Submitted:
30 August 2023
Posted:
01 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Neutropenia with Reduced Bone Marrow Reserve
2.1. Cyclic Neutropenia
2.2. Shwachman-Diamond Syndrome
2.3. Kostmann Syndrome
2.4. Chédiak–Higashi Syndrome
2.5. Myelokathexis
2.6. Reticular Dysgenesis
2.7. Dyskeratosis Congenita
3. Secondary Neutropenia with Reduced Bone Marrow Reserve
3.1. Drug-Induced Neutropenia
3.2. T Cell Large Granular Lymphocytic Leukemia
3.3. Nutritional Deficiency
3.4. Viral Infections
4. Neutropenia with Normal Bone Marrow Reserve
4.1. Chronic Benign Neutropenia of Infancy and Childhood
4.2. Non-Immune Chronic Benign Neutropenia
4.3. Benign Familial Neutropenia
4.4. Autoimmune Neutropenia
4.4.1. Primary Autoimmune Neutropenia
4.4.2. Secondary Autoimmune Neutropenia
4.5. Alloimmune Neutropenia
4.6. Drug-Induced Neutropenia (Antibody-Mediated)
4.7. Infection-Related Neutropenia (Antibody-Mediated)
4.8. Hypersplenism
4.9. Maternal Hypertension
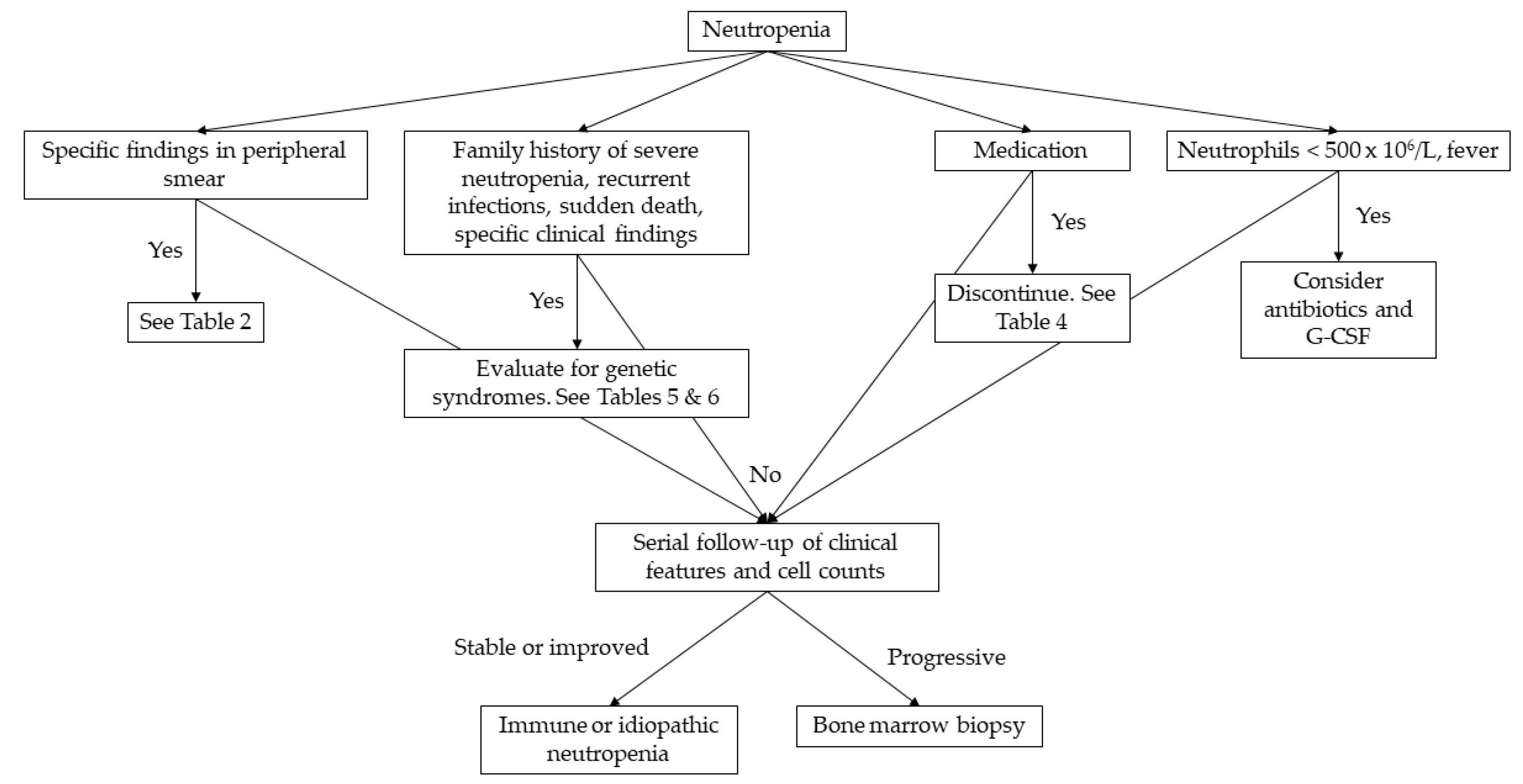
5. Diagnostic Approach to Neutropenia
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Celkan, T.; Koc, B.S. Approach to the Patient with Neutropenia in Childhood. Türk Pediatr. Arşivi 2015, 50, 136–144. [Google Scholar] [CrossRef]
- Boxer, L.; Dale, D.C. Neutropenia: Causes and Consequences. Semin. Hematol. 2002, 39, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.; Saffarzadeh, M.; Weber, A.N.R.; Rieber, N.; Radsak, M.; von Bernuth, H.; Benarafa, C.; Roos, D.; Skokowa, J.; Hartl, D. Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury. PLoS Pathog. 2015, 11, e1004651. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.L. How I Investigate Neutropenia. Int. J. Lab. Hematol. 2020, 42 Suppl 1, 121–132. [Google Scholar] [CrossRef]
- Denic, S.; Showqi, S.; Klein, C.; Takala, M.; Nagelkerke, N.; Agarwal, M.M. Prevalence, Phenotype and Inheritance of Benign Neutropenia in Arabs. BMC Blood Disord. 2009, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Haddy, T.B.; Rana, S.R.; Castro, O. Benign Ethnic Neutropenia: What Is a Normal Absolute Neutrophil Count? J. Lab. Clin. Med. 1999, 133, 15–22. [Google Scholar] [CrossRef]
- Walkovich, K.; Boxer, L.A. How to Approach Neutropenia in Childhood. Pediatr. Rev. 2013, 34, 173–184. [Google Scholar] [CrossRef]
- Dale, D.C.; Bolyard, A.A.; Aprikyan, A. Cyclic Neutropenia. Semin. Hematol. 2002, 39, 89–94. [Google Scholar] [CrossRef]
- Fink-Puches, R.; Kainz, J.T.; Kahr, A.; Urban, C.; Smolle, J.; Kerl, H. Granulocyte Colony-Stimulating Factor Treatment of Cyclic Neutropenia with Recurrent Oral Aphthae. Arch. Dermatol. 1996, 132, 1399–1400. [Google Scholar] [CrossRef]
- Hammond, W.P.; Price, T.H.; Souza, L.M.; Dale, D.C. Treatment of Cyclic Neutropenia with Granulocyte Colony-Stimulating Factor. N. Engl. J. Med. 1989, 320, 1306–1311. [Google Scholar] [CrossRef]
- Coccia, P.; Ruggiero, A.; Attinà, G.; Maurizi, P.; Lazzareschi, I.; Molinari, F.; Riccardi, R.; Ruggiero, A. Shwachman Diamond Syndrome: An Emergency Challenge. Signa Vitae 2007, 2, 10. [Google Scholar] [CrossRef]
- Dror, Y.; Freedman, M.H. Shwachman-Diamond Syndrome. Br. J. Haematol. 2002, 118, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Lyu, B.; Lyu, W.; Zhang, X. Kostmann Syndrome With Neurological Abnormalities: A Case Report and Literature Review. Front. Pediatr. 2020, 8, 586859. [Google Scholar] [CrossRef] [PubMed]
- Roques, G.; Munzer, M.; Barthez, M.-A.C.; Beaufils, S.; Beaupain, B.; Flood, T.; Keren, B.; Bellanné-Chantelot, C.; Donadieu, J. Neurological Findings and Genetic Alterations in Patients with Kostmann Syndrome and HAX1 Mutations. Pediatr. Blood Cancer 2014, 61, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Donadieu, J.; Fenneteau, O.; Beaupain, B.; Mahlaoui, N.; Chantelot, C. Congenital Neutropenia: Diagnosis, Molecular Bases and Patient Management. Orphanet J. Rare Dis. 2011, 6, 26. [Google Scholar] [CrossRef]
- Ajitkumar, A.; Yarrarapu, S.; Ramphul, K. Higashi Syndrome. [Updated 2023 Feb 13] Available online:. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507881/ (accessed on 6 July 2023).
- Aprikyan, A.A.G.; Liles, W.C.; Park, J.R.; Jonas, M.; Chi, E.Y.; Dale, D.C. Myelokathexis, a Congenital Disorder of Severe Neutropenia Characterized by Accelerated Apoptosis and Defective Expression Ofbcl-x in Neutrophil Precursors. Blood 2000, 95, 320–327. [Google Scholar] [CrossRef]
- Hoenig, M.; Lagresle-Peyrou, C.; Pannicke, U.; Notarangelo, L.D.; Porta, F.; Gennery, A.R.; Slatter, M.; Cowan, M.J.; Stepensky, P.; Al-Mousa, H.; et al. Reticular Dysgenesis: International Survey on Clinical Presentation, Transplantation, and Outcome. Blood 2017, 129, 2928–2938. [Google Scholar] [CrossRef]
- Hauck, F.; Klein, C. Pathogenic Mechanisms and Clinical Implications of Congenital Neutropenia Syndromes. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 596–606. [Google Scholar] [CrossRef]
- Moore, D.C. Drug-Induced Neutropenia: A Focus on Rituximab-Induced Late-Onset Neutropenia. P T 2016, 41, 765–768. [Google Scholar]
- Rose, M.G.; Berliner, N. T-Cell Large Granular Lymphocyte Leukemia and Related Disorders. Oncologist 2004, 9, 247–258. [Google Scholar] [CrossRef]
- Newburger, P.E.; Dale, D.C. Evaluation and Management of Patients with Isolated Neutropenia. Semin. Hematol. 2013, 50, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.Y.; Varma, C.P. V; Bhatt, S. An Infant with Chronic Severe Neutropenia. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef]
- Papadaki, H.A.; Charoulakis, N.Z.; Eliopoulos, D.G.; Psyllaki, M.; Eliopoulos, G.D. Patients with Non-Immune Chronic Idiopathic Neutropenia Syndrome Have Increased Splenic Volume on Ultrasonography. Clin. Lab. Haematol. 2001, 23, 111–117. [Google Scholar] [CrossRef]
- Autrel-Moignet, A.; Lamy, T. Autoimmune Neutropenia. Presse Med. 2014, 43, e105–e118. [Google Scholar] [CrossRef] [PubMed]
- Dale, D.C. How I Manage Children with Neutropenia. Br. J. Haematol. 2017, 178, 351–363. [Google Scholar] [CrossRef]
- Marks, P.W. Hematologic Manifestations of Liver Disease. Semin. Hematol. 2013, 50, 216–221. [Google Scholar] [CrossRef]
- Christensen, R.D.; Yoder, B.A.; Baer, V.L.; Snow, G.L.; Butler, A. Early-Onset Neutropenia in Small-for-Gestational-Age Infants. Pediatrics 2015, 136, e1259–67. [Google Scholar] [CrossRef]
- Mouzinho, A.; Rosenfeld, C.R.; Sanchez, P.J.; Risser, R. Effect of Maternal Hypertension on Neonatal Neutropenia and Risk of Nosocomial Infection. Pediatrics 1992, 90, 430–435. [Google Scholar] [CrossRef]
- Gosselin, R.C.; Adcock, D.; Dorgalaleh, A.; Favaloro, E.J.; Lippi, G.; Pego, J.M.; Regan, I.; Siguret, V. International Council for Standardization in Haematology Recommendations for Hemostasis Critical Values, Tests, and Reporting. Semin. Thromb. Hemost. 2020, 46, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.; Julia, N. Hematology. In The Harriet Lane Handbook; Tschudy, M.M., Arcara, K.M., Eds.; Elsevier Mosby: Philadelphia, 2012; p. 333. [Google Scholar]
- Andrès, E.; Mourot-Cottet, R.; Maloisel, F.; Séverac, F.; Keller, O.; Vogel, T.; Tebacher, M.; Weber, J.-C.; Kaltenbach, G.; Gottenberg, J.-E.; et al. Idiosyncratic Drug-Induced Neutropenia & Agranulocytosis. QJM 2017, 110, 299–305. [Google Scholar] [CrossRef]
- Huber, M.; Andersohn, F.; Bronder, E.; Klimpel, A.; Thomae, M.; Konzen, C.; Meyer, O.; Salama, A.; Schrezenmeier, H.; Hildebrandt, M.; et al. Drug-Induced Agranulocytosis in the Berlin Case-Control Surveillance Study. Eur. J. Clin. Pharmacol. 2014, 70, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Casique, N.; Tong, H.Y.; Borobia, A.M.; Carcas, A.J.; Frías, J.; Ramírez, E. Non-Chemotherapy-Induced Agranulocytosis Detected by a Prospective Pharmacovigilance Program in a Tertiary Hospital. Basic Clin. Pharmacol. Toxicol. 2015, 117, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.R. Non-Chemotherapy Drug-Induced Neutropenia: Key Points to Manage the Challenges. Hematol. Am. Soc. Hematol. Educ. Progr. 2017, 2017, 187–193. [Google Scholar] [CrossRef]
- Donadieu, J.; Beaupain, B.; Fenneteau, O.; Bellanné-Chantelot, C. Congenital Neutropenia in the Era of Genomics: Classification, Diagnosis, and Natural History. Br. J. Haematol. 2017, 179, 557–574. [Google Scholar] [CrossRef]
- National Institute of Allergy and Infectious Diseases. Congenital Neutropenia Syndromes. Available online: https://www.niaid.nih.gov/diseases-conditions/congenital-neutropenia-syndromes (accessed on 12 June 2023).
- Wang, J.; Zhang, H.; Wang, Y.; Liang, L.; Yang, Z. Severe Congenital Neutropenia Caused by ELANE Gene Mutation: A Case Report and Literature Review. Medicine (Baltimore). 2022, 101, e31357. [Google Scholar] [CrossRef] [PubMed]
- ELANE Elastase, Neutrophil Expressed [ Homo Sapiens (Human) ] Gene ID: 1991. Available online: https://www.ncbi.nlm.nih.gov/gene/1991 (accessed on 15 May 2023).
- Horwitz, M.; Benson, K.F.; Person, R.E.; Aprikyan, A.G.; Dale, D.C. Mutations in ELA2, Encoding Neutrophil Elastase, Define a 21-Day Biological Clock in Cyclic Haematopoiesis. Nat. Genet. 1999, 23, 433–436. [Google Scholar] [CrossRef]
- Dale, D.C.; Person, R.E.; Bolyard, A.A.; Aprikyan, A.G.; Bos, C.; Bonilla, M.A.; Boxer, L.A.; Kannourakis, G.; Zeidler, C.; Welte, K.; et al. Mutations in the Gene Encoding Neutrophil Elastase in Congenital and Cyclic Neutropenia. Blood 2000, 96, 2317–2322. [Google Scholar] [CrossRef]
- Triot, A.; Järvinen, P.M.; Arostegui, J.I.; Murugan, D.; Kohistani, N.; Dapena Díaz, J.L.; Racek, T.; Puchałka, J.; Gertz, E.M.; Schäffer, A.A.; et al. Inherited Biallelic CSF3R Mutations in Severe Congenital Neutropenia. Blood 2014, 123, 3811–3817. [Google Scholar] [CrossRef]
- Devriendt, K.; Kim, A.S.; Mathijs, G.; Frints, S.G.; Schwartz, M.; Van Den Oord, J.J.; Verhoef, G.E.; Boogaerts, M.A.; Fryns, J.P.; You, D.; et al. Constitutively Activating Mutation in WASP Causes X-Linked Severe Congenital Neutropenia. Nat. Genet. 2001, 27, 313–317. [Google Scholar] [CrossRef]
- Auer, P.L.; Teumer, A.; Schick, U.; O’Shaughnessy, A.; Lo, K.S.; Chami, N.; Carlson, C.; de Denus, S.; Dubé, M.-P.; Haessler, J.; et al. Rare and Low-Frequency Coding Variants in CXCR2 and Other Genes Are Associated with Hematological Traits. Nat. Genet. 2014, 46, 629–634. [Google Scholar] [CrossRef]
- Boocock, G.R.B.; Morrison, J.A.; Popovic, M.; Richards, N.; Ellis, L.; Durie, P.R.; Rommens, J.M. Mutations in SBDS Are Associated with Shwachman-Diamond Syndrome. Nat. Genet. 2003, 33, 97–101. [Google Scholar] [CrossRef]
- Stepensky, P.; Chacón-Flores, M.; Kim, K.H.; Abuzaitoun, O.; Bautista-Santos, A.; Simanovsky, N.; Siliqi, D.; Altamura, D.; Méndez-Godoy, A.; Gijsbers, A.; et al. Mutations in EFL1, an SBDS Partner, Are Associated with Infantile Pancytopenia, Exocrine Pancreatic Insufficiency and Skeletal Anomalies in AShwachman-Diamond like Syndrome. J. Med. Genet. 2017, 54, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Dickinson, R.; Bigley, V. Haematopoietic and Immune Defects Associated with GATA2 Mutation. Br. J. Haematol. 2015, 169, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Boztug, K.; Appaswamy, G.; Ashikov, A.; Schäffer, A.A.; Salzer, U.; Diestelhorst, J.; Germeshausen, M.; Brandes, G.; Lee-Gossler, J.; Noyan, F.; et al. A Syndrome with Congenital Neutropenia and Mutations in G6PC3. N. Engl. J. Med. 2009, 360, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Veiga-da-Cunha, M.; Gerin, I.; Chen, Y.T.; Lee, P.J.; Leonard, J. V; Maire, I.; Wendel, U.; Vikkula, M.; Van Schaftingen, E. The Putative Glucose 6-Phosphate Translocase Gene Is Mutated in Essentially All Cases of Glycogen Storage Disease Type I Non-A. Eur. J. Hum. Genet. 1999, 7, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Barth, P.G.; Wanders, R.J.; Vreken, P.; Janssen, E.A.; Lam, J.; Baas, F. X-Linked Cardioskeletal Myopathy and Neutropenia (Barth Syndrome) (MIM 302060). J. Inherit. Metab. Dis. 1999, 22, 555–567. [Google Scholar] [CrossRef]
- Gorlin, R.J.; Gelb, B.; Diaz, G.A.; Lofsness, K.G.; Pittelkow, M.R.; Fenyk, J.R. WHIM Syndrome, an Autosomal Dominant Disorder: Clinical, Hematological, and Molecular Studies. Am. J. Med. Genet. 2000, 91, 368–376. [Google Scholar] [CrossRef]
- Boztug, K.; Järvinen, P.M.; Salzer, E.; Racek, T.; Mönch, S.; Garncarz, W.; Gertz, E.M.; Schäffer, A.A.; Antonopoulos, A.; Haslam, S.M.; et al. JAGN1 Deficiency Causes Aberrant Myeloid Cell Homeostasis and Congenital Neutropenia. Nat. Genet. 2014, 46, 1021–1027. [Google Scholar] [CrossRef]
- Kolehmainen, J.; Black, G.C.M.; Saarinen, A.; Chandler, K.; Clayton-Smith, J.; Träskelin, A.-L.; Perveen, R.; Kivitie-Kallio, S.; Norio, R.; Warburg, M.; et al. Cohen Syndrome Is Caused by Mutations in a Novel Gene, COH1, Encoding a Transmembrane Protein with a Presumed Role in Vesicle-Mediated Sorting and Intracellular Protein Transport. Am. J. Hum. Genet. 2003, 72, 1359–1369. [Google Scholar] [CrossRef]
- Person, R.E.; Li, F.-Q.; Duan, Z.; Benson, K.F.; Wechsler, J.; Papadaki, H.A.; Eliopoulos, G.; Kaufman, C.; Bertolone, S.J.; Nakamoto, B.; et al. Mutations in Proto-Oncogene GFI1 Cause Human Neutropenia and Target ELA2. Nat. Genet. 2003, 34, 308–312. [Google Scholar] [CrossRef]
- Kostmann, R. Infantile Genetic Agranulocytosis; Agranulocytosis Infantilis Hereditaria. Acta Paediatr. Suppl. 1956, 45, 1–78. [Google Scholar] [CrossRef]
- Klein, C.; Grudzien, M.; Appaswamy, G.; Germeshausen, M.; Sandrock, I.; Schäffer, A.A.; Rathinam, C.; Boztug, K.; Schwinzer, B.; Rezaei, N.; et al. HAX1 Deficiency Causes Autosomal Recessive Severe Congenital Neutropenia (Kostmann Disease). Nat. Genet. 2007, 39, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Huizing, M.; Scher, C.D.; Strovel, E.; Fitzpatrick, D.L.; Hartnell, L.M.; Anikster, Y.; Gahl, W.A. Nonsense Mutations in ADTB3A Cause Complete Deficiency of the Beta3A Subunit of Adaptor Complex-3 and Severe Hermansky-Pudlak Syndrome Type 2. Pediatr. Res. 2002, 51, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Bohn, G.; Allroth, A.; Brandes, G.; Thiel, J.; Glocker, E.; Schäffer, A.A.; Rathinam, C.; Taub, N.; Teis, D.; Zeidler, C.; et al. A Novel Human Primary Immunodeficiency Syndrome Caused by Deficiency of the Endosomal Adaptor Protein P14. Nat. Med. 2007, 13, 38–45. [Google Scholar] [CrossRef]
- Volpi, L.; Roversi, G.; Colombo, E.A.; Leijsten, N.; Concolino, D.; Calabria, A.; Mencarelli, M.A.; Fimiani, M.; Macciardi, F.; Pfundt, R.; et al. Targeted Next-Generation Sequencing Appoints C16orf57 as Clericuzio-Type Poikiloderma with Neutropenia Gene. Am. J. Hum. Genet. 2010, 86, 72–76. [Google Scholar] [CrossRef]
- Vilboux, T.; Lev, A.; Malicdan, M.C. V; Simon, A.J.; Järvinen, P.; Racek, T.; Puchalka, J.; Sood, R.; Carrington, B.; Bishop, K.; et al. A Congenital Neutrophil Defect Syndrome Associated with Mutations in VPS45. N. Engl. J. Med. 2013, 369, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Makaryan, V.; Rosenthal, E.A.; Bolyard, A.A.; Kelley, M.L.; Below, J.E.; Bamshad, M.J.; Bofferding, K.M.; Smith, J.D.; Buckingham, K.; Boxer, L.A.; et al. TCIRG1-Associated Congenital Neutropenia. Hum. Mutat. 2014, 35, 824–827. [Google Scholar] [CrossRef]
- Delépine, M.; Nicolino, M.; Barrett, T.; Golamaully, M.; Lathrop, G.M.; Julier, C. EIF2AK3, Encoding Translation Initiation Factor 2-Alpha Kinase 3, Is Mutated in Patients with Wolcott-Rallison Syndrome. Nat. Genet. 2000, 25, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.; Smith, L.; Wibrand, F.; Ravn, K.; Bross, P.; Thiffault, I.; Christensen, M.; Atherton, A.; Farrow, E.; Miller, N.; et al. CLPB Variants Associated with Autosomal-Recessive Mitochondrial Disorder with Cataract, Neutropenia, Epilepsy, and Methylglutaconic Aciduria. Am. J. Hum. Genet. 2015, 96, 258–265. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Ziętkiewicz, S.; Kousi, M.; Szklarczyk, R.; Haack, T.B.; Gersting, S.W.; Muntau, A.C.; Rakovic, A.; Renkema, G.H.; Rodenburg, R.J.; et al. CLPB Mutations Cause 3-Methylglutaconic Aciduria, Progressive Brain Atrophy, Intellectual Disability, Congenital Neutropenia, Cataracts, Movement Disorder. Am. J. Hum. Genet. 2015, 96, 245–257. [Google Scholar] [CrossRef]
- Abdollahpour, H.; Appaswamy, G.; Kotlarz, D.; Diestelhorst, J.; Beier, R.; Schäffer, A.A.; Gertz, E.M.; Schambach, A.; Kreipe, H.H.; Pfeifer, D.; et al. The Phenotype of Human STK4 Deficiency. Blood 2012, 119, 3450–3457. [Google Scholar] [CrossRef] [PubMed]
- Witzel, M.; Petersheim, D.; Fan, Y.; Bahrami, E.; Racek, T.; Rohlfs, M.; Puchałka, J.; Mertes, C.; Gagneur, J.; Ziegenhain, C.; et al. Chromatin-Remodeling Factor SMARCD2 Regulates Transcriptional Networks Controlling Differentiation of Neutrophil Granulocytes. Nat. Genet. 2017, 49, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.P.; Rosenberg, P.S.; Brody, L.C. Clinical and Molecular Features Associated with Biallelic Mutations in FANCD1/BRCA2. J. Med. Genet. 2007, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Age | WBC Mean (95% CI) |
Neu* Mean (95% CI) [%] |
Lymph Mean (95% CI) [%] |
Mono Mean [%] |
Eos Mean [%] |
|---|---|---|---|---|---|
| Birth | 18100 (9000-30000) | 11000 (6000-26000) [61] | 5500 (2000-11000) [31] | 1100 [6] | 400 [2] |
| 12 hr | 22800 (13000-38000) | 15500 (6000-28000) [68] | 5501 (2000-11000) [24] | 1200 [5] | 500 [2] |
| 24 hr | 18900 (9400-34000) | 11500 (5000-21000) [61] | 5800 (2000-11500) [31] | 1100 [6] | 500 [2] |
| 1 wk | 12200 (5000-21000) | 5500 (1500-10000) [45] | 5000 (2000-17000) [41] | 1100 [9] | 500 [4] |
| 2 wk | 11400 (5000-20000) | 4500 (1000-9500) [40] | 5500 (2000-17000) [48] | 1000 [9] | 400 [3] |
| 1 mo | 10800 (5000-19500) | 3800 (1000-8500) [35] | 6000 (2500-16500) [56] | 700 [7] | 300 [3] |
| 6 mo | 11900 (6000-17500) | 3800 (1000-8500) [32] | 7300 (4000-13500) [61] | 600 [5] | 300 [3] |
| 1 yr | 11400 (6000-17500) | 3500 (1500-8500) [31] | 7000 (4000-10500) [61] | 600 [5] | 300 [3] |
| 2 yr | 10600 (6000-17000) | 3500 (1500-8500) [33] | 6300 (3000-9500) [59] | 500 [5] | 300 [3] |
| 4 yr | 9100 (5500-15500) | 3800 (1500-8500) [42] | 4500 (2000-8000) [50] | 500 [5] | 300 [3] |
| 6 yr | 8500 (5000-14500) | 4300 (1500-8000) [51] | 3500 (1500-7000) [42] | 400 [5] | 200 [3] |
| 8 yr | 8300 (4500-13500) | 4400 (1500-8000) [53] | 3300 (1500-6800) [39] | 400 [4] | 200 [2] |
| 10 yr | 8100 (4500-13500) | 4400 (1500-8500) [54] | 3100 (1500-6500) [38] | 400 [4] | 200 [2] |
| 16 yr | 7800 (4500-13000) | 4400 (1800-8000) [57] | 2800 (1200-5200) [35] | 400 [5] | 200 [3] |
| 21 yr | 7400 (4500-11000) | 4400 (1800-7700) [59] | 2500 (1000-4800) [34] | 300 [4] | 200 [3] |
| Finding | Disorders |
|---|---|
| Blasts | Leukemia |
| Nucleated erythrocytes | Hemolytic anemia, blood loss |
| Hypersegmented neutrophils | Vitamin B12 deficiency, folic acid deficiency |
| Neutrophils with pycnotic nuclei | Myelokathexis |
| Neonate | Infant/ Child | Adult |
|---|---|---|
| Infection | Infection | Idiosyncratic drug reactions |
| Maternal hypertension | Autoimmune neutropenia | Infections |
| Maternal antibodies | Neoplasms replacing the bone marrow | Neoplasms replacing the bone marrow |
| Constitutional neutropenia disorders | Idiosyncratic drug reactions | Myeloblastic therapies |
| Cyclic neutropenia | Collagen vascular disorders | Collagen vascular disorders |
| Kostmann syndrome | Immunodeficiency disorders | Immunodeficiency disorders |
| Chédiak–Higashi syndrome | Myeloablative therapies | |
| Constitutional neutropenia disorders | ||
| Megaloblastic anemia | ||
| Copper deficiency |
| Medications | |
|---|---|
| Amoxicillin | Metronidazole |
| Benzylpenicillin | Noramidopyrine |
| Carbamazepine | Piperacillin-tazobactam |
| Carbimazole | Quetiapine |
| Cefepime | Salazopyrine |
| Cefotaxime | Sulfamethoxazole/trimethoprim |
| Ceftriaxone | Sulfasalazine |
| Ciprofloxacin | Tacrolimus |
| Clindamycin | Teicoplanin |
| Clozapine | Thiamazole |
| Cotrimoxazole | Ticlopidine |
| Ibuprofen | Tobramycin |
| Levetiracetam | Torsemide |
| Linezolid | Valganciclovir |
| Meropenem | Vancomycin |
| Metamizole (dipyrone) | Venlafaxine |
| Gene | Inheritance/ location | Syndromes |
|---|---|---|
| ELANE [40,41] | Dominant/ 19q13.3 | Severe congenital neutropenia, cyclic neutropenia |
| CSF3R [42] | Dominant/ 1p35-p34.3 | Germline mutation of CSF3R |
| WAS [43] | X Linked/ Xp11.4-p11.21 | Severe congenital neutropenia |
| CXCR2 [44] | Recessive/ 2q35 | Chronic neutropenia |
| SBDS [45] | Recessive/ 7q11.22 | Shwachman-Diamond syndrome |
| EFL1 [46] | Recessive/ 15q25.2 | EFL1 syndrome |
| GATA2 [47] | Dominant/ 3q21.3 | GATA2 syndrome |
| G6PC3 [48] | Recessive/ 17q21 | Severe congenital neutropenia |
| SLC37A4 [49] | Recessive/ 11q23.3 | Glycogen storage type Ib |
| TAZ [50] | X Linked/ Xq28 | Barth Disease |
| CXCR4 [51] | Dominant/ 2q21 | WHIM syndrome |
| JAGN1 [52] | Recessive/ 3p25.3 | Severe congenital neutropenia |
| VPS13B [53] | Recessive/ 8q22-q23 | Cohen syndrome |
| GFI1 [54] | Dominant/ 1p22 | Severe congenital neutropenia |
| HAX1 [55,56] | Recessive/ 1q21.3 | Kostmann disease |
| AP3B1 [57] | Recessive/ 5q14.1 | Hermansky–Pudlak syndrome type 2 |
| LAMTOR2 [58] | Recessive/ 1q21 | Chronic neutropenia |
| USB1 [59] | Recessive/ 16q21 | Poikiloderma type Clericuzio |
| VPS45 [60] | Recessive/ 1q21.2 | Severe congenital neutropenia |
| TCIRG1 [61] | Dominant/ 11q13.2 | Severe congenital neutropenia |
| EIF2AK3 [62] | Recessive/ 2p11.2 | EIF2AK3/ Wolcott-Rallison syndrome |
| CLPB [63,64] | Recessive/ 11q13.4 | CLPB syndrome |
| STK4 [65] | Recessive/ 20q13 | STK4 (MST1) syndrome |
| SMARCD2 [66] | Recessive/ 17q23 | SMARCD2 |
| System | Findings | Disorders |
|---|---|---|
| Eyes | Congenital cataract | CLPB syndrome Charcot-Marie-Tooth |
| Retinochoroidal dystrophy | Cohen disease | |
| Heart | Arrythmias | G6PC3 Neutropenia |
| Dilated cardiomyopathy | Barth disease | |
| Cardiomyopathy | Shwachman-Diamond syndrome | |
| Various cardiac abnormalities | Shwachman-Diamond syndrome WHIM syndrome (Tetralogy Fallot) Neutropenia G6PC3 STK4 (MST1) deficiency |
|
| Skin | Skin xerosis eczema | Shwachman-Diamond syndrome |
| Prominent superficial veins | G6PC3 Neutropenia | |
| Poikiloderma | SCN with poikiloderma type Clericuzio | |
| Partial or complete albinism | Hermansky Pudlak type 2 AP14 defect Chédiak–Higashi disease Griscelli disease |
|
| Fine, sparse, and light-coloured hair | Cartilage hair hypoplasia GATA2 | |
| Lymphoedema | GATA2 syndrome | |
| Skin angiomatosis | TCIRG1 SCN | |
| Petechiae (thrombocytopenia) | Shwachman-Diamond syndrome GATA2 syndrome |
|
| Hyperpigmentation on the trunk, neck, and intertriginous areas, café au lait spots, and hypopigmented areas | Fanconi Anemia | |
| Musculoskeletal system | Weakness | G6PC3 Neutropenia Axonal Charcot-Marie-Tooth disease Shwachman-Diamond syndrome |
| Metaphyseal dysplasia | Shwachman-Diamond syndrome Cartilage-hair hypoplasia |
|
| Facial dysmorphia | Cohen disease | |
| Palatal cleft | Shwachman-Diamond syndrome | |
| Hyperlaxity | Cohen disease | |
| Short stature and various skeletal abnormalities | Fanconi Anemia | |
| Central nervous system | Mental retardation | Kostmann disease |
| Epilepsy | Shwachman-Diamond syndrome Cohen disease CLPB syndrome VPS45 syndrome |
|
| Metabolic system | Type I diabetes | Wolcott-Rallison |
| Fasting intolerance and glycogenesis | Glycogen storage disease type Ib | |
| 3-methyl glucagonic acid | Barth disease CLPB syndrome |
|
| Ear | Inner ear defect | GFI1/severe chronic neutropenia GATA2 syndrome |
| Urogenital system | Uropathy | G6PC3 Neutropenia GATA2 syndrome |
| Cryptorchidism | Cohen disease G6PC3 Neutropenia |
|
| Nephromegaly | VPS45 syndrome | |
| Findings of non-bacterial infections | HPV | WHIM syndrome GATA2 syndrome STK4 deficiency |
| Mycobacterial | GATA2 syndrome WHIM syndrome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





