Submitted:
02 September 2023
Posted:
04 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and apparatus
2.2. Cultivation of Ganoderma mycelia
2.3. HPLC analysis of the triterpenes 1-19
2.4. Isolation of compounds 1-5 from YK-01 mycelium
2.5. Determination of ganoderic acid T (10) and S (12)
2.5.1. Sample pretreatment
2.5.2. Calibration curve
2.5.3. Recovery tests
2.5.4. Limit of detection and limit of quantitation
2.5.5. Accuracy and precision
3. Results
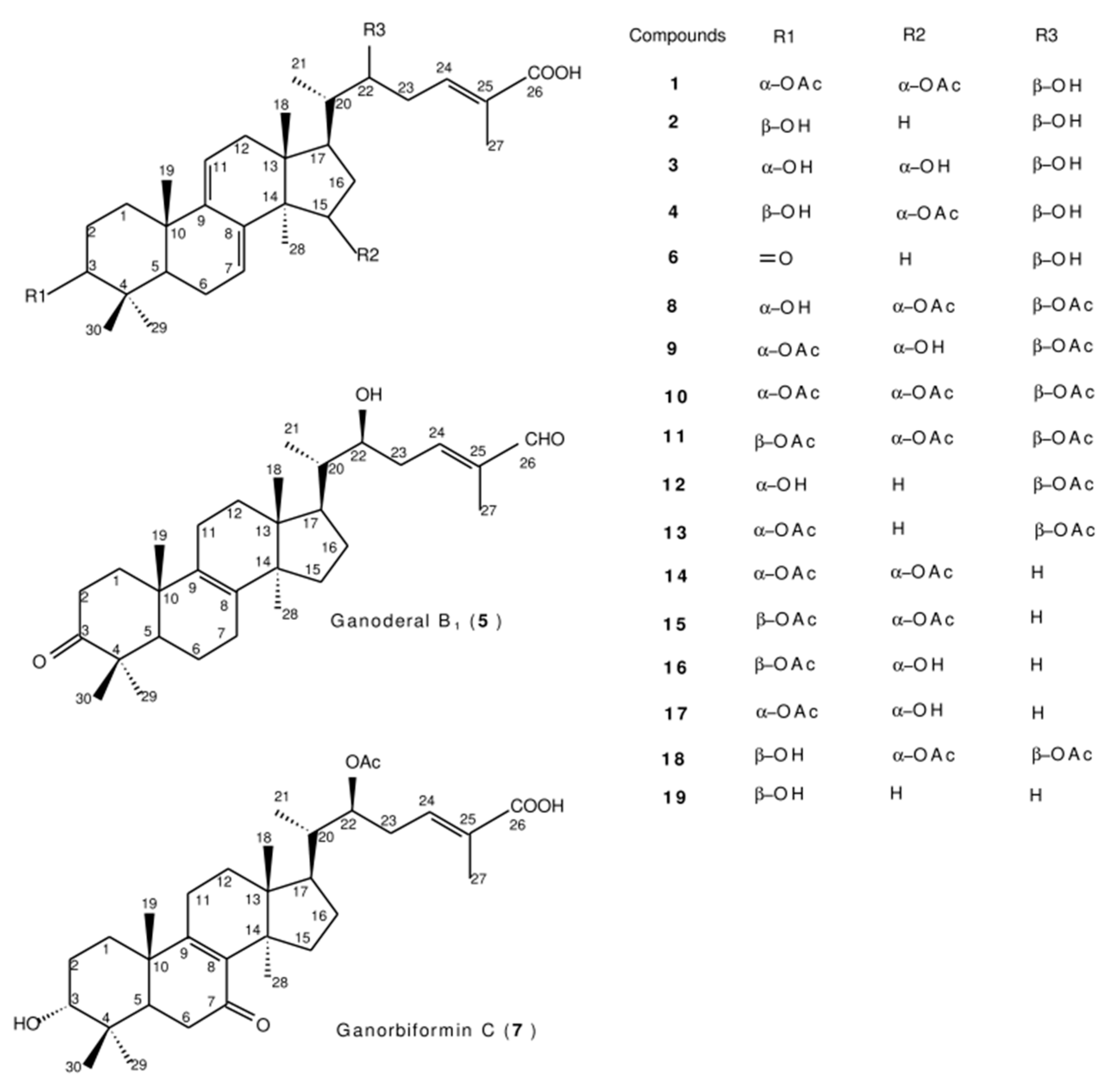
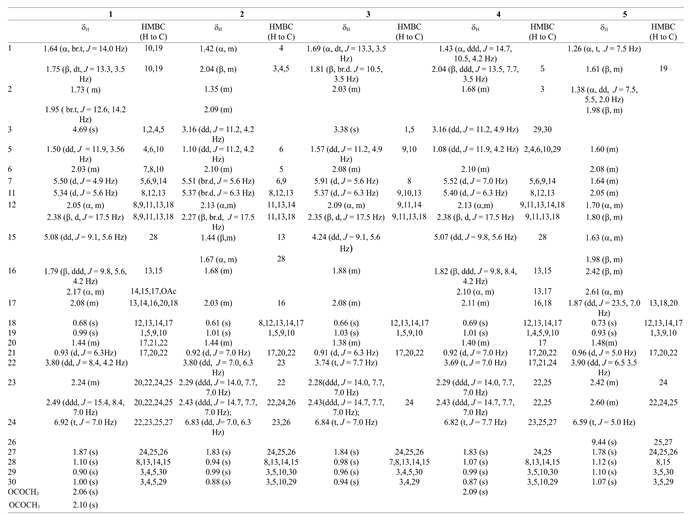
3.1. Structural identifications of the new triterpenes 1~5
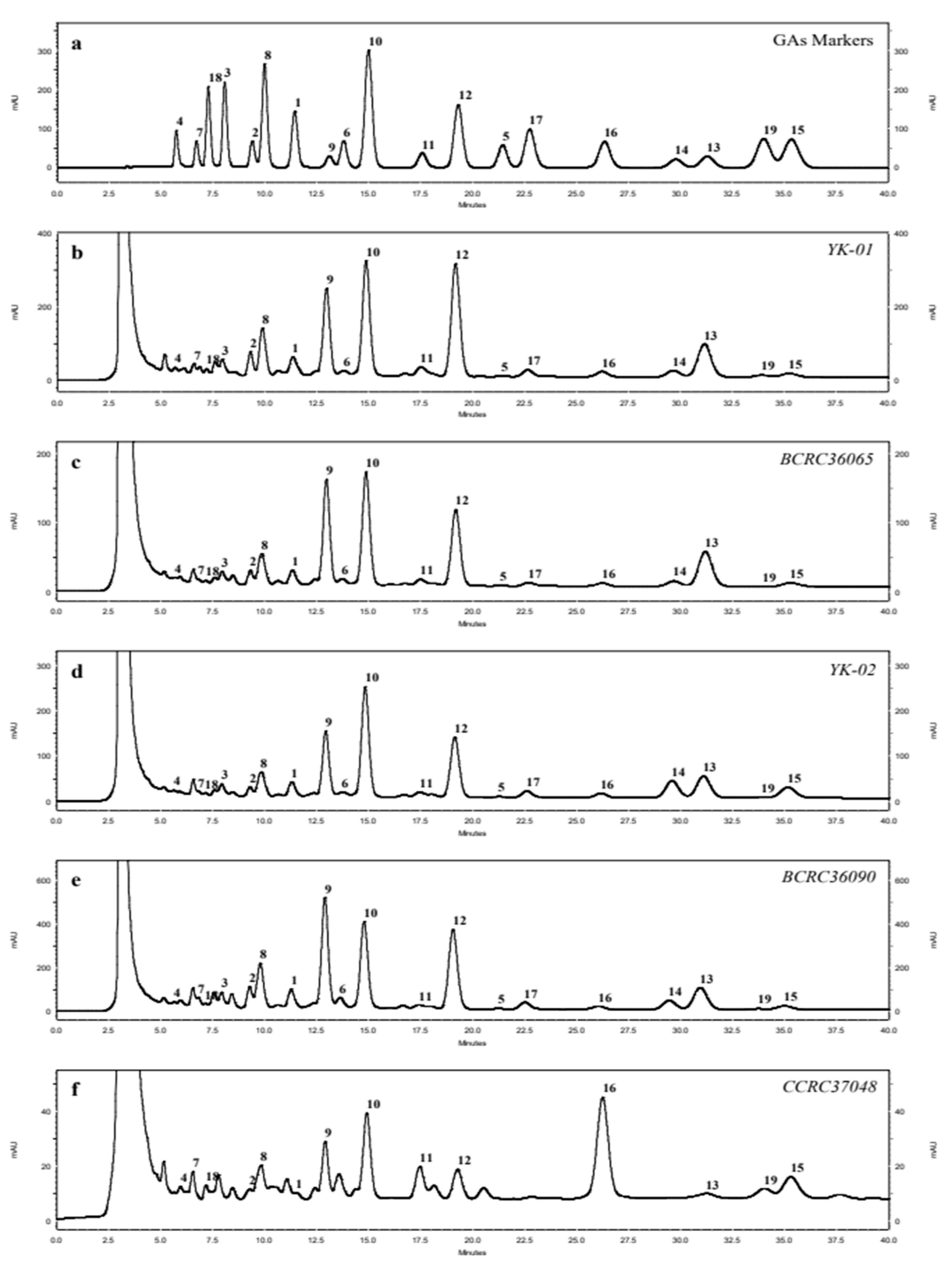
3.2. HPLC fingerprint profiles of triterpenes from Ganoderma mycelia
3.3. Methods for validation and quantitation of ganoderic acid T (10) and S (12)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cör, D.; Knez, Ž.; Hrnčič, M.K. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: a review. Molecules 2018, 23, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.H.; Ju, H.Y.; Sheu, K.C. Simple Fourier transform (FT)-IR and reverse-phase HPLC identification methods of commercial Ganoderma products. J. Chin. Chem. Soc. 2001, 48, 1207–1210. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, H.Z.; Sun, X.F.; Zhao, H.J.; Wu, L.F.; Zhu, D. , Yang, G.H.; Xin, Y.N.; Mao, L.Z.; Zhang, G.M. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.H.; Chen, K.D. Determination of ganoderic acids in triterpenoid constituents of Ganoderma tsugae. J. Food Drug Anal. 2003, 11, 195–200. [Google Scholar] [CrossRef]
- Chen, D.H.; Wang, J.Y.; Chen, M.T.; Chen, K.D. HPLC fingerprint profiles of lucidenic acids from Ganoderma lucidum (lingzhi). J. Chin. Chem. Soc. 2022, 69, 950–959. [Google Scholar] [CrossRef]
- You, B.J.; Lee H,Z. ; Chung, K.R.; Lee, M.H.; Huang, M.J.; Tien, N.; Chgen, C.W.; Kuo, Y.H. Enhanced production of ganoderic acids and cytotoxicity of Ganoderma lucidum using solid-medium culture. Biosci. Biotechnol. Biochem. 2012, 76, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.H.; Liu, J.W; Zhong, J.J. Ganoderic acid T inhibits tumor invasion in vitro and in vivo through inhibition of MMP expression. Pharmacol. Rep. 2010, 62, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.H.; Huang, C.P.; Chen, D.H.; Chen, K.D. Lin, S.B. Ganoderma triterpenoid ganoderic acid T inhibits growth and metastasis of A549 lung adenocarcinoma in vitro and in vivo. J. Chin. Oncl. Soc. 2009, 25, 413–420. [Google Scholar]
- Chyr, R.; Shiao, M.S. Liquid chromatographic characterization of the triterpenoid patterns in Ganoderma lucidum and related species. J. Chromatogr. 1991, 542, 327–336. [Google Scholar] [CrossRef]
- Hirotani, M.; Asaka, I.; Ino, C.; Furuya, T.; Shiro, M. Ganoderic acid derivatives and ergosta-4,7,22-triene-3,6-dione from Ganoderma lucidum. Phytochemistry 1987, 26, 2797–2803. [Google Scholar] [CrossRef]
- Lin, L.J.; Shiao, M.S. Seven new triterpenes from Ganoderma lucidum. J. Nat. Prod. 1988, 51, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Shiao, M.S.; Lin, L.J.; Yeh, S.F. Triterpenes in Ganoderma lucidum. Phytochemistry 1988, 27, 873–875. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Gao, X.X.; Yang, Y.C.; Chen, G.; Hou, G.L.; Huo, X.T.; Jia, X.M.; Wang, A.H.; Hu, G.S. Lanostane-type triterpenoids from the mycelial mat of Ganoderma lucidum and their hepatoprotective activities. Phytochemistry 2022, 198, 113131. [Google Scholar] [CrossRef] [PubMed]
- Nishitoba, T.; Sato, H.; Oda, K.; Sakamura, S. Novel triterpenoids and a steroid from the fungus Ganoderma luicidum. Agric. Biol. Chem. 1988, 52, 211–216. [Google Scholar]
- Paterson, R.R.M.; Lima, N. Failed PCR of Ganoderma type specimens affects nomenclature. Phytochemistry 2015, 114, 16–17. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention Inc. USP NSP39-NF34, Maryland, USA, 2016; pp. 6641-6647.
- Galappaththi, M.C.A.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D. Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A review of Ganoderma triterpenoids and their bioactivities. Biomolecules 2023, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Nishitoba, T.; Sato, H.; Oda, K.; Sakamura, S. Novel mycelial components, ganoderic acid Mg, Mh, Mi, Mj and Mk, from the fungus Ganoderma lucidum. Agric. Biol. Chem. 1987, 51, 1149–1153. [Google Scholar]
- Hu, G.S.; Zhai, M.H.; Niu, R.; Xu, X.Q.; Liu, Q.; Jia, J.M. Optimization of culture condition for ganoderic acid production in Ganoderma lucidum liquid static culture and design of a suitable bioreactor. Molecules 2018, 23, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Zhao, W.; Zhong, J.J. Biotechnological production and application of ganoderic acids. Appl. Microbiol. Biotechnol. 2010, 87, 457–466. [Google Scholar] [CrossRef] [PubMed]
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| No. | |||||
| 1 | 30.6 (t) | 37.1 (t) | 31.2 (t) | 37.1 (t) | 30.8 (t) |
| 2 | 23.1 (t) | 28.3 (t) | 26.7 (t) | 28.5 (t) | 27.6 (t) |
| 3 | 78.1 (d) | 79.6 (d) | 76.8 (d) | 79.6 (d) | 217.9 (s) |
| 4 | 36.5 (s) | 38.6 (s) | 38.4 (s) | 38.6 (s) | 36.9 (s) |
| 5 | 44.0 (d) | 50.7 (d) | 44.3 (d) | 50.5 (d) | 51.2 (d) |
| 6 | 22.8 (t) | 24.1 (t) | 24.0 (t) | 24.0 (t) | 26.3 (t) |
| 7 | 121.2 (d) | 121.6 (d) | 122.6 (d) | 122.6 (d) | 19.4 (t) |
| 8 | 140.1 (s) | 143.9 (s) | 142.4 (s) | 141.7 (s) | 133.3 (s) |
| 9 | 145.9 (s) | 147.5 (s) | 147.9 (s) | 147.4 (s) | 135.1 (s) |
| 10 | 37.3 (s) | 39.8 (s) | 38.5 (s) | 39.8 (s) | 44.5 (s) |
| 11 | 115.6 (d) | 117.4 (d) | 116.7 (d) | 117.4 (d) | 21.0 (t) |
| 12 | 38.0 (t) | 39.1 (t) | 39.8 (t) | 39.3 (t) | 31.0 (t) |
| 13 | 43.9 (s) | 44.8 (s) | 45.4 (s) | 45.1 (s) | 47.4 (s) |
| 14 | 51.4 (s) | 51.5 (s) | 53.4 (s) | 52.6 (s) | 49.9 (s) |
| 15 | 77.3 (d) | 32.6 (t) | 75.2 (d) | 78.8 (d) | 36.0 (t) |
| 16 | 36.4 (t) | 28.5 (t) | 39.9 (t) | 37.4 (t) | 35.2 (t) |
| 17 | 45.2 (d) | 48.6 (d) | 46.4 (d) | 46.5 (d) | 46.8 (d) |
| 18 | 15.9 (q) | 16.2 (q) | 16.5 (q) | 16.4 (q) | 15.8 (q) |
| 19 | 22.6 (q) | 23.3 (q) | 23.3 (q) | 23.3 (q) | 24.2 (q) |
| 20 | 40.8 (d) | 42.4 (d) | 42.3 (d) | 42.1 (d) | 41.4 (d) |
| 21 | 11.5 (q) | 12.1 (q) | 12.2 (q) | 12.1 (q) | 11.7 (q) |
| 22 | 72.5 (d) | 73.4 (d) | 73.4 (d) | 73.2 (d) | 72.8 (d) |
| 23 | 35.2 (t) | 35.8 (t) | 35.8 (t) | 35.7 (t) | 34.6 (t) |
| 24 | 141.0 (d) | 141.0 (d) | 141.0 (d) | 140.8 (d) | 151.3 (d) |
| 25 | 128.9 (s) | 130.1 (s) | 130.1 (s) | 130.2 (s) | 140.6 (s) |
| 26 | 171.3 (s) | 171.7 (s) | 171.5 (s) | 173.1 (s) | 195.2 (d) |
| 27 | 12.4 (q) | 12.8 (q) | 12.7 (q) | 12.8 (q) | 9.5 (q) |
| 28 | 18.5 (q) | 26.2 (q) | 18.0 (q) | 18.9 (q) | 18.7 (q) |
| 29 | 27.8 (q) | 28.8 (q) | 28.9 (q) | 28.8 (q) | 26.2 (q) |
| 30 | 22.4 (q) | 16.5 (q) | 23.4 (q) | 16.5 (q) | 21.3 (q) |
| OAc | 170.8 (s) | 171.6 (s) | |||
| OAc | 170.8 (s) | ||||
| OCOCH3 | 21.3 (q) | 21.2 (q) | |||
| OCOCH3 | 21.4 (q) |
 |
| Linear regression calibration curves | ||||||||
| 10 | 12 | |||||||
| R2 | 0.9984 | 0.9995 | ||||||
| Linear range (g/mL) | 25-3000 | 25-1000 | ||||||
| LOD (Limit of Detection, g/mL) | 2.2 | 2.1 | ||||||
| LOQ (Limit of Quantitation, g/mL) | 6.4 | 6.4 | ||||||
| Intraday and Interday precision | ||||||||
| Concentration (g/mL) | Mean ± SD (RSD, %) | |||||||
| 10 | 12 | |||||||
| Intraday | Interday | Intraday | Interday | |||||
| 100.0 | 101.8 ± 0.15 (0.15) | 100.7 ± 0.06 (0.06) | 101.8 ± 0.42 (0.41) | 100.9 ± 0.11 (0.11) | ||||
| 400.0 | 406.9 ± 1.63 (0.40) | 403.6 ± 0.26 (0.06) | 407.4 ± 0.48 (0.12) | 401.9 ± 0.36 (0.09) | ||||
| 800.0 | 813.8 ± 0.36 (0.04) | 808.0 ± 0.72 (0.09) | 814.7 ± 1.74 (0.21) | 803.9 ± 3.55 (0.44) | ||||
| Recovery tests | ||||||||
| amount added (g/mL) | amount measured (g/mL) , Mean ± SD |
Recovery (%) | RSD (%) | |||||
| 10 | 12 | 10 | 12 | 10 | 12 | |||
| 0 (placebo) | 1216.0 ± 9.60a | 368.1 ± 3.63a | ||||||
| 100.0 | 1322.3 ± 2.39b | 473.6 ± 1.58b | 106.3 | 105.5 | 2.25 | 1.50 | ||
| 400.0 | 1636.7 ± 6.78b | 777.0 ± 11.35b | 105.2 | 102.2 | 1.61 | 2.78 | ||
| 800.0 | 2067.3 ± 13.14b | 1139.3 ± 5.91b | 106.4 | 96.4 | 1.54 | 0.77 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




