W. Innovation in neglected tropical disease drug discovery and development. Infectious diseases of poverty 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.; Bruhn, H.; Gaworski, I.; Fleischer, B.; Leippe, M. NK-lysin and its shortened analog NK-2 exhibit potent activities against Trypanosoma cruzi. Antimicrobial agents and chemotherapy 2003, 47, 607–613. [Google Scholar] [CrossRef]
- Souza, A.L.; Faria, R.X.; Calabrese, K.S.; Hardoim, D.J.; Taniwaki, N.; Alves, L.A.; De Simone, S.G. Temporizin and temporizin-1 peptides as novel candidates for eliminating Trypanosoma cruzi. PLoS One 2016, 11, e0157673. [Google Scholar] [CrossRef]
- Kleschenko, Y.E.; Karpenko, L.; Villalta, F. Effects of human defensin-α1 on Trypanosoma cruzi trypomastigotes in vitro. Bulletin of experimental biology and medicine 2010, 149, 731. [Google Scholar] [CrossRef]
- Pinto, E.G.; Pimenta, D.C.; Antoniazzi, M.M.; Jared, C.; Tempone, A.G. Antimicrobial peptides isolated from Phyllomedusa nordestina (Amphibia) alter the permeability of plasma membrane of Leishmania and Trypanosoma cruzi. Experimental parasitology 2013, 135, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Brand, G.D.; Leite, J.R.S.; Silva, L.P.; Albuquerque, S.; Prates, M.V.; Azevedo, R.B.; Carregaro, V.; Silva, J.S.; Sá, V.C.; Brandao, R.A. Dermaseptins from Phyllomedusa oreades andPhyllomedusa distincta: Anti-Trypanosoma Cruzi Activity Without Cytotoxicity To Mammalian Cells. Journal of Biological Chemistry 2002, 277, 49332–49340. [Google Scholar] [CrossRef]
- Adade, C.M.; Oliveira, I.R.; Pais, J.A.; Souto-Padrón, T. Melittin peptide kills Trypanosoma cruzi parasites by inducing different cell death pathways. Toxicon 2013, 69, 227–239. [Google Scholar] [CrossRef]
- Freire, K.A.; Torres, M.D.T.; Lima, D.B.; Monteiro, M.L.; Martins, A.M.C.; Oliveira Jr, V.X. Wasp venom peptide as a new antichagasic agent. Toxicon 2020, 181, 71–78. [Google Scholar] [CrossRef] [PubMed]
- El-Dirany, R.; Shahrour, H.; Dirany, Z.; Abdel-Sater, F.; Gonzalez-Gaitano, G.; Brandenburg, K.; Martinez de Tejada, G.; Nguewa, P.A. Activity of anti-microbial peptides (AMPs) against Leishmania and other parasites: an overview. Biomolecules 2021, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A new era of antibiotics: the clinical potential of antimicrobial peptides. International journal of molecular sciences 2020, 21, 7047. [Google Scholar] [CrossRef]
- Robles-Loaiza, A.A.; Pinos-Tamayo, E.A.; Mendes, B.; Teixeira, C.; Alves, C.; Gomes, P.; Almeida, J.R. Peptides to tackle leishmaniasis: Current status and future directions. International Journal of Molecular Sciences 2021, 22, 4400. [Google Scholar] [CrossRef] [PubMed]
- Lewies, A.; Wentzel, J.F.; Jacobs, G.; Du Plessis, L.H. The potential use of natural and structural analogues of antimicrobial peptides in the fight against neglected tropical diseases. Molecules 2015, 20, 15392–15433. [Google Scholar] [CrossRef]
- Price, R.L. Determining the effect of treatment with an exogenous host defence peptide on Mycobacterium marinum-zebrafish infection. 2017.
- Zahedifard, F.; Lee, H.; No, J.H.; Salimi, M.; Seyed, N.; Asoodeh, A.; Rafati, S. Anti-leishmanial activity of Brevinin 2R and its Lauric acid conjugate type against L. major: In vitro mechanism of actions and in vivo treatment potentials. PLoS neglected tropical diseases 2019, 13, e0007217. [Google Scholar]
- Rivas, L.; Luque-Ortega, J.R.; Andreu, D. Amphibian antimicrobial peptides and Protozoa: lessons from parasites. Biochimica et Biophysica Acta (BBA)-Biomembranes 2009, 1788, 1570–1581. [Google Scholar] [CrossRef]
- Salas-Sarduy, E.; Niemirowicz, G.T.; José Cazzulo, J.; Alvarez, V.E. Target-based screening of the Chagas box: setting up enzymatic assays to discover specific inhibitors across bioactive compounds. Current Medicinal Chemistry 2019, 26, 6672–6686. [Google Scholar] [CrossRef]
- Fieck, A.; Hurwitz, I.; Kang, A.S.; Durvasula, R. Trypanosoma cruzi: synergistic cytotoxicity of multiple amphipathic anti-microbial peptides to T. cruzi and potential bacterial hosts. Experimental parasitology 2010, 125, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Sabia Junior, E.F.; Menezes, L.F.S.; de Araújo, I.F.S.; Schwartz, E.F. Natural occurrence in venomous arthropods of antimicrobial peptides active against protozoan parasites. Toxins 2019, 11, 563. [Google Scholar] [CrossRef]
- Richter, A.; Sutherland, D.; Ebrahimikondori, H.; Babcock, A.; Louie, N.; Li, C.; Coombe, L.; Lin, D.; Warren, R.L.; Yanai, A. Associating Biological Activity and Predicted Structure of Antimicrobial Peptides from Amphibians and Insects. Antibiotics 2022, 11, 1710. [Google Scholar] [CrossRef]
- Telleria, E.L.; Tinoco-Nunes, B.; Leštinová, T.; de Avellar, L.M.; Tempone, A.J.; Pitaluga, A.N.; Volf, P.; Traub-Csekö, Y.M. Lutzomyia longipalpis antimicrobial peptides: Differential expression during development and potential involvement in vector interaction with microbiota and leishmania. Microorganisms 2021, 9, 1271. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; He, X.; Zhang, P.; Shen, C.; Mwangi, J.; Xu, C.; Mo, G.; Lai, R.; Zhang, Z. In vitro and in vivo antimalarial activity of LZ1, a peptide derived from snake cathelicidin. Toxins 2019, 11, 379. [Google Scholar] [CrossRef]
- Adade, C.M.; Souto-Padrón, T. Venoms as sources of novel anti-parasitic agents. Toxins and Drug Discovery 2015, 1–31. [Google Scholar]
- Rapposelli, S.; Gaudio, E.; Bertozzi, F.; Gul, S. Protein–protein interactions: Drug discovery for the future. Frontiers in Chemistry 2021, 9, 811190. [Google Scholar] [CrossRef] [PubMed]
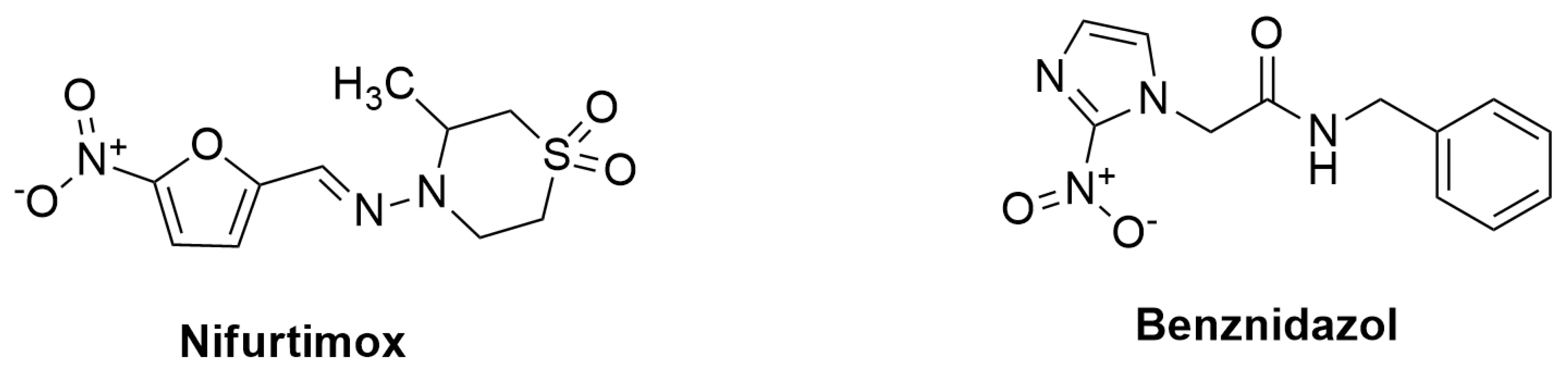
| No | Diseases | Species | Types | Geographic distributions | References |
|---|---|---|---|---|---|
| 1 |
Leishmaniasis |
Leishmania (L.) Aethiopica | Cutaneous and mucocutaneous leishmaniasis | Ethiopia, Kenya | [37] |
| 2 | L. Tropica | Visceral and cutaneous leishmaniasis | Eastern and Northern India, Central Asia, East and South Africa, Middle East | [37] | |
| 3 | L. Amazonensis | Cutaneous and mucocutaneous leishmaniasis | Brazil, Bolivia, Venezuela | [38] | |
| 4 | L. Infantum | Visceral and cutaneous leishmaniasis | Mexico, Brazil, Bolivia, Venezuela, Northern Africa, Middle East, Mediterranean regions, Southern Europe, Central Asia | [39] | |
| 5 | L. Donovani | Post Kala azar dermal leishmaniasis, visceral and cutaneous leishmaniasis | India, Myanmar, Nepal, Sri Lanka, Middle East, China, Ethiopia, Kenya, Sudan | [39] | |
| 6 | L. Major | Cutaneous and mucocutaneous leishmaniasis | Central Asia, Middle East, and Central, Northern and West Africa | [40] | |
| 7 | L. Mexicana | Cutaneous and visceral leishmaniasis | United States of America, Venezuela, Ecuador, Peru, Brazil | [38] | |
| 8 | L. Venezuelensis | Cutaneous leishmaniasis | Northern and Southern America, Venezuela | [41] | |
| 9 | L. Braziliensis | Cutaneous and mucocutaneous leishmaniasis | Amazon stretch, Brazil, Southern America, Bolivia, Peru, Venezuela | [42] | |
| 10 | L. Guyanensis | Cutaneous and mucocutaneous leishmaniasis | Southern America, French Guiana, Suriname, Brazil | [43] | |
| 11 | L. Panamensis | Cutaneous and mucocutaneous leishmaniasis | Panama, South and Northern America, Brazil, Ecuador, Columbia, Venezuela | [43] | |
| 12 | L. Lainsoni | Cutaneous leishmaniasis | French Guiana, Peru, Bolivia, Brazil | [44] | |
| 13 | L. Naffi | Cutaneous leishmaniasis | French Guiana, Brazil | [44] | |
| 14 | L. Lindenberg | Cutaneous leishmaniasis | Brazil | [45] | |
| 15 | L. Peruviana | Cutaneous and mucocutaneous leishmaniasis | Peru, Bolivia, Amazon | [46] | |
| 16 | L. Shawi | Cutaneous leishmaniasis | Brazil | [47] | |
| 17 | L. Martiniquensis | Visceral and cutaneous leishmaniasis | Martinique, Thailand, France, Germany, Switzerland, Myanmar | [48] | |
| 18 | L. Siamensis | Visceral and cutaneous leishmaniasis | Central Europe, Thailand, United States of America | [49] | |
| 19 | L. Colombiensis | Visceral and cutaneous leishmaniasis | Columbia | [50] | |
| 20 | African Trypanosomiasis (aka Sleeping Sickness) | T. brucei | Acute and chronic infections | Eastern, Western, Southern and Central Africa | [51] |
| 21 | Chagas disease (aka American trypanosomiasis) | T. cruzi | Acute and chronic infections | Bolivia, Argentina, Paraguay, Ecuador, El Salvador, and Guatemala | [52] |
| Disease | Drug | Route | Advantages | Disadvantages | Toxicity | References |
|---|---|---|---|---|---|---|
| Leishmaniasis | Miltefosine | Oral 5-100 mg/kg/day for 28 days | No hospitalization | Need allometric administration in children | Gastrointestinal complications, teratogenic | [157,158,159] |
| Leishmaniasis | Paromomycin | Parenteral (im) 15 mg/kg/ day for 21 days | Low cost | Poor results against African VL as monotherapy | Pain in the injection site, hepatotoxicity | [160,161] |
| Leishmaniasis | Pentamidine | Slow infusion (iv) 4 mg/kg monthly for 12 months | Use in HIV positive co-infections | Multiple adverse effects | Insulin-dependent diabetes, myocarditis, nephrotoxicity | [162] |
| Leishmaniasis | SbV - paromomycin combination |
Parenteral (im) SbV 20 mg/ kg/day + paromomycin for 17 days | Reduce the number of injections | Require hospitalization | Problems regarding SbV administration | [157,163] |
| Leishmaniasis | Amphotericine B deoxycholate | Slow infusion (iv) 1 mg/kg/day for 30 days |
Effective against SbV resistant strains |
Require hospitalization | Nephrotoxicity | [164,165] |
| Leishmaniasis | SbV -based drugs | Parenteral (im) 20 mg/kg/day for 28-30 days | Low cost | Drug resistance in Bihar (India), PKDL | Pain in the injection site, cardiotoxicity, pancreatitis | [166,167] |
| Leishmaniasis | AmBisome | Slow infusion (iv) 10 mg/kg single dose | Effective at single dose |
Costly, chemically unstable | Fever during infusion, back pain, nephrotoxicity | [168,169] |
| Chagas disease | Benznidazole (BZL) | It is given for 60 days on daily basis at 5-7 mg/kg, and 10 mg/kg for adults and children, respectively | Lower side effects than Nifurtimox, better tolerance by children, and more effective during the acute phase of the disease. | Low solubility, toxic and several side effects | Low bioavailability and drug effectiveness, chronic effects | [170,171,172,173] |
| Chagas disease | Nifurtimox (NFX) | 8-10 mg/Kg daily in three divided doses for adults, and 15-20 mg/kg daily in four divided doses for children during 60 to 90 days | Better tolerance by children and more effectiveness during the acute phase of the disease. | Toxic and have side effects, causes gastrointestinal, maladies (nausea, vomiting, abdominal pain) effects |
Have higher toxicity and adverse effect than BZL, and it affects the pancreases and heart via increasing of oxidative stress | [174.175.176] |
| Peptide | Source | Sequence | Study model | IC50 µg/mL | Reference |
| NK-2 | Synthetic peptide | KILRGVCKKIMRTFLRRISKDILTGKK | In vitro | - | [217] |
| Temporizin-1 | Synthetic peptide | FLPLWLWLWLWLWKLK | In vitro | - | [218] |
| Defensin-α1 | Human | ACYCRIPACIAGERRYGTCIYQGRLWAFCC | In vitro | - | [219] |
| Phylloseptin 7 | Phyllomedusa nordestina (Frog) | FLSLIPHAINAVSAIAKHF | In vitro | 0.34 | [220] |
| DS 01 | Phyllomedusa oreades (Frog) | GLWSTIKQKGKEAAIAAAKAAGQAALGAL | In vitro | - | [221] |
| Melittin | Apis mellifera (Bee) | - | In vitro | 2.44 | [222] |
| Polybia-CP | Polybia paulista (Wasp) | ILGTILGLLSKL | In vitro | - | [223] |
| Hmc 364–382 | Penaeus monodon (Shrimp) | NVQYYGALHNTAHIVLGRQ | In vitro | 4.79 | [223] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





