Submitted:
02 September 2023
Posted:
05 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
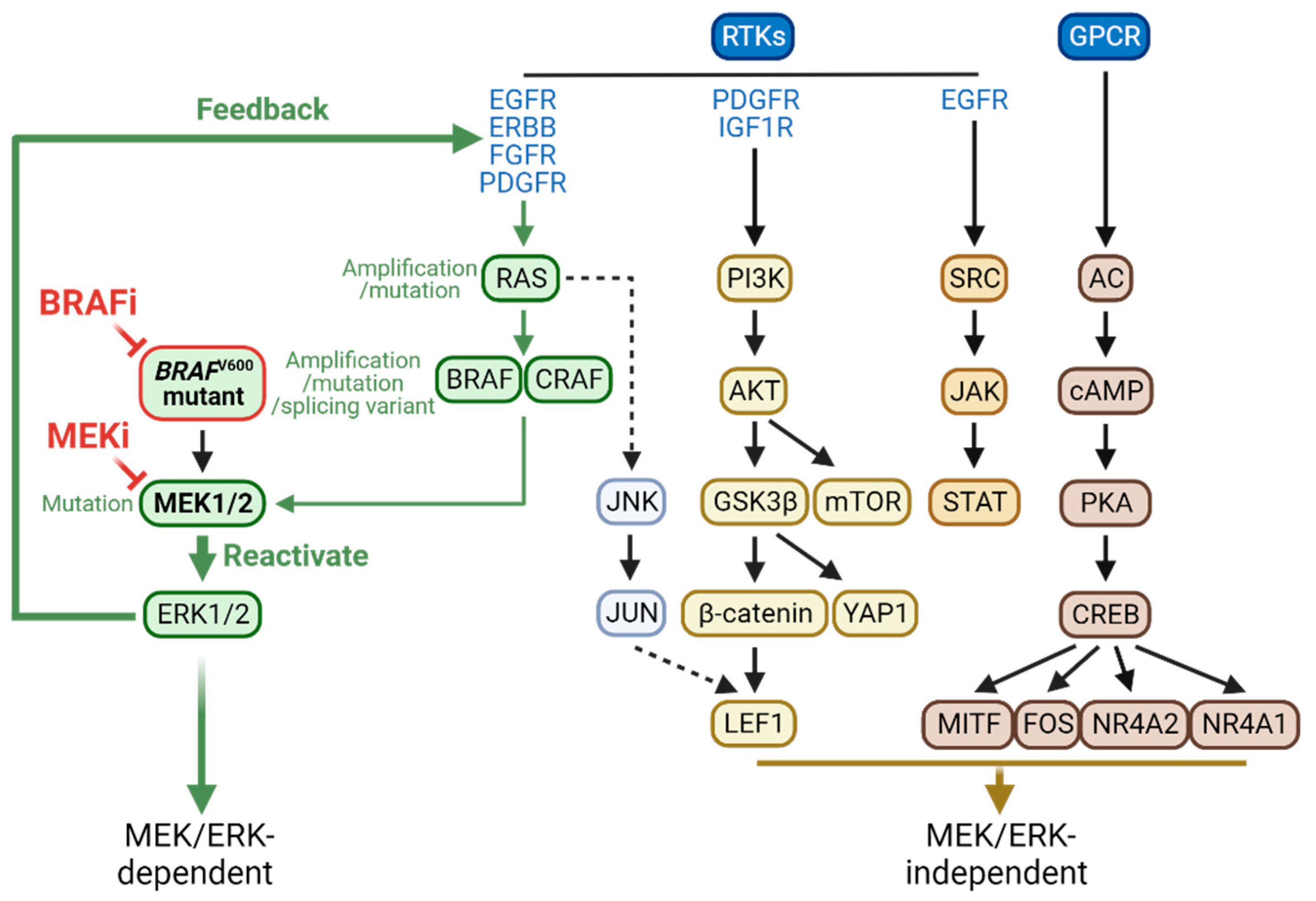
2. MEK/ERK-dependent resistance mechanisms
2.1. MEK/ERK-dependent adaptive resistance
2.2. MEK/ERK-dependent acquired resistance
3. MEK/ERK-independent resistance mechanisms
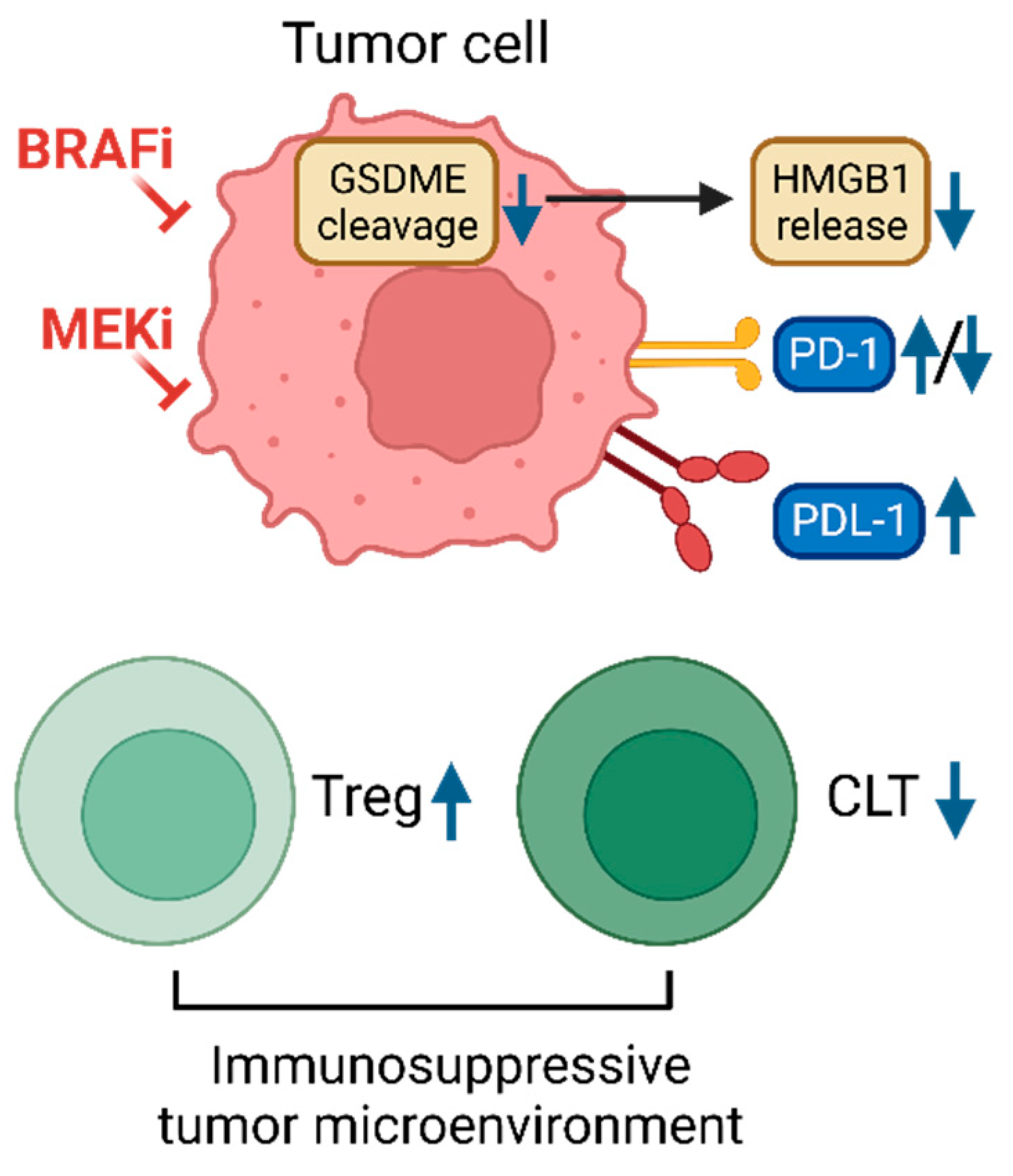
4. Co-evolution of intra-tumoral immunity
5. Future perspectives and conclusion
| Drugs | Tumor types | Source of study | Alterations for resistance | Resistance types | Consequence | Reference |
|---|---|---|---|---|---|---|
| Dabra/Tram* | Melanoma | Patient biopsy | BRAF amplification, NRAS mutations, MEK2C125S | Acquired | ERK1/2 reactivation | [39] |
| Dabra/Tram | Melanoma | Patient biopsy |
BRAF splicing isoform lacking exons 2-10, MEK2Q60P, Somatic mutations of ETS, SAMD4B |
Acquired | ERK1/2 reactivation | [40] |
| Dabra/Tram | Melanoma | Patient biopsy | Activating BRAF in-frame deletion | Acquired | ERK1/2 reactivation | [41] |
| Dabra/Tram | Melanoma | Patient biopsy, cell lines | AKT1Q79K that activates PI3K-AKT signaling, PDGFR- β upregulation | Adaptive | MEK/ERK-independent resistance | [63] |
| Dabra/Tram | Melanoma | Patient biopsy | MCL-1 overexpression, activation of survival pathway | Adaptive | MEK/ERK-independent resistance | [69] |
| Dabra/Tram | Colorectal cancer | Patient biopsy | KRAS amplification, BRAF amplification, MEK1F53L | Acquired | ERK1/2 reactivation | [28] |
| Dabra/Tram | Colorectal cancer | Patient biopsy | KRASG12C, BRAFV600E allele frequency increase | Acquired | ERK1/2 reactivation | [38] |
| Dabra/Tram | Melanoma | Cell lines, PDX model, biopsy | Increase of IGF1R/IR expression | Acquired | MEK/ERK-independent resistance | [66] |
| PLX4720/PD0325901 | Melanoma | Cell lines, PDX model | Rebound of mTOC1 pathway | Acquired | AKT or ERK contributes to the activation of mTORC1 depending on PTEN status | [62] |
| PLX4720/Tram Dabra/Tram |
Melanoma | Cell lines, PDX model | Upregulation of ATF4 | Acquired | ERK1/2 reactivation | [55] |
| PLX4720/PD0325901 | Melanoma | Synergetic mouse model, cell lines, | Failed to induce GSDME, decreased intra-tumoral T cell infiltration |
Acquired | MEK/ERK-independent resistance | [99] |
| BRAFi/EGFRi (dabrafenib + panitumumab), BRAFi/EGFRi/MEKi (dabrafenib + panitumumab + trametinib) | Colorectal cancer | Patient biopsy, cell lines | One or more RAS mutations (KRAS or NRAS) | Acquired | ERK1/2 reactivation | [57] |
| PLX4720+ AZD6244 | Melanoma | Gain of function screen, Patient biopsy | GPCR-PKA-cAMP, CREB phosphorylation | Adaptive | MEK/ERK-independent resistance | [72] |
| PLX4720+ AZD6244 | Melanoma | Gain of function screen, patient biopsy | c-Fos, NR4A1, NR4A2, MITF, activation of MEK/ERK downstream effectors | Intrinsic, adaptive, acquired | MEK/ERK-independent resistance | [72] |
| Vemurafenib only or Vemurafenib/Tram |
Melanoma | Cell lines, Patient biopsy | Decreased ability to induce IFNγ release by CD8+ TILs | Acquired | Decreases T cell activation | [100] |
| Vemurafenib only or Vemurafenib/Tram |
Melanoma | Cell lines, Patient biopsy | Decreased TOP1 expression | Acquired | unclear | [107] |
| Drug | Tumor types | Source of study | Alterations for resistance | Resistance types | Consequence | Reference |
|---|---|---|---|---|---|---|
| Vemurafenib | Melanoma | Patient biopsy, cell lines | PDGFR-β upregulation, NRASQ61K | Acquired | ERK1/2 reactivation | [32] |
| Dabrafenib | Melanoma | Cell lines | MEK1K59del, NRASQ61K and/or NRASA146T with and without MEK1P387S | Acquired | ERK1/2 reactivation | [27] |
| SB590885 | Melanoma | Patient biopsy, cell lines | IGF1R-PI3K-AKT activation | Acquired | MEK/ERK-independent resistance | [65] |
| Dabrafenib or vemurafenib | Melanoma | Patient biopsy | RAS mutations, mutant BRAF amplification, and alternative splicing | Acquired | ERK1/2 reactivation | [59] |
| Dabrafenib or vemurafenib | Melanoma | Patient biopsy | AKT1E17K and AKT1Q79K | Acquired | MEK/ERK-independent resistance | [59] |
| Vemurafenib | Melanoma | Cell lines | FGFR3-Ras activation | Acquired | ERK1/2 reactivation | [108] |
| Vemurafenib | Melanoma | Cell lines | SHOC-2/Sur-8 expression for N-Ras/C-Raf interaction | Acquired | ERK1/2 reactivation | [109] |
| Vemurafenib | Melanoma | Cell lines | Bcl-2 modifying factor (BMF) downregulation, increased eIF4F complex formation, reprogrammed translation | Acquired, adaptive | MEK/ERK-independent resistance | [71] |
| Vemurafenib | Melanoma | Cell lines | Relief of feedback inhibition of mitogenic signaling | Adaptive | ERK1/2 reactivation | [110] |
| Vemurafenib | Melanoma | Patient biopsy, Cell lines | c-JUN upregulation, LEF1 and SPRY4 downregulation, activation of downstream effector | Acquired, adaptive | MEK/ERK-independent resistance | [73] |
| Vemurafenib | NSCLC, Melanoma | Cell lines, Patient biopsy | YAP upregulation, activation of downstream effectors | Intrinsic, adaptive | MEK/ERK-independent resistance | [80] |
| PLX4720 | Melanoma | Gain of function screen | MAP3K8/COT/TPL-2 | Secondary tumor development | ERK1/2 reactivation | [111] |
| PLX4720 | Melanoma | Cell lines | BH-3 only protein silencing, activation of survival pathway | Acquired | MEK/ERK-independent resistance | [70] |
| Vemurafenib | Melanoma | Cell lines, Patient biopsy | EGFR-SFK-STAT3, activation of downstream effector | Acquired, adaptive | ERK1/2 reactivation | [77] |
| Vemurafenib | Melanoma | Cell lines | Activation of MAPKs and the PI3K pathways, enhanced NRAS expression | Acquired | Activation of all the three MAPKs, ERK, JNK, and p38 | [37] |
| Vemurafenib | Melanoma | Cell lines | Upregulated AXL in PTEN wild-type cells | Acquired | Hyperactivation of AXL/AKT and ERK pathways | [61] |
| Vemurafenib | Melanoma | Cell lines | Upregulated PERK in PTEN-inactivated | Acquired | Hyperactivation of ERK pathway | [56] |
| Vemurafenib | Thyroid cancer | Cell lines | ERBB/HER3 transcription, autocrine secretion of neuregulin 1 | Adaptive | ERK1/2 reactivation | [30] |
| Vemurafenib | Colorectal cancer | Cell lines | EGFR activation | Adaptive | ERK1/2 reactivation | [29] |
| Selumetinib | Colorectal cancer | Cell lines | KRAS or BRAF amplification | Acquired | ERK1/2 reactivation | [47] |
| Selumetinib | Melanoma | Patient biopsy | MEK1P124L | Acquired | ERK1/2 reactivation | [46] |
| Selumetinib | Melanoma | Patient biopsy, cell lines | c-MET up-expression, LEF1 down-expression, YAP1 signature enrichment | Acquired | ERK1/2 reactivation | [81] |
| Selumetinib | Colorectal cancer | Cell lines | BRAF amplification | Acquired | ERK1/2 reactivation | [112] |
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer Genome Atlas, N., Genomic Classification of Cutaneous Melanoma. Cell, 2015. 161(7): p. 1681-96.
- Cheng, L., et al., Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward precision medicine. Mod Pathol, 2018. 31(1): p. 24-38. [CrossRef]
- Cohen, Y., et al., BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst, 2003. 95(8): p. 625-7. [CrossRef]
- Gonsalves, W.I., et al., Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst, 2014. 106(7). [CrossRef]
- Xu, X., et al., High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res, 2003. 63(15): p. 4561-7.
- Karoulia, Z., E. Gavathiotis, and P.I. Poulikakos, New perspectives for targeting RAF kinase in human cancer. Nat Rev Cancer, 2017. 17(11): p. 676-691. [CrossRef]
- Wu, P.K. and J.I. Park, MEK1/2 Inhibitors: Molecular Activity and Resistance Mechanisms. Semin Oncol, 2015. 42(6): p. 849-62.
- Flaherty, K.T., et al., Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med, 2010. 363(9): p. 809-19.
- Flaherty, K.T., et al., Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med, 2012. 367(18): p. 1694-703. [CrossRef]
- Long, G.V., et al., Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet, 2015. 386(9992): p. 444-51. [CrossRef]
- Hauschild, A., et al., Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet, 2012. 380(9839): p. 358-65. [CrossRef]
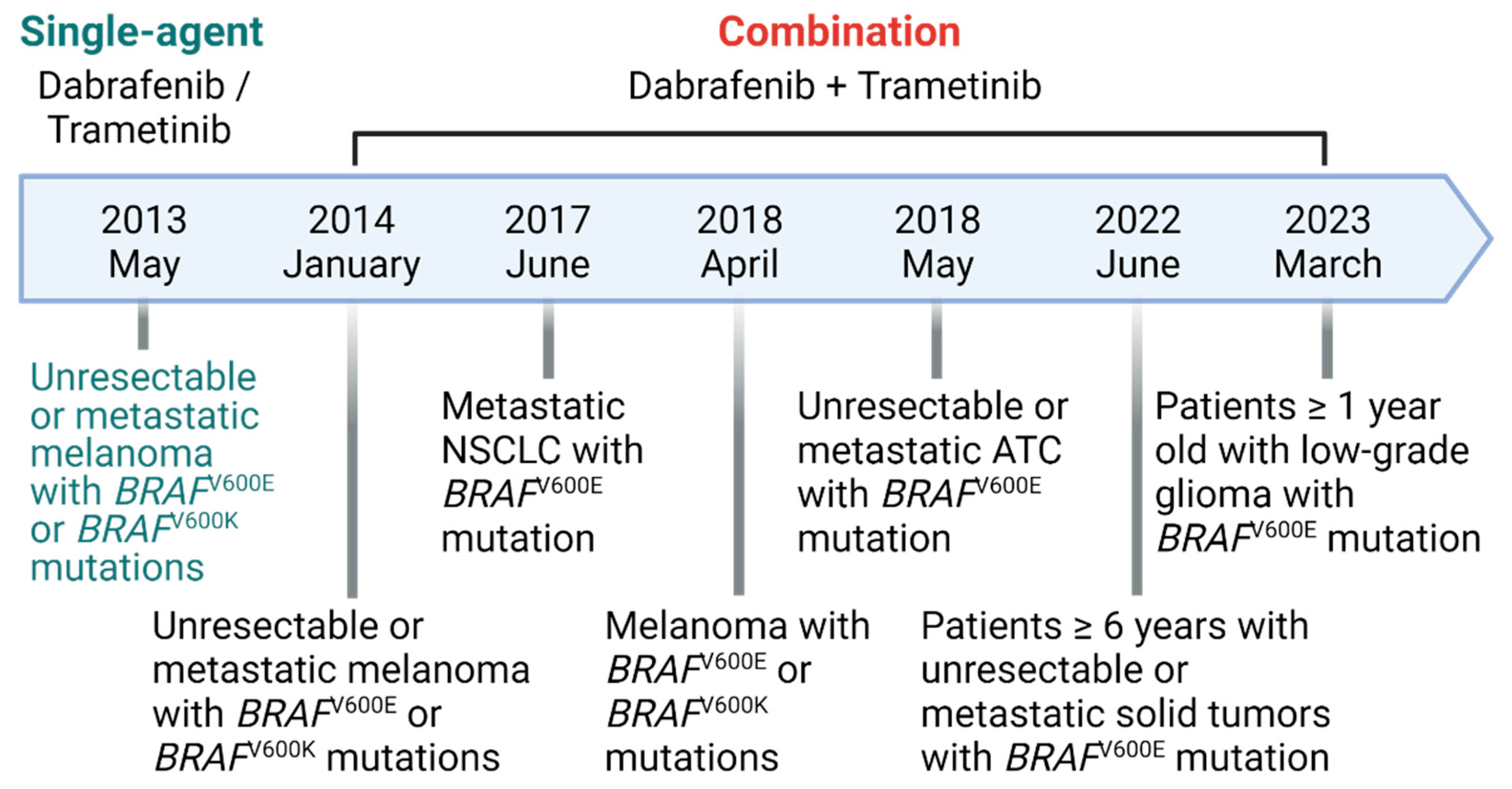
- Salama, A.K.S., et al., Dabrafenib and Trametinib in Patients With Tumors With BRAF(V600E) Mutations: Results of the NCI-MATCH Trial Subprotocol H. J Clin Oncol, 2020. 38(33): p. 3895-3904.
- FDA. FDA approves dabrafenib plus trametinib for adjuvant treatment of melanoma with BRAF V600E or V600K mutations. 2018; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dabrafenib-plus-trametinib-adjuvant-treatment-melanoma-braf-v600e-or-v600k-mutations.
- FDA. FDA approves new uses for two drugs administered together for the treatment of BRAF-positive anaplastic thyroid cancer. 2018; Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-uses-two-drugs-administered-together-treatment-braf-positive-anaplastic-thyroid.
- FDA. FDA grants regular approval to dabrafenib and trametinib combination for metastatic NSCLC with BRAF V600E mutation. 2017; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-dabrafenib-and-trametinib-combination-metastatic-nsclc-braf-v600e.
- Corcoran, R.B., et al., Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol, 2015. 33(34): p. 4023-31. [CrossRef]
- Kopetz, S., et al., Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol, 2015. 33(34): p. 4032-8. [CrossRef]
- FDA. FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. 2022; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid.
- FDA. FDA approves dabrafenib with trametinib for pediatric patients with low-grade glioma with a BRAF V600E mutation. 2023; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dabrafenib-trametinib-pediatric-patients-low-grade-glioma-braf-v600e-mutation.
- Puzanov, I., P. Burnett, and K.T. Flaherty, Biological challenges of BRAF inhibitor therapy. Mol Oncol, 2011. 5(2): p. 116-23. [CrossRef]
- Solit, D.B. and N. Rosen, Resistance to BRAF inhibition in melanomas. N Engl J Med, 2011. 364(8): p. 772-4. [CrossRef]
- Villanueva, J., A. Vultur, and M. Herlyn, Resistance to BRAF inhibitors: unraveling mechanisms and future treatment options. Cancer Res, 2011. 71(23): p. 7137-40. [CrossRef]
- Alcala, A.M. and K.T. Flaherty, BRAF inhibitors for the treatment of metastatic melanoma: clinical trials and mechanisms of resistance. Clin Cancer Res, 2012. 18(1): p. 33-9. [CrossRef]
- Paraiso, K.H., et al., Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer, 2010. 102(12): p. 1724-30. [CrossRef]
- Sala, E., et al., BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Mol Cancer Res, 2008. 6(5): p. 751-9. [CrossRef]
- Hatzivassiliou, G., et al., ERK inhibition overcomes acquired resistance to MEK inhibitors. Mol Cancer Ther, 2012. 11(5): p. 1143-54.
- Greger, J.G., et al., Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther, 2012. 11(4): p. 909-20.
- Ahronian, L.G., et al., Clinical Acquired Resistance to RAF Inhibitor Combinations in BRAF-Mutant Colorectal Cancer through MAPK Pathway Alterations. Cancer Discov, 2015. 5(4): p. 358-67.
- Prahallad, A., et al., Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature, 2012. 483(7387): p. 100-3. [CrossRef]
- Montero-Conde, C., et al., Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov, 2013. 3(5): p. 520-33.
- Fattore, L., et al., Combination of antibodies directed against different ErbB3 surface epitopes prevents the establishment of resistance to BRAF/MEK inhibitors in melanoma. Oncotarget, 2015. 6(28): p. 24823-41. [CrossRef]
- Nazarian, R., et al., Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature, 2010. 468(7326): p. 973-7. [CrossRef]
- Heidorn, S.J., et al., Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell, 2010. 140(2): p. 209-21. [CrossRef]
- Hatzivassiliou, G., et al., RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature, 2010. 464(7287): p. 431-5. [CrossRef]
- Poulikakos, P.I., et al., RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature, 2010. 464(7287): p. 427-30.
- Cabanillas, M.E., et al., Acquired Secondary RAS Mutation in BRAF(V600E)-Mutated Thyroid Cancer Patients Treated with BRAF Inhibitors. Thyroid, 2020. 30(9): p. 1288-1296.
- Lidsky, M., et al., Mitogen-activated protein kinase (MAPK) hyperactivation and enhanced NRAS expression drive acquired vemurafenib resistance in V600E BRAF melanoma cells. J Biol Chem, 2014. 289(40): p. 27714-26. [CrossRef]
- Oddo, D., et al., Molecular Landscape of Acquired Resistance to Targeted Therapy Combinations in BRAF-Mutant Colorectal Cancer. Cancer Res, 2016. 76(15): p. 4504-15.
- Long, G.V., et al., Increased MAPK reactivation in early resistance to dabrafenib/trametinib combination therapy of BRAF-mutant metastatic melanoma. Nat Commun, 2014. 5: p. 5694. [CrossRef]
- Wagle, N., et al., MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer Discov, 2014. 4(1): p. 61-8.
- Johnson, D.B., et al., BRAF internal deletions and resistance to BRAF/MEK inhibitor therapy. Pigment Cell Melanoma Res, 2018. 31(3): p. 432-436. [CrossRef]
- Chen, S.H., et al., Oncogenic BRAF Deletions That Function as Homodimers and Are Sensitive to Inhibition by RAF Dimer Inhibitor LY3009120. Cancer Discov, 2016. 6(3): p. 300-15.
- Foster, S.A., et al., Activation Mechanism of Oncogenic Deletion Mutations in BRAF, EGFR, and HER2. Cancer Cell, 2016. 29(4): p. 477-493. [CrossRef]
- Niu, Y., Y. Zhang, and X. Yao, Resistance mechanism of the oncogenic beta3-alphaC deletion mutation in BRAF kinase to dabrafenib and vemurafenib revealed by molecular dynamics simulations and binding free energy calculations. Chem Biol Drug Des, 2019. 93(2): p. 177-187.
- Wagle, N., et al., Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol, 2011. 29(22): p. 3085-96.
- Emery, C.M., et al., MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A, 2009. 106(48): p. 20411-6.
- Little, A.S., et al., Amplification of the driving oncogene, KRAS or BRAF, underpins acquired resistance to MEK1/2 inhibitors in colorectal cancer cells. Sci Signal, 2011. 4(166): p. ra17.
- Askari, N., et al., MAP-quest: could we produce constitutively active variants of MAP kinases? Mol Cell Endocrinol, 2006. 252(1-2): p. 231-40.
- Emrick, M.A., et al., Constitutive activation of extracellular signal-regulated kinase 2 by synergistic point mutations. J Biol Chem, 2001. 276(49): p. 46469-79. [CrossRef]
- Emrick, M.A., et al., The gatekeeper residue controls autoactivation of ERK2 via a pathway of intramolecular connectivity. Proc Natl Acad Sci U S A, 2006. 103(48): p. 18101-6. [CrossRef]
- Levin-Salomon, V., et al., Isolation of intrinsically active (MEK-independent) variants of the ERK family of mitogen-activated protein (MAP) kinases. J Biol Chem, 2008. 283(50): p. 34500-10. [CrossRef]
- Wu, P.K., A. Becker, and J.I. Park, Growth Inhibitory Signaling of the Raf/MEK/ERK Pathway. Int J Mol Sci, 2020. 21(15). [CrossRef]
- Wu, P.K., et al., Active ERK2 is sufficient to mediate growth arrest and differentiation signaling. FEBS J, 2015. 282(6): p. 1017-30. [CrossRef]
- Smorodinsky-Atias, K., N. Soudah, and D. Engelberg, Mutations That Confer Drug-Resistance, Oncogenicity and Intrinsic Activity on the ERK MAP Kinases-Current State of the Art. Cells, 2020. 9(1).
- Ojha, R., et al., ER Translocation of the MAPK Pathway Drives Therapy Resistance in BRAF-Mutant Melanoma. Cancer Discov, 2019. 9(3): p. 396-415. [CrossRef]
- Qin, Y., et al., PERK mediates resistance to BRAF inhibition in melanoma with impaired PTEN. NPJ Precis Oncol, 2021. 5(1): p. 68. [CrossRef]
- Hazar-Rethinam, M., et al., Convergent Therapeutic Strategies to Overcome the Heterogeneity of Acquired Resistance in BRAF(V600E) Colorectal Cancer. Cancer Discov, 2018. 8(4): p. 417-427.
- Song, Y., et al., Targeting RAS-RAF-MEK-ERK signaling pathway in human cancer: Current status in clinical trials. Genes Dis, 2023. 10(1): p. 76-88.
- Shi, H., et al., Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov, 2014. 4(1): p. 80-93. [CrossRef]
- Solit, D.B. and N. Rosen, Towards a unified model of RAF inhibitor resistance. Cancer Discov, 2014. 4(1): p. 27-30. [CrossRef]
- Zuo, Q., et al., AXL/AKT axis mediated-resistance to BRAF inhibitor depends on PTEN status in melanoma. Oncogene, 2018. 37(24): p. 3275-3289. [CrossRef]
- Wang, B., et al., Targeting mTOR signaling overcomes acquired resistance to combined BRAF and MEK inhibition in BRAF-mutant melanoma. Oncogene, 2021. 40(37): p. 5590-5599. [CrossRef]
- Shi, H., et al., A novel AKT1 mutant amplifies an adaptive melanoma response to BRAF inhibition. Cancer Discov, 2014. 4(1): p. 69-79. [CrossRef]
- Perna, D., et al., BRAF inhibitor resistance mediated by the AKT pathway in an oncogenic BRAF mouse melanoma model. Proc Natl Acad Sci U S A, 2015. 112(6): p. E536-45. [CrossRef]
- Villanueva, J., et al., Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell, 2010. 18(6): p. 683-95.
- Patel, H., et al., IGF1R/IR Mediates Resistance to BRAF and MEK Inhibitors in BRAF-Mutant Melanoma. Cancers (Basel), 2021. 13(22). [CrossRef]
- Barras, D., et al., BRAF V600E Mutant Colorectal Cancer Subtypes Based on Gene Expression. Clin Cancer Res, 2017. 23(1): p. 104-115. [CrossRef]
- Middleton, G., et al., BRAF-Mutant Transcriptional Subtypes Predict Outcome of Combined BRAF, MEK, and EGFR Blockade with Dabrafenib, Trametinib, and Panitumumab in Patients with Colorectal Cancer. Clin Cancer Res, 2020. 26(11): p. 2466-2476.
- Fofaria, N.M., et al., Overexpression of Mcl-1 confers resistance to BRAFV600E inhibitors alone and in combination with MEK1/2 inhibitors in melanoma. Oncotarget, 2015. 6(38): p. 40535-56.
- Shao, Y. and A.E. Aplin, BH3-only protein silencing contributes to acquired resistance to PLX4720 in human melanoma. Cell Death Differ, 2012. 19(12): p. 2029-39. [CrossRef]
- Boussemart, L., et al., eIF4F is a nexus of resistance to anti-BRAF and anti-MEK cancer therapies. Nature, 2014. 513(7516): p. 105-9. [CrossRef]
- Johannessen, C.M., et al., A melanocyte lineage program confers resistance to MAP kinase pathway inhibition. Nature, 2013. 504(7478): p. 138-42. [CrossRef]
- Ramsdale, R., et al., The transcription cofactor c-JUN mediates phenotype switching and BRAF inhibitor resistance in melanoma. Sci Signal, 2015. 8(390): p. ra82. [CrossRef]
- Kong, X., et al., Cancer drug addiction is relayed by an ERK2-dependent phenotype switch. Nature, 2017. 550(7675): p. 270-274. [CrossRef]
- Kitai, H., et al., Epithelial-to-Mesenchymal Transition Defines Feedback Activation of Receptor Tyrosine Kinase Signaling Induced by MEK Inhibition in KRAS-Mutant Lung Cancer. Cancer Discov, 2016. 6(7): p. 754-69.
- Brighton, H.E., et al., New Mechanisms of Resistance to MEK Inhibitors in Melanoma Revealed by Intravital Imaging. Cancer Res, 2018. 78(2): p. 542-557. [CrossRef]
- Girotti, M.R., et al., Inhibiting EGF receptor or SRC family kinase signaling overcomes BRAF inhibitor resistance in melanoma. Cancer Discov, 2013. 3(2): p. 158-67.
- Ebbing, E.A., et al., Stromal-derived interleukin 6 drives epithelial-to-mesenchymal transition and therapy resistance in esophageal adenocarcinoma. Proc Natl Acad Sci U S A, 2019. [CrossRef]
- Manore, S.G., et al., IL-6/JAK/STAT3 Signaling in Breast Cancer Metastasis: Biology and Treatment. Front Oncol, 2022. 12: p. 866014. [CrossRef]
- Lin, L., et al., The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet, 2015. 47(3): p. 250-6. [CrossRef]
- Hugo, W., et al., Non-genomic and Immune Evolution of Melanoma Acquiring MAPKi Resistance. Cell, 2015. 162(6): p. 1271-85. [CrossRef]
- Kim, M.H., et al., Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J, 2016. 35(5): p. 462-78. [CrossRef]
- Muranen, T., et al., ERK and p38 MAPK Activities Determine Sensitivity to PI3K/mTOR Inhibition via Regulation of MYC and YAP. Cancer Res, 2016. 76(24): p. 7168-7180. [CrossRef]
- Fisher, M.L., et al., Inhibition of YAP function overcomes BRAF inhibitor resistance in melanoma cancer stem cells. Oncotarget, 2017. 8(66): p. 110257-110272. [CrossRef]
- Haq, R., et al., Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Cancer Cell, 2013. 23(3): p. 302-15. [CrossRef]
- Haq, R., D.E. Fisher, and H.R. Widlund, Molecular pathways: BRAF induces bioenergetic adaptation by attenuating oxidative phosphorylation. Clin Cancer Res, 2014. 20(9): p. 2257-63. [CrossRef]
- Smith, M.P., et al., The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFalpha. Cancer Discov, 2014. 4(10): p. 1214-29.
- Van Allen, E.M., et al., The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov, 2014. 4(1): p. 94-109. [CrossRef]
- Smith, M.P., et al., Inhibiting Drivers of Non-mutational Drug Tolerance Is a Salvage Strategy for Targeted Melanoma Therapy. Cancer Cell, 2016. 29(3): p. 270-284. [CrossRef]
- Ullmann, T.M., et al., Dual inhibition of BRAF and MEK increases expression of sodium iodide symporter in patient-derived papillary thyroid cancer cells in vitro. Surgery, 2020. 167(1): p. 56-63. [CrossRef]
- Leboulleux, S., et al., A Phase II Redifferentiation Trial with Dabrafenib-Trametinib and 131I in Metastatic Radioactive Iodine Refractory BRAF p.V600E-Mutated Differentiated Thyroid Cancer. Clin Cancer Res, 2023. 29(13): p. 2401-2409.
- Schubert, L., et al., MAPK Pathway Inhibitors in Thyroid Cancer: Preclinical and Clinical Data. Cancers (Basel), 2023. 15(3). [CrossRef]
- Frederick, D.T., et al., BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res, 2013. 19(5): p. 1225-31.
- Hong, A., et al., Durable Suppression of Acquired MEK Inhibitor Resistance in Cancer by Sequestering MEK from ERK and Promoting Antitumor T-cell Immunity. Cancer Discov, 2021. 11(3): p. 714-735. [CrossRef]
- Ribas, A., et al., Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med, 2019. 25(6): p. 936-940. [CrossRef]
- Ascierto, P.A., et al., Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med, 2019. 25(6): p. 941-946. [CrossRef]
- Dummer, R., et al., Combined PD-1, BRAF and MEK inhibition in advanced BRAF-mutant melanoma: safety run-in and biomarker cohorts of COMBI-i. Nat Med, 2020. 26(10): p. 1557-1563. [CrossRef]
- Tian, J., et al., Combined PD-1, BRAF and MEK inhibition in BRAF(V600E) colorectal cancer: a phase 2 trial. Nat Med, 2023. 29(2): p. 458-466.
- Erkes, D.A., et al., Mutant BRAF and MEK Inhibitors Regulate the Tumor Immune Microenvironment via Pyroptosis. Cancer Discov, 2020. 10(2): p. 254-269. [CrossRef]
- Pieper, N., et al., Evolution of melanoma cross-resistance to CD8(+) T cells and MAPK inhibition in the course of BRAFi treatment. Oncoimmunology, 2018. 7(8): p. e1450127. [CrossRef]
- Dummer, R., et al., Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600-Mutant Unresectable or Metastatic Melanoma. J Clin Oncol, 2022. 40(13): p. 1428-1438.
- Maeda, T., T. Yanagi, and H. Ujiie, Lessons from clinical trials on triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF-mutant melanoma. Ann Transl Med, 2023. 11(9): p. 326. [CrossRef]
- Ascierto, P.A. and R. Dummer, Immunological effects of BRAF+MEK inhibition. Oncoimmunology, 2018. 7(9): p. e1468955. [CrossRef]
- Dry, J.R., et al., Transcriptional pathway signatures predict MEK addiction and response to selumetinib (AZD6244). Cancer Res, 2010. 70(6): p. 2264-73.
- Loboda, A., et al., A gene expression signature of RAS pathway dependence predicts response to PI3K and RAS pathway inhibitors and expands the population of RAS pathway activated tumors. BMC Med Genomics, 2010. 3: p. 26. [CrossRef]
- Phadke, M., et al., Dabrafenib inhibits the growth of BRAF-WT cancers through CDK16 and NEK9 inhibition. Mol Oncol, 2018. 12(1): p. 74-88. [CrossRef]
- Oliveira, E.A., et al., TOP1 modulation during melanoma progression and in adaptative resistance to BRAF and MEK inhibitors. Pharmacol Res, 2021. 173: p. 105911.
- Yadav, V., et al., Reactivation of mitogen-activated protein kinase (MAPK) pathway by FGF receptor 3 (FGFR3)/Ras mediates resistance to vemurafenib in human B-RAF V600E mutant melanoma. J Biol Chem, 2012. 287(33): p. 28087-98.
- Kaplan, F.M., et al., SHOC2 and CRAF mediate ERK1/2 reactivation in mutant NRAS-mediated resistance to RAF inhibitor. J Biol Chem, 2012. 287(50): p. 41797-807.
- Lito, P., et al., Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell, 2012. 22(5): p. 668-82. [CrossRef]
- Johannessen, C.M., et al., COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature, 2010. 468(7326): p. 968-72.
- Corcoran, R.B., et al., BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal, 2010. 3(149): p. ra84. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





