Submitted:
02 September 2023
Posted:
05 September 2023
You are already at the latest version
Abstract
Keywords:
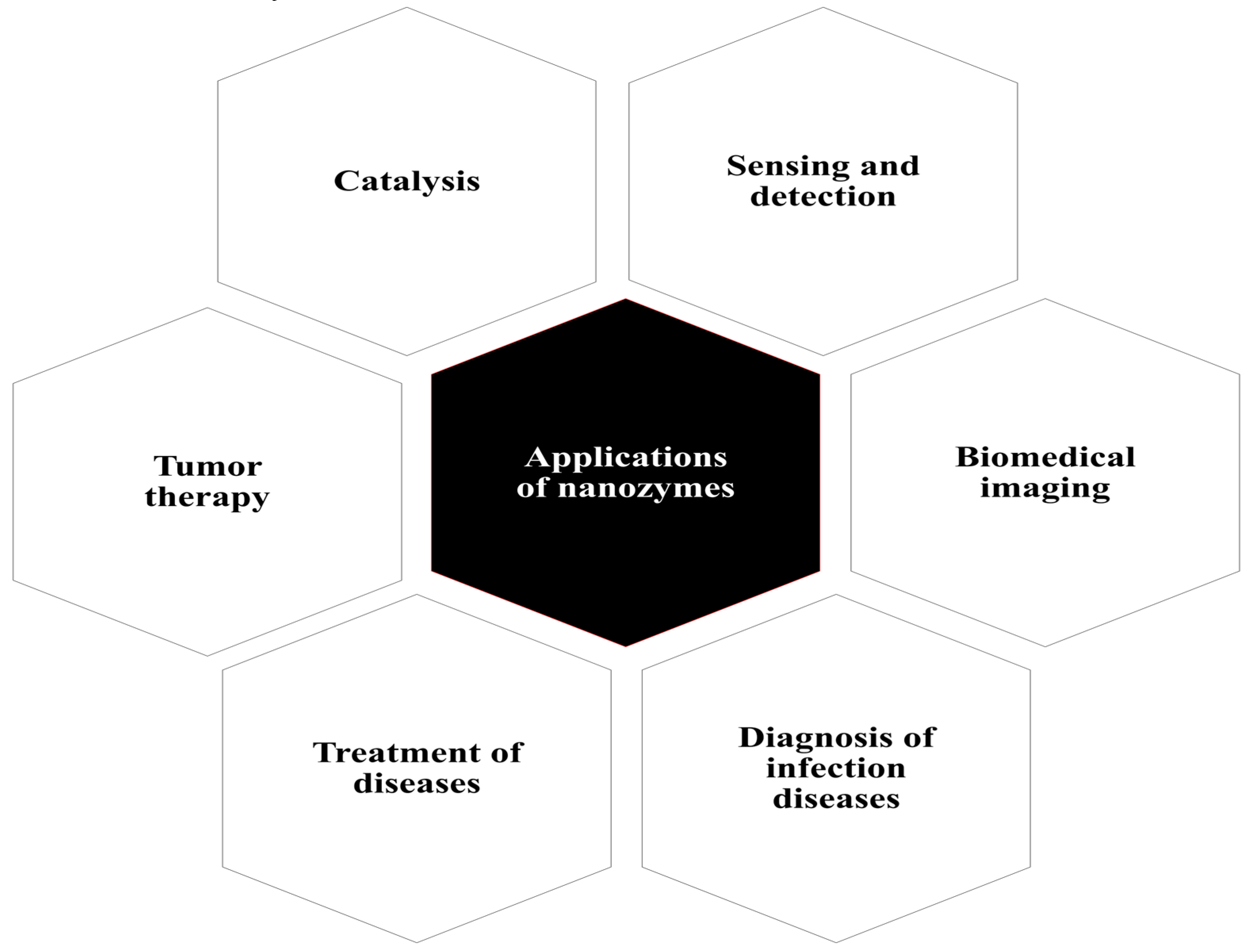
1. Nanozymes: Introduction
2. Historical Review of Nanozyme-Based Sensors
3. Perspectives
- ✓
- Developing nanozymes with higher catalytic efficiency and higher substrate affinity in their native form.
- ✓
- Developing nanozymes with intrinsic activity of commercial enzymes such as lipase and urease for application in industrial process in real world.
- ✓
- Extending the multinanozyme systems for improving sensitivity and selectivity of nanozymatic sensors
- ✓
- Design of biocompatible nanozymes with drug-like properties for treatment of diseases with minimal side effects.
- ✓
- Developing simple surface modification of nanozymes for enhancing their specificity.
- ✓
- Evaluating biochemical behavior of nanozymes for better understanding their best performances
- ✓
- Developing reusable nanozymes with high cycling stability and simple recovery suitable for real practical applications
- ✓
- Design of specific nanozyme-based sensors compared of current selective sensors
- ✓
- etc.
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2018, 48, 1004–1076. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Yan, X.; Fan, K. Structure and activity of nanozymes: Inspirations for de novo design of nanozymes. Mater. Today 2020, 41, 81–119. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, X.; Fan, K. Nanozymes Inspired by Natural Enzymes. Accounts Mater. Res. 2021, 2, 534–547. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Wei, H. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond. Inorg. Chem. Front. 2015, 3, 41–60. [Google Scholar] [CrossRef]
- Jiao, L.; Yan, H.; Wu, Y.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. When nanozymes meet single-atom catalysis. Angew. Chem. 2020, 132, 2585–2596. [Google Scholar]
- Liu, B.; Liu, J. Surface modification of nanozymes. Nano Res. 2017, 10, 1125–1148. [Google Scholar] [CrossRef]
- Wang, D.; Jana, D.; Zhao, Y. Metal–Organic Framework Derived Nanozymes in Biomedicine. Accounts Chem. Res. 2020, 53, 1389–1400. [Google Scholar] [CrossRef]
- Dong, S.; Dong, Y.; Jia, T.; Liu, S.; Liu, J.; Yang, D.; Lin, J. GSH-depleted nanozymes with hyperthermia-enhanced dual enzyme-mimic activities for tumor nanocatalytic therapy. Adv. Mater. 2020, 32, 2002439. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.R.H. Low-temperature destructive hydrodechlorination of long-chain chlorinated paraffins to diesel and gasoline range hydrocarbons over a novel low-cost reusable ZSM-5@Al-MCM nanocatalyst: A new approach toward reuse instead of common mineralization. Chem. Pap. 2023, 77, 4963–4977. [Google Scholar] [CrossRef]
- Hormozijangi, S.R.; Akhond, M. High throughput green reduction of tris (p-nitrophenyl) amine at ambient temperature over homogenous AgNPs as H-transfer catalyst. J. Chem. Sci. 2020, 132, 1–8. [Google Scholar]
- Dehghani, Z.; Akhond, M.; Jangi, S.R.H.; Absalan, G. Highly sensitive enantioselective spectrofluorimetric determination of R-/S-mandelic acid using l-tryptophan-modified amino-functional silica-coated N-doped carbon dots as novel high-throughput chiral nanoprobes. Talanta 2024, 266, 124977. [Google Scholar]
- Jangi, S.R.H.; Gholamhosseinzadeh, E. Developing an ultra-reproducible and ultrasensitive label-free nanoassay for L-methionine quantification in biological samples toward application in homocystinuria diagnosis. Chem. Pap. 2023, 1–13. [Google Scholar] [CrossRef]
- Jangi, S.R.H.; Akhond, M. Ultrasensitive label-free enantioselective quantification of d-/l-leucine enantiomers with a novel detection mechanism using an ultra-small high-quantum yield N-doped CDs prepared by a novel highly fast solvent-free method. Sens. Actuators B Chem. 2021, 339, 129901. [Google Scholar]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Jangi, S.R.H. Synthesis and characterization of magnesium-based metal-organic frameworks and investigating the effect of coordination solvent on their biocompatibility. 2023.
- Hormozi Jangi, S.R. Biochemical characterization of enzyme-like silver nanoparticles toward nanozyme-catalysed oxidation reactions. Micromater. Interfaces 2023, 1, 2170. [Google Scholar]
- Hormozi Jangi, S.R. Evaluation of Biochemical Behavior and Stability of Gold Nanoparticles with High Intrinsic Peroxidase-Like Activity. Petro. Chem. Indus. Intern. 2023, 6, 234–239. [Google Scholar]
- Jangi, S.R.H. Introducing a High Throughput Nanozymatic Method for Eco-Friendly Nanozyme-Mediated Degradation of Methylene Blue in Real Water Media. Sustain. Chem. Eng. 2023, 90–99. [Google Scholar] [CrossRef]
- Hormozi Jangi, S.R.; Dehghani, Z. Kinetics and biochemical characterization of silver nanozymes and investigating impact of storage conditions on their activity and shelf-life. Chem. Res. Nanomater. 2023, 1, 25–33. [Google Scholar]
- Jangi, S.R.H. Determining kinetics parameters of bovine serum albumin-protected gold nanozymes toward different substrates. Qeios 2023. [Google Scholar] [CrossRef]
- Jangi, S.R.H. Effect of daylight and air oxygen on nanozymatic activity of unmodified silver nanoparticles: Shelf-stability. Qeios 2023. [Google Scholar] [CrossRef]
- Ahmadi-Leilakouhi, B.; Jangi, S.R.H.; Khorshidi, A. Introducing a novel photo-induced nanozymatic method for high throughput reusable biodegradation of organic dyes. Chem. Pap. 2022, 77, 1033–1046. [Google Scholar] [CrossRef]
- Hormozi Jangi, S.R. A Comparative Study on Kinetics Performances of BSA-gold Nanozymes for Nanozyme-mediated Oxidation of 3,3',5,5'-Tetramethylbenzidine and 3,3'-Diaminobenzidine. Biochem. Mol. Biol. J. 2023, 9, 21. [Google Scholar]
- Jangi, S.R.H.; Akhond, M. High throughput urease immobilization onto a new metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) for water treatment and safe blood cleaning. Process. Biochem. 2021, 105, 79–90. [Google Scholar] [CrossRef]
- Jangi, S.R.H.; Akhond, M. Introducing a covalent thiol-based protected immobilized acetylcholinesterase with enhanced enzymatic performances for biosynthesis of esters. Process. Biochem. 2022, 120, 138–155. [Google Scholar] [CrossRef]
- Jangi, S.R.H.; Akhond, M.; Dehghani, Z. High throughput covalent immobilization process for improvement of shelf-life, operational cycles, relative activity in organic media and enzymatic kinetics of urease and its application for urea removal from water samples. Process. Biochem. 2019, 90, 102–112. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Lu, W.; Guo, Y.; Zhang, J.; Yue, Y.; Fan, L.; Li, F.; Dong, C.; Shuang, S. A High Catalytic Activity Nanozyme Based on Cobalt-Doped Carbon Dots for Biosensor and Anticancer Cell Effect. ACS Appl. Mater. Interfaces 2022, 14, 57206–57214. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, D.; Wang, Y.; Li, H.; Zhang, Y.; Chen, H.; Li, X.; Huo, M. Nanozymes-recent development and biomedical applications. J. Nanobiotechnol. 2022, 20, 1–18. [Google Scholar] [CrossRef]
- Tang, G.; He, J.; Liu, J.; Yan, X.; Fan, K. Nanozyme for tumor therapy: Surface modification matters. Exploration 2021, 1, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, H.; Hou, Y.; Wang, X.; Xue, C.; Li, W.; Cai, K.; Zhao, Y.; Luo, Z. State-of-the-art iron-based nanozymes for biocatalytic tumor therapy. Nanoscale Horizons 2019, 5, 202–217. [Google Scholar] [CrossRef]
- Yu, R.; Wang, R.; Wang, Z.; Zhu, Q.; Dai, Z. Applications of DNA-nanozyme-based sensors. Anal. 2021, 146, 1127–1141. [Google Scholar] [CrossRef]
- Chang, Y.; Gao, S.; Liu, M.; Liu, J. Designing signal-on sensors by regulating nanozyme activity. Anal. Methods 2020, 12, 4708–4723. [Google Scholar] [CrossRef]
- Arshad, F.; Mohd-Naim, N.F.; Chandrawati, R.; Cozzolino, D.; Ahmed, M.U. Nanozyme-based sensors for detection of food biomarkers: A review. RSC Adv. 2022, 12, 26160–26175. [Google Scholar] [CrossRef]
- Jangi, A.R.H.; Jangi, M.R.H.; Jangi, S.R.H. Detection mechanism and classification of design principles of peroxidase mimic based colorimetric sensors: A brief overview. Chin. J. Chem. Eng. 2020, 28, 1492–1503. [Google Scholar] [CrossRef]
- Jangi, S.R.H.; Akhond, M.; Absalan, G. A novel selective and sensitive multinanozyme colorimetric method for glutathione detection by using an indamine polymer. Anal. Chim. Acta 2020, 1127, 1–8. [Google Scholar] [CrossRef]
- Jangi, S.R.H.; Davoudli, H.K.; Delshad, Y.; Jangi, M.R.H.; Jangi, A.R.H. A novel and reusable multinanozyme system for sensitive and selective quantification of hydrogen peroxide and highly efficient degradation of organic dye. Surf. Interfaces 2020, 21, 100771. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, S.; Li, Y.; Liu, J. Facile Synthesis of Iron and Nitrogen Co-Doped Carbon Dot Nanozyme as Highly Efficient Peroxidase Mimics for Visualized Detection of Metabolites. Molecules 2023, 28, 6064. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.R.H.; Akhond, M. Synthesis and characterization of a novel metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) and its application for constructing a reusable nanozyme-based sensor for selective and sensitive glutathione quantification. Microchem. J. 2020, 158, 105328. [Google Scholar] [CrossRef]
- Ray, S.; Biswas, R.; Banerjee, R.; Biswas, P. A gold nanoparticle-intercalated mesoporous silica-based nanozyme for the selective colorimetric detection of dopamine. Nanoscale Adv. 2019, 2, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wei, Y.; Liu, Z.; Nie, C.; Ye, Y. Engineering of 2D artificial nanozyme-based blocking effect-triggered colorimetric sensor for onsite visual assay of residual tetracycline in milk. Microchim. Acta 2022, 189, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Akhond, M.; Jangi, S.R.H.; Barzegar, S.; Absalan, G. Introducing a nanozyme-based sensor for selective and sensitive detection of mercury(II) using its inhibiting effect on production of an indamine polymer through a stable n-electron irreversible system. Chem. Pap. 2019, 74, 1321–1330. [Google Scholar] [CrossRef]
- Chen, J.; Wu, W.; Huang, L.; Ma, Q.; Dong, S. (2019). Self-indicative gold nanozyme for H2O2 and glucose sensing. Chem.–A Eur. J. 2019, 25, 11940–11944. [Google Scholar] [CrossRef]
- Jangi, S.R.H.; Dehghani, Z. Spectrophotometric quantification of hydrogen peroxide utilizing silver nanozyme. 2023.
- Jangi, S.R.H.; Akhond, M.; Absalan, G. A field-applicable colorimetric assay for notorious explosive triacetone triperoxide through nanozyme-catalyzed irreversible oxidation of 3, 3′-diaminobenzidine. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef]
- Singh, M.; Weerathunge, P.; Liyanage, P.D.; Mayes, E.; Ramanathan, R.; Bansal, V. Competitive Inhibition of the Enzyme-Mimic Activity of Gd-Based Nanorods toward Highly Specific Colorimetric Sensing of l-Cysteine. Langmuir 2017, 33, 10006–10015. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Zhou, X.; Sun, H.; Su, X. Fluorescence sensing strategy for xanthine assay based on gold nanoclusters and nanozyme. Sensors Actuators B: Chem. 2022, 358, 131488. [Google Scholar] [CrossRef]
- Heo, N.S.; Song, H.P.; Lee, S.M.; Cho, H.-J.; Kim, H.J.; Huh, Y.S.; Kim, M.I. Rosette-shaped graphitic carbon nitride acts as a peroxidase mimic in a wide pH range for fluorescence-based determination of glucose with glucose oxidase. Microchim. Acta 2020, 187, 1–11. [Google Scholar] [CrossRef]
- Hormozi Jangi, S.R. A Brief Overview on Clinical and Epidemiological Features, Mechanism of Action, and Diagnosis of Novel Global Pandemic Infectious Disease, Covid-19, And its Comparison with Sars, Mers, And H1n1. World J. Clin. Med. Img. 2023, 2, 45–52. [Google Scholar]
- Jangi, S.R.H. Natural Polyphenols of Pomegranate and Black Tea Juices can Combat COVID-19 through their SARS-CoV-2 3C-like Protease-inhibitory Activity. Qeios. 2023. [Google Scholar]
- Liang, C.; Liu, B.; Li, J.; Lu, J.; Zhang, E.; Deng, Q.; Li, T. (2021). A nanoenzyme linked immunochromatographic sensor for rapid and quantitative detection of SARS-CoV-2 nucleocapsid protein in human blood. Sens. Actuators B Chem. 2021, 349, 130718. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ye, W.; Yang, Q.; Yu, J.; Wang, Q.; Zhou, P.; Wang, C.; Xue, D.; Zhao, S. Three-dimensional hierarchical porous PtCu dendrites: A highly efficient peroxidase nanozyme for colorimetric detection of H2O2. Sens. Actuators B Chem. 2016, 230, 721–730. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, P.; Kumar, N.; Nara, S. Colorimetric sensing of malathion using palladium-gold bimetallic nanozyme. Biosens. Bioelectron. 2017, 92, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, L.; Gao, C.; Zhao, C.; Wang, Y.; Wang, J. Hemin immobilized into metal–organic frameworks as an electrochemical biosensor for 2, 4, 6-trichlorophenol. Nanotechnology 2018, 29, 074003. [Google Scholar] [CrossRef]
- Chen, Y.; Ni, D.; Yang, X.; Liu, C.; Yin, J.; Cai, K. Microwave-assisted synthesis of honeycomblike hierarchical spherical Zn-doped Ni-MOF as a high-performance battery-type supercapacitor electrode material. Electrochim. Acta 2018, 278, 114–123. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, C.; Niu, Y.; Chen, J.; Zhao, Y.; Zhang, Y.; Gao, R.; He, J. Target triggered cleavage effect of DNAzyme: Relying on Pd-Pt alloys functionalized Fe-MOFs for amplified detection of Pb2+. Biosens. Bioelectron. 2018, 101, 297–303. [Google Scholar] [CrossRef]
- Li, Y.; Yu, C.; Yang, B.; Liu, Z.; Xia, P.; Wang, Q. Target-catalyzed hairpin assembly and metal-organic frameworks mediated nonenzymatic co-reaction for multiple signal amplification detection of miR-122 in human serum. Biosens. Bioelectron. 2018, 102, 307–315. [Google Scholar] [CrossRef]
- Lopa, N.S.; Rahman, M.; Ahmed, F.; Sutradhar, S.C.; Ryu, T.; Kim, W. A base-stable metal-organic framework for sensitive and non-enzymatic electrochemical detection of hydrogen peroxide. Electrochimica Acta 2018, 274, 49–56. [Google Scholar] [CrossRef]
- Wang, X.; Qin, L.; Zhou, M.; Lou, Z.; Wei, H. Nanozyme Sensor Arrays for Detecting Versatile Analytes from Small Molecules to Proteins and Cells. Anal. Chem. 2018, 90, 11696–11702. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Sheng, Y.; Xu, J.; Li, Y.; Lu, X.; Zhu, Y.; Duan, X.; Wen, Y. In-situ reduction of Ag+ on black phosphorene and its NH2-MWCNT nanohybrid with high stability and dispersibility as nanozyme sensor for three ATP metabolites. Biosens. Bioelectron. 2019, 145, 111716. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Han, L.; Wang, X.; Li, W.; Guo, H.; Wei, H. Nanozyme Sensor Arrays Based on Heteroatom-Doped Graphene for Detecting Pesticides. Anal. Chem. 2020, 92, 7444–7452. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Q.; Wang, X.; Zhu, Y.; Zhou, X.; Wei, H. Gold alloy-based nanozyme sensor arrays for biothiol detection. Anal. 2020, 145, 3916–3921. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Pelalak, R.; Pishnamazi, M.; Marjani, A.; Albadarin, A.B.; Sarkar, S.M.; Shirazian, S. Synthesis of multi-organo-functionalized fibrous silica KCC-1 for highly efficient adsorption of acid fuchsine and acid orange II from aqueous solution. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Butova, V.V.; Pankin, I.A.; Burachevskaya, O.A.; Vetlitsyna-Novikova, K.S.; Soldatov, A.V. New fast synthesis of MOF-801 for water and hydrogen storage: Modulator effect and recycling options. Inorganica Chim. Acta 2020, 514, 120025. [Google Scholar] [CrossRef]
- He, H.; Li, L.; Liu, Y.; Kassymova, M.; Li, D.; Zhang, L.; Jiang, H.-L. Rapid room-temperature synthesis of a porphyrinic MOF for encapsulating metal nanoparticles. Nano Res. 2020, 14, 444–449. [Google Scholar] [CrossRef]
- Kang, K.; Wang, B.; Ji, X.; Liu, Y.; Zhao, W.; Du, Y.; Guo, Z.; Ren, J. Hemin-doped metal–organic frameworks based nanozyme electrochemical sensor with high stability and sensitivity for dopamine detection. RSC Adv. 2021, 11, 2446–2452. [Google Scholar] [CrossRef]
- Hermosilla, E.; Seabra, A.B.; Lourenço, I.M.; Ferreira, F.F.; Tortella, G.; Rubilar, O. Highly sensitive oxidation of MBTH/DMAB by MnFe2O4 nanoparticles as a promising method for nanozyme-based sensor development. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.; Wu, X.; Xu, S.; Liu, M.; Cao, X.; Huang, H. MnO2 nanozyme-mediated CRISPR-Cas12a system for the detection of SARS-CoV-2. ACS Appl. Mater. Interfaces 2022, 14, 50534–50542. [Google Scholar] [CrossRef]
- He, M.; Xu, X.; Wang, H.; Wu, Q.; Zhang, L.; Zhou, D.; Liu, H. Nanozyme-Based Colorimetric SARS-CoV-2 Nucleic Acid Detection by Naked Eye. Small 2023, 19, 208167. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Jiang, M.; Hui, Y.; Huang, Y.; Kong, W.; Zhu, W.; Ji, L. Colorimetric immunosensing using liposome encapsulated MnO2 nanozymes for SARS-CoV-2 antigen detection. Biosens. Bioelectron. 2023, 239, 115623. [Google Scholar] [CrossRef] [PubMed]
- Vafabakhsh, M.; Dadmehr, M.; Noureini, S.K.; Es' haghi, Z.; Malekkiani, M.; Hosseini, M. Colorimetric detection of COVID-19 using aptasenor based on biomimetic peroxidase like activity of ChF/ZnO/CNT nano-hybrid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 301, 122980. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




